Abstract
Attenuated skin blood flow (SkBF) is often assumed to impair core temperature (Tc) regulation. Profound pharmacologically-induced reductions in SkBF (~85%) lead to impaired sweating but whether the smaller attenuations in SkBF (~20%) more associated with ageing and certain diseases lead to decrements in sweating and maximum heat loss potential is unknown. Seven healthy males (28±4y) completed a 30-min equilibration period at 41°C and a vapour pressure (Pa) of 2.57 kPa followed by incremental steps in Pa of 0.17 kPa every 6-min to 5.95 kPa. Differences in heat loss potential were assessed by identifying the critical vapour pressure (Pcrit) at which an upward inflection in Tc occurred. Three separate treatments elicited changes in plasma volume to achieve three distinct levels of SkBF: control (CON), diuretic-induced iso-osmotic dehydration to lower SkBF (DEH), and continuous saline infusion to maintain SkBF (SAL). Tc, mean skin temperature (Tsk), heart rate, mean laser-Doppler flux (forearm, thigh; LDFmean), mean local sweat rate (forearm, thigh; LSRmean), and metabolic rate were measured. In DEH, a 14.2±5.7% lower plasma volume resulted in a ~20% lower LDFmean (DEH: 139±23, CON: 176±22, SAL: 186±22 PU; P=0.034). However, LSRmean and whole-body sweat losses were unaffected by treatment throughout (P>0.482). Pcrit for Tc was similar between treatments (CON: 5.05±0.30, DEH: 4.93±0.16, SAL: 5.12±0.10 kPa; P=0.166). Further, no differences were observed in the Tsk−Ta gradient, metabolic rate, or changes in Tc (P>0.197). In conclusion, a ~20% reduction in SkBF alters neither sweat rate nor the upper limit for heat loss from the skin during non-encapsulated passive heat stress.
Keywords: core temperature, critical vapour pressure, cutaneous vascular conductance
INTRODUCTION
In response to rising core and/or skin temperatures, reflex and locally-mediated mechanisms initiate cutaneous vasodilatation, leading to greater skin blood flow (SkBF). Since internal heat flux to the skin surface is governed primarily by the core-to-skin temperature gradient and SkBF, it is reasonable to suggest that physiological conditions impairing cutaneous vasodilatation and/or SkBF, can diminish the rate of whole-body heat loss from the skin to the external environment (Johnson & Proppe, 1996; Charkoudian, 2010).
Fundamentally, heat exchange between the skin and the environment is driven by the physical properties of the skin-air interface; specifically, dry heat transfer is governed by the skin-air temperature gradient, while evaporative heat loss is dependent on the skin-air vapour pressure gradient and the area of skin sweat coverage. Under fixed ambient conditions, attenuated SkBF could thereby influence heat exchange with the environment through changes in mean skin temperature (Tsk) and/or diminished sweat production. Exposure to very high ambient temperatures favours dry heat gain, but higher Tsk secondary to greater SkBF would theoretically assist core temperature regulation by reducing the negative skin-air temperature gradient. With respect to a possible relationship between SkBF and sweating, local ischaemia following arterial occlusion abolishes reflex sweating in warm and hot environments (Randall et al., 1948; Elizondo, 1973), and noradrenaline-induced reductions in SkBF attenuate the rate of local sweat production by as much as 50% for a given change in core temperature (ΔTc) during passive heating in a water-perfused suit (Wingo et al., 2010). It must be noted however that the ~85–100% reductions in SkBF achieved with noradrenaline infusion and local ischaemia are well above the ~20% reduction in SkBF typically reported with ageing (Minson et al., 1998), disease (Carberry et al., 1992; Cui et al., 2005; Green et al., 2006; Wick et al., 2006; Sokolnicki et al., 2009), or dehydration (Nadel et al., 1980; Montain & Coyle, 1992a, 1992b). Therefore, whether reductions in SkBF of a more physiologically relevant magnitude diminish sweating and thereby maximum evaporative heat loss, have not to the best of our knowledge been previously examined.
Many previous studies have examined cutaneous vasodilatory and SkBF responses during exercise with submaximal thermolytic requirements (i.e., compensable conditions) or during passive heating in encapsulated conditions such as a water-perfused garment, which yields an extremely high Tsk under the suit while local skin temperature at the SkBF measurement site is held constant. However, in order to assess the upper limit for heat loss from the skin surface, a non-encapsulated approach that identifies the transition from compensability to uncompensability must be adopted, such as an incremental humidity protocol consisting of short-duration steps of increasing ambient vapour pressure at a constant air temperature and metabolic rate. Above a critical ambient vapour pressure (Pcrit), an upward inflection in core temperature is observed, indicating that the thermolytic requirements—a combination of metabolic heat production and any dry heat gain from the environment—have exceeded the maximum rate of heat loss that can be physiologically attained via evaporation. Values for Pcrit can then be compared between different conditions in which SkBF levels are manipulated to assess whether skin surface heat loss is meaningfully altered. Historically, this approach has been used to define critical environmental limits between populations (Kamon et al., 1978; Kenney & Zeman, 2002; Dougherty et al., 2010), at various air velocities and ambient temperatures (Belding & Kamon, 1973; Kamon & Avellini, 1979; Ravanelli et al., 2015), and with different clothing ensembles (Belding & Kamon, 1973; Kamon et al., 1978; Kenney et al., 1993), and may be especially useful to assess whether physiological differences in SkBF are sufficient to alter maximum heat loss potential in a non-encapsulated heat stress condition.
The current investigation sought to determine if physiologically relevant reductions in SkBF attenuate sweating rates and whole-body heat loss to the extent that the Pcrit for core temperature is ultimately lowered. It was hypothesized that (i) diminished SkBF would result in lower sweat rates and a lower Pcrit for core temperature inflection, and (ii) the maintenance of a higher SkBF would preserve sweat rates and the Pcrit for core temperature inflection.
METHODS
Subjects
Seven healthy young males completed the study (age: 28 ± 4 years; body mass: 82.3 ± 17.2 kg; height: 1.76 ± 0.07 m; body surface area: 1.98 ± 0.23 m2). Based on previously reported data (Ravanelli et al., 2015), and using conventional β (0.20) and α (0.05) values, a sample size of seven subjects was estimated to be sufficient to detect a significant difference in the Pcrit for core temperature (G*power version 3.1.9.2). Subjects were non-smokers; reported no history of cardiovascular, metabolic, respiratory, or neurological disease; were not taking any medications; and were not acclimated to the heat. The protocol was explained to each subject before obtaining written informed consent, and a complete medical history was obtained prior to testing. The experimental protocol was approved by the University of Ottawa Health Sciences and Science Research Ethics Board and the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas. All procedures conformed to the standards set forth by the Declaration of Helsinki.
Instrumentation and Measurements
To assess SkBF, cutaneous red blood cell flux was measured using laser-Doppler velocimetry (Moor Instruments Ltd., Devon, UK) on the dorsal forearm and the mid-point of the anterior thigh. Skin blood flow data are reported as the mean of both sites as absolute laser-Doppler flux in arbitrary perfusion units (LDFmean), as a percentage of maximum laser-Doppler flux (%LDFmax), as cutaneous vascular conductance (CVCmean), which was calculated as the quotient of LDFmean and mean arterial pressure (MAP), and as the percentage of the maximum cutaneous vascular conductance (%CVCmax). Additionally, forearm blood flow (FBF) was measured via venous occlusion plethysmography (Whitney, 1953) with an indium-gallium strain gauge (Hokanson, Bellevue, WA, USA) every 12 min during the first 72 min of the protocol, and then every 6 min thereafter. Forearm vascular conductance (FVC) was subsequently calculated as the quotient FBF and MAP. Maximum laser-Doppler flux was assessed by heating each measurement site for ~45 min by setting the local heater to 44°C. Arterial blood pressures were measured with an electrosphygmomanometer (Tango, SunTech Medical Instruments, Inc., Raleigh, NC, USA), with MAP values calculated as (pulse pressure/3) + diastolic blood pressure. Heart rate was taken from an electrocardiogram (Solar 8000M, GE Medical Systems, Madison, WI, USA). Venous blood samples (4 ml) were collected from a forearm vein in lithium-heparin tubes (BD Vacutainer, Franklin Lakes, NJ, USA) and analysed in triplicate for haemoglobin concentration (Hemoximeter, OSM3, Radiometer, Copenhagen, Denmark), haematocrit, and plasma osmolality (Micro-Osmometer, Model 3MO plus, Advanced Instruments Inc., Norwood, MA, USA). Changes in blood, cell, and plasma volumes were estimated with the equations of Dill and Costill (1974).
Oesophageal temperature was measured with a general-purpose thermocouple probe (Mon-a-therm, Mallinckrodt Medical, St. Louis, MO, USA) inserted to a maximum depth of 40 cm, which is a level estimated to be adjacent to the left ventricle and aorta (Mekjavic & Rempel, 1990). Skin temperatures were measured on the chest, shoulder, abdomen, lower back, thigh, and calf using thermocouples secured to the skin with soft cloth medical tape (Medipore, 3M), and Tsk was calculated as a weighted average of these six sites (Taylor et al., 1989). Oesophageal and skin temperatures were measured with thermocouple readers (Sable Systems International, Las Vegas, NV, USA). Gastrointestinal temperature was also measured in six subjects using a telemetric pill (HQ Inc., Palmetto, FL, USA) ingested ~4 h prior to baseline data collection.
Local sweat rates were measured using two 4.1-cm2 ventilated capsules placed adjacent to each laser-Doppler probe. Nitrogen gas was supplied to each capsule at a flow rate of 300 ml min−1, and the effluent air was analysed for vapour concentration using capacitance hygrometry (Vaisala, Vantaa, Finland). Local sweat rate for each site was calculated as the product of the vapour concentration and flow rate relative to the skin surface area covered by a sweat capsule (mg·cm−2·min−1). Local sweat rate (LSRmean) values are reported as the average of the two sites.
Expired gases were analysed with a metabolic cart (TrueOne 2400, Parvo Medics, Sandy, UT, USA). The rate of oxygen uptake (VO2) and the respiratory exchange ratio (RER) were used to determine metabolic rate (M) via indirect calorimetry:
Where: ec and ef are the energetic equivalents for carbohydrate (21.13 kJ L O2−1) and fat (19.62 kJ L O2−1), respectively. Dry heat exchange was calculated as the sum of convection (C) and radiation (R) using the following equations, which assumed negligible clothing insulation:
The value of the convective heat transfer coefficient (hc) was taken as 3.1 W m−2 K−1 since air velocity inside the thermal chamber was measured to be <0.2 m s−1 (Mitchell, 1974). Ambient temperature (Ta) and mean radiant temperature (Tr) were assumed to be equal. Ambient temperatures were measured in degrees Celsius, but converted to Kelvin degrees to calculate dry heat transfer coefficients. The radiant heat transfer coefficient (hr) was calculated as:
Here, ε is the emissivity of the skin, assumed to be 0.95 (no dimensions); σ is the Stefan-Boltzmann constant (5.67·10−8 W m−2 K−4); Ar AD−1 is the effective radiant surface area (non-dimensional), which was taken as 0.7 (Guibert & Taylor, 1952; Kerslake, 1972).
Experimental Protocol
In a randomized, counterbalanced order separated by at least 3 days, subjects performed three experimental trials: a control condition (CON) in which no treatment was administered, a diuretic-induced iso-osmotic dehydration trial (DEH) with the goal of lowering SkBF relative to CON, and a continuous saline infusion trial (SAL) with the goal of elevating SkBF relative to CON. Prior to experimentation, subjects were instructed to avoid cold, allergy, and anti-inflammatory medicines for 36 h, alcohol consumption and exercise for 24 h, and caffeine intake for 12 h. They were also asked to consume plenty of water the night before and the morning of each visit to ensure adequate hydration, and to eat breakfast before leaving for the laboratory.
Subjects arrived at the lab between 0800 and 0900. Nude body mass, standing height, and urine specific gravity (USG) were first recorded, with euhydration accepted at a USG ≤ 1.025 (Kenefick & Cheuvront, 2012). A forearm venous catheter was then inserted, and after 30 min of supine rest, an initial blood sample was drawn. In DEH, 40–80 mg of furosemide was then administered orally, after which the subjects rested for ~3.5 h to allow the drug to take effect (Ikegawa et al., 2011). Furosemide was selected to induce hypovolemia without any change in plasma osmolality, which can independently affect sweating responses (Fortney et al., 1984). In CON and SAL, subjects rested for an equivalent duration to ensure that heating commenced at a similar time of day during each visit. Drinking water was provided ad libitum during the initial rest period in CON and SAL only, but a small volume of fluid was ingested in DEH to facilitate insertion of the oesophageal temperature probe during instrumentation. Urine output was also recorded during this period. Nude body mass was measured again at the end of the rest period.
Following instrumentation, subjects entered the chamber. The experimental protocol began with a 30-min baseline equilibration period at 41°C, 33% RH (2.57 kPa) during which steady-state sweat rates and core temperature were attained. After, an incremental humidity protocol began with Pa increased by 0.17 kPa every 6 min from 2.57 to 5.95 kPa (maximum: 20 stages or 120 min). Throughout baseline and the incremental humidity protocol Ta was controlled at 41.0 ± 0.1°C. This Ta was selected to elicit skin temperatures similar to those achieved in encapsulated protocols (e.g. water-perfused suit studies), and induce high sweating rates that would facilitate examination of a link between SkBF and LSR. The pattern of change in Pa over time was nearly identical between trials, resulting in steps of 0.17 ± 0.01 kPa per 6-min stage. Termination criteria were (i) completion of the 20th stage, (ii) reaching a 1.0°C ΔTc from baseline, (iii) hypotension (systolic blood pressure < 80 mmHg), or (iv) voluntary withdrawal due to excessive discomfort. Trials were conducted in an upright seated position (n=4) on a steel-framed mesh chair (height: 83 cm) or in a supine position (n=3) on a hospital bed. Experimentation was conducted initially in the upright seated posture, but because of episodes of hypotension in some DEH trials, testing was completed in a supine posture by the remaining three subjects to minimize the possibility of syncope. Each subject conducted his trials in the same posture. During SAL, 0.9% saline was infused continuously at a rate of 0.05 ml kg−1min−1 throughout the incremental humidity trial (total: 479 ± 105 ml). Core and skin temperatures, local sweat rates, skin blood flow, and heart rate were measured throughout. Blood pressure was measured at the end of each stage; expired gases were sampled at baseline and every four stages. Upon completion of the incremental humidity protocol, a final blood sample was drawn and the thermal chamber was cooled. Local heating of the laser-Doppler measurement sites was performed for ~45 min to induce maximum LDF values. A final nude body mass measurement was then performed.
Data Analysis
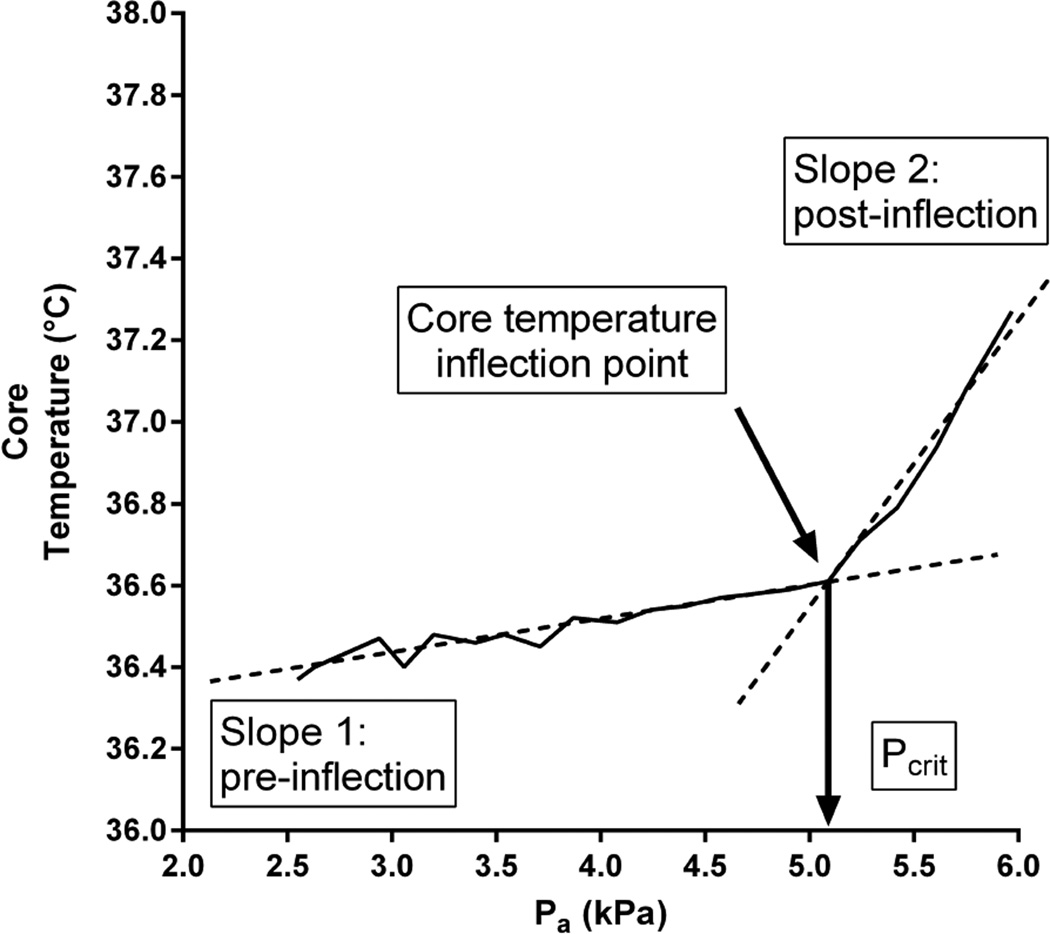
Continually measured variables were sampled at 25 Hz (MP150, Biopac Systems, Inc., Santa Barbara, CA). For each 6-min stage, data were averaged over the final 2 min. In accordance with recent work, these values were subsequently used to define Pcrit for core temperature and heart rate using segmental regression analysis (Ravanelli et al., 2015).This latter statistical approach is based on the biphasic core temperature response during an incremental humidity protocol: the first phase is a baseline phase during which the slope of the relationship between core temperature and Pa is approximately zero, and an “inflected” phase during which core temperature rises abruptly and linearly from its baseline level as the heat loss requirement has exceeded heat loss potential due to increasing Pa. The segmental regression analysis identifies the Pa value at the intersection between these two slopes, which is then accepted as Pcrit (see Figure 1). The core temperature Pcrit was determined from oesophageal temperature in six subjects, and gastrointestinal temperature in one subject.
Figure 1.
Representative core temperature data from the incremental humidity protocol, demonstrating the determination of acritical ambient vapour pressure (Pcrit). After 30-min baseline at 41°C with 2.57 kPa (30%RH), ambient vapour pressure was elevated by 0.17 kPa (~2% RH) every 6 min while air temperature remained fixed. Core temperature stayed relatively stable despite the rising ambient vapour pressure. At Pcrit, core temperature inflects and rises rapidly. The value of Pcrit is identified as the intersection of the two slopes (Slope 1, pre-inflection; Slope 2, post-inflection), determined via segmental regression analysis.
Statistical Analysis
Data are presented as means ± standard deviations. A one-way repeated measured analysis of variance (ANOVA), using the independent factor of treatment (3 levels: CON, DEH, SAL), was employed to compare USG, fluid intake and urine output during rest, whole-body sweat loss, Pcrit values, and metabolic rate. A two-way repeated measures ANOVA, with the independent factors of treatment (3 levels: CON, DEH, SAL) and time, was also employed to compare changes in blood and plasma volumes and plasma osmolality (4 levels: initial, baseline, at 60 min, and at the end of the protocol); LDFmean, %LDFmax, CVCmean, %CVCmax, LSRmean, Tsk, ΔTc, Tsk−Ta, and dry heat exchange (15 levels: baseline and every 6-min stage to ~5.0 kPa or 84 min of the protocol); as well as FBF and FVC (8 levels: baseline and every 2 stages to ~4.8 kPa or 78 min of the protocol). A Greenhouse-Geisser correction was applied if the assumption of sphericity had been violated. In the event of a significant time-by-treatment interaction, post-hoc analysis was performed using Tukey’s range test for multiple comparisons. All remaining statistical analyses were performed with Prism 6 for Windows (GraphPad, Software, La Jolla, CA, USA). Alpha was set at the 0.05 level.
RESULTS
Initial Rest Period
Initial USG values upon arrival were not different between trials (CON: 1.014 ± 0.007, DEH: 1.012 ± 0.007, SAL: 1.013 ± 0.006; P = 0.633). Ad libitum fluid intake in CON (613 ± 239 ml) and SAL (614 ± 184 ml) was greater than in DEH (253 ± 109 ml) (P = 0.002). Diuretic ingestion resulted in greater urine output in DEH (1997 ± 633 ml) compared to CON (566 ± 361 ml) and SAL (878 ± 507 ml) (P < 0.001). Consequently, blood volume declined to a greater extent during this time in DEH compared to CON and SAL due to a contraction of plasma volume, but not cell volume (P < 0.001, Table 1). Fluid intake, urine output, changes in blood, cell, and plasma volumes, and body mass changes were not different between CON and SAL during the initial rest period (P > 0.168).
Table 1.
Changes in blood volumes, plasma osmolality, and body mass throughout experimentation.
| Rest | Baseline | 60 min | End | ||
|---|---|---|---|---|---|
| CON | Δ Blood volume (%) | – | −1.7 ± 1.3 a | −2.3 ± 1.8 ab | −6.1 ± 2.9 ab |
| Δ Cell volume (%) | – | 0.5 ± 2.2 | −0.2 ± 2.0 | −0.7 ± 3.4 | |
| Δ Plasma volume (%) | – | −3.4 ± 2.0 a | −4.1 ± 3.0 ab | −10.4 ± 3.4 ab | |
| Plasma osmolality (mOsm kg−1) | 296 ± 5 | 293 ± 5 | 295 ± 3 | 297 ± 4 | |
| Δ Body mass (kg) | – | −0.3 ± 0.5 a | – | −1.1 ± 0.3 | |
| Δ Body mass (%) | – | −0.4 ± 0.6 a | – | −1.4 ± 0.4 | |
| DEH | Δ Blood volume (%) | – | −8.1 ± 2.5 ac | −9.0 ± 3.5 ac | −11.8 ± 2.8 ac |
| Δ Cell volume (%) | – | −0.5 ± 2.1 | −1.3 ± 1.5 | −1.8 ± 2.1 | |
| Δ Plasma volume (%) | – | −14.2 ± 5.7 ac | −15.3 ± 6.8 ac | −19.7 ± 5.1 ac | |
| Plasma osmolality (mOsm kg−1) | 293 ± 5 | 293 ± 4 | 296 ± 7 | 298 ±5 | |
| Δ Body mass (kg) | – | −2.0 ± 0.6 ac | – | −0.9 ± 0.4 | |
| Δ Body mass (%) | – | −2.4 ± 0.7 ac | – | −1.1 ± 0.6 | |
| SAL | Δ Blood volume (%) | – | −0.7 ± 2.6 c | 1.2 ± 3.4 bc | −1.6 ± 3.1bc |
| Δ Cell volume (%) | – | 0.6 ± 1.9 | 0.8 ± 2.2 | −0.3 ± 1.7 | |
| Δ Plasma volume (%) | – | −1.6 ± 5.0 c | 1.5 ± 6.8 bc | −2.7 ± 5.3 bc | |
| Plasma osmolality (mOsm kg−1) | 294 ± 4 | 290 ± 2 | 294 ± 5 | 296 ± 5 | |
| Δ Body mass (kg) | – | −0.4 ± 0.4 c | – | −1.1 ± 0.3 | |
| Δ Body mass (%) | – | −0.5 ± 0.5 c | – | −1.3 ± 0.4 |
Data are mean ± SD for seven subjects. CON, control condition without treatment; DEH, diuretic-induced dehydration; SAL, continuous saline infusion. Note: changes in blood, cell, and plasma volumes are expressed as percentage changes from rest; changes in body mass are expressed as absolute and percentage changes from rest to baseline (i.e. from arrival until the start of the incremental humidity protocol, “Baseline”) and from baseline to the end of the incremental humidity protocol (“End”).
a, b, and c indicate differences between CON and DEH, CON and SAL, and DEH and SAL, respectively (P < 0.05).
Incremental Humidity Protocol
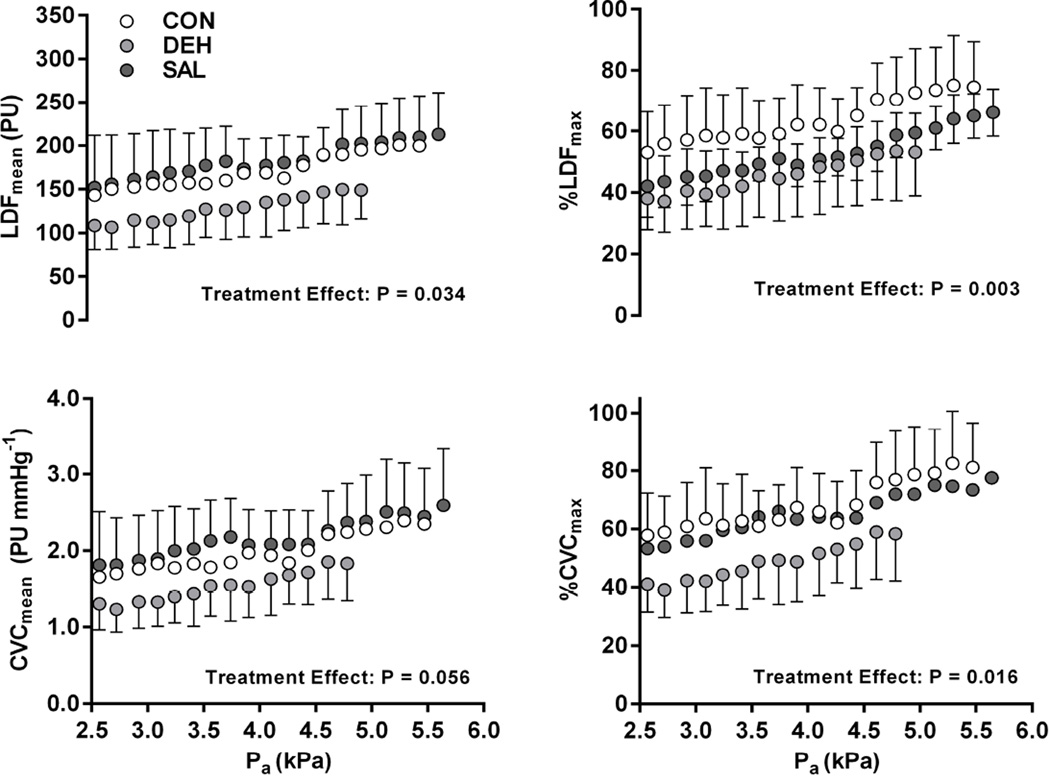
The greater reduction in blood and plasma volumes during DEH persisted throughout the incremental humidity protocol (P < 0.001), but no differences in plasma osmolality were observed between treatments (P = 0.546) (Table 1). This greater hypovolemia in DEH mitigated the increase in laser-Doppler SkBF during the protocol (Figure 2). Significant main effects of treatment were found for LDFmean (P = 0.034), %LDFmax (P = 0.003), and %CVCmax (P = 0.016), while CVCmean tended to show an effect of treatment (P = 0.056). Treatment-related effects resulted in an ~20% difference in each laser-Doppler index of SkBF between DEH and CON, as well as DEH and SAL (Figure 2), while no differences were observed between CON and SAL treatments. A similar pattern of change in all indices of SkBF were observed between forearm and thigh sites. No effect of treatment was evident for FBF and FVC (P ≥ 0.769).
Figure 2.
Laser-Doppler derived skin blood flow responses in a control condition without treatment (CON), following diuretic following diuretic-induced dehydration (DEH), and with continuous saline infusion (SAL). Four indices of skin blood flow are presented: mean absolute laser-Doppler flux (LDFmean), mean laser-Doppler flux expressed as a percentage of maximum values (%LDFmax), mean cutaneous vascular conductance (CVCmean), and mean cutaneous vascular conductance expressed as a percentage of maximum values (%CVCmax). Analyses were performed for seven subjects up to ~5 kPa (84 min), after which early termination criteria were met at different times. A significant main effect of treatment was observed for LDFmean, %LDFmax, and %CVCmax (P < 0.05). A trend towards an effect of treatment on CVCmean was also observed.
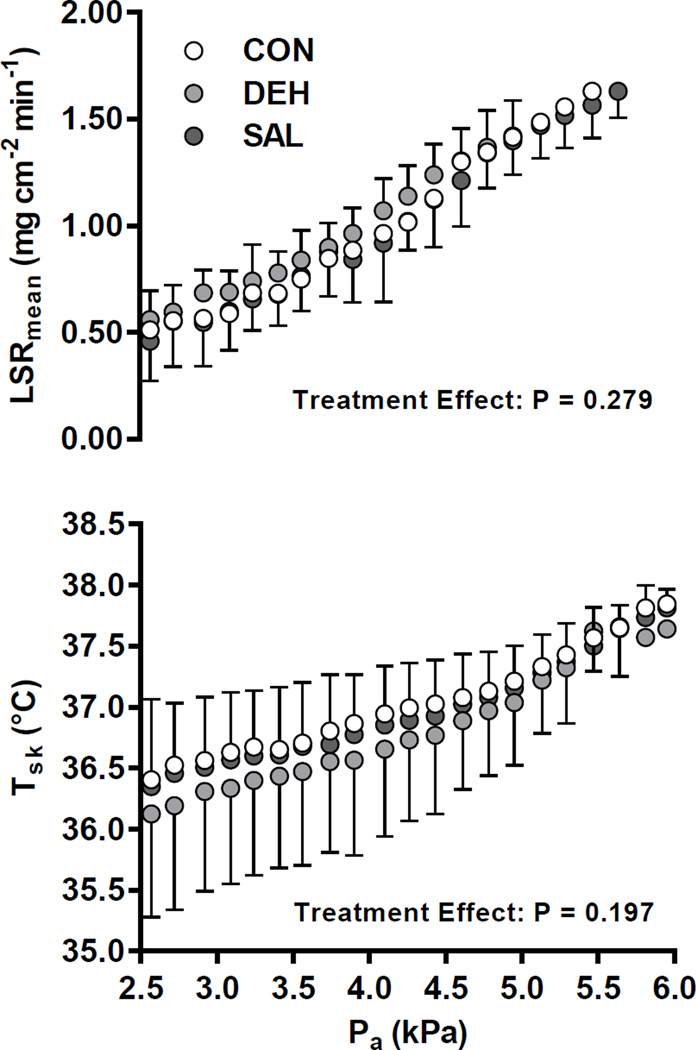
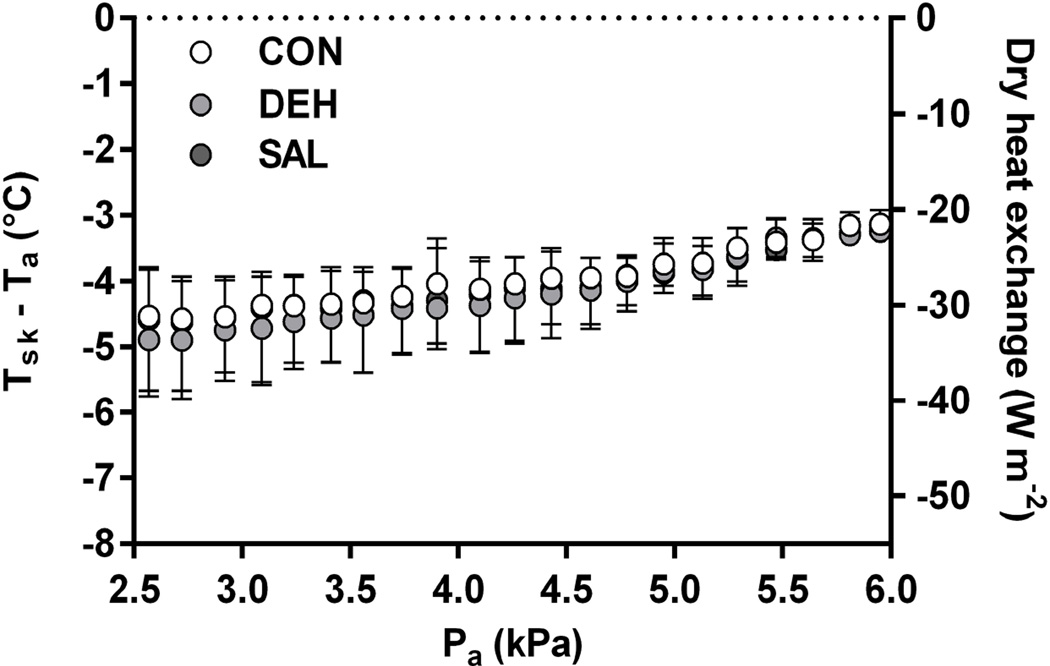
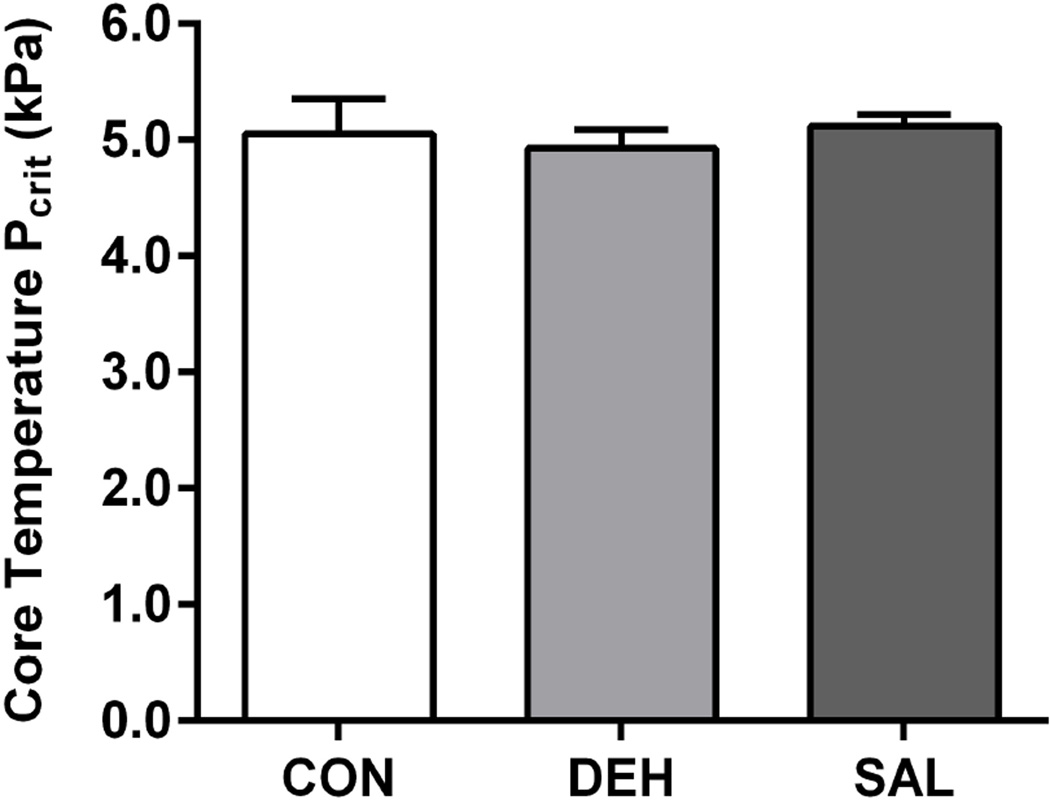
Despite the attenuation of SkBF, no treatment effect was observed for LSRmean (P = 0.279, Figure 3), while changes in body mass, indicating whole-body sweat loss, were not different between conditions (P = 0.482; Table 1). Similarly, no effects of treatment on Tsk (Figure 3), the Tsk − Ta thermal gradient, and the calculated rate of dry heat exchange (Figure 4) were found between trials (P > 0.193). Moreover, metabolic rate was not different between treatments, averaging 119 ± 13, 115 ± 15, and 109 ± 18 W in CON, DEH, and SAL, respectively (P = 0.121). Consequently, Pcrit for core temperature was not different across treatments (P = 0.166, Figure 5). A similar rate of increase in ΔTc was also observed post-inflection between trials (CON: 0.020 ± 0.003°C min−1, DEH: 0.019 ± 0.004°C min−1, SAL: 0.018 ± 0.002°C min−1; P = 0.353).
Figure 3.
Local sweating and skin temperature responses throughout the incremental humidity protocol in a control condition without treatment (CON), following diuretic-induced dehydration (DEH), and with continuous saline infusion (SAL). LSRmean, mean local sweat rate (2 sites: forearm, thigh); Tsk, mean skin temperature; Pa, ambient vapour pressure. Analysis was performed up to ~5 kPa (84 min) in seven subjects. Beyond this point, early termination criteria were met at different times. No treatment effect was observed for LSRmean and Tsk.
Figure 4.
The skin-air temperature gradient and the corresponding rate of dry heat exchange throughout the incremental humidity protocol in a control condition without treatment (CON), following diuretic-induced dehydration (DEH), and with continuous saline infusion (SAL). Tsk, mean skin temperature; Ta, ambient temperature; Pa, ambient vapour pressure. Data are for seven subjects. Note that negative values for dry heat exchange indicate dry heat gain. No effect of treatment was observed for Tsk − Ta and dry heat exchange (P > 0.193).
Figure 5.
Critical ambient vapour pressures (Pcrit) for core temperature in a control condition without treatment (CON), following diuretic-induced dehydration (DEH), and with continuous saline infusion (SAL) for seven subjects. No effect of treatment on Pcrit for core temperature was observed (P = 0.166).
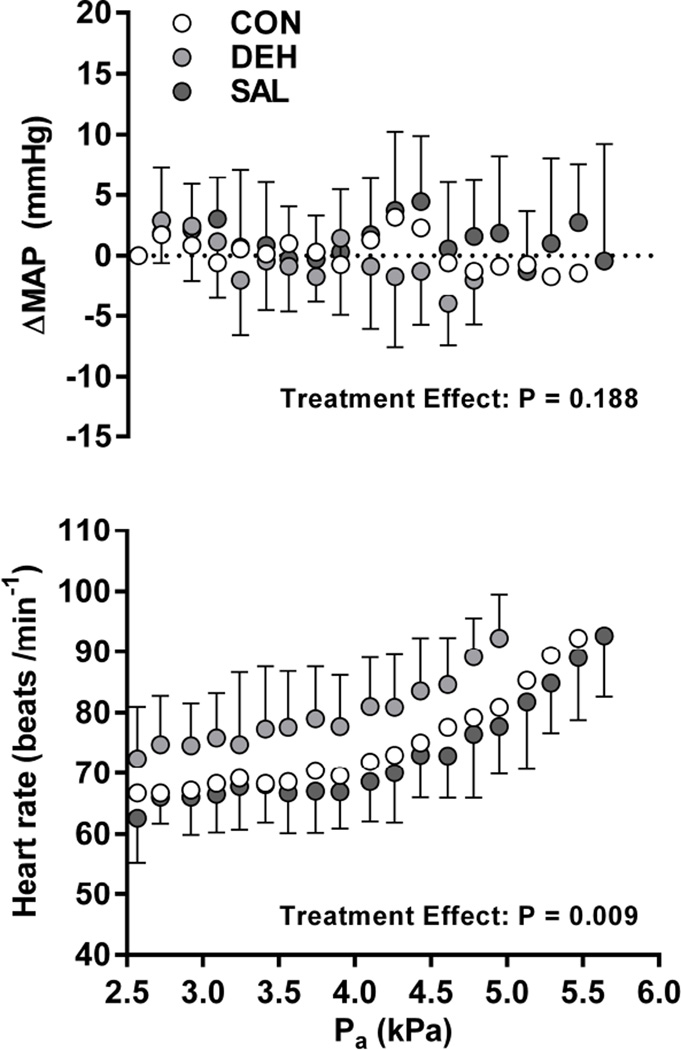
Baseline MAP was not different between treatments (CON: 87 ± 7, DEH: 84 ± 7, SAL: 84 ± 10 mmHg; P = 0.740), and no differences in ΔMAP were observed during the protocol (Figure 6; P = 0.189). Volume depletion caused a significantly higher HR throughout DEH compared to CON and SAL (P = 0.009, Figure 6), amounting to a consistent difference of 8 ± 2 beats/min in CON versus DEH, and 11 ± 2 beats min−1 in SAL versus DEH.
Figure 6.
Mean arterial pressure (MAP) and heart rate responses during the incremental humidity protocol in a control condition without treatment (CON), following diuretic following diuretic-induced dehydration (DEH), and with continuous saline infusion (SAL). ΔMAP, change in mean arterial pressure from baseline; Pa, ambient vapour pressure. Analyses were performed for seven subjects up to ~5 kPa (84 min), after which early termination criteria were met at different times. A significant main effect of treatment was observed for heart rate, but not ΔMAP.
DISCUSSION
In the present study, the influence of physiologically-relevant differences in SkBF on sweating and skin surface heat loss potential were assessed by employing an incremental humidity protocol that enabled us to identify the critical ambient vapour pressure (Pcrit) above which Tc could no longer be compensated. That is, above Pcrit, rates of metabolic heat production and dry heat gained from a 41°C environment could no longer be balanced by the total rate of evaporative heat loss from the skin. Using an iso-osmotic volume-depletion model, a ~20% reduction in SkBF had no effect on sweating (Figure 4) and ultimately the Pcrit for core temperature (Figure 6) compared to control and volume-infusion treatments, suggesting that sudomotor output and the upper limit of heat dissipation from the skin is not sufficiently compromised by an attenuated SkBF within the parameters presently employed.
Changes in body mass (−2.4%) and plasma volume (−14.0%) following oral furosemide administration, but prior to heat exposure, were consistent with those reported previously (Romero et al., 2011; Ikegawa et al., 2011). It is particularly noteworthy that the subsequent reduction in SkBF (Figure 3) was similar to that observed with exercise- (Montain & Coyle, 1992a) and diuretic-induced (Ikegawa et al., 2011) hypohydration compared to euhydration. Despite the lower SkBF in DEH (Figure 3), LSR (Figure 4), whole-body sweat rate, and Pcrit (Figure 6) were similar relative to CON and SAL, suggesting that maximum heat dissipation was not affected by lower SkBF. At a constant metabolic rate and ambient temperature, a difference in heat loss potential (i.e., Pcrit) secondary to altered SkBF would theoretically arise from changes in the physical properties of the skin surface—Tsk, maximum skin wettedness, and/or evaporative efficiency—that alter heat exchange with the environment. Specifically, if attenuated SkBF caused a lower Tsk, a wider skin-air temperature gradient would follow and the rate of dry heat gain (convection and radiation) would rise. Similarly, if SkBF compromised sweat production and/or efficiency, the rate of evaporative heat loss would be reduced. In the present study, since ambient temperature was constant (41°C) and Tsk was not different between treatments, no differences in the skin-air temperature gradient and the rate of dry heat exchange were observed (Figure 5). Furthermore, it stands to reason that evaporative heat loss was similar between conditions given the absence of any differences in local and whole-body sweating responses, Tsk, and the Pcrit for core temperature (Figures 4–6). Although a functional relationship between SkBF and sweating has been reported previously (Wingo et al., 2010), the supra-physiological reduction in local SkBF induced pharmacologically (noradrenaline infusion) in the study of Wingo et al. cannot be compared to the whole-body observations reported in the present study. Importantly, we show that physiologically-relevant reductions in SkBF, such as those that occur with ageing (Kenney et al., 1997; Minson et al., 1998), and disease (Kenney & Kamon, 1984; Green et al., 2006), are unlikely to affect whole-body heat loss potential.
Although this is the first study to investigate a direct link between altered SkBF and the potential for skin surface heat loss, previous data suggest that high levels of SkBF may not be advantageous for core temperature regulation. For example, a 15% reduction in FVC did not alter local sweat rates, Tsk, or ΔTc in hypoxic versus normoxic conditions (Miyagawa et al., 2011), or following a 2.6% reduction in body mass with diuretic-induced hypohydration (Ikegawa et al., 2011) during exercise. Additionally, chronic heart failure patients with a 20% lower CVC demonstrated similar ΔTc and Tsk (sweat rate was not measured) compared to healthy controls during non-encapsulated passive heat stress (38°C, 50% RH) (Green et al., 2006). Similarly, a 40% reduction in FBF in older versus younger individuals resulted in similar ΔTc, Tsk, or sweat rates during exercise in the heat despite similarities in the rate of metabolic heat production, body size, and aerobic fitness between groups (Kenney, 1988). Although this has not been a consistent finding in aged versus younger groups (Kenney et al., 1997), it is important to note that elderly individuals also demonstrate attenuated sudomotor responses and rates of evaporative heat loss, which perhaps contribute to greater ΔTc independently of any parallel reductions in SkBF (Anderson & Kenney, 1987; Larose et al., 2013). Collectively, current and previous data suggest that core temperature is regulated similarly despite a ≥15% lower SkBF, provided that sweating and evaporative heat loss are not physiologically limited. It follows that populations demonstrating concurrent vasodilatory and sudomotor impairments, such as the elderly (Minson et al., 1998), type-2 diabetics (Wick et al., 2006; Sokolnicki et al., 2009), skin graft recipients (Davis et al., 2007, 2009), and spinal cord injury victims (Freund et al., 1984), may be at greater risk of thermal injury during severe heat stress due to problems associated with sudomotor function and/or evaporation, and perhaps not due to impairments of skin blood flow per se.
In many previous studies, physiological regulation of cutaneous vasodilatation and SkBF has been examined during passive heating to a fixed elevation in core temperature using a water-perfused suit [e.g., (Freund et al., 1984; Minson et al., 1998; Wick et al., 2006; Davis et al., 2007, 2010)], which controls skin temperature under the suit at a set level (~38°C) and simultaneously impedes evaporative heat loss over most of the skin surface. Given the mostly encapsulated nature of water-perfusion suit studies, the influence of SkBF on sweating and whole-body heat loss cannot be established with this experimental approach. Similarly, studies conducted during exercise (Montain & Coyle, 1992a, 1992b) or non-encapsulated passive heat stress (Green et al., 2006) with submaximal heat loss requirements would not necessarily observe any effect on core temperature as any reductions in skin surface heat loss due to lower SkBF are likely to be physiologically compensated by increased sweat production and evaporation. In contrast, the incremental humidity protocol used presently permits the evaluation of whole-body heat loss potential in non-encapsulated conditions of high environmental heat stress. The basis for this approach lies in examining how various factors/interventions affect the heat loss side of the conceptual heat balance equation. Since the rate of heat storage is equal to the difference between rates of heat production and loss, Pcrit represents the threshold ambient vapour pressure above which thermal balance (i.e., a rate of heat storage equal to zero) cannot be maintained. Therefore, a shift in Pcrit at a fixed metabolic heat production and ambient temperature, attributed to the factor/intervention in question, directly indicates a change in maximum whole-body skin surface heat loss. The current experiment represents a novel use of this classic protocol (Belding & Kamon, 1973).
Although the current iteration of the incremental humidity protocol used similar stepwise increases in Pa (~0.17 kPa per stage) and stage durations (6 min) as in previous studies (Belding & Kamon, 1973; Kenney et al., 1993; Ravanelli et al., 2015), it could be argued that the present approach may lack the sensitivity required to detect differences in Pcrit secondary to a reduction in SkBF. One way to demonstrate the sensitivity of the current protocol is through the effect of stepwise increases in Pa on the skin wettedness required for heat balance (Gagge, 1937). Theoretically, skin wettedness reflects the fraction of the skin surface that must be covered with sweat to achieve a certain rate of evaporative heat loss, while maximum skin wettedness refers to the value of skin wettedness at the threshold for compensability (i.e., Pcrit). Presently, stepwise increases in Pa of 0.17 kPa corresponded to average elevations in skin wettedness of 0.05 ± 0.02 (range: 0.02–0.10). Given that interventions (e.g. heat acclimation) alter maximum skin wettedness by ~0.15, a possible shift in the maximum skin wettedness by < 0.05 (and thus Pcrit) by an attenuated SkBF response would have been trivial. Furthermore, a lack of any effect of lowered SkBF on Pcrit is supported by a similar rate of change in core temperature above Pcrit. If a true shift in Pcrit to a lower ambient vapour pressure due to lowered SkBF was not detected using the present analysis, a higher rate of change in core temperature would have been expected above Pcrit in the DEH trial; however, this was not the case.
Continuous or rapid saline infusion has been an effective approach to elevate SkBF above non-infusion levels, resulting in a higher FBF during exercise (Nose et al., 1990). The lack of any effect of saline infusion (0.05 ml kg−1 min−1) on indices of SkBF, LSRmean, Tsk, and ultimately ΔTc (Figures 2 and 5) in the present study is perhaps explained by the relatively modest infusion rate compared to previous studies. While a higher infusion rate could have shifted Pcrit to a higher value by affecting SkBF and sweating, this possibility requires further investigation.
Estimates of SkBF are typically expressed in a variety of ways, including absolute LDF or FBF, CVC or FVC values that are normalized to arterial blood pressure, or as a percentage of maximum LDF or CVC. While indices of SkBF expressed relative to blood pressure or a percentage of maximum flux indicate aspects of vasomotor control, it must be recognized that heat exchange between cutaneous blood and skin tissue will be dependent on the absolute level of blood flow through cutaneous vessels, as well as the blood-skin thermal gradient, not absolute CVC or CVC normalized to maximum values. Absolute SkBF was represented herein by the LDFmean value, which was consistently lower in DEH, while %LDFmax, CVCmean, and %CVCmax were also included given the conventional use of these parameters (Figure 2). Regardless of how it was expressed, interpretation of the SkBF data was similar. Absolute SkBF was also estimated using venous occlusion plethysmography to determine FBF in the present study; however, unlike previous investigations using a volume-depletion experimental model (Nadel et al., 1980; Fortney et al., 1983), no treatment effect was detected. Previously, Johnson et al. (1984) demonstrated good agreement between LDF and FBF values measured during passive heating. However, in that study, the experimental conditions were very different to those of the present investigation; specifically, LDF and FBF measurements would have been performed on an arm exposed to the ambient air, with skin much cooler than skin under the heating garment (Johnson et al., 1984). As such, LDF and FBF values would have represented reflex drive for vasodilatation only. While the severity of the heat stress imposed in the present study would have likely induced reflex- and locally-mediated cutaneous vasodilation, there is no conspicuous reason for the present discrepancy between laser-Doppler and plethysmographic measurements, unless alterations in cutaneous blood flow were balanced by changes in blood flow through underlying tissues. Nevertheless, the consistent attenuation of LDFmean observed among all subjects during DEH in the present study (Figure 2) suggests that blood flow in cutaneous vascular beds was consistently altered as expected.
Limitations and Future Research
Due to instances of hypotension following volume depletion, body posture was adjusted from an upright seated position to a supine posture (note: seven total subjects were tested, with four seated in the upright and three supine). Although postural differences with heat stress influence baroreflex control of SkBF (Lind et al., 1968), it should be noted that (i) all analyses were performed within-subject with the position of a given subject consistent between all three trials; (ii) perturbations that unload baroreceptors do not appear to alter thermoregulatory sweat rate (Wilson et al., 2005; Kenny et al., 2010; Lynn et al., 2012); and (iii) comparisons within posture still revealed no effect of treatment on Pcrit for core temperature, metabolic rate, whole-body sweat rate, LSRmean, and Tsk; (iv) the slightly higher Pcrit for core temperature in the upright seated position (CON: 5.2 ±0.1, DEH: 5.0 ± 0.2, SAL: 5.2 ± 0.1 kPa; n=4) compared to the supine posture (CON: 4.9 ±0.4, DEH: 4.9 ± 0.1, SAL: 5.1 ± 0.1 kPa; n=3) can be explained by the greater effective skin area from which heat loss could occur while seated.
The applicability of the present findings is limited to healthy young male subjects at one high ambient temperature (~41°C), under resting conditions, and with minimal clothing. Future studies should examine the implications of lower SkBF on physiological compensability to environmental heat stress in special populations with well-known impairments of cutaneous vasodilatation, between sexes, at different ambient temperatures, with various clothing ensembles, and even during exercise at different metabolic rates.
Conclusion
The present study examined whether physiologically relevant reductions in SkBF alter sweating and subsequently lower the potential for skin surface heat loss during non-encapsulated passive whole-body heat stress. Despite a ~20% lower SkBF achieved using a volume-depletion model, sweating and heat loss potential were not altered compared to control and continuous saline infusion treatments.
New Findings.
What is the central question of this study?
Does attenuated skin blood flow (SkBF) diminish sweating and reduce the critical environmental limit for heat balance, which indicates maximum heat loss potential, during severe heat stress?
What is the main finding and its importance?
Iso-osmotic hypovolaemia attenuated SkBF by ~20%, but did not result in different sweating rates, mean skin temperatures, or critical environmental limits for heat balance compared to control and volume-infusion treatments, suggesting that lower levels of skin blood flow commonly observed in aged and diseased populations may not diminish maximum whole-body heat dissipation.
Acknowledgments
This study was performed by M.N. Cramer in partial fulfilment of the degree Doctor of Philosophy from the University of Ottawa. The authors are grateful for the participation of all volunteers; to Amy Adams, M.S., Naomi Kennedy, R.N., and Drs. Paula Poh, Steven Romero, and Zachary Schlader for assistance with data collection; and to Dr. Robert Kenefick for his valuable input in the development of the current protocol.
Funding: This research was supported by National Institutes of Health R01GM068865 (C.G.C), Department of Defense W81XWH-12-1-0152 (C.G.C.), and by a Discovery Grant (#386143-2010) from the Natural Sciences and Engineering Research Council (O.J). M.N.C. was supported by an Excellence Scholarship and a Student Mobility Bursary from the Faculty of Graduate and Postdoctoral Studies at University of Ottawa, as well as an Ontario Graduate Scholarship (OGS). D.G. was supported by a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship.
ABBREVIATIONS
- C
rate of convective heat exchange
- CON
control trial
- CVC
cutaneous vascular conductance
- DEH
dehydration trial
- FBF
forearm blood flow
- FVC
forearm vascular conductance
- LDF
laser Doppler flux
- LSR
local sweat rate
- M
metabolic rate
- MAP
mean arterial pressure
- Pcrit
critical ambient vapour pressure
- R
rate of radiant heat exchange
- SAL
saline infusion trial
- SkBF
skin blood flow
- Tc
core temperature
- Tsk
mean skin temperature
- VO2
rate of oxygen uptake
Footnotes
Competing interests: The authors report no competing interests.
Author contributions: All experiments were conducted at the Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital of Dallas. M.N.C., D.G., C.G.C, and O.J. conceived and designed experiments; M.N.C. and D.G. conducted experiments; M.N.C analysed data; M.N.C., D.G., C.G.C, and O.J. interpreted data; M.N.C. drafted manuscript; M.N.C., D.G., C.G.C, and O.J. edited, revised, and approved final draft of the manuscript.
REFERENCES
- Anderson RK, Kenney WL. Effect of age on heat-activated sweat gland density and flow during exercise in dry heat. J Appl Physiol. 1987;63:1089–1094. doi: 10.1152/jappl.1987.63.3.1089. [DOI] [PubMed] [Google Scholar]
- Belding HS, Kamon E. Evaporative coefficients for prediction of safe limits in prolonged exposures to work under hot conditions. Fed Proc. 1973;32:1598–1601. [PubMed] [Google Scholar]
- Carberry PA, Shepherd AM, Johnson JM. Resting and maximal forearm skin blood flows are reduced in hypertension. Hypertension. 1992;20:349–355. doi: 10.1161/01.hyp.20.3.349. [DOI] [PubMed] [Google Scholar]
- Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol. 2010;109:1221–1228. doi: 10.1152/japplphysiol.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Arbab-Zadeh A, Prasad A, Durand S, Levine BD, Crandall CG. Effects of heat stress on thermoregulatory responses in congestive heart failure patients. Circulation. 2005;112:2286–2292. doi: 10.1161/CIRCULATIONAHA.105.540773. [DOI] [PubMed] [Google Scholar]
- Davis SL, Korkmas MA, Crandall CG, Frohman EM. Impaired sweating in multiple sclerosis leads to increased reliance on skin blood flow for heat dissipation (Abstract) FASEB J. 2010;24:991.25. [Google Scholar]
- Davis SL, Shibasaki M, Low DA, Cui J, Keller DM, Purdue GF, Hunt JL, Arnoldo TBD, Kowalske KJ, Crandall CG. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res. 2007;28:427–434. doi: 10.1097/BCR.0B013E318053D312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SL, Shibasaki M, Low DA, Cui J, Keller DM, Wingo JE, Purdue GF, Hunt JL, Arnoldo BD, Kowalske KJ, Crandall CG. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery. J Burn Care Res. 2009;30:675–685. doi: 10.1097/BCR.0b013e3181abfd43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Dougherty KA, Chow M, Kenney WL. Critical environmental limits for exercising heat-acclimated lean and obese boys. Eur J Appl Physiol. 2010;108:779–789. doi: 10.1007/s00421-009-1290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizondo RS. Local control of eccrine sweat gland function. Fed Proc. 1973;32:1583–1587. [PubMed] [Google Scholar]
- Fortney SM, Wenger CB, Bove JR, Nadel ER. Effect of blood volume on forearm venous and cardiac stroke volume during exercise. J Appl Physiol. 1983;55:884–890. doi: 10.1152/jappl.1983.55.3.884. [DOI] [PubMed] [Google Scholar]
- Fortney SM, Wenger CB, Bove JR, Nadel ER. Effect of hyperosmolality on control of blood flow and sweating. J Appl Physiol. 1984;57:1688–1695. doi: 10.1152/jappl.1984.57.6.1688. [DOI] [PubMed] [Google Scholar]
- Freund PR, Brengelmann GL, Rowell LB, Halar E. Attenuated skin blood flow response to hyperthermia in paraplegic men. J Appl Physiol. 1984;56:1104–1109. doi: 10.1152/jappl.1984.56.4.1104. [DOI] [PubMed] [Google Scholar]
- Gagge AP. A New Physiological Variable Associated with Sensible and Insensible Perspiration. Am J Physiol. 1937;120:277–287. [Google Scholar]
- Green DJ, Maiorana AJ, Siong JHJ, Burke V, Erickson M, Minson CT, Bilsborough W, O’Driscoll G. Impaired skin blood flow response to environmental heating in chronic heart failure. Eur Heart J. 2006;27:338–343. doi: 10.1093/eurheartj/ehi655. [DOI] [PubMed] [Google Scholar]
- Guibert A, Taylor CL. Radiation area of the human body. J Appl Physiol. 1952;5:24–37. doi: 10.1152/jappl.1952.5.1.24. [DOI] [PubMed] [Google Scholar]
- Ikegawa S, Kamijo Y, Okazaki K, Masuki S, Okada Y, Nose H. Effects of hypohydration on thermoregulation during exercise before and after 5-day aerobic training in a warm environment in young men. J Appl Physiol. 2011;110:972–980. doi: 10.1152/japplphysiol.01193.2010. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology. Environmental Physiology. Bethesda, MD: American Physiological Society; 1996. pp. 215–243. MD: Am. Physiol. Soc. 1996. [Google Scholar]
- Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol. 1984;56:798–803. doi: 10.1152/jappl.1984.56.3.798. [DOI] [PubMed] [Google Scholar]
- Kamon E, Avellini B. Wind speed limits to work under hot environments for clothed men. J Appl Physiol. 1979;46:340–345. doi: 10.1152/jappl.1979.46.2.340. [DOI] [PubMed] [Google Scholar]
- Kamon E, Avellini B, Krajewski J. Physiological and biophysical limits to work in the heat for clothed men and women. J Appl Physiol. 1978;44:918–925. doi: 10.1152/jappl.1978.44.6.918. [DOI] [PubMed] [Google Scholar]
- Kenefick RW, Cheuvront SN. Hydration for recreational sport and physical activity. Nutr Rev. 2012;70(Suppl 2):S137–S142. doi: 10.1111/j.1753-4887.2012.00523.x. [DOI] [PubMed] [Google Scholar]
- Kenney WL. Control of heat-induced cutaneous vasodilatation in relation to age. Eur J Appl Physiol Occup Physiol. 1988;57:120–125. doi: 10.1007/BF00691250. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Kamon E. Comparative physiological responses of normotensive and essentially hypertensive men to exercise in the heat. Eur J Appl Physiol. 1984;52:196–201. doi: 10.1007/BF00433392. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Mikita DJ, Havenith G, Puhl SM, Crosby P. Simultaneous derivation of clothing-specific heat exchange coefficients. Med Sci Sports Exerc. 1993;25:283–289. [PubMed] [Google Scholar]
- Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol. 1997;272:H1609–H1614. doi: 10.1152/ajpheart.1997.272.4.H1609. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Zeman MJ. Psychrometric limits and critical evaporative coefficients for unacclimated men and women. J Appl Physiol. 2002;92:2256–2263. doi: 10.1152/japplphysiol.01040.2001. [DOI] [PubMed] [Google Scholar]
- Kenny GP, Gagnon D, Shiff D, Armstrong R, Journeay WS, Kilby D. Influence of nonthermal baroreceptor modulation of heat loss responses during uncompensable heat stress. Eur J Appl Physiol. 2010;108:541–548. doi: 10.1007/s00421-009-1255-7. [DOI] [PubMed] [Google Scholar]
- Kerslake DM. The Stress of Hot Environments. London: Cambridge University Press; 1972. [PubMed] [Google Scholar]
- Larose J, Wright HE, Sigal RJ, Boulay P, Hardcastle S, Kenny GP. Do older females store more heat than younger females during exercise in the heat? Med Sci Sports Exerc. 2013;45:2265–2276. doi: 10.1249/MSS.0b013e31829d24cc. [DOI] [PubMed] [Google Scholar]
- Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol. 1968;25:268–276. doi: 10.1152/jappl.1968.25.3.268. [DOI] [PubMed] [Google Scholar]
- Lynn AG, Gagnon D, Binder K, Boushel RC, Kenny GP. Divergent roles of plasma osmolality and the baroreflex on sweating and skin blood flow. Am J Physiol Regul Integr Comp Physiol. 2012;302:R634–R642. doi: 10.1152/ajpregu.00411.2011. [DOI] [PubMed] [Google Scholar]
- Mekjavic IB, Rempel ME. Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol. 1990;69:376–379. doi: 10.1152/jappl.1990.69.1.376. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Mitchell D. Convective heat transfer from man and other animals. In: Monteith JL, Mounts LE, editors. Heat Loss from Animals and Man. London: Butterworths; 1974. [Google Scholar]
- Miyagawa K, Kamijo Y-I, Ikegawa S, Goto M, Nose H. Reduced hyperthermia-induced cutaneous vasodilation and enhanced exercise-induced plasma water loss at simulated high altitude (3,200 m) in humans. J Appl Physiol. 2011;110:157–165. doi: 10.1152/japplphysiol.00950.2010. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Coyle EF. Fluid ingestion during exercise increases skin blood flow independent of increases in blood volume. J Appl Physiol. 1992a;73:903–910. doi: 10.1152/jappl.1992.73.3.903. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Coyle EF. Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J Appl Physiol. 1992b;73:1340–1350. doi: 10.1152/jappl.1992.73.4.1340. [DOI] [PubMed] [Google Scholar]
- Nadel ER, Fortney SM, Wenger CB. Effect of hydration state of circulatory and thermal regulations. J Appl Physiol. 1980;49:715–721. doi: 10.1152/jappl.1980.49.4.715. [DOI] [PubMed] [Google Scholar]
- Nose H, Mack GW, Shi XR, Morimoto K, Nadel ER. Effect of saline infusion during exercise on thermal and circulatory regulations. J Appl Physiol. 1990;69:609–616. doi: 10.1152/jappl.1990.69.2.609. [DOI] [PubMed] [Google Scholar]
- Randall WC, Deering R, Dougherty I. Reflex sweating and the inhibition of sweating by prolonged arterial occlusion. J Appl Physiol. 1948;1:53–59. doi: 10.1152/jappl.1948.1.1.53. [DOI] [PubMed] [Google Scholar]
- Ravanelli NM, Hodder SG, Havenith G, Jay O. Heart rate and body temperature responses to extreme heat and humidity with and without electric fans. JAMA. 2015;313:724–725. doi: 10.1001/jama.2015.153. [DOI] [PubMed] [Google Scholar]
- Romero SA, Moralez G, Rickards CA, Ryan KL, Convertino VA, Fogt DL, Cooke WH. Control of cerebral blood velocity with furosemide-induced hypovolemia and upright tilt. J Appl Physiol. 2011;110:492–498. doi: 10.1152/japplphysiol.01060.2010. [DOI] [PubMed] [Google Scholar]
- Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol. 2009;106:566–570. doi: 10.1152/japplphysiol.91289.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Whitney RJ. The measurement of volume changes in human limbs. J Physiol. 1953;121:1–27. doi: 10.1113/jphysiol.1953.sp004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick DE, Roberts SK, Basu A, Sandroni P, Fealey RD, Sletten D, Charkoudian N. Delayed threshold for active cutaneous vasodilation in patients with Type 2 diabetes mellitus. J Appl Physiol. 2006;100:637–641. doi: 10.1152/japplphysiol.00943.2005. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Mean body temperature does not modulate eccrine sweat rate during upright tilt. J Appl Physiol Bethesda Md 1985. 2005;98:1207–1212. doi: 10.1152/japplphysiol.00648.2004. [DOI] [PubMed] [Google Scholar]
- Wingo JE, Low DA, Keller DM, Brothers RM, Shibasaki M, Crandall CG. Skin blood flow and local temperature independently modify sweat rate during passive heat stress in humans. J Appl Physiol. 2010;109:1301–1306. doi: 10.1152/japplphysiol.00646.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]