Abstract
Background
The global prevalence of Cimex lectularius infestations has challenged current intervention efforts, as pyrethroid resistance has become ubiquitous, availability of labeled insecticides for bed bugs is limited, and non-chemical treatment options, such as heat, are often unaffordable. We evaluated representative insecticides toward the goal of developing a novel, ingestible liquid bait for hematophagous arthropods.
Results
LC50 values were estimated for adult males and first instar nymphs of an insecticide-susceptible strain for abamectin, clothianidin, fipronil and indoxacarb, after ingestion from an in vitro feeder. LD50 values were calculated based on the ingested blood volume. Ingested abamectin, clothianidin and fipronil caused rapid mortality in both life stages. Fipronil was ∼43-fold more effective by ingestion than by topical application. Indoxacarb and its bioactive metabolite decarbomethoxyllated JW062 (DCJW) were ineffective at causing bed bug mortality even at concentrations as high as 1000 ng mL−1 blood.
Conclusions
Fipronil, clothianidin and abamectin have potential for being incorporated into a liquid bait for bed bug control; indoxacarb and DCJW were not effective. Bed bugs are a good candidate for an ingestible liquid bait because systemic formulations generally require less active ingredient than residual sprays, they remain contained and more effectively target hematophagous arthropods.
Keywords: Bed bug, Cimex lectularius, mortality, systemic control, liquid bait
1 Introduction
The incidence and prevalence of bed bug infestations are on the rise worldwide. There does not appear to be a human environment that is unacceptable to bed bugs1 and because Cimex lectularius accepts a variety of vertebrate hosts, bed bug reservoirs can be sustained in pet shops and confined animal production farms. Infestations can have significant economic impacts on households, the hospitality industry and poultry farms.2 The public health impacts of bed bugs are arguably as important, not only because they are obligatory blood feeders with potential to vector pathogens,3 but also because of the anxiety, sleeplessness and ostracism associated with infestations.4 Bed bug saliva can cause skin irritation, ranging from mild annoyance to severe dermatitis, and immune responses can occur soon after feeding or days or even weeks later.5 Itching may be so severe that chronic scratching often leads to secondary infection(s).6
Bed bug infestations are often controlled with insecticide sprays, heat treatments, fumigation and sometimes freezing. However, each of these approaches has significant constraints and short-comings. Pyrethroids are the most common class of insecticides labelled for bed bug control, but resistance to pyrethroids has become widespread globally.7-10 Bioassays of field-collected populations confirm extensive resistance to pyrethroids,11 but its pervasiveness is especially highlighted by molecular analyses showing that >80% and >95% of USA and European bed bug populations, respectively, carried one and/or both mutations in the voltage-gated sodium channel gene, conferring target-site based pyrethroid resistance.11, 12 Multiple molecular mechanisms that underlie resistance to pyrethroids also include differential gene expression of carboxylesterases, P450s, glutathione-S-transferases and cuticular protein genes that contribute to a thicker cuticle.9, 11, 13-16 Combinations of pyrethroids with other classes of insecticides (e.g., imidacloprid/β-cyfluthrin or acetamiprid/bifenthrin) also tend to be less effective due to pyrethroid resistance.17 Overall, the pervasiveness of pyrethroid resistance, and lack of reliable alternative insecticides have resulted in repetitive applications by pest controllers, which ultimately results in large amounts of residual insecticides in the home environment.18
Baits have been successfully deployed against major groups of urban pests, including ants, cockroaches, termites and wasps.19-21 Bait formulations have several noteworthy advantages. They are slower acting which allows time for foraging insects to return to their harborage and potentially deliver the active ingredient (AI) – often in a palatable excretion – to nestmates or aggregations,22 providing secondary effects of the insecticide.23, 24 Baits often include attractants and phagostimulants that facilitate intake, and the formulation tends to protect the AI from environmental factors (e.g., UV) giving it longer residual activity.22 Baits can be formulated to be insect-specific and they can be placed in desired locations to more effectively target the pest. Finally, ingested insecticides (like pharmaceuticals) tend to be more bioavailable and hence more effective at a lower dose than other formulations. Because baits require greater concerted effort, including reduction of competing food sources, they are generally a preferred formulation in the heterogeneous built environment more than in vast agricultural fields where plants, often in monocultures, are the preferred food.
In plant protection, systemic insecticides are effectively used to control a variety of hemipteran species with piercing/sucking mouthparts.25, 26 AIs are taken up by the plant, transported in phloem and are thus ingested in a liquid medium. An advantage of this system is that attractants and phagostimulants are not necessary because the host plant serves this function. A similar strategy has been exceptionally successful with vertebrate hosts in veterinary medicine, where systemic insecticides are used to protect pets and farm animals from ectoparasites. AIs such as afoxolaner,27 members of the avermectin family,28 and a combination of spinosad and milbemycin oxime29 are ingested by dogs, cats and livestock to control blood feeding arthropods, such as fleas and ticks.
Bed bugs are known to feed on a variety of vertebrate hosts associated with humans and human-made structures.3 For example, C. lectularius populations thrive in poultry farms30, 31 and associate with bats in roosts within urban structures.12 Their hemimetabolous development and the absolute requirement for a blood meal by both sexes and all mobile life stages3 (unlike fleas, mosquitoes, and various flies including sand flies) make a baiting system targeting bed bugs particularly appealing. Whether artificial formulations or live hosts are considered as baits, it is essential to identify effective insecticides that would kill bed bugs at low concentrations and would be compatible with these prospective approaches.
We report the evaluation of four insecticides with different modes of action as potential AIs for incorporation into an artificial liquid baiting system or a live baiting system. None of the following insecticides are currently labeled for bed bug control. Abamectin is a member of the avermectin (macrocylic lactones) family which are naturally derived from the soil bacterium, Streptomyces avermitilis.32 It has insecticidal, nematicidal and acaricidal properties, and it targets the γ-aminobutyric (GABA) receptors and glutamate gated chloride channels, resulting in paralysis.33, 34 Clothianidin is a neonicotinoid and an agonist to the nicotinic acetylcholine receptors (nAChR).35, 36 Fipronil is a phenylpyrazole that can act by contact or systemically and, like abamectin, affects the GABA receptors and chloride channels in the central and peripheral nervous system.33 However, unlike abamectin, fipronil causes hyperexcitation of the nerves.37 Indoxacarb is an oxadiazine and is unique in that it must be bioactivated within the insect and blocks sodium channels,38 consequently pseudoparalysis occurs and neural activity ceases.39
All four of these insecticides are active on insects with piercing/sucking mouthparts. Fipronil and indoxacarb are used in topical treatments for dogs and cats. Fipronil has been shown to cause mortality in fleas that feed on treated mice40 and indoxacarb not only causes mortality in adult fleas, but also interrupts the development of flea eggs and larvae upon contact with treated cats.41 Clothianidin has systemic activity in crop protection against many species with piercing/sucking mouthparts.42 Lastly, the avermectin class is widely known for its systemic activity. Ivermectin kills mosquitoes and causes sublethal effects such as lower fecundity of females that fed on treated cattle.43 A recent study showed that ivermectin also caused mortality in bed bugs fed on treated mice and humans.44
2 Materials and Methods
2.1 Bed bugs and rearing procedures
Cimex lectularius colonies of the Harold Harlan strain (HH; also known as Fort Dix, collected in 1973), which is known to be susceptible to pyrethroid insecticides, were maintained in an incubator at 27°C, ∼50% RH, and LD 12:12, and all experimental insects were maintained in the same conditions. Colonies were fed defibrinated rabbit blood in an artificial feeding system, as used in Romero and Schal (Fig. 1A).45 Briefly, custom-fabricated water-jacketed glass condensers (Prism Research Glass, Raleigh, NC) served as feeders. An internal reservoir (blood chamber) was surrounded by an outer reservoir (circulating water chamber) connected to a thermal circulator that circulated heated water through the condenser to maintain blood near human body temperature (38.4°C). The reservoir held up to ∼4 mL of blood, but 2 mL were sufficient to eliminate air from the feeder. Nescofilm® (Alfresa Pharma Corporation, Osaka, Japan) was stretched across the bottom of the feeder and blood was introduced through an opening at the top. Feeders were connected in series so several colonies could be fed concurrently. Bed bugs were housed in containers or vials with paper inserts for harborage and plankton netting (BioQuip Products, Rancho Dominguez, CA) on the top through which all life stages could feed.
Figure 1.
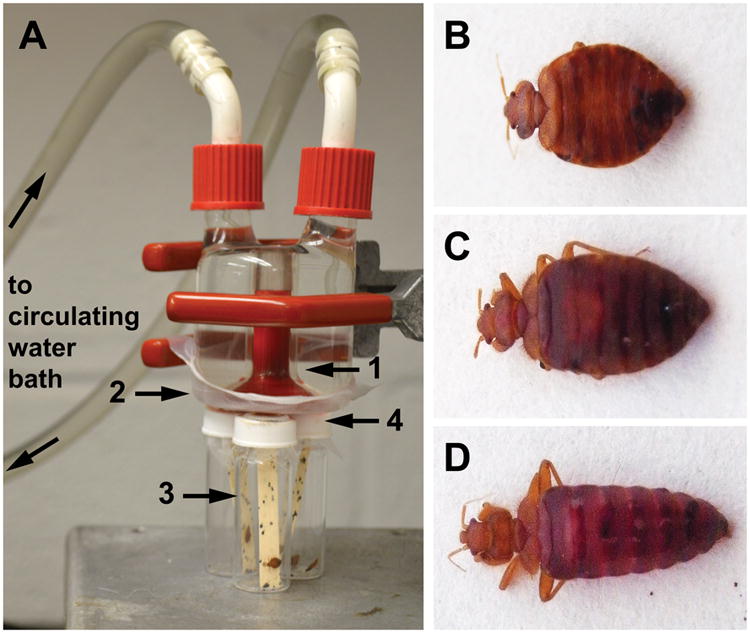
(A) Apparatus used to feed bed bugs insecticide-treated defibrinated rabbit blood. Blood was placed into the internal reservoir of a custom-fabricated glass condenser (1) heated with a circulating water bath and held in place by a ductile, malleable, thermoplastic paraffin film (2). Bed bugs were placed in glass vials containing Manila paper (3) and capped with plankton screen (4) through which they could insert their mouthparts. (B–D) Unfed (B), partially fed (C) and fully fed (D) adult male bed bugs. Only fully fed individuals were included in our assays.
All bed bugs were starved for 7–10 days prior to feeding or topical application assays. We used adult males because they were more readily obtained and their physiological state is relatively independent of reproductive cycles. In comparison, we also tested first instar nymphs because they are much smaller and therefore take a much smaller blood meal.
2.2 Measurement of blood meal volume
Starved adult male bed bugs (5 males per replicate, 5 replicates, total n=25) and first instar nymphs (10 per replicate, 5 replicates, total n=50) were placed into 4 mL glass vials and weighed prior to feeding. They were allowed to feed for 10 min on defibrinated rabbit blood. Only fully fed individuals (determined visually) (Fig. 1D) were then reweighed and the mass gain per individual was adjusted by the number of individuals that fed. We converted mass to blood volume without making any corrections for the specific gravity of blood. However, assuming a specific gravity of blood of 1.0506 at 37°C,46 our volumetric estimates may be inflated by ∼5%.
2.3 In vitro feeding assays
2.3.1 Dimethyl sulfoxide (DMSO) dose response
DMSO was used as the vehicle for all insecticides. Various amounts of DMSO were added to defibrinated rabbit blood to obtain final concentrations of DMSO of 0% (blood only), 0.25%, 0.5%, 0.75% and 1%. Only adult males were used in this assay. We used 3 replicates of 10 males for each concentration of DMSO. Each replicate was placed in a 4 mL glass vial with a strip of Manila folder (clinging substrate) that reached the top of the vial to facilitate feeding.
Each group was allowed to feed for 30 min. After the first 15 min a glass Pasteur pipette was inserted into the internal reservoir and the blood was agitated. After 30 min of feeding, only fully engorged bed bugs were separated and monitored daily for mortality for 7 days.
2.3.2 Insecticide in vitro feeding bioassays
Technical grade abamectin (98-99%, Whitmire [now BASF], Research Triangle Park, NC), fipronil (88.7%, Aventis [now Bayer], Research Triangle Park, NC), clothianidin (99.5%, Chem Service Inc., West Chester, PA), indoxacarb (98.5%, Chem Service Inc., West Chester, PA) and DCJW (active form of indoxacarb, 98%, DuPont, Wilmington, DE) were dissolved in DMSO to make stock solutions of 10 mg AI mL−1 DMSO and then serially diluted in DMSO to obtain the desired concentrations.
For each insecticide concentration we used 28–30 adult males and 24–30 first instar nymphs per treatment group, with two control groups (defibrinated rabbit blood and defibrinated rabbit blood plus 0.75% DMSO) and 6 treatment groups for each life stage tested. All feedings were made in the scotophase, 2–8 hrs after lights-off.
2.4 Topical application assays
Fipronil and indoxacarb were dissolved in acetone to make a stock solution of 20 mg AI mL−1 and then diluted in acetone. For each insecticide we used 30 adult males per dose (solvent control group and 5 treatment groups; n=180). Each replicate of 10 males was placed in a 4 mL vial, immobilized on ice ∼10–15 min, bed bugs were placed ventral side up onto a filter paper (Whatman #1, 55 mm) in a petri dish (Falcon®, 60×15 mm), and 0.5 μL of acetone or one of the pesticide doses in acetone was applied onto the thorax, between the coxae, using a Hamilton syringe held in a manual micro-applicator (Hamilton Co., Reno, NV). The acetone was allowed to evaporate and the Petri dish lid was then taped in place to prevent the bugs from escaping. All applications were made in the scotophase, 2–8 hrs after lights-off. Individuals were monitored and mortality was recorded daily for 5 days.
2.5 Statistical Analysis
Probit analysis was used to determine the concentration required to kill 50% of test insects (LC50) of each insecticide on days 1–7 using PoloPlus© (LeOra Software Company, Petaluma CA). This software was also used to estimate the dose required to kill 50% of test insects (LD50) values from topical application assays.
3 Results
3.1 Ingested blood volume by adult males and first instars
All adult bed bugs fed to repletion (Table 1), and each adult male consumed a mean of 3.92 ± 0.21 μL (SE, n=25) of defibrinated rabbit blood (without DMSO). The blood meal represented 147.4% of the unfed body mass of an average adult male and resulted in ∼2.5-fold greater body mass of fed males. Fully engorged first instar nymphs (47 of 50) consumed 0.46 ± 0.04 μL (n=47) of blood, representing 219% of their unfed body mass, or a ∼3.2-fold greater body mass after feeding. Thus, there was nearly a tenfold difference in the blood meal volume ingested by first instars and adult males, coincident with a tenfold difference in their unfed body mass (Table 1). Therefore, on a mass AI per body mass basis, the dose-response curves of insecticides are expected to be similar for these two life stages.
Table 1.
Relationship between in vitro blood meal volume and body mass of unfed adult males and first instars.
| Life Stage | Number of bugs (n) | Number of bugs fully fed (%) | Unfed body mass (mg) mean ± SE | Blood meal size (μL) mean ± SE | Increase in body mass (%) |
|---|---|---|---|---|---|
| Adult males | 25 | 25 (100) | 2.66 ± 0.33 | 3.92 ± 0.21 | 147% |
| First instars | 50 | 47 (94) | 0.21 ± 0.04 | 0.46 ± 0.04 | 219% |
3.2 In vitro feeding assays
3.2.1 Effect of DMSO on bed bug mortality
A dose-response study was conducted to determine the concentration of DMSO in defibrinated rabbit blood that could be used in subsequent dose-response studies with insecticides. Most of the bed bugs fully engorged even on the highest concentration of DMSO (Fig. 2). However, there was a dramatic increase in mortality as DMSO concentration increased >0.75%. Thus, 0.75% DMSO in defibrinated rabbit blood was deemed suitable because it caused only 10% mortality 7 days post ingestion (n=30 bed bugs), which was the same as the blood-only control.
Figure 2.
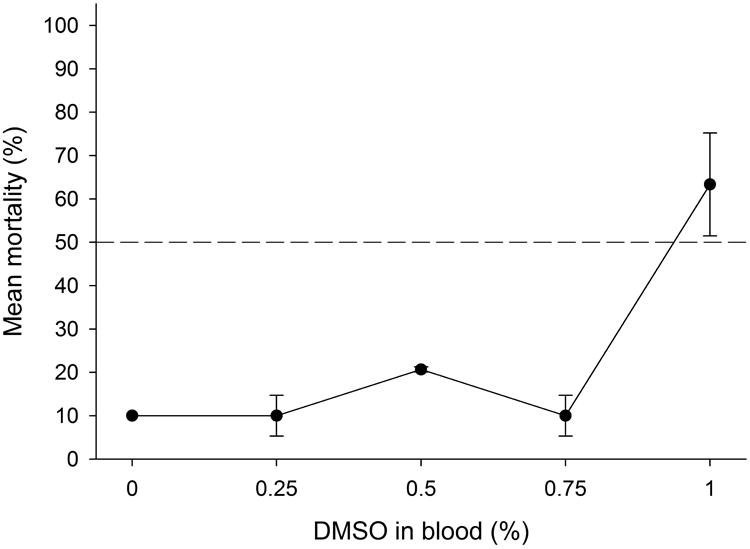
Dose-response for DMSO in defibrinated rabbit blood, showing adult male bed bug mortality 7 days after ingestion. Each mean (± SE) represents 3 replicates of 10 bed bugs each.
3.2.2 In vitro feeding assays with insecticides
The results of the in vitro feeding bioassays are shown in Table 2 and Fig. 3. Mortality was recorded for 7 days (supplemental information, Figs. S1-S4) but only results for day 3 are shown. Generally, the HH bed bugs readily accepted and ingested the insecticides offered in rabbit blood, even at their highest concentrations (Tables S1-S4). The order of toxicity of the four insecticides against fully engorged bed bugs was determined to be fipronil > clothianidin > abamectin ≫> indoxacarb for both adult males and first instars based on results of probit analysis. We also estimated the LD50 ingested by adult males and first instars (LC50 × blood volume ingested); these LD50 values may be compared to the results of topical applications.
Table 2.
In vitro feeding bioassay results 3 days after ingestion of four different insecticides by bed bugs of the Harold Harlan strain.
| Active ingredient | Life stage | n | LC50 Slope (± SE) | LC50 (95% CI)a (ng mL−1 blood) | LD50 b (ng insect−1) | LD50 c (ng mg−1 fed body mass) |
|---|---|---|---|---|---|---|
| Abamectin | Adult males | 240 | 7.79 (1.66) | 32.59 (27.14–37.88)d | 0.127 | 0.019 |
| First instars | 229 | 3.96 (0.61) | 22.10 (17.87–26.87)d | 0.010 | 0.015 | |
| Clothianidin | Adult males | 238 | 2.64 (0.51) | 14.19 (9.12–18.91) | 0.056 | 0.008 |
| First instars | 228 | 1.59 (0.24) | 20.66 (4.40–49.75) | 0.010 | 0.015 | |
| Fipronil | Adult males | 240 | 2.45 (0.43) | 13.37 (9.51–21.29)d | 0.052 | 0.008 |
| First instars | 234 | 7.16 (1.35) | 6.84 (5.89–7.99)d | 0.003 | 0.004 | |
| Indoxacarb | Adult males | 216 | - | - | ||
| First instars | 232 | - | - |
LC50 values and 95% confidence intervals. Values are in ng AI mL−1 defibrinated rabbit blood.
LD50 values are in ng AI insect−1 and were calculated based on the observation that each adult male bed bug consumed 3.9 μL blood and each first instar ingested 0.46 μL blood (LC50 × blood volume consumed).
LD50 values are in ng AI per mg fed body mass and were determined based on a fed body mass value of 6.58 mg for each adult male bed bug and 0.67 mg for each first instar (LD50 × fed body mass).
Significant differences between the LC50 values of males and nymphs due to non-overlap of 95% CI for the two life stages tested.
Figure 3.
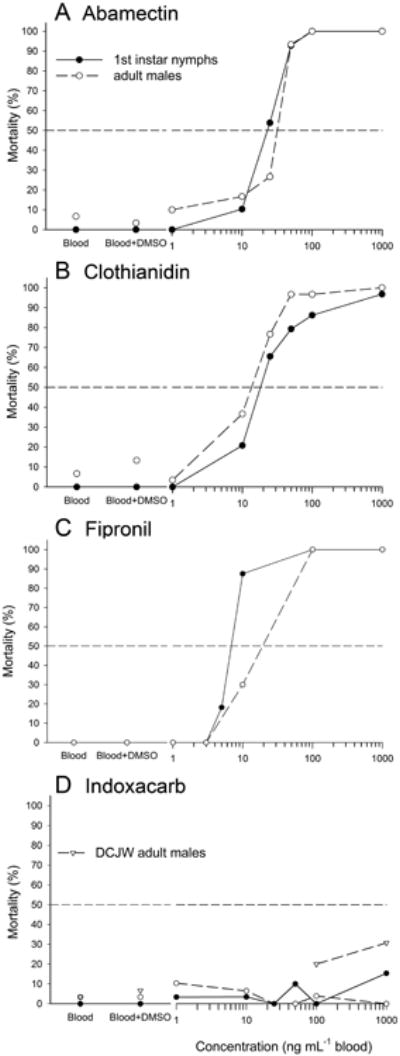
Mortality dose-response curves for abamectin, clothianidin, fipronil and indoxacarb, three days post ingestion, for both first instar nymphs (solid line) and adult males (dashed line). Each insecticide was dissolved in DMSO and mixed with defibrinated rabbit blood to a final concentration of 0.75% DMSO.
Fipronil, clothianidin and abamectin were highly effective at causing 100% mortality in both adult males and first instars by day 3 (Fig. 3, Table 2). HH strain bed bugs accepted fipronil, with 85–100% fully engorging (Table S3). Fipronil was most effective with an LC50 value of 13.4 ng mL−1 blood and 6.84 ng mL−1 blood for adult males and first instars, respectively, corresponding to LD50 values of only 52 pg male−1 (3 pg nymph−1) and 8 pg mg−1 fed male body mass (0.4 pg mg−1 fed nymph body mass); fipronil caused 100% mortality at 100 ng mL−1 blood in both adult males and first instars.
Both adult males and first instar nymphs readily accepted clothianidin, with 97-100% fully engorging at all concentrations (Table S2). A concentration of 1000 ng mL−1 blood clothianidin caused 100% mortality in adult males and first instars by day 4 (Fig. S2). The LC50 values for adult males and first instars 3 days after ingestion of clothianidin were similar to fipronil (Table 2). There were no appreciable changes in mortality between days 3 and 7 for both males and first instars (Fig. S2).
Abamectin was readily ingested by males and nymphs, with 93-100% of bed bugs fully engorging even on the highest concentrations of abamectin (Table S1). Abamectin caused 100% mortality at 100 ng mL−1 blood in both adult males and first instars, with LC50 values ∼2.5-fold higher than for fipronil and clothianidin for adult males. LC50 values for first instars were similar for clothianidin and ∼3-fold higher compared to fipronil (Table 2). Like clothianidin, abamectin mortality increased only slightly between days 3 and 7 (Fig. S1).
Indoxacarb was variably ingested by the HH bed bugs. All first instar nymphs fully engorged even on 1000 ng indoxacarb mL−1 blood, but 67–93% of adult males fully engorged on 25–1000 ng indoxacarb mL−1 blood (Table S4). Surprisingly, ingested indoxacarb was ineffective, with only <20% mortality at the highest concentration (1000 ng mL−1 blood; ∼4 ng male−1 or ∼0.5 ng nymph−1) on day 3 (Fig. 3) and <90% mortality by day 7 (Fig. S4). Probit analysis could not be performed with indoxacarb, but adult males were more susceptible to it than first instars. The active metabolite of indoxacarb, DCJW, was slightly more effective, causing 20% and 30% mortality by day 3 at concentrations of 100 and 1000 ng mL−1 blood, respectively (Fig. 3).
For clothianidin, the LC50 values were not significantly different for adult males and first instar nymphs, based on overlap of the 95% confidence intervals of both life stages. Using the same criterion, first instars were significantly more sensitive to fipronil and abamectin than adult males (Table 2). However, on a per mg body mass basis the clothianidin LD50 values were similar for males and first instars, 19 and 15 pg mg−1, respectively.
3.3 Topical Applications
Mortality of HH strain males was monitored for 5 days (Fig. S5), but only day 3 results are shown in Table 3 for each insecticide tested. Fipronil again was highly effective at causing bed bug mortality 3 days after topical application; the LD50 was 2.21 ng male−1 (Table 3, Fig. 4) compared to 0.052 ng male−1 by ingestion (Table 2).
Table 3.
Topical application bioassay results, 3 days after application, with two different insecticides tested against adult males of the Harold Harlan population.
| Technical Insecticide | n | Slope (±SE) | LD50a (95% CI) |
|---|---|---|---|
| Fipronil | 180 | 3.89 (0.93) | 2.21 (1.54–2.87) |
| Indoxacarb | 180 | - | - |
LD50 values (ng technical insecticide per male) were derived from Probit analysis on day 3.
Figure 4.
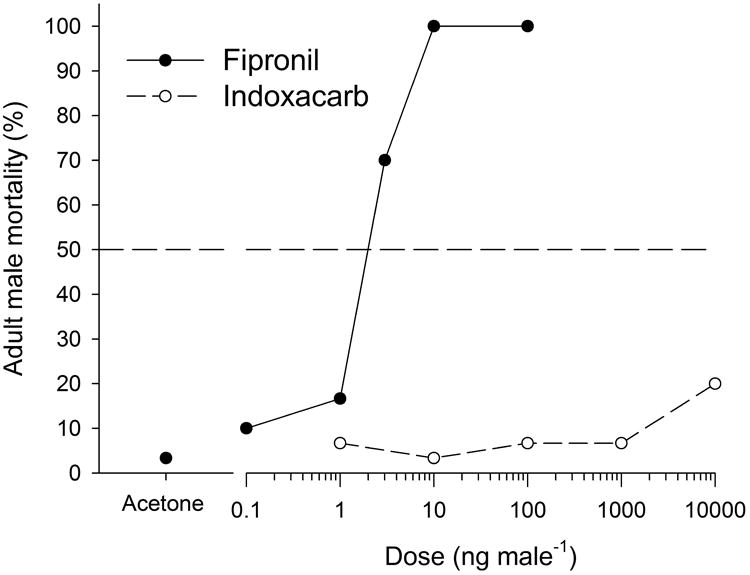
Dose-response mortality curves for topical applications of fipronil and indoxacarb to adult male bed bugs, 3 days post application.
Indoxacarb caused some bed bug mortality 3 or 7 days after ingestion (Table 2, Fig. S4) or 5 days after topical application (Fig. S5), but only at extremely high doses of 3.9 ng insect−1 and 10,000 ng insect−1, respectively. Doses as high as 10 μg indoxacarb killed only <60% of the HH bed bugs 5 days after topical application.
4 Discussion and Conclusions
4.1 Ingestible insecticides for bed bug control
Overall, three of the four insecticides we assayed by ingestion – abamectin, clothianidin and fipronil – proved to be highly effective at causing bed bug mortality. Patterns of mortality were similar for adult males and first instar nymphs of the Harold Harlan population, an insecticide-susceptible strain that has been maintained in laboratory culture since 1973. On the other hand, indoxacarb and DCJW were surprisingly ineffective at causing bed bug mortality by ingestion, and topical applications confirmed that this insecticide is ineffective against bed bugs. The same lot of indoxacarb was tested on the German cockroach, Blattella germanica, and it was found to be effective,47 indicating that the differential efficacy of indoxacarb is related to its species-specific processing. It is unlikely that reduced cuticular penetration of indoxacarb was involved because it was ineffective by ingestion. It is also unlikely that indoxacarb was rapidly catabolized because it is a pro-insecticide and its major metabolite, DCJW, was also ineffective. Although indoxacarb is particularly effective on lepidopteran larvae, it is much less potent, especially on a per mg body mass basis, on piercing sucking insects, beetles and even cockroaches.38 Notably, while indoxacarb was active on Aedes aegypti mosquito larvae, it failed to kill adult female mosquitoes.48 Likewise, indoxacarb was ineffective by topical application on first instars of the kissing bug Triatoma infestans,49 suggesting that it may be generally ineffective on hematophagous insects. Nevertheless, topical spot-on applications of indoxacarb were shown to be highly effective against cat fleas on cats.50 We considered the possibility that bed bugs lack the enzymes – presumably esterases – necessary to cleave indoxacarb to its N-decarbomethoxyllated metabolite which is the active blocker of sodium-dependent action potentials. However, DCJW, the active metabolite, was only slightly more effective by ingestion than indoxacarb. Clearly, further research is needed to understand why indoxacarb was ineffective on bed bugs and whether its activity can be synergized in an ingestible formulation.
Oral administration of fipronil was much more effective than topical application, as expected. Based on calculations of LD50 values, C. lectularius males were ∼43-fold more sensitive to fipronil by ingestion than by topical application, with remarkably high toxicity of 8 pg per mg fed body mass by ingestion. The LC50 value for fipronil on bed bugs was 10-fold lower than for afoxolaner on fleas; monthly treatments of dogs with oral dosages of 2.5 mg kg−1 afoxolaner maintains a plasma concentration >100 ng mL−1, and results in 100% efficacy on fleas.27 These results support the notion that effective AIs can be combined with phagostimulants45 in a liquid bait that would use much less AI than is often used in residual applications of insecticides.
Overall, our results with an in vitro feeding system provide support for the concept that liquid baits should be developed as a novel and valuable insecticide delivery system against bed bugs and other hematophagous arthropods. Our experiments only evaluated the responses of adult males and first instar nymphs. Future studies should investigate adult females and sub-lethal effects on fecundity, egg viability, egg hatch and the ability of bed bugs to refeed after sublethal exposure to insecticides. Also, recently collected field populations should be examined to evaluate their susceptibility to these insecticides via ingestion and topical application. The HH strain bed bugs readily accepted and ingested even the highest concentrations of these insecticides, but the palatability of these and other AIs should be tested on recently collected bed bugs. Moreover, more insecticides representing these and other classes with different modes of action should be evaluated to assess their potential inclusion in a liquid baiting system. Finally, this research addresses only one component, the AI dissolved in blood, as a proof-of-principle for prospective fully synthetic liquid baiting formulations. Before this concept can be implemented, attractants (including heat), phagostimulants and other formulation ingredients will need to be explored.
5 Summary
The goal of the study was to screen several common AIs with different modes of action for their potential inclusion in a liquid baiting system. Moreover, we sought to compare two modes of delivery of insecticides, ingestion and contact. A baiting system could serve as a safer alternative to residual insecticides because less AI would be used in containerized formulations placed in desired locations. Three AIs, abamectin, clothianidin and fipronil, were highly effective by ingestion. However, indoxacarb and DCJW were ineffective by either ingestion or topical application. The concept of an artificial liquid or live baiting system has high appeal, and these results highlight its potential as well.
Supplementary Material
Acknowledgments
We would like to thank Harold Harlan for the original HH colony, Rick Santangelo for maintaining the Cimex lectularius colonies, Daniel Cordova (DuPont Crop Protection) for a gift of DCJW, Ayako Wada-Katsumata and Zachary DeVries for assisting with statistical analyses and photography. The work was supported in part by a National Institutes of Health funded Initiative for Maximizing Student Diversity (IMSD) fellowship to AS, grants from the US Department of Housing and Urban Development (grant NCHHU-0017-13), and Alfred P. Sloan Foundation (2013-5-35 MBE) to CS, NIEHS (P30ES025128) to the Center for Human Health and the Environment, and the Blanton J. Whitmire Endowment at North Carolina State University.
References
- 1.Kells SA. Bed bugs: a systemic pest within society. Am Entomol. 2006;52:107–110. [Google Scholar]
- 2.Reinhardt K, Siva-Jothy MT. Biology of the bed bugs (Cimicidae) Annu Rev Entomol. 2007;52:351–374. doi: 10.1146/annurev.ento.52.040306.133913. [DOI] [PubMed] [Google Scholar]
- 3.Usinger RL. Monograph of Cimicidae (Hemiptera, Heteroptera) Entomological Society of America; College Park, MD: 1966. [Google Scholar]
- 4.Susser SR, Perron S, Fournier M, Jacques L, Denis G, Tessier F, et al. Mental health effects from urban bed bug infestation (Cimex lectularius L.): a cross-sectional study. BMJ Open. 2012;2:e000838. doi: 10.1136/bmjopen-2012-000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doggett SL, Dwyer DE, Peñas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev. 2012;25:164–192. doi: 10.1128/CMR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol. 2004;43:430–433. doi: 10.1111/j.1365-4632.2004.02115.x. [DOI] [PubMed] [Google Scholar]
- 7.Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol. 2007;44:175–178. doi: 10.1603/0022-2585(2007)44[175:IRITBB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Kilpinen O, Kristensen M, Jensen KMV. Resistance differences between chlorpyrifos and synthetic pyrethroids in Cimex lectularius population from Denmark. Parasitol Res. 2011;109:1461–1464. doi: 10.1007/s00436-011-2423-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhu F, Gujar H, Gordon JR, Haynes KF, Potter MF, Palli SR. Bed bugs evolved unique adaptive strategy to resist pyrethroid insecticides. Sci Rep. 2013;3:e1456. doi: 10.1038/srep01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies TGE, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol. 2012;26:241–254. doi: 10.1111/j.1365-2915.2011.01006.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhu F, Wigginton J, Romero A, Moore A, Ferguson K, Palli R, et al. Widespread distribution of knockdown resistance mutations in the bed bug, Cimex lectularius (Hemiptera: Cimicidae), populations in the United States. Arch Insect Biochem Physiol. 2010;73:245–257. doi: 10.1002/arch.20355. [DOI] [PubMed] [Google Scholar]
- 12.Booth W, Balvín O, Vargo EL, Vilímová J, Schal C. Host association drives genetic divergence in the bed bug, Cimex lectularius. Mol Ecol. 2015;24:980–992. doi: 10.1111/mec.13086. [DOI] [PubMed] [Google Scholar]
- 13.Adelman ZN, Kilcullen KA, Koganemaru R, Anderson MA, Anderson TD, Miller DM. Deep sequencing of pyrethroid-resistant bed bugs reveals multiple mechanisms of resistance within a single population. PLoS One. 2011;6:e26228. doi: 10.1371/journal.pone.0026228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koganemaru R, Miller DM, Adelman ZN. Robust cuticular penetration resistance in the common bed bug (Cimex lectularius L.) correlates with increased steady-state transcript levels of CPR-type cuticle protein genes. Pestic Biochem Physiol. 2013;106:190–197. [Google Scholar]
- 15.Lilly DG, Latham SL, Webb CE, Doggett SL. Cuticle thickening in a pyrethroid-resistant strain of the common bed bug, Cimex lectularius L. (Hemiptera: Cimicidae) PLoS One. 2016;11:e0153302. doi: 10.1371/journal.pone.0153302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benoit JB, Adelman ZN, Reinhardt K, Dolan A, Poelchau M, Jennings EC, et al. Unique features of a global human ectoparasite identified through sequencing of the bed bug genome. Nat Commun. 2016;7:e10165. doi: 10.1038/ncomms10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell BE, Miller DM. Insecticide resistance in eggs and first instars of the bed bug, Cimex lectularius (Hemiptera: Cimicidae) Insects. 2015;6:122–132. doi: 10.3390/insects6010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangloff-Kaufmann J, Hollingsworth C, Hahn J, Hansen L, Kard B, Waldvogel M. Bed bugs in America: a pest management industry survey. Am Entomol. 2006;52:105–106. [Google Scholar]
- 19.Gore JC, Zurek L, Santangelo RG, Stringham SM, Watson DW, Schal C. Water solutions of boric acid and sugar for management of German cockroach populations in livestock production systems. J Econ Entomol. 2004;97:715–720. doi: 10.1093/jee/97.2.715. [DOI] [PubMed] [Google Scholar]
- 20.Klotz J, Greenberg L, Venn EC. Liquid boric acid bait for control of the Argentine ant (Hymenoptera: Formicidae) J Econ Entomol. 1998;91:910–914. [Google Scholar]
- 21.Silverman J, Roulston TH. Acceptance and intake of gel and liquid sucrose compositions by the Argentine ant (Hymenoptera: Formicidae) J Econ Entomol. 2001;94:511–515. doi: 10.1603/0022-0493-94.2.511. [DOI] [PubMed] [Google Scholar]
- 22.Buczkowski G, Schal C. Emetophagy: fipronil-induced regurgitation of bait and its dissemination from German cockroach adults to nymphs. Pestic Biochem Physiol. 2001;71:147–155. [Google Scholar]
- 23.Buczkowski G, Scherer CW, Bennett GW. Horizontal transfer of bait in the German cockroach: indoxacarb causes secondary and tertiary mortality. J Econ Entomol. 2008;101:894–901. doi: 10.1603/0022-0493(2008)101[894:htobit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Silverman J, Vitale G, Shapas T. Hydramethylnon uptake by Blattella germanica (Orthoptera: Blattellidae) by coprophagy. J Econ Entomol. 1991;84:176–180. doi: 10.1093/jee/84.1.176. [DOI] [PubMed] [Google Scholar]
- 25.Elbert A, Overbeck H, Iwaya K, Tsuboi S. Brighton Crop Protection Conference, Pests and Diseases-1990 Vol 1. British Crop Protection Council; 1990. Imidacloprid, a novel systemic nitromethylene analogue insecticide for crop protection; pp. 21–28. [Google Scholar]
- 26.Horowitz A, Mendelson Z, Weintraub P, Ishaaya I. Comparative toxicity of foliar and systemic applications of acetamiprid and imidacloprid against the cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) Bull Entomol Res. 1998;88:437–442. [Google Scholar]
- 27.Shoop WL, Hartline EJ, Gould BR, Waddell ME, McDowell RG, Kinney JB, et al. Discovery and mode of action of afoxolaner, a new isoxazoline parasiticide for dogs. Vet Parasitol. 2014;201:179–189. doi: 10.1016/j.vetpar.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Shoop WL, Mrozik H, Fisher MH. Structure and activity of avermectins and milbemycins in animal health. Vet Parasitol. 1995;59:139–156. doi: 10.1016/0304-4017(94)00743-v. [DOI] [PubMed] [Google Scholar]
- 29.Holmstrom SD, Totten ML, Newhall KB, Qiao M, Riggs KL. Pharmacokinetics of spinosad and milbemycin oxime administered in combination and separately per os to dogs. J Vet Pharmacol Ther. 2012;35:351–364. doi: 10.1111/j.1365-2885.2011.01333.x. [DOI] [PubMed] [Google Scholar]
- 30.Steelman CD, Szalanski AL, Trout R, McKern JA, Solorzano C, Austin JW. Susceptibility of the bed bug Cimex lectularius L.(Heteroptera: Cimicidae) collected in poultry production facilities to selected insecticides. J Agric Urban Entomol. 2008;25:41–51. [Google Scholar]
- 31.Axtell R, Arends J. Ecology and management of arthropod pests of poultry. Annual review of entomology. 1990;35:101–126. doi: 10.1146/annurev.en.35.010190.000533. [DOI] [PubMed] [Google Scholar]
- 32.Campbell WC. Ivermectin and abamectin. Springer Science & Business Media; 2012. [Google Scholar]
- 33.Nauen R, Bretschneider T. New modes of action of insecticides. Pestic Outlook. 2002;13:241–245. [Google Scholar]
- 34.Stumpf N, Nauen R. Biochemical markers linked to abamectin resistance in Tetranychus urticae (Acari: Tetranychidae) Pestic Biochem Physiol. 2002;72:111–121. [Google Scholar]
- 35.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45:247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- 36.Elbert A, Haas M, Springer B, Thielert W, Nauen R. Applied aspects of neonicotinoid uses in crop protection. Pest Manage Sci. 2008;64:1099–1105. doi: 10.1002/ps.1616. [DOI] [PubMed] [Google Scholar]
- 37.Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, et al. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res. 2015;22:5–34. doi: 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wing KD, Sacher M, Kagaya Y, Tsurubuchi Y, Mulderig L, Connair M, et al. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Protect. 2000;19:537–545. [Google Scholar]
- 39.Silver KS, Song W, Nomura Y, Salgado VL, Dong K. Mechanism of action of sodium channel blocker insecticides (SCBIs) on insect sodium channels. Pestic Biochem Physiol. 2010;97:87–92. doi: 10.1016/j.pestbp.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santora KA, Zakson-Aiken M, Rasa C, Shoop W. Development of a mouse model to determine the systemic activity of potential flea-control compounds. Vet Parasitol. 2002;104:257–264. doi: 10.1016/s0304-4017(01)00627-6. [DOI] [PubMed] [Google Scholar]
- 41.Guerino F, Qureshi T, Hair J, Young D, Fourie J. Indoxacarb kills developing stages of fleas in the environment of treated cats. Proceedings of the BSAVA World Congress. 2012 [Google Scholar]
- 42.Uneme H. Chemistry of clothianidin and related compounds. J Agric Food Chem. 2011;59:2932–2937. doi: 10.1021/jf1024938. [DOI] [PubMed] [Google Scholar]
- 43.Fritz M, Siegert P, Walker E, Bayoh M, Vulule J, Miller J. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae sl. Ann Trop Med Parasitol. 2009;103:539–547. doi: 10.1179/000349809X12459740922138. [DOI] [PubMed] [Google Scholar]
- 44.Sheele JM, Anderson JF, Tran TD, Teng YA, Byers PA, Ravi BS, et al. Ivermectin causes Cimex lectularius (bedbug) morbidity and mortality. J Emerg Med. 2013;45:433–440. doi: 10.1016/j.jemermed.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Romero A, Schal C. Blood constituents as phagostimulants for the bed bug Cimex lectularius L. J Exp Biol. 2014;217:552–557. doi: 10.1242/jeb.096727. [DOI] [PubMed] [Google Scholar]
- 46.Trudnowski RJ, Rico RC. Specific gravity of blood and plasma at 4 and 37 degrees C. Clin Chem. 1974;20:615–616. [PubMed] [Google Scholar]
- 47.Ko AE, Bieman DN, Schal C, Silverman J. Insecticide resistance and diminished secondary kill performance of bait formulations against German cockroaches (Dictyoptera: Blattellidae) Pest Manage Sci. 2016;72:1778–1784. doi: 10.1002/ps.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul A, Harrington LC, Scott JG. Evaluation of novel insecticides for control of dengue vector Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2006;43:55–60. doi: 10.1603/0022-2585(2006)043[0055:EONIFC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.Carvajal G, Mougabure-Cueto G, Toloza AC. Toxicity of non-pyrethroid insecticides against Triatoma infestans (Hemiptera: Reduviidae) Mem Inst Oswaldo Cruz. 2012;107:675–679. doi: 10.1590/s0074-02762012000500015. [DOI] [PubMed] [Google Scholar]
- 50.Dryden MW, Payne PA, Smith V, Heaney K, Sun F. Efficacy of indoxacarb applied to cats against the adult cat flea, Ctenocephalides felis, flea eggs and adult flea emergence. Parasit Vectors. 2013;6:126. doi: 10.1186/1756-3305-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


