Abstract
Objective To evaluate visual outcomes and potential complications for optic nerve decompression using an endoscopic endonasal approach (EEA) for fibrous dysplasia.
Design Retrospective chart review of patients with fibrous dysplasia causing extrinsic compression of the canalicular segment of the optic nerve that underwent an endoscopic endonasal optic nerve decompression at the University of Pittsburgh Medical Center from 2010 to 2013.
Main Outcome Measures The primary outcome measure assessed was best-corrected visual acuity (BCVA) with secondary outcomes, including visual field testing, color vision, and complications associated with the intervention.
Results A total of four patients and five optic nerves were decompressed via an EEA. All patients were symptomatic preoperatively and had objective findings compatible with compressive optic neuropathy: decreased visual acuity was noted preoperatively in three patients while the remaining patient demonstrated an afferent pupillary defect. BCVA improved in all patients postoperatively. No major complications were identified.
Conclusion EEA for optic nerve decompression appears to be a safe and effective treatment for patients with compressive optic neuropathy secondary to fibrous dysplasia. Further studies are required to identify selection criteria for an open versus an endoscopic approach.
Keywords: endoscopic, endonasal, optic nerve decompression, fibrous dysplasia
Introduction
Fibrous dysplasia is a rare disease with reported prevalence between 1/100,000 to 1/1,000,000.1 The underlying pathophysiology is secondary to a somatic mutation of the GNAS gene ultimately leading to amplified proliferation of the osteogenic cell lineage in bone marrow.2 3 4 Phenotypic presentations are variable depending on the timing of the mutation in development and range from isolated monostotic fibro-osseous involvement to more severe forms with polyostotic involvement, endocrine abnormalities, and cutaneous pathology. Involvement of the craniofacial bones is common and affects 50 to 100% of individuals with the polyostotic form.5 Disease progression after adolescence is rare and typically minor,6 though clinical deterioration has been reported later in life.1 Pathologic involvement of the orbit and optic canal has potential to cause compressive optic neuropathy with subsequent decline in visual acuity and visual field (VF) deficits. Interestingly, several studies have shown that despite radiographic evidence of optic nerve involvement by disease, only a minority become symptomatic.7 8 While there has been controversy regarding the optimal management, it has become increasingly agreed upon that intervention should only occur if the disease process becomes symptomatic,9 typically from cranial nerve compression. No currently available medical therapies have been identified that can halt or reverse this compressive effect,1 10 11 rendering surgical intervention the only viable option for successful management of this component of the disease.
Optic nerve decompression has been available for many years as a means to treat or temporize compressive pathology of the optic nerve. Transcranial and transfacial access are the classical approaches for surgical intervention, though as early as 1998, endoscopic transnasal approaches have been described.12 Improvements in endoscopic instrumentation, anatomic understanding, and increased experience have led to further advances in endoscopic endonasal approaches (EEAs). There currently is an abundance of literature available regarding the use of endoscopic decompression for traumatic optic neuropathy (TON) and while its utility in this setting is controversial given its unproven efficacy, the safety of the approach is well documented. Contrary to this, the literature is relatively sparse with the use of the EEA for optic nerve decompression to treat other benign pathology of the canalicular segment. Given the benign nature of these lesions, the risk of treatment must not outweigh the potential benefits. The present report seeks to review a single institution's experience with regards to visual outcomes and complications of endoscopic endonasal decompression of the optic nerve for fibrous dysplasia causing compressive optic neuropathy.
Methods
Following institutional review board approval, a retrospective chart review was performed on patients operated on at the University of Pittsburgh Medical Center from 2010 to 2013. All patients who underwent EEA for optic nerve decompression for fibrous dysplasia with pre- and postoperative ophthalmologic examinations were included for analysis. Patients with other benign or malignant pathology or who underwent concurrent transcranial approaches were excluded.
The primary outcome assessed was visual acuity. Best-corrected visual acuity (BCVA) was categorized as normal (20/20) or mild (20/25–20/60), moderate (20/70–20/160), severe (20/200–20/400), and profound (20/500–20/1000) deficits. VF testing was evaluated relative to preoperative status. Additional data points collected included optical coherence tomography (OCT) and color vision measured by Ishihara color plate testing if available. Charts were reviewed for any postoperative complications.
Surgical Technique
All EEAs were performed as “team” surgery with both an otolaryngologist-head and neck surgeon and neurosurgeon working in conjunction (“2 surgeon, 4 hands” technique). Image guidance with a high-resolution maxillofacial computed tomography angiogram was utilized in all cases. The nasal cavity was decongested with topical oxymetazoline (0.05%) or epinephrine (1:1,000) according to the surgeon's preference. All cases were performed via uninarial access unless bilateral decompressions were performed. The ipsilateral middle turbinate was medialized or resected as necessary to provide adequate access. An ipsilateral sphenoethmoidectomy was performed in standard fashion exposing the skull base and lamina papyracea. A maxillary antrostomy was performed in some cases to aid in identification of the orbital limits. Key landmarks within the sphenoid sinus were identified when possible, including the internal carotid artery (ICA) and optic nerve prominence, the lateral opticocarotid recess, and sella turcica. A posterior medial orbital decompression was then performed while maintaining intact periorbita (Fig. 1). The thicker bone surrounding the orbital apex was then thinned with the drill (3–4 mm coarse diamond bit) and dissected free (Fig. 2). Drilling of bone overlying the optic canal and orbital apex was performed with a self-irrigating drill or with copious handheld irrigation to prevent any thermal injury to the underlying nerve. The bone overlying the optic canal was thinned and carefully dissected free to the tuberculum, optic strut and paraclinoidal ICA, taking extreme care to avoid injury. At least 180 degrees of bone removal was performed to be considered adequate (Fig. 3). In general, a 270-degree bony decompression was the goal of the intervention. Improved access for visualization and instrumentation can be improved by adding a contralateral sphenoidotomy with removal of the sphenoid rostrum. A 2-mm drill bit may be necessary when drilling the medial anterior clinoid, optic strut and superior surface of the optic canal. Representative pre- and postoperative CT scans are shown in Fig. 4 demonstrating the degree of bony decompression. The optic nerve sheath was not violated in any case. The surgical defect was covered with a free mucosal graft (if available from the previously resected middle turbinate) or fibrin glue. Nonabsorbable packing was not used in any case.
Fig. 1.
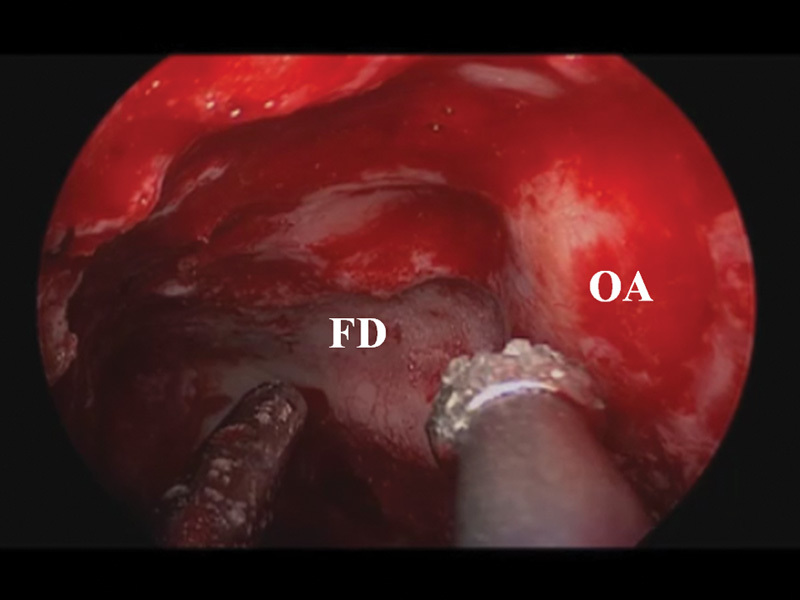
Endoscopic view of left sphenoid sinus and orbital apex. Abnormal bone overlying the orbital apex and optic nerve is drilled using copious irrigation. FD, fibrous dysplasia; OA, orbital apex.
Fig. 2.

Decompressed left orbital apex. Thinned bone overlying the left optic nerve dissected free with fine dissector. PS, planum sphenoidale; ON, optic nerve; OA, orbital apex.
Fig. 3.

Final decompression demonstrating greater than 180 degrees of bony decompression of the optic nerve. ON, optic nerve; OA, orbital apex.
Fig. 4.

Preoperative (A, B) and postoperative (C, D) CT scans of representative patient. Symptomatic encasement of left optic nerve (arrowheads) underwent wide bony decompression (arrows). No intervention was performed on the encased and asymptomatic right optic nerve. CT, computed tomography.
Results
Four patients were identified meeting the study criteria. One patient had bilateral fibrous dysplasia involvement requiring bilateral decompression for a total of five optic nerve decompressions. There were two males and two females with a median age of 19.5 years (range: 16–47 years). While the majority of patients were adolescents and young adults, the single outlier is a 47-year-old female with stable, long-standing fibrous dysplasia who developed optic nerve compression after starting a bisphosphonate for osteoporosis 8 months earlier. She discontinued the medication after 3 months, but her vision did not improve after cessation of the medication. The median duration of time from the onset of signs or symptoms to treatment was 37 days (range: 8–75 days).The mean follow-up for the cohort was 14.8 months.
All decompressed optic nerves demonstrated an improvement in visual function, see Table 1. Decreased visual acuity (three mild, one profound) was noted preoperatively in four eyes while the remaining patient demonstrated an afferent pupillary defect consistent with early compressive optic neuropathy. BCVA improved in all patients with preoperative impairment and all nerves demonstrated normal visual acuity postoperatively. VF testing was ultimately improved in one nerve, stable in three nerves and was incomplete for one optic nerve. Color vision was found to be improved or stable in all patients. OCT data were available for four optic nerves with a median retinal nerve fiber layer thickness of 100 µm (range: 83–135 µm). One patient required revision functional endoscopic sinus surgery secondary to cicatrix formation leading to chronic sinusitis 6 months following optic nerve decompression. No other postoperative complications were noted. One patient returned 10 months following endoscopic decompression with a mild decrease in ipsilateral visual acuity, relative afferent pupillary defect, and VF defect. Repeat imaging demonstrated stable appearing fibrous dysplasia lateral to the optic nerve that was unable to be addressed during the prior EEA. He underwent a pterional craniotomy and microsurgical anterior clinoidectomy achieving a 360-degree decompression of the optic nerve with restoration of normal visual function.
Table 1. Visual outcomes.
| Patient | Age (y) | Involved eye | Time (d) to intervention | BCVA preop | BCVA postop | Visual fields | Color vision | OCT-RNFL (mean, µm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 47 | OS | 75 | 20/50 | 20/20 | Improved | Stable | 83 |
| 2 | 18 | OS | 60 | 20/20 | 20/15 | Stable | Stable | 97 |
| 3 | 16 | OS | 14 | 20/500 | 20/20 | n/a | Improved | n/a |
| 4 | 21 | OD | 8 | 20/25 | 20/20 | Stable | Stable | 103 |
| OS | 8 | 20/25 | 20/20 | Stable | Stable | 135 |
Abbreviations: BCVA, best-corrected visual acuity; n/a; not applicable; OCT-RNFL, optical coherence tomography - retinal nerve fiber layer; OD, right eye; OS, left eye; postop, postoperative; preop, preoperative.
Discussion
Visual impairment is the most common neurological sequela of skull base fibrous dysplasia.13 Management of this component of the disease has been a source of controversy. While many thought medical intervention may have an impact on the course of the disease, no pharmacological therapy has demonstrated success (notably biphosphonates) in halting or reversing progressive bony disease.1 10 11 Involvement of the optic nerve is common in craniofacial fibrous dysplasia. In a large study of 87 patients by Cutler et al,7 83% of optic nerves demonstrated > 50% encasement and 61% had circumferential encasement. Only 12% of those patients with complete nerve encasement had an optic neuropathy, while no patient with less than 100% encasement demonstrated impaired visual function. Others have demonstrated that even apparent narrowing of the optic canal on diagnostic imaging is inaccurate in predicting those patients that will suffer from compressive optic neuropathy.8 Prior reports have advocated for prophylactic decompression,14 15 though a recent meta-analysis has revealed improved clinical outcomes with expectant management as opposed to prophylactic decompression with long-term stable vision occurring in 95 versus 75.6% of patients, respectively.9 While numerous studies have demonstrated the potential benefit from therapeutic intervention through transcranial approaches, the role of less invasive treatments such as endoscopic decompression is still being defined.
A sound understanding of ventral skull base anatomy is critical for successful endoscopic endonasal decompression. The optic nerve travels from an anterolateral to posteromedial direction until reaching the chiasm and is divided into four parts; the intraocular, orbital, canalicular, and intracranial segments. We have recently proposed further subdivision of the canalicular segment into true canalicular (true osseous optic canal) and preforaminal (under the falciform ligament) subsegments, with distinct surgical implications for exposure and decompression at each subsegment (unpublished data). While any of these segments is potentially at risk, the canalicular segment is particularly prone to compressive injury given its rigid bony confines. The optic nerve at the preforaminal subsegment is, however, protected by cerebrospinal fluid extending from the optic-carotid intracranial cistern. The use of image-guidance navigation systems can be a critical aid during surgery, though it should not be relied upon to define anatomy for the surgeon. Detailed knowledge of the neighboring neurovascular structures is critical for safe surgery. It is important to decompress the posterior orbit and sella as early steps as this allows for relatively safe landmarks from which to proceed with decompression. Also, if not obscured by disease, the lateral opticocarotid recess provides a reliable landmark within the sphenoid sinus (or possibly a posterior ethmoid cell in the presence of an Onodi cell) for identifying the paraclinoidal ICA inferiorly and the canalicular segment of the optic nerve superiorly. Also, important is the relationship of the ophthalmic artery relative to the nerve. Any compromise of this blood supply could lead to visual loss. Anatomic studies16 17 have found that in the vast majority of individuals, the artery arises from the ICA, though in a minority, it can arise from branches of the ICA or even branches from the external carotid artery.17 Within the canalicular segment, the ophthalmic artery most commonly lays inferior or inferomedial to the nerve and moves laterally to be located inferolateral to the nerve at the intraorbital os. At the intracranial os, there is significantly greater variability in its location.16 17 While the artery typically runs within the optic nerve sheath, in a small subset of patients the artery may travel in a separate bony canal inferior to the nerve16; it is potentially at risk during decompression and thus detailed preoperative imaging is important in alerting the surgeon to this anatomic variation.
The definitive role and indications for endoscopic optic nerve decompression are still being defined. Much of the early work was done to treat TON. These works provided much of the original descriptions of the procedure and established its safety. While endoscopic decompression is still performed for TON, numerous studies have failed to demonstrate conclusive evidence that it improves outcomes18 and thus it remains a controversial indication. Contrary to TON, endoscopic decompression of the optic nerve for other benign pathologies causing compressive optic neuropathy of the canalicular segment has only rarely been reported in the literature19 20 21 and only in the form of case reports or small case series. While benign lesions affecting the optic nerve can lead to optic neuropathy and visual impairment, the risk:benefit ratio of surgical treatment must be weighed on a case by case basis. Possible complications, though rare, include cerebrospinal fluid leak (which can be limited by avoiding nerve sheath incisions), nasal morbidity, carotid injury, and worsening vision/blindness. Current contraindications include severe neural atrophy in which decompression is unlikely to make any significant impact, asymptomatic patients with no signs of compressive optic neuropathy, and lack of advanced endoscopic expertise and equipment.
The current report demonstrates that endoscopic decompression of the optic nerve for fibrous dysplasia is potentially safe and effective for stabilizing or restoring visual impairment in select patients. Early outcomes in all patients demonstrated either complete resolution or improvement of preoperative visual impairment, though long-term outcomes are lacking. No major complications were noted, though one patient did require revision sinus surgery for chronic sinusitis due to cicatrix formation. Despite wide variation in the timing from the onset of signs/symptoms to presentation and treatment, all patients demonstrated benefit in terms of their visual outcomes. This coincides with other series (open, transcranial)22 23 that have noted a poor correlation between duration of symptoms before intervention and visual outcomes following decompression. Perhaps a better predictor of success may be OCT data, specifically retinal nerve fiber layer thickness as some have suggested.24 25 26
While this study demonstrates a benefit of endoscopic decompression in select patients, there are several limitations. First, it is a small study population. Benign pathology of the canalicular segment of the optic nerve that is amenable to surgical decompression is rare. The limited number of patients and interventions prevents making definitive statements about the overall efficacy and safety. The current study only evaluated patients affected by fibrous dysplasia and whether this can be extrapolated to those with other fibro-osseous lesions is unknown. The follow-up in the current study is limited. As demonstrated by one patient, there is the possibility for recurrent compressive optic neuropathy following endoscopic endonasal decompression. Although there was not disease progression on imaging, his visual acuity and color perception worsened on ophthalmologic testing and prompted a lateral decompression via craniotomy. The prevalence of the need for additional interventions as well as timing following an endoscopic approach is unknown. Additional work is required in the future to fully define the role of endoscopic optic nerve decompression for fibrous dysplasia. More concrete indications need to be determined to enable more widespread acceptance. While randomized controlled trials are unrealistic given the rarity of these disorders, additional reporting of outcomes is needed to critically analyze and determine those likely to benefit from intervention. While endoscopic optic nerve decompression does appear to have an emerging role in the management of these lesions, this does not mean that open cranial approach is now obsolete. The potential benefits of improved visualization, preserved olfaction, more rapid recovery, lack of scarring, decreased operative stress,27 and lack of cerebral retraction19 would favor an endoscopic approach in some settings, though it is not universal. Location of the pathology (medial vs. lateral) is perhaps the most critical determinant of approach used as well as surgeon experience and comfort with a particular technique. While the use of endoscopic endonasal decompression is expected to grow with further clarification of its indications, it is not expected to completely supplant traditional transcranial approaches.
Conclusion
Fibrous dysplasia is a rare disease that can lead to compressive optic neuropathy and subsequent visual impairment. Only patients with signs and/or symptoms of compressive optic neuropathy are candidates for surgery. The EEA for optic nerve decompression appears to be a safe and effective means of treating these patients. It is unknown if endoscopic decompression can be successfully applied to most or all patients with this disease component or rather if only a select subgroup is likely to benefit and requires further study with longer follow-up. While transcranial approaches are expected to continue to have a role in the management of these patients, the EEA shows promise and may become a preferred option for therapeutic intervention.
Financial Support
None.
Footnotes
Conflict of Interest None.
References
- 1.Dumitrescu C E, Collins M T. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. doi: 10.1186/1750-1172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinstein L S, Shenker A, Gejman P V, Merino M J, Friedman E, Spiegel A M. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325(24):1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 3.Bianco P, Riminucci M, Majolagbe A. et al. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res. 2000;15(1):120–128. doi: 10.1359/jbmr.2000.15.1.120. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P, Robey Pg. Diseases of bone and the stromal cell lineage. J Bone Miner Res. 1999;14(3):336–341. doi: 10.1359/jbmr.1999.14.3.336. [DOI] [PubMed] [Google Scholar]
- 5.Valentini V, Cassoni A, Marianetti T M, Terenzi V, Fadda M T, Iannetti G. Craniomaxillofacial fibrous dysplasia: conservative treatment or radical surgery? A retrospective study on 68 patients. Plast Reconstr Surg. 2009;123(2):653–660. doi: 10.1097/PRS.0b013e318196bbbe. [DOI] [PubMed] [Google Scholar]
- 6.DeKlotz T R, Kim H J, Kelly M, Collins M T. Sinonasal disease in polyostotic fibrous dysplasia and McCune-Albright Syndrome. Laryngoscope. 2013;123(4):823–828. doi: 10.1002/lary.23758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler C M Lee J S Butman J A et al. Long-term outcome of optic nerve encasement and optic nerve decompression in patients with fibrous dysplasia: risk factors for blindness and safety of observation Neurosurgery 20065951011–1017., discussion 1017–1018 [DOI] [PubMed] [Google Scholar]
- 8.Lee J S, FitzGibbon E, Butman J A. et al. Normal vision despite narrowing of the optic canal in fibrous dysplasia. N Engl J Med. 2002;347(21):1670–1676. doi: 10.1056/NEJMoa020742. [DOI] [PubMed] [Google Scholar]
- 9.Amit M, Collins M T, FitzGibbon E J, Butman J A, Fliss D M, Gil Z. Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia—a meta-analysis. PLoS ONE. 2011;6(9):e25179. doi: 10.1371/journal.pone.0025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plotkin H, Rauch F, Zeitlin L, Munns C, Travers R, Glorieux F H. Effect of pamidronate treatment in children with polyostotic fibrous dysplasia of bone. J Clin Endocrinol Metab. 2003;88(10):4569–4575. doi: 10.1210/jc.2003-030050. [DOI] [PubMed] [Google Scholar]
- 11.DiMeglio L A. Bisphosphonate therapy for fibrous dysplasia. Pediatr Endocrinol Rev. 2007;4 04:440–445. [PubMed] [Google Scholar]
- 12.Luxenberger W, Stammberger H, Jebeles J A, Walch C. Endoscopic optic nerve decompression: the Graz experience. Laryngoscope. 1998;108(6):873–882. doi: 10.1097/00005537-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Dumont A S, Boulos P T, Jane J A Jr, Ellegala D B, Newman S A, Jane J A Sr. Cranioorbital fibrous dysplasia: with emphasis on visual impairment and current surgical management. Neurosurg Focus. 2001;10(5):E6. [PubMed] [Google Scholar]
- 14.Papay F A Morales L Jr Flaharty P et al. Optic nerve decompression in cranial base fibrous dysplasia J Craniofac Surg 1995615–10., discussion 11–14 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y R Breidahl A Chang C N Optic nerve decompression in fibrous dysplasia: indications, efficacy, and safety Plast Reconstr Surg 199799122–30., discussion 31–33 [DOI] [PubMed] [Google Scholar]
- 16.Locatelli M, Caroli M, Pluderi M. et al. Endoscopic transsphenoidal optic nerve decompression: an anatomical study. Surg Radiol Anat. 2011;33(3):257–262. doi: 10.1007/s00276-010-0734-1. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Wang J, Jing X, Zhang W, Zhang X, Qiu Y. Transsphenoidal optic nerve decompression: an endoscopic anatomic study. J Craniofac Surg. 2008;19(6):1670–1674. doi: 10.1097/SCS.0b013e31818b4316. [DOI] [PubMed] [Google Scholar]
- 18.Robinson D Wilcsek G Sacks R Orbit and orbital apex Otolaryngol Clin North Am 2011444903–922., viii [DOI] [PubMed] [Google Scholar]
- 19.Kong D-S, Shin H J, Kim H Y. et al. Endoscopic optic canal decompression for compressive optic neuropathy. J Clin Neurosci. 2011;18(11):1541–1545. doi: 10.1016/j.jocn.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 20.Pletcher S D, Metson R. Endoscopic optic nerve decompression for nontraumatic optic neuropathy. Arch Otolaryngol Head Neck Surg. 2007;133(8):780–783. doi: 10.1001/archotol.133.8.780. [DOI] [PubMed] [Google Scholar]
- 21.Sia D IT, Chan W O, Wormald P J, Davis G, Selva D. Decompression of benign orbital apex lesion via medial endoscopic approach. Orbit. 2012;31(5):344–346. doi: 10.3109/01676830.2012.678920. [DOI] [PubMed] [Google Scholar]
- 22.Bulters D O, Shenouda E, Evans B T, Mathad N, Lang D A. Visual recovery following optic nerve decompression for chronic compressive neuropathy. Acta Neurochir (Wien) 2009;151(4):325–334. doi: 10.1007/s00701-009-0192-x. [DOI] [PubMed] [Google Scholar]
- 23.Sleep T J, Hodgkins P R, Honeybul S, Neil-Dwyer G, Lang D, Evans B. Visual function following neurosurgical optic nerve decompression for compressive optic neuropathy. Eye (Lond) 2003;17(5):571–578. doi: 10.1038/sj.eye.6700439. [DOI] [PubMed] [Google Scholar]
- 24.Jacob M, Raverot G, Jouanneau E. et al. Predicting visual outcome after treatment of pituitary adenomas with optical coherence tomography. Am J Ophthalmol. 2009;147(1):64–7000. doi: 10.1016/j.ajo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Miller N R. Predicting visual recovery following optic nerve decompression for chronic optic neuropathy. Acta Neurochir (Wien) 2009;151(12):1729. doi: 10.1007/s00701-009-0404-4. [DOI] [PubMed] [Google Scholar]
- 26.Moon C H, Hwang S C, Ohn Y-H, Park T K. The time course of visual field recovery and changes of retinal ganglion cells after optic chiasmal decompression. Invest Ophthalmol Vis Sci. 2011;52(11):7966–7973. doi: 10.1167/iovs.11-7450. [DOI] [PubMed] [Google Scholar]
- 27.Metson R Pletcher S D Endoscopic orbital and optic nerve decompression Otolaryngol Clin North Am 2006393551–561., ix [DOI] [PubMed] [Google Scholar]


