Abstract
Objectives The objective of this study was to evaluate region-specific surgical instrument kinematics among novice and experienced surgeons performing endoscopic endonasal skull base surgery.
Design Cadaveric experimental study.
Setting Tertiary academic center.
Participants Two novice and two experienced surgeons performed eight endoscopic total ethmoidectomies and sphenoidotomies using an optically tracked microdebrider.
Main Outcome Measures Time-stamped Euclidian coordinates were recorded. Cumulative instrument travel, mean linear velocity and acceleration, and mean angular velocities were calculated in the anterior ethmoid, posterior ethmoid, and sphenoid sinus regions.
Results Mean cumulative instrument travel (standard deviation) was highest in the posterior ethmoid region for both novice and experienced surgeons (9,795 mm [1,664] vs. 3,833 mm [1,080]). There was a trend in mean linear and angular velocities, and acceleration with increasing magnitudes for experienced surgeons compared with novices. Among experienced surgeons, we observed a trend of decreasing yaw velocity during the approach to the surgical target.
Conclusions We present a novel method of evaluating surgical instrument motion with respect to anatomical regions of the skull base during endoscopic endonasal skull base surgery. These data may be used in the development of surgical monitoring and training systems to optimize patient safety.
Keywords: surgical instrument motion, kinematics, skull base, surgical safety, surgical training
Introduction
Understanding the objective metrics of surgical instrument motion is critical for optimizing surgical safety during endoscopic endonasal skull base and sinus surgery, evaluating and designing surgical instruments, evaluating trainee performance, and peer review of surgical performance. Objective surgical instrument kinematics, that is, the properties of motion in an object, depend on the procedure or surgical exercise being performed.1 As a result, alternative methods for analyzing surgical instrument motion have been investigated, including evaluation of unstructured instrument motion.2 One aspect common to all of surgery is respect of anatomical boundaries. During endoscopic endonasal approaches to the sella and pituitary gland, our group has observed instrument movements involving longer excursions in the anterior ethmoid region compared with shorter, more compact, and precise movements during the sphenoidotomy and while accessing the dorsum sella. We believe that augmentation of instrument kinematics is a result of the proximity to critical neurovascular structures. However, we currently lack methods for evaluating surgical motion with respect to anatomical regions and critical structures.
Surgical motion analysis is currently limited to evaluation using video review and/or objective analyses using probabilistic models, descriptive models, or aggregate metrics of performance over an entire procedure. Recent work employing expert video review of laparoscopic procedures found that surgical skill correlates with patient outcomes.3 However, video review is time consuming and inefficient as the lone method for instrument motion and skill analysis during surgical procedures. Objective instrument motion analysis automates and complements video review. Aggregate objective motion metrics have been observed to differentiate surgical skill level when comparing cumulative instrument travel over the course of a procedure.4 5 6 7 8 Other aggregate objective measures that have been studied include velocity and time to completion.9 Instrument gestures, such as reaching for a needle, have also been explored as a method for evaluating differences between expert and intermediate level surgeons during robotic training tasks.10 Previous surgical motion investigations have largely been performed in bench-top models for practical reasons including to develop models for further research. Clinical generalizability is limited with bench-top models, and further analysis of instrument kinematics in surgical procedures is warranted.
The purpose of this study was to develop and evaluate a method for measuring endoscopic instrument kinematics within three anatomical regions of the nasal cavity during an approach to the pituitary gland. Based on prior observations during endoscopic endonasal skull base approaches to the pituitary, we anticipated that surgical instrument kinematic parameters would vary by anatomical region and that kinematic parameters would vary with experience. Exploring instrument kinematics during surgical procedures in actual patients or high-fidelity models (e.g., cadavers) increases the generalizability of kinematic measurements to clinical scenarios including the development of surgical warning systems and surgical training tools. A cadaveric model was employed to mimic tissue conditions during live surgery. Cadaveric models have been used in previous work evaluating surgical instrument motion.11
Methods
After obtaining approval by the University of Washington Institutional Review Board (IRB 51785), two experienced otolaryngologists and two junior otolaryngology resident surgeons were enrolled in the study. Participants received a standardized orientation to the surgical procedure and did not review the computed tomography (CT) scan prior to surgical performance. Participants were made aware that the objective of this study was to develop a method for evaluating surgical instrument motion for patient safety and training purposes. Each participant was asked to perform a bilateral anterior ethmoidectomy, posterior ethmoidectomy, and sphenoidotomy as they would normally do to provide surgical access to the sella using a zero-degree endoscope and an optically tracked microdebrider.
Experimental Setup
A fresh cadaver cephalus was placed in fixation using a Mayfield Skull Clamp (Integra LifeSciences, Plainsboro, New Jersey, United States). The cadaver specimen was registered to the respective preoperative CT scan using the iNtellect Cranial Navigation System (Stryker, Kalamazoo, Michigan, United States). Optical instrument tracking was performed by fixing a Stryker Universal Tracker to a microdebrider (Medtronic, Minneapolis, Minnesota, United States) via a clamp. The microdebrider was registered to the cadaver specimen. An endoscopic video tower was positioned in front of the navigation screen to prevent participants from using the navigation for the surgical procedure. A study coordinator visualized the navigation monitor to verify instrument tracking and warn the operating surgeons when optical occlusion occurred. Each surgeon performed the aforementioned procedures on one cadaver in the bilateral nasal cavities until self-determination of completion of the ethmoidectomy and sphenoidotomy. All procedures were performed in a surgical simulation laboratory located at Harborview Medical Center.
Data Capture and Processing
Dense time-stamped microdebrider Euclidian coordinates were recorded at a rate of 10 samples per second and were stored in a text file within the navigation computer. Following completion of the procedure, the coordinate log was transferred to a laboratory laptop equipped with MATLAB (R2015A, MathWorks, Natick, Massachusetts, United States). Data were converted by our custom surgical planning software system into an ASCII file format and were then imported into MATLAB. Anatomical boundaries were defined via CT from each cadaver specimen using open source software (3D Slicer, www.slicer.org). These boundaries included the anterior ethmoid region defined from the head of the middle turbinate to the junction of the vertical and horizontal segments of the basal lamella, posterior ethmoid region defined as the junction of the vertical and horizontal segments of the basal lamella to the sphenoid face, and sphenoid region from 1 cm anterior to the sphenoid face to the clivus. Time-stamped Euclidian coordinates were divided by region (Fig. 1a, b). Coordinates were used to calculate cumulative instrument travel, mean velocity, and mean acceleration. To calculate angular velocity, the difference between the instrument and optical tracker vectors was calculated at each time point. The angle between sequential vectors was calculated. Angular velocity, including mean pitch and yaw velocities, was calculated by determining the change in angle divided by Δt. The change in time (Δt) between each consecutive data sample was defined by calculating the inverse of the sampling rate:
Fig. 1.
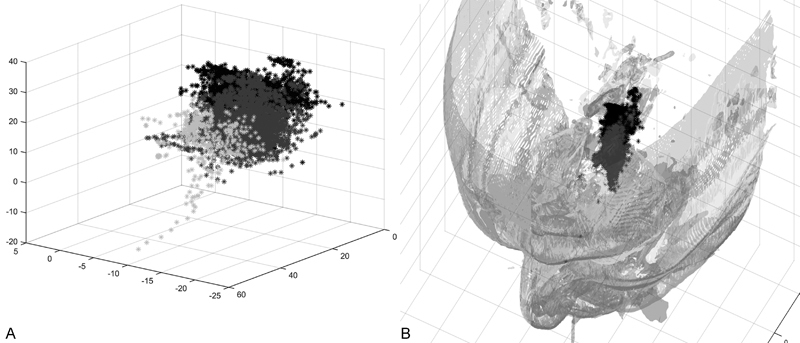
Time-stamped instrument coordinates. (A) Representative microdebrider coordinates in the time domain. (B) Region-specific microdebrider coordinates illustrated within their respective locations of the nasal cavity. Light gray points, anterior ethmoid coordinates; medium gray points, posterior ethmoid coordinates; dark gray points, sphenoid coordinates.
between samples or 100 ms between samples. To capture consecutive time points within a region, we excluded instantaneous velocities with a Δt of > 100 ms from the analyses. An instantaneous velocity with a Δt > 100 ms would imply nonconsecutive data samples. Kinematic parameters were calculated for each anatomical region and exported to an Excel spreadsheet (Microsoft Corporation, Redmond, Washington, United States).
Statistical Analysis
Descriptive statistics were performed using Stata 14 statistical programming software (Stata Statistical Software, Release 14, 2015, StataCorp., College Station, Texas, United States). Summary statistics were generated for each region and skill level. Inferential hypothesis testing was not performed as the primary goal of this study was to develop and refine methods for evaluating instrument kinematics with respect to sinus and skull base structures.
Results
Cumulative distance traveled by the microdebrider tip was calculated for each surgeon including two novices (n = 4 sides) and two experienced surgeons (n = 4 sides) on a total of four cadaver specimens (Fig. 2). Among novices, mean (standard deviation, SD) travel was greatest during the posterior ethmoidectomy at 9,795 mm (1,664) compared with the experienced surgeons whose mean cumulative travel was 3,833 mm (1,080). Cumulative microdebrider travel for the novices was 1,785 mm (535.1) in the anterior ethmoid region and 1,453 mm (693.1) in the sphenoid. Experienced surgeon instrument travel during the anterior ethmoidectomy was 820.9 mm (410.7), and during the sphenoidotomy it was 2,633 mm (1,449).
Fig. 2.
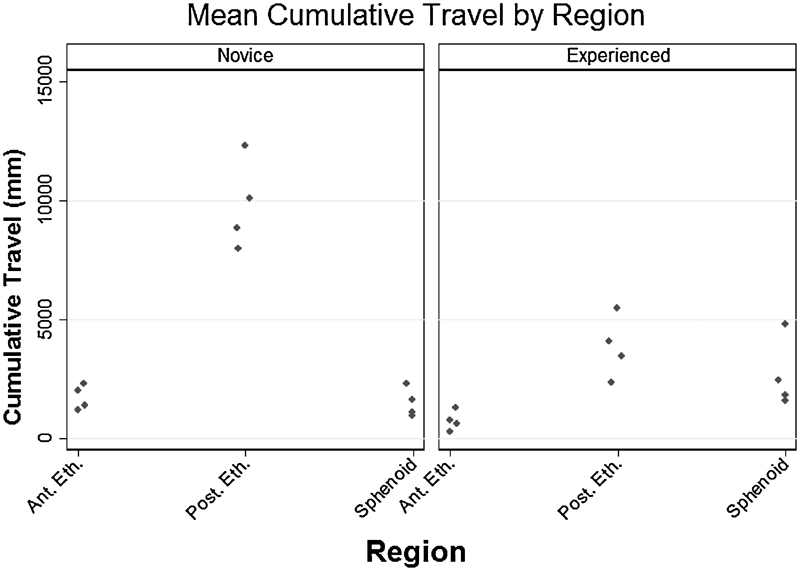
Mean cumulative instrument travel by region. Mean cumulative instrument travel (mm) is represented for novice procedures (left panel; n = 4) and experienced surgeon procedures (right panel; n = 4). Points represent individual means from each procedure within respective anatomical regions. Ant. Eth., anterior ethmoid region; Post. Eth., posterior ethmoid region; Sphenoid, sphenoid region.
The mean (SD) microdebrider velocities for experienced surgeons were 2.2 cm/s (0.75), 2.5 cm/s (0.59), and 2.6 cm/s (0.52) for the anterior ethmoidectomy, posterior ethmoidectomy, and the sphenoidotomy, respectively (Fig. 3a, left panel). Among the novice surgeons, mean velocities were 1.5 cm/s (0.41), 1.4 cm/s (0.38), and 1.3 cm/s (0.27) for the anterior ethmoid, posterior ethmoid, and sphenoid regions, respectively (Fig. 3a, right panel). Mean accelerations by region for the experienced surgeons were 1,150 cm/s2 (634), 903 cm/s2 (276), and 877 cm/s2 (177), and for the novice surgeons, they were 744 cm/s2 (383), 227 cm/s2 (51.1), and 171 cm/s2 (62.1) for the anterior ethmoid, posterior ethmoid, and sphenoid regions, respectively (Fig. 3b).
Fig. 3.
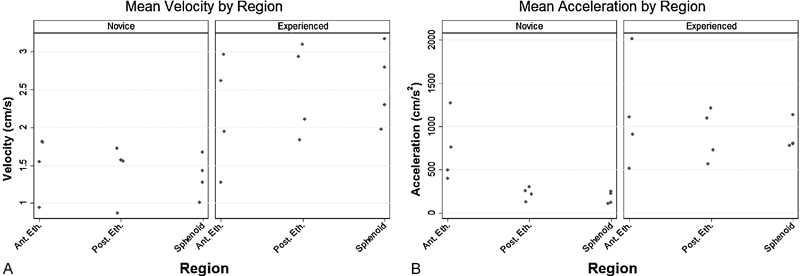
Mean instrument linear velocity and acceleration by region. (A) Mean instrument linear velocity (cm/s) by region for novice procedures (left panel; n = 4) and experienced surgeons (right panel; n = 4). (B) Mean instrument linear acceleration (cm/s2) by region for novice procedures (left panel; n = 4) and experienced surgeons (right panel; n = 4). Points represent individual means from each procedure within respective anatomical regions. Ant. Eth., anterior ethmoid region; Post. Eth., posterior ethmoid region; Sphenoid, sphenoid region.
Mean angular velocities were calculated by region for each participant. Angular velocity represents rotational movements over time. Angular velocity may change with instrument location along the skull base due to physical constraints of the nasal cavity and proximity of critical neurovascular structures. Among the experienced surgeons, mean (SD) instrument pitch velocities, angular velocity in the sagittal plane, were 12.6 deg/s (1.74), 13.7 deg/s (2.03), and 12.1 deg/s (1.80), whereas among the novice surgeons, mean pitch velocities, angular velocity in the axial plane, were 8.57 deg/s (2.20), 7.89 deg/s (1.61), and 6.90 deg/s (1.35) for the anterior ethmoid, posterior ethmoid, and sphenoid regions, respectively (Fig. 4a). Mean (SD) instrument yaw velocities among the experienced surgeons were 22.1 deg/s (4.17), 17.8 deg/s (2.38), and 14.4 deg/s (2.36) in contrast to the novice surgeons at 9.71 deg/s (2.91), 8.65 deg/s (2.25), and 6.21 deg/s (1.49) for the anterior ethmoid, posterior ethmoid, and sphenoid regions, respectively (Fig. 4b).
Fig. 4.
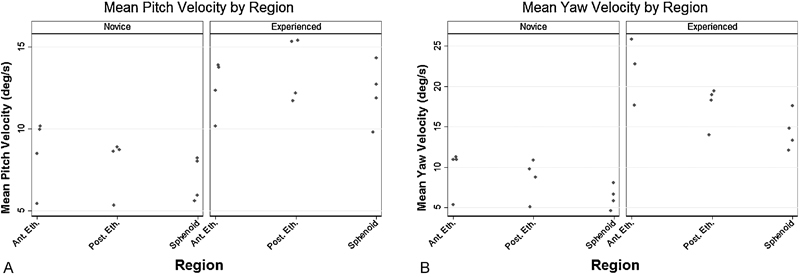
Mean instrument angular velocity by region. (A) Mean instrument pitch velocity (deg/s) by region for novice procedures (left panel; n = 4) and experienced surgeons (right panel; n = 4). (B) Mean instrument yaw velocity (deg/s) by region for novice procedures (left panel; n = 4) and experienced surgeons (right panel; n = 4). Points represent individual means from each procedure within respective anatomical regions. Ant. Eth., anterior ethmoid region; Post. Eth., posterior ethmoid region; Sphenoid, sphenoid region.
Discussion
In this study, we illustrated a novel paradigm for evaluating surgical instrument motion with respect to intranasal and skull base anatomical domains. Our principal findings include increased instrument travel in the posterior ethmoid for novice compared with experienced surgeons, higher linear and angular velocity magnitudes among experienced surgeons compared with novice surgeons, and decreasing yaw velocity as the pituitary is approached among experienced surgeons. These data suggest that instrument motion varies with level of experience as more experienced surgeons operate with higher magnitude linear and angular velocities and acceleration, and lower overall instrument travel indicative of greater efficiency and knowledge of surrounding neurovascular structures. Experienced surgeons are more aware of the surrounding anatomy and augment their movements as suggested by our yaw velocity findings. Collectively, our method of region-based objective instrument kinematic analysis provides a platform for quantifying surgical therapy.
To our knowledge, this is the first study to evaluate instrument kinematics with respect to anatomical regions. Previous investigators measured linear velocity on a da Vinci robotic surgical system (Intuitive Surgical, Sunnyvale, California, United States) during a bead transfer task to compare novice and expert surgeons and observed that linear velocity ranged from 0.8 to 10 cm/s for novices and 0.08 to 12.1 cm/s for experts.9 In the more confined space of the nasal cavity, we noted mean linear velocities dependent on anatomical region ranging from 1.3 to 1.5 cm/s for novices and 2.2 to 2.6 cm/s for experienced surgeons, suggesting that anatomical constraints and proximity to critical neurovascular structures are key factors in assessing instrument kinematics. Verner et al found that angular velocity averaged 14.7 deg/s for novices and 16.5 deg/s for experts, whereas we noted mean angular velocities close to 9 deg/s in the anterior ethmoid region for novices and up to 22.1 deg/s in the anterior ethmoid region for experts.9 Aggregate instrument kinematics may be more generalizable when analyzed with respect to anatomical regions, allowing for more granular evaluation of surgical skill and efficiency.
We also noted differences in surgical instrument kinematics stratified by anatomical region among surgeons with different amounts of experience. Experienced surgeons attenuated their yaw velocities between the anterior ethmoid and sphenoid sinus region decreasing from 22.1 to 14.4 deg/s (Fig. 4b, right panel). Experienced surgeons may decrease yaw velocity, or side-to-side motion, given the tight confines of the posterior ethmoid air cells compared with the anterior ethmoid region, which allows broader, sweeping motions. Moreover, yaw motion in the posterior ethmoid air cells is attenuated as the surgeon must assess proximity to the skull base prior to debridement of a candidate air cell. Yaw velocity in the sphenoid region may decrease even further given the proximity to the internal carotid artery and optic nerve while performing the sphenoidotomy. In comparison, novice surgeons operated with a more constant mean yaw velocity between the anterior ethmoid and sphenoid sinus regions decreasing from 9.71 to 6.21 deg/s (Fig. 4b, left panel). Generally, lower yaw velocities among novice surgeons may reflect uncertainty of anatomical landmarks and the location of the skull base. Prior investigators noted similar observations in a robotic surgical simulation where novices showed excess, more erratic, and slower movements compared with experts.9
Our data also suggest differences in efficiency based on experience. Experienced surgeons operated with higher yaw velocity and acceleration in the anterior ethmoid where the main structures at risk are the orbit and nasolacrimal duct, whereas novices used more constant and lower kinematic magnitudes throughout the nasal cavity. Similarly, previous research by Rosen et al in a laparoscopic trainer observed that novices used higher force and torque magnitudes compared with experts during tissue manipulation, whereas experts used sufficient force and torque magnitudes to safely accomplish the task.12 13 Ahmidi et al also performed a study evaluating surgical instrument and eye motion patterns observing that expert surgeon's tool path was more simple in structure and directed to the target compared with novice instrument and eye motion patterns.11 Our region-specific kinematic data were in agreement with Ahmidi et al's work as we noted higher instrument travel with more constant kinematic features by novice surgeons, suggesting less directed and less efficient instrument motion as a result of uncertainty and less experience. In contrast to prior studies, this study evaluated instrument kinematics during actual operative procedures in a cadaver model. We also illustrated the value of measuring anatomical region-specific instrument kinematics for differentiating skill level during surgical approaches to the skull base, which add to the existing literature and suggest a complementary technique for assessing surgical efficiency during training and live surgical procedures.
While endoscopic endonasal surgery has drastically reduced the rate of major complications such as vascular injury during the transsphenoidal approach to pituitary tumors, these devastating injuries continue to occur in at least 0.2 to 1.4% of cases.14 No study has demonstrated that image guidance, a user-dependent modality, decreases complication rates during endoscopic endonasal surgery.15 A recent analysis of internal carotid artery injuries during endoscopic endonasal sinus and skull base surgery for both inflammatory cases (e.g., polyposis) and neoplasm (e.g., pituitary adenoma) found that reported injuries tended to occur during entry into the sphenoid sinus, while removing bone around the sphenoid sinus, and during drilling along the skull base among other causes.16 Understanding instrument motion in key areas of the endoscopic endonasal approach may allow us to develop real-time monitoring systems with the capacity to detect high-risk movements. For example, linear and/or angular velocities outside of the characteristic range of a surgeon in a given anatomical location could trigger a warning system. Movements with slower velocity than expected could reflect uncertainty of the location of skull base in the setting of distorted anatomy. Movements with high velocity in close proximity to critical neurovascular structures could also trigger an alarm.
Limitations of this study include the small sample size. The intent of this exploratory analysis was to develop a novel, yet complementary technique for analyzing surgical instrument motion. We were also interested in determining the necessary sample size to accordingly power subsequent research in this area. Our power analysis revealed that the largest sample size needed to detect a difference in linear velocity between novice and experienced surgeons of 0.673 cm/s (i.e., for the anterior ethmoid region) is 14 subjects per group. In comparison, to detect a difference in mean yaw velocities between the anterior ethmoid and sphenoid regions of 7.7 deg/s, we would need a sample size of five subjects per group. Another limitation is the lack of kinematic analysis of other instruments. Differences in instrument kinematics between novices and experts may have been masked by the microdebrider. While the main goal of this study was to develop and evaluate a method for assessing instrument kinematics with respect to anatomical structures, a future studies will evaluate additional instruments to characterize differences by instrument type. We have developed a novel method for objectively assessing endoscopic endonasal surgical motion and determined the sample size needed for future validation studies of this technique. Currently, work is underway to validate this technique in actual transnasal skull base surgery at our institution.
Conclusion
Our findings provide evidence that the measurement of anatomical region-specific instrument kinematics may be used during endoscopic endonasal skull base surgery to assess surgical skill in real time. Region-specific instrument kinematics provide information that could be used in the design of real-time intraoperative monitoring applications running in the background that assess surgical efficiency. Instrument kinematics could also be used for designing surgical warning systems that detect variations in surgeon-specific kinematics such as trepidation due to uncertainty of the location of skull base due to severe inflammation or disease obscuring normal landmarks. Further investigation is warranted to fully elucidate region- and surgeon-specific instrument kinematics.
Acknowledgments
The authors would like to acknowledge grant support from the following grants: T32DC000018 (PI: Edward Weaver) and R21 EB 016122–02 (PI: Blake Hannaford).
References
- 1.Oropesa I, Sánchez-González P, Lamata P. et al. Methods and tools for objective assessment of psychomotor skills in laparoscopic surgery. J Surg Res. 2011;171(1):e81–e95. doi: 10.1016/j.jss.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 2.Ahmidi N, Poddar P, Jones J D. et al. Automated objective surgical skill assessment in the operating room from unstructured tool motion in septoplasty. Int J CARS. 2015;10(6):981–991. doi: 10.1007/s11548-015-1194-1. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer J D, Finks J F, O'Reilly A. et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369(15):1434–1442. doi: 10.1056/NEJMsa1300625. [DOI] [PubMed] [Google Scholar]
- 4.Datta V, Mackay S, Mandalia M, Darzi A. The use of electromagnetic motion tracking analysis to objectively measure open surgical skill in the laboratory-based model. J Am Coll Surg. 2001;193(5):479–485. doi: 10.1016/s1072-7515(01)01041-9. [DOI] [PubMed] [Google Scholar]
- 5.Datta V, Mandalia M, Mackay S, Chang A, Cheshire N, Darzi A. Relationship between skill and outcome in the laboratory-based model. Surgery. 2002;131(3):318–323. doi: 10.1067/msy.2002.120235. [DOI] [PubMed] [Google Scholar]
- 6.Bann S D, Khan M S, Darzi A W. Measurement of surgical dexterity using motion analysis of simple bench tasks. World J Surg. 2003;27(4):390–394. doi: 10.1007/s00268-002-6769-7. [DOI] [PubMed] [Google Scholar]
- 7.Dosis A, Aggarwal R, Bello F. et al. Synchronized video and motion analysis for the assessment of procedures in the operating theater. Arch Surg. 2005;140(3):293–299. doi: 10.1001/archsurg.140.3.293. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal R, Grantcharov T, Moorthy K. et al. An evaluation of the feasibility, validity, and reliability of laparoscopic skills assessment in the operating room. Ann Surg. 2007;245(6):992–999. doi: 10.1097/01.sla.0000262780.17950.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verner L, Oleynikov D, Holtmann S, Haider H, Zhukov L. Measurements of the level of surgical expertise using flight path analysis from da Vinci robotic surgical system. Stud Health Technol Inform. 2003;94:373–378. [PubMed] [Google Scholar]
- 10.Lin H C, Shafran I, Yuh D, Hager G D. Towards automatic skill evaluation: detection and segmentation of robot-assisted surgical motions. Comput Aided Surg. 2006;11(5):220–230. doi: 10.3109/10929080600989189. [DOI] [PubMed] [Google Scholar]
- 11.Ahmidi N, Ishii M, Fichtinger G, Gallia G L, Hager G D. An objective and automated method for assessing surgical skill in endoscopic sinus surgery using eye-tracking and tool-motion data. Int Forum Allergy Rhinol. 2012;2(6):507–515. doi: 10.1002/alr.21053. [DOI] [PubMed] [Google Scholar]
- 12.Rosen J, Brown J D, Barreca M, Chang L, Hannaford B, Sinanan M. The Blue DRAGON—a system for monitoring the kinematics and the dynamics of endoscopic tools in minimally invasive surgery for objective laparoscopic skill assessment. Stud Health Technol Inform. 2002;85:412–418. [PubMed] [Google Scholar]
- 13.Rosen J, Solazzo M, Hannaford B, Sinanan M. Objective laparoscopic skills assessments of surgical residents using Hidden Markov Models based on haptic information and tool/tissue interactions. Stud Health Technol Inform. 2001;81:417–423. [PubMed] [Google Scholar]
- 14.Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84(8):843–849. doi: 10.1136/jnnp-2012-303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunkaraneni V S, Yeh D, Qian H, Javer A R. Computer or not? Use of image guidance during endoscopic sinus surgery for chronic rhinosinusitis at St Paul's Hospital, Vancouver, and meta-analysis. J Laryngol Otol. 2013;127(4):368–377. doi: 10.1017/S0022215113000261. [DOI] [PubMed] [Google Scholar]
- 16.Chin O Y, Ghosh R, Fang C H, Baredes S, Liu J K, Eloy J A. Internal carotid artery injury in endoscopic endonasal surgery: a systematic review. Laryngoscope. 2016;126(3):582–590. doi: 10.1002/lary.25748. [DOI] [PubMed] [Google Scholar]


