Abstract
Background
Underage drinking is widely recognized as a leading public health and social problem for adolescents in the United States. Being able to identify at-risk children before they initiate heavy alcohol use could have immense clinical and public health implications; however, few investigations have explored individual-level precursors of adolescent substance use. This prospective investigation used machine learning with demographic, neurocognitive, and neuroimaging data in substance-naïve adolescents to predict alcohol use initiation by age 18.
Materials and Methods
Participants (N=137) were healthy substance-naïve adolescents (ages 12–14) who underwent neuropsychological testing and structural and functional magnetic resonance imaging (sMRI and fMRI), then were followed annually. By age 18, 70 youth (51%) initiated moderate-to-heavy alcohol use and 67 remained non-users. Random forests classification generated individual alcohol use outcome predictions based on demographic, neuropsychological, sMRI, and fMRI data.
Results
The final random forests model was 74% accurate, with good sensitivity (74%) and specificity (73%) and included 34 predictors contributing to alcohol use by age 18, including several demographic and behavioral factors (being male, higher socioeconomic status, early dating, more externalizing behaviors, positive alcohol expectancies), worse executive functioning, and thinner cortices and less brain activation in diffusely distributed regions of the brain. Inclusion of neuropsychological, sMRI, and fMRI data significantly increased the prediction accuracy of the model.
Discussion
Identification of at-risk youth is not validated for clinical use. Its value is for research to address brain mechanisms that predispose to early drinking.
INTRODUCTION
Underage drinking is widely recognized as one of the leading public health and social problem for adolescents in the United States. This is concerning, as teen drinking is very common in the United States, with approximately 66% of 18 year olds reporting alcohol use (1). Adverse consequences of adolescent drinking include higher rates of violence, missing school, drunk driving, riding with a drunk driver, suicide, and risky sexual behavior and accounts for over 5,000 deaths per year (2). Thus, being able to identify at-risk children before they initiate heavy alcohol use could have immense clinical and public health implications. However, few investigations have been conducted to gain a greater understanding of individual differences that could lead to adolescent substance use.
Previous findings have suggested that a mix of social, psychological, and biological mechanisms contribute to alcohol use during adolescence (for review (3–5). Demographic risk factors for alcohol initiation include being male, having higher levels of psychological problems and externalizing behaviors, and having positive expectations about the effects of alcohol (5). Neuropsychological and neuroimaging data may provide quantification of underlying behavioral mechanisms of substance use risk. Several studies suggest that poorer performance on tests of executive functioning (6), as well as less brain activation compared to controls during tasks of working memory, inhibition, and reward processing can be used predict which youth will initiate alcohol use during adolescence (7–11). Additionally, less volume in brain regions involved in impulsivity, reward sensitivity, and decision-making appear to influence initiation of alcohol and other substance use during adolescence (7, 11). Understanding factors involved in the initiation and escalation of alcohol use during adolescence could provide crucial information for preventions and interventions.
Machine learning approaches (12–17) are increasingly being used to generate predictions based on complex data like brain imaging (18, 19). Random forests (20) is a machine learning tool that is one of the most consistently robust predictive techniques, yielding superior performance in independent replication (21). The random forests technique consists of a complex partitioning of the predictor variable space and can be used when the number of predictor variables is much larger than the number of subjects, as is typically the case with neuroimaging data. Moreover, random forests has a low tendency to over-fit, and the stepwise partitioning of the predictor space can yield high-order interactions among many predictor variables that cannot be identified using other classification procedures (22). Random forests models have successfully been used to detect a number of clinical outcomes and predict behaviors (23), but have not been used to generate substance use outcome predictions.
The present study uses random forests models in combination with multimodal imaging and neuropsychological test data to predict which substance-naïve adolescents would initiate moderate to heavy alcohol use by age 18. Based on previous research, initiation of substance use was expected to be associated with key demographic factors (e.g., being male, endorsing more externalizing behaviors and psychopathology, having positive expectancies about alcohol use), neuropsychological performance (e.g., poorer performance on executive functioning tasks), and thinner cortices and less brain activation in key brain regions involved in executive functioning and decision making. Unlike other studies that focused on initiation of any alcohol use (11), we were interested in factors that predicted a pattern of more frequent and intense alcohol use, as these have been associated with poorer cognitive (7, 24) and social (5) outcomes.
MATERIALS AND METHODS
Participants
Participants were 137 healthy 12–14 year-olds (44% female) from a larger ongoing neuroimaging study recruited through flyers sent to households of students attending local middle schools (see Table 1). Extensive screening and background information were obtained from the youth, their biological parent, and one other parent or close relative. All primary informants lived with the youth. The study protocol was executed in accordance with the standards approved by the University of California, San Diego Human Research Protections Program.
Table 1.
Demographic, psychological, and neuropsychological variables for adolescents who remained non-users (n=67) or transitioned into moderate to heavy substance use (n=70) patterns by approximately age 18 (N = 137).
| Continuous Non-Users (N=67) M (SD) or % | Moderate-Heavy Alcohol Initiators (N=70) M (SD) or % | t-test or χ2 | df | p-value | Cohen’s D | |
|---|---|---|---|---|---|---|
| DEMOGRAPHIC/FAMILY VARIABLES | ||||||
| Sex (% female)* | 60% | 29% | 13.48 | 1, N=137 | <.001 | .661 |
| Baseline age (range: 12–14) | 13.44 (0.69) | 13.52 (0.77) | .62 | 135 | .537 | .109 |
| Follow-up age (range: 16–19)+ | 18.20 (0.66) | 18.20 (0.57) | .07 | 135 | .943 | .000 |
| Race (% Caucasian) | 58% | 77% | 5.63 | 1, N=137 | .018 | .414 |
| Baseline Hollingshead Index of Social Position score (socioeconomic status) | 24.03 (16.75) | 21.11 (11.50) | 1.19 | 135 | .235 | .203 |
| Family history density of alcohol or drug use disorder | 0.17 (0.28) | 0.18 (0.31) | .14 | 135 | .887 | .034 |
| Baseline Pubertal Development Scale (girls only; n=60) total | 15.05 (3.15) | 14.95 (3.15) | .12 | 58 | .908 | .032 |
| Baseline Pubertal Development Scale (boys only; n=77) total | 10.81 (3.33) | 11.20 (3.37) | .48 | 75 | .632 | .116 |
| Baseline grade in school | 6.76 (.74) | 7.00 (0.87) | 1.73 | 135 | .086 | .297 |
| Birth order | 1.52 (0.79) | 1.64 (0.76) | 0.91 | 135 | .364 | .155 |
| Baseline % living with both parents | 79% | 81% | 0.12 | 1, N=137 | .732 | .059 |
| Baseline % youth with biological parents married to each other | 78% | 77% | 0.00 | 1, N=137 | .948 | .000 |
| BASELINE YOUTH BEHAVIOR, MOOD, AND COGNITION | ||||||
| % initiated dating by age 14* | 22% | 61% | 21.37 | 1, N=137 | <.001 | .860 |
| % involved in extracurricular activities | 82% | 89% | 1.15 | 1, N=137 | .283 | .184 |
| Hours of video games played per week | 3.10 (4.95)a | 2.47 (4.04)a | .72 | 106 | .472 | .139 |
| Grade point average (4.0 scale)* | 3.65 (0.45) | 3.47 (0.55) | 2.14 | 135 | .034 | .358 |
| CBCL Externalizing disorder T-score | 41.09 (7.12)b | 40.42 (7.64)b | .52 | 128 | .606 | .091 |
| CBCL Internalizing T-score | 44.48 (8.80)b | 42.11 (7.65)b | 1.65 | 128 | .102 | .287 |
| CBCL Withdrawn T-score | 52.00 (3.79)b | 51.42 (2.69)b | 1.00 | 128 | .319 | .176 |
| CBCL Somatic Complaints T-score | 52.45 (4.13)b | 51.79 (3.15)b | 1.04 | 128 | .302 | .180 |
| CBCL Anxious/depressed T-score | 51.61 (3.60)b | 51.11 (2.60)b | .92 | 128 | .361 | .159 |
| CBCL Social Problems T-score | 51.33 (3.62)b | 50.55 (1.64)b | 1.60 | 128 | .113 | .278 |
| CBCL Thought Problems T-score | 51.98 (3.90)b | 51.03 (2.31)b | 1.70 | 128 | .091 | .296 |
| CBCL Attention Problems T-score | 51.05 (2.16)b | 51.20 (2.81)b | .34 | 128 | .734 | .060 |
| CBCL Delinquent Behavior T-score | 50.59 (1.84)b | 50.98 (2.53)b | 1.01 | 128 | .317 | .176 |
| CBCL Aggressive Behavior T-score | 50.75 (2.06)b | 50.68 (2.11)b | .19 | 128 | .852 | .034 |
| CBCL Total Problem T-score | 39.83 (9.75)b | 37.83 (8.83)b | 1.22 | 128 | .223 | .215 |
| Conduct Disorder Questionnaire total score* | 0.45 (1.08) | 1.17 (1.88) | 2.75 | 135 | .007 | .470 |
| Beck Depression Inventory II total score | 1.39 (2.75)c | 1.25 (2.81)c | .30 | 132 | .765 | .050 |
| State Trait Anxiety Inventory total score | 26.22 (6.66)d | 26.68 (6.48)d | .40 | 130 | .690 | .070 |
| AEQ total* | 79.14 (32.23) | 92.45 (29.75) | 2.51 | 135 | .013 | .429 |
| AEQ Global Positive total | 37.81 (8.37) | 40.83 (9.53) | 1.97 | 135 | .051 | .336 |
| AEQ Social Behavior Change total* | 36.92 (7.41) | 41.23 (7.94) | 3.28 | 135 | .001 | .561 |
| AEQ Improved Performance total | 16.55 (4.57) | 16.96 (4.98) | .50 | 135 | .621 | .086 |
| AEQ Sexual Enhancement total | 20.15 (5.06) | 21.00 (4.07) | 1.09 | 135 | .279 | .185 |
| AEQ Impaired Performance total | 98.41 (10.08) | 96.39 (10.28) | 1.16 | 135 | .247 | .198 |
| AEQ Increased Arousal total | 24.24 (5.44) | 24.96 (4.78) | .82 | 135 | .414 | .141 |
| AEQ Relaxation total | 41.88 (9.83) | 43.87 (7.90) | 1.31 | 135 | .193 | .293 |
| BASELINE SUBSTANCE USE | ||||||
| CDDR baseline lifetime smoking days | 0.03 (0.17) | 0.11 (0.65) | 1.03 | 135 | .305 | .168 |
| CDDR baseline lifetime drinking days | 0.00 (0.00) | 0.31 (1.50) | 1.72 | 135 | .089 | .292 |
| CDDR baseline lifetime marijuana use days | 0.00 (0.00) | 0.09 (0.44) | 1.59 | 135 | .115 | .289 |
| Total number of repetitions excluded during fMRI | 4.46 (6.77) | 7.03 (8.82) | 1.90 | 134 | .060 | .327 |
| FOLLOW-UP SUBSTANCE USE | ||||||
| Age of first use of alcohol* | 16.54 (1.27)e | 15.46 (1.60) | 3.09 | 94 | .003 | .748 |
| Lifetime alcohol use occasions* | 1.52 (2.87)e | 69.01 (110.58) | 4.99 | 135 | .000 | .863 |
| Peak drinks on an occasion in past year* | 0.46 (0.96)e | 9.30 (4.60) | 15.41 | 135 | .000 | 2.66 |
| Lifetime marijuana use occasions* | 0.06 (0.38)f | 108.61 (256.94) | 3.46 | 135 | .001 | .597 |
| % reporting >30 lifetime marijuana use occasions | 0% | 29% | ||||
| Lifetime other drug use | 0.00 (0.00) | 7.04 (38.05) | 1.52 | 135 | .132 | .261 |
| Domain assessed | Continuous Non-Users (N=67) M (SD) | Moderate-Heavy Alcohol Initiators (N=70) M (SD) | t-test | DF | p-value | Cohen’s D | |
|---|---|---|---|---|---|---|---|
| BASELINE NEUROPSYCHOLOGICAL TEST VARIABLES | |||||||
| Digit Vigilance total time to complete (seconds) | Sustained attention | 225.06 (52.80)g | 219.59 (41.46)g | .67 | 132 | .504 | .115 |
| WASI Block Design raw | Spatial perception, visual abstract processing, and problem solving | 47.27 (13.81)g | 42.86 (13.13)g | 1.89 | 132 | .060 | .327 |
| WASI Matrix Reasoning raw* | Nonverbal abstract problem solving, inductive reasoning | 27.80 (3.62)h | 26.41 (3.34)h | 2.31 | 131 | .023 | .399 |
| WASI Vocabulary raw | Word knowledge, verbal concept formation | 53.02 (7.09)g | 51.96 (7.06)g | .87 | 132 | .389 | .150 |
| WASI Similarities raw | Abstract verbal reasoning | 35.17 (4.24)g | 34.31 (5.07)g | 1.06 | 132 | .292 | .184 |
| D-KEFS Trails Number-Letter Switching time to complete (seconds) | Flexibility of thinking on a visual-motor task | 70.60 (19.45) | 77.46 (28.75) | 1.63 | 135 | .106 | .279 |
| D-KEFS Towers Total Achievement Score raw | Spatial planning, rule learning | 17.04 (2.68) | 17.24 (2.73) | .43 | 135 | .669 | .074 |
| D-KEFS Color Word Interference Inhibition time to complete (seconds) | Inhibitory functioning | 57.39 (14.25) | 59.60 (13.12) | .95 | 135 | .346 | .161 |
| D-KEFS Color Word Interference Inhibition/Switching time to complete (seconds) | Inhibition/cognitive flexibility | 65.81 (15.81) | 63.91(14.39) | .73 | 135 | .465 | .126 |
| Rey-Osterrieth Complex Figure (ROCF) copy accuracy | Visuospatial functioning; organization | 29.35 (3.02)g | 28.63 (3.01)g | 1.39 | 132 | .168 | .239 |
| ROCF delay accuracy | Spatial memory | 18.09 (4.12)i | 17.77 (4.26)i | .43 | 130 | .665 | .076 |
| WISC-III Digits Forward raw | Attention | 9.91 (1.84)g | 10.17 (1.94)g | .81 | 132 | .421 | .138 |
| WISC-III Digits Backward raw | Short term memory, attention | 6.44 (1.74)g | 6.19 (2.05)g | .76 | 132 | .447 | .131 |
| WISC-III Arithmetic raw | Computational skills, auditory memory, attention, problem solving | 22.23 (3.36)g | 22.46 (3.3 4)g | .39 | 132 | .701 | .069 |
| WISC-III Coding raw | Visual motor speed, visual memory, sustained effort and attention | 61.20 (10.88)g | 59.37 (10.75)g | .98 | 132 | .329 | .169 |
| WISC-III Mazes raw | Planning, perceptual organization, visual-motor coordination and speed, nonverbal reasoning | 23.48 (3.71)g | 22.77 (3.20)g | 1.19 | 132 | .235 | .205 |
| WAIS-IV Letter-Number Sequence raw | Attention, auditory short-term memory, and sequencing ability | 10.69 (1.94)j | 10.09 (1.88)j | 1.65 | 108 | .103 | .314 |
| Hooper Visual Organization Test total raw | Visuospatial functioning and spatial integration | 25.32 (2.26)j | 24.94 (2.40)j | .86 | 108 | .392 | .163 |
| CVLT list A total 1 to 5 raw | Verbal learning and memory | 55.91 (8.01)g | 54.51 (6.29)g | 1.12 | 132 | .263 | .194 |
| CVLT list A Trial 1 raw | Verbal learning and memory | 7.72 (1.93)g | 7.37 (1.64)g | 1.13 | 132 | .262 | .195 |
| CVLT list A Trial 5 raw | Verbal learning and memory | 12.92 (1.68)g | 12.74 (1.57)g | .64 | 132 | .525 | .111 |
| CVLT short delay free raw | Verbal learning and memory | 11.87 (2.07)g | 11.74 (2.29)g | .33 | 132 | .743 | .060 |
| CVLT short delay cued raw | Verbal learning and memory | 12.15 (1.94)k | 12.19 (1.70)k | .12 | 129 | .907 | .022 |
| CVLT long delay free raw | Verbal learning and memory | 12.11 (1.91)l | 12.30 (1.79)l | .60 | 130 | .550 | .103 |
| CVLT long delay cued raw | Verbal learning and memory | 12.52 (1.80)l | 12.54 (1.75)l | .04 | 130 | .968 | .011 |
| WRAT-3 Reading raw | Premorbid functioning and intellectual capacity | 45.44 (4.14)g | 44.67 (4.04)g | 1.08 | 132 | .280 | .188 |
CBCL=Child Behavior Checklist; AEQ=Alcohol Expectancy Questionnaire; CDDR =Customary Drinking and Drug Use Record; WASI=Wechsler Abbreviated Scale of Intelligence; D-KEFS=Delis-Kaplan Executive Function System; ROCF=Rey-Osterrieth Complex Figure; WISC-III=Wechsler Intelligence Scale for Children, 3rd edition; WAIS-IV=Wechsler Adult Intelligence Scale; CVLT= California Verbal Learning Test; WRAT-3= Wide Range Achievement Test-3 Reading scores
Age range: All controls in this sample were at least 17 years of age, to all sufficient time to transition into alcohol use. 16-year-old substance users were included, as it was clear that by age 16 they were already using.
p < .05
N=108, Continuous Non-User n=55, Alcohol Initiators n=63
N=130, Continuous Non-User n=64, Alcohol Initiators n=66
N=134, Continuous Non-User n=66, Alcohol Initiators n=68
N=132, Continuous Non-User n=64, Alcohol Initiators n=68
n=26
n=2
N=134, Continuous Non-User n=64, Alcohol Initiators n=70
N=133, Continuous Non-User n=64, Alcohol Initiators n=69
N=132, Continuous Non-User n=62, Alcohol Initiators n=70
N=110, Continuous Non-User n=55, Alcohol Initiators n=55
N=131, Continuous Non-User n=63, Alcohol Initiators n=68
N=132, Continuous Non-User n=63, Alcohol Initiators n=69
Strict exclusionary criteria for project entry included: experience with alcohol or drugs, defined as ≥10 total days in their life on which drinking had occurred, or > 2 drinks in a week (i.e., two drinks on one occasion or one drink on two occasions in the same week); ≥3 lifetime experiences with marijuana and any use in the past three months; ≥5 lifetime cigarette uses; and any history of other intoxicant use; any suggestion of prenatal alcohol (>2 drinks during a given week) or any illicit drug exposure; premature birth (i.e., born prior to 35th gestational week); history of any neurological or DSM-IV Axis I disorder, determined by the NIMH Diagnostic Interview Schedule for Children –version 4.0, head trauma or loss of consciousness (>2 minutes), chronic medical illness, learning disability or mental retardation, or use of medications potentially affecting the brain; contraindication to MRI (e.g., braces); inadequate comprehension of English; and non-correctable sensory problems.
Measures
Substance use measures
At baseline and follow-ups, the Customary Drinking and Drug Use Record (25) was administered to obtain quantity and frequency of lifetime and recent (past year) alcohol, marijuana, and other drug use, withdrawal/hangover symptoms, and DSM-IV abuse and dependence criteria. Breathalyzer and urine toxicology screens confirmed self-report data at baseline. Substance use information was updated every six months via phone or in-person after the participant’s baseline assessment. Parent and/or informant (sibling, friend, roommate) report of youth substance use was collected as collateral evidence.
Demographic information
The Structured Clinical Interview (26) was administered to youth and ascertained information about the child’s sex, age, race, academic functioning (i.e., grade point average; GPA on a 4.0 scale), grade in school, family characteristics (i.e., birth order, living situation, parent’s marital status), dating status (e.g., never dated vs. history of dating), involvement in extracurricular activities, and hours of video games played per week.
Socioeconomic status
Socioeconomic background information (i.e., educational attainment, occupation, and salary of each parent) was obtained from parents and converted to a Hollingshead Index of Social Position score (27).
Family background
At baseline, the Family History Assessment Module (28) was administered to parents and youth and ascertained familial density of alcohol and drug use disorders in first- and second-degree relatives. Family history density scores were calculated by adding 0.5 for each biological parent and 0.25 per biological grandparent endorsed by either youth or parent as having AUD or SUD for a possible score of 0 to 4.
Pubertal Development
The Pubertal Development Scale (29) ascertained current level of pubertal development for girls and boys separately with five sex-specific items, with scores ranging from 1 (prepubertal) to 4 (postpubertal). Participants in this sample were, on average early-to mid-pubertal at baseline, and late- to post-pubertal at follow-up.
Psychopathology and mood
The parent-administered Child Behavior Checklist (CBCL; (30) provided age- and gender-normed continuous measures of externalizing and internalizing psychopathology. T-scores from the following CBCL subscales were used in analyses: withdrawn, somatic complaints, anxious/depressed, social problems, thought problems, attention problems, delinquent behavior, aggressive behavior scores, and three summary score representing externalizing, internalizing, and total problems. Youth completed the Conduct Disorder Questionnaire (31), which determined DSM-IV diagnostic criteria for conduct disorder; total symptom count was used in analyses. Beck Depression Inventory-II (BDI-II; (32) and State-Trait Anxiety Inventory (STAI; (33) assessed recent depressive and anxiety state symptoms in youth.
Alcohol Expectancy Questionnaire-Adolescent Version
Youth completed the Alcohol Expectancy Questionnaire-Adolescent version (AEQ-A; (34), which was developed to assess beliefs about the anticipated effects of alcohol. This version yields a total score (AEQ Total Score) and seven empirically derived factor scores indicating expectations for the effects of drinking alcohol including: (1) global positive changes (AEQ Global Positive), (2) enhancement/impedance of social behavior (AEQ Social Behavior Change), (3) improvement in cognitive/behavioral functioning (AE Q Improved Performance), (4) enhancement of sexuality (AEQ Sexual Enhancement), (5) deterioration in cognitive/behavioral functioning (AEQ Impaired Performance), (6) increased arousal, (AEQ Increased Arousal) and (7) promotion of relaxation/tension reduction (AEQ Relaxation).
Neurocognition
A comprehensive neuropsychological battery was completed by youth at baseline to assess cognitive functioning on several cognitive domains that could potentially affect initiation of alcohol and marijuana use during adolescence. See Table 1 for neuropsychological test and domains assessed and Supplementary References for neuropsychological testing material citations.
Follow-up procedures
At baseline, 12 to 14 year old youth were administered a baseline interview, neuropsychological testing, and structural and functional neuroimaging session. Every 6 months, over-the-phone or in-person interviews assessed current substance use and psychiatric functioning. At baseline, all participants were considered controls, and had never had more than 10 lifetime alcohol use occasions, with never more than 1 drink per occasion, and no more than 3 lifetime marijuana use episodes. Ninety-seven percent of the sample had never used alcohol and 98% had never used marijuana. Rigorous follow-up procedures were utilized to ensure excellent follow-up rates (96%). At follow-up (age ~18), participants were classified as continuous controls (baseline control who maintained abstinence over the follow-up, defined as 0–4 drinks on an occasion and <12 lifetime drinking occasions; 61% continued to remain completely alcohol-naïve at follow-up) or moderate-heavy drinking initiators (baseline control who transitioned into moderate or heavy alcohol use, defined as 3–29 drinks on an occasion and 3–834 drinking occasions; see Figure 1 for classification). Participants who had fewer drinking days, but drank significantly on those occasions (e.g., 3 lifetime occasions, 15 drinks per occasion) were classified as moderate-heavy drinkers to capture the fact they had initiated significant levels of alcohol use. Sixty-seven participants were classified as continuous controls, while 70 (51%) were moderate-heavy drinking initiators (see Table 1; 27% met criteria for moderate drinking, while 73% met criteria for heavy drinking). Continuous non-users were at least 17 years of age at follow-up, to allow sufficient time to transition into alcohol use. Sixteen-year-old substance users were included, as it was clear that substance use had onset by that age. For this sample, rates of alcohol initiation were consistent with the general US adolescent population (1).
Figure 1.
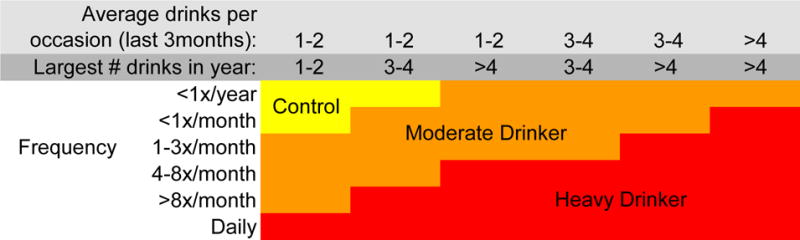
Substance use classification chart. “Control” indicates “continuous non-user”. Reprint from Squeglia et al., 2015; American Journal of Psychiatry. “Largest # drinks in year” refers to the largest number of alcoholic beverages consumed on one occasion in the past year”.
Procedures
Image acquisition
High-resolution anatomical and functional images were collected at the UC San Diego Center for fMRI on a 3-Tesla CXK4 short bore Excite-2 MR system (General Electric, Milwaukee, WI) with an 8-channel phase-array head coil. Participants were placed comfortably on the scanner table and the head was stabilized within the head coil using foam cushions (NoMoCo, La Jolla, CA). Scan sessions involved a 10-second scout scan to assure good head placement and slice selection covering the whole brain followed by a high-resolution T1-weighted sequence using a sagittally-acquired spoiled gradient recalled sequence (FOV 24 cm, 256 × 256 × 192 matrix, .94 × .94 × 1 mm voxels, 176 slices, TR=20 ms, TE=4.8 ms; flip angle 12°, acquisition time 7:26 minutes). BOLD response contrast was measured with T2*-weighted axially acquired echo-planar images (FOV=24 cm, 64 × 64 matrix, 3.75 × 3.75 × 3.8 mm voxels, 32 slices, TE=30 ms, TR=2000 ms, flip angle 90°, ramped bandwidth 250 KHz). Field maps were acquired to minimize warping and signal dropout (~4 minutes total) and employed 2 different echo times to assess field inhomogeneities and signal distortions under the same grid parameters as echo-planar images were acquired.
Visual Working Memory Task
All participants were administered the same fast event related visual working memory task (35) during fMRI acquisition, which has been shown to predict future initiation of alcohol use (8). Participants were required to indicate whether dot arrays presented with a 2000ms inter-stimulus interval were identical or differed (i.e., one dot was of a different color). Each subject completed 30 trials of each level of complexity (2, 4, or 6 dots) presented randomly, in addition to 69 null trials of 2000 ms each interspersed to provide an optimized fast-event related sequence (256 repetitions in all; 8 minutes and 32 seconds). The 6-dot condition is considered supra-span (i.e., higher than most people’s working memory span) and the 2-dot condition is sub-span (i.e., well within most people’s working memory load capacity (36). None of the 137 total runs used during analysis had performance at or below chance level (50%) on the 2-dot (i.e., low-capacity/easy condition). A greater BOLD response contrast (i.e., larger fit coefficient) to the 6-dot (supra-span) relative to the 2-dot (sub-span) condition was interpreted as more cognitive energy expended to complete the challenging supra-span trials.
Data Analysis
Structural image processing
FreeSurfer (version 5.0, surfer.nmr.mgh.harvard.edu) was used for cortical surface reconstruction and cortical thickness estimation (37, 38) of the high-resolution T1-weighted MR data. The FreeSurfer program utilizes a series of automated imaging algorithms to produce measures of cortical thickness. One rater (LMS), blind to participant characteristics, followed the reconstruction procedures (http://surfer.nmr.mgh.harvard.edu/fswiki/RecommendedReconstruction) to identify and correct any errors made during the cortical reconstruction. Following inspection, an automated parcellation procedure divided each hemisphere into 32 independent cortical regions based on gyral and sulcal features (39). See Table 2 for list of parcellated brain regions. Cortical thickness estimates of each region were extracted for subsequent statistical analyses.
Table 2.
List of variables entered into each model: Model 1 initially included Demographic/Family and Youth Behavior, Mood, and Cognition variables. Model 2 initially included all of the variables from Model 1 + Neuropsychological Testing Variables. Model 3 initially included all of the Variables from Models 1 + 2 + Neuroimaging variables [cortical thickness and blood oxygen level dependent (BOLD) response during a visual working memory task]. Grayed-out boxes indicate variables that were not selected for the final model. Demographic and neuropsychological variables predicting initiation into alcohol use by age 18 are marked with an X. For neuroimaging data (Model 3), Desikan (39) brain region location is specified using R=right hemisphere, L=left hemisphere, as well as the neuroimaging index CT=cortical thickness and BOLD=BOLD response contrast during a visual working memory task (6-dot supra-span relative to the 2-dot sub-span condition).
| Model 1: | Model 2: | Model 3: | |
|---|---|---|---|
| DEMOGRAPHIC/FAMILY VARIABLES | |||
| 1. Sex | X | X | X |
| 2. Baseline age | |||
| 3. Follow-up age | X | X | X |
| 4. Race | |||
| 5. Hollingshead Index of Social Position score (socioeconomic status) | X | X | X |
| 6. Family history density of alcohol or drug use disorder | |||
| 7. Pubertal Development Scale total | X | ||
| 8. Grade in school | |||
| 9. Birth order | |||
| 10. Living with both parents | |||
| 11. Parents’ marital status | |||
| YOUTH BEHAVIOR, MOOD, AND COGNITION | |||
| 12. Dating status | X | X | X |
| 13. Child involvement in extracurricular activities | |||
| 14. Hours of video games per week | |||
| 15. Grade point average | X | ||
| 16. CBCL Externalizing T-score | |||
| 17. CBCL Internalizing T-score | |||
| 18. CBCL Withdrawn T-score | |||
| 19. CBCL Somatic complaints T-score | |||
| 20. CBCL Anxious/depressed T-score | |||
| 21. CBCL Social Problems T-score | |||
| 22. CBCL Thought Problems T-score | |||
| 23. CBCL Attention Problems T-score | |||
| 24. CBCL Delinquent Behavior T-score | |||
| 25. CBCL Aggressive Behavior T-score | |||
| 26. CBCL Total Problem T-score | X | ||
| 27. Conduct Disorders Questionnaire total | X | X | X |
| 28. Beck Depression Inventory-II total | |||
| 29. State Trait Anxiety Inventory total | |||
| 30. AEQ Total Score | X | X | X |
| 31. AEQ Global Positive total | X | X | X |
| 32. AEQ Social Behavior Change total | X | X | X |
| 33. AEQ Improved Performance total | |||
| 34. AEQ Sexual Enhancement total | |||
| 35. AEQ Impaired Performance total | |||
| 36. AEQ Increased Arousal total | |||
| 37. AEQ Relaxation total | |||
| 38. CDDR baseline lifetime smoking days (< 5 for all participants) | |||
| 39. CDDR baseline lifetime drinking days (<10) | |||
| 40. CDDR baseline lifetime marijuana use days (<3) | |||
| 41. Repetitions excluded from fMRI series due to motion | X | ||
| NEUROPSYCHOLOGICAL TESTING VARIABLES | |||
| 1. Digit Vigilance total time to complete (sec) | X | X | |
| 2. WASI Block Design raw | X | X | |
| 3. WASI Matrix Reasoning raw | X | X | |
| 4. WASI Vocabulary raw | X | ||
| 5. WASI Similarities raw | |||
| 6. D-KEFS Trails Condition 4 (Number-Letter Switching) time to complete (sec) | X | ||
| 7. D-KEFS Towers Total Achievement Score raw | |||
| 8. D-KEFS Color Word Interference Inhibition time to complete (sec) | |||
| 9. D-KEFS Color Word Interference Inhibition/Switching time to complete (sec) | |||
| 10. ROCF copy accuracy | |||
| 11. ROCF delay accuracy | |||
| 12. WISC-III Digits Forward raw | |||
| 13. WISC-III Digits Backward raw | |||
| 14. WISC-III Arithmetic raw | |||
| 15. WISC-III Coding raw | |||
| 16. WISC-III Mazes raw | |||
| 17. WAIS-IV Letter-Number Sequence raw | |||
| 18. Hooper Visual Organization Test total raw | |||
| 19. CVLT list A total 1 to 5 raw | |||
| 20. CVLT list A Trial 1 raw | |||
| 21. CVLT list A Trial 5 raw | |||
| 22. CVLT short delay free raw | |||
| 23. CVLT short delay cued raw | |||
| 24. CVLT long delay free raw | |||
| 25. CVLT long delay cued raw | |||
| 26. WRAT-3 Reading raw | |||
| CORTICAL THICKNESS AND BOLD REGIONS | |||
| Based on Desikan atlas (39); 34 regions per hemisphere, cortical thickness and fMRI measures for each region listed; 34 × 2 × 2=136 total variables | |||
| 1. Banks of superior temporal sulcus | L-CT | ||
| 2. Caudal anterior cingulate | R-BOLD | ||
| 3. Caudal middle frontal | |||
| 4. Cuneus | |||
| 5. Entorhinal | |||
| 6. Fusiform | |||
| 7. Inferior parietal | |||
| 8. Inferior temporal | |||
| 9. Isthmus cingulate | |||
| 10. Lateral occipital | L-CT | ||
| 11. Lateral orbitofrontal | |||
| 12. Lingual | L-CT | ||
| 13. Medial orbitofrontal | |||
| 14. Middle temporal | R-CT, L-BOLD | ||
| 15. Parahippocampal | |||
| 16. Paracentral | |||
| 17. Pars opercularis | |||
| 18. Pars orbitalis | R-CT | ||
| 19. Pars triangularis | |||
| 20. Pericalcarine | |||
| 21. Postcentral | |||
| 22. Posterior cingulate | R-BOLD | ||
| 23. Precentral | |||
| 24. Precuneus | R-CT, L-BOLD, R-BOLD | ||
| 25. Rostral anterior cingulate | L-CT | ||
| 26. Rostral middle frontal | R-CT | ||
| 27. Superior frontal | R-CT | ||
| 28. Superior parietal | L-CT, R-CT | ||
| 29. Superior temporal | R-BOLD | ||
| 30. Supramarginal | L-CT | ||
| 31. Frontal pole | R-CT, R-BOLD | ||
| 32. Temporal pole | R-CT | ||
| 33. Transverse temporal | L-CT | ||
| 34. Insula |
CBCL=Child Behavior Checklist; AEQ=Alcohol Expectancy Questionnaire; CDDR =Customary Drinking and Drug Use Record; WASI=Wechsler Abbreviated Scale of Intelligence; D-KEFS=Delis-Kaplan Executive Function System; ROCF=Rey-Osterrieth Complex Figure; WISC-III=Wechsler Intelligence Scale for Children, 3rd edition; WAIS-IV=Wechsler Adult Intelligence Scale; CVLT= California Verbal Learning Test; WRAT-3= Wide Range Achievement Test-3 Reading scores
Functional image processing
Analysis of Functional NeuroImages (AFNI; (40) was used to process functional images. Artifact and aberrant signal levels were examined in each repetition of each slice using an automated program developed by the UCSD Laboratory of Cognitive Neuroimaging. Motion in time series data were corrected by registering each acquisition to the maximally stable base volume with an iterated least squares algorithm (41) to estimate three rotational and three displacement parameters for each participant. An output file specifying adjustments made controlled for spin history effects in analyses if no significant task-correlated motion was found. To evaluate task-related motion, the reference vector was correlated with the six motion parameters for each dataset. Datasets with significant task-correlated or bulk motion (>2mm) were excluded from analyses. Two trained raters then scanned the time series en cine to omit any remaining repetitions with visually discernible motion; if more than 15% of repetitions in a task were discarded, the run was not used (n=10, not described in this paper).
Raw time series data were standardized to percent signal change from baseline, and deconvolution was conducted with a reference function that convolved the behavioral stimuli with a hemodynamic response model, while covarying for linear trends and motion correction, ignoring the first three repetitions (42). This resulted in a functional image in which every voxel contains a fit coefficient representing the change in signal across behavioral conditions, as well as percent signal change and threshold statistics. Standardized Talairach transformations were made for each high-resolution anatomical image, and functional datasets were warped in accordance to manage individual anatomical variability. Functional data were resampled into isotropic voxels (3 mm3), and a spatial smoothing Gaussian filter (full-width half maximum 5 mm) was applied to minimize the influence of individual anatomic variability. Co-registration of structural images to functional images was performed with a mutual information registration program (41) that robustly handles images with different signal characteristics and of different spatial resolutions.
Volumetric and functional image alignment
The AFNI SUrface MApper (SUMA; (43) program was used to align segmented volumetric and functional datasets to the same template space. SUMA programs allow for fine control over the mapping between volume and surface domains produced by the FreeSurfer segmentation process while maintaining a direct link to volumetric data from which surface models and data originated. Combination of functional and structural neuroimaging data using SUMA is described in detail in Squeglia et al (44). BOLD response values, averaged across the parcellation regions derived from FreeSurfer (39), were imported from AFNI to SPSS.
Statistical analysis
Independent sample t-tests or Chi-square tests (for dichotomous variables) compared differences between groups (Table 1). Random forests classification was implemented in R statistics (http://cran.r-project.org; randomForest library) to predict alcohol initiator status, with missing data handled using the rfimpute function (see Table 1 for sample sizes per variable). Default parameters for the randomForest function were used, with the exception of expanding the number of trees to 2000 (45).
Random forests classification has been described in detail elsewhere (20, 23). Briefly, random forests classification has two primary parameters: the number of trees (2000 used in these analyses) and the number of variables tried at each node (as recommended (20), the square root of the total number of variables). In addition, trees were grown to the maximum possible number of nodes such that all participants in the bootstrap training sample were accurately classified. Variable selection was accomplished using permutation importance scores, defined as the mean decrease in model accuracy when a predictor variable’s values are randomly permuted. Specifically, the random forests algorithm was first run 500 times on the entire set of possible predictors, to generate stable importance scores for each predictor (based on the median score across the 500 repetitions). The removal of poor performing variables can increase overall accuracy by increasing the relevance of included data to outcome prediction. Therefore, these importance scores were used to select which predictor variables would be included in a more parsimonious final model. Because negative permutation importance scores are due to random variation around zero of the poor predictor variables (22), only variables with an importance score greater than the magnitude of the most negative score were used in the final model (22, 23).
Importantly, this technique utilizes bootstrapped cross-validation to reduce overfitting. In addition, using variable importance in the initial models to select predictors, and reporting accuracy, sensitivity, and specificity only from the final model avoids the problem of “double dipping”, i.e. the repeated extraction of test statistics from the same sample. This approach is consistent with Ball et al., 2014 (23).
Three sequential models were built in order to compare the following sets of variables (see Table 2): 1) demographic/behavioral variables only, 2) demographic/behavioral and neuropsychological test variables, and 3) demographic/behavioral, neuropsychological, and neuroimaging variables. See Supplementary Materials for models were run on 26 neuropsychological and 136 neuroimaging variables separately. The relatively wide range in baseline age (12 to 14 years) could have biased findings since those enrolled at age 14 survived two more years without initiating alcohol use; this was accounted for by including baseline and follow-up age in the models. McNemar’s Chi-squared test was used to compare the three models. Fifty-one percent of participants (n=70) transitioned into moderate to heavy alcohol use (using Figure 1 classification) by age 18. Therefore, model accuracy for each random forests model was also compared to the base response rate using McNemar’s Chi-squared test.
RESULTS
Demographics model
The initial model was comprised of 41 demographic and psychological variables (see Table 2) as predictors of moderate-heavy alcohol initiation. Eleven variables met criteria for inclusion in the final model: sex, age at follow-up, socioeconomic status, pubertal development, dating status, GPA, Child Behavior Checklist total problems, Conduct Disorder Questionnaire total problems count, AEQ total score, AEQ Global Positive total, and AEQ Social Behavior Change total. The final model with these variables yielded accuracy of 62%, which was not statistically different from the base rate (51%) of youth transitioning into moderate-heavy drinking (p=.07). Sensitivity was 0.60, and specificity was 0.64. The positive predictive value (PPV) was 64% and the negative predictive value (NPV) was 61%. The positive likelihood ratio of 1.67 (95% CI: 1.15, 2.43) and the negative likelihood ratio of 0.62 (95% CI: 0.44, 0.87) were statistically significantly different from each other and from 1.0 (p<0.05).
Demographics and neuropsychological performance model
After adding neuropsychological test variables (26 variables; see Table 2) to the first model (41 variables), 13 out of the 67 total variables met criteria for inclusion in the final model; 8 of 11 from the previous model: sex; age at follow-up; socioeconomic status; dating status; Conduct Disorder Questionnaire total problems count; AEQ total score, AEQ Global Positive total, and AEQ Social Behavior Change total; and 5 additional variables: Digit Vigilance Test total time; WASI Block Design, Matrix Reasoning, and Vocabulary total raw scores; and D-KEFS Trails Condition 4 (Number-Letter Switching) time to complete. The final model with these variables yielded accuracy of 69%, which was not statistically significantly different from the model based on demographics only (p=.12), but was significantly different from the model based on initiation rate alone (p=.004). Sensitivity was 0.67, and specificity was 0.70. PPV was 70% and NPV was 67%. The positive likelihood ratio of 2.25 (95% CI: 1.50, 3.36) and the negative likelihood ratio of 0.47 (95% CI: 0.32, 0.68) were statistically significantly different from each other and from 1.0 (p<0.05).
Demographics, neuropsychological performance, and neuroimaging model
After including the neuroimaging data (see Table 2; cortical thickness and BOLD response for each of the 68 brain regions), 34 out of the 203 total variables met criteria for inclusion in the final model (see Table 2 and Figure 2). The final model with these variables yielded accuracy of 74%, which was statistically significantly a better model fit based on base rates (p<.001) and demographic information alone (p=.03); however, it was not statistically different from the neuropsychological model (p=.30). Sensitivity was 0.74 and specificity was 0.73. PPV was 74% and NPV was 73%. The positive likelihood ratio of 2.77 (95% CI: 1.82, 4.20) and the negative likelihood ratio of 0.35 (95% CI: 0.23, 0.54) were statistically significantly different from each other and from 1.0 (p<0.05). Sixteen out of 19 moderate drinkers were correctly classified as drinkers (84%), suggesting the model was able to accurately predict transition to both moderate and heavy drinking status.
Figure 2.
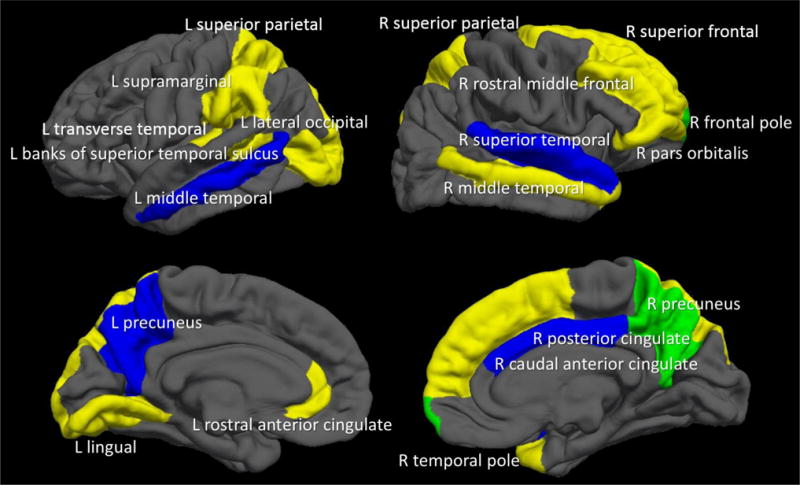
Twenty brain regions that predicted alcohol initiation by age ~18. YELLOW= Cortical thickness regions included in the final model; BLUE= BOLD response regions included in the final model; GREEN (where yellow and blue regions overlapped) = Cortical thickness and BOLD response in the same brain region. In regards to neuroimaging data, thinner cortices (in 13 out of the 15 regions) and less BOLD response contrast (in all 7 regions) predicted initiation of moderate-to-heavy drinking by age 18.
Further Investigation of Model 3
The precise contribution of each variable to the outcome prediction is complex, due to the high-order interactions critical to the success of random forests. However, main effects can be investigated straightforwardly. As shown in Table 3, alcohol initiators had less brain activation contrast between supra- and sub-span conditions than continuous non-using controls in all 7 brain regions and thinner cortices in 13 out of 15 brain regions in the final model. The lingual gyrus and lateral occipital gyrus were thicker in future alcohol initiators. Neuropsychological test variables that predicted future initiation of drinking included: faster Digit Vigilance time and poorer performance on Block Design and Matrix Reasoning. Demographic predictors of initiating alcohol use included: being male, higher socioeconomic status, starting to date at an earlier age (by age 14), greater endorsement of conduct disorder-related behaviors, higher positive alcohol expectancies (i.e., higher AEQ Global Positive, Social Behavior change, and Total scores), and more motion repetitions during the fMRI task. See Table 3 for variables, in order of importance, in the final model (Model 3), including which variables were statistically different between continuous non-users and moderate-heavy alcohol initiators. Importantly, while each variable by itself may not differentiate continuous controls from drinkers (as shown by the p-values in Table 3), all variables included in the model contribute to accurate prediction via interaction effects, supporting the importance of using statistical techniques such as random forests that can model these complex, high-order interaction terms.
Table 3.
Variables, in order of importance, in the final model (Model 3), including demographic, neuropsychological, and structural and functional neuroimaging data. Importance was defined as the mean decrease in model accuracy when the variable was permuted. In random forests analyses, the contribution of each variable to the outcome prediction is complex given the high-order interactions critical to the success of this technique. While some group differences on individual variables are statistically non-significant or would not survive control for multiple comparisons, each variable contributes significantly to the overall success of the predictive model when allowed to interact with other variables. The group differences and effect sizes are presented to better understand the direction and magnitude of the relationship.
| Continuous Non-Users (N=67) M (SD) or % | Moderate-Heavy Alcohol Initiators (N=70) M (SD) or % | p-value | Variable importance | Cohen’s D | |
|---|---|---|---|---|---|
| 1. L supramarginal CT | 2.96 (0.14) | 2.89 (0.13) | .002 | 10.01 | .518 |
| 2. Sex (% female) * | 60% | 29% | >.001 | 9.99 | .661 |
| 3. R posterior cingulate BOLD | −0.63 (3.90) | −2.19 (3.60) | .016 | 8.84 | .416 |
| 4. R superior temporal BOLD | −1.17 (3.60) | −1.89 (2.83) | .194 | 8.45 | .222 |
| 5. % dating at baseline * | 22% | 61% | >.001 | 8.08 | .860 |
| 6. Socioeconomic status | 24.03 (16.75) | 21.11 (11.50) | .235 | 7.44 | .203 |
| 7. L transverse temporal CT | 2.78 (0.21) | 2.68 (0.21) | .006 | 7.37 | .476 |
| 8. R pars orbitalis CT | 3.02 (0.22) | 2.92 (0.19) | .005 | 7.28 | .487 |
| 9. WASI Matrix Reasoning raw | 27.80 (3.62) | 26.41 (3.34) | .023 | 7.11 | .399 |
| 10. WASI Block Design raw | 47.27 (13.81) | 42.86 (13.13) | .060 | 6.43 | .327 |
| 11. R rostral middle frontal CT | 2.53 (0.10) | 2.49 (0.11) | .058 | 6.06 | .381 |
| 12. L middle temporal BOLD | −1.11 (2.56) | −1.62 (2.47) | .238 | 5.83 | .203 |
| 13. R superior parietal CT | 2.60 (0.12) | 2.55 (0.10) | .016 | 5.76 | .453 |
| 14. L lingual CT | 2.26 (0.16) | 2.28 (0.13) | .367 | 5.62 | .137 |
| 15. R precuneus CT | 2.82 (0.11) | 2.78 (0.11) | .049 | 5.42 | .364 |
| 16. R caudal anterior cingulate BOLD | 2.40 (4.90) | 0.43 (4.66) | .017 | 5.24 | .412 |
| 17. AEQ Social Behavior Change total * | 36.92 (7.41) | 41.23 (7.94) | .001 | 5.23 | .561 |
| 18. R temporal pole CT | 3.88 (0.30) | 3.76 (0.37) | .041 | 5.18 | .356 |
| 19. R precuneus BOLD | −1.46 (5.64) | −3.06 (4.90) | .078 | 4.81 | .303 |
| 20. Repetitions excluded from fMRI series due to motion | 4.46 (6.77) | 7.03 (8.82) | .060 | 4.44 | .327 |
| 21. L lateral occipital CT | 2.45 (0.16) | 2.46 (0.14) | .774 | 4.41 | .067 |
| 22. Conduct Disorder Questionnaire total | 0.45 (1.08) | 1.17 (1.88) | .007 | 4.04 | .470 |
| 23. R frontal pole BOLD | 2.02 (4.74) | 0.50 (7.54) | .161 | 3.91 | .241 |
| 24. R frontal pole CT | 3.10 (0.28) | 2.99 (0.33) | .038 | 3.80 | .360 |
| 25. L rostral anterior cingulate CT | 3.20 (0.30) | 3.12 (0.26) | .107 | 3.71 | .285 |
| 26. AEQ Total Score | 79.14 (32.23) | 92.45 (29.75) | .013 | 2.88 | .429 |
| 27. R middle temporal CT | 3.26 (0.14) | 3.22 (0.18) | .192 | 2.85 | .248 |
| 28. L banks superior temporal sulcus CT | 2.85 (0.19) | 2.80 (0.16) | .104 | 2.67 | .285 |
| 29. L precuneus BOLD | −2.03 (5.85) | −3.46 (5.17) | .130 | 2.63 | .260 |
| 30. L superior parietal CT | 2.60 (0.12) | 2.5 (0.11) | .015 | 2.55 | .869 |
| 31. AEQ Global Positive total | 37.81 (8.37) | 40.83 (9.53) | .051 | 2.42 | .336 |
| 32. Digit Vigilance completion time (seconds) | 225.06 (52.80) | 219.59 (41.46) | .504 | 2.36 | .115 |
| 33. Right superior frontal CT | 3.05 (0.15) | 3.01 (0.13) | .076 | 2.19 | .285 |
| 34. Follow-up age | 18.20 (0.66) | 18.20 (0.57) | .943 | 1.22 | .000 |
AEQ=Alcohol Expectancy Questionnaire; WASI=Wechsler Abbreviated Scale of Intelligence
For neuroimaging data, R=right hemisphere, L=left hemisphere, CT=cortical thickness (mm), and BOLD=blood oxygen level dependent response during a visual working memory task (% signal change during 6-dot vs. 2-dot condition).
Group differences that survive Bonferonni correction (p<.001).
DISCUSSION
This study aimed to address an important public health issue: generating individual-level predictions of who is at an elevated risk for initiating alcohol use during adolescence. Findings show that a mix of demographic, neuropsychological, and brain imaging indices were able to predict which 12–14 year-olds would initiate moderate-to-heavy alcohol use by approximately age 18. Notably, the overall model was 74% accurate in predicting a behavior that is influence by a multitude of factors, not all of which could be collected in one study. Specifically, demographic factors revealed that youth who were male and from higher socioeconomic backgrounds were more likely to initiate drinking by ~18. In terms of early adolescent behavior and cognitions, those who reported dating, had more externalizing behaviors, and believed alcohol would affect them positively (particularly in social settings) by age 14 initiated alcohol use by age ~18 at higher rates. In terms of neuropsychological functioning, adolescents who showed poorer performance on executive functioning tests and were faster on sustained attention tests (perhaps indicating impulsivity) during early adolescence had higher rates of alcohol initiation, consistent with previous findings (46). The neuroimaging features of thinner cortices and less BOLD response contrast to a cognitive challenge by age 14 contributed to risk of moderate to heavy drinking before age ~18, consistent with previous findings (5, 8, 11). Interestingly, more head movement (yet still within the acceptable limits to be included in analyses) while in the scanner was included in the final model, perhaps representing a phenotypic marker of impulsivity. Baseline alcohol, cigarette, and marijuana use was not predictive of substance use initiation; however, this is not surprising given our sample was almost completely substance naïve at baseline (97% had never tried alcohol). These findings build on previous reports (11), with a specific focus on predicting patterns of more frequent and intense alcohol use as opposed to initiation alone.
The addition of neuroimaging indices to the predictive model significantly increased accuracy, with 5 of the 10 most important predictors being MRI and fMRI variables (see Table 3). Morphometry or activation of twenty diffusely distributed brain regions substantially contributed to alcohol initiation (see Figure 1). Cortical thickness and BOLD response prediction regions did not overlap, except in the right precuneus and right frontal pole, similar to previous studies showing that structural and functional maturation tend to show distinct developmental trajectories during early adolescence (44). More “mature” neural functioning (i.e., thinner cortices and less BOLD response contrast) was related to greater rates of transitioning into substance use, which is consistent with previous findings (8–10, 47). This “pseudomaturity” in at risk-youth has also been observed in other behavioral studies, including a 33-year longitudinal study that found more mature behavior during childhood (based on psychiatrist ratings) predicted greater nicotine dependence in adulthood (48). Early maturation of neural features could be considered a vulnerability for youth, increasing the likelihood of engaging in sensation-seeking behaviors at an earlier age. Neurodevelopmentally precocious youth may have a greater tendency to initiate and escalate risk-taking behaviors (e.g., early dating, substance use) relative to peers. Longitudinal studies with three or more time points will be needed to elucidate the trajectory of youth with these different outcomes.
Consistent with epidemiological data, alcohol was the most commonly used substance in this sample (1). However, significant marijuana use was reported among the moderate-to-heavy alcohol initiators. We chose to focus on alcohol initiation specifically as only 15% of our overall sample (29% of alcohol initiators) endorsed more than 30 lifetime occasions of marijuana use, and most alcohol initiators (80%) used alcohol before trying marijuana. However, it is likely that the reported risk factors confer risk not only to use of alcohol, but also marijuana and other illicit substances, and potentially additional risky behaviors. Larger studies and additional years of follow-up will indicate the extent to which these predictive features are replicated, predictive specifically of substance use or to problem behavior more broadly, and if, as participants age, these features predict addiction.
Strengths of this study include the relatively large sample size, extensive neuropsychological and multimodal neuroimaging data, and utilization of a robust machine learning technique to identify risk factors for adolescent alcohol use. Limitations of this study include the lack of an independent replication sample. Nevertheless, random forests is a robust statistical technique that is suggested to be superior to other machine learning techniques (21), and includes bootstrapped cross-validation, with accuracy determined only for the out-of-bag sample to reduce overfitting. In addition, double dipping was avoided by using a different metric to select variables (via variable importance scores) than to evaluate the model (via accuracy, sensitivity, specificity). Regardless, future work should seek to replicate these predictors. To this end, we are publishing the random forests scripts (see Supplementary Materials) that we used in the study so other groups can replicate our findings on their own datasets (see Supplementary Materials). In random forests analyses, the contribution of each variable to the outcome prediction is complex given the high-order interactions critical to the success of this technique. While some group differences on individual variables are statistically non-significant or would not survive control for multiple comparisons (Table 3), each variable contributes significantly to the overall success of the predictive model when allowed to interact with other variables. The group differences are presented to better understand the direction of the relationship. Genotyping was not included in this study. While previous findings suggest a nominal role of genetics in adolescent alcohol initiation compared to other personality and environmental factors (11), future studies should explore potential genetic risk factors associated with alcohol use and risk-taking generally. The participants in this sample came from a relatively high SES, which may limit generalizability to low SES youth; published scripts will allow for replication in more diverse samples (see Supplemental Materials). A limitation inherent to fMRI is that the BOLD findings are task dependent and only have sensitivity to detect regions engaged by the task. Therefore, it is possible that functional activity in different regions would be predictive of future alcohol use if a different task were used. There is a large quantity/frequency range covered across the moderate-heavy alcohol initiator category, and predictors might vary across the severity of this continuum. While most of our drinkers were not drinking frequently, they tended to drink in large quantities (average of >9 drinks on peak occasion in the past year), suggesting that we were capturing risky drinking behaviors in this group. Continued follow-up of this sample, as some youth transition into alcohol use disorders, will help clarify which predictors are most important in identifying problematic drinking.
The results provide evidence that multi-modal neuroimaging data, as well as neuropsychological testing, can be used to generate predictions of future behaviors with significantly better accuracy than demographic information alone. Understanding neurocognitive factors that predate substance use initiation is crucial to specifying the consequences of substance use on brain development, as well as identifying at-risk youth and potential targets of preventive efforts. The random forests scripts (see Supplementary Materials) used in this study are now published to allow for other groups to easily replicate findings, in hopes that a final, validated model can be used clinically to predict adolescent alcohol use.
Supplementary Material
Acknowledgments
Special thanks to the Adolescent Brain Imaging Project lab and the participating schools in the San Diego Unified School District and their families, as well as Lindsay Meredith for assistance with preparation of this manuscript.
Funding Support: R01 AA13419, U01 DA041089, and U01 AA021692 (Tapert); K12 DA031794 (Squeglia); T32 DA031098 (McKenna); T32 AA013525 (Nguyen-Louie); R01 DA016663, P20 DA027843 (Paulus).
References
- 1.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 1975–2014: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2015. [Google Scholar]
- 2.Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- 3.Squeglia LM, Jacobus J, Tapert SF. The effect of alcohol use on human adolescent brain structures and systems. Handbook of Clinical Neurology. 2014;125:501–510. doi: 10.1016/B978-0-444-62619-6.00028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Squeglia LM, Gray KM. Alcohol and drug use and the developing brain. Current Psychiatry Reports. 2016;18:46. doi: 10.1007/s11920-016-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121:S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squeglia LM, Jacobus J, Nguyen-Louie TT, Tapert SF. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology. 2014;28:782–790. doi: 10.1037/neu0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain development in heavy-drinking adolescents. American Journal of Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: Influence of initiating heavy drinking. Journal of Studies on Alcohol and Drugs. 2012;73:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, Zucker RA. Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug and Alcohol Dependence. 2014;141:51–57. doi: 10.1016/j.drugalcdep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Barker GJ, Bokde AL, Büchel C, Carvalho FM, Conrod PJ, Flor H, Fauth-Bühler M, Frouin V, Gallinat J, Gan G, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Martinot JL, Nees F, Ortiz N, Paillère-Martinot ML, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Ströhle A, Schumann G, Garavan H, IMAGEN Consortium Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passos IC, Mwangi B, Kapczinski F. Big data analytics and machine learning: 2015 and beyond. The lancet Psychiatry. 2016;3:13–15. doi: 10.1016/S2215-0366(15)00549-0. [DOI] [PubMed] [Google Scholar]
- 13.Patel MJ, Andreescu C, Price JC, Edelman KL, Reynolds CF, 3rd, Aizenstein HJ. Machine learning approaches for integrating clinical and imaging features in late-life depression classification and response prediction. Int J Geriatr Psychiatry. 2015;30:1056–1067. doi: 10.1002/gps.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavagnino L, Amianto F, Mwangi B, D’Agata F, Spalatro A, Zunta-Soares GB, Abbate Daga G, Mortara P, Fassino S, Soares JC. Identifying neuroanatomical signatures of anorexia nervosa: a multivariate machine learning approach. Psychol Med. 2015;45:2805–2812. doi: 10.1017/S0033291715000768. [DOI] [PubMed] [Google Scholar]
- 15.Hajek T, Cooke C, Kopecek M, Novak T, Hoschl C, Alda M. Using structural MRI to identify individuals at genetic risk for bipolar disorders: a 2-cohort, machine learning study. J Psychiatry Neurosci. 2015;40:316–324. doi: 10.1503/jpn.140142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato JR, Moll J, Green S, Deakin JF, Thomaz CE, Zahn R. Machine learning algorithm accurately detects fMRI signature of vulnerability to major depression. Psychiatry Res. 2015;233:289–291. doi: 10.1016/j.pscychresns.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shouval R, Bondi O, Mishan H, Shimoni A, Unger R, Nagler A. Application of machine learning algorithms for clinical predictive modeling: a data-mining approach in SCT. Bone marrow transplantation. 2014;49:332–337. doi: 10.1038/bmt.2013.146. [DOI] [PubMed] [Google Scholar]
- 18.Pariyadath V, Stein EA, Ross TJ. Machine learning classification of resting state functional connectivity predicts smoking status. Frontiers in Human Neuroscience. 2014;8:425. doi: 10.3389/fnhum.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brodersen KH, Deserno L, Schlagenhauf F, Lin Z, Penny WD, Buhmann JM, Stephan KE. Dissecting psychiatric spectrum disorders by generative embedding. NeuroImage: Clinical. 2013;4:98–111. doi: 10.1016/j.nicl.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breiman L. Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 21.Qi Y, Bar-Joseph Z, Klein-Seetharaman J. Evaluation of different biological data and computational classification methods for use in protein interaction prediction. Proteins: Structure, Function, and Bioinformatics. 2006;63:490–500. doi: 10.1002/prot.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychological Methods. 2009;14:323–348. doi: 10.1037/a0016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ball TM, Stein MB, Ramsawh HJ, Campbell-Sills L, Paulus MP. Single-subject anxiety treatment outcome prediction using functional neuroimaging. Neuropsychopharmacology. 2014;39:1254–1261. doi: 10.1038/npp.2013.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 26.Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied & Preventive Psychology. 1994;3:61–73. [Google Scholar]
- 27.Hollingshead AB. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- 28.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JIJ, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 29.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17 doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 30.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 31.Brown SA, Gleghorn A, Schuckit MA, Myers MG, Mott MA. Conduct disorder among adolescent alcohol and drug abusers. Journal of Studies on Alcohol. 1996;57:314–324. doi: 10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 33.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 34.Brown SA, Christiansen BA, Goldman MS. The Alcohol Expectancy Questionnaire: An instrument for the assessment of adolescent and adult alcohol expectancies. Journal of Studies on Alcohol. 1987;48:483–491. doi: 10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- 35.Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. Journal of Studies on Alcohol. 2004;65:692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- 36.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- 37.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 39.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 41.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 42.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 43.Saad ZS, Reynolds RC. SUMA. NeuroImage. 2012;62:768–773. doi: 10.1016/j.neuroimage.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Squeglia LM, McKenna BS, Jacobus J, Castro N, Sorg SF, Tapert SF. BOLD response to working memory not related to cortical thickness during early adolescence. Brain Research. 2013;1537:59–68. doi: 10.1016/j.brainres.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genuer R, Poggi JM, Tuleau-Malot C. Variable selection using random forests. Pattern Recognition Letters. 2010;31:2225–2236. [Google Scholar]
- 46.Peeters M, Janssen T, Monshouwer K, Boendermaker W, Pronk T, Wiers R, Vollebergh W. Weaknesses in executive functioning predict the initiating of adolescents’ alcohol use. Developmental Cognitive Neuroscience. 2015;16:139–146. doi: 10.1016/j.dcn.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Developmental Cognitive Neuroscience. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellanos FX. personal communication.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


