Introduction
Perforating dermatosis is a heterogeneous group of disorders characterized by transepidermal elimination of dermal components such as collagen, elastin, or fibrin. Acquired perforating dermatosis usually develops in adulthood in association with comorbid disease such as diabetes mellitus (DM) or renal failure. We present a diffuse and giant variant with an elastic fiber predominant elimination pattern, which has rarely been described.
Case report
A 60-year-old Chinese woman presented for a 6-month history of an intensely pruritic skin eruption that began on her right arm and spread to become generalized. Lesions started as red papules that subsequently grew in size. The patient had end-stage renal disease (ESRD) requiring hemodialysis for the last 9 years and a history of hyperparathyroidism after subtotal parathyroidectomy.
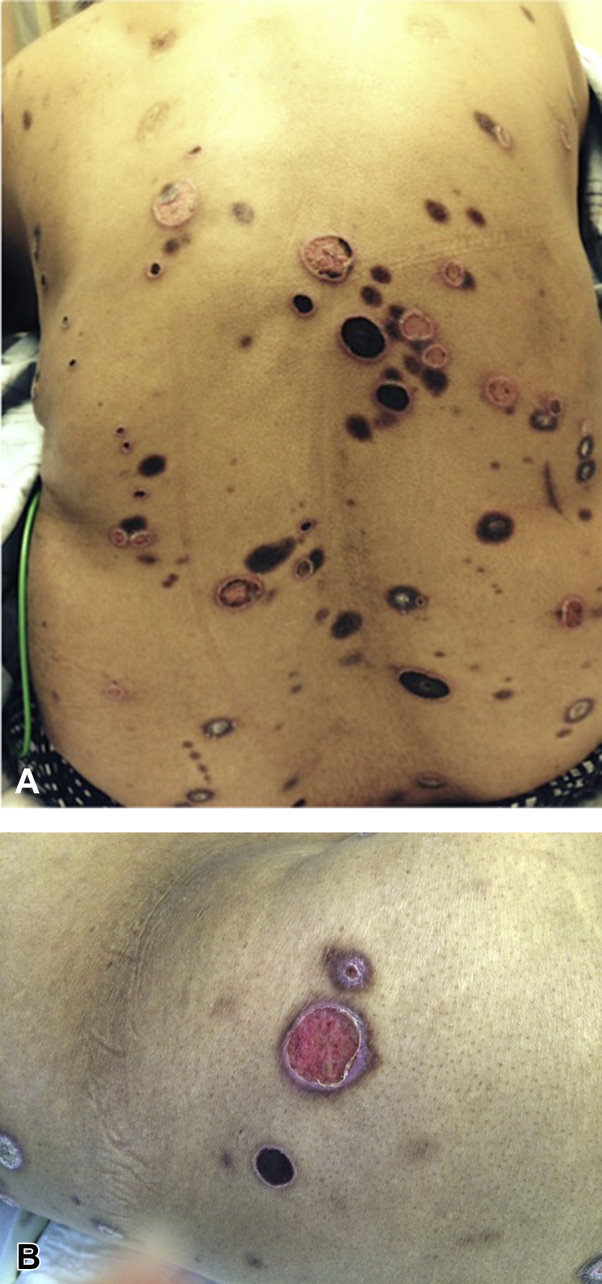
On physical examination, the patient had various sized, annular plaques with sharply demarcated pink-to-violaceous borders—some with a central crater and some with a central eschar on her trunk and extremities (Fig 1, A and B). Tissue cultures failed to grow any microorganisms. Histopathologic examination from a characteristic lesion found markedly degenerative elastic fibers in the superficial dermis with a striking pattern of transepithelial elimination through the epidermis and into the stratum corneum along with evidence of trauma characterized by a superficial myofibroblastic response of collagen deposition (Fig 2, A and B). Overall, the findings were typical for an acquired perforating dermatosis by a dominant pattern of elastic fiber transepidermal elimination. The patient was treated with topical corticosteroids and oral doxepin with mild improvement. Narrow band ultraviolet B phototherapy was declined by the patient. She was awaiting renal transplant.
Fig 1.
Giant variant of acquired perforating dermatosis. A, Photograph from clinical examination shows a generalized eruption. B, Note the annular plaques with a central crater or an eschar.
Fig 2.
A, Marked degeneration of elastic fibers in the superficial dermis and transepidermal elimination through a hyperplastic epidermis. B, Higher magnification of degenerated hypereosinophilic elastic fibers. (A and B, Hematoxylin-eosin stain.)
Discussion
Perforating dermatosis is a heterogeneous group of disorders characterized by transepidermal elimination of dermal components such as collagen, elastin, or fibrin.
Classically, there are 4 subtypes based on both clinical and histologic factors: reactive perforating collagenosis (RPC), elastosis perforans serpiginosa (EPS), perforating folliculitis, and Kyrle disease. In 1989, Rapini et al1 coined the term acquired perforating dermatosis (APD) as an entity distinct from the classic 4. APD denotes those perforating eruptions that arise in adulthood in the setting of comorbid diseases, such as DM and pruritus of renal failure. APD may histologically resemble any of the four classic subtypes or may exhibit an overlap of elimination patterns.1 RPC and Kyrle disease–like forms are more commonly reported than perforating folliculitis and EPS-like forms.2, 3 However, it is proposed to encompass all forms of APD instead of subclassifying them, as pathologic findings may vary from lesion to lesion, and manipulation of the lesions by patients frequently alters the histologic changes.
Clinically, APD presents as local or generalized 1- to 10-mm papules with a central keratotic crater. Although lesions may present anywhere on the body, there is a slight predilection for the lower extremities.2, 3 Symptoms of APD consist mostly of pruritus, and rarely pain.2, 3 The mean age of presentation is at the fourth to fifth decade of life, although any age may be affected with no gender predilection.2, 3, 4 The duration of disease varies from a few weeks to 8 years.2, 4 Our patient's clinical presentation is characterized by large plaques of 1 to 2 cm in diameter. It closely resembles the rare giant variant of APD first described by Hoque et al.5 Only 5 patients with this presentation have been reported in the literature.5, 6 Three of the 5 patients were Asian. All patients had pruritus. Trunk and extremities were preferred sites. Disease duration before the diagnosis of APD varied from 3 months to 5 years (mean = 23 months), likely because of its unusual presentation (Table I).5, 6
Table I.
Clinical features of reported giant variant of acquired perforating dermatosis
| Patient | Age (y), sex, race | Distribution | Associated disorders | Duration before diagnosis | Histologic features |
|---|---|---|---|---|---|
| 1 | 77, F, Asian | Lower legs | DM type I | 1 y | RPC-like |
| 2 | 70, M, Asian | Trunk, extremities | DM type II, proteinuria, hypertension | 3 mo | RPC-like |
| 3 | 37, M, white | Trunk, extremities | DM type I, ESRD, renal transplant | 3 y | RPC-like |
| 4 | 70, F, white | Trunk, extremities | CRF, hypertension, breast cancer | 5 mo | RPC-like |
| 5 | 57, M, Asian | Trunk, extremities, face | DM type II, CRF with hydronephrosis | 5 y | RPC-like |
| Our patient | 60, F, Asian | Trunk, extremities | ESRD on dialysis, hyperparathyroidism | 6 mo | EPS-like |
Differential diagnoses of perforating dermatosis include disorders of papules and nodules with a central keratotic crater or crust-like prurigo nodularis or multiple keratoacanthomas. EPS may resemble other annular or serpiginous disorders, such as granuloma annulare, sarcoid, and porokeratosis.
APD is often associated with at least one concomitant systemic disease. DM and chronic renal failure (CRF) are the most common, followed by hypertension, hepatitis, hypothyroidism, and chronic obstructive pulmonary disease.2, 3 The 5 patients with giant variant of APD had either longstanding history of DM or kidney diseases (Table I).5, 6 Although APD is rare, an incidence between 4.5% and 11% is reported in patients receiving maintenance hemodialysis.7, 8 They generally present with an RPC-like form.9 The EPS-like form of APD in patients with chronic kidney disease, as in our patient, is rare.
Conversely, classic or primary perforating dermatosis may be caused by either genetic or acquired abnormalities of collagen or elastic fibers. Classic EPS predominantly occurs in children. One quarter of cases occur in association with genetic disorders, including Down syndrome, Ehlers-Danlos, osteogenesis imperfecta, pseudoxanthoma elasticum, Marfan syndrome, and Rothmund Thompson syndrome. Drug-induced EPS has also been well documented secondary to penicillamine therapy, often in the setting of Wilson's disease.
The precise etiology and pathogenesis of APD is unknown. Superficial trauma, microvasculopathy in DM, calcium deposition in skin, and genetic predisposition are proposed to underlie the pathogenesis of APD. One theory for EPS in particular suggests that a trigger may induce the aggregation of altered elastic fibers and, in turn, initiate transepidermal elimination. In vitro and in vivo studies find elevations of elastin receptor in the epidermis surrounding the degenerating elastic fibers in EPS. Blocking receptors with an elastin receptor antibody attenuates the transepidermal elimination of the elastic fibers. This finding indicates that elastin receptor may play a role in the pathogenesis of EPS.10, 11
Many treatments for APD have been attempted. These include phototherapy, destruction with cryotherapy, curettage, electrocautery, excision, and medical treatments with imiquimod, allopurinol, topical or intralesional corticosteroids, and topical or oral retinoids. No single gold standard therapy exists that shows consistent success within current literature. In our patient, follow-up was insufficient to render whether treatment was effective.
Acknowledgments
The authors thank Magalie Bruneus, MD, for the clinical photos and critical review of the manuscript. No financial compensation was provided.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Rapini R.P., Herbert A.A., Drucker C.R. Acquired perforating dermatosis. Evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125(8):1074–1078. doi: 10.1001/archderm.125.8.1074. [DOI] [PubMed] [Google Scholar]
- 2.Kim S.W., Kim M.S., Lee J.H. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26(2):162–171. doi: 10.5021/ad.2014.26.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saray Y., Seckin D., Bilezikci B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20(6):679–688. doi: 10.1111/j.1468-3083.2006.01571.x. [DOI] [PubMed] [Google Scholar]
- 4.Akoglu G., Emre S., Sungu N., Kurtoglu G., Metin A. Clinicopathological features of 25 patients with acquired perforating dermatosis. Eur J Dermatol. 2013;23(6):864–871. doi: 10.1684/ejd.2013.2237. [DOI] [PubMed] [Google Scholar]
- 5.Hoque S.R., Ameen M., Holden C.A. Acquired reactive perforating collagenosis: four patients with a giant variant treated with allopurinol. Br J Dermatol. 2006;154(4):759–762. doi: 10.1111/j.1365-2133.2005.07111.x. [DOI] [PubMed] [Google Scholar]
- 6.Gnanaraj P., Venugopal V., Sangitha C., Rajagopalan V., Pandurangan C.N. A giant variant of acquired reactive perforating collagenosis associated with hydronephrosis: successful treatment with allopurinol. Int J Dermatol. 2009;48(2):204–206. doi: 10.1111/j.1365-4632.2009.03801.x. [DOI] [PubMed] [Google Scholar]
- 7.Hood A.F., Hardegen G.L., Zarate A.R., Nigra T.P., Gelfand M.C. Kyrle's disease in patients with chronic renal failure. Arch Dermatol. 1982;118(2):85–88. [PubMed] [Google Scholar]
- 8.Morton C.A., Henderson I.S., Jones M.C., Lowe J.G. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135(5):671–677. [PubMed] [Google Scholar]
- 9.Hari Kumar K.V., Prajapati J., Pavan G., Parthasarathy A., Jha R., Modi K.D. Acquired perforating dermatoses in patients with diabetic kidney disease on hemodialysis. Hemodial Int. 2010;14(1):73–77. doi: 10.1111/j.1542-4758.2009.00405.x. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto N., Tajima S., Ishibashi A. Elastin peptides induce migration and terminal differentiation of cultured keratinocytes via 67 kDa elastin receptor in vitro: 67 kDa elastin receptor is expressed in the keratinocytes eliminating elastic materials in elastosis perforans serpiginosa. J Invest Dermatol. 2000;115(4):633–639. doi: 10.1046/j.1523-1747.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto N., Akagi A., Tajima S. Expression of the 67-kDa elastin receptor in perforating skin disorders. Br J Dermatol. 2002;146(1):74–79. doi: 10.1046/j.1365-2133.2002.04550.x. [DOI] [PubMed] [Google Scholar]