Abstract
Background.
The aim of this paper is to present a clinical case of laparoscopic repair of a uterine scar defect, to assess the effectiveness of treatment reviewing the latest literature sources, and to provide recommendations of uterine scar defect management.
Materials and methods.
We report the case of a 33-year-old woman with an insufficient uterine scar and one-year history of secondary infertility. Following this, she underwent corrective laparoscopic repair, successfully got pregnant two months later and carried pregnancy to full term. We discuss the prevalence of caesarean scar defects, their clinical symptoms, diagnostic methods, various treatment techniques, and their outcomes.
Results and conclusion.
Caesarean scar defects, insufficient uterine scars, isthmocele or scar dehiscence following a caesarean section involve myometrial discontinuity at the site of a scar previous caesarean section. These anatomical defects associated with prolonged menstrual bleeding, chronic pelvic pain, dysmenorrhea, dyspareunia and secondary infertility. Laparoscopic repair of the uterine scar defect is an effective method of treatment of secondary infertility. Patients with a previous history of caesarean section who present complaints of secondary infertility, need a detailed evaluation of the uterine scar before planning future pregnancies
Keywords: uterine scar defect, caesarean section, secondary infertility, laparoscopy, Rendezvous technique
Abstract
LAPAROSKOPINIS GIMDOS RANDO DEFEKTO ATSTATYMAS – SĖKMINGAS ANTRINIO NEVAISINGUMO GYDYMO METODAS: KLINIKINIS ATVEJIS IR LITERATŪROS APŽVALGA
Santrauka
Tikslas. Pristatyti pacientės, kuriai buvo atliktas laparoskopinis gimdos rando atstatymas, klinikinį atvejį siekiant įvertinti šio gydymo metodo efektyvumą ir pateikti gimdos rando defekto gydymo rekomendacijas.
Medžiaga ir metodai. Straipsnyje pateikiamas 33 metų amžiaus moters su gimdos rando defektu ir vienų metų antriniu nevaisingumu klinikinis atvejis. Pacientei atliktas laparoskopinis gimdos rando atstatymas, po kurio, praėjus 2 mėnesiams, sėkmingai pastojo ir išnešiojo vaisių iki gimdymo termino. Straipsnyje aptariame gimdos rando defekto dažnį, sukeliamus klinikinius simptomus, diagnostikos metodus, įvairias gydymo taktikas ir jų išeitis.
Rezultatai ir išvados. Gimdos rando defektas, dar įvardijamas kaip gimdos rando nepakankamumas, istmocelė arba rando „prasivėrimas“ - tai miometro nevientisumas, esantis rando po atliktos cezario pjūvio operacijos srityje. Šis anatominis defektas susijęs su ilgomis menstruacijomis, tepliojimais tarp menstruacijų, lėtiniu dubens skausmu, dismenorėja, dispareunija ir antriniu nevaisingumu. Laparoskopinis gimdos rando atstatymas – efektyvus gydymo būdas siekiant atkurti vaisingumą.
Raktažodžiai: gimdos rando defektas, cezario pjūvio operacija, antrinis nevaisingumas, laparoskopija, histeroskopija, Rendezvous technika
INTRODUCTION
Caesarean section is the most frequently performed surgery in obstetrics, which has increased in incidence over the recent decades. The frequency of caesarean section reaches 25.7% in non-developed countries of the world. In developed countries such as Sweden, Italy, or the USA the frequency of caesarean section varies from 16.3% to 38.2% (1, 2). The annual rate of caesarean section in the Obstetrics and Gynaecology Centre of Vilnius University Hospital Santariškių Klinikos is 23.4%. Caesarean section is an inevitable and beneficial operation in many high-risk cases of pregnancy, however, at the same time, it relates to possible complications due to the uterine scar insufficiency (3).
The uterine scar insufficiency is diagnosed in about 1.9% of women who have undergone a caesarean section (4). The uterine scar defect, also called the uterine scar insufficiency, isthmocele, or scar dehiscence (“opening”), is a breakdown of myometrium along the scar defect (3). One of the possible complications of this pathology is secondary infertility (5-7). However, 92% of women successfully get pregnant after endoscopic treatment of the scar defect (8).
The paper presents a clinical case of secondary infertility, resulting from uterine scar insufficiency. Literature review is provided, examining the incidence of uterine scar insufficiency, its symptoms, diagnosis and the strategy of treatment.
A CLINICAL CASE
A 33-year-old female presented with an inability to conceive for one year. She underwent a caesarean section due to labour dystocia six years ago. The laboratory and instrumental tests were performed to find out the reason of infertility. Hormonal blood tests showed no changes (Table).
Table.
Results of hormonal blood tests
| HORMONES | RESULT |
|---|---|
| Follicle-stimulating Hormone (FSH) | 4.62 IU/l |
| Luteinizing Hormone (LH) | 8.54 IU/l |
| Estradiol (E2) | 160.2 pmol/l |
| Prolactin (PRL) | 164.1 mIU/l |
| Testosterone (T) | 1.26 nmol/l |
| Sex Hormone Binding Globulin (SHBG) | 68.96 nmol/l |
| Free Androgen Index (FAI) | 1.83% |
| Thyrotropin (TSH) | 2.77 mIU/l |
A transvaginal sonography revealed both ovaries with no visible pathology, endometrium 9 mm thick. A hysterosalpingography confirmed normal bilateral fallopian tubes and a saddle-shaped uterus. A cervical smear was taken to check against sexually transmitted diseases. Ureaplasma parvum was detected by the PCR method and treated for ten days with doxycycline 100 mg. After the treatment clomiphene citrate was used to stimulate the ovaries, chorionic α- gonadotropin for ovulation induction, and the intrauterine insemination was performed. After this procedure the woman got pregnant, however, she had a miscarriage on the 6th week of pregnancy.
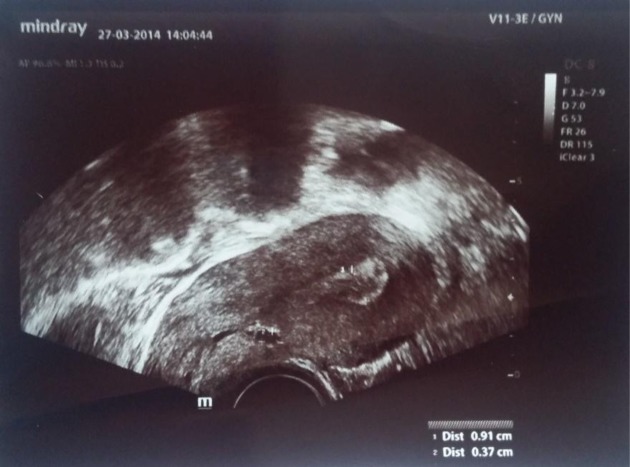
During a repeated transvaginal sonography, the uterine scar of the past caesarean section was evaluated: the endometrial contour at the isthmus was deformed and the anterior uterine wall was deficient up to 0.8 cm, which led to a suspected scar insufficiency (Figure). Hysteroscopic or laparoscopic repair of the scar was recommended.
At first a hysteroscopy was performed and the following was found: at the isthmus of the uterus there was a deepening which could not be fully explored during the hysteroscopy. Then it was decided to perform a laparoscopy and the following was found: uterine adnexa without visible pathology, the uterus of a normal size, a scar of the previous caesarean section visible at the anterior wall of the uterus at the isthmus. The scar was opened with a small incision and the edges were renewed and closed with 2-0 vicryl sutures.
Figure.
Ultrasound evaluation. Scar insufficiency
The woman got pregnant spontaneously two months after surgery. At the beginning of regular contractions on the 38th week of gestation, it was decided to complete the delivery by an emergency caesarean section. The caesarean section was performed under spinal anaesthesia; a male baby of 3490 g weight and 51 cm length was born, with an Apgar score of 9/10. The amniotic fluid was clear. The placenta was attached to the posterior wall and weighed 600 g. The uterus was closed by a double-layer suture. There were no complications during the surgery.
Infusion therapy and narcotic analgesics were prescribed after the surgery. The postoperative period was uneventful. The wound healed in the normal manner. The patient was discharged on the fourth day after the operation.
DISCUSSION
Uterine scar defects associated with abnormal bleeding symptoms have already been mentioned in literature since 1975 (9). In 1995, the obstetrician gynaecologist Morris was the first to describe the uterine scar insufficiency in women after caesarean section, to analyse in detail the anatomical and histological deformities and changes of scar tissue at the isthmus area of the anterior uterine wall (10).
The meta-analysis of delivery after a previous caesarean section found that incidence of uterine scar defect was 1.9% (11). In the other studies the frequency of scar defect varies from 0.6 to 3.8%, depending on diagnostic methods (2, 11, 14).
Risk factors for uterine scar are suturing of myometrium by a one-layer seam, more than one caesarean section, retroflexed uterus, incision in cervical area, etc. (8, 12, 13). Weaker scar formation was noticed in women with preeclampsia (14).
The most commonly mentioned symptoms related to the scar insufficiency are prolonged menstrual bleeding, intermenstrual bleeding. Rare symptoms are chronic pelvic pain, dysmenorrhea, dyspareunia, infertility (5, 15, 16). The intensity of symptoms is directly related to the defect size. Small uterine scar defects can be asymptomatic (4). Women with larger scar defects usually complain of longer duration of bleeding, they are more likely to suffer from a complex of symptoms (13, 18).
In our presented clinical case the main complaint of the patient was secondary infertility. There are two main mechanisms that can cause secondary infertility to the patient with a uterine scar defect: bleeding from the defect of the scar area and retention of menstrual blood in the scar area (2, 17). Sperm movement towards the ovum is disrupted due to bleeding from the uterine scar, and blastocyst can be washed out from the uterine cavity. Retention of blood in the scar area harms the quality of the sperm, cervical mucus, and interferes with successful implantation (2, 13, 19).
In most cases the scar defect is diagnosed to women who are investigated due to the above-mentioned symptoms (8). Visualization methods such as hysterography, sonohysterography, and transvaginal ultrasound can be used for the evaluation of the integrity of the anterior wall of the uterus (1, 15). An ultrasound examination is considered to be the first diagnostic method (8). In the sonographic examination the uterine scar defect is identified by “niche” term meaning a triangular hypoechogenic zone in the assumed uterine scar area (14). The apex of the triangular zone is usually oriented to the anterior wall of the uterus, and the base points to the uterine cavity or the cervical canal (8). The remaining thickness of solid myometrium can be measured when diagnosing the defect (1). The defect depth and breadth can be evaluated during hysterography and sonohysterography (13, 22).
In 2015, the obstetrician gynaecologist Tanimura with co-authors described new diagnostic criteria of secondary infertility for determination of the conditional uterine scar defect:
Retention of blood in the uterine scar or the uterine cavity during the period from the end of menstruation to ovulation;
Unsuccessful attempts to become pregnant after two or more procedures of artificial insemination or due to other unknown cause of infertility (17).
Literature mentions some treatment options of the uterine scar defect. However, there is no exact treatment algorithm. Both hysteroscopic scar tissue resection and laparoscopic restoration of the uterine wall are mentioned to be successful methods of treatment (11, 17, 20). Results of many studies point to advantages of laparoscopic wall recovery when treating uterine scar insufficiency (12). Such results are explained as incomplete removal of scar tissue during hysteroscopy (8, 13). In our presented case, successful treatment of secondary infertility was achieved with laparoscopic surgery: by removing a fibrous uterine scar and renewing the edges of the uterine scar. Prior to this, diagnostic hysteroscopy was performed, during which a deepening at the isthmus of the uterus was found, which could not be examined fully during the hysteroscopy. The combined technique when both methods are being applied is called the Rendezvous technique. The characteristic feature of the surgery is the “Halloween sign”. This is a visualization of a defect during laparoscopy when the hysteroscope with a light source is located behind a defect. This helps to detect the exact width, depth, and localization of the defect (21).
It is recommended to get pregnant at least three months after the surgical intervention. According to literature data, patients usually get pregnant in a natural way in 12–24 months (8). In all cases, it is recommended to complete the pregnancy by a caesarean section, however, there are cases mentioned of successful delivery through natural delivery paths (1).
RECOMMENDATIONS
If a patient who is planning to get pregnant suffers from symptoms characterized by the uterine scar insufficiency and if the thickness of the residual myometrium is measured to be bigger than 3 mm, then it is recommended to monitor the lower uterine segment by ultrasound. When the thickness of the residual myometrium is measured to be less than 3 mm, then it is recommended to perform a planned laparoscopy during which the scar tissue is resected and the edges of the scar are renewed (13).
If a patient expresses complaints but does not seek to get pregnant, it is recommended to perform an operative hysteroscopy (2).
If the scar defect is diagnosed by chance and the patient does not have any of these symptoms, surgery is not recommended (2).
If a patient gets pregnant after uterine scar repair, then it is recommended to monitor the lower uterine segment by ultrasound each month from the 20th to the 32nd weeks of pregnancy and then weekly until delivery (2).
It is recommended to end a pregnancy at 38-39 weeks of gestation by a planned caesarean section. If premature delivery and tocolysis is unsuccessful, then it is recommended to end the pregnancy by an urgent caesarean section (2).
Greta Bakavičiūtė, Sabina Špiliauskaitė, Audronė Meškauskienė, Diana Ramašauskaitė
References
- Drouin O, Bergeron T, Beaudry A, Demers S, Roberge S, Bujold E. Ultrasonographic evaluation of uterine scar niche before and after laparoscopic Surgical Repair: a case report. AJP Rep. 2014; 4: 65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta ML, Donnez J, Squifflet J, Jadoul P, Darii N, Donnez O. Laparoscopic repair of post-cesarean section uterine scar defects diagnosed in nonpregnant women. J Minim Invasive Gynecol. 2013; 20: 386–91. [DOI] [PubMed] [Google Scholar]
- Wang CB, Chiu WW, Lee CY, Sun YL, Lin YH, Tseng CJ. Cesarean scar defect: correlation between Cesarean section number, defect size, clinical symptoms and uterine position. Ultrasound Obstet Gynecol. 2009; 34: 85–9. [DOI] [PubMed] [Google Scholar]
- Klemm P, Koehler C, Mangler M, Schneider U, Schneider A. Laparoscopic and vaginal repair of uterine scar dehiscence following cesarean section as detected by ultrasound. J Perinat Med. 2005; 33: 324–31. [DOI] [PubMed] [Google Scholar]
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013; 20: 562–72. [DOI] [PubMed] [Google Scholar]
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013; 20: 562–72. [DOI] [PubMed] [Google Scholar]
- Florio P Filippeschi M Moncini I Marra E Franchini M, Gubbini G. Hysteroscopic treatment of the cesarean-induced isthmocele in restoring infertility. Curr Opin Obstet Gynecol. 2012; 24: 180–6. [DOI] [PubMed] [Google Scholar]
- Van der Voet LF Vervoort AJ Veersema S BijdeVaate AJ Brölmann HA Huirne JA. Minimally invasive therapy for gynaecological symptoms related to a niche in the caesarean scar : a systematic review. BJOG. 2014; 121: 145–56. [DOI] [PubMed] [Google Scholar]
- Stewart KS, Evans TW. Recurrent bleeding from the lower segment scar – a late complication of Caesarean section. Br J Obstet Gynaecol. 1975; 82: 682–6. [DOI] [PubMed] [Google Scholar]
- Morris H.Surgical pathology of the lower uterine segment caesarean section scar: is the scar a source of clinical symptoms? Int J Gynecol Pathol. 1995; 14: 16–20. [DOI] [PubMed] [Google Scholar]
- La Rosa MF McCarthy S Richter C Azodi M. Robotic repair of uterine dehiscence. JSLS. 2013; 17: 156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikhareva Osser O Valentin L. Risk factors for incomplete healing of the uterine incision after caesarean section. BJOG. 2010; 117: 1119–26. [DOI] [PubMed] [Google Scholar]
- Schepker N GarciaRocha GJ von VersenHoynck F Hillemanns P Schippert C. Clinical diagnosis and therapy of uterine scar defects after caesarean section in nonpregnant women. Arch Gynecol Obstet. 2014; 291: 1417–23. [DOI] [PubMed] [Google Scholar]
- Bij de Vaate AJ, van der Voet LF, Naji O, Witmer M, Veersema S, Brölmann HA. et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014; 43: 372–82. [DOI] [PubMed] [Google Scholar]
- Roberge S, Boutin A, Chaillet N, Moore L, Jastrow N, Demers S. et al. Systematic review of cesarean scar assessment in the nonpregnant state: imaging techniques and uterine scar defect. Amer J Perinatol. 2012; 29: 465–72. [DOI] [PubMed] [Google Scholar]
- La Sala AP, Berkeley AS. Primary cesarean section and subsequentfertility. Am J Obstet Gynecol. 1987; 157: 379–83. [DOI] [PubMed] [Google Scholar]
- Tanimura S, Funamoto H, Hosono T, Shitano Y, Nakashima M, Ametani Y. et al. New diagnostic criteria and operative strategy for cesarean scar syndrome: Endoscopic repair for secondary infertility caused by cesarean scar defect. J Obstet Gynaecol Res. 2015; 41: 1363–9. [DOI] [PubMed] [Google Scholar]
- Api M, Boza A, Gorgen H, Api O. Should cesarean scar defect be treated laparoscopically? A case report and review of the literature. J Minim Invasive Gynecol. 2015; 22: 1145–52. [DOI] [PubMed] [Google Scholar]
- Roberge S, Boutin A, Chaillet N, Moore L, Jastrow N, Demers Set al. Systematic review of cesarean scar assessment in the nonpregnant state: imaging techniques and uterine scar defect. Am J Perinatol. 2012; 29: 465–71. [DOI] [PubMed] [Google Scholar]
- Gubbini G, Centini G, Nascetti D, Marra E, Moncini I, Bruni L. et al. Surgical hysteroscopic treatment of cesareaninduced isthmocele in restoring fertility: prospective study. J Minim Invasive Gynecol. 2011; 18: 234–7. [DOI] [PubMed] [Google Scholar]
- Nirgianakis K, Oehler R, Mueller M. The Ren dezvous technique for treatment of caesarean scar defects: a novel combined endoscopic approach. Surg Endosc. 2016; 30: 770–1. [DOI] [PubMed] [Google Scholar]
- Fabres C, Aviles G, De La Jara C, Escalona J, Muñoz JF, Mackenna A. et al. The cesarean delivery scar pouch: clinical implications and diagnostic correlation between transvaginal sonography and hysteroscopy. J Ultrasound Med. 2003; 22: 695–700. [DOI] [PubMed] [Google Scholar]