Abstract
Objectives This study aims to report tumor control rates and cranial nerve function after low dose (11.0 Gy) Gamma knife radiosurgery (GKRS) in patients with vestibular schwannomas.
Methods A retrospective chart review was performed on 30 consecutive patients with vestibular schwannomas treated from March 2004 to August 2010 with GKRS at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. The marginal dose for all patients was 11.0 Gy prescribed to the 50% isodose line. Median follow-up time was 42 months. The median treatment volume was 0.53 cm3. Hearing data were obtained from audiometry reports before and after radiosurgery.
Results The actuarial progression free survival (PFS) based on freedom from surgery was 100% at 5 years. PFS based on freedom from persistent growth was 91% at 5 years. One patient experienced tumor progression requiring resection at 87 months. Serviceable hearing, defined as Gardner–Robertson score of I–II, was preserved in 50% of patients. On univariate and multivariate analyses, only higher mean and maximum dose to the cochlea significantly decreased the proportion of patients with serviceable hearing.
Conclusion Vestibular schwannomas can be treated with low doses (11.0 Gy) of GKRS with good tumor control and cranial nerve preservation.
Keywords: neuroma, acoustic, radiosurgery, vestibular schwannoma
Introduction
Vestibular schwannomas are cranial nerve sheath tumors that arise from the vestibular branch of the eighth cranial nerve in the internal acoustic meatus, the cerebellopontine angle, or both.1 The incidence is approximately 0.6 per 100,000 person years and they represent about 6% of intracranial tumors.2 While these tumors are benign and usually slow growing, they can eventually cause a range of symptoms including hearing loss, tinnitus, vertigo, and facial nerve dysfunction. One option for patients with tumors < 1.5 cm in diameter that are relatively asymptomatic is observation with serial hearing tests and magnetic resonance imaging (MRI) scans.3 Alternatively, tumors can be treated with microsurgery or radiation therapy with photons or protons. Progression rates following stereotactic radiosurgery (SRS) are similar to recurrence and progression rates seen following surgery and fractionated radiotherapy respectively.4 5 6 7 8 9 Progressive tumors are treated with either microsurgery or radiosurgery.3
Tumors < 3cm in diameter can be treated initially with single fraction SRS (range, 12–16 Gy), fractionated radiation therapy (range, 50–54 Gy) or proton therapy.10 There are no direct comparisons among these modalities and outcomes with respect to tumor control and hearing preservation have been similar.9 10 11 Tumor control rates following SRS, defined as freedom from surgery, range from 91 to 95% over 3 years, and hearing preservation rates range from 25 to 78%. Currently, most Gamma knife (Elekta Instrument AB Stockholm, Sweden) centers treating vestibular schwannomas prescribe doses of 12 to 13 Gy to the 50% isodose line. Given the good clinical results following all of the therapeutic options described above, many centers treating this benign tumor have attempted to reduce prescribed radiation doses to limit treatment-related hearing loss and other cranial nerve complications.1 4 7 12 13 14 15 16 The Thomas Jefferson group has shown that tumor control rates and cranial nerve preservation were excellent following a lower dose of 46.8 Gy compared with 50.4 Gy delivered with a fractionated stereotactic radiation therapy technique.11 In a similar attempt, we have treated all vestibular schwannoma patients at our center with Gamma knife radiosurgery (GKRS) to 11.0 Gy prescribed to the 50% isodose line. Prescription to the 50% isodose line is typical of GKRS and, compared with typical linear accelerator-based SRS, results in higher doses within the tumor itself. Any difference in tumor dose between GKRS and what might be delivered with linear accelerator-based SRS is an incidental result of these differences in prescription to the tumor periphery. Given the unique dosimetric advantages of GKRS, by prescribing to a lower isodose line (50%) compared with linear accelerator-based SRS, we were hopeful that the resultant dose escalation within the target volume would compensate for any reduction in therapeutic effect that might be caused by reduction in prescribed dose from 12 to 13 to 11 Gy. This study reports the tumor control rates and cranial nerve function preservation rates in these patients.
Methods
Between March 2004 and August 2011, 30 consecutive patients were treated with GKRS for vestibular schwannoma at the Robert H. Lurie Cancer Center of Northwestern University. None of the patients had previously undergone treatment. Patient and treatment outcome data were collected retrospectively from patient charts and were recorded with respect to age, sex, previous intervention, MRI dates, initial tumor volume and diameter, margin dose, tumor growth, need for resection subsequent intervention, audiometry data, and NF2 status.
Radiosurgery Technique
SRS was performed using Model 4-C or Perfexion Leksell Gamma knife (Elekta Instrument AB Stockholm). After stereotactic frame application, target delineation was performed using high-resolution contrast-enhanced MRI. The GKRS plan was performed using multiple isocenters to deliver conformal radiation treatment. The GKRS plan was jointly reviewed by a neurosurgeon, radiation oncologist, and medical physicist. The doses to the brainstem and the cochlea were evaluated. The median tumor volume was 0.53 cm3 (range, 0.025–4.7 cm3). The marginal dose for all patients was 11.0 Gy prescribed to the 50% isodose line. Multiple isocenters (range: 1–17, median: 7) were used to deliver conformal radiosurgery. The cochlear dose depends on the lateral extent of the tumor and also on the size and number of shots used for treatment planning. For acoustic neuromas we only use 4 and 8 mm shots and we used blocking to reduce the cochlear dose. Patient records were examined for evidence of cranial neuropathies and complications from GKRS based on evaluations of the treating physician.
Follow-Up
Follow-up time was calculated from the date of GKRS to the most recent MRI or otolaryngology (ENT) visit, whichever was later. Clinical and MRI follow-up was performed at 3 months, then every 6 months for the first 2 years, and annually thereafter.
Tumor Progression
Progression free survival (PFS) was defined as: (1) freedom from repeat surgical intervention and (2) freedom from persistent tumor growth after radiosurgery.13 17 Tumor size was calculated assuming spherical dimensions as length × width × height × 0.52.18 19
Hearing Preservation
Audiometry results were reviewed each of the patients treated at Northwestern, and audiometry results pre- and postradiosurgery were available for 18 of 30 patients. Pure tone audiometry decibel levels, speech recognition threshold (SRT), and word recognition score (WRS) were tabulated. The Gardner–Robertson (GR) score20 was calculated from SRT and WRS to obtain an assessment of overall hearing because this is the standard used in studies of hearing in vestibular schwannomas. Serviceable hearing was defined as a GR score of I–II, which is based on both SRT ≤ 50 dB and WRS ≥ 50%. If the WRS and SRT did not lead to the same categorization, the higher (worse hearing) GR score was used. The serviceable hearing preservation rate was defined as the percentage of patients who maintained serviceable hearing at follow-up. The GR score preservation rate was defined as the percentage of patients that maintained the same GR at follow-up. Tinnitus is a quality of life issue for patients, however, it is a subjective measure of hearing. Given the limitations of collecting subjective information in a retrospective study, we decided to limit our discussion of hearing toxicity to the results from formal hearing tests.
Facial Nerve Function
Facial nerve damage before and after treatment was evaluated based on the examining physician's determination of the presence or absence of facial nerve deficit. Facial nerve function was measured using the House–Brackmann index (HB).21 Values, where possible, were based either on reported HB index, or estimated retrospectively based on charted physical examination findings of facial nerve dysfunction. Consistent with prior studies, facial nerve dysfunction following GKRS was defined as facial nerve functioning HB score III or greater in patients who had HB score of I–II before GKRS.4 An estimate of the House–Brackmann index was calculated based on charted physical examination finding. The rate of new facial nerve dysfunction was calculated as the percentage of patients with new facial nerve dysfunction or worsening of the HB index at follow-up.
Trigeminal Neuropathy
Trigeminal neuropathy was defined as new facial numbness or trigeminal neuralgia as reported in the patient chart. It was recorded as either present or absent. The rate of new trigeminal neuropathy was calculated as the percentage of patients with trigeminal neuropathy at follow-up.
Vestibular Nerve Dysfunction
Vestibular nerve dysfunction was assessed by the presence or absence of balance difficulty before and after treatment as noted in the patient examination records. The vestibular nerve dysfunction was new if it was reported after but not before GKRS, and it was reported as worse if the patient record explicitly stated that the functioning was worse after GKRS. Results of vestibular nerve dysfunction are reported as the composite of new or worse vertigo or unsteadiness following GKRS.
Statistical Analysis
The clinical data were tabulated using Microsoft Excel (Excel 14.2.3, Microsoft Corporation, Redmond, Washington). Statistical analysis, correlation analysis, multivariate analysis, and Kaplan–Meier survival curves were computed using GraphPad Prism (GraphPad Software, Inc., San Diego, California) and R (R Foundation for statistical computing, Vienna, Austria). Using the Clopper–Pearson interval 95% confidence intervals (CI) were calculated with R. Survival curves were compared using the log-rank test for significance. Statistical significance was set arbitrarily at p < 0.05. Univariate and multivariate analyses were performed using a Cox proportional hazards model. In addition, Fisher exact test with a two-tailed p value was used to compare hearing outcomes for mean cochlear doses above or below 12 Gy and for max cochlear doses above or below 6 Gy. The Institutional Review Board at the Lurie Comprehensive Cancer Center of Northwestern University approved of data collection, analysis, and publication of this study, and the study was performed within ethical standards. The Kaplan–Meier survival method was used to calculate PFS based on freedom from repeat intervention. Survival proportions were calculated using the Kaplan and Meier method in GraphPad prism. When survival proportions were 100%, confidence intervals were calculated using the Wald method based on the number of patients at risk. Patient characteristics are tabulated in Table 1.
Table 1. Patient characteristics.
| Patient characteristics | Median (where appropriate) | Range |
|---|---|---|
| Patients (N) | 30 | |
| Men | 13 | 10 |
| Women | 17 | |
| Age (y) | 51 | 16–83 |
| Dose (Gy) | 11.0 | 11.0 |
| Mean cochlear dose | 6.0 | 2.2–10.1 |
| Maximum cochlear dose | 12.0 | 3.9–18.0 |
| Volume (cm3) | 0.53 | 0.025–4.7 |
| Follow-up (mo) | 42 | 6–87 |
| Time to MRI (mo) | ||
| 1st | 3.7 | |
| 2nd | 9.4 | |
| 3rd | 18 | |
| 4th | 28 | |
| Audiogram available | 18 | |
| Required resection | 1 | |
Abbreviation: GY, Gray; mo, months; MRI, magnetic resonance imaging; N, number of patients.
Results
No complications such as hydrocephalus, radionecrosis, or tumor cystic cavitation were noted in any patient. One patient (3.3%) had a complication of vestibular neuritis (new dizziness) following GKRS and was treated with steroids. There were no treatment-related deaths. Other treatment-related side effects are outlined below.
Facial Neuropathy
Out of 30 patients, 2 (7%) patients had some facial nerve dysfunction before GKRS (HB of 3 or more). Zero percent of patients with normal (HB 1–2) facial nerve functioning before GKRS had new or worse facial nerve dysfunction (HB 3 or more) after GKRS. Results are summarized in Table 2.
Table 2. Neuropathies following SRS.
| Percentage | 95% CI | |
|---|---|---|
| Vestibular neuropathy | 7 | 1–21 |
| Vestibular improved | 92 | 62–99 |
| CN VII neuropathy | 0 | 0–12 |
| CN V neuropathy | 3.3 | 0–17 |
| Complications | 3.3 | 0–17 |
Abbreviations: CI, confidence interval; CN, cranial nerve; SRS, stereotactic radiosurgery.
Note: Percentage of patients with new CN V, VII, or vestibular VIII neuropathies after SRS. CN VII neuropathy is defined as new symptoms or worsening of the House–Brackmann index. One complication, vestibular neuritis, treated with steroids.
Vestibular Neuropathy
Vestibular neuropathy was noted in 12 of 30 patients before GKRS, and 11 of these 12 patients (92%) showed improvement after GKRS. One patient reported unsteadiness immediately following treatment, and one patient reported new dizziness.
Trigeminal Neuropathy
No patient had trigeminal neuralgia following GKRS and one patient reported new facial numbness. Results are shown in Table 2.
Tumor Control
Tumor relapse occurred in 1 out of 30 patients (3.3%). The single vestibular schwannoma progression in this series required surgical intervention for persistent tumor growth 87 months after radiosurgery. Persistent growth for this tumor started at 48 months and was seen in three subsequent MRIs over 37 months. The 5-year PFS for freedom from surgical intervention was 100% (Fig. 1A). The 5-year PFS based on freedom from persistent growth was 91% (Fig. 1B, Table 3). Around 31% of the patients experienced tumor growth following GKRS (Fig. 1C). Around 28% of patients experienced temporary tumor enlargement on follow-up MRI without progression. Twelve patients (40%) experienced a decrease in tumor size a median of 14 months after treatment. Enlargement occurred a median of 4.4 months after GKRS. The median temporary volumetric enlargement was 0.12 cm3 or 56% of the pretreatment volume. Four of these patients experienced a decrease in tumor size following initial tumor enlargement. The other two patients were found to have stable tumors on all subsequent MRIs for a total of 38 and 41 months after enlargement. Neither radionecrosis nor edema was seen in the patients in this series.
Fig. 1.
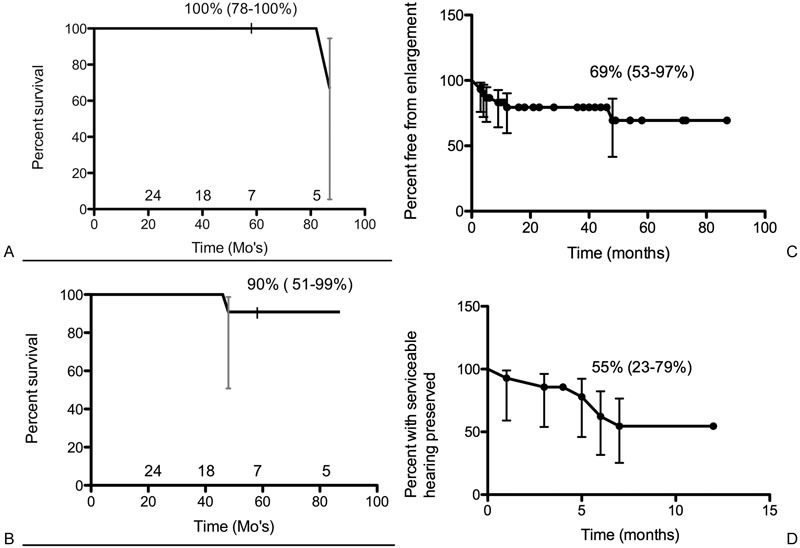
(A) Progression free survival based on freedom from intervention, 95% confidence interval, and number of patients at risk are shown. (B) Progression free survival based on freedom from persistent growth, 95% confidence interval, and number of patients at risk are shown. (C) Freedom from tumor enlargement and 95% confidence interval are shown. (D) Percentage of patients with preserved serviceable hearing and 95% confidence interval are shown.
Table 3. Five-year PFS.
| % or median | 95% CI or range | |
|---|---|---|
| PFS freedom from surgery | 100%a | 68–100 |
| PFS freedom from persistent growth | 91% | 51–99 |
Abbreviations: CI, confidence interval; PFS, progression free survival.
One patient required surgical resection for persistent growth at 87 months.
Dosimetry
The dose for all patients was 11.0 Gy prescribed to the 50% isodose line. The average cochlear volume was 0.068 cm3 (range, 0.016–0.128 cm3). The average minimum cochlear dose was 2.7 Gy (range, 0.5–5.6 Gy). The mean dose to 50% of the cochlea was 5.7 Gy (range, 2.2–10.1 Gy). The average maximum cochlear dose was 12.0 Gy (range, 3.8–18.0 Gy). An illustrative case is shown in Fig. 2.
Fig. 2.
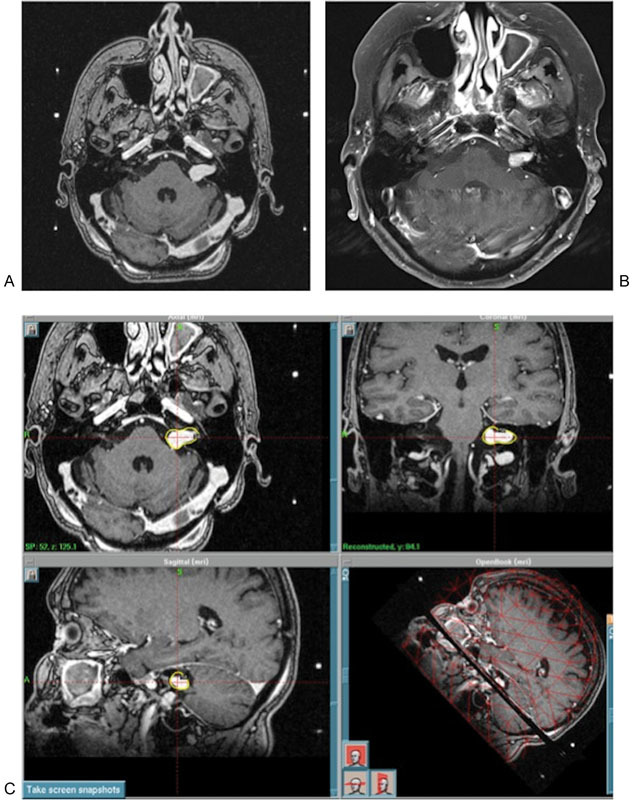
Illustrative case: 68-year-old woman found to have a small left canalicular mass on workup for tinnitus. On follow-up the next year, the mass had grown, and the patient had progressive hearing loss. She elected for radiosurgery, and underwent Gamma knife radiosurgery (Elekta Instrument AB Stockholm, Sweden) with a dose of 11.0 Gy to the 50% isodose line. At 5-year follow-up, there was no progression, and the lesion had shrunk approximately 25%. (A) Pretreatment MRI, (B) 5-year follow-up MRI, (C) treatment plan. MRI, magnetic resonance imaging.
Hearing Preservation
A total of 18 patients had audiometry data available before and after GKRS. Out of these, 61% patients had serviceable hearing before GKRS and 33% of all patients had serviceable hearing after GKRS. Of the 11 patients with serviceable hearing before GKRS, 55% maintained serviceable after GKRS (Fig. 1D). Of the 18 patients with some level of speech discrimination before GKRS (GR I–IV), 100% maintained a GR of at least IV. Around 6% (1/18) of patients showed an improvement in GR score. Results are shown in Table 4.
Table 4. Hearing preservation.
| Percentage | 95% CI | |
|---|---|---|
| Serviceable pre-SRS | 61 | 36–83 |
| Serviceable post-SRS | 33 | 13–59 |
| GR score preserved | 50 | 30–74 |
| GR improved | 6 | 0–27 |
| Serviceable preserved | 55 | 23–79 |
Abbreviations: CI, confidence interval; GR, Gardner–Robertson; SRS, stereotactic radiosurgery.
Note: Serviceable hearing: GR score of I–II. GR level preserved: Percentage of patients who maintained the same GR scores before and after SRS. Serviceable preserved: Percentage of patients who maintained GR score of I–II after SRS.
Serviceable hearing was preserved in 100 versus 13% of patients receiving a mean dose to the cochlea of less than 6 versus 6 Gy or more, respectively (p = 0.005, n = 13). Among patients with preserved serviceable hearing, half received a mean cochlear dose of < 4 Gy and half received a mean cochlear dose of 4 to 10 Gy. GR score was maintained in 100 versus 18% of patients receiving a mean dose to the cochlea of less than 6 versus 6 Gy or more, respectively (p = 0.001, n = 19). Results are shown in Table 5.
Table 5. Hearing preservation and mean cochlear dose.
| Mean < 6 Gy (%) | Mean > 6 Gy (%) | p Value | |
|---|---|---|---|
| Serviceable preserved | 100 | 13 | 0.005 |
| GR Preserved | 100 | 18 | 0.001 |
Abbreviations: GR, Gardner–Robertson; SRS, stereotactic radiosurgery.
Note: Serviceable hearing: GR score of I–II. GR level preserved: Percentage of patients who maintained the same GR scores before and after SRS. Serviceable preserved: Percentage of patients who maintained GR score of I–II after SRS.
Serviceable hearing was preserved in 80 versus 25% of patients receiving a maximum dose to the cochlea of less than 12 versus 12 Gy or more, respectively (p = 0.10, n = 13). Among patients who had preserved serviceable hearing, two-thirds received a maximum cochlear dose of < 10 Gy. All patients who did not have preserved serviceable hearing had received a maximum cochlear dose of > 11 Gy. GR score was maintained in 89 versus 20% of patients receiving a maximum dose to the cochlea of less than 12 versus 12 Gy or more, respectively (p = 0.01, n = 19). Results are shown in Table 6.
Table 6. Hearing preservation and maximum cochlear dose.
| Maximum < 12 Gy (%) | Maximum > 12 Gy (%) | p Value | |
|---|---|---|---|
| Serviceable preserved | 80 | 25 | 0.10 |
| GR Preserved | 89 | 20 | 0.01 |
Abbreviations: GR, Gardner–Robertson; SRS, stereotactic radiosurgery.
Note: Serviceable hearing: GR score of I–II. GR level preserved: Percentage of patients who maintained the same GR scores before and after SRS. Serviceable preserved: Percentage of patients who maintained GR score of I–II after SRS.
Univariate and Multivariate Analyses
Univariate and multivariate analyses were performed using a Cox proportional hazards model. Three independent variables were modeled: tumor progression, tumor expansion, and hearing preservation. Each of these independent variables was modeled with respect to age and tumor volume. Hearing preservation was further modeled with respect to transient tumor expansion and mean cochlear dose. A similar analysis was done on hearing preservation with maximum cochlear dose instead of mean cochlear dose. Dose was not modeled as an independent variable because it was uniform throughout the study population. There were no significant correlations in any of the three models with respect to age, tumor volume, or transient tumor expansion (p > 0.05). However, on univariate and multivariate analyses, mean and maximum cochlear dose had a statistically significant detrimental impact on hearing preservation. The results from these analyses are shown in Tables 7 and 8.
Table 7. Multivariate proportional hazard models.
| Persistent growth | Progression free survival | GR hearing preservation | GR hearing preservation | |
|---|---|---|---|---|
| Variable | p Value | p Value | p Value | p Value |
| Age | > 0.99 | 0.70 | 0.53 | 0.94 |
| Volume | > 0.99 | 0.73 | 0.96 | 0.72 |
| Expansion | 0.53 | 0.62 | ||
| Mean cochlear dose | 0.03 | |||
| Maximum cochlear dose | 0.017 |
Abbreviations: GR, Gardner–Robertson.
Note: The independent variables are shown in columns and dependent values are shown in rows.
Table 8. Univariate proportional hazard model hearing preservation.
| Variable | HR | p Value |
|---|---|---|
| Mean cochlear dose | 0.72 | < 0.01 |
| Maximum cochlear dose | 0.53 | < 0.01 |
Abbreviation: HR, hazard ratio.
Note: Independent variable is Gardner–Robertson hearing preservation.
Discussion
In the past two decades, SRS for vestibular schwannomas has gained favor over microsurgery because of its improved side effect profile and similar rate of tumor control.1 16 18 There are no randomized trials comparing the two techniques, however, and efficacy data relies on retrospective studies. Based on these data, SRS is now widely considered a viable alternative to microsurgery for the treatment of vestibular schwannomas,1 5 22 and surgery is still used for tumors > 3 cm in diameter.1 As discussed earlier, the high tumor control rates have prompted neurosurgeons and radiation oncologists to reduce prescribed SRS doses to improve hearing outcomes and cranial nerve function. SRS doses have been reduced from a median of 16 Gy in older studies to 12.5 Gy in more recent reports with similar rates of tumor control.1 4 5 7 13 15 23 Moreover, these lower doses have been associated with better cranial nerve function and hearing preservation rates.8
Dose Selection and Tumor Control Rates
The characteristics of our patients are summarized in Table 1 and are similar to those found in other studies. In a review by Yang et al of 74 studies that included more than 4,000 patients,8 the mean age was 52, the mean tumor volume was 3.9 cm3, and the mean follow-up time was 44 months. The mean GKRS dose was 14 Gy and 98% of patients received 12 to 14 Gy. Major differences between our study and the review by Yang et al are the lower prescribed median dose of 11 Gy and smaller median tumor volume of 0.53 cm3 in our series.
Another review by Yang et al showed the combined results of 5,825 patients treated for vestibular schwannoma with SRS.24 The mean tumor control rate in that review was 94% after a median follow-up time of 34 months, and the mean dose was 16 Gy. For patients treated with ≤ 12.5 Gy, the mean tumor control rates were 94 ± 3% after a median follow-up time of 34 months. Other studies report a tumor control of 91 to 95% over 3 years.4 5 6 7 8 There have been multiple small studies reporting favorable outcomes for patients treated with 12 Gy or less. Aiba and coworkers treated 25 patients with vestibular schwannoma to 12 Gy and reported an actuarial PFS of 96% at 60 months.25 Zacest and coworkers treated 84 patients to a median of 12 Gy and their PFS based on freedom from surgery was 98% at 65 months.26 Several groups reduced doses even further. Paek et al treated 25 patients to a median of 12 Gy (range, 11–14 Gy) and found a PFS based on freedom from surgery of 100% at 45 months.27 Later, Uyama and coworkers reported the results of 51 patients also treated at Osaka City General Hospital to a median of 12 Gy (range, 8–12 Gy). These results included six patients treated to 11 Gy or less, with two treated to 8 Gy, and four treated to 10 Gy.28 Patients with larger tumors were treated with lower doses to the tumor margin. Overall, the PFS based on freedom from surgery was 96% at a median of 60 months. Separate results were not reported for the tumors treated to 11 Gy or less, although one of the two retreated tumors was initially treated with 12 Gy. Régis et al at Hôpital de la Timone in Marseille reported results of 3,050 patients treated from 1992 to 2011 of whom 2,336 had follow-up of 3 years or more. The mean dose was 12.3 Gy. The authors reported that all patients with serviceable hearing (GR I–II) were treated with 11 Gy, and 46% of the patients in this series had serviceable hearing. However, results were not reported separately for patients receiving 11 Gy. The tumor control rate was 97.5% at 3 years.29 Petit et al at the University of Maryland treated 47 patients with a median of 12 Gy (range, 10–15 Gy) and the actuarial PFS based on freedom from surgery was 96% (range, 92–100%) after a median of 43 months.30 Inoue treated 20 patients to a median of 12 Gy (range, 10–12 Gy) including 4 patients to 10 Gy and 2 patients to 11 Gy.31 The PFS based on freedom from surgery was 95% at 60 months. Based on the earlier data from Osaka City Hospital and the University of Maryland, and in an attempt to reduce morbidity from treatment for Vestibular Schwannoma (VS), patients at Northwestern were treated with lower doses of 11 Gy. Our results of 5-year PFS of 100 and 91% freedom from persistent tumor growth are comparable with results from prior studies of patients treated with lower dose SRS as summarized above.
Hearing Preservation
Hearing preservation rates, defined as preservation of serviceable hearing scores of I–II using the GR scale,20 range from 35 to 78% in other studies.1 4 5 12 13 14 15 16 24 32 Yang et al conducted a review of hearing preservation based on 74 studies that included 2,083 patients with intact hearing treated with SRS for vestibular schwannoma.7 Overall, 57% of patients had preserved serviceable hearing based on GR. The mean dose of SRS was 16 Gy and patients were followed for a median follow-up time of 34.3 months. When stratified according to dose, patients with ≤ 12.5 Gy had significantly higher hearing preservation rates (59 vs. 53%). There was no statistically significant difference in hearing preservation when patients were stratified according to tumor size (< or > 1.5 cm3) or by age (< 65 or > 65 years old). In this study, 55% maintained serviceable hearing, comparable to prior reports of 51% in a large review study.8 Direct comparisons between this and other studies on the effect of dose on hearing preservation are not possible due to the retrospective study design. Several factors have been associated with worse hearing including dose, tumor size, and dose to the cochlea, all of which are associated with total dose.8 16 33 These data, along with other studies and within the limits of study design, support using lower doses to preserve hearing.8 9 13
Prior studies have variably demonstrated a link between hearing preservation and factors including dose, tumor volume expansion, and age.8 33 34 35 36 37 Univariate and multivariate analysis were performed for the patients in our study. However, no correlation for PFS or hearing preservation with respect to age or tumor volume was found. This may be due to the small sample size, and the occurrence of only one patient requiring further intervention after tumor progression.
Other studies have proposed minimizing dose to the cochlea to reduce hearing loss. Results from such studies have shown variable results.33 35 Tamura et al at Hôpital de la Timone found that, among patients with GR score of I before treatment, a dose to the cochlea of < 4 Gy was associated with improved hearing on multivariate analysis.35 In this study, univariate and multivariate analyses demonstrated that hearing preservation was statistically significantly increased in patients receiving lower mean or maximum dose to the cochlea.
Facial Nerve Preservation
Yang et al reported a review of 23 articles on facial nerve preservation following SRS for vestibular schwannoma.24 The study excluded patients with facial nerve dysfunction before GKRS defined as HB III or worse. Overall, 1,908 patients were included in the analysis and the mean dose was 13.1 Gy. Of those patients, 96.2% had preserved facial nerve function after a median follow-up time of 43 months. The authors found a statistically significant improvement in facial nerve preservation for patients ≤ 60 years of age (96.8 vs. 89.4%), tumors ≤ 1.5 cm3 (99.5 vs. 95.5%), and doses ≤ 13 Gy (98.5 vs. 94.7%). In this report only one (3.3%) of our patients had cranial nerve VII neuropathy after GKRS, compared with 0.5 to 17% in past studies.4 13 22 29 38 The rate of cranial neuropathies in our study is on the lower end of past reported figures for patients treated with lower dose SRS.
Summary and Study Limitations
We have given 11 Gy to all patients and continue to do so. The objective was to reduce the dose to the adjacent normal tissues including the cochlea and the fifth cranial nerve and reduce treatment-related side effects. Unlike the series by Régis et al we did not choose this dose based on hearing status but rather to see if we could further reduce the dose safely without compromising tumor control rates. This report is our review of the tumor control rates in a cohort of patients consecutively treated with 11 Gy. We concede that most centers continue to deliver 12 to 12.5 Gy for acoustic neuromas treated with GKRS.
Our study, despite the limitations of a small number of patients and a relatively short follow-up after radiosurgery, compares favorably to other published reports on low doses of SRS and has demonstrated excellent tumor control rates and good cranial nerve function preservation. Furthermore, we have shown that reducing mean and maximum cochlear dose can result in higher hearing preservation rates. More clinical data and longer follow-up are required to confirm that good cranial nerve function preservation and tumor control can be maintained after treatment with lower doses of GKRS.
Conclusion
This report demonstrates that low-dose GKRS (11 Gy) for vestibular schwannomas can result in similar tumor control rates and cranial nerve function preservation compared with higher dose radiosurgery. Furthermore, we have shown that reductions in cochlear dose can lead to improved hearing preservation rates. Further studies are required to confirm long-term maintenance of tumor control and cranial nerve function in these patients.
Note
There was no funding received for this work. There are no financial, institutional, or personal interest disclosures to report.
References
- 1.Karpinos M, Teh B S, Zeck O. et al. Treatment of acoustic neuroma: stereotactic radiosurgery vs. microsurgery. Int J Radiat Oncol Biol Phys. 2002;54(5):1410–1421. doi: 10.1016/s0360-3016(02)03651-9. [DOI] [PubMed] [Google Scholar]
- 2.Propp J M, McCarthy B J, Davis F G, Preston-Martin S. Descriptive epidemiology of vestibular schwannomas. Neuro-oncol. 2006;8(1):1–11. doi: 10.1215/S1522851704001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conley G S, Hirsch B E. Stereotactic radiation treatment of vestibular schwannoma: indications, limitations, and outcomes. Curr Opin Otolaryngol Head Neck Surg. 2010;18(5):351–356. doi: 10.1097/MOO.0b013e32833c71a2. [DOI] [PubMed] [Google Scholar]
- 4.Arthurs B J, Lamoreaux W T, Mackay A R. et al. Gamma knife radiosurgery for vestibular schwannomas: tumor control and functional preservation in 70 patients. Am J Clin Oncol. 2011;34(3):265–269. doi: 10.1097/COC.0b013e3181dbc2ab. [DOI] [PubMed] [Google Scholar]
- 5.Kondziolka D, Lunsford L D, McLaughlin M R, Flickinger J C. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339(20):1426–1433. doi: 10.1056/NEJM199811123392003. [DOI] [PubMed] [Google Scholar]
- 6.Shin Y J, Lapeyre-Mestre M, Gafsi I. et al. Neurotological complications after radiosurgery versus conservative management in acoustic neuromas: a systematic review-based study. Acta Otolaryngol. 2003;123(1):59–64. doi: 10.1081/0036554021000028084. [DOI] [PubMed] [Google Scholar]
- 7.Yang I, Aranda D, Han S J. et al. Hearing preservation after stereotactic radiosurgery for vestibular schwannoma: a systematic review. J Clin Neurosci. 2009;16(6):742–747. doi: 10.1016/j.jocn.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Yang I, Sughrue M E, Han S J. et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg. 2010;112(4):851–859. doi: 10.3171/2009.8.JNS0985. [DOI] [PubMed] [Google Scholar]
- 9.Combs S E, Welzel T, Kessel K. et al. Hearing preservation after radiotherapy for vestibular schwannomas is comparable to hearing deterioration in healthy adults and is accompanied by local tumor control and a highly preserved quality of life (QOL) as patients' self-reported outcome. Radiother Oncol. 2013;106(2):175–180. doi: 10.1016/j.radonc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Amichetti M, Amelio D, Minniti G. Radiosurgery with photons or protons for benign and malignant tumours of the skull base: a review. Radiat Oncol. 2012;7:210. doi: 10.1186/1748-717X-7-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champ C E, Shen X, Shi W. et al. Reduced-dose fractionated stereotactic radiotherapy for acoustic neuromas: maintenance of tumor control with improved hearing preservation. Neurosurgery. 2013;73(3):489–496. doi: 10.1227/NEU.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 12.Baschnagel A M, Chen P Y, Bojrab D. et al. Hearing preservation in patients with vestibular schwannoma treated with Gamma Knife surgery. J Neurosurg. 2013;118(3):571–578. doi: 10.3171/2012.10.JNS12880. [DOI] [PubMed] [Google Scholar]
- 13.Flickinger J C, Kondziolka D, Niranjan A, Maitz A, Voynov G, Lunsford L D. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004;60(1):225–230. doi: 10.1016/j.ijrobp.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013;118(3):557–565. doi: 10.3171/2012.10.JNS12523. [DOI] [PubMed] [Google Scholar]
- 15.Lunsford L D Niranjan A Flickinger J C Maitz A Kondziolka D Radiosurgery of vestibular schwannomas: summary of experience in 829 cases J Neurosurg 2005102(Suppl):195–199. [PubMed] [Google Scholar]
- 16.Wolbers J G, Dallenga A H, Mendez Romero A, van Linge A. What intervention is best practice for vestibular schwannomas? A systematic review of controlled studies. BMJ Open. 2013;3(2):e001345. doi: 10.1136/bmjopen-2012-001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollock B E, Link M J, Foote R L. Failure rate of contemporary low-dose radiosurgical technique for vestibular schwannoma. J Neurosurg. 2009;111(4):840–844. doi: 10.3171/2009.3.JNS08949. [DOI] [PubMed] [Google Scholar]
- 18.Foote K D, Friedman W A, Buatti J M, Meeks S L, Bova F J, Kubilis P S. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg. 2001;95(3):440–449. doi: 10.3171/jns.2001.95.3.0440. [DOI] [PubMed] [Google Scholar]
- 19.Miller R C, Foote R L, Coffey R J. et al. Decrease in cranial nerve complications after radiosurgery for acoustic neuromas: a prospective study of dose and volume. Int J Radiat Oncol Biol Phys. 1999;43(2):305–311. doi: 10.1016/s0360-3016(98)00397-6. [DOI] [PubMed] [Google Scholar]
- 20.Gardner G, Robertson J H. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97(1):55–66. doi: 10.1177/000348948809700110. [DOI] [PubMed] [Google Scholar]
- 21.House J W, Brackmann D E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 22.Kaylie D M, Horgan M J, Delashaw J B, McMenomey S O. A meta-analysis comparing outcomes of microsurgery and gamma knife radiosurgery. Laryngoscope. 2000;110(11):1850–1856. doi: 10.1097/00005537-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Andrews D W, Werner-Wasik M, Den R B. et al. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas: comparison of two dose cohorts. Int J Radiat Oncol Biol Phys. 2009;74(2):419–426. doi: 10.1016/j.ijrobp.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Yang I, Sughrue M E, Han S J. et al. Facial nerve preservation after vestibular schwannoma Gamma Knife radiosurgery. J Neurooncol. 2009;93(1):41–48. doi: 10.1007/s11060-009-9842-3. [DOI] [PubMed] [Google Scholar]
- 25.Iwai Y, Yamanaka K, Kubo T, Aiba T. Gamma knife radiosurgery for intracanalicular acoustic neuromas. J Clin Neurosci. 2008;15(9):993–997. doi: 10.1016/j.jocn.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Roos D E, Potter A E, Zacest A C. Hearing preservation after low dose linac radiosurgery for acoustic neuroma depends on initial hearing and time. Radiother Oncol. 2011;101(3):420–424. doi: 10.1016/j.radonc.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Paek S H, Chung H T, Jeong S S. et al. Hearing preservation after gamma knife stereotactic radiosurgery of vestibular schwannoma. Cancer. 2005;104(3):580–590. doi: 10.1002/cncr.21190. [DOI] [PubMed] [Google Scholar]
- 28.Iwai Y Yamanaka K Shiotani M Uyama T Radiosurgery for acoustic neuromas: results of low-dose treatment Neurosurgery 2003532282–287., discussion 287–288 [DOI] [PubMed] [Google Scholar]
- 29.Régis J, Carron R, Delsanti C. et al. Radiosurgery for vestibular schwannomas. Neurosurg Clin N Am. 2013;24(4):521–530. doi: 10.1016/j.nec.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Petit J H Hudes R S Chen T T Eisenberg H M Simard J M Chin L S Reduced-dose radiosurgery for vestibular schwannomas Neurosurgery 20014961299–1306., discussion 1306–1307 [DOI] [PubMed] [Google Scholar]
- 31.Inoue H K Low-dose radiosurgery for large vestibular schwannomas: long-term results of functional preservation J Neurosurg 2005102(Suppl):111–113. [PubMed] [Google Scholar]
- 32.Régis J, Pellet W, Delsanti C. et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97(5):1091–1100. doi: 10.3171/jns.2002.97.5.1091. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y H, Kim D G, Han J H. et al. Hearing outcomes after stereotactic radiosurgery for unilateral intracanalicular vestibular schwannomas: implication of transient volume expansion. Int J Radiat Oncol Biol Phys. 2013;85(1):61–67. doi: 10.1016/j.ijrobp.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 34.Nagano O, Higuchi Y, Serizawa T. et al. Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg. 2008;109(5):811–816. doi: 10.3171/JNS/2008/109/11/0811. [DOI] [PubMed] [Google Scholar]
- 35.Tamura M Carron R Yomo S et al. Hearing preservation after gamma knife radiosurgery for vestibular schwannomas presenting with high-level hearing Neurosurgery 2009642289–296., discussion 296 [DOI] [PubMed] [Google Scholar]
- 36.van Eck A T Horstmann G A Increased preservation of functional hearing after gamma knife surgery for vestibular schwannoma J Neurosurg 2005102(Suppl):204–206. [PubMed] [Google Scholar]
- 37.Wowra B Muacevic A Jess-Hempen A Hempel J M Müller-Schunk S Tonn J C Outpatient gamma knife surgery for vestibular schwannoma: definition of the therapeutic profile based on a 10-year experience J Neurosurg 2005102(Suppl):114–118. [PubMed] [Google Scholar]
- 38.Yamakami I, Uchino Y, Kobayashi E, Yamaura A. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: a systematic review of outcome and risk of three therapeutic options. Neurol Res. 2003;25(7):682–690. doi: 10.1179/016164103101202075. [DOI] [PubMed] [Google Scholar]


