Abstract
Point-of-care (POC) diagnostics play an important role in delivering healthcare, particularly for clinical management and disease surveillance in both developed and developing countries. Currently, the majority of POC diagnostics utilize paper substrates owing to their affordability, disposability, and mass production capability. Recently, flexible polymer substrates have been investigated due to their enhanced physicochemical properties, potential to be integrated into wearable devices with wireless communications for personalized health monitoring, and ability to be customized for POC diagnostics. Here, we focus on the latest advances in developing flexible substrate-based diagnostic devices, including paper and polymers, and their clinical applications at the POC.
Keywords: Flexible substrate, paper, polymer, wearable devices, point-of-care diagnostics
Point of care diagnostics
Point-of-care (POC, see Glossary) diagnostics play an important role in guiding timely patient care in primary care settings, because they are inexpensive, rapid, simple-to-use, and instrument-independent [1–4]. Owing to the advantages over laboratory-based diagnostics, POC diagnostics are used for the prevention and control of infectious diseases and monitoring of health conditions in non-laboratory settings, where well-trained technicians and sophisticated laboratory infrastructure are not available [5, 6]. For example, paper-based POC diagnostics such as glucose strips or pregnancy tests can be used by end-users at home, without requiring formal training. Continued research in paper-based diagnostics focuses on enhancing sensitivity, minimizing batch-to-batch variation, and increasing multiplexing capability. Challenges include overcoming considerable variation in liquid evaporation and sample retention, which compromise the sensitivity and reproducibility of paper-based diagnostics. In addition, it is difficult to capture quantifiable information, particularly for multiplexed paper-based POC diagnostics [1, 7]. Therefore, a great deal of research effort focuses on overcoming these challenges and creating next-generation POC diagnostic tools to meet the need for patient care and personalized healthcare monitoring in non-hospital and home settings.
Flexible polymers have been recently used in POC diagnostics because of their enhanced and adjustable physiochemical properties such as transparency, heat conductivity, or electrochemical resistance [8–10]. Examples of polymers include polydimethylsiloxane (PDMS), poly(methyl methacrylate) (PMMA), polypropylene (PP), polyimide (PI), hydrogel, and polystyrene (PS), which are transparent and can be used in optical detection platforms such as photonic crystal-based sensing [11], surface plasmon resonance (SPR) [12] or photonic nanosensing [13]. Other polymers, such as polyethylene terephthalate (PET), are generally stable at temperatures up to 180°C and have high heat conductivity [14], which is ideal for temperature-controlled biological reactions. Polymers such as polycarbonate (PC), PET, cyclic olefin copolymer (COC), and poly(methyl methacrylate) (PMMA) are electrochemically inert and can be used for developing electrochemical-based diagnostic devices [15]. Unlike paper, flexible polymers do not absorb liquid and thus enable more precise delivery of materials for detection. Lastly, and most importantly, flexible polymers can be engineered thin and bendable, potentially allowing for the integration of sensors within wearable devices or textiles so as to achieve conformal contact to the curvilinear surfaces of the human body for potential personalized healthcare monitoring at the POC [16–19].
In this review, we aim to summarize the latest advances in developing flexible substrate-based devices for POC diagnostics. Paper-based diagnostic devices are also discussed in this review from the perspective of latest advances in multiplexing and enhancing sensitivity for POC diagnostics. Polymer-based flexible devices are discussed in detail with regard to their capability to detect/measure targeted biomarkers from biological fluids or monitoring physiological parameters [16, 17, 20] and integration into wearable devices. Emerging smartphone and computer-based wireless technologies offer unprecedented ease of data collection, analysis, storage and communication, and they have been increasingly tethered with wearable devices for POC applications. An overview of the latest advances in developing flexible substrate-based POC diagnostics is illustrated in Figure 1.
Figure 1.

Overview of flexible substrate-based devices for POC diagnostics.
Flexible substrate-based POC devices for POC diagnostics
In this section, we first present recent advances in improving the multiplexing capability and sensitivity of paper-based POC diagnostics, and then we summarize the development of flexible polymer-based POC diagnostics for sensing biological targets, and their potential applications to be developed into wearable devices.
To improve multiplexing capability, a lateral flow diagnostic microarray was used to enable simultaneous detection of 15 IgE antibodies from human serum [21]. In this array, 15 allergens were well positioned on paper substrates, and their specific IgE antibodies were detected from 35 patient serum samples with a sensitivity comparable to a commercial test, i.e., ImmunoCAP [21]. This strategy overcame the drawback of traditional lateral flow strips for detecting multiple targets due to “downstream effect” that causes reduction of signal intensity at the downstream detection zones. Alternatively, a paper-based vertical flow microarray (VFMA) was developed to improve multiplexing capability [22]. In this report, 10 allergens were first micro-spotted on a porous nitrocellulose membrane, and this special configuration allowed the detection of their allergen-specific IgE antibodies in human serum without signal interference. The VFMA assay had a high concordance with ImmunoCAP with a linearity of 0.89 among 31 patient samples. Recently, fluorescence labeling was used to increase the multiplexing capability as demonstrated in detection of up to 13 types of human papillomavirus from PCR products using a portable fluorescence lateral flow reader [23].
The development of paper-based diagnostic assays (μPADs) also facilitated multiplexed testing using multiple microchannel configurations [24–28]. For instance, a μPAD-based assay was initially developed to detect glucose and bovine serum albumin (BSA) in artificial urine samples with a limit of detection of 5 mM and 0.75 μM, respectively [29]. Further, a liver function μPAD was developed to semi-quantitate the level of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) for drug-related hepatotoxicity monitoring in resource-limited settings [26]. μPADs are similar to lateral flow assays in that they are inexpensive, equipment-free, and can be used for colorimetric visualization, but also include the capability to multiplex and can be scaled-up with different micro-fabrication methods such as photolithography [25], inkjet etching [30], plasma etching [31] and wax printing [32]. Although these manufacturing methods seem more complicated than fabrication of lateral low-based assays, μPADs are still regarded as one of the ideal detection platforms for resource-limited settings.
To increase the sensitivity of paper-based POC diagnostics, a variety of nanotechnologies have been employed including surface enhanced Raman scattering (SERS), surface plasmonic resonance among others [33–36]. Despite superior sensitivity, SERS has not yet been translated to POC diagnostics potentially due to the complexity in preparing SERS substrates. Recently, a SERS array was created on paper substrates using an ordinary ball pen filled with plasmonic inks consisting of Au nanospheres, Au nanorods, and Ag nanorods [37]. This paper-based SERS array showed high uniformity and long stability. In a real-world application, this sensor detected thiabendazole, a fungicide and parasiticide, at 20 ppm. Self-assembled arrays of Au nanoparticles on common chromatographic paper were also developed for SERS [38]. The array of evenly distributed Au nanoparticles was prepared using an oil/water interface and then transferred to paper with a well-designed geometry to create a large number of hot spots at the tip via capillary force. This paper-based SERS array successfully detected 10 fM of malachite green from fishpond water. This POC SERS array is easy-to-prepare, inexpensive (less than 3 cents), and sensitive, showing the potential for environmental and food safety monitoring in the field. In another study, high density of nanowires was patterned on paper substrates for electrical sensing [39].
Flexible polymers, owing to enhanced physicochemical properties, have been increasingly used as substrates for developing POC diagnostics. Table 1 lists some clinical diagnostic applications of flexible substrate-based POC devices. For instance, a sensitive and wearable flexible organic thin-film electrode was reported for analysis of glucose and uric acid in human saliva samples (Figure 2A) [40]. In this study, Pt electrodes and poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate) (PEDOT:PSS)) were printed on a PET substrate to form organic electrochemical transistors (OECTs). Pt electrodes were coated with a composite film of graphene, Nafion and polyaniline (PANI) for selective detection of hydrogen peroxide. OECTs were employed to detect glucose and uric acid at a detection limit of 30 and 10 nM, respectively, and demonstrated biocompatibility, ease of fabrication with different conductive materials, and mechanical robustness during bending tests up to 1000 times. In another study, a carbon nanotube (CNT)-based flexible graphite electrode was developed for detection of glucose with a detection limit of 30 μM [41]. The device was robust and not affected by mechanical stress up to 200 cycles of bending, so it may be suitable for continued development as a sensitive and wearable device for glucose monitoring.
Table 1.
Clinical diagnostic applications of flexible substrate-based POC devices for detection of targeted species.
| Analyte | Dynamic range | Limit of Detection | Sample Type | Number of samples | Detection Modality | Substrate | Thickness | Dimension | Refs | |
|---|---|---|---|---|---|---|---|---|---|---|
| Uric acid and Glucose | 100 nM-500μM for uric acid; NR for glucose. | 10 nM and 30 nM for uric acid and glucose, respectively. | PBS | NR | Amperometric | Polyethylene terephthalate | 50 μm | NR | [40] | |
| Glucose | 0.05–1.0 mM | 30 μM | Serum | NR | Amperometric | Flexible Graphite | NR | NR | [41] | |
| Cortisol | 10–200 ng/mL | 1 ng/mL | Sweat | NR | EIS | Polyamide | NR | NR | [42] | |
| Ascorbic acid, dopamine, and uric acid. | 20–400 μM, 0.5–35 μM and 0.5–35 μM, respectively. | 2.0 μM, 0.01 μM and 0.02 μM, respectively. | PBS | 5 urine samples | DPV | Flexible Graphite | NR | 1×3 cm | [44] | |
| HIV-1 | NR | 106 copies/mL | PBS | NR | Impedance spectroscopy | Polyester film | NR | NR | [45] | |
| E. coli | NR | 4.5 × 107 CFU/mL | Culture media | NR | Impedance spectroscopy | Acetate sheet | NR | NR | [46] | |
| Type II diabetes Leu7Pro gene | NR | Single mismatch | PCR DNA | NR | Melting Curve Analysis | Polyethylene Naphthalate | 125 μm | NR | [47] | |
| Glucose | 0.1–0.6 mM | NA | PBS | NR | Amperometric | Polyimide film | ~1.5 μm | NR | [50] | |
| Lactate | 0.1–0.5 mM | NA | Unstimulated human saliva | NR | Amperometric | Polyethylene terephthalate | NR | NR | [51] | |
| Glucose | 3–100 μM | 3μM | Interstitial fluid under the epidermis | 7 | Amperometric | Tattoo base paper | NR | NR | [54] | |
| Lactate | 1–20 mM | 1 mM | Sweat | 10 | Amperometric | Tattoo base paper | NR | NR | [55] | |
| pH | 4.35–8 | NR | Interstitial fluid (wound) | NR | Potentiometric | Polyaniline | NR | NR | [56] | |
| NADH | 0–100 μM | NR | PBS | NR | Chronoamperometry | Textile | NR | 1.5×15 mm | [59] | |
| H. pylori | Up to 106 cells | ~ 100 cells | Tooth enamel | NR | Electrical resistance | Silk fibroin film | 50 μm | NR | [71] | |
| Sodium | 0.1–100 mM | 0.1 mM | Sweat | 10 | Potentiometric | Polyethylene terephthalate | NR | NR | [73] | |
NR: not reported; PBS: phosphate-buffered saline; EIS: electrochemical impedance spectroscopy; DPV: different pulse voltammetry; CFU: colony forming unit.
Figure 2.

Flexible substrate-based POC diagnostic devices. (A) Flexible OECTs for detection of uric acid and glucose in saliva [40]. (B) Flexible nanoporous polyamide-based electrical sensors with detection of cortisol in sweat [42]. (C) A flexible polyester film-based electrical sensor for detection of HIV-1 [45]. (D) A lab-on-a-foil biosensor for detection of single nucleotide polymorphism in type II diabetes Leu7Pro gene via melting curve analysis [47]. Figures are adapted with permission from References as shown.
In another study, a flexible nanoporous substrate-based biosensor was developed to detect and quantify the level of cortisol in sweat (Figure 2B) [42]. Cortisol is an important multi-functional hormone in controlling metabolism, bone formation, and immune system, and its imbalance is highly related to psychological stress and a number of clinical outcomes [43]. This flexible sensor consisted of three electrodes deposited on flexible polyamide substrates with nanoporous structures. The semiconductive nanoporous structures, when interacting with liquid electrolytes, formed an electrical double layer, which significantly improved charge storage capacity, and thus the sensitivity and dynamic range of the electrical sensor. A zinc oxide (ZnO) thin film was also deposited to form an active region for cortisol antibody immobilization. This flexible sensor exhibited a linear dynamic range of 10–200 ng/mL and a detection limit of 1 ng/mL for detecting in human sweat samples. This flexible cortisol sensor can be turned into a wearable device for non-invasively monitoring the level of cortisol in a clinically relevant range. In another report, a flexible graphite electrochemical detection based diagnostic assay was recently reported for the analysis of dopamine (DA) and uric acid (UA) in urine samples [44].
Flexible substrate-based diagnostic devices have also been developed for analysis of various intact/pathogenic microorganisms. A flexible polyester based diagnostic device was demonstrated for detection of HIV-1 (Figure 2C) [45]. Via impedance magnitude spectra-based electrical sensing, HIV-1 was successfully detected at a level of 106 copies/mL, showing the feasibility of detecting high HIV-1 viremia at the stage of acute HIV-1 infection. In another study, Escherichia coli (E. coli) was detected using an inexpensive, label-free, flexible substrate-based diagnostic device [46]. In this device, graphene was assembled on acetate film, and interdigitated gold electrodes were deposited on graphene to facilitate impedance spectroscopy. Although no capturing moieties were needed, this device only detected E. coli with a limit of detection of 4.5 × 107 cfu/mL.
DNA analysis has also been miniaturized into flexible substrate-based POC diagnostics. For example, a flexible lab-on-a-foil microfluidic device was developed for the detection of single nucleotide polymorphism (SNP) in Type II diabetes biomarker Leu7Pro, based on melting curve analysis (MCA) (Figure 2D) [47]. The device was made of polyethylene naphthalate (PEN) foil with an integrated Cu thin film mesh heater, which was coated with parylene C. After PCR amplification, fluorescence-labeled single-stranded DNA amplicons hybridized to their specific probes, which were immobilized on the Cu thin film mesh heater. An increase in temperature on the mesh heater dissociated fluorescent DNA amplicons from their probes, leading to reduction of fluorescence intensity and detection of SNP in Leu7Pro. However, the gene of Leu7Pro was amplified using a conventional PCR instrument prior to MCA analysis on-chip, making this device unsuitable for POC testing [47]. On-chip amplification of bacterial DNA was also demonstrated on a flexible cassette [48]. The flexible cassette consisted of three layers of polyethylene ribbons with punched holes. These layers were bonded together via double-sided adhesive, and white paper was attached the bottom of the flexible device to form sample reservoirs and to facilitate subsequent colorimetric detection. The flexible cassette containing bacteria was rolled onto a collector reel for LAMP amplification at 65 °C in less than 1 hour. This flexible cassette detected E. coli and S. aureus were detected at 30 and 200 CFU/mL, respectively.
Wearable devices for personalized healthcare monitoring
In this section, we divide wearable device-based POC diagnostics into the two groups: 1) wearable devices for detection of targeted species (e.g., glucose, pH, metabolites, electrolytes and pathogens) from biological fluids (e.g., tear, saliva, and sweat), and 2) wearable devices for monitoring physiological parameters such as temperature, motion and stress.
For detection of glucose in tears, a flexible amperometric sensor integrated in a contact lens was reported [49]. This glucose sensor was microfabricated by depositing Ti/Pd/Pt metal electrodes on a PET film and then shaped into a contact lens. Subsequently, a titania sol-gel film was applied on the surface to immobilize glucose oxidase, and a Nafion film was used to remove interfering agents such as ascorbic acid, lactate and urea. This contact lens-shaped wearable glucose sensor exhibited detection of limit of 0.01 mM and a good linearity over a clinically relevant range of 0.1–0.6 mM in tears. In another study, a printable ultrathin conformal biosensor was fabricated by coating In2O3 solutions on ultrathin polyimide films (Figure 3A) [50]. The ultrathin polyimide film allowed for highly conformal contact to curvilinear surfaces, and high density In2O3 enabled chemical modification with target-specific functional groups. This hybrid material-based flexible field-effect transistor showed current responses to 0.1–0.4 mM of glucose. These two wearable glucose sensors showed great potential to non-invasively measure glucose levels in tears for management of patients with diabetes at the POC.
Figure 3.
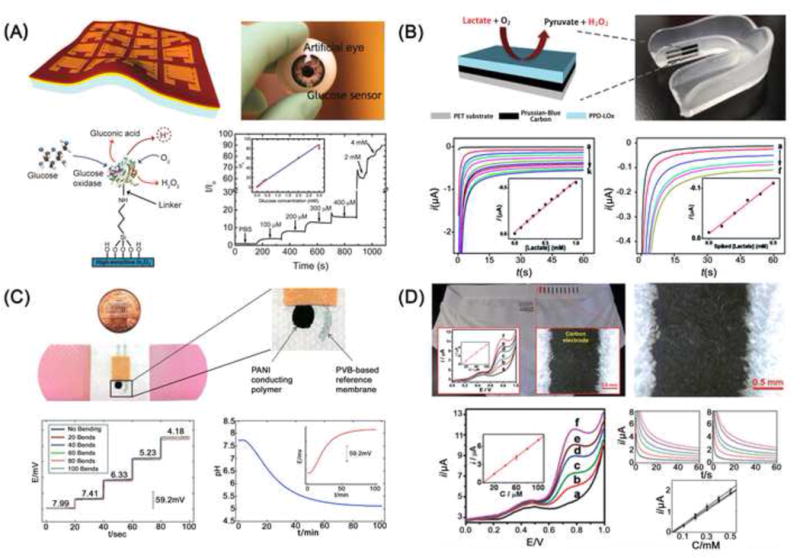
Wearable devices for measuring physiological bio-analytes. (A) A ultrathin In2O3-based conformal glucose biosensor [50]. (B) A PET-based mouthguard lactate biosensor [51]. (C) A bandage-based wearable pH sensor [56]. (D) A textile-based amperometric sensors [59]. Figures are adapted with permission from References as shown.
Saliva testing shows great promise for the detection of metabolites such as glucose, lactate, uric acid and cholesterol for personalized healthcare monitoring. Recently, a wearable biosensor was integrated into a mouthguard for detection of lactate in saliva (Figure 3B) [51]. This mouthguard biosensor consisted of 3 electrodes printed on a flexible PET substrate. The working electrode was coated with a poly(o-phenylenediamine) film to immobilize lactate oxidase. Lactate was oxidized by lactate oxidase, and the resultant hydrogen peroxide was oxidized by a Prussian-Blue transducer. Chronoamperometric measurements were made to quantify the concentration of lactate in human saliva, exhibiting a linear dynamic range of 0.1–0.5 mM. A similar mouthguard-based biosensor was subsequently developed for detection of salivary uric acid [52].
Tattoo-based wearable devices have been developed for sensing targeted species from sweat or interstitial fluids on skin [53]. Recently, a wearable amperometric biosensor was integrated into temporary tattoos for noninvasively measuring interstitial glucose in human skin [54]. In this tattoo-based glucose meter, an additional pair of iontophoresis electrodes were utilized to extract interstitial glucose to the epidermis, where interstitial glucose was amperometrically measured between a glucose oxidase-modified working electrode and a reference/counter electrode. For device fabrication, a conductive silver/silver chloride (Ag/AgCl) ink, a Prussian blue conductive carbon ink, and a transparent insulator ink were sequentially printed on a tattoo base paper. Glucose oxidase was then functionalized on the surface of Prussian blue electrodes. The flexible tattoo-based electrode detected interstitial glucose at a detection limit of 3 μM. Although this tattoo-based glucose meter was non-invasive, it took 10 minutes for reverse iontophoresis and 5 minutes for measurement, which is longer and less user-friendly for POC testing than finger-prick based glucose meter. Similarly, a wearable tattoo-based electrochemical enzymatic biosensor was developed for monitoring the level of lactate in human sweat during physical activity [55].
Recently, wearable pH sensors have been developed to assess chronic wound healing. For example, a bandage-based wearable sensor was constructed by screen-printing one Ag/AgCl reference electrode and a carbon electrode on a conductive polyaniline membrane and polyvinyl butyral (PVB) polymer, respectively [56]. These screen-printed electrodes were assembled on a commercial adhesive bandage (Figure 3C). This wearable potentiometric sensor exhibited a Nernstian behavior to pH ranging from 5.5–8 with good reproducibility against mechanical stress. In another report, a flexible pH electrochemical sensor array was developed on polyaniline, and it showed a linear dynamic range of pH from 4–10 [57]. Recently, a flexible hydrogel fiber-based dressing has been developed for monitoring pH during wound healing [58]. In this study, mesoporous microparticles loaded with pH sensitive dyes were incorporated into flexible hydrogel fibers via spin coating. The pH hydrogel fibers were then attached to a transparent medical tape to create a wearable pH sensor, which exhibited responses to pH ranging from 5.5–9. Clearly, these studies demonstrate the feasibility of developing wearable pH sensors for monitoring the wound healing process at the POC.
Flexible biosensors have also been integrated into textiles. Amperometric sensors fabricated by screen-printing technology on the elastic waist of underwear were reported (Figure 3D) [59]. This textile-based sensor not only demonstrated satisfactory mechanical robustness, but also showed good sensing capability for detection of ferrocyanide, hydrogen peroxide and nicotinamide adenine dinucleotide reduced dipotassium salt (NADH). Recently, a new way of fabricating flexible electrochemical sensors using textile weaving was performed [60]. The electrochemical sensors were made of silk yarn which was pre-coated with conducting inks and reagents. The modified silk yarn was woven into textile forming different electrodes. In contrast to traditional screen-printing technology, fabrication of electrochemical sensors using a simple handloom significantly lowered the cost of reagent consumption and requirement for manufacturing facilities, and thus the cost of sensors (<2 cents per test). As demonstrated, the electrochemical sensor detected blood glucose and hemoglobin within clinically relevant ranges using chronoamperometry and differential pulse voltammetry, respectively. However, the requirement for sample preprocessing and data recording significantly limits their POC application for detection of blood glucose and hemoglobin as wearable devices.
Flexible materials are attractive for developing epidermal electronics to measure electrophysiological activities and other physiological parameters. For example, a flexible, thin (~50 μm) polyester sheet based sensor was used to record electroencephalograms (EEGs), electrocardiograms (ECGs), and electromyograms (EMGs) [61]. The flexible substrate provided conformal contact to the skin via van der Waals forces and was not affected by skin motion. Furthermore, an ultra-thin and wearable device was developed for continuously measuring skin temperature to facilitate health/wellness assessment (Figure 4A) [62]. In this study, microscale temperature sensors simultaneously acted as micro-heaters (actuators) that were integrated in elastomeric PI in an array format with either thin Au (50 nm in thickness) or PIN diodes embedded in a silicon membrane (320 nm in thickness). The integrated PI device offered mechanical properties and device geometries that not only matched human skin for soft and comfortable contact, but also reduced measurement artifacts due to relative motion between sensors and the skin. These wearable temperature sensors had a measurement precision comparable to infrared cameras, enabling close monitoring of temperature-related physiological changes due to mental activity or physical motion, as well as monitoring thermal conductivity associated with hydration and/or perspiration at the POC.
Figure 4.

Wearable devices for measuring physiological parameters. (A) An ultrathin conformal thermal sensor for detection of skin temperature at rest and during mental and physical stimulation [62]. (B) A flexible stain-gauge sensor with reversible interlocking nanofibers for detection of human heartbeat [63]. (C) A graphene-based electrical sensor for detection of H. pylori with wireless recording [71]. (D) A flexible bio-integrated electronic device for on-skin acquiring electrocardiograms (ECGs) and electroencephalograms (EEGs) signals [72]. Figures are adapted with permission from References as shown.
To reduce design complexity, a wearable skin-like strain-gauge sensor with a configuration of two interlocked arrays was developed for highly sensitive measurement of pressure, shear, and torsion (Figure 4B) [63]. The flexible device was fabricated by sandwiching two layers of identical nanohairs, consisting of Pt-coated nanofibers at a high density, into two thin PDMS substrates (~500 μm). These two layers of nanofibers were reversely bonded via an interlocking mechanism for sensing mechanical stimuli. Upon mechanical stress, such as pressure, shear, or torsion, the two layers of nanofibers generated an electrical resistance signal and measured with a strain gauge sensor. The inexpensive flexible strain-gauge sensor accurately measured pulses with robust tolerance to bending up to 10,000 cycles.
As another example, a wearable bio-integrated system was developed for temperature and mechanical sensing, as well as temperature-controlled drug delivery [64]. This multifunctional system consisted of a resistive random access memory (RRAM) array, a temperature sensor, strain sensors, electroresistive sensors, and mesoporous-silica nanoparticles loaded with drugs on a flexible hydrocolloid patch. These modules enable sensing of movement such as tremors (e.g., Parkinson’s disease, epilepsy), data storage and analysis, and feedback therapy. Clearly, wearable electronics hold great promise for monitoring health conditions in real time and can be even integrated with feedback therapy to enable treatment in POC settings.
Wireless communication for wearable devices
Smartphone and computer-based wireless electronics have been tethered with wearable devices to acquire sensing signals, analyze data, and report and transfer clinical results for POC applications [58, 65–70]. For example, a wearable biosensor attached to tooth enamel was recently developed for detection of bacteria in saliva (Figure 4C) [71]. This wearable biosensor consisted of graphene-based interdigitated electrodes and an inductive coil antenna printed onto silk thin-film substrates. Dissolution of silk thin-film allowed intimate attachment of the flexible biosensor to irregular surface of tooth enamel. The capture of bacteria by antimicrobial peptides on the sensor surface resulted in change in conductivity of the graphene film, which was monitored using an inductively coupled radio frequency readout unit. The wireless electronics-based wearable sensor successfully detected ~ 100 H. pylori in 1 μL of human saliva, and detected S. aureus at a detection limit of 1 bacterium per microliter. This wearable graphene sensor offered sensitive, specific, and label-free detection of bacteria on non-planar surfaces with wireless sensing capability, which can be used as POC diagnostics for continuous monitoring bacterial infection in a non-invasive mode.
For continuous monitoring physiological activities on skin, a sophisticated bio-integrated flexible device was developed with capability to transmit radio frequency (RF) data (Figure 4D) [72]. This ultrathin skin-like device was based on a silicon oligomer Sylgard 184, consisting of microfluidic channels, circuits, sensors, and radios in a multi-layer interconnected network to filter, amplify and transmit electrophysicological signals. As demonstrated, this bio-integrated device successfully recorded the QRS complex in ECG signals with high single-to-noise ratio in a wireless mode, which was comparable to standard wired connections. This device also successfully documented low frequency EEG signals during mental math performed by a subject counting backwards from 200 by 7s with eyes closed. This device is small (1.9 × 2.9 cm2) and can be integrated with thermal and motion sensors, and so can be potentially used as POC diagnostics for neonates monitoring in intensive care units or home settings.
Recently, a tattoo-based wearable device with real-time wireless signal transduction was developed for continuously monitoring the level of sodium on the skin [73]. The concentration of sodium in sweat is indicative of electrolyte imbalance under physiological or pathological conditions. This tattoo-based potentiometric sensor had two electrodes and one custom designed transceiver for wireless signal transduction. Nernstian responses were observed for physiological concentrations of sodium in sweat ranging from 30–110 mM. For on-body characterization, this sensor clearly demonstrated real-time sodium dynamics during exercise. In another study, the same group developed wearable mouthguard biosensor with an integrated Bluetooth Low Energy (BLE) chipset for continuous monitoring or uric acid in saliva [52]. The demonstrated device was fabricated by using a screen-printed flexible PET electrode that was integrated with a potentiostat, microcontroller, and miniaturized on-chip BLE communication system for low-power operation. The developed platform transferred sensing data through wireless transmission to standard smartphones, laptops, and other consumer electronics for on-demand data processing. These studies clearly demonstrated the feasibility for integrating wireless electronics with wearable devices for continuously monitoring physiological conditions.
Concluding remarks and Future Perspectives
So far, flexible substrates with enhanced physiochemical properties have been increasingly integrated into flexible biosensors and wearable devices to allow for detecting/measuring targeted biomarkers from biological fluids and physiological parameters for personalized healthcare monitoring at the POC. Flexible substrate-based POC diagnostics, particularly with integrated graphene-based biosensors and OFETs, not only significantly enhance sensitivity, but also allow for continuous on-body health monitoring in a non-invasive mode. Improved human-device interfaces are becoming possible due to the incorporation of mobile-based technologies for signal acquisition, data analysis, and result reporting directly at the POC without specialized equipment. Moreover, integration of flexible sensors into textiles incorporated into wireless communication data collection (e.g., bluetooth technologies) will create unprecedented opportunities for real-time, continuous on-body monitoring of physiological parameters during daily activities. Furthermore, back-end cloud services allow for long-term signal collection and data mining, which would be helpful for advanced medical research for early diagnosis of cancer, Parkinson’s disease and other chronic diseases. Thus, we envision that next generation flexible substrate-based POC devices will leverage mobile health (mHealth) technologies and fast-advancing nanotechnologies to achieve early diagnosis, treatment monitoring, and disease surveillance in the future establishing a better base-line for human-beings at a personalized level. However, flexible substrate-based POC diagnostics and wearable devices are still at the early stage of development, and key challenges remain to be addressed (see Outstanding Questions). For example, current flexible polymer-based devices are generally coupled with sophisticated optical or electrical setups for sensitive detection, which is less ideal for POC testing. Although emerging smartphone and computer-based wireless technologies have significantly improved the portability and human-device interface of flexible polymer-based POC diagnostics, technical challenges such as signal drift, variation in lightening, device miniaturization, and body temperature fluctuation need to be addressed for electrochemical sensing and optical sensing. More importantly, commercialization challenges remain a significant hurdle for translating microfluidics-based wearable devices into clinical practice such as lack of standardization on materials, poor integration from sample preparation to result report, and uneven regulatory requirement for POC device approval [74, 75]. Limited funding and insufficient access to clinical samples also constrict product refinement and systematic clinical validation of flexible substrate-based POC diagnostics and wearable devices prior to entry to the market. Therefore, joint efforts are needed to advance POC diagnostics and wearable devices to be affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and deliverable to end users (ASSURED) [2]. Towards this goal, fully integrated wearable sensor arrays have been developed to simultaneously analyze sweat metabolites and electrolytes, as well as temperature without external analysis [76].
Trends Box.
Flexible substrates: Owing to enhanced optical, physical and chemical properties, as well as affordability and amenity to mass production, flexible polymer-based substrates are increasingly being used to develop next-generation POC diagnostic devices with enhanced performances.
Wearable devices: Due to excellent bending tolerance, flexible polymer substrates are highly attractive for developing wearable diagnostic devices with integrated thermal, electrical, and/or optical sensors for real-time monitoring of health conditions in POC settings.
Cloud-based POC diagnostics: Mobile- or computer- based wireless systems can be easily integrated with on-body devices to enable real-time personalized healthcare monitoring and information exchange in a remote mode, offering a new, viable and affordable paradigm for better health care delivery at the POC.
Outstanding Questions.
How to leverage the characteristics of flexible polymers as substrates for developing next-generation POC diagnostic devices that can overcome the limitations of paper-based POC devices?
What substrate materials and device architecture can provide sample-to-answer capability in POC diagnostic devices?
How to develop an integrated wearable device at low cost for real-time detection of biological analytes and continuous monitoring of physiological parameters at the POC?
How to enable mobile-based tele-diagnostics and wireless communications systems to facilitate health services and to establish disease surveillance networks at the POC?
Acknowledgments
Dr. Wang acknowledges the supported by the Fundamental Research Funds for the Central Universities (2015QNA7026) from China. Dr. Demirci would like to acknowledge R01 AI093282, R01 GM108584, R01 DE02497101, R01 AI081534, R21 Al113117, R21 Al110277, and U54 EB015408.
Glossary
- Organic electrochemical transistors (OECTs)
one type of highly flexible organic thin-film transistors that can be used for potentiometric sensing
- Point-of-care (POC)
patient care in the emergency room, in primary clinics, at home, or in other non-hospital settings where diagnosis can be made and treatment can be administered
- Surface enhanced Raman scattering (SERS)
a spectroscopic approach that can detect chemical and biological species down to a single molecule without labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS
Dr. U Demirci is a founder of, and has an equity interest in: (i) DxNow Inc., a company that is developing microfluidic and imaging technologies for point-of-care diagnostic solutions, and (ii) Koek Biotech, a company that is developing microfluidic IVF technologies for clinical solutions. U.D.’s interests were viewed and managed in accordance with the conflict of interest policies.
References
- 1.Wang S, et al. Advances in addressing technical challenges of point-of-care diagnostics in resource-limited settings. Expert review of molecular diagnostics. 2016;16:449–459. doi: 10.1586/14737159.2016.1142877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mabey D, et al. Diagnostics for the developing world. Nature reviews Microbiology. 2004;2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 3.Gubala V, et al. Point of Care Diagnostics: Status and Future. Analytical chemistry. 2012;84:487–515. doi: 10.1021/ac2030199. [DOI] [PubMed] [Google Scholar]
- 4.Yager P, et al. Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 5.Yetisen AK, et al. Paper-based microfluidic point-of-care diagnostic devices. Lab on a chip. 2013;13:2210–2251. doi: 10.1039/c3lc50169h. [DOI] [PubMed] [Google Scholar]
- 6.Tian T, et al. Distance-based microfluidic quantitative detection methods for point-of-care testing. Lab on a chip. 2016;16:1139–1151. doi: 10.1039/c5lc01562f. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, et al. Advances in paper-based point-of-care diagnostics. Biosensors & bioelectronics. 2014;54:585–597. doi: 10.1016/j.bios.2013.10.075. [DOI] [PubMed] [Google Scholar]
- 8.Focke M, et al. Lab-on-a-Foil: microfluidics on thin and flexible films. Lab on a Chip. 2010;10:1365–1386. doi: 10.1039/c001195a. [DOI] [PubMed] [Google Scholar]
- 9.Pang C, et al. Recent advances in flexible sensors for wearable and implantable devices. Journal of Applied Polymer Science. 2013;130:1429–1441. [Google Scholar]
- 10.Ren KN, et al. Materials for Microfluidic Chip Fabrication. Accounts Chem Res. 2013;46:2396–2406. doi: 10.1021/ar300314s. [DOI] [PubMed] [Google Scholar]
- 11.Shafiee H, et al. Nanostructured Optical Photonic Crystal Biosensor for HIV Viral Load Measurement. Scientific reports. 2014;4 doi: 10.1038/srep04116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokel O, et al. Portable Microfluidic Integrated Plasmonic Platform for Pathogen Detection. Scientific reports. 2015;5 doi: 10.1038/srep09152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yetisen AK, et al. Reusable, Robust, and Accurate Laser-Generated Photonic Nanosensor. Nano letters. 2014;14:3587–3593. doi: 10.1021/nl5012504. [DOI] [PubMed] [Google Scholar]
- 14.Holden MT, et al. Photolithographic Synthesis of High-Density DNA and RNA Arrays on Flexible, Transparent, and Easily Subdivided Plastic Substrates. Analytical chemistry. 2015;87:11420–11428. doi: 10.1021/acs.analchem.5b02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutz S, et al. Microfluidic lab-on-a-foil for nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA) Lab on a Chip. 2010;10:887–893. doi: 10.1039/b921140c. [DOI] [PubMed] [Google Scholar]
- 16.Bandodkar AJ, Wang J. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 2014;32:363–371. doi: 10.1016/j.tibtech.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Hammock ML, et al. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Advanced materials. 2013;25:5997–6037. doi: 10.1002/adma.201302240. [DOI] [PubMed] [Google Scholar]
- 18.Liao CZ, et al. Flexible Organic Electronics in Biology: Materials and Devices. Advanced materials. 2015;27:7493–7527. doi: 10.1002/adma.201402625. [DOI] [PubMed] [Google Scholar]
- 19.Vilela D, et al. Flexible sensors for biomedical technology. Lab on a chip. 2016;16:402–408. doi: 10.1039/c5lc90136g. [DOI] [PubMed] [Google Scholar]
- 20.Xu TB, et al. High resolution skin-like sensor capable of sensing and visualizing various sensations and three dimensional shape. Scientific reports. 2015;5 doi: 10.1038/srep12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinnasamy T, et al. A lateral flow paper microarray for rapid allergy point of care diagnostics. Analyst. 2014;139:2348–2354. doi: 10.1039/c3an01806g. [DOI] [PubMed] [Google Scholar]
- 22.Chinnasamy T, et al. Point-of-Care Vertical Flow Allergen Microarray Assay: Proof of Concept. Clinical Chemistry. 2014;60:1209–1216. doi: 10.1373/clinchem.2014.223230. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, et al. Fluorescent probe-based lateral flow assay for multiplex nucleic acid detection. Analytical chemistry. 2014;86:5611–5614. doi: 10.1021/ac5010458. [DOI] [PubMed] [Google Scholar]
- 24.Martinez AW, et al. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19606–19611. doi: 10.1073/pnas.0810903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez AW, et al. Diagnostics for the developing world: microfluidic paper-based analytical devices. Analytical chemistry. 2010;82:3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 26.Pollock NR, et al. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Science translational medicine. 2012;4:152ra129. doi: 10.1126/scitranslmed.3003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan L, et al. Barcode-like paper sensor for smartphone diagnostics: an application of blood typing. Analytical chemistry. 2014;86:11362–11367. doi: 10.1021/ac503300y. [DOI] [PubMed] [Google Scholar]
- 28.Dungchai W, et al. Electrochemical detection for paper-based microfluidics. Analytical chemistry. 2009;81:5821–5826. doi: 10.1021/ac9007573. [DOI] [PubMed] [Google Scholar]
- 29.Martinez AW, et al. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angewandte Chemie International Edition. 2007;46:1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe K, et al. Inkjet-printed microfluidic multianalyte chemical sensing paper. Analytical chemistry. 2008;80:6928–6934. doi: 10.1021/ac800604v. [DOI] [PubMed] [Google Scholar]
- 31.Li X, et al. Paper-based microfluidic devices by plasma treatment. Analytical chemistry. 2008;80:9131–9134. doi: 10.1021/ac801729t. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, et al. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis. 2009;30:1497–1500. doi: 10.1002/elps.200800563. [DOI] [PubMed] [Google Scholar]
- 33.Polavarapu L, et al. Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles. J Mater Chem C. 2014;2:7460–7476. [Google Scholar]
- 34.Polavarapu L, Liz-Marzan LM. Towards low-cost flexible substrates for nanoplasmonic sensing. Phys Chem Chem Phys. 2013;15:5288–5300. doi: 10.1039/c2cp43642f. [DOI] [PubMed] [Google Scholar]
- 35.Zhuo Y, Cunningham BT. Label-Free Biosensor Imaging on Photonic Crystal Surfaces. Sensors-Basel. 2015;15:21613–21635. doi: 10.3390/s150921613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaster RS, et al. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nature nanotechnology. 2011;6:314–320. doi: 10.1038/nnano.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polavarapu L, et al. Pen-on-paper approach toward the design of universal surface enhanced Raman scattering substrates. Small. 2014;10:3065–3071. doi: 10.1002/smll.201400438. [DOI] [PubMed] [Google Scholar]
- 38.Zhang K, et al. Multifunctional Paper Strip Based on Self-Assembled Interfacial Plasmonic Nanoparticle Arrays for Sensitive SERS Detection. ACS applied materials & interfaces. 2015;7:16767–16774. doi: 10.1021/acsami.5b04534. [DOI] [PubMed] [Google Scholar]
- 39.Mostafalu P, Sonkusale S. A high-density nanowire electrode on paper for biomedical applications. Rsc Adv. 2015;5:8680–8687. [Google Scholar]
- 40.Liao C, et al. Flexible organic electrochemical transistors for highly selective enzyme biosensors and used for saliva testing. Advanced materials. 2015;27:676–681. doi: 10.1002/adma.201404378. [DOI] [PubMed] [Google Scholar]
- 41.Kim WS, et al. A flexible, nonenzymatic glucose biosensor based on Ni-coordinated, vertically aligned carbon nanotube arrays. RSC Advances. 2014;4:48310–48316. [Google Scholar]
- 42.Munje RD, et al. Flexible nanoporous tunable electrical double layer biosensors for sweat diagnostics. Scientific reports. 2015;5:14586. doi: 10.1038/srep14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaushik A, et al. Recent advances in cortisol sensing technologies for point-of-care application. Biosensors & bioelectronics. 2014;53:499–512. doi: 10.1016/j.bios.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 44.Cai W, et al. Electrochemical determination of ascorbic acid, dopamine and uric acid based on an exfoliated graphite paper electrode: A high performance flexible sensor. Sensors and Actuators B: Chemical. 2014;193:492–500. [Google Scholar]
- 45.Shafiee H, et al. Paper and flexible substrates as materials for biosensing platforms to detect multiple biotargets. Scientific reports. 2015;5:8719. doi: 10.1038/srep08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basu PK, et al. Graphene based E. coli sensor on flexible acetate sheet. Sensors and Actuators B: Chemical. 2014;190:342–347. [Google Scholar]
- 47.Ohlander A, et al. Genotyping of single nucleotide polymorphisms by melting curve analysis using thin film semi-transparent heaters integrated in a lab-on-foil system. Lab on a Chip. 2013;13:2075–2082. doi: 10.1039/c3lc50171j. [DOI] [PubMed] [Google Scholar]
- 48.Safavieh M, et al. A simple cassette as point-of-care diagnostic device for naked-eye colorimetric bacteria detection. Analyst. 2014;139:482–487. doi: 10.1039/c3an01859h. [DOI] [PubMed] [Google Scholar]
- 49.Yao H, et al. A contact lens with embedded sensor for monitoring tear glucose level. Biosensors & bioelectronics. 2011;26:3290–3296. doi: 10.1016/j.bios.2010.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rim YS, et al. Printable Ultrathin Metal Oxide Semiconductor-Based Conformal Biosensors. Acs Nano. 2015;9:12174–12181. doi: 10.1021/acsnano.5b05325. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, et al. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst. 2014;139:1632–1636. doi: 10.1039/c3an02359a. [DOI] [PubMed] [Google Scholar]
- 52.Kim J, et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosensors & bioelectronics. 2015;74:1061–1068. doi: 10.1016/j.bios.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bandodkar AJ, et al. Tattoo-Based Wearable Electrochemical Devices: A Review. Electroanal. 2015;27:562–572. [Google Scholar]
- 54.Bandodkar AJ, et al. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Analytical chemistry. 2014;87:394–398. doi: 10.1021/ac504300n. [DOI] [PubMed] [Google Scholar]
- 55.Jia W, et al. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Analytical chemistry. 2013;85:6553–6560. doi: 10.1021/ac401573r. [DOI] [PubMed] [Google Scholar]
- 56.Guinovart T, et al. Bandage-Based Wearable Potentiometric Sensor for Monitoring Wound pH. Electroanal. 2014;26:1345–1353. [Google Scholar]
- 57.Rahimi R, et al. A low-cost flexible pH sensor array for wound assessment. Sensors and Actuators B: Chemical. 2016;229:609–617. [Google Scholar]
- 58.Tamayol A, et al. Flexible pH-Sensing Hydrogel Fibers for Epidermal Applications. Advanced healthcare materials. 2016;5:711–719. doi: 10.1002/adhm.201500553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang YL, et al. Thick-film textile-based amperometric sensors and biosensors. Analyst. 2010;135:1230–1234. doi: 10.1039/b926339j. [DOI] [PubMed] [Google Scholar]
- 60.Choudhary T, et al. Woven electrochemical fabric-based test sensors (WEFTS): a new class of multiplexed electrochemical sensors. Lab on a chip. 2015;15:2064–2072. doi: 10.1039/c5lc00041f. [DOI] [PubMed] [Google Scholar]
- 61.Kim DH, et al. Epidermal electronics. Science. 2011;333:838–843. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- 62.Webb RC, et al. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nature materials. 2013;12:938–944. doi: 10.1038/nmat3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pang C, et al. A flexible and highly sensitive strain-gauge sensor using reversible interlocking of nanofibres. Nature materials. 2012;11:795–801. doi: 10.1038/nmat3380. [DOI] [PubMed] [Google Scholar]
- 64.Son D, et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nature nanotechnology. 2014;9:397–404. doi: 10.1038/nnano.2014.38. [DOI] [PubMed] [Google Scholar]
- 65.Coskun AF, et al. A personalized food allergen testing platform on a cellphone. Lab on a chip. 2013;13:636–640. doi: 10.1039/c2lc41152k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang S, et al. Integration of cell phone imaging with microchip ELISA to detect ovarian cancer HE4 biomarker in urine at the point-of-care. Lab on a chip. 2011;11:3411–3418. doi: 10.1039/c1lc20479c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yetisen AK, et al. A smartphone algorithm with inter-phone repeatability for the analysis of colorimetric tests. Sensor Actuat B-Chem. 2014;196:156–160. [Google Scholar]
- 68.Steinhubl SR, et al. The emerging field of mobile health. Science translational medicine. 2015;7 doi: 10.1126/scitranslmed.aaa3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Preechaburana P, et al. Biosensing with cell phones. Trends Biotechnol. 2014;32:351–355. doi: 10.1016/j.tibtech.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Wang S, et al. Micro-a-fluidics ELISA for rapid CD4 cell count at the point-of-care. Scientific reports. 2014;4:3796. doi: 10.1038/srep03796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mannoor MS, et al. Graphene-based wireless bacteria detection on tooth enamel. Nat Commun. 2012;3:763. doi: 10.1038/ncomms1767. [DOI] [PubMed] [Google Scholar]
- 72.Xu S, et al. Soft Microfluidic Assemblies of Sensors, Circuits, and Radios for the Skin. Science. 2014;344:70–74. doi: 10.1126/science.1250169. [DOI] [PubMed] [Google Scholar]
- 73.Bandodkar AJ, et al. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosensors and bioelectronics. 2014;54:603–609. doi: 10.1016/j.bios.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 74.Volpatti LR, Yetisen AK. Commercialization of microfluidic devices. Trends Biotechnol. 2014;32:347–350. doi: 10.1016/j.tibtech.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Chin CD, et al. Commercialization of microfluidic point-of-care diagnostic devices. Lab on a chip. 2012;12:2118–2134. doi: 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]
- 76.Gao W, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]


