Abstract
Objective
Causes of placebo effects in antidepressant trials have been inferred from observational studies and meta-analyses, but their mechanisms have not been directly established. The goal of this study was to examine in a prospective, randomized controlled trial (RCT) whether patient expectancy mediates placebo effects in antidepressant studies.
Method
Adult outpatients with Major Depressive Disorder (MDD) were randomized to Open or Placebo-controlled citalopram treatment. Following measurement of pre- and post-randomization expectancy, subjects were treated with citalopram or placebo for 8 weeks. Independent samples t tests determined whether patient expectancy differed between the Open and Placebo-controlled Groups, and mixed-effects models assessed Group effects on Hamilton Rating Scale for Depression (HRSD) scores over time while controlling for treatment assignment. Finally, mediation analyses tested whether between-Group differences in patient expectancy mediated the Group effect on HRSD scores.
Results
Post-randomization expectancy scores were significantly higher in the Open Group (12.1 ± 2.1) as compared to the Placebo-controlled Group (11.0 ± 2.0, t=2.32, df 45, p=0.03). Mixed-effects modeling revealed a significant Week × Group interaction (F(1,296)=8.61, p=0.0036), indicating that HRSD scores for citalopram-treated subjects declined at a faster rate in the Open Group compared to the Placebo-controlled Group. Patient expectations post-randomization partially mediated Group effects on Week 8 HRSD (p=0.046).
Conclusions
Patient expectancy is a significant mediator of placebo effects in antidepressant trials. Expectancy-related interventions should be investigated as a means of controlling placebo responses in antidepressant clinical trials and improving patient outcome in clinical treatment.
Keywords: antidepressants, placebo effect, clinical trials, pharmacotherapy, expectancy
INTRODUCTION
Placebo responses in antidepressant trials have become a critical issue for the development of novel therapeutics and the treatment of patients in clinical settings. On the one hand, increasing placebo response complicates efforts to detect signals of efficacy for new agents in the drug development setting. The average difference observed in published antidepressant trials between medication and placebo decreased from an average of 6 points on the Hamilton Rating Scale for Depression (HRSD) in 1982 to 3 points in 2008 (1). Consequently, for most currently approved antidepressants, less than half of the efficacy trials filed with the Food and Drug Administration for regulatory approval found active drug superior to placebo (2-3). On the other hand, practicing clinicians know that many patients will not experience sustained remission of their depression with currently available treatments (4). Because non-pharmacologic elements of medication treatment (i.e., placebo effects and supportive care) likely cause a substantial portion of the observed response (5-6), optimizing the therapeutic components leading to placebo response has the potential to significantly improve treatment outcomes in clinical practice.
Given the potential benefits to be realized from modulating the amplitude of placebo response in patient care and pharmacologic research, understanding the mechanisms of action of placebo response is critically important. Placebo effects are defined as the therapeutic consequences of receiving a substance or undergoing a procedure that are not caused by any inherent powers of the substance or procedure (7). As such, they are conceptually distinct from other factors contributing to observed placebo response (i.e., the proportion of subjects assigned to placebo who manifest ≥ 50% decrease in baseline symptoms), such as regression to the mean, spontaneous improvement, and rater bias (8). In many cases placebo effects appear to be cognitively mediated by patient expectancy (9), which refers to an individual’s belief about whether and how much they will improve as the consequence of a treatment intervention. The most common procedures for experimentally manipulating expectancies and measuring their causal effects include comparing placebo to no-treatment control conditions or else administering a drug in an open versus hidden manner (10). However, ethical considerations prevent the use of these procedures in patients with Major Depressive Disorder (MDD).
As an alternative approach, we have argued that placebo effects can be studied meaningfully without resorting to unethical forms of deception by comparing medication response between placebo-controlled trials (i.e., one or more medications compared to placebo) and active comparator trials (i.e., one or more medications with no placebo group) (11). In adults and older adults with MDD, mean medication response rates in comparator trials are significantly greater than the mean medication response rates in placebo-controlled trials (12-13). Patients in comparator trials know they have a 100% chance of receiving an active medication, which may increase their expectancy of improvement, leading to enhanced placebo effects and greater observed antidepressant response. Consistent with these results, Papakostas and Fava (2009) reported that the probability of receiving placebo in a clinical trial correlated inversely with antidepressant and placebo response (14), and Sinyor et al (2010) found that medication response was significantly higher in drug-drug (i.e., comparator) studies (65.4%) compared to drug-drug-placebo studies (57.7%) and drug-placebo studies (51.7%) (15). These retrospective analyses suggest, though do not provide causal evidence, that the design of a clinical trial shapes patients’ expectancies of improvement during the trial, which in turn influence response to antidepressant medication and placebo.
In a prior pilot study, we found that depressed patients receiving citalopram under comparator conditions had significantly greater expectancy and improved clinical outcome compared to patients receiving placebo-controlled citalopram (16). The present study follows up these findings in a larger trial designed to provide causal evidence for patient expectancy as a source of placebo effects in antidepressant clinical trials. Outpatients with MDD were randomized to open administration (i.e., 100% probability) of citalopram vs. placebo-controlled administration (i.e., 50% probability) of citalopram, and expectancy and depressive symptom scores were followed over 8 weeks of acute treatment. We hypothesized that subjects randomized to open citalopram would have greater expectancy of improvement and experience greater reductions in depressive symptoms compared to subjects randomized to placebo-controlled citalopram. Further, we hypothesized that patient expectancy would mediate the depression outcome difference between these medication-treated groups at study endpoint, such that increases in expectancy would produce greater reductions in depression symptoms. Based upon the rationale that older age may be associated with structural and functional brain changes influencing patient expectancy, we planned to explore whether age moderated the magnitude of expectancy effects observed.
METHOD
Subjects
This study was conducted in the Adult and Late Life Depression Research Clinic at the New York State Psychiatric Institute (NYSPI) and approved by the NYSPI Institutional Review Board. All participants met eligibility criteria and signed informed consent for the study. Below we report clinical findings from the study, but functional magnetic resonance imaging (fMRI) data were also collected and will be presented in subsequent papers.
Eligible subjects were men and women aged 24-65 years old who met Diagnostic and Statistical Manual IV (DSM-IV) (17) criteria for non-psychotic MDD, had a 24-item HRSD score ≥ 16, were right-handed, had no contraindications to MRI, were using appropriate contraception if they were women of child-bearing age, and were willing to and capable of providing informed consent and complying with study procedures. Subjects were excluded from participation if they were diagnosed with a current comorbid Axis I DSM IV disorder (other than Nicotine Dependence, Adjustment Disorder, or Anxiety Disorders), substance abuse or dependence within the past 12 months, or a lifetime history of psychosis or mania. Other exclusion criteria included the presence of significant suicidality (HRSD suicide item > 2), a history of allergic or adverse reaction to citalopram, non-response to adequate trial of citalopram within the current depressive episode, current treatment with psychotherapy, Clinical Global Impressions (CGI) score of 7 at baseline, current or recent (within the past 4 weeks) treatment with any psychoactive medications, or acute, severe, or unstable medical illness.
Study Design
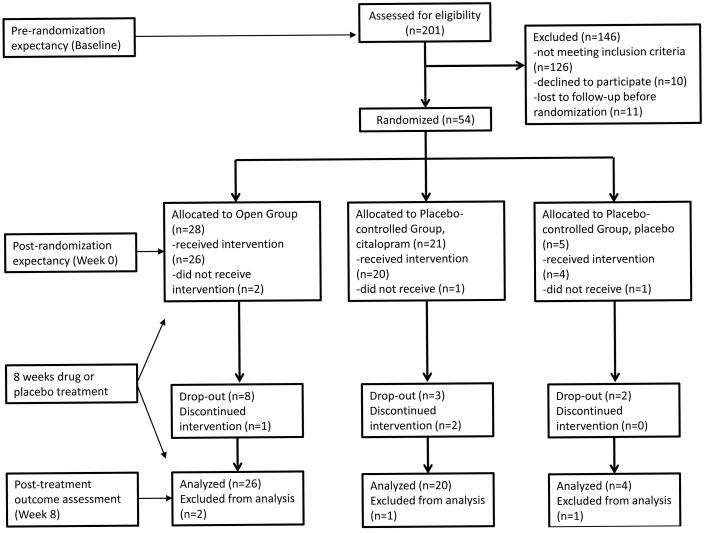
Subjects were enrolled in an 8-week duration antidepressant clinical trial randomizing participants to a Placebo-controlled Group or Open Group (see Figure 1). Subjects were initially evaluated at a baseline visit, where eligibility was assessed and pre-randomization expectancy and depression scores were measured. Approximately one week later, subjects returned for a Week 0 visit during which they were randomized, post-randomization expectancy and depression scores were measured, and either medication or placebo was distributed. Thus, both pre- and post-randomization expectancy were measured prior to subjects receiving any study medication. Subjects then began study medication and returned for 8 weekly visits.
Figure 1.
CONSORT flow diagram with timing of assessments.
Expectancy Manipulation
Expectancy was manipulated via instructions to participants about the probability of receiving active medication as compared to placebo. As depicted in Figure 1, subjects at baseline had what they perceived to be a 75% probability of receiving active antidepressant medication. Pre-randomization expectancy was measured with subjects having this knowledge. Then, subjects were randomized either to the Placebo-controlled Group (50% chance of receiving active treatment) or Open Group (100% chance of receiving active treatment) and informed of the results of this randomization. Post-randomization expectancy was measured with subjects having this additional information. Subjects within the Placebo-controlled Group were randomized to medication or placebo with a 4:1 ratio favoring medication, which maximized the sample sizes for the primary comparison of interest (i.e., Placebo-controlled citalopram vs. Open citalopram). This second randomization within the Placebo-controlled Group was blinded, and neither subjects nor outcome assessors were aware of the 4:1 randomization schedule or the specific treatment assignment to medication or placebo
While subjects were informed of their Group assignment (since this was the study’s means of manipulating expectancy), outcome assessors were blinded to Group. To conceal Group assignment from raters in this study, we developed a scripted method of instructing subjects not to divulge this information and study raters not to request it. We also conducted checks throughout each subject’s participation in the study to determine whether the blind was broken. Subjects in the Placebo-controlled Group were informed: “You have been randomly assigned to the Placebo-controlled Group of the study. This means that there is a chance you will receive the antidepressant medication citalopram for the duration of the study. Citalopram has been proven effective for the treatment of depression in patients like you. There is also a chance you will receive placebo for the duration of the study. A placebo is a sugar pill that is not specifically effective for depression. Neither you, nor your doctors, will know whether you are receiving citalopram or placebo. If it can be avoided, please do not reveal to anyone in the study the Group to which you have been assigned.” Subjects in the Open Group were informed: “You have been randomly assigned to the Open Group of the study. This means that there is a 100% chance you will receive the antidepressant medication citalopram for the duration of the study. Citalopram has been proven effective for the treatment of depression in patients like you. You will not be receiving any placebo pills for the duration of the study. While you are aware that you are receiving actual antidepressant medication and not placebo, other study personnel do not know whether you are taking citalopram or placebo. If it can be avoided, please do not reveal to anyone in the study the Group to which you have been assigned.”
Antidepressant Medication
Following the randomization procedures described above, subjects were prescribed citalopram 20mg per day or pill placebo. We selected citalopram for use in this study based on its established efficacy in MDD, a favorable side effect profile, minimal drug-drug interactions, and cost effectiveness (18-19). If subjects did not meet remission criteria (HRSD ≤ 7) after 4 weeks, the citalopram dose was increased to 40mg for the remaining 4 weeks of the study. Subjects were aware of the potential for dosage increase at Week 4 and that individuals randomized to placebo would simply continue to receive placebo. Subjects unable to tolerate the increased dose of medication had their dosage reduced to the maximum previously tolerated dose. Absence of remission was selected to trigger dosage increases, because remission is a clinically meaningful outcome denoting the absence of significant depressive symptoms and reduced risks of recurrent depression and adverse medical outcomes (20-21). In the case of severe insomnia, subjects were permitted zolpidem 10mg per day.
Study Assessments
At baseline, patients were screened for significant medical problems with a medical history and physical examination, a blood sample for screening laboratories, an electrocardiogram, and a urine toxicology. Vital signs were recorded at baseline and weekly thereafter.
A Structured Clinical Interview Diagnostic for DSM-IV TR (SCID) (22) was performed at baseline to confirm subject eligibility. The 24-item HRSD (i.e., the version containing items for diurnal variation, depersonalization, paranoid feelings, helplessness, hopelessness, and worthlessness) was performed at every study visit, and change on the HRSD was defined a priori as the primary clinical outcome measure. Response (≥ 50% decrease in baseline HRSD score) and remission (Week 8 HRSD ≤ 7) were defined for secondary analyses. Other weekly assessments included the Hamilton Anxiety Rating Scale (HARS), Quick Inventory of Depressive Symptomatology 16-item self-report (QIDS 16-SR), the CGI-Severity and CGI-Improvement scales, a rating scale for treatment-emergent side effects, weekly pill counts, and a clinician and patient-rated blind assessment.
Expectancy was measured before randomization to Group (baseline) and after randomization to Group but before medication administration (Week 0). The measure used to assess expectancy was a modified version of the Treatment Credibility and Expectancy Scale (CES) (23), which is a widely used measure of expectancy and has demonstrated good psychometric properties in multiple studies (24-25). For our measure of expectancy, we use the subset of CES items pertaining to expectancy (i.e., rather than treatment credibility), and we have previously demonstrated this modified CES to be sensitive to differences in expectancy when subjects know they are receiving active medication as opposed to possibly receiving placebo (16). These items ask respondents “I believe the chances of my depression being completely better at the end of this study are…” and “Compared with now, I think my depression at the end of this study will be…” Both questions are rated on Likert scales from 1-7 and summed to create the score. Anchors correspond to Very Poor (Much Worse), Somewhat Poor (Worse), Slightly Poor (Worse), No Different, Slightly Good (Better), Somewhat Good (Better), Very Good (Much Better)
Data Analysis
To test for balance between randomized groups in demographic and clinical measures, two-sample t-tests were used for continuous measures and Fisher’s exact tests for categorical variables. Associations between demographic factors and pre-randomization expectancy were tested using one-way ANOVA. Within-group tests of change in expectancy from pre- to post-randomization were obtained by regressing pre-and post-randomization expectancy as repeated measures predicted by group, time (pre- vs post-), and group by time interaction, and Week 0 HRSD values. The effect of randomized group on expectancy was tested by regressing post-randomization expectancy values on randomization group and Week 0 HRSD.
Chi-square tests compared response and remission rates between the two Groups. Mixed Effects modeling was used to test group differences in HRSD scores over time, including Group, week, and their interaction as predictors, while also adjusting for gender, age, ethnicity, and Week 0 HRSD, as covariates, as well as an indicator for whether or not the subject was given placebo. Post-hoc contrasts from a mixed effects model treating week as categorical were used to test mean Group differences at each follow-up week. To explore whether age moderated Group differences, an age (treated as continuous) by Group by week interaction was also added to the model. Contrasts were formed from the model for Group differences at fixed ages (i.e. 25, 45, 55, 65) to facilitate interpretability of the age interaction effect.
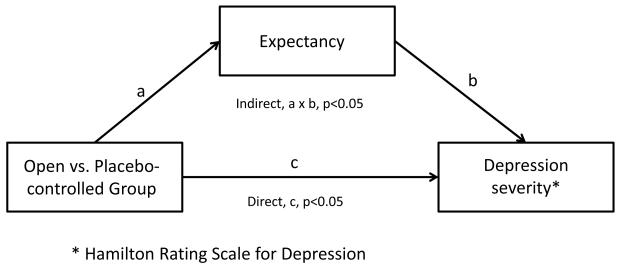
Tests for mediation of the Group effect on HRSD scores were conducted using Structural Equation Modeling (SEM), with Group as a predictor of both post-randomization expectancy (path a in Figure 2) and week 8 HRSD scores (path c in Figure 2), and with post-randomization expectancy also predicting week 8 HRSD score (path b in Figure 2). Models for both post-randomization expectancy and Week 8 HRSD controlled for Week 0 HRSD, and the model for week 8 HRSD additionally controlled for an indicator of whether the patient received placebo or medication to account for the therapeutic effects of citalopram. To investigate whether the mediation effect of expectancy varied as a function of self-report vs. rater-administered outcome measures, the analyses were repeated using Week 8 QIDS as the outcome. SEM was performed using MPlus version 7.3. All other analyses were conducted using SAS 9.4. Statistical significance was determined at p<0.05 level.
Figure 2.
Mediation of Group effect on depression severity scores by patient expectancy.
RESULTS
Subject Disposition and Characteristics
The final CONSORT diagram for the study also is shown in Figure 1. 55 subjects participated in the study, of whom 4 were lost to follow up prior to taking study medication and were excluded from the analyses. Participants in the Open Group were 41.4 ± 12.0 years old, 34.6% male, and had 24-item HRSD 25.7 ± 5.5, QIDS SR 19.7 ± 5.2, and CGI-Severity 4.4 ± 0.6, while participants receiving citalopram in the Placebo-controlled Group were 43.8 ± 10.7 years old, 54.2% male, and had 24-item HRSD 25.7 ± 4.1, QIDS SR 19.8 ± 7.2, and CGI-Severity 4.3 ± 0.5 (see Table 1). Participants receiving placebo in the Placebo-controlled Group were 34.3 ± 10.2 years old, 25% male, and had 24-item HRSD 23.8 ± 2.8, QIDS SR 17.8 ± 7.5, and CGI-Severity 4.3 ± 0.6. No significant differences were observed between subjects receiving citalopram in the Placebo-controlled and Open Groups on the bases of demographic data or clinical characteristics.
Table 1.
Clinical and demographic characteristics of included subjects.
| Characteristic | Open Group, citalopram (n=26) |
Placebo- controlled Group, citalopram (n=20) |
Placebo- controlled Group, placebo (n=4) |
p Value |
|---|---|---|---|---|
|
| ||||
| Age (years) | 41.4 ± 12.0 | 45.7 ± 10.0 | 34.3 ± 10.2 | 0.140 |
|
| ||||
| Gender (% male) | 34.6 | 54.2 | 25.0 | 0.467 |
|
| ||||
| Ethnicity (% Hispanic) |
19.2 | 12.5 | 75.0 | 0.613 |
|
| ||||
| Race | 0.379 | |||
| % White | 50.0 | 66.7 | 75.0 | |
| % African- American |
29.2 | 25.0 | 0.0 | |
| % Other | 20.8 | 8.3 | 25.0 | |
|
| ||||
| Duration of current depressive episode (weeks) |
285.2 ± 459.8 | 155.1 ± 432.8 | 77.3 ± 69.6 | 0.525 |
|
| ||||
| Number of prior antidepressant medications |
1.3 ± 1.2 | 2.0 ± 2.0 | 1.3 ± 1.0 | 0.319 |
|
| ||||
| 24-item HRSD | 25.7 ± 5.5 | 26.1 ± 4.2 | 23.8 ± 2.8 | 0.679 |
|
| ||||
| QIDS SR | 19.7 ± 5.2 | 20.2 ± 7.3 | 17.8 ± 7.5 | 0.774 |
|
| ||||
| CGI-Severity | 4.4 ± 0.6 | 4.3 ± 0.5 | 4.3 ± 0.6 | 0.877 |
|
| ||||
| HARS | 13.2 ± 4.9 | 15.1 ± 4.8 | 16.7 ± 9.2 | 0.381 |
HRSD = Hamilton Rating Scale for Depression
QIDS SR = Quick Inventory of Depressive Symptomatology Self Report
CGI = Clinical Global Impressions
HARS = Hamilton Anxiety Rating Scale
Expectancy Manipulation
Mean pre-randomization expectancy scores, which did not differ significantly between Groups (p=0.301), were 11.2 ± 1.1 for the Open Group (range 7.0-14.0) and 10.8 ± 1.9 for Placebo-controlled Group (range 7.0-12.0). Pre-randomization expectancy did not differ between subjects on the basis of sex (F[1,48]=0.511, p=0.478), ethnicity (F[1,48]=0.272, p=0.604), or educational attainment (F[4,44]=0.220, p=0.926). Adjusting for Week 0 HRSD, the randomization to Group significantly increased expectancy scores in the Open Group (t=3.45, df 23, p=0.002), whereas no significant change was present in expectancy scores in the Placebo-controlled Group (t=0.45, df 23, p=0.654). Post-randomization expectancy scores were significantly higher in the Open Group (12.1 ± 2.1, range 6.0-14.0) compared to the Placebo-controlled Group (11.0 ± 2.0, range 6.0-12.0; t=2.32, df 45, p=0.03), when adjusted for Week 0 HRSD. Thus, the experimental design succeeded in manipulating expectancies.
Clinical Outcomes
Response rates were 25% for Placebo-controlled placebo, 45% for Placebo-controlled citalopram, and 53.8% for Open citalopram (Pearson Χ2=1.276, df 2, p=0.528). Remission rates were 0% for Placebo-controlled placebo, 20% for Placebo-controlled citalopram, and 34.6% for Open citalopram (Pearson Χ2=2.783, df 2, p=0.249).
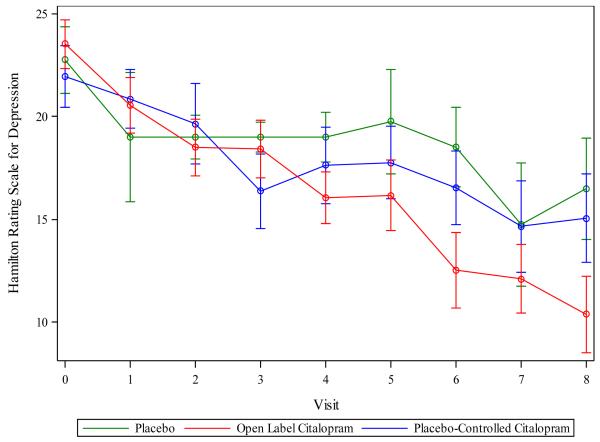
In the mixed model of HRSD scores containing variables for Group, Week, Week × Group, Placebo indicator, and Week 0 HRSD, the Week × Group interaction was significant (F(1,296)=8.61, p =0.0036). HRSD scores declined over time at a faster rate in the Open Group compared to the citalopram and placebo-treated subjects in the Placebo-controlled Group (see Figure 3). No benefit of citalopram vs. placebo was observed (F[1,296]=0.01, p=0.99), likely because very few subjects received placebo (n=4). Examination of weekly contrasts showed that subjects in the Open Group had significantly lower HRSD scores starting in Week 4 and continuing through Week 8. Adjusting for covariates, the average subject treated with citalopram in the Open Group experienced 6.2 points of additional improvement by Week 8 compared to the average subject treated with citalopram in the Placebo-controlled Group. Repeating the analyses using the 17-item HRSD resulted in a similar pattern of results.
Figure 3.
Change in depressive symptoms over time for study conditions.
Because we anticipated that the presence of vascular lesions and executive dysfunction (which were not directly measured in this study) may influence the magnitude of expectancy effects, we explored whether age moderated the Group effect on HRSD score by adding the three-way interaction term week × age × Group to the model of HRSD scores. This interaction was significant (F(1,294)=5.24, p=0.0228). Testing weekly contrasts showed that the week × Group effect decreased as age increased. At age 25, the average between-Group HRSD difference at Week 8 was 11.2 (p=0.0016, favoring the Open Group), compared to 5.6 (p=0.0013) at age 45, 2.8 (p=0.248) at age 55, and −0.04 (p=0.992, favoring the Placebo-controlled Group) at age 65. To test whether additional participant characteristics were relevant to depressive outcomes, we also adjusted this model of HRSD scores for sex, education, and ethnicity, but adding these variables did not substantively affect the results. Of note, the age moderation of the Group effect on HRSD score was not accounted for by older participants having longer duration, lower expectancy depressive episodes. Age was not significantly correlated with duration of current depressive episode (Pearson r = 0.062, p = 0.677), and duration of current episode was not significantly correlated with either baseline expectancy scores (Pearson r = 0.117, p = 0.430) or the pre-post randomization change in expectancy (Pearson r = 0.093, p =0.528).
Mediation of Clinical Outcomes by Expectancy
The standardized effects of Group on post-randomization expectancy score (a=0.268, p=0.038) and of post-randomization score on Week 8 HRSD score (b=−0.279, p=0.005) controlling for Group were each significant (see Figure 2). The direct (i.e., unmediated) effect of Group on the Week 8 HRSD was c=−0.311 (p=0.012). Thus, the indirect effect (i.e., mediated effect) of Group on Week 8 HRSD through post-randomization expectancy score was a × b=−0.075 (p=0.046). These values indicate that post-randomization expectancy is a partial mediator of the Group effect on Week 8 HRSD score.
In the mediation analyses using Week 8 QIDS as the outcome, the standardized effects of Group on post-randomization expectancy score (a=0.360, p=0.001) and of post-randomization score on Week 8 HRSD score (b=−0.238, p=.036) remained significant, while the direct effect of Group on Week 8 QIDS was c=0.07 (p=0.646). Thus, the indirect effect of Group on QIDS through expectancy was a × b=−0.086 (p=0.102), which, while similar to the indirect effect for HRSD in size, did not reach statistical significance at the 0.05 level.
DISCUSSION
The primary findings of this study were that subjects randomized to open citalopram had significantly greater improvement in depressive symptoms compared to those receiving placebo-controlled citalopram, and patient expectancy partially mediated these differences. Strikingly, despite receiving the identical antidepressant medication, being treated by the same study clinicians, and visiting the same treatment site, depressed subjects who knew they were receiving citalopram improved on average 6 HRSD points more than subjects receiving citalopram who were aware they had a chance of receiving placebo. This difference between citalopram outcomes under high vs. low expectancy conditions is greater in magnitude than the typically observed differences between drug and placebo in antidepressant trials (26-27), testifying to the powerful influence of expectancy-based placebo effects on depressive symptoms.
By providing the first prospective, causal evidence for patient expectancy as a mediator of placebo effects in antidepressant clinical trials, this study confirms data from our pilot work and extends the findings of previous investigations. Leuchter et al (2014) reported a study in which participants were randomized to supportive care alone, supportive care plus placebo, and supportive care plus antidepressant medication (28). While the randomization process did not affect expectancy, these investigators found that the pill taking conditions were associated with more symptom improvement compared to supportive care alone. Greater patient expectancy was associated with greater improvement in the placebo condition, but not the medication condition. Prior observational analyses from the NIMH Treatment of Depression Collaborative Study and a single-blind trial of reboxetine showed that higher patient expectation of improvement predicted greater likelihood of depression response (29-30). More broadly, these findings can be compared with neuroimaging studies in which expectations of increased pain relief have additive and dissociable effects to opiate analgesics on reported pain and pain-related brain responses (31-32).
The differential improvement between the Open and Placebo-controlled Groups in this study became apparent starting at Week 4, and this time course generates hypotheses for future study regarding the mechanisms by which expectancy influences depressive symptoms. One possibility is that expectancies may directly modulate depression-associated dysfunctions in the brain, perhaps akin to what is observed in neuroimaging studies of placebo analgesia (9) or in conditions in which participants are asked to consciously regulate their emotional responses to stimuli (33). Alternatively, expectancy may lead to more gradual depression improvement by mean of indirect mechanisms, such as increasing behavioral activation, improving medication compliance, or enhancing the therapeutic alliance between patient and doctor (34-36). Given the delayed time course, data from this study appear to suggest that indirect mechanisms are likely to play a role in mediating expectancy effects on depression.
To the extent that expectancy mediates placebo responses in antidepressant studies, limiting patient expectancy may be a useful strategy to reduce placebo response in Phase III trials. For example, designs in which patients have a higher probability of receiving placebo (i.e., 50%) may be preferable to designs randomizing patients to multiple active treatment arms and placebo. Conversely, the optimal strategy in clinical practice may be to combine active medication with a presentation that enhances patient expectancy, leading to greater medication response. Updated clinical management techniques may involve educating patients about the effectiveness of the prescribed medication and utilizing a confident and enthusiastic interpersonal style. Such presentations have been shown to enhance placebo effects for medical treatments, as in a recent study of irritable bowel syndrome (37).
Intriguingly, we found evidence of diminished expectancy effects in older subjects, particularly those 55 and older. Late-Life Depression is associated with decreased response to antidepressant medications (38) and is often accompanied by structural brain changes (i.e., white matter hyperintensities [39-41]) and executive dysfunction (42-43). Given these data, one might speculate whether a loss of expectancy-related placebo effects may underlie the diminished antidepressant response often observed in Late-Life Depression. Patients with executive dysfunction may have difficulty updating and maintaining appropriate treatment expectancies in response to the information they are provided about the treatment being received. Even if they are able to form expectancies, vascular damage to frontostriatal tracts may limit the top-down modulation of limbic and striatal structures necessary for expectancies to produce change in depressive symptoms.
As in all studies, the results reported here should be interpreted in light of relevant limitations. Our study design did not permit us to differentiate the main effects of patient expectancy from expectancy × medication interactions. This limitation is unavoidable in depression studies given the ethical difficulties posed by using methodologies such as the balanced placebo study design, which allows expectancy and medication effects to be disambiguated (44). The deception inherent to this strategy, particularly informing depressed patients that they will receive medication while actually giving them placebo is problematic. Our study methods replicated 3 of the 4 cells of a balanced placebo design, allowing us to conclude that medication plus expectancy produced greater depressive symptom improvement than medication alone. Other limitations were posed by the relatively small sample size of the study, which did not hamper our ability to obtain significant results, and the asymmetric randomization strategy pursued in the Placebo-controlled group. The few number of subjects assigned to placebo prohibited us from meaningfully testing the medication effect (i.e., citalopram vs. placebo) or testing the influence of expectancy on placebo response.
In summary, this prospective, randomized study of placebo effects in antidepressant treatments is the first to experimentally demonstrate placebo effects in MDD that is not confounded by artifacts such as spontaneous remission, natural history, or regression to the mean. We found patient expectancy to be a significant mediator of these placebo effects. Developing interventions capable of modifying patient expectancy may reduce placebo response in the drug development setting and improve medication response in community treatment.
Acknowledgments
Disclosures: This study was funded by NIMH K23 MH085236 (Rutherford). Dr. Rutherford had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Rutherford reports receiving consulting fees from Pfizer. Dr. Wager received consulting fees from Janssen. This paper has not been previously presented.
Footnotes
Mr. Choo and Drs. Wall, Brown, Peterson, Kirsch, and Roose have no disclosures or conflicts of interest to report.
Contributor Information
Bret R Rutherford, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute, 1051 Riverside Drive, Box 98, New York, NY 10032, 646 774 8660 (telephone).
Melanie M. Wall, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute.
Patrick J. Brown, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute.
Tse-Hwei Choo, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute.
Tor D. Wager, Department of Psychology and Neuroscience and the Institute of Cognitive Science, University of Colorado at Boulder.
Bradley S. Peterson, Institute for the Developing Mind, Children’s Hospital Los Angeles, Keck School of Medicine at the University of Southern California.
Sarah Chung, New York State Psychiatric Institute.
Irving Kirsch, Harvard Medical School, Beth Israel Deaconess Medical Center.
Steven P. Roose, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute.
REFERENCES
- 1.Khan A, Bhat A, Kolts R, Thase ME, Brown W. Why has the Antidepressant-Placebo Difference in Antidepressant Clinical Trials Diminished over the Past Three Decades? CNS Neuroscience & Therapeutics. 2010;16:217–226. doi: 10.1111/j.1755-5949.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan A, Khan S, Brown WA. Are placebo controls necessary to test new antidepressants and anxiolytics? Int J Neuropsychopharmacol. 2002;5:193–197. doi: 10.1017/S1461145702002912. [DOI] [PubMed] [Google Scholar]
- 3.Hooper M, Amsterdam JD. Do clinical trials reflect drug potential? A review of FDA evaluation of new antidepressants. 39th Annual NCDEU Meeting; Boca Raton. June 11–14, 1998. [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 5.Kirsch I, Sapirstein G. Listening to prozac but hearing placebo: A meta-analysis of antidepressant medication. Prev Treat. 1998 posted at http://journals.apa.org/prevention/volumeI/pre0010002a.html. [Google Scholar]
- 6.Kirsch I, Deacon BJ, Huedo-Medina TB. Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration. PLoS Medicine. 2008;5:260–268. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychol Bull. 2004;30:324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- 8.Huedo-Medina TB, Kirsch I, Middlemass J, Klonizakis M, Siriwardena AN. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012:345. doi: 10.1136/bmj.e8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in fmri in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 10.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta ZK. Neurobiological Mechanisms of the Placebo Effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutherford BR, Roose SP. A Model of Placebo Effects in Antidepressant Clinical Trials. Am J Psychiatry. 2013;170:723–733. doi: 10.1176/appi.ajp.2012.12040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutherford BR, Sneed JR, Roose SP. Does Study Design Affect Outcome? The Effects of Placebo Control and Treatment Duration in Antidepressant Trials. Psychother Psychosom. 2009;78:172–181. doi: 10.1159/000209348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sneed JR, Rutherford BR, Rindskopf D, Roose SP. Design Makes a Difference: A Meta-Analysis of Antidepressant Response Rates in Placebo-Controlled versus Comparator Trials in Late-Life Depression. Am J Geri Psychiatry. 2008;16:65–73. doi: 10.1097/JGP.0b013e3181256b1d. [DOI] [PubMed] [Google Scholar]
- 14.Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharm. 2009;19:34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Sinyor M, Levitt AJ, Cheung AH, Schaffer A, Kiss A, Dowlati Y, Lanctot KL. Does Inclusion of a Placebo Arm Influence Response to Active Antidepressant Treatment in Randomized Controlled Trials? Results from Pooled and Meta-Analyses. J Clin Psychiatry. 2010;71:270–279. doi: 10.4088/JCP.08r04516blu. [DOI] [PubMed] [Google Scholar]
- 16.Rutherford BR, Marcus SM, Wang P, Sneed JR, Pelton GH, Devanand DP, Duan N, Roose SP. A Randomized, Prospective Pilot Study of Patient Expectancy and Antidepressant Outcome. Psychol Med. 2013;43:975–982. doi: 10.1017/S0033291712001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., Revised American Psychiatric Publishing, Inc.; Washington, DC: 2000. [Google Scholar]
- 18.Feighner JP, Overo K. Multicenter, placebo-controlled, fixed-dose study of citalopram in moderate-to-severe depression. J Clin Psychiatry. 1999;60:824–830. doi: 10.4088/jcp.v60n1204. [DOI] [PubMed] [Google Scholar]
- 19.Patris M, Bouchard JM, et al. Citalopram versus fluoxetine: a double-blind, controlled, multicentre, phase III trial in patients with unipolar major depression treated in general practice. Int Clin Psychopharm. 1996;11:129–136. [PubMed] [Google Scholar]
- 20.Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy N, Paykel ES. Residual symptoms at remission from depression: impact on long-term outcome. J Affect Disord. 2004;80:135–144. doi: 10.1016/S0165-0327(03)00054-5. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) American Psychiatric Press, Inc.; Washington, D.C.: 1996. [Google Scholar]
- 23.Borkovec TD, Nau SD. Credibility of Analogue Therapy Rationales. J Behav Ther Exp Psychiat. 1972;3:257–260. [Google Scholar]
- 24.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiat. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 25.Borkovec TD, Costello E. Efficacy of Applied Relaxation and Cognitive-Behavioral Therapy in the Treatment of Generalized Anxiety Disorder. J Consult Clin Psychol. 1993;61:611–619. doi: 10.1037//0022-006x.61.4.611. [DOI] [PubMed] [Google Scholar]
- 26.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo Response in Studies of Major Depression: Variable, Substantial, and Growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 27.Rutherford BR, Cooper TM, Persaud A, Brown PJ, Sneed JR, Roose SP. Less is More in Antidepressant Clinical Trials: A Meta-Analysis of the Effect of Visit Frequency on Treatment Response and Drop-out. J Clin Psychiatry. 2013;74:703–715. doi: 10.4088/JCP.12r08267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leuchter AF, Hunter AM, Tartter M, Cook IA. Role of pill-taking, expectation and therapeutic alliance in the placebo response in clinical trials for major depression. Br J Psychiatry. 2014;205:443–449. doi: 10.1192/bjp.bp.113.140343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer B, Pilkonis PA, Krupnick JL, Egan MK, Simmens SJ, Sotsky SM. Treatment Expectancies, Patient Alliance, and Outcome: Further Analyses from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 2002;70:1051–1055. [PubMed] [Google Scholar]
- 30.Krell HV, Leuchter AF, Morgan M, Cook IA, Abrams M. Subject Expectations of Treatment Effectiveness and Outcome of Treatment with an Experimental Antidepressant. J Clin Psychiatry. 2004;65:1174–1179. doi: 10.4088/jcp.v65n0904. [DOI] [PubMed] [Google Scholar]
- 31.Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. J Neurosci. 2012;32:8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The Effect of Treatment Expectation on Drug Efficacy: Imaging the Analgesic Benefit of the Opioid Remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 33.Ochsner KN, Gross JJ. The cognitive control of emotion. Trands Cog Neurosci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Dimidjian S, Hollon SD, Dobson KS, et al. Randomized Trial of Behavioral Activation, Cognitive Therapy, and Antidepressant Medication in the Acute Treatment of Adults with Major Depression. J Consult Clin Psychol. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- 35.Martin DJ, Garske JP, Davis K. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. J Consult Clin Psychol. 2000;68:438–450. [PubMed] [Google Scholar]
- 36.Sexton H. Exploring a psychotherapeutic change sequence relating process to intersessional and posttreatment outcome. J Consult Clin Psychol. 1993;61:128–136. doi: 10.1037//0022-006x.61.1.128. [DOI] [PubMed] [Google Scholar]
- 37.Kaptchuk T, Kelley JM, Conboy LA, et al. Components of the Placebo Effect: A randomized controlled trial in irritable bowel syndrome. BMJ. 2008;336:998–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothschild AJ. The diagnosis and treatment of late-life depression. J Clin Psychiatry. 1996;57:5–11. [PubMed] [Google Scholar]
- 39.Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry. 1999;7:309–316. [PubMed] [Google Scholar]
- 40.Simpson SW, Jackson A, Baldwin RC, Burns A. Subcortical hyperintensities in late-life depression: acute response to treatment and neuropsychological impairment. Int Psychogeriatr. 1997;9:257–275. doi: 10.1017/s1041610297004432. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien J, Ames D, Chiu E, Schweitzer I, Desmond P, Tress B. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow-up study. BMJ. 1998;317:982–984. doi: 10.1136/bmj.317.7164.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey A, Hull J. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57:285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 44.Rohsenow DJ, Marlatt GA. The Balanced Placebo Design: Methodological Considerations. Addict Behav. 1981;6:107–122. doi: 10.1016/0306-4603(81)90003-4. [DOI] [PubMed] [Google Scholar]