Abstract
We describe the construction, structural properties and enzymatic substrate abilities of a series of circular DNA oligonucleotides that are entirely composed of the C-rich human telomere repeat, (CCCTAA)n. The nanometer-sized circles range in length from 36 to 60 nt, and act as templates for synthesis of human telomere repeats in vitro. The circles were constructed successfully by the application of a recently developed adenine-protection strategy, which allows for cyclization/ligation with T4 DNA ligase. Thermal denaturation studies showed that at pH 5.0, all five circles form folded structures with similar stability, while at pH 7.0 no melting transitions were seen. Circular dichroism spectra at the two pH conditions showed evidence for i-motif structures at the lower pH value. The series was tested as rolling circle templates for a number of DNA polymerases at pH = 7.3–8.5, using 18mer telomeric primers. Results showed that surprisingly small circles were active, although the optimum size varied from enzyme to enzyme. Telomeric repeats ≫1000 nt in length could be synthesized in 1 h by the Klenow (exo-) DNA polymerase. The results establish a convenient way to make long human telomeric repeats for in vitro study of their folding and interactions, and establish optimum molecules for carrying this out.
INTRODUCTION
The study of the structures of human telomeres and their interactions with proteins is widely recognized as important because of the relevance of telomeres and their capping state to human aging and cancer (1,2). Telomeric DNA multimers have the possibility of folding into multiple structures, including several topological variants of quadruplexes (3). In addition, it is becoming increasingly evident that, in addition to secondary structure, higher-order structures are biologically important as well (4). For example, the question of how adjacent folded quadruplexes interact in longer telomeric repeats is now under investigation (5). Natural human telomeres are thousands of nucleotides in length, while synthetic DNAs can be made only up to roughly 100 nt. Thus, for study of higher-order telomeric structures, efficient tools for elongation of telomeric DNAs are necessary. Unfortunately, telomerase enzyme preparations exhibit low activity in vitro, requiring amplification steps to observe the very short elongations that typically occur (6,7). For these reasons, it would be useful if new methods for elongating telomeres were available.
Recently, we reported that a 54mer DNA nanometer-sized circle containing the C-rich repeat of human telomeres is able to template the elongation of the complementary, G-rich strand (8). Commercially available DNA polymerases were used in this biomimetic mechanism, and repeating DNAs up to several thousands of nucleotides in length were produced. This early result, however, raised a number of important questions. Among these are, can smaller circular templates be constructed, and are they active as polymerase substrates? This issue is important because smaller oligonucleotides are more readily synthesized than large ones. A second question is whether such a mechanism might be active using DNA polymerases from human cells, which would be useful in mechanistic and structural studies in vivo (9). This possibility would require that DNA polymerases from the nucleus of the cell can utilize these unusual synthetic templates. Finally, since the circles themselves contain C-rich telomeric sequences that are also known to fold when in linear form (10,11), there is the question of whether they form secondary structures that might inhibit this mechanism. The current study addresses these issues. The results show that telomeric C-rich circles can in fact be constructed as small as 36 nt in length. The data suggest that the telomeric circles can indeed form pH-dependent folded structures, but despite this are able to template the synthesis of long telomeric repeats. The experiments reveal that surprisingly small circles can be active in telomere synthesis, including polymerases from nuclear extracts.
MATERIALS AND METHODS
Synthesis of DNA nanocircles
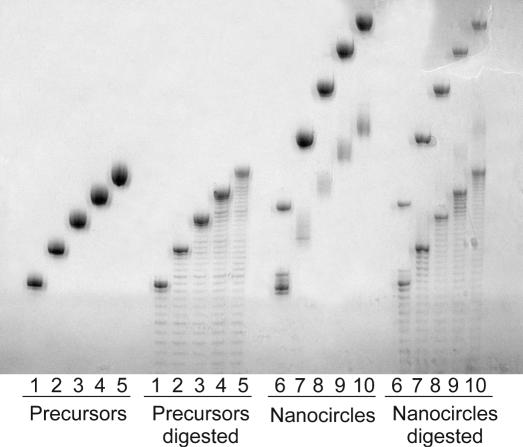
Circularization of the linear precursor oligonucleotides was carried out using T4 DNA ligase (New England Biolabs). The corresponding precursor (1 μM) was combined with a 18mer ligation splint (GTTAGGGTTAGGGTTAGG, 1.5 μM) in 50 mM Tris, pH 7.5, 10 mM MgCl2. After denaturation for 5 min at 75°C, the solution was allowed to slowly reach room temperature. ATP, DTT and BSA were added to final concentrations of 100 μM, 10 mM and 25 μg/ml, respectively. The reaction was initiated by adding T4 DNA ligase to a final concentration of 800 U/ml. The reaction was incubated for 12 h at room temperature, followed by heat inactivation of the ligase and dialysis against water. The reaction mixture was concentrated, followed by purification of circular oligonucleotides by 15% PAGE. Confirmation of circularity was provided by nicking with S1 endonuclease. Initial cleavage of circle produces a single band with the mobility of the linear precursor (see Figure 2). The faint smear with a greater mobility in the circular samples is believed to be a folded conformation of the circle sequences.
Figure 2.
Nuclease studies showing circular structures. Lane 1, 36 nt precircle; lane 2, 42 nt precircle; lane 3, 48 nt precircle; lane 4, 54 nt precircle; lane 5, 60 nt precircle; lane 6, 36 nt nanocircle; lane 7, 42 nt nanocircle; lane 8, 48 nt nanocircle; lane 9, 54 nt nanocircle; and lane 10, 60 nt nanocircle. Partial digestions were carried out by incubation with S1 nuclease.
Because of the unusual repeating sequences being ligated, successful cyclization required protection of central adenines in each sequence with N6-dimethylacetamidine, and the remaining bases with more labile protecting groups. This modified nucleotide was prepared according to the method described by McBride et al. (12). The modified and orthogonally deprotected DNA oligonucleotides were synthesized as described previously (13). Additional details are given in the Supplementary Material.
Polymerase extension reactions
All extension reactions were carried out in a final volume of 20 μl containing 100 nM DNA nanocircle, traces of 5′-32P-labeled primer (GGGTTA)3 and 1 mM dNTPs. The reactions were started by the addition of the respective polymerase or cell extracts and incubated at 37°C. After 1 h, the reactions were quenched in formamide loading buffer and analyzed by 15% PAGE.
Calf thymus polymerase α. A total of 3 U of calf thymus DNA polymerase α (Chimerx) were applied in 50 mM Tris buffer, pH 7.4, 5 mM MgCl2, 0.3 mg/ml BSA and 1 mM DTT.
Human polymerase β. A total of 3.2 U of human DNA polymerase β (Chimerx) were used in 50 mM Tris buffer, pH 8.5, 100 mM KCl, 10 mM MgCl2, 0.4 mg/ml BSA and 1 mM DTT.
Klenow Fragment. A total of 8 U of Klenow Fragment lacking 3′-5′-exonuclease activity (New England Biolabs) were used in 50 mM Tris buffer, pH 7.5, 5 mM MgCl2 and 7.5 mM DTT.
Nuclear cell extracts. A total of 4.7 U of HeLaScribe Nuclear Extract (Promega) were incubated at 37°C in 50 mM Tris buffer, pH 8.5, 100 mM KCl, 10 mM MgCl2, 0.4 mg/ml BSA and 1 mM DTT.
Thermal denaturation experiments
Solutions for thermal denaturation studies were prepared as 1 ml samples of 1 μM concentration. For pH 5, 50 mM NaOAc and for pH 7, 10 mM PIPES buffer were used. Details and plots are available in the Supplementary Material.
Circular dichroism measurements
Solutions for measuring circular dichroism (CD) spectra were prepared as 1 ml samples of 1 μM concentration. For pH 5, 50 mM NaOAc and for pH 7, 10 mM PIPES buffer were used. Details are available in the Supplementary Material.
RESULTS
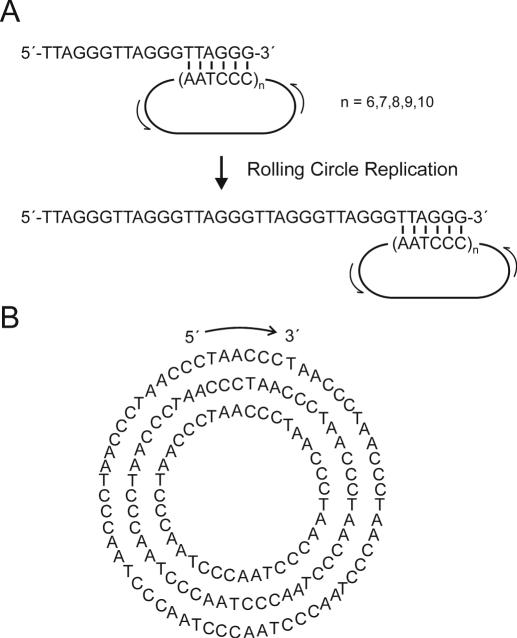
Synthesis of circular single-stranded DNAs composed of highly repetitive sequences is difficult since hybridization of splints to assist in enzymatic ligations does not necessarily occur at the ends of the corresponding circle precursor as desired. To prevent complications from the repeating sequence, an orthogonal protecting group strategy, involving dimethylacetamidine protecting groups on selected adenine residues, was employed (13). Circularization of linear, 5′-phosphorylated precursors was accomplished using T4 DNA ligase and a ligation splint DNA that hybridizes to both ends of the precursor oligonucleotides. Using this strategy, five different nanocircles ranging from 6 to 10 repeats of the hexamer sequence (TAACCC) were synthesized, resulting in circle sizes of 36–60 nt (Figure 1). Confirmation of circularity was performed by the nuclease method (see Figure 2).
Figure 1.
Mechanism of telomere repeat elongation by DNA nanocircles. (A) Circular, single-stranded DNA composed of the C-rich human telomeric sequence binds to the 3′ end of the G-rich complement, corresponding to the sequence at the end of a telomere. When incubated with DNA polymerases, the nanocircles act as templates in a rolling circle replication, resulting in elongation of the G-rich telomeric sequence by hundreds or thousands of nucleotides. (B) DNA nanocircles consisting of 6 to 10 repeats of the hexameric sequence (resulting in circle sizes of 36, 42, 48, 54 and 60 nt) were characterized. Sequences of circles 36, 48 and 60 are shown.
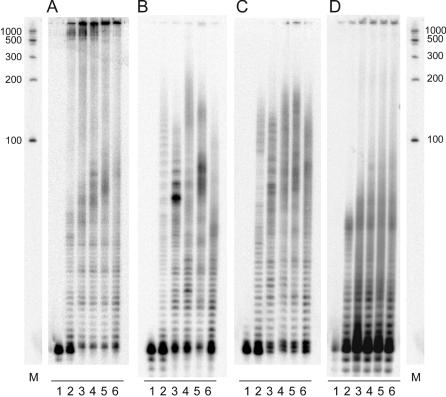
Next, we tested the different sizes of nanocircles with purified DNA polymerases for their ability to elongate a primer composed of three repeats of the G-rich human telomeric sequence (GGGTTA)3 (Figure 3A–C). Not surprisingly, variations in efficiency of rolling circle elongation of the telomeric sequence were observed with different polymerases. Using Klenow Fragment of Escherichia coli DNA polymerase I (lacking 3′-5′-exonuclease activity), circles composed of 42 and 48 nt showed the best performance in the extension reactions (Figure 3A). After 1 h, even the smallest nanocircle (36mer) showed extensive elongation of the primer to very long products composed of 1000 and more nucleotides. Although also facilitating substantial elongation of the telomeric primer, mammalian polymerases such as calf thymus polymerase α (Figure 3B) and human polymerase β (Figure 3C) were not able to produce as long extension products as the prokaryotic polymerase. Specifically, the 48 and 54mer nanocircles (representing 8 and 9 repeats of the sequence TAACCC) were most efficient, resulting in the synthesis of elongated telomeric DNAs with a maximum length of 200–300 nt after 1 h. Although the medium-sized 42–54 nt circles showed the strongest activity with these purified polymerases, even the smallest case, the 36mer, did show significant activity with all three enzymes.
Figure 3.
Extension of a human telomeric primer using C-rich DNA nanocircles. A radioactive primer composed of three repeats of the G-rich human telomeric sequence (GGGTTA)3 was incubated with (A) Klenow Fragment of E.coli DNA polymerase I, lacking 3′–5′-exonuclease activity; (B) calf thymus polymerase alpha; (C) human polymerase beta; and (D) HeLa nuclear cell extracts. Reactions further contained 100 nM DNA nanocircle, traces of 5′-32P-labeled primer (GGGTTA)3, and 1 mM dNTPs, and were incubated at 37°C for 1 h. Lane 1, control reaction lacking DNA nanocircle; lane 2, 36 nt circle; lane 3, 42 nt circle; lane 4, 48 nt circle; lane 5, 54 nt circle; lane 6, 60 nt circle; and M, size marker.
Recent studies have suggested that a natural alternative telomere-lengthening mechanism may operate with circular telomere repeats in eukaryotic cells (14,15). To explore the viability of the current very small templates in such a mechanism, we then tested nuclear HeLa cell extracts as a source of cellular polymerases (Figure 3D). With a control reaction lacking a circular telomeric template, no elongation of the primer was observed. This result showed that no residual telomerase activity from the cells is observed under these conditions. Although telomerase is expressed in HeLa cell lines (16), the activity is low and remains undetected without further amplification, such as by the TRAP assay (17–19). Relatively strong nuclease activity of the nuclear cell extracts was observed, resulting in digestion of the radioactive primer in a time-dependent manner, especially in the absence of DNA nanocircles. In contrast to the control experiment, with all reactions containing DNA nanocircles, significant primer elongation was observed, with strongly varying efficiency depending on circle size. It has been shown that circular, single-stranded DNAs smaller than 36 nt are able to perform rolling circle replication when incubated with bacterial and phage DNA polymerases (20–24). Nevertheless, when incubated with HeLa nuclear extracts, only very short extension products were observed with the 36mer. With all other DNA nanocircles tested (42–60 nt), at least small amounts of long elongation products ranging from 200 to over 1000 nt were seen.
To better understand the different behavior of the varying circle sizes, we investigated the structure of the DNA nanocircles employed in this study. Under certain conditions, the linear C-rich human telomeric sequence is known to fold into i-motif structures with protonated C-C+-base pairs (10,25,26). The topological limits of such small circles such as those in this study might provide a useful context for controlling and studying the folding of C-rich telomeric sequences. Earlier studies by Chan (27) have shown that very small DNA circles containing C-rich sequences can indeed form i-motif structures, although they were not composed of the human telomeric C-rich sequence. On the other hand, highly structured nanocircles would represent unsatisfactory templates for rolling circle replication, since such structure might well compete with proper hybridization of C-rich circle with the G-rich primer strand.
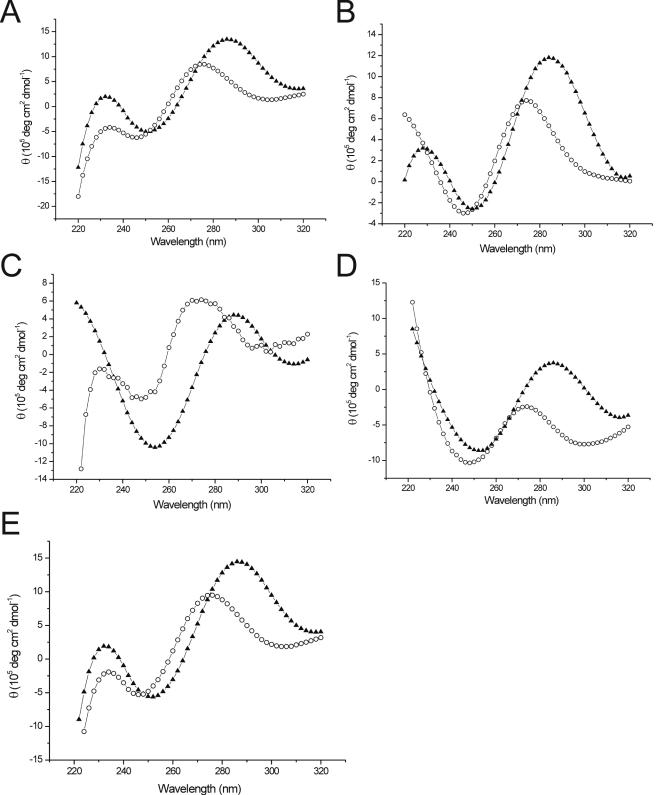
To investigate whether significant helical structures were formed by the DNA nanocircles, thermal melting curves were determined by ultraviolet absorption at 260 nm for all sizes of circles at pH 5 and pH 7 (Figure S1, Supplementary Material). Interestingly, pronounced structures were only observed under acidic conditions. This is in accordance with possible formation of i-motif structures (10). Surprisingly, all transitions fell into a relatively narrow window of 42–47°C. The melting temperatures appeared to alternate, with maxima at uneven repeats of the telomeric sequence (42 and 54 nt, respectively). To identify the nature of the structures formed under the acidic conditions, CD spectroscopy was applied, and spectra were measured for all circle sizes at pH 5 and pH 7. The results are shown in Figure 4. For all circles, measurements at pH 5 revealed a maximum at 285–288 nm, which is characteristic for i-motif structures (28,29). At pH 7, the maximum shifted toward 275 nm for all investigated circle sizes, consistent with the absence of an i-motif. These values almost exactly match results that were reported recently for short, linear DNAs composed of the C-rich, human telomeric sequence at pH 5.5 and pH 7 (30). In our series, the CD spectra are all very similar, although the spectral region below 240 nm shows some differences.
Figure 4.
CD spectra of C-rich telomeric DNA circles having the sizes 36, 42, 48, 54, and 60 nt (A–E), at low (pH = 5.0; closed triangles) and high (pH = 7.0; open circles) pH. The measurements were carried out in buffered solution (10 mM PIPES, pH 7.0 or 50 mM NaOAc, pH 5.0) at 1 μM concentration.
Taken together, the structural data suggest that all sizes of these telomeric DNA nanocircles probably adopt i-motif structures, or at least C+C duplexes, at acidic pH. We also investigated the linear precursors of the telomeric circles. In contrast to the circular species, a small fraction of the linear sequences was found to be folded into i-motif at pH 7 (data not shown). At neutral pH, there is no evidence of stable folds of such structures within the circular cases. This is consistent with their proficiency as templates for extending telomeric sequences.
DISCUSSION
The present results offer a viable approach, perhaps the only one currently available, for the synthesis of human telomeric G-rich strands of a length much greater than is accessible from a DNA synthesizer. If highly active and pure human telomerase ribonucleoproteins were available, it might well be useful in making such G-rich strands. Unfortunately, although the enzyme has been cloned, it has not been made active in pure form (31). The enzyme can be partially purified by selective affinity methods, but the activity remains quite dilute (32). Thus, the elongation of telomeres by this enzyme requires amplification steps to be observed, and does not offer the possibility either of long repeats or of preparative quantities that might be needed in studies of telomere folding and interactions. In any case, the current approach, once a template circle is in hand, is extremely simple to carry out, requiring only a commercial polymerase, a deoxynucleoside triphosphate mixture and a short primer. Previous data have shown that labels or reporters such as fluorophores can also be incorporated into the G-rich repeat (8).
Importantly, circle sizes as small as 36 nt show efficient elongation of the primer if incubated with isolated polymerases. The finding that smaller circle sizes are as effective as larger constructs is of particular importance, since synthesis of circular single-stranded oligonucleotides composed of repeating sequences remains challenging (13). When HeLa cell nucleus extracts are applied, a minimum circle size of 42 nt is required in order to obtain longer elongation products. In general, Klenow Fragment, the only prokaryotic DNA polymerase tested, showed more efficient elongation than the mammalian polymerases, with a large fraction of long products. The structural studies carried out on the circular templates suggest that all the circles are prone to form i-motifs, but these structures appear to form only at pH values lower than neutral pH.
The present circular DNAs also offer a new molecular context for the study of intramolecular folding of the C-rich strand of the human telomere repeat. Closed circular topologies are useful in promoting intramolecular folding and simultaneously preventing intermolecular associations. These are known to be complicating factors in the study of folding of both C-rich and G-rich sequences containing the human telomeric repeat (10,11). Our preliminary studies have shown apparently well-behaved melting behavior, which can be problematic for linear telomere repeats. Thus, further structural studies of selected circles in this series are warranted.
Future work will be aimed at studying the effects of DNA modifications on this biomimetic elongation, and at employing DNA nanocircles for elongating human telomeres in living cells. Altering telomere length in vivo without the need for expressing telomerase could be very useful for applications, such as tissue engineering and therapy of age-related diseases, and could be used for studying structure and function of human telomeres in their natural environment.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Acknowledgments
ACKNOWLEDGEMENTS
J.S.H. was supported by a fellowship of the Deutsche Forschungsgemeinschaft. This work was supported by the US National Institutes of Health (GM069763).
REFERENCES
- 1.Blasco M.A. (2003) Mammalian telomeres and telomerase: why they matter for cancer and aging. Eur. J. Cell Biol., 82, 441–446. [DOI] [PubMed] [Google Scholar]
- 2.Djojosubroto M.W., Choi,Y.S., Lee,H.W. and Rudolph,K.L. (2003) Telomeres and telomerase in aging, regeneration and cancer. Mol. Cells, 15, 164–175. [PubMed] [Google Scholar]
- 3.Neidle S. and Parkinson,G.N. (2003) The structure of telomeric DNA. Curr. Opin. Struct. Biol., 13, 275–283. [DOI] [PubMed] [Google Scholar]
- 4.Simonsson T. (2001) G-quadruplex DNA structures—variations on a theme. Biol. Chem., 382, 621–628. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson G.N., Lee,M.P. and Neidle,S. (2002) Crystal structure of parallel quadruplexes from human telomeric DNA. Nature, 417, 876–880. [DOI] [PubMed] [Google Scholar]
- 6.Stewart S.A., Hahn,W.C., O'Connor,B.F., Banner,E.N., Lundberg,A.S., Modha,P., Mizuno,H., Brooks,M.W., Fleming,M., Zimonjic,D.B. et al. (2002) Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc. Natl Acad. Sci. USA, 99, 12606–12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artandi S.E., Alson,S., Tietze,M.K., Sharpless,N.E., Ye,S., Greenberg,R.A., Castrillon,D.H., Horner,J.W., Weiler,S.R., Carrasco,R.D. et al. (2002) Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc. Natl Acad. Sci. USA, 99, 8191–8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindstrom U.M., Chandrasekaran,R.A., Orbai,L., Helquist,S.A., Miller,G.P., Oroudjev,E., Hansma,H.G. and Kool,E.T. (2002) Artificial human telomeres from DNA nanocircle templates. Proc. Natl Acad. Sci. USA, 99, 15953–15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stansel R.M., de Lange,T. and Griffith,J.D. (2001) T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J., 20, 5532–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan A.T. and Mergny,J.L. (2002) Human telomeric DNA: G-quadruplex, i-motif and Watson–Crick double helix. Nucleic Acids Res., 30, 4618–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyoshi D., Matsumura,S., Li,W. and Sugimoto,N. (2003) Structural polymorphism of telomeric DNA regulated by pH and divalent cation. Nucleosides Nucleotides Nucleic Acids, 22, 203–221. [DOI] [PubMed] [Google Scholar]
- 12.McBride L.J., Kierzek,R., Beaucage,S.L. and Caruthers,M.H. (1986) Amidine protecting groups for oligonucleotide synthesis. J. Am. Chem. Soc., 108, 2040–2048. [Google Scholar]
- 13.Lindstrom U.M. and Kool,E.T. (2002) An orthogonal oligonucleotide protecting group strategy that enables assembly of repetitive or highly structured DNAs. Nucleic Acids Res., 30, e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regev A., Cohen,S., Cohen,E., Bar-Am,I. and Lavi,S. (1998) Telomeric repeats on small polydisperse circular DNA (spcDNA) and genomic instability. Oncogene, 17, 3455–3461. [DOI] [PubMed] [Google Scholar]
- 15.Tomaska L., McEachern,M.J. and Nosek,J. (2004) Alternatives to telomerase: keeping linear chromosomes via telomeric circles. FEBS Lett., 567, 142–146. [DOI] [PubMed] [Google Scholar]
- 16.Sawant S.G., Gregoire,V., Dhar,S., Umbricht,C.B., Cvilic,S., Sukumar,S. and Pandita,T.K. (1999) Telomerase activity as a measure for monitoring radiocurability of tumor cells. FASEB J., 13, 1047–1054. [DOI] [PubMed] [Google Scholar]
- 17.Szatmari I. and Aradi,J. (2001) Telomeric repeat amplification, without shortening or lengthening of the telomerase products: a method to analyze the processivity of telomerase enzyme. Nucleic Acids Res., 29, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim N.W., Piatyszek,M.A., Prowse,K.R., Harley,C.B., West,M.D., Ho,P.L., Coviello,G.M., Wright,W.E., Weinrich,S.L. and Shay,J.W. (1994) Specific association of human telomerase activity with immortal cells and cancer. Science, 266, 2011–2015. [DOI] [PubMed] [Google Scholar]
- 19.Wright W.E., Shay,J.W. and Piatyszek,M.A. (1995) Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res., 23, 3794–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fire A. and Xu,S.Q. (1995) Rolling replication of short DNA circles. Proc. Natl Acad. Sci. USA, 92, 4641–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frieden M., Pedroso,E. and Kool,E.T. (1999) Tightening the belt on polymerases: evaluating the physical constraints on enzyme substrate size. Angew. Chem. Int. Ed., 38, 3654–3657. [PubMed] [Google Scholar]
- 22.Baner J., Nilsson,M., Mendel-Hartvig,M. and Landegren,U. (1998) Signal amplification of padlock probes by rolling circle replication. Nucleic Acids Res., 26, 5073–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lizardi P.M., Huang,X.H., Zhu,Z.R., Bray-Ward,P., Thomas,D.C. and Ward,D.C. (1998) Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nature Genet., 19, 225–232. [DOI] [PubMed] [Google Scholar]
- 24.Liu D.Y., Daubendiek,S.L., Zillman,M.A., Ryan,K. and Kool,E.T. (1996) Rolling circle DNA synthesis: small circular oligonucleotides as efficient templates for DNA polymerases. J. Am. Chem. Soc., 118, 1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanaori K., Shibayama,N., Gohda,K., Tajima,K. and Makino,K. (2001) Multiple four-stranded conformations of human telomere sequence d(CCCTAA) in solution. Nucleic Acids Res., 29, 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phan A.T., Gueron,M. and Leroy,J.L. (2000) The solution structure and internal motions of a fragment of the cytidine-rich strand of the human telomere. J. Mol. Biol., 299, 123–144. [DOI] [PubMed] [Google Scholar]
- 27.Li T., Liu,D., Chen,J., Lee,A.H., Qi,J. and Chan,A.S. (2001) Construction of circular oligodeoxyribonucleotides on the new structural basis of i-motif. J. Am. Chem. Soc., 123, 12901–12902. [DOI] [PubMed] [Google Scholar]
- 28.Pataskar S.S., Dash,D. and Brahmachari,S.K. (2001) Intramolecular i-motif structure at acidic pH for progressive myoclonus epilepsy (EPM1) repeat d(CCCCGCCCCGCG)n. J. Biomol. Struct. Dyn., 19, 307–313. [DOI] [PubMed] [Google Scholar]
- 29.Bishop G.R. and Chaires,J.B. (2002) In Jones,R.A. (ed.), Current Protocols in Nucleic Acid Chemistry. Wiley & Sons, Hoboken, NJ, Vol. 2, pp. 7.11.17. [Google Scholar]
- 30.Li W., Miyoshi,D., Nakano,S. and Sugimoto,N. (2003) Structural competition involving G-quadruplex DNA and its complement. Biochemistry, 42, 11736–11744. [DOI] [PubMed] [Google Scholar]
- 31.Bickenbach J.R., Vormwald-Dogan,V., Bachor,C., Bleuel,K., Schnapp,G., Boukamp,P., Rodi,H.P., Rettig,W.J., Schnapp,A. and Damm,K. (1998) Telomerase is not an epidermal stem cell marker and is downregulated by calcium. One-step affinity purification protocol for human telomerase. J. Invest. Dermatol., 111, 1045–1052. [DOI] [PubMed] [Google Scholar]
- 32.Bachand F. and Autexier,C. (1999) Functional reconstitution of human telomerase expressed in Saccharomyces cerevisiae. J. Biol. Chem., 274, 38027–38031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.