Abstract
Background
Prader-Willi Syndrome (PWS) is associated with hyperphagia and hyperghrelinemia with major morbidity due to obesity without effective medical treatment targeting hyperphagia. Exenatide [Byetta (synthetic Exendin-4); AstraZeneca, Wilmington DE] is a GLP-1 receptor agonist which reduces appetite and weight, and may be an effective treatment in PWS.
Objective
To determine the effect of a 6-mo trial of exenatide on appetite, weight, and gut hormones in youth with PWS.
Methods
Ten overweight and obese subjects with PWS (13-25 yr) were recruited for a 6-mo open-label, non-randomized, longitudinal study conducted at Children's Hospital Los Angeles. Exenatide was given using standard diabetes dosing without dietary modifications. Weight, BMI, truncal fat, appetite, and plasma acylated ghrelin were measured over 6 mo. Mixed meal tolerance tests were done at 0 and 6 mo.
Results
Appetite scores significantly decreased from baseline (32.2 ± 8.7) after 1, 3, and 6 mo of treatment (27.5 ± 8.8, 25.4 ± 9.3, 25.4 ± 7.2, respectively; p=0.004). Hemoglobin A1c decreased significantly after treatment, but weight, BMI z-score, and adiposity did not. There was no significant change in ghrelin.
Conclusion
This is the first longitudinal investigation of the effects of exenatide in subjects with PWS. It was effective in decreasing appetite, without change in weight or BMI in the short term. Larger, controlled, longer-term trials in patients with PWS are needed to confirm the efficacy and safety of exenatide, and to evaluate whether its use might induce weight loss when given in conjunction with behavioral modification.
Keywords: Prader-Willi Syndrome, exenatide, Byetta, hyperphagia, appetite, ghrelin
Introduction
Prader-Willi Syndrome (PWS) occurs in 1:15,000 to 1:30,000 births worldwide (1) due to loss of the paternal allele at 15q11.2-13 (2). Between birth and 2 years, affected children typically manifest hypotonia, delayed motor development, and failure-to-thrive. After this age, an insatiable appetite and progressive obesity ensue (3). Major morbidity and mortality in PWS are due to complications from uncontrolled obesity (4). Relentless food-seeking behavior in PWS can be disruptive to home and school activities, causing families social and psychological turmoil. Currently, there is no effective medical therapy of hyperphagia in individuals with PWS.
PWS's obesity phenotype is unique, including delayed meal termination, earlier return of hunger, increased food consumption, hoarding and stealing of food, pica, delayed gastric emptying, and reduced vomiting (5, 6). Decreased resting energy expenditure compared to the general population leads to weight gain without appropriate food restriction (7). Weight control is difficult, and current treatment includes behavioral modifications, calorie reduction, exercise, close supervision, and restriction from food and money (8). Although the etiology of hyperphagia in PWS is not fully elucidated, it has been postulated that the distinctive hyperghrelinemia in PWS is related to their obesity. Ghrelin is an orexigenic hormone produced predominantly in the stomach with circulating concentrations normally elevated when fasting and decreased after eating. Elevated ghrelin levels in rodents and humans are associated with increased appetite, food consumption, and weight gain (9, 10). Multiple studies show elevated plasma ghrelin levels in subjects with PWS at all ages (11, 12, 13, 14). As hyperphagia seems to be the primary basis of obesity in PWS, therapy targeting components underlying hyperphagia is needed to alleviate the burden of obesity in PWS.
Exenatide [Byetta™; AstraZeneca, Wilmington DE] is a GLP-1 receptor agonist and incretin-mimetic FDA-approved as adjunctive treatment of type 2 diabetes in adults. In addition, exenatide has resulted in persistent weight loss in animals and obese adults with and without type 2 diabetes (15, 16). Modest reductions in body mass index (BMI) in obese adolescents without diabetes on exenatide have been shown, although the same reduction was not seen in the placebo group during an open label extension (17). Exendin-4 (exenatide) has decreased ghrelin levels up to 74% for a period of 8 hr after administration to fasted rodents (18). Additionally, in obese subjects, intravenous exenatide decreased hyperactivation of brain responses to food cues in areas associated with reward and appetite, suggesting a central mechanism in appetite regulation (19). A case report showed successful weight loss in a female with PWS treated with exenatide (20). Another study showed increased satiety in adults with PWS after a single dose of exenatide, with fewer side effects in PWS subjects than obese controls, although ghrelin levels were not affected (21). Despite these promising data, there have been no published studies on long-term use of exenatide in a large population of individuals with PWS. To determine if exenatide decreases appetite and weight in subjects with PWS, we designed a pilot study to examine its effects in adolescents and young adults with PWS over a 6-mo period.
Methods
Subjects
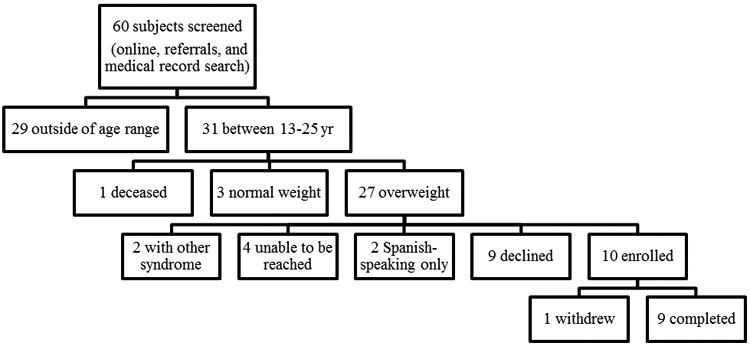
The study was conducted at Children's Hospital Los Angeles (CHLA). Potential subjects were identified from endocrinology clinics at CHLA and Children's Hospital of Orange County (CHOC), and through the Prader-Willi Syndrome Association (http://www.pwsausa.org/). Enrollment criteria included: age 13-25 yr, body mass index (BMI) ≥ 85th percentile for subjects <18 yr or ≥ 25 kg/m2 for subjects ≥ 18 yr, genetic confirmation of PWS. Subjects were excluded if they had: previously used GLP-1 receptor agonists, other syndromic illness, prior medical or family history of pancreatitis or renal failure, change in doses of testosterone, estrogen, or growth hormone > 25% of dose/kg/day within the past 6 mo. No subjects were on appetite or weight altering medications other than metformin in those with type 2 diabetes. Once consented, subjects were seen for an initial screening visit which included review of past medical history, physical examination, and safety laboratory tests (comprehensive metabolic profile, amylase, lipase, and serum hCG if female). Ten subjects (4 male/6 female, age range 14.7-24.6 yr, age mean 18 yr, age median 16 yr) were recruited (Figure 1). Race of subjects were: 6 non-Hispanic white, 1 non-Hispanic black, 1 Mexican-American, and 2 other or mixed race. Nine subjects completed the study, and one subject withdrew after 1 mo due to transportation difficulties. Three subjects (including withdrawal subject) had type 2 diabetes at enrollment. Diabetes medication doses (rapid-acting insulin, long-acting insulin, and metformin) were stable at least 3 months prior to enrollment and no changes were made during the study. Reasons for refusal to enroll in the study included: difficulty with travel (n=2), unwillingness to participate in research (n=6), unwillingness to take injectable medications (n=1).
Figure 1. Screening and Enrollment of Subjects.
Study Procedures
The study protocol was approved by the Committee on Clinical Investigations, the institutional review board of CHLA, and registered on clinicaltrials.gov (NCT01444898) prior to enrollment. Signed parental consents and age-appropriate assents were obtained. Study visits occurred at baseline, and 1, 3, and 6 mo after starting exenatide, each in the morning after > 8-hr fast. Each visit included a history, physical examination, appetite questionnaire, and safety- and obesity-related laboratory tests [hemoglobin A1c (HbA1c), insulin, acylated ghrelin, leptin, and pancreatic polypeptide (PP)]. Standing heights (without shoes) were determined at each visit using a digital wall-mounted stadiometer, accurate to within 0.1 cm, and weight was measured using a digital scale to the nearest 0.1 kg, with the subjects in light clothing.
At baseline and 6-mo visits, subjects underwent a mixed meal tolerance test (MMTT). Boost® was given at 6 mL/kg up to 360 mL. Blood was drawn for glucose, acylated ghrelin, and PP measurements at baseline (fasting) and +15, +60, +120 min. Dual X-ray absorptiometry (DEXA) evaluation for body composition was done at baseline and 6 mo (Hologic Delphi W Bone Densitometer [Bedford, MA]). Truncal fat was analyzed as total body fat was unable to be measured accurately in subjects larger than scan perimeters.
As subjects had adult weights > 69 kg at baseline, standard FDA-approved adult diabetes dosing of exenatide was used; 5 mcg twice daily subcutaneous (SQ) injections by pen device was started on the evening of the baseline visit, increased to 10 mcg twice daily at the 1-mo visit, and continued thereafter. Exenatide was given 60 min prior to morning and evening meals. Subjects received the dose of exenatide the evening prior to the 1-, 3-, and 6-mo study visits but not in the morning.
Hyperphagia Questionnaire
A syndrome-specific validated hyperphagia questionnaire (22) was completed by the same caretaker familiar with the subject's daily routine. It was done at the beginning of each visit, including those with a MMTT, and scored according to previously published guidelines. The scores were tallied as a total, and sub-divided into behavior, drive, and severity components.
Study Medication Adherence
Subjects returned used pens at follow-up study visits and adherence was inferred by calculating the percent of medication used. Categorization included: high (≥ 80% medication used), moderate (50-80% medication used), low (<50% medication used).
Assays
Comprehensive metabolic panel analytes, amylase, lipase, insulin (using chemiluminescent immunoassay), hCG, and HbA1c were measured at Quest Laboratories (West Hills, CA) using commercially available assays. Acylated ghrelin, leptin, and PP were measured using enzyme-linked immunosorbent assays (EMD Millipore, Billerica, MA). Samples for acylated ghrelin were collected in EDTA tubes containing 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride and hydrochloric acid was later added. Samples were stored at -80° C for batch analyses.
Safety Monitoring
At each visit, adverse event monitoring was done. Serum amylase and lipase levels were measured to evaluate pancreatic function. Renal function was monitored by calculating estimated creatinine clearance using the Cockcroft-Gault formula (23).
Statistics
Prior to analysis, distributions were evaluated for normality and natural log transformation was performed to analyze data not normally distributed. Data are presented as mean ± SD unless not normally distributed, in which case they are presented as median with intra-quartile ranges (25th and 75th percentiles). Within-subject changes between visits were analyzed by mixed model repeated measures. When the overall F-test for difference among visits was significant, Dunnett-adjusted pairwise comparisons were made between baseline and each subsequent visit. MMTT data was analyzed both by linear mixed model measures as well as area-under-the-curve (AUC). Statistical analysis was done with SAS/STAT® software v9.2 (Cary, NC).
Results
Anthropometrics and Adiposity
Exenatide treatment was not associated with a significant change in mean weight, BMI, or BMI z-score over time (Table 1, Supplemental Figure 1). There was no significant change in mean BMI percent change from baseline at 1 mo (0.6% ± 1.8%, p=0.4), 3 mo (0.6% ± 2.9%, p=0.5), or 6 mo (1.3% ± 2%, p=0.08). There was no significant change in adiposity from baseline as measured by DEXA. Mean baseline truncal fat was 20 ± 4.6 kg and mean percent truncal fat 46.3 ± 6.4%. Mean 6-mo truncal fat was 19.3 ± 4.2 kg and mean percent truncal fat 44.7 ± 6.8 % (p=0.4 and 0.1, respectively).
Table 1. Anthropometrics, Fasting HbA1c, Insulin, Leptin, Acylated Ghrelin, and PP over Time †.
| Baseline | 1 mo | 3 mo | 6 mo | P-Value across Visits | |
|---|---|---|---|---|---|
| Weight (kg) | 102.5 ± 18.6 | 103.3 ± 18.3 | 101.6 ± 19.2 | 102.0 ± 17.7 | 0.07 |
| BMI (kg/m2) | 41.7 (34.1, 55.0) | 41.8 (33.9, 54.9) | 41.5 (34.5, 46.5) | 43.1 (33.7, 46.4) | 0.9* |
| BMI Z-Score | 3.5 ± 1.1 | 3.5 ± 1.1 | 3.3 ± 1.1 | 3.6 ± 0.7 | 0.8 |
| HbA1c (%) | 5.9 (5.6, 7.9) | 5.8 (5.1, 5.9) | 5.8 (5.3, 6.0) | 5.6 (5.4, 5.9) | 0.04* |
| Insulin (uIU/mL) | 10.5 (6.0, 21.0) | 14.5 (5.0, 42.0) | 12.5 (9.0, 15.0) | 13.5 (6.5, 18.0) | 0.8* |
| Leptin (ng/mL) | 36.4 ± 18.3 | 30.0 ± 18 | 27.5 ± 13.3 | 29.0 ± 14.5 | 0.2 |
| Acy Ghr (pg/mL) | 362.3 (259.0, 740.7) | 378.5 (273.9, 644.6) | 503.9 (375.5, 828.3) | 625.3 (358.5, 767.2) | 0.2* |
| PP (pg/mL) | 89 (31.0, 662.0) | 104.0 (67.0, 468.0) | 138.0 (58.0, 496.0) | 104.0 (25.0, 423.0) | 0.04*€ |
Abbreviations: BMI, body mass index; Acy Ghr, acylated ghrelin
Data expressed as mean ±SD or median (IQR) for non-normally distributed variables
P-value from analysis of log-transformed values
No significant differences from baseline; p-value most likely due to differences among subsequent time points, which were not included in post hoc pairwise comparisons
Appetite Scores
Total appetite scores significantly decreased from baseline (32.2 ± 8.7) after 1, 3, and 6 mo of treatment (27.5 ± 8.8, 25.4 ± 9.3, and 25.4 ± 7.2, respectively; p=0.004). This reduction was primarily driven by decreases in behavior and drive sub-scores, as no significant difference in change of severity score was seen (Figure 2).
Figure 2.
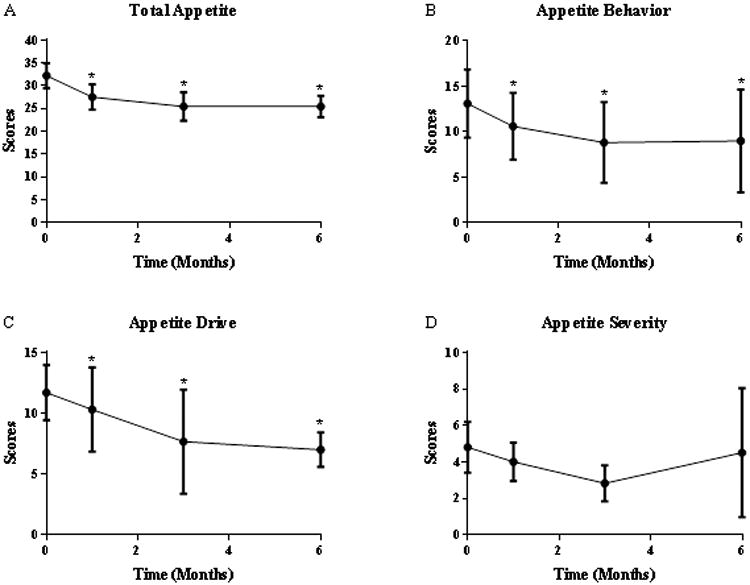
Appetite Scores Before and After Treatment with Exenatide. Shown as mean ± SD.
(A) Total appetite scores, (B) Appetite behavior scores, (C) Appetite drive scores, and (D) Appetite severity scores. *denotes scores that were significantly different from Time 0.
Obesity-Related laboratory Tests
There was a significant decrease in mean HbA1c from baseline after 1, 3, and 6 mo of treatment. There was no significant change in fasting insulin, leptin, PP, or acylated ghrelin from baseline at any visit (Table 1). There was no difference noted in MMTT results for visits 1 and 4 based on mixed model analyses for acylated ghrelin, PP or glucose (p-values 0.6, 0.8, and 0.9 respectively; data not shown). There was no difference in excursion of acylated ghrelin, PP, or glucose during the MMTT based on AUC data (p-values 0.5, 0.7, and 0.3 respectively; data not shown).
Safety Laboratory Test Results and Serious Adverse Events
All subjects had normal baseline amylase, lipase, and creatinine with no significant change in levels after treatment. There were no serious adverse events during the study. One subject reported abdominal cramping and diarrhea for a short period due to acute infectious gastroenteritis. Another subject experienced mild abdominal discomfort and diarrhea after doubling the dose of exenatide for one day. There were no other reports of gastroenterological symptoms.
Adherence and Confounding Factors
Examination of returned pens showed ≥ 70% high adherence suggesting that non-adherence did not impact our results. However, we found multiple confounding factors during the study including change in eating schedule during the school year vs. vacation, use of psychotropic medications (one subject had adjustment of anti-depressive medications), change in caretaker supervision, and unforeseen orthopedic procedures leading to decreased activity.
Discussion
This pilot study investigated the effects of exenatide on weight and appetite in overweight youths with PWS in which we compared weight, BMI, adiposity, and appetite scores over 6 mo. We chose a medication-only intervention without incorporating diet or lifestyle modifications to evaluate if a therapy targeting hyperphagia would be a beneficial and safe option for weight control in individuals with PWS. We found a significant decrease in appetite scores, specifically in the behavior and drive categories, which were noted as early as 1 mo after initiation of treatment. Nonetheless, there was no effect on weight, BMI, or adiposity over 6 mo.
We also measured circulating levels of acylated ghrelin, PP, and leptin during treatment with exenatide. Hyperghrelinemia is well-documented in PWS and is hypothesized to contribute to the hyperphagic state of the condition (11, 12, 13, 14). Pancreatic polypeptide is an anorectic hormone released by the pancreas and levels are normally lower in the fasting state and increase after eating. Studies have shown deficiency of PP in PWS which may also contribute to hyperphagia (24, 25). In our study, there was no significant change in acylated ghrelin or PP during treatment despite a decrease in appetite scores which does not further elucidate the role these hormones may play in regulating appetite in PWS, suggesting that hyperphagia in this condition is multifactorial. We also considered that the lack of change may have been due to the waning effect of the evening dose of exenatide as concentrations are usually measurable for about 10 hours. It may also be postulated that if effects wane between doses, fluctuations in appetite suppression may occur during the day. Longer-acting GLP-1 agonists may alleviate these difficulties.
The one case report documenting the long-term effects of exenatide in an individual with PWS showed significant decrease in weight, but did not comment on the diet or exercise regimen during the treatment (20). Two other case reports on the use of a longer-acting GLP-1 receptor agonist, liraglutide, in patients with PWS (26, 27) showed significant weight loss after the initiation of treatment; however, only Senda, et al (27) discussed concomitant lifestyle modifications. A recent publication of 6 adults with PWS and type 2 diabetes treated with either exenatide or liraglutide showed a trend in improved BMI and glycemic control over 24 months that was not statistically significant (28). The combination of these reports as well as our findings, suggests that there is a need to incorporate lifestyle modifications in addition to appetite-suppressing medication.
Similar to the previously mentioned case reports, we saw a significant decrease in HbA1c during treatment. This is an expected outcome of GLP-1 receptor agonists and highlights the additional benefit of improved glycemic control with this class of medication and indicates good compliance during the course of the study.
In our small cohort, we found no evidence of safety concerns with exenatide use. The most commonly reported side effects are nausea, vomiting, diarrhea, dizziness, headache, jitteriness, and acidic stomach. During the study, we had one complaint of drug-related nausea and abdominal pain when a subject took twice the recommended dose. This suggests that exenatide is well-tolerated in individuals with PWS, as previously suggested by Sze, et al (21). In light of reports of pancreatitis, pancreatic cancer, and pancreatic hyperplasia in individuals receiving GLP-1 receptor agonist therapy, we monitored for symptoms of pancreatic disease and measured levels of circulating pancreatic enzymes during treatment (29, 30). We found no change in serum amylase or lipase levels. In addition, other studies have commented on a hypothetical increased risk of gastric rupture in individuals with PWS treated with exenatide due to delayed gastric emptying (21). Accordingly, we counseled our subjects and caregivers about the symptoms and risk of gastric rupture. During the study, there were no complaints of symptoms associated with pancreatitis or gastric rupture. Thus, it appears that exenatide can be used safely in this population.
We recognize the limitations to our study, the first of which is our small sample size. It is challenging to recruit qualified subjects with a rare disorder and, with a larger sample size, we may have been able to detect a change in weight or adiposity. As our recruitment area was small, this may have created some referral bias although we did attempt to open recruitment to subjects outside of CHLA. We recognize that this study was not randomized or placebo-controlled. Also, the short length of our study may have impacted our ability to see weight loss and therefore a longer term study is needed. We did not attempt to introduce standardized lifestyle modifications. With the lack of scientific data on the efficacy and safety of exenatide in PWS, we felt it was prudent to start with a pilot study to see if further studies are warranted. As such, we also did not introduce lifestyle modifications which could themselves influence weight loss results and, rather, focused on the effects of the addition of only exenatide. In addition, we noted multiple confounding factors during the study, including variations in eating schedule, psychotropic medications, caretaker supervision, and activity. Some of these factors were unavoidable, but others could be controlled for in future studies. The subjective collection of appetite measures may lead to inaccuracy of results and future studies should consider the addition of objective methods such as food intake measurement. With the decrease in appetite scores, a change in weight or adiposity would have been expected but the above factors may have impacted our ability to detect change; therefore, future studies controlling for these limitations may yield different results. Despite these limitations, this is the first study to systematically examine the long-term effects of exenatide on weight and appetite in a group of subjects with PWS. However, it is clear that medication alone is not an effective method of controlling weight in PWS.
This 6-mo pilot study of overweight and obese youths with PWS showed that exenatide was well-tolerated and resulted in a significant decrease in appetite and HbA1c, but no decrease in weight, BMI, adiposity, acylated ghrelin, or PP. Obesity in PWS is multifactorial and requires a multidimensional treatment strategy. Our findings suggest that larger, controlled studies are required to further study the efficacy and safety of exenatide in subjects with PWS. Longer-acting formulations of GLP-1 agonists may alleviate some of the challenges that limited our study and warrant further exploration.
Supplementary Material
Supplemental Figure 1. BMI of Individual Subjects Over Time, Individual BMI trajectories for each subject.
What is already known about this subject?
-Prader-Willi Syndrome (PWS) has a unique phenotype of obesity which includes hyperphagia and hyperghrelinemia without any effective medical therapy for the excessive appetite.
-Exenatide [Byetta™; AstraZeneca, Wilmington DE] is a GLP-1 receptor agonist shown to result in weight loss in obese individuals with and without type 2 diabetes.
-There are case reports that report weight loss with the use of GLP-1 receptor agonists in PWS, but there have been no longitudinal studies.
What does this study add?
-This pilot study is the first longitudinal study to evaluate the effects of a GLP-1 receptor agonist on a group of youths with PWS.
-The study gives evidence that exenatide leads to decreased appetite in youths with PWS.
-Exenatide was well-tolerated by this cohort and may be a treatment option for patients with PWS.
Acknowledgments
Drug was provided by AstraZeneca (formerly Amylin). Support was in part by the NIH NCRR CTSI Grant 1UL1RR031986 and performed at the CHLA CTSI. PS and DJ conceived and carried out experiments, SM and MG conceived experiments, CA analyzed data, IH carried out experiments. All authors were involved in writing the paper and had final approval of the submitted and published versions. The authors would like to thank study participants and Dr. Joyce Richey at the University of Southern California Metabolic Assay Core and Ehsan Ehsanipour for their contributions with running assays.
Funding: Study drug was provided by AstraZeneca (formerly Amylin Pharmaceuticals). This study was supported in part by the NIH NCRR CTSI Grant 1UL1RR031986 as well as by CHLA Department of Endocrine Merit Fund 8030-RR1000019-00.
Footnotes
Disclosures: P. Salehi is involved in the study “Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial of ZGN-440 (Subcutaneous Beloranib in Suspension) in Obese Subjects with Prader-Willi Syndrome to Evaluate Total Body Fat Mass, Food-Related Behavior, and Safety over 6 Months)” sponsored by Zafgen, Inc; has consulted for Stratas Partners. M. Geffner is a clinical trial consultant for Daiichi-Sankyo; serves on advisory boards for Ipsen, Pfizer, and Sandoz; has research contracts with Eli Lilly, Inc., Novo Nordisk, and Versartis; has lectured on behalf of Sandoz; serves on a data safety monitoring board for Tolmar Inc.; and receives royalties from McGraw Hill and UpToDate.
Conflicts of Interest: P. Salehi has a research contract with Zafgen, Inc; has consulted for Stratas Partners. M. Geffner is a clinical trial consultant for Daiichi-Sankyo; on advisory boards for Ipsen, Pfizer, and Sandoz; has research contracts with Eli Lilly, Inc., Novo Nordisk, and Versartis; has lectured for Sandoz; serves on a data safety monitoring board for Tolmar Inc.; receives royalties from McGraw Hill and UpToDate
References
- 1.Lionti T, Reid SM, White SM, Rowell MM. A population-based profile of 160 Australians with Prader-Willi syndrome: Trends in diagnosis, birth prevalence and birth characteristics. Am J Med Genet A. 2014;167A:371–378. doi: 10.1002/ajmg.a.36845. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls RD, Knoll JH, Butler MG, Karam S, Lalande M. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature. 1989;342:281–285. doi: 10.1038/342281a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holm VA, Cassidy SB, Butler MG, et al. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91:398–402. [PMC free article] [PubMed] [Google Scholar]
- 4.Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet. 2001;38:792–798. doi: 10.1136/jmg.38.11.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland AJ, Treasure J, Coskeran P, Dallow J, Milton N, Hillhouse E. Measurement of excessive appetite and metabolic changes in Prader-Willi syndrome. Int J Obes Relat Metab Disord. 1993;17:527–532. [PubMed] [Google Scholar]
- 6.Whittington J, Holland A, Webb T, Butler J, Clarke D, Boer H. Relationship between clinical and genetic diagnosis of Prader-Willi syndrome. J Med Genet. 2002;39:926–932. doi: 10.1136/jmg.39.12.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A. 2011;155A:1040–1049. doi: 10.1002/ajmg.a.33951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93:4183–4197. doi: 10.1210/jc.2008-0649. [DOI] [PubMed] [Google Scholar]
- 9.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 10.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE, Clement K, Purnell JQ, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8:643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 12.Haqq AM, Farooqi IS, O'Rahilly S, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88:174–178. doi: 10.1210/jc.2002-021052. [DOI] [PubMed] [Google Scholar]
- 13.Paik KH, Jin DK, Song SY, et al. Correlation between fasting plasma ghrelin levels and age, body mass index (BMI), BMI percentiles, and 24-hour plasma ghrelin profiles in Prader-Willi syndrome. J Clin Endocrinol Metab. 2004;89:3885–3889. doi: 10.1210/jc.2003-032137. [DOI] [PubMed] [Google Scholar]
- 14.Feigerlova E, Diene G, Conte-Auriol F, et al. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93:2800–2805. doi: 10.1210/jc.2007-2138. [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 16.Rosenstock J, Klaff LJ, Schwartz S, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33:1173–1175. doi: 10.2337/dc09-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly AS, Rudser KD, Nathan BM, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167:355–360. doi: 10.1001/jamapediatrics.2013.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Tilve D, Gonzalez-Matias L, Alvarez-Crespo M, et al. Exendin-4 potently decreases ghrelin levels in fasting rats. Diabetes. 2007;56:143–151. doi: 10.2337/db05-0996. [DOI] [PubMed] [Google Scholar]
- 19.van Bloemendaal L, RG IJ, Ten Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–4196. doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 20.Seetho IW, Jones G, Thomson GA, Fernando DJ. Treating diabetes mellitus in Prader-Willi syndrome with Exenatide. Diabetes Res Clin Pract. 2011;92:e1–2. doi: 10.1016/j.diabres.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Sze L, Purtell L, Jenkins A, et al. Effects of a single dose of exenatide on appetite, gut hormones, and glucose homeostasis in adults with Prader-Willi syndrome. J Clin Endocrinol Metab. 2011;96:E1314–1319. doi: 10.1210/jc.2011-0038. [DOI] [PubMed] [Google Scholar]
- 22.Dykens EM, Maxwell MA, Pantino E, Kossler R, Roof E. Assessment of hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring) 2007;15:1816–1826. doi: 10.1038/oby.2007.216. [DOI] [PubMed] [Google Scholar]
- 23.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 24.Zipf WB, O'Dorisio TM, Cataland S, Dixon K. Pancreatic polypeptide responses to protein meal challenges in obese but otherwise normal children and obese children with Prader-Willi syndrome. J Clin Endocrinol Metab. 1983;57:1074–1080. doi: 10.1210/jcem-57-5-1074. [DOI] [PubMed] [Google Scholar]
- 25.Tomita T, Greeley G, Jr, Watt L, Doull V, Chance R. Protein meal-stimulated pancreatic polypeptide secretion in Prader-Willi syndrome of adults. Pancreas. 1989;4:395–400. doi: 10.1097/00006676-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Cyganek K, Koblik T, Kozek E, Wojcik M, Starzyk J, Malecki MT. Liraglutide therapy in Prader-Willi syndrome. Diabet Med. 2011;28:755–756. doi: 10.1111/j.1464-5491.2011.03280.x. [DOI] [PubMed] [Google Scholar]
- 27.Senda M, Ogawa S, Nako K, Okamura M, Sakamoto T, Ito S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr J. 2012;59:889–894. doi: 10.1507/endocrj.ej12-0074. [DOI] [PubMed] [Google Scholar]
- 28.Fintini D, Grugni G, Brufani C, Bocchini S, Cappa M, Crino A. Use of GLP-1 receptor agonists in Prader-Willi Syndrome: report of six cases. Diabetes Care. 2014;37:e76–77. doi: 10.2337/dc13-2575. [DOI] [PubMed] [Google Scholar]
- 29.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–2604. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. BMI of Individual Subjects Over Time, Individual BMI trajectories for each subject.


