Abstract
Bacteriolytic therapy using the anaerobic bacterium Clostridium novyi-NT (C. novyi-NT) is considered as a promising approach for the treatment of solid tumors. Image-guided intra-tumoral administration of C. novyi-NT spores may serve to improve therapeutic efficacy while potentially reducing toxicity (compared to systemic administration approaches). To this end, we demonstrate an approach to label C. novyi-NT spores with branched gold nanoparticles (BGNP) thus permitting intra-procedural X-ray CT visualization of spore delivery to solid tumors upon intra-tumoral injection. BGNP-coated C. novyi-NT spores were prepared using a simple electrostatic deposition method. In PC3 prostate tumor-bearing mouse model, BGNP-coated spores were injected into the hypoxic center of each tumor with CT confirmation of intra-tumoral delivery. Follow-up immunohistochemical analyses were used to validate the bacteriolytic efficacy of these BGNP-coated C. novyi-NT spores in the same animal model. BGNP-coating had no impact upon spore germination and proliferation rates and in vivo results demonstrated significant bacteriolytic anti-tumor efficacy. These BGNP-coated spores should importantly permit intra-procedural guidance to optimize and confirm targeted intra-tumoral delivery in patients with visceral organ tumors.
Keywords: CT image-guided therapy, Bacteriolytic tumor therapy, Clostridium novyi-NT, Gold nanoparticles, Spores
Graphical Abstract
Branched gold nanoparticles (BGNP)-coated Clostridium novyi-NT (C. novyi-NT) spores were developed for CT-guided bacteriolytic tumor therapy. The BGNP-coated spores were successfully injected into a tumor site under CT image guidance. As a result, a strong anti-tumor effect was observed in PC3 prostate tumor-bearing mouse model.

The innate and/or adaptive resistance of many types of cancer cells has limited tumor response to a broad range of different therapeutic options[1] including both radiation and chemotherapies. Recently, bacteriolytic therapy with anaerobic spores has received a great deal of attention given the generally favorable toxicity profile and strong oncolytic impact with minimal tumor resistance, particularly within hypoxic tissues.[2] The first use of bacterial cancer therapy was reported by William Coley 100 years ago; since that time, significant progress has been made to improve therapeutic efficacy with the advancement of recombinant DNA technology.[3] More recently, Vogelstein and colleagues demonstrated that the spore-forming obligate anaerobe Clostridium novyi (specifically C. novyi-NT, an attenuated clone derived from C. novyi after elimination of its lethal toxin[2]) can serve as a potent therapeutic agent for the treatment of solid tumors refractory to conventional therapeutic approaches.[4] Bacteriolytic therapy using C. novyi-NT spores is currently undergoing clinical evaluation in a human Phase I trial for patients with treatment-refractory tumors (NCT01924689).[3, 5]
The efficacy of bacteriolytic therapy with C. novyi-NT spores has been demonstrated for a broad range of tumor etiologies, using either systemic administration approaches or percutaneous intra-tumoral injection.[3, 6] Intra-tumoral injection affords several advantages including a) the ability to increase the number of spores that ultimately reach the targeted tumor(s) and b) the potential to reduce systemic exposure thus limiting side-effect toxicities. Prior percutaneous protocols have involved digital palpation of surface tumors to guide injection catheter placement and/or ultrasound guidance without a means to immediately visualize spore deposition within the targeted tumors following administration.[5] X-ray computed tomography (CT) is widely used in interventional radiology settings to guide the placement of biopsy needles and ablation electrodes during the diagnosis and treatment of solid tumors within visceral organs. CT guidance approaches should be ideal for intra-procedural optimization of injection catheter placement during bacteriolytic therapy; however, native C. novyi-NT spores are not sufficiently radio-opaque to permit in vivo visualization. Ultimately, visual confirmation of spore delivery to the tumor(s) may be critical for patient-specific procedural optimization and/or early prediction of therapeutic outcomes.[7]
To this end, we developed an approach to coat C. novyi-NT spores with branched gold nanoparticles (BGNP) for x-ray computed tomography (CT)-guided bacteriolytic therapy. CT approaches offer unlimited penetration depth, high spatial resolution, and multi-planar reconstruction for 3-D visualization.[8] Gold nanoparticles (GNP) have a high X-ray attenuation coefficient given high atomic number and electron density (z = 79, q = 19.3 g/cm3)[9]; GNP have been considered an ideal CT contrast agent given well-established biocompatibility. In recent studies,[10] exogenous cell labeling with GNP permitted CT imaging of cell migration and examination of resulting biodistribution. Recently, our group developed a unique deoxycholate bile acid directed method for efficient, biocompatible synthesis of BGNP.[11] BGNP are particularly promising CT contrast agents given the large relative surface (compared to spherical GNP) that can yield strong x-ray attenuation characteristics. For our studies (Scheme 1a), the peptidoglycan walls of C. novyi-NT spores were electrostatically coated with functionalized BGNP. Following in vitro characterization studies, our BGNP-coated C. novyi-NT spores were injected into mouse PC-3 tumors (prostate cancer xenograft model) to validate the potential for non-invasive CT visualization and confirm bacteriolytic efficacy with comparison to PC-3 tumors treated with native (uncoated) spores.
Scheme 1.
Schematic illustration of (a) BGNP-coated C. novyi-NT spores for CT-guided intra-tumor infusion and tracking, and (b) synthetic route for preparation of BGNP-coated C. novyi-NT spores through electrostatic deposition.
C. novyi-NT spores were isolated by use of a Percoll density gradient column (See Supporting Information, Figure S1)[12] prior to the following studies. To prepare the BGNP-coated C. novyi-NT spores, an electrostatic deposition method (Scheme 1b) was adopted. Electrostatic deposition is commonly used for cell-surface engineering given facile nature of the procedure, efficient synthetic yield, and relatively minimal observed toxicities.[13] The surface of C. novyi-NT spores is negatively charged due to the N-acetylemuramic acid (NAM) moieties on the peptidoglycan membrane.[14] The negatively charged C. novyi-NT spores were functionalized with positively charged small molecular-weight branched polyethylenimine (bPEI, 600 Da) to avoid the undesirable cytotoxicity observed with high molecular-weight bPEI.[15] After cationic bPEI polymer functionalization, the surface charge of C. novyi-NT spores was significantly increased from negative (~ −26 mV) to positive (~ +38 mV) (Figure 1a). Next, BGNP were synthesized according to our previous reported protocol through a deoxycholate bile acid directed mechanism.[11] As shown in Figure 1b, highly branched GNP were successfully synthesized and their characteristic absorption band observed across a broad range of visible and near infrared (NIR) wavelengths. These results were consistent with our previous report.[11] To confirm the superior radio-opaque characteristics of our BGNP, CT numbers (Hounsfield units, HU) were measured for phantoms produced using increasing, matched concentrations of spherical GNP and our BGNP; latter measurements were performed using a pre-clinical micro CT system (Mediso NanoPET/CT®, See Supporting Information, Figure S2). BGNP demonstrated significantly higher CT numbers than spherical GNP at concentrations above 6 mg/mL. This result may be attributed to the branch-shaped geometry of BGNP which results in a greater surface area than spherical counterpart of equivalent size.[16] Next, the surface charge of the BGNP needed to be modified to complex with the positively-functionalized C. novyi-NT spores; thus, the synthesized BGNP were modified with 4-mercaptobenzoic acid (4-MCBA) via ligand exchange and ionization.[17] After ligand exchange with 4-MCBA, the BGNP exhibited highly negative surface charge, approximately −37 mV (Figure 1a). Finally, the synthesized negatively-charged BGNP were deposited on the positively-charged spore-walls through electrostatic interaction. As shown in Figure 1a, the BGNP-coated C. novyi-NT spores exhibited negative surface charge attributable to the immobilization of the negative-charged BGNP on the surface of the spores. To optimize labeling efficiency, a certain amount of C. novyi-NT spores (3.89 × 108 spores) was labeled with various concentrations (0.08 – 1.32 mg/mL) of BGNP. As a result, the highest BGNP-labeling efficacy (~91 %) was achieved when 0.66 mg/ml of BGNP was added in C. novyi-NT spores (3.89 × 108 spores) (“BGNP-labeled spores 4” in Table S1). The optimized labeling condition was selected for further study. Additionally, UV-Vis absorption spectra after BGNP immobilization was measured to observe optical property changes (Figure 1b). NIR absorption for BGNP-coated C. novyi-NT was observed within the spectral absorption region that was the same trend as observed for the BGNP alone. The slight absorption increase between 400 to 700 nm may be due to light absorption of the native spore itself. To investigate morphological changes after BGNP deposition, we performed scanning electronic microscopy (SEM). While native C. novyi-NT spores have a smooth spore wall structure (Figure 1d), BGNP deposition upon the spore walls was observed for the coated C. novyi-NT spores (Figure 1e). The germination and proliferation of C. novyi-NT spores was not affected by bPEI polymer functionalization and BGNP deposition (See Supporting Information, Figure S3). To demonstrate CT contrast effects of the BGNP-coated spores, an in vitro CT phantom study was performed after BGNP labeling (Figure 1f). While no CT contrast signal was observed in control group (e.g., spores only, and water), in the BGNP-coated spore group, significantly enhanced CT contrast was observed, which suggested the feasibility of using CT imaging to monitor in vivo delivery of our BGNP-coated spores. Overall, in vitro results indicated that BGNP coating of the C. novyi-NT spore wall was successfully achieved through electrostatic deposition. To the best of our knowledge, this is the first demonstration of GNP-coating of C. novyi-NT spores for CT image-guided bacteriolytic therapy.
Figure 1.
In vitro physico-chemical characterization of BGNP-coated C. novyi-NT spores. (a) Zeta-potential measurement of (1) native C. novyi-NT spores, (2) cationic PEI-coated spores, (3) anionic BGNP, and (4) BGNP-coated C. novyi-NT spores. (B) UV-Vis spectrum of BGNP, BGNP-coated C. novyi-NT spores, and native C. novyi-NT spores. (c–e) SEM images of (c) BGNP (Inset: TEM image of BGNP, scale bar: 50 nm), (d) native C. novyi-NT spores, and (e) BGNP-coated C. Novyi-NT spores (Red dot line indicates the outline of one BGNP-coated C. novyi-NT spore). (f) In vitro CT phantom of BGNP-coated C. novyi-NT spores (The red arrow indicates region of hyper-attenuated CT signal from the BGNP-coated spores).
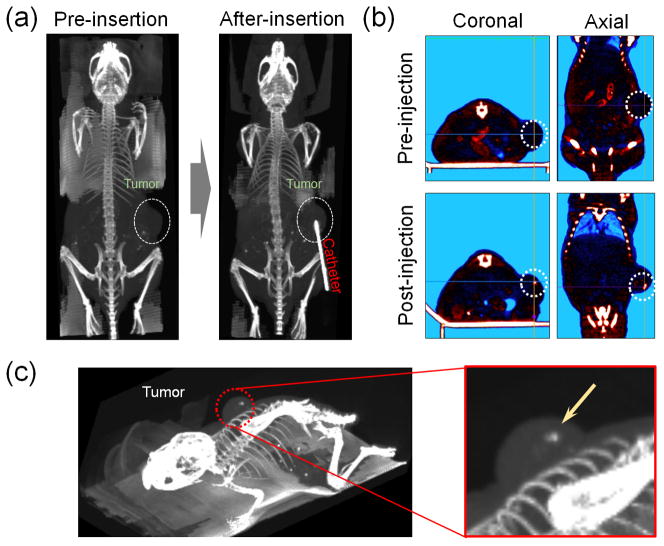
To evaluate in vivo imaging capabilities and therapeutic efficacy of BGNP-coated C. novyi-NT spores, we used a human prostate cancer cell (PC3) xenograft mouse model. Because common therapeutic option for prostate cancer is localized intervention,[18] we selected prostate cancer to assess feasibility for our CT image-guided bacteriolytic therapy. PC3 human prostate xenograft tumors were generated by inoculating PC3 human prostate cancer cells into BALB/c nude mice. BGNP-coated C. novyi-NT spores were infused via percutaneous intra-tumoral injection in this PC3 mouse model (dose of spores was 3 × 108/mice). Precise injection of spores into solid tumors may be critical to achieve efficient germination of the C. novyi-NT anaerobes within the targeted hypoxic tissue regions.[5] We performed CT image-guided injections of the BGNP-coated C. novyi-NT spores into the center of each tumor at position anticipated to be hypoxic and necrotic. Before positioning the radio-opaque injection catheter, we could readily observe the location of the PC3 tumor on right flank within the CT images (Figure. 2a, pre-insertion). Then, the injection catheter was inserted and position was adjusted such that the tip was located at the center of tumor (Figure. 2a, post-insertion). As shown in Figure 2b, the needle-catheter position can be readily observed within the CT images. The BGNP-coated C. novyi-NT spores were then infused and the injection catheter removed. Spore deposition was observed as a punctate region of hyper-attenuation within the center of each tumor, visible within both 2D axial images three-dimensional renderings of the tumor volume (Figure 2c). The mean ROI value of the injected site was approximately 505 (SD, ±298). These results indicate that CT methods should will permit precise image-guided delivery of BGNP-coated spores to targeted lesions. CT guidance offers tremendous advantages over optical and MR imaging methods providing complementary anatomic information (tumor location and size) while also permitting rapid image acquisition rates, particularly well-suited for real-time procedural guidance.[9, 19]
Figure 2.
In vivo CT-guided bacteriolytic therapy in PC3 tumor-bearing mouse model. (a) In vivo CT-guided placement of injection-catheter, (b) In vivo CT images of PC3 tumor following intra-tumoral injection of BGNP-coated C. novyi-NT spores (injection-catheter removed), and (c) 3D-rendered CT images of mouse following injection of BGNP-coated C. novyi-NT spores (Yellow arrow indicates hyper-attenuated region due to deposition of the BGNP-coated spores).
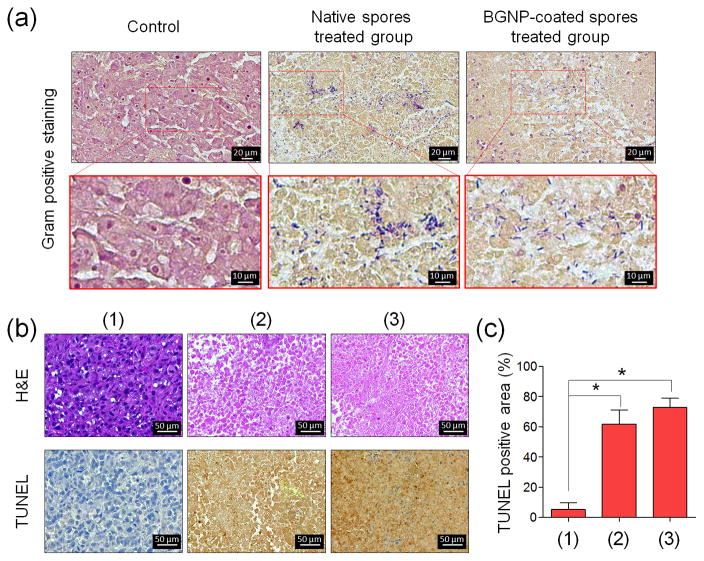
Finally, the therapeutic efficacy of BGNP-coated C. novyi-NT spores was evaluated with histological staining of tumor tissues harvested at 48 hours after infusion. Germination of C. novyi-NT bacteria in tumor tissues was confirmed with gram-positive staining (Figure 3a). Vegetative forms of C. novyi-NT were observed in both tumors treated with native C. novyi-NT as well as tumor treated with BGNP-coated C. novyi-NT spore. This result indicates that the germination capability of C. novyi-NT was not affected by BGNP deposition on spore walls, which is consistent with in vitro germination study (See Supporting Information, Figure S3). Furthermore, H&E and TUNEL staining analysis were performed to evaluate liquefaction (oncolysis) induced therapeutic effects within the tumor tissues (Figure 3b and c). H&E-stained tumor tissues demonstrated a significantly increased number of necrotic tumor cells in both tumors treated with native spores and BGNP-coated spores. For quantitative immunohistochemistry analyses (Figure 3c), TUNEL positive tumor cells were also much increased in both spore treated groups, regardless of BGNP coating. Few TUNEL positive cell were observed in non-treated control group tumors. These results indicate that BGNP-coated C. novyi-NT spores were able to retain innate anti-tumoral efficacy, most likely through direct oncolytic effects,[20] regardless of BGNP incorporation. However, we did observe that the therapeutic effect of both native C. novyi-NT spores and BGNP-coated C. novyi-NT spores was limited to hypoxic central regions of the tumors as C. novyi-NT can survive only in hypoxic condition (See Supporting Information, Figure S4). The vascularized rim of solid tumors has been well established as a major cause of tumor resistance to bacteriolytic therapy with anaerobic spores.[4, 21] To overcome this limitation, combinational approaches have been investigated with chemotherapy or radiotherapy used to eradicate the remaining normoxic regions within solid tumors.[12a, 21] Our approach using BGNP-coated spores could potentiate the development of salient alternative combinations. BGNP can serve as potent radiosensitizers and/or serve as highly efficient for transducers for photothermal therapy (PTT). Additionally, the BGNP coating could serve as a functional carrier for concurrent intra-tumoral delivery of cytotoxic drugs. Regarding long-term toxicity, although clinical signs of toxicities include lethargy, weight loss, and abscessation was manageable by administration of antibiotics in the large animal model and human,[3–5] long-term risk for mutagenesis of C. novyi-NT should be evaluated in the future study.
Figure 3.
In vivo anti-tumor efficacy of BGNP-coated C. novyi-NT spores. (a) Representative gram-positive staining images of non-treated tumor tissue (Control) and tumor tissue treated with native C. novyi-NT spores or BGNP-coated C. novyi-NT spores. (b) Representative H&E and TUNEL staining from (1) non-treated tumor tissue (Control) and tumor tissue treated with (2) native C. novyi-NT spores or (3) BGNP-coated C. novyi-NT spores. (c) Quantitative analysis of TUNEL positive tumor cells from (1) the non-treated tumor tissues (Control) and tumor tissues treated with (2) native C. novyi-NT spores or (3) BGNP-coated C. novyi-NT spores (n=3, *p<0.001).
In conclusion, in this study, we demonstrated a new type of BGNP-coated C. novyi-NT spore for efficient CT-image guided bacteriolytic tumor therapy. BGNP-coated C. novyi-NT spores were successfully prepared with electrostatic deposition methods. In a PC3 human prostate tumor xenograft mouse model, the BGNP-coated spores were injected into a tumor site under CT image guidance. The infused BGNP-coated spores exhibited strong therapeutic efficacy consistent with that observed for native C. novyi-NT spores suggesting that the innate anti-tumoral function was preserved. Based on these results, we believe that our BGNP-coated C. novyi-NT spores have the potential to not only facilitate CT image-guided bacteriolytic procedures but also to serve as a novel delivery platform for a combinational tumor therapy.
Supplementary Material
Figure S1. Percoll density gradient column for isolation of C. Novyi-NT spores. The density gradient column was performed using 70 and 55 % percoll in PBS (150 mM) solution with centrifugation (3000 rpm, for 15 minutes). Upon completion, C. Novyi-NT spores were successfully purified in bottom layer of the density gradient column.
Figure S2. Evaluation of the x-ray CT imaging properties of the BGNP. (a) CT images of phantoms prepared with increasing matched concentrations of sphere-shaped gold nanoparticles (GNP) and BGNP. (b) Plot of CT number versus concentration of nanoparticles (sphere shaped GNP or BGNP).
Figure S3. Bacterial growth of native C. Novyi-NT spores and BGNP-coated C. Novyi-NT spores. The bacterial growth test was performed in Oxyrase (2 wt%) containing culture media at 37 °C. The concentration of vegetative C. novyi-NT bacteria was calculated using the formula 1 OD600 = 1×109 cells/mL.[4]
Figure S4. A low magnification H&E and TUNEL stained tissue image from tumor tissues treated with BGNP-coated C. Novyi-NT spores. Significant tumor cell death was observed at hypoxic center of tumor.
Table S1. Characterization of BGNP-coated C. novyi-NT spores
Acknowledgments
The financial supports from NIH/NCI-R01CA141047 (Larson), R21CA173491 (Kim), and R21CA185274 (Kim), and NIH/NIBIB-R21EB017986 (Kim) are greatly acknowledged. This work was also supported by the Center for Translational Imaging and Mouse Histology and Phenotyping Laboratory at Northwestern University.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Wooram Park, Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, 60611, USA
Dr. Soojeong Cho, Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, 60611, USA
Dr. Xiaoke Huang, Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, 60611, USA
Prof. Andrew C. Larson, Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, 60611, USA. Robert H. Lurie Comprehensive Cancer Center, Chicago, IL 60611, USA. Department of Biomedical Engineering, Northwestern University, Evanston, IL 60208, USA. Department of Electrical Engineering and Computer Science, Evanston, IL 60208, USA. International Institute of Nanotechnology (IIN), Northwestern University, Evanston, IL 60208, USA
Prof. Dong-Hyun Kim, Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, 60611, USA. Robert H. Lurie Comprehensive Cancer Center, Chicago, IL 60611, USA
References
- 1.a) Geng L, Donnelly E, McMahon G, Lin PC, Sierra-Rivera E, Oshinka H, Hallahan DE. Cancer Res. 2001;61:2413. [PubMed] [Google Scholar]; b) Gottesman MM. Annu Rev Med. 2002;53:615. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal N, Bettegowda C, Cheong I, Geschwind J-F, Drake CG, Hipkiss EL, Tatsumi M, Dang LH, Diaz LA, Pomper M, Abusedera M, Wahl RL, Kinzler KW, Zhou S, Huso DL, Vogelstein B. Proc Natl Acad Sci U S A. 2004;101:15172. doi: 10.1073/pnas.0406242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staedtke V, Roberts NJ, Bai R-Y, Zhou S. Genes Dis. 2016;3:144. doi: 10.1016/j.gendis.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz LA, Cheong I, Foss CA, Zhang X, Peters BA, Agrawal N, Bettegowda C, Karim B, Liu G, Khan K, Huang X, Kohli M, Dang LH, Hwang P, Vogelstein A, Garrett-Mayer E, Kobrin B, Pomper M, Zhou S, Kinzler KW, Vogelstein B, Huso DL. Toxicol Sci. 2005;88:562. doi: 10.1093/toxsci/kfi316. [DOI] [PubMed] [Google Scholar]
- 5.Roberts NJ, Zhang L, Janku F, Collins A, Bai R-Y, Staedtke V, Rusk AW, Tung D, Miller M, Roix J, Khanna KV, Murthy R, Benjamin RS, Helgason T, Szvalb AD, Bird JE, Roy-Chowdhuri S, Zhang HH, Qiao Y, Karim B, McDaniel J, Elpiner A, Sahora A, Lachowicz J, Phillips B, Turner A, Klein MK, Post G, Jr, Diaz LA, Riggins GJ, Papadopoulos N, Kinzler KW, Vogelstein B, Bettegowda C, Huso DL, Varterasian M, Saha S, Zhou S. Sci Transl Med. 2014;6:249ra111. doi: 10.1126/scitranslmed.3008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Krick EL, Sorenmo KU, Rankin SC, Cheong I, Kobrin B, Thornton K, Kinzler KW, Vogelstein B, Zhou S, Diaz LA., Jr Am J Vet Res. 2012;73:112. doi: 10.2460/ajvr.73.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Staedtke V, Bai RY, Sun W, Huang J, Kibler KK, Tyler BM, Gallia GL, Kinzler K, Vogelstein B, Zhou S, Riggins GJ. Oncotarget. 2015;6:5536. doi: 10.18632/oncotarget.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng L, Zhang Z, Khazaie K, Saha S, Lewandowski RJ, Zhang G, Larson AC. PloS one. 2014;9:e116204. doi: 10.1371/journal.pone.0116204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Y, Wang J, Ke H, Wang S, Dai Z. Biomaterials. 2013;34:4794. doi: 10.1016/j.biomaterials.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J, Carey TE, Kopelman R. Nano Lett. 2008;8:4593. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Astolfo A, Arfelli F, Schültke E, James S, Mancini L, Menk RH. Nanoscale. 2013;5:3337. doi: 10.1039/c3nr34089a. [DOI] [PubMed] [Google Scholar]; b) Meir R, Shamalov K, Betzer O, Motiei M, Horovitz-Fried M, Yehuda R, Popovtzer A, Popovtzer R, Cohen CJ. ACS nano. 2015;9:6363. doi: 10.1021/acsnano.5b01939. [DOI] [PubMed] [Google Scholar]
- 11.Kim D-H, Larson AC. Biomaterials. 2015;56:154. doi: 10.1016/j.biomaterials.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Proc Natl Acad Sci. 2001;98:15155. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Plomp M, McCaffery JM, Cheong I, Huang X, Bettegowda C, Kinzler KW, Zhou S, Vogelstein B, Malkin AJ. J Bacteriol. 2007;189:6457. doi: 10.1128/JB.00757-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Akin D, Sturgis J, Ragheb K, Sherman D, Burkholder K, Robinson JP, Bhunia AK, Mohammed S, Bashir R. Nat Nanotechnol. 2007;2:441. doi: 10.1038/nnano.2007.149. [DOI] [PubMed] [Google Scholar]; b) Kempaiah R, Chung A, Maheshwari V. Acs Nano. 2011;5:6025. doi: 10.1021/nn201791k. [DOI] [PubMed] [Google Scholar]; c) Park JH, Hong D, Lee J, Choi IS. Acc Chem Res. 2016;49:792. doi: 10.1021/acs.accounts.6b00087. [DOI] [PubMed] [Google Scholar]; d) Yang SH, Lee T, Seo E, Ko EH, Choi IS, Kim BS. Macromol Biosci. 2012;12:61. doi: 10.1002/mabi.201100268. [DOI] [PubMed] [Google Scholar]
- 14.a) Warth AD, Strominger JL. Proc Natl Acad Sci U S A. 1969;64:528. doi: 10.1073/pnas.64.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Paredes-Sabja D, Setlow P, Sarker MR. Trends Microbiol. 2011;19:85. doi: 10.1016/j.tim.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 15.a) Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. Biomaterials. 2003;24:1121. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]; b) Yim H, Park W, Kim D, Fahmy TM, Na K. Biomaterials. 2014;35:9912. doi: 10.1016/j.biomaterials.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Yuan H, Fales AM, Register JK, Vo-Dinh T. Front Chemistry. 2015;3 doi: 10.3389/fchem.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Liu Y, Purich DL, Wu C, Wu Y, Chen T, Cui C, Zhang L, Cansiz S, Hou W, Wang Y, Yang S, Tan W. J Am Chem Soc. 2015;137:14952. doi: 10.1021/jacs.5b08533. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu Y, Chen T, Wu C, Qiu L, Hu R, Li J, Cansiz S, Zhang L, Cui C, Zhu G, You M, Zhang T, Tan W. J Am Chem Soc. 2014;136:12552. doi: 10.1021/ja5060324. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ling D, Hyeon T. Small. 2013;9:1450–1466. doi: 10.1002/smll.201202111. [DOI] [PubMed] [Google Scholar]
- 18.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, D’Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, Higano CS, Kraus SR, Moul JW, Tangen S. AUA Prostate Cancer Clinical Guideline Update Panel. J Urol. 2007;177:2106. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Huang P, Bao L, Zhang C, Lin J, Luo T, Yang D, He M, Li Z, Gao G, Gao B, Fu S, Cui D. Biomaterials. 2011;32:9796. doi: 10.1016/j.biomaterials.2011.08.086. [DOI] [PubMed] [Google Scholar]
- 20.a) Bettegowda C, Huang X, Lin J, Cheong I, Kohli M, Szabo SA, Zhang X, Diaz LA, Velculescu VE, Parmigiani G, Kinzler KW, Vogelstein B, Zhou S. Nat Biotechnol. 2006;24:1573. doi: 10.1038/nbt1256. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cheong I, Huang X, Bettegowda C, Diaz LA, Kinzler KW, Zhou S, Vogelstein B. Science. 2006;314:1308. doi: 10.1126/science.1130651. [DOI] [PubMed] [Google Scholar]
- 21.Bettegowda C, Dang LH, Abrams R, Huso DL, Dillehay L, Cheong I, Agrawal N, Borzillary S, McCaffery JM, Watson EL, Lin KS, Bunz F, Baidoo K, Pomper MG, Kinzler KW, Vogelstein B, Zhou S. Proc Natl Acad Sci U S A. 2003;100:15083. doi: 10.1073/pnas.2036598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Percoll density gradient column for isolation of C. Novyi-NT spores. The density gradient column was performed using 70 and 55 % percoll in PBS (150 mM) solution with centrifugation (3000 rpm, for 15 minutes). Upon completion, C. Novyi-NT spores were successfully purified in bottom layer of the density gradient column.
Figure S2. Evaluation of the x-ray CT imaging properties of the BGNP. (a) CT images of phantoms prepared with increasing matched concentrations of sphere-shaped gold nanoparticles (GNP) and BGNP. (b) Plot of CT number versus concentration of nanoparticles (sphere shaped GNP or BGNP).
Figure S3. Bacterial growth of native C. Novyi-NT spores and BGNP-coated C. Novyi-NT spores. The bacterial growth test was performed in Oxyrase (2 wt%) containing culture media at 37 °C. The concentration of vegetative C. novyi-NT bacteria was calculated using the formula 1 OD600 = 1×109 cells/mL.[4]
Figure S4. A low magnification H&E and TUNEL stained tissue image from tumor tissues treated with BGNP-coated C. Novyi-NT spores. Significant tumor cell death was observed at hypoxic center of tumor.
Table S1. Characterization of BGNP-coated C. novyi-NT spores