Abstract
To determine the clinical significance of minimal residual disease (MRD) in patients with prognostically relevant subtypes of childhood acute lymphoblastic leukemia (ALL), we analyzed data from 488 patients treated in St. Jude Total Therapy Study XV with treatment intensity based mainly on MRD levels measured during remission induction. MRD levels on day 19 predicted treatment outcome for patients with hyperdiploid >50 ALL, NCI standard-risk B-ALL or T-cell ALL, while MRD levels on day 46 were prognostic for patients with NCI standard-risk or high-risk B-ALL. Patients with t(12;21)/(ETV6-RUNX1) or hyperdiploidy >50 ALL had the best prognosis; those with a negative MRD on day 19 had a particularly low risk of relapse: 1.9% and 3.8%, respectively. Patients with NCI high-risk B-ALL or T-cell ALL had an inferior outcome; even with undetectable MRD on day 46, cumulative risk of relapse was 12.7% and 15.5%, respectively. Among patients with NCI standard-risk B-ALL, the outcome was intermediate overall but was poor if MRD was ≥1% on day ≥19 or MRD was detectable at any level on day 46. Our results indicate that the clinical impact of MRD on treatment outcome in childhood ALL varies considerably according to leukemia subtype and time of measurement.
INTRODUCTION
In childhood acute lymphoblastic leukemia (ALL), levels of minimal residual disease (MRD) measured during treatment reflect the overall response to chemotherapy and hence the aggregate effect of the leukemia and host factors that regulate treatment efficacy in each patient.1 Thus, MRD constitutes the single most important prognostic indicator in ALL, as shown in numerous pediatric and adult studies.1–15 Indeed, recent studies have demonstrated that personalized treatment based on MRD can improve ALL outcome by identifying patients who can be successfully managed with low-intensity and low-toxicity regimens as well as those who require intensified treatment to avert relapse.16–20 For example, in the St. Jude Total Therapy Study XV, MRD-directed treatment improved outcome for patients with Philadelphia chromosome-like ALL and hypodiploid ALL, subtypes historically associated with a poor prognosis.21,22 Likewise, in the AIEOP-BFM ALL 2000 study, MRD-directed therapy improved outcome for patients with intrachromosomal amplification of chromosome 21.20
The prognostic importance and therapeutic impact of MRD in patients with specific leukemia subtypes who already received MRD-directed therapy are not completely defined. The AIEOP-BFM ALL 2000 study used MRD measurements by polymerase chain reaction (PCR) at the end of induction protocol IA (day 33) and at the end of induction consolidation protocol IB (day 78) to prospectively inform treatment decisions.2,4 Negative MRD on day 33 remained a predictor of a better treatment outcome in both T-cell and B-ALL, while negative MRD at later time point (day 78) was more predictive of favorable outcome of T-cell ALL than B-ALL.2 In the St. Jude Total Therapy XV study, MRD was measured by flow cytometry and/or PCR on days 19 and 46 of remission induction therapy.19,23 We found that MRD-directed treatment improved outcome overall19,23 and noted that MRD continued to be prognostically important for patients with some high-risk subtypes of ALL such as Philadelphia chromosome-like ALL or hypodiploid ALL.21,22,
In this study, we sought to further elucidate the impact of MRD during remission induction on the major ALL subtypes, in the context of MRD-directed treatment. We postulated that, combined with presenting features, negative MRD on day 19 (after 2 weeks of induction treatment with 4 drugs) would help identifying very low risk patients that could be candidates for future treatment reduction, while positive MRD on day 46 (at the end of remission induction treatment with 7 drugs) would identify patients who will require more intensive or novel therapy for cure. The knowledge gained from this analysis should be useful in the design of future, increasingly personalized, treatment protocols.
MATERIALS AND METHODS
Patients
Between June 2000 and October 2007, 498 consecutive patients (1 to 18 years of age) with newly diagnosed ALL were enrolled in the St. Jude Total Therapy XV study (ClinicalTrials.gov, number NCT00137111).23 The protocol was approved by the institutional review boards, and written informed consent was obtained from the patients, parents, or guardians, with assent from the patients, as appropriate.
Diagnosis, MRD Measurement and Risk Classification
The diagnosis of ALL was based on the morphologic, immunophenotypic, and genetic features of the leukemic cells, as described previously.24 MRD was determined by multiparameter flow cytometry, polymerase chain reaction (PCR) analysis or both in bone marrow specimens collected on day 19 and at the end (around day 46) of remission induction.25,26 Either method could routinely detect ALL cells with a sensitivity of 0.01% or better and results were generally concordant.25–28 In the few cases with discrepant results, we used the highest MRD value for risk assignment. In the single patient with no available markers, MRD was monitored by RT-PCR amplification of the MLL-AF9/(KMT2A-MLLT3) fusion transcript.
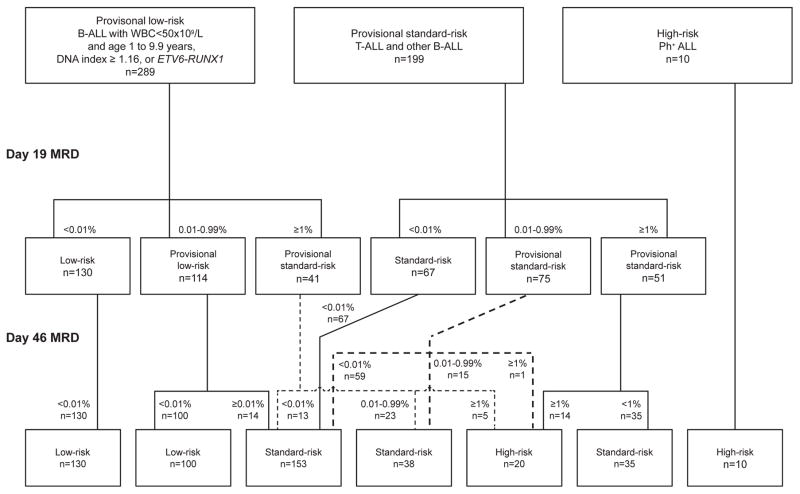
Initial risk classification was based on the National Cancer Institute [NCI] risk group criteria (which emphasize presenting age and leukocyte count), leukemic cell immunophenotype, cytogenetics and molecular genetics; the final risk status was determined by the level of MRD during and after remission induction therapy (Figure 1). Patients with B-ALL who were between 1 and 10 years old and had a leukocyte count <50 x 109/L (NCI standard-risk), had a leukemic cell DNA index ≥1.16 (or hyperdiploidy>50), or had the t(12;21)/(ETV6-RUNX1) without a CNS3 status or testicular leukemia were provisionally classified as having low-risk ALL. Patients with the Philadelphia chromosome (BCR-ABL1) were considered to have high-risk ALL, while the remaining patients, including those with NCI high-risk B-ALL status (age ≥10 years or a leukocyte count ≥50 x 109/L) but without t(12;21)/(ETV6-RUNX1) or DNA index ≥1.16 (or hyperdiploidy>50) were provisionally classified to have standard-risk (intermediate-risk) ALL. Any patient with MRD ≥1% on day 19 of remission induction or 0.01% to 0.99% on day 46 was considered to have standard-risk ALL. Patients with MRD ≥1% upon completion of remission induction were assigned to the high-risk group.
Figure 1.
CONSORT flow diagram. Patients were classified provisionally as having low, standard- or high-risk ALL based on presenting features and subsequently, on day 19 and day 46 of remission induction, as having low-, standard- or high-risk ALL based on minimal residual disease level (MRD) levels.
Treatment
Details of the treatment regimen of Total Therapy Study XV are described elsewhere.23 In brief, after 4 days of treatment with methotrexate, conventional remission induction with prednisone, vincristine, daunorubicin, and Escherichia coli asparaginase (Elspar) was initiated in all patients. Those with MRD ≥1% on day 19 of remission induction, regardless of the provisional risk group, received three additional doses of asparaginase between day 19 and 26. All patients received cyclophosphamide, mercaptopurine and cytarabine between days 26 and 39. Upon completion of remission induction (around day 46), consolidation therapy started with four courses of high-dose methotrexate (targeted steady-state concentration of 33 μM for low-risk and 65 μM for standard- or high-risk patients) and intrathecal therapy together with nightly mercaptopurine given over 8 weeks. Low-risk and standard- or high-risk patients then received risk-directed continuation therapy for 120 weeks for girls and 146 weeks for boys, including two reinduction treatments (reinduction I, weeks 7–9; reinduction II, weeks 17–19). High-risk patients were offered the option of allogeneic hematopoietic cell transplantation when they achieved a negative MRD status (<0.01%) after subsequent treatment. All patients received early triple intrathecal therapy with additional doses given to those with T-cell ALL with a leukocyte count ≥50 x 109/L, B-cell ALL with a leukocyte count >100 x 109/L, the Philadelphia chromosome (BCR-ABL1), MLL (KMT2A) rearrangement, hypodiploidy, a CNS2 or CNS3 status, or traumatic lumbar puncture with blasts on cytospin. No patient received prophylactic cranial irradiation.
Statistical Analysis
Event-free and overall survival rates were estimated by the method of Kaplan-Meier, and were compared by the log-rank test. The duration of event-free survival was defined as the time from diagnosis of ALL until the date of failure (induction failure, relapse, death in remission from any cause, or the development of a second malignancy) or until the date of last contact. Patients who did not attain complete remission were considered to have treatment failure at time zero. The cumulative risk of relapse was estimated according to the method of Kalbfleisch and Prentice,29 and compared with Gray’s test;30 deaths in remission and the development of second neoplasms were considered competing events. The 95% confidence interval (CI) was computed by using the asymptotic normality approximation; a nonparametric method was applied if the sample size was small. In this analysis, the 10 patients with Philadelphia chromosome (BCR-ABL1)-positive ALL were not included because the patient number was too small, and most patients did not receive ABL tyrosine kinase inhibitor which is a treatment currently regarded as standard. The MRD levels at each time point were categories into three groups: <0.01%, 0.01%–1%, and >1%; they were regarded as unordered in the analysis. To determine the association between individual leukemia subtypes and outcome in comparisons that identified significant differences among multiple subtypes within each MRD category, we removed the subtype with the worst outcome and performed the analysis with the remaining subtypes; this procedure was repeated until there was no longer a statistical difference at the 0.05 level. Outcome data used in the analysis was last updated on February 4, 2016. The median follow-up for patients remaining alive in continuous remission was 10.5 years (interquartile range, 1.3 to 15.3 years). At the time of analysis, 88% of the survivors had had a follow-up visit within 2 years; only 3.3% of the patients lacked a documented contact within the preceding 5 years.
RESULTS
Overall treatment outcome and prognostic impact of genetic subtypes
Of the 488 evaluable patients, 482 attained complete remission after induction chemotherapy. Major adverse events that occurred subsequently included 49 relapses (30 hematologic, 12 isolated CNS, 5 combined CNS and hematologic and 2 testicular), 3 second malignancies, and 11 deaths in remission. For the entire cohort of 488 patients, the 10-year cumulative risk of relapse was 11.6% (95% CI, 8.7 to 14.4%), the 10-year event-free survival 85.8% (81.5–90.1%), and the 10-year overall survival 92.5% (89.2–95.8%). As shown in Table 1, the lowest cumulative risk of any relapse and the highest event-free survival rates were observed among patients with ALL denoted by either t(12;21)/(ETV6-RUNX1) (n=94) or hyperdiploidy >50 chromosomes (n =124). The highest relapse rates and worst event-free survival rates were recorded among patients with T-cell ALL (n=76) or NCI high-risk B-ALL (n=95). The remaining patients, including those with t(1;19)/(TCF3-PBX1) (n=28) or NCI standard-risk B-ALL (n=71) had an intermediate outcome. Notably, all 3 adverse events that occurred in patients with t(1;19)/(TCF3-PBX1) ALL were CNS relapses; these were treated successfully, resulting in the overall survival of 100% among patients with this genetic feature.
Table 1.
Comparison of 10-year rates of event-free and overall survival and cumulative risk of any relapse by leukemia subtype
| Provisional Risk Status | Leukemia Subtype | No. Patients | Outcome | ||
|---|---|---|---|---|---|
| EFS (%) | Overall Survival (%) | Risk of Any Relapse (%) | |||
| Low | t(12;21)/(ETV6-RUNX1) | 94 | 95.8(89.2–98.4) | 97.6(90.7–99.4) | 4.2(0.2–8.3) |
| Hyperdiploidy >50 | 124 | 91.3(84.3–95.3) | 96.8(91.6–98.8) | 7.8(2.8–12.9) | |
| Other NCI standard-risk B-ALL | 71 | 85.4(74.5–91.9) | 92.8(83.5–96.9) | 14.6(6.1–23.0) | |
| Standard | t(1;19)/(TCF3-PBX1) | 28 | 89.3(70.4–96.4) | 100.0 | 10.7(0.0–22.4) |
| T-ALL | 76 | 73.5(62.0–82.0)* | 84.1(73.6–90.6)* | 19.9(10.8–28.9)* | |
| NCI high-risk B-ALL | 95 | 78.2(68.3–85.4)* | 85.8(76.8–91.5)* | 15.3(7.9–22.7)* | |
| P <0.001 | P= 0.0004 | P= 0.0011 | |||
EFS, event-free survival,
data contributing to the significant difference in the comparison in a given column.
Prognostic impact of MRD during remission induction therapy
Among the 478 patients with MRD measurements on day 19 of remission induction, outcome was significantly worse for the 92 who had MRD ≥1%. These patients had a 10-year cumulative risk of relapse of 26.6 % (17.0 to 36.2%) versus 7.6% (4.9 to 10.3%) for the 386 with either a negative MRD finding (<0.01%; n=197) or low levels of MRD (0.01 to 0.99%; n=189) (P<0.001). Correspondingly, the 10-year event-free and overall survival were also worse: 66.8% (55.6–75.7%) versus 90.6% (87.2–93.1%, P<0.001), and 81.0% (71.2 to 87.7%) versus 95.7% (93.1 to 97.4%, P<0.001), respectively. Significant associations between day 19 MRD and treatment outcome were also noted for patients with hyperdiploid >50 ALL, NCI standard-risk B-ALL, or T-cell ALL (Table 2). Thus, patients with hyperdiploid >50 ALL and MRD ≥1% on day 19 had a higher cumulative risk of relapse (23.5%, 1.4–45.6%) and a poorer event-free survival rate (76.5%, 47.1–90.9%) than the remaining patients within this subgroup. Nevertheless, most patients who relapsed could be salvaged, leading to an overall survival of 90.0% (65.6–97.4%). By contrast, patients with either T-cell ALL or NCI standard-risk B-ALL who had day 19 MRD ≥1% not only had a poorer event-free survival [55.0% (32.4–72.9%) and 52.7% (26.7–73.2%), respectively] but also a poorer overall survival [68.5% (45.0–83.6%) and 76.7% (49.2–90.6%)].
Table 2.
Comparison of 10-year event-free and overall survival rates and cumulative risk of any relapse among leukemia subtypes based on MRD level on day 19 of remission induction*
| Provisional Risk Status | Leukemia Subtype | MRD <0.01% | MRD 0.01%–0.99% | MRD ≥1% | P value | |||
|---|---|---|---|---|---|---|---|---|
| N | EFS (%) | No. | EFS (%) | No. | EFS (%) | |||
| Low | t(12;21)/(ETV6-RUNX1) | 54 | 98.1(87.6–99.7) | 36 | 91.7(76.3–97.2) | 3 | 100.0 | 0.310 |
| Hyperdiploidy >50 | 54 | 94.3(83.4–98.1) | 48 | 95.8(84.4–98.9) | 20 | 76.5(47.1–90.9) † | 0.056 | |
| Other NCI standard-risk B-ALL | 22 | 90.5(67.0–97.5) | 30 | 96.7(78.6–99.5) | 18 | 52.7(26.7–73.2)† | <0.001 | |
| Standard | t(1;19)/(TCF3-PBX1) | 15 | 93.3(61.3–99.0) | 12 | 91.7(53.9–98.8) | 1 | 0 (CNS relapse) | 1.000 |
| T–ALL | 26 | 84.6(64.0–93.9) | 25 | 80.0(58.4–91.1) ** | 23 | 55.0(32.4–72.9)† | 0.072 | |
| NCI high-risk B-ALL | 26 | 76.6(55.1–88.7) ** | 38 | 81.2(64.5–90.6) ** | 27 | 77.2(56.1–89.1) | 0.930 | |
| P=0.023 | P=0.036 | P=0.164 (excluding TCF-PBX1) | ||||||
| No. | Overall survival (%) | No. | Overall survival (%) | No. | Overall survival (%) | |||
| Low | t(12;21)/(ETV6-RUNX1) | 54 | 97.5(83.5–99.6) | 36 | 97.2(81.9–99.6) | 3 | 100.0 | 1.000 |
| Hyperdiploidy >50 | 54 | 98.1(87.6–99.7) | 48 | 100.0 | 20 | 90.0(65.6–97.4)† | 0.069 | |
| Other NCI standard-risk B-ALL | 22 | 100.0 | 30 | 96.7(78.6–99.5) | 18 | 76.7(49.2–90.6)† | 0.002 | |
| Standard | t(1;19)/(TCF3-PBX1) | 15 | 100.0 | 12 | 100.0 | 1 | 100.0 | 1.000 |
| T-ALL | 26 | 92.3(72.6–98.0) | 25 | 92.0(71.6–97.9) | 23 | 68.5(45.0–83.6)† | 0.042 | |
| NCI high-risk B-ALL | 26 | 92.3(72.6–98.0) | 38 | 83.6(67.1–92.3) ** | 27 | 84.1(62.9–93.7) | 0.639 | |
| P=0.369 | P=0.015 | P=0.30 (excluding TCF-PBX1) | ||||||
| No. | Risk of Relapse (%) | No. | Risk of relapse (%) | No. | Risk of relapse (%) | |||
| Low | t(12;21)/(ETV6-RUNX1) | 54 | 1.9(0.0–5.5) | 36 | 8.3(0.0–17.5) | 3 | 0 | 0.320 |
| Hyperdiploidy >50 | 54 | 3.8(0.0–9.1) | 48 | 4.2(0.0–9.9) | 20 | 23.5(1.4–45.6)† | 0.026 | |
| Other NCI standard-risk B-ALL | 22 | 9.5(0.0–22.4) | 30 | 3.3(0.0–9.9) | 18 | 41.8(16.5–67.1)† | 0.001 | |
| Standard | t(1;19)/(TCF3-PBX1) | 15 | 6.7(0.0–19.7) | 12 | 8.3(0.0–24.7) | 1 | 0 | 1.000 |
| T-ALL | 26 | 7.7(0.0–18.2) | 25 | 20.0(4.0–36.0) | 23 | 31.3(11.4–51.1) | 0.122 | |
| NCI high-risk B-ALL | 26 | 23.4(6.6–40.3) ** | 38 | 8.1(0.0–17.1) | 27 | 15.2(1.1–29.2) | 0.349 | |
| P = 0.017 | P = 0.216 | P = 0.343 (excluding TCF-PBX1) | ||||||
EFS, event-free survival rate, overall survival rate and relapse risk are reported as mean percentages with 95% confidence intervals. Ten patients had missing MRD data on day 19 of remission induction.
Data contributing to significant or borderline significant differences in the comparison in a given column.
Data contributing to significant or borderline significant differences in the comparison across each row.
Among patients with an excellent initial response to chemotherapy, as shown by MRD negativity (<0.01%) on day 19, those with t(12;21)/(ETV6-RUNX1) or hyperdiploidy >50 ALL had a particularly low risk of relapse: 1.9% and 3.8%, respectively. By contrast, despite a negative MRD on day 19, patients with NCI high-risk B-ALL had a higher cumulative risk of relapse (23.4%, 6.6–40.3%) and a poorer event-free survival (76.6%, 55.1–88.7%). Low levels of day 19 MRD (0.01% and 0.99%) predicted a poorer event-free survival in patients with T-cell ALL (80.0%, 58.4–91.1%) and NCI high-risk B-ALL (81.2%, 64.5–90.6%).
Prognostic impact of MRD at the end of remission induction therapy
MRD levels upon completion of remission induction therapy (day 46) were measured in 482 of the 488 patients. They were predictive of a higher risk of relapse, and poorer event-free and overall survival in patients with NCI standard-risk or NCI high-risk B-ALL (Table 3). Among patients with negative MRD on day 46, those with T-cell ALL or NCI high-risk B-ALL had a higher risk of relapse [15.5% (5.5–25.5%), and 12.7% (4.4–21.1%), respectively], poorer event-free survival [78.7% (64.9–87.6%), and 84.1% (72.4–91.1%)], and an inferior overall survival [86.4% (73.6–93.3%) and 92.0% (81.9–96.6%)] than did patients with other leukemia subtypes (Table 3).
Table 3.
Comparison of 10-year event-free and overall survival rates and cumulative risk of any relapse among leukemia subtypes based on MRD level on day 46 of remission induction*
| Provisional Risk Status | Leukemia Subtype | MRD <0.01% | MRD 0.01%–0.99% | MRD≥1% | P value | |||
|---|---|---|---|---|---|---|---|---|
| No. | EFS (%) | No | EFS (%) | No. | EFS (%) | |||
| Low | t(12;21)/(ETV6-RUNX1) | 85 | 95.3(87.9–98.2) | 9 | 100.0 | 0 | 1.000 | |
| Hyperdiploidy>50 | 106 | 92.7(85.1–96.5) | 14 | 85.7(53.9–96.2) | 2 | 100.0 | 0.433 | |
| Other NCI standard-risk B-ALL | 54 | 94.4(83.6–98.2) | 14 | 60.1(28.2–81.5)† | 3 | 0† | <0.001 | |
| Standard | t(1;19)/(TCF3-PBX1) | 26 | 88.5(68.4–96.1) | 2 | 100.0 | 0 | 1.000 | |
| T-ALL | 52 | 78.7(64.9–87.6) ** | 15 | 66.7(37.5–84.6) | 7 | 57.1(17.2–83.7) | 0.245 | |
| NCI High-risk B-ALL | 64 | 84.1(72.4–91.1) ** | 21 | 68.9(43.3–84.7) | 8 | 50.0(15.2–77.5) † | 0.031 | |
| P = 0.005 | P = 0.266 | P = 0.261 | ||||||
| No. | Overall survival (%) | No | Overall survival (%) | No. | Overall survival (%) | |||
| Low | t(12;21)/(ETV6-RUNX1) | 85 | 97.3(89.7–99.3) | 9 | 100.0 | 0 | 0.774 | |
| Hyperdiploidy>50 | 106 | 99.1(93.5–99.9) | 14 | 85.7(53.9–96.2)† | 2 | 100.0 | 0.036 | |
| Other NCI standard-risk B-ALL | 54 | 98.1(87.4–99.7) | 14 | 84.6(51.2–95.9)† | 3 | 33.3(0.9–77.4)† | <0.001 | |
| Standard | t(1;19)/(TCF3-PBX1) | 26 | 100.0 | 2 | 100.0 | 0 | 1.000 | |
| T-ALL | 52 | 86.4(73.6–93.3) ** | 15 | 80.0(50.0–93.1) | 7 | 85.7(33.4–97.9) | 0.860 | |
| NCI high-risk B-ALL | 64 | 92.0(81.9–96.6)** | 21 | 68.7(42.9–84.6)† | 8 | 75.0(31.5–93.1)† | 0.031 | |
| P = 0.002 | P = 0.419 | P = 0.088 | ||||||
| No. | Risk of relapse (%) | No | Risk of relapse (%) | No. | Risk of relapse (%) | |||
| Low | t(12;21)/(ETV6-RUNX1) | 85 | 4.7(0.2–9.2) | 9 | 0 | 0 | 1.000 | |
| Hyperdiploidy>50 | 106 | 6.3(1.3–11.4) | 14 | 14.3(0.0–33.3) | 2 | 0 | 0.093 | |
| Other NCI standard B-ALL | 54 | 5.6(0.0–11.9) | 14 | 39.9(10.3–69.5)† | 3 | 66.7(0.0–100)† | 0.002 | |
| Standard | t(1;19)/(TCF3-PBX1) | 26 | 11.5(0.0–24.1) | 2 | 0 | 0 | 1.000 | |
| T-ALL | 52 | 15.5(5.5–25.5) ** | 15 | 20.0(0.0–41.1) | 7 | 42.9(2.4–83.3) | 0.163 | |
| NCI high-risk B-ALL | 64 | 12.7(4.4–21.1) ** | 21 | 15.7(0.0–32.7) | 8 | 37.5(0.9–74.1)† | 0.085 | |
| P = 0.087 | P = 0.395 | P = 0.630 | ||||||
EFS, event-free survival rate, survival rate and relapse risk are reported as mean percentages with 95% confidence intervals. Six patients had missing MRD data on day 46 of remission induction.
Data contributing to significant or borderline significant differences in the comparison in a given column.
Data contributing to significant or borderline significant differences in the comparison across each row.
DISCUSSION
MRD measurements can be used to refine the treatment of ALL by identifying patients who require more intensive therapy while sparing others from the risk of unnecessary treatment-related toxicities. Hence, this response-adapted therapy is increasingly becoming the favored contemporary approach to clinical management of ALL. However, the amount of leukemic cells that persist during treatment retains prognostic significance even in treatment protocols based on this strategy. We found that MRD levels measured on day 19 (which reflect the short-term response to prednisone, vincristine, daunorubicin and asparaginase) and on day 46 (which reflect response to the preceding four drugs plus cyclophosphamide, cytarabine and mercaptopurine) had prognostic relevance for patients with ALL but their clinical utility varied for different patient subgroups. The MRD measurement on day 19 was particularly useful for patients with favorable presenting features as it identified candidates for treatment de-intensification, while day 46 MRD helped identifying patients at an increased risk of relapse within the NCI standard- or high-risk B-ALL, categories which include clinically and biologically heterogeneous forms of ALL.
It should be noted that while MRD levels were significantly associated with cumulative risk of any relapse, event-free survival and overall survival, they did not correlate with CNS relapse in our Total Therapy Study XV.23 Total Therapy Study XV has relatively low rate of hematological relapse; while isolated and any CNS relapses accounted for 17 of 49 relapses in the study, the cumulative risk of CNS relapse (2.7%) was actually comparable to those (0.9 to 4.0%) of other contemporary clinical trials.31 In fact, despite total omission of prophylactic cranial irradiation, the 5-year event-free survival and overall survival rates in Total Therapy Study XV were at least comparable to those of other contemporary studies.31,32
Patients with t(12;21)/(ETV6-RUNX1) and those with hyperdiploid >50 clearly had the best treatment result in our response-adapted protocol, with a 10-year event-free survival of 95.8% and 91.3%, respectively. This excellent outcome is particularly notable because 187 of 216 (86.6%) of patients with either genotype were treated on the low-risk arm of our clinical trial. Those who achieved negative MRD on day 19 had a cumulative risk of relapse of only 1.9% and 3.8%, respectively, suggesting that patients with these forms of ALL and rapid response should have priority for trials testing treatment reduction strategies to improve quality of life. It should be noted that as many as 16% of patients with hyperdiploid >50 ALL had MRD ≥1% on day 19, and they had a high risk of relapse (23.5%). It was reported that most patients with hyperdiploid >50 ALL refractory to remission induction treatment can be cured with intensive chemotherapy.33 Similarly, in our study, 6 of 8 patients with relapsed hyperdiploid>50 ALL survived for 7+ to 9+ years after retrieval therapy. Nevertheless, treatment for relapse is often associated with substantial late sequelae. Therefore, improved treatment strategies that may avoid relapse are needed for the subset of hyperdiploid >50 ALL patients with day 19 MRD ≥1%.
In the AIEOP-BFM 2000 study, a risk classification based on MRD measurements on days 33 and 78 was also predictive of outcome among patients with t(12;21)/(ETV6-RUNX1) or hyperdiploid >50 ALL treated with MRD-guided therapy.4 Thus, 5-year event-free survival rates for patients with t(12;21)/(ETV6-RUNX1) was 93.7% if they were at standard risk according to MRD and 81.7% if at intermediate risk; for patients with hyperdiploid >50 patients, the rates were 93.7% and 79.8%. Although these rates were excellent, we argue that an earlier measurement performed during remission induction therapy might have identified patients treated in their protocol with an even higher probability of remaining in continuous remission. Indeed, when Basso et al.6 examined the value of flow cytometry measurements on day 15 in a subset of patients enrolled in the AIEOP-BFM 2000 study, they found that this additional determination could further refine prognostic classification among patients with different ALL subtypes. For example, in patients with t(12;21)/(ETV6-RUNX1), 5-year cumulative incidence of relapse was 6.3% if MRD was <0.1% on day 15. Together with the results of our study, these data provide a compelling support to the use of early MRD determinations to refine risk-classification and treatment.
Excluding patients with t(12;21)/(ETV6-RUNX1) or hyperdiploidy >50, the remaining patients with NCI standard-risk B-ALL had an event-free survival of 85.4% and an overall survival of 92.8%. MRD measurements were particularly useful in determining prognosis among patients classified by these criteria. Patients with MRD ≥1% on day 19 or any detectable MRD on day 46 had a higher risk of relapse, with poorer event-free and overall survival rates (Tables 2 and 3). These patients would clearly require further treatment intensification or perhaps an entirely novel treatment approach. By contrast, patients with negative day 46 MRD had excellent event-free and overall survival rates of 94.4% and 98.1%. In line with these findings, a recent Medical Research Council UKALL study reported event-free survival of 91% and overall survival of 97% for low-risk patients with negative MRD at the end of induction treatment; as a caveat, their analysis included patients with t(12;21)/(ETV6-RUNX1) or hyperdiploidy >50 ALL, and some with NCI high-risk ALL.34
At the low end of the chemosensitivity spectrum for patients with B-lineage ALL were those with NCI high-risk B-ALL, who had an inferior treatment outcome overall. MRD testing was also informative for this group: outcome was especially poor among patients with MRD ≥1% on day 46, who had an event-free survival of 50%, despite treatment with allogeneic hematopoietic cell transplantation. Similarly, in the Children’s Oncology Group AAL0232 study, the 5-year event-free survival for patients with high-risk B-ALL was 44% if the MRD level at the end of remission induction was 1% to <10%, and 26% if it was ≥10%.35 T-cell ALL patients also had an inferior outcome in this study and the event-free survival was only 55% in those with day 19 MRD ≥1%. Such low rate resembled that of the small subset of T-cell patients with MRD ≥1% on day 46 who underwent allogeneic hematopoietic cell transplantation (57.1%), and that reported by the BFM-ALL 2000 investigators for their MRD high-risk group (49.8%).2 Even though MRD negativity on day 46 was associated with a lower risk of relapse in patients with NCI high-risk B-ALL or T-cell ALL, we would argue that such relapse rates are still unacceptably high. Since all patients with these two leukemia subtypes had been treated with intensive chemotherapy and a relatively high proportion had received allogeneic hematopoietic cell transplantation, further increase in treatment intensity is not feasible and novel approaches should be evaluated. Whether use of the newer and more sensitive method of detecting ultra-low MRD levels28,36 can identify a highly curable group of patients with NCI high-risk B-ALL or T-cell ALL remains to be determined.
An exception to the continuing prognostic value of MRD within subgroups receiving MRD-guided therapy were patients with t(1;19)/(TCF3-PBX1) ALL, all of whom received intensive chemotherapy in the standard-risk arm of Total Therapy Study XV. They had an intermediate outcome in terms of event-free survival (89.3%) because of their increased risk of isolated CNS relapse (3 among 28 patients).37 However, since isolated CNS relapse is highly curable in patients who have not received prophylactic cranial irradiation,23 all patients with t(1;19)(TCF3-PBX1) were long-term survivors in this study. In our current clinical trial, we have intensified triple intrathecal therapy for these patients.
With the increasing availability of new agents for ALL, an ever more refined risk algorithm based on MRD levels and leukemia subtypes is needed to develop optimal treatment strategies. Our study demonstrates that the impact of MRD varies according to time of measurement and leukemia subtype, even in the context of contemporary response-adapted therapy. On the basis of the findings of this study and those reported in the literature,2,4,6 we would recommend that patients with favorable presenting features and undetectable MRD after two weeks remission induction therapy receive de-intensified therapy, while novel approaches should be considered for patients with high levels of MRD at the end of induction therapy. The time points of MRD determination and remission induction treatment vary among study groups. Therefore, our results should provide a useful guideline, which can be adapted by individual study groups to their most effective MRD thresholds and monitoring intervals.
Acknowledgments
This work was supported by grants (CA21765, CA36401, and GM115279) from the National Institutes of Health, and by the American Lebanese Syrian Associated Charities.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing conflict of interests.
AUTHOR CONTRIBUTIONS
C-H.P., and D.C designed the study, reviewed and interpreted data, and wrote the paper; C-H.P., S,J., W.P.B., J.T.S., R.C.R., J.E.R., H.I., T.A.G., and W.H.L. enrolled patients, and revised the manuscript; D.P., and C.C. provided statistical expertise, analyses, and data interpretation; S.C.R. performed genetic analyses, and E.C-S. minimal residual disease determination. All authors made substantial contributions to the concept, design and conduct of the clinical trial, were involved in the writing and critical revision of the manuscript, and gave final approval of the revision to be submitted.
References
- 1.Campana D. Minimal residual disease monitoring in childhood acute lymphoblastic leukemia. Curr Opin Hematol. 2012;19(4):313–8. doi: 10.1097/MOH.0b013e3283543d5c. [DOI] [PubMed] [Google Scholar]
- 2.Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grümayer R, Möricke A, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118(8):2077–84. doi: 10.1182/blood-2011-03-338707. [DOI] [PubMed] [Google Scholar]
- 3.Bowman WP, Larsen EL, Devidas M, Linda SB, Blach L, Carroll AJ, et al. Augmented therapy improves outcome for pediatric high risk acute lymphocytic leukemia: results of Children's Oncology Group trial P9906. Pediatr Blood Cancer. 2011;57(4):569–77. doi: 10.1002/pbc.22944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grümayer R, Möricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–14. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 5.Stow P, Key L, Chen X, Pan Q, Neale GA, Coustan-Smith E, et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115(23):4657–63. doi: 10.1182/blood-2009-11-253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basso G, Veltroni M, Valsecchi MG, Dworzak MN, Ratei R, Silvestri D, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27(31):5168–74. doi: 10.1200/JCO.2008.20.8934. [DOI] [PubMed] [Google Scholar]
- 7.Sutton R, Venn NC, Tolisano J, Bahar AY, Giles JE, Ashton LJ, et al. Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia. Br J Haematol. 2009;146(3):292–9. doi: 10.1111/j.1365-2141.2009.07744.x. [DOI] [PubMed] [Google Scholar]
- 8.Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111(12):5477–85. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavé H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer--Childhood Leukemia Cooperative Group. N Engl J Med. 1998;339(9):591–8. doi: 10.1056/NEJM199808273390904. [DOI] [PubMed] [Google Scholar]
- 10.van Dongen JJ, Seriu T, Panzer-Grümayer ER, Biondi A, Pongers-Willemse MJ, Corral L, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352(9142):1731–8. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- 11.Coustan-Smith E, Behm FG, Sanchez J, Boyett JM, Hancock ML, Raimondi SC, et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet. 1998;351(9102):550–4. doi: 10.1016/S0140-6736(97)10295-1. [DOI] [PubMed] [Google Scholar]
- 12.Ribera JM, Oriol A, Morgades M, Montesinos P, Sarrà J, González-Campos J, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32(15):1595–604. doi: 10.1200/JCO.2013.52.2425. [DOI] [PubMed] [Google Scholar]
- 13.Beldjord K, Chevret S, Asnafi V, Huguet F, Boulland ML, Leguay T, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739–49. doi: 10.1182/blood-2014-01-547695. [DOI] [PubMed] [Google Scholar]
- 14.Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL) Blood. 2009;113(18):4153–62. doi: 10.1182/blood-2008-11-185132. [DOI] [PubMed] [Google Scholar]
- 15.Paganin M, Fabbri G, Conter V, Barisone E, Polato K, Cazzaniga G, et al. Postinduction minimal residual disease monitoring by polymerase chain reaction in children with acute lymphoblastic leukemia. J Clin Oncol. 2014;32(31):3553–8. doi: 10.1200/JCO.2014.56.0698. [DOI] [PubMed] [Google Scholar]
- 16.Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Houghet R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. doi: 10.1016/S1470-2045(12)70600-9. [DOI] [PubMed] [Google Scholar]
- 17.Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809–18. doi: 10.1016/S1470-2045(14)70243-8. [DOI] [PubMed] [Google Scholar]
- 18.Yeoh AE, Ariffin H, Chai EL, Kwok CS, Chan YH, Ponnudurai K, et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol. 2012;30(19):2384–92. doi: 10.1200/JCO.2011.40.5936. [DOI] [PubMed] [Google Scholar]
- 19.Pui CH, Pei D, Coustan-Smith E, Jeha S, Cheng C, Bowman WP, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol. 2015;16(4):465–74. doi: 10.1016/S1470-2045(15)70082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attarbaschi A, Panzer-Grümayer R, Mann G, Möricke A, König M, Mecklenbräuker A, et al. Minimal residual disease-based treatment is adequate for relapse-prone childhood acute lymphoblastic leukemia with an intrachromosomal amplification of chromosome 21: the experience of the ALL-BFM 2000 trial. Klin Padiatr. 2014;226(6–7):338–43. doi: 10.1055/s-0034-1387795. [DOI] [PubMed] [Google Scholar]
- 21.Roberts KG, Pei D, Campana D, Payne-Turner D, Li Y, Cheng C, et al. Outcomes of children with BCR-ABL1-like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol. 2014;32(27):3012–20. doi: 10.1200/JCO.2014.55.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullighan CG, Jeha S, Pei D, Payne-Turner D, Coustan-Smith E, Roberts KG, et al. Outcome of children with hypodiploid ALL treated with risk-directed therapy based on MRD levels. Blood. 2015;126(26):2896–9. doi: 10.1182/blood-2015-09-671131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pui CH, Evans WE. Acute lymphoblastic leuikemia. N Engl J Med. 1998;339(9):605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 25.Coustan-Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M, et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117(23):6267–76. doi: 10.1182/blood-2010-12-324004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stow P, Key L, Chen X, Pan Q, Neale GA, Coustan-Smith E, et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115(23):4657–63. doi: 10.1182/blood-2009-11-253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neale GA, Coustan-Smith E, Stow P, Pan Q, Chen X, Pui CH, et al. Comparative analysis of flow cytometry and polymerase chain reaction for the detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2004;18(5):934–8. doi: 10.1038/sj.leu.2403348. [DOI] [PubMed] [Google Scholar]
- 28.Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173–80. doi: 10.1182/blood-2012-07-444042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: Wiley; 2002. pp. 1–439. [Google Scholar]
- 30.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–54. [Google Scholar]
- 31.Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J Clin Oncol. 2015;33(27):2938–48. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vora A, Andreano A, Pui CH, Hunger SP, Schrappe M, Moericke A, et al. Influence of Cranial Radiotherapy on Outcome in Children With Acute Lymphoblastic Leukemia Treated With Contemporary Therapy. J Clin Oncol. 2016;34(9):919–26. doi: 10.1200/JCO.2015.64.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrappe M, Hunger SP, Pui CH, Saha V, Gaynon PS, Baruchel A, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366(15):1371–81. doi: 10.1056/NEJMoa1110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartram J, Wade R, Vora A, Hancock J, Mitchell C, Kinsey S, et al. Excellent outcome of minimal residual disease-defined low-risk patients is sustained with more than 10 years follow-up: results of UK paediatric acute lymphoblastic leukaemia trials 1997–2003. Arch Dis Child. 2016;101(5):449–54. doi: 10.1136/archdischild-2015-309617. [DOI] [PubMed] [Google Scholar]
- 35.Borowitz MJ, Wood BL, Devidas M, Loh ML, Raetz EA, Salzer WL, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232. Blood. 2015;126(8):964–71. doi: 10.1182/blood-2015-03-633685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu D, Sherwood A, Fromm JR, Winter SS, Dunsmore KP, Loh ML, et al. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med. 2012;4(134):134ra63. doi: 10.1126/scitranslmed.3003656. [DOI] [PubMed] [Google Scholar]
- 37.Jeha S, Pei D, Raimondi SC, Onciu M, Campana D, Cheng C, Sandlund JT, et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. 2009;23(8):1406–9. doi: 10.1038/leu.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]