There is no single accepted treatment approach for mantle cell lymphoma (MCL). Currently, as a general paradigm, young, fit patients receive intensive therapy, usually with high dose chemotherapy and autologous stem cell support (ASCT) consolidation. The more prevalent older or less fit patient often receives less intensive therapy (reviewed in 1). R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) has remained a commonly used initial therapy for MCL, yielding high response rates but with limited durability (2), and still serves as a comparator in recently published studies (3-5). The treatment landscape for MCL when E1499 was designed in the early 2000’s often included R-CHOP for 6 cycles, with consolidative ASCT in a selected subset of patients, followed by observation. Maintenance rituximab was not generally applied. Given that relatively few MCL patients actually proceed to ASCT, ECOG-ACRIN designed its earlier trials in MCL to encompass the general MCL population.
The phase 2 E1499 trial, the first MCL trial performed by ECOG-ACRIN, was designed to test the hypothesis that a single dose of radioimmunotherapy (RIT) consolidation with 90Y-ibritumomab tiuxetan after only 4 cycles of R-CHOP as initial therapy for MCL would be safe, well-tolerated and improve time-to-treatment failure (TTF) compared with historical R-CHOP data. E1499 met its primary endpoint with TTF at 1.5 years of 69% (95% CI 58-78%) (6) and treatment was well-tolerated. Here we report 10 year follow-up data on this uniformly-treated cohort of patients with MCL.
As we previously reported (6), 56 eligible patients with previously untreated MCL and adequate organ function were enrolled between November 2003 and February 2005. Median age at enrollment was 60 (range 33-83) years, 73% were male and MIPI was low in 50%, intermediate in 27% and high in 21%. Pathology material for all cases was centrally reviewed. Therapy consisted of standard dose R-CHOP given every 3 weeks for only 4 cycles. Patients were re-staged with CT imaging 3-4 weeks after the 4th cycle was administered. Those whose disease had not progressed proceeded to receive a standard administration of 90Y-ibritumomab tiuxetan (7). Per the approved methodology, 0.4 mCi/kg (maximum 32 mCi) was infused, preceded by rituximab 250 mg/m2 1 week prior to, and again on the day of, RIT. All planned therapy was administered to 51 patients (91%). The reason for not receiving RIT in five patients was disease progression in 3, 1 inter-current death from myocardial infarction and 1 patient preference.
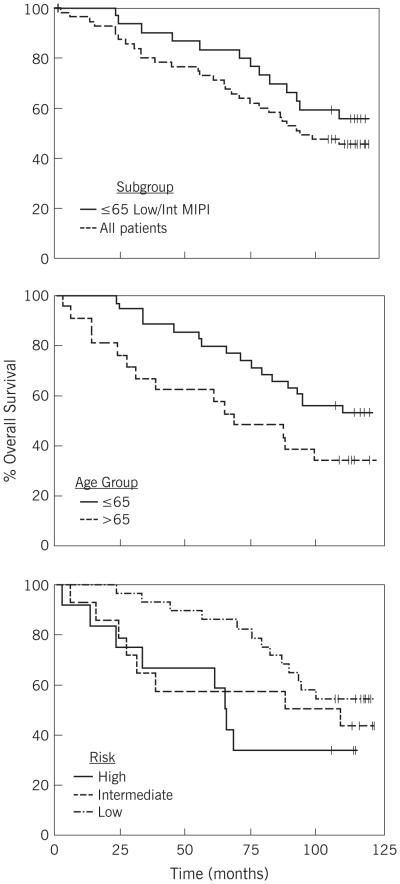
Median follow-up is now 9.8 years (May 2015 data cut-off). Response rates and duration are similar to previously reported values. The overall objective response rate (ORR) after all treatment was 82% (55% complete remission CR/CRu). Median TTF is 34 months, compared with the historical target of 18 months (2). By MIPI scores, TTF was 36 months for low (n=28), 25 months for intermediate (n=15) and 10 months for high MIPI (n=12). For patients who achieved CR, median TTF was 37 months vs. 14 months for all other patients (p = 0.01). Median overall survival (OS) for the entire cohort of 56 eligible patients is 7.9 years. For those under 65 years of age at entry on study, median OS has not been reached at 10 years, compared with 5.7 years for those 65 years or older (p = 0.07). OS for patients with low MIPI scores also has not been reached at 10 years, is 8.2 years for intermediate MIPI and 5.5 years for those with high baseline MIPI. Although TTF was longer for patients who achieved CR/CRu, OS did not differ significantly (p=0.19).
No unexpected short- or long-term toxicity has been reported. In contrast, RIT consolidation after more intense induction was reportedly more toxic (8). While extensive long-term toxicity monitoring has not been carried out in our study, all second malignancies are captured. There has been no additional therapy-related myeloid neoplasia (t-MN) other than the one previously reported t-MN which occurred subsequent to additional therapy. Other reported malignancies include: non-small cell lung cancer (N=2), bladder cancer (N=1), ampullary adenocarcinoma (N=1) and resected localized non-melanoma skin cancers (N=2).
Long-term follow-up of this cohort of patients treated with a simple 4 month outpatient regimen continues to suggest a benefit for RIT consolidation in prolonging TTF, with median TTF of almost 3 years, compared with a full course of R-CHOP without maintenance as reported in other studies having TTF or PFS of < 2 years. Specifically, though in somewhat older populations, in the LYM-3002 trial (4) median PFS was 14 months for six cycles of R-CHOP alone (compared with 25 months for six cycles of VR-CAP), and 22 months for six cycles of R-CHOP in the StiL trial (3). Ongoing rituximab maintenance after eight cycles of R-CHOP is another effective consolidation strategy with 57% of patients progression-free at 4 years (5). RIT may be viewed as an alternative to maintenance rituximab following induction therapy, but testing this hypothesis may not be of high priority with newer agents available.
Overall survival (OS) is an important endpoint in an incurable disease such as MCL. Median OS for our entire cohort is 7.9 years. For OS, age was the single most important prognostic factor in this cohort. MIPI was also important. Patients with high MIPI had short TTF, however, their OS suggests that additional therapies were effective even in this high risk group. We do not have uniformly assessed Ki67 data for evaluation. As novel agents rapidly move into first-line regimens, often based on early assessments of efficacy based on response rates and duration (9), but not OS, these long-term data will inform benchmark expectations of OS in MCL.
As MIPI is highly dependent on age, most younger patients have low or intermediate MIPI. Thus, as expected, patients under age 65 in our trial (N=35), who today would generally be considered for intensive treatment approaches, commonly had low or intermediate MIPI (N = 30 of 35). Specifically examining this subset of patients, that is age ≤ 65 years with low or intermediate MIPI, median OS has not been reached at 10 years. OS in these young patients treated in our study with a short course of simple outpatient therapy is comparable to contemporaneous studies using intense initial therapy (10-15) (Table). This indicates the need for cautious interpretation of apparent prolonged OS in uncontrolled trials with various regimens as patient selection, in particular age and MIPI score, play an important role.
Table.
Overall survival reported with intense regimens for mantle cell lymphoma compared with E1499.
| Regimen (reference) |
N | Median Age (yr) |
MIPI % Low/Int/High |
Overall Survival | |
|---|---|---|---|---|---|
| Younger | Older | ||||
| R-HyperCVAD/R-MA Chihara 10 Merli 11 Bernstein 12 |
97 60 49 |
61 57 57 |
50/28/18 60/31/ 9 55/31/14 |
66% @ 10 yr (age < 65) 73% @ 5 yr (age ≤ 66) 69% @ 5 yr (age ≤ 65) |
24% @ 10 yr (age ≥ 65) 29% @ 5 yr (age > 65) |
| Nordic2, Geisler 13
MaxiCHOP/R-HiDAC->SCT |
160 | 56 | 51/26/23 | 60% @ 10 yr (age ≤ 60) 49% @ 10 yr (age 61 -65) |
|
| R-CHOP ± R-DHAP-> SCT European MCL Younger Hoster 14 |
454 | 55 |
Low 61 Int 24 High 14 |
By MIPI, @ 5 yr (age ≤ 66) 84% 58% 40% |
|
| CALGB 59909: sequential chemotherapy -> SCT Damon 15 |
78 | 57 | 53/31/15 | 64% @ 5 yr (age < 70) | |
| E1499 | 56 | 60 | 50/27/12 | 56% @ 10 yr (age ≤ 65) | 33% @ 10 yr (age > 65) |
SCT = high dose therapy with autologous stem cell support
yr = years; = intermediate
We conclude, with 10 year follow-up for overall survival of this uniformly treated cohort, that a brief, < 4 month therapeutic regimen consisting of R-CHOP for 4 cycles followed by one administration of 90Y-RIT is an active regimen for initial treatment of MCL.
figure.
Overall survival for patients treated on E1499 protocol.
Top panel: Red line entire cohort, Blue line patients ≤ 65 years of age with low or intermediate MIPI score;
Middle panel by age: Red age ≤ 65 years, Blue age > 65 years;
Bottom panel by MIPI: Red high, blue intermediate, and green low.
ACKNOWLEDGEMENTS
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180794, CA180820, CA180853, CA189859, CA180816, CA180855, CA180799, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. 90Y-ibritumomab tiuxetan was supplied by Spectrum Pharmaceuticals.
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180794, CA23318, CA66636, CA180820, CA21115, CA180853, CA27525, CA17145, CA189859, CA14958, CA180816, CA180855, CA180833, CA180799, CA21076, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflict of interest statement:
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180794, CA180820, CA180853, CA189859, CA180816, CA180855, CA180799, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. 90Y-ibritumomab tiuxetan was supplied by Spectrum Pharmaceuticals.
REFERENCES
- 1.Spurgeon SE, Till BG, Martin P, Goy AH, Dreyling MP, Gopal AK, et al. Recommendations for Clinical Trial Development in Mantle Cell Lymphoma. J Natl Cancer Inst. doi: 10.1093/jnci/djw263. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard OM, Gribben JG, Neuberg DS, Grossbard M, Poor C, Janicek MJ, Shipp MA. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20:1288–94. doi: 10.1200/JCO.2002.20.5.1288. [DOI] [PubMed] [Google Scholar]
- 3.Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, et al. Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–10. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 4.Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372:944–53. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 5.Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Trneny M, Geisler CH, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367:520–31. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- 6.Smith MR, Li H, Gordon L, Gascoyne RD, Paietta E, Forero-Torres A, et al. Phase II study of R-CHOP followed by 90Y-ibritumomab tiuxetan in untreated mantle cell lymphoma (MCL): 5 year follow-up of Eastern Cooperative Oncology Group E1499. J Clin Oncol. 2012;30:3119–3126. doi: 10.1200/JCO.2012.42.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skarbnik AP, Smith MR. Radioimmunotherapy in mantle cell lymphoma. Best Pract Res Clin Haematol. 2012;25:201–10. doi: 10.1016/j.beha.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arranz R, García-Noblejas A, Grande C, Cannata-Ortiz J, Sánchez JJ, García-Marco, et al. First-line treatment with rituximab-hyperCVAD alternating with rituximab-methotrexate-cytarabine and followed by consolidation with 90Y-ibritumomab-tiuxetan in patients with mantle cell lymphoma. Results of a multicenter, phase 2 pilot trial from the GELTAMO group. Haematologica. 2013;98:1563–70. doi: 10.3324/haematol.2013.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chihara D, Cheah CY, Westin JR, Fayad LE, Rodriguez MA, Hagemeister FB, et al. Rituximab plus hyper-CVAD alternating with MTX/Ara-C in patients with newly diagnosed mantle cell lymphoma: 15-year follow-up of a phase II study from the MD Anderson Cancer Center. Br. J. Haematol. 2016;172:80–88. doi: 10.1111/bjh.13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merli F, Luminari S, Ilariucci F, Petrini M, Visco C, Ambrosetti A, et al. Rituximab plus HyperCVAD alternating with high dose cytarabine and methotrexate for the initial treatment of patients with mantle cell lymphoma, a multicentre trial from Gruppo Italiano Studio Linfomi. Br. J. Haematol. 2012;156:346–53. doi: 10.1111/j.1365-2141.2011.08958.x. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein SH, Epner E, Unger JM, Leblanc M, Cebula E, Burack R, et al. A phase II multicenter trial of hyperCVAD MTX/Ara-C and rituximab in patients with previously untreated mantle cell lymphoma; SWOG 0213. Ann. Oncol. 2013;24:1587–93. doi: 10.1093/annonc/mdt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisler CH, Kolstad A, Laurell A, Jerkeman M, Räty R, Andersen NS, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br. J. Haematol. 2012;158:355–62. doi: 10.1111/j.1365-2141.2012.09174.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoster E, Klapper W, Hermine O, Kluin-Nelemans HC, Walewski J, van Hoof A, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol. 2014;32:1338–46. doi: 10.1200/JCO.2013.52.2466. [DOI] [PubMed] [Google Scholar]
- 15.Damon LE, Johnson JL, Niedzwiecki D, Cheson BD, Hurd DD, Bartlett NL, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J. Clin. Oncol. 2009;27:6101–8. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]