Abstract
We present a case of a patient with elevated His lead capture thresholds and intermittent recruitment of the left bundle. The patient underwent a non-invasive electrophysiology study and was determined to have a left bundle branch block due to post-repolarization refractoriness. The nature of bundle branch block can have important implications for optimal patient selection and device programming in the emerging field of His bundle pacing.
Keywords: Left bundle branch block, his bundle, pacemaker, block, resynchronization therapy
Case report
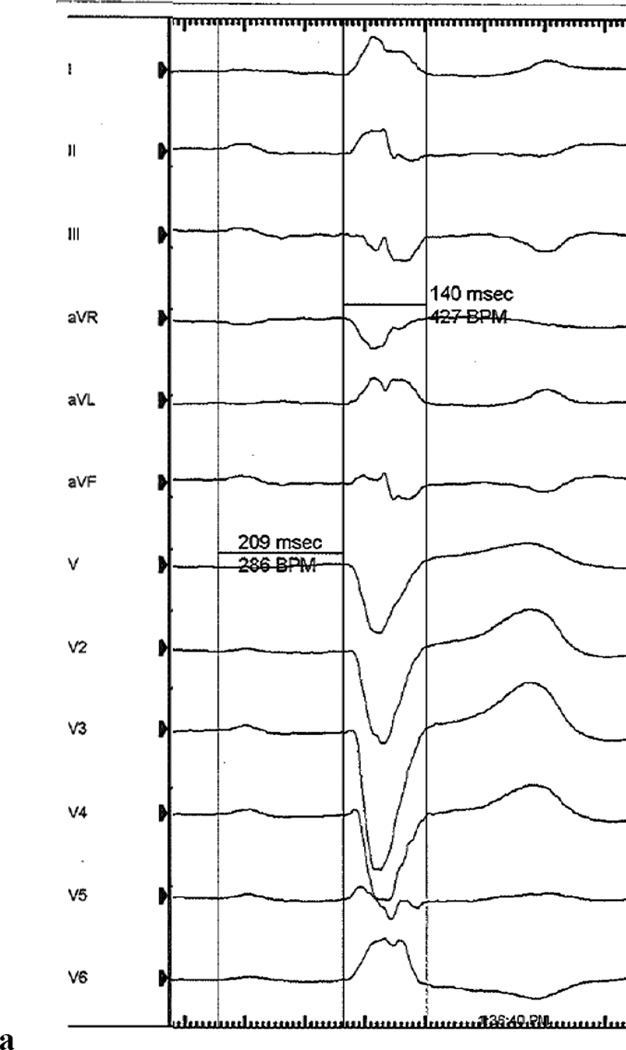
A 71 year old man with a history of coronary artery disease and hypertension presented for evaluation of syncope. The patient had experienced 4 years of pre-syncopal symptoms without palpitations or prodrome. Two months prior to evaluation, the patient experienced exertional syncope with atypical chest pain in the setting of new onset lower extremity edema and exertional dyspnea. A 12-lead ECG demonstrated first degree heart block (PR=209ms) and a left bundle branch block (LBBB) with a QRS duration of 140ms (Figure 1a). A cardiac MRI demonstrated normal left ventricular size, an ejection fraction of 55%, dyssynchronous left ventricular contraction consistent with a LBBB, and no evidence of scar as determined by delayed enhancement assessment. Based on the history of unexplained syncope and a LBBB, the patient was referred for implantation of a permanent pacemaker.
Figure 1.
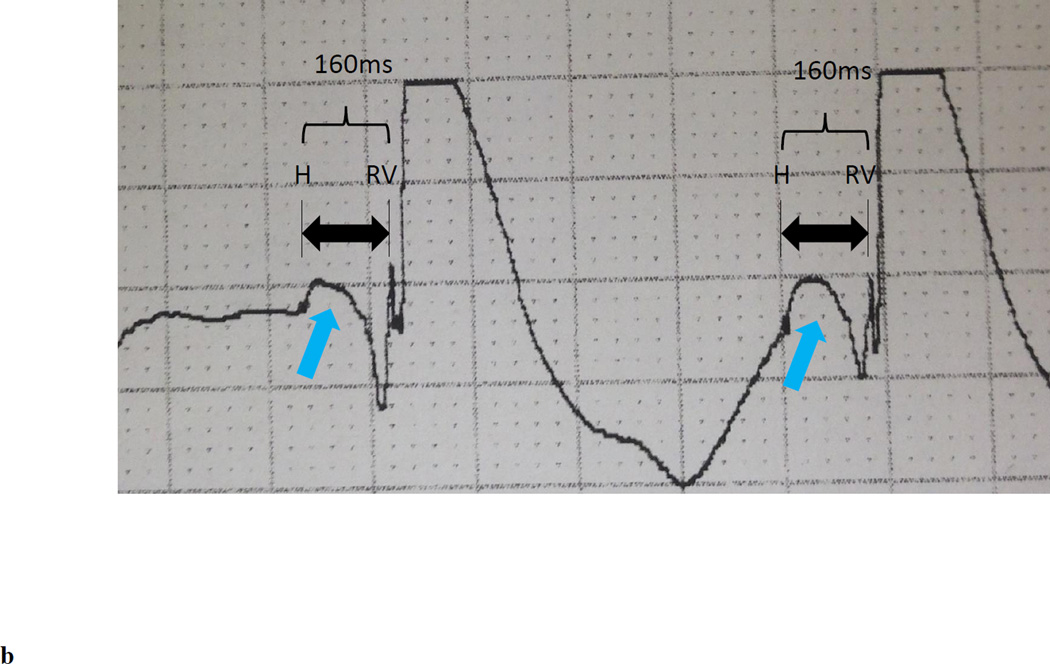
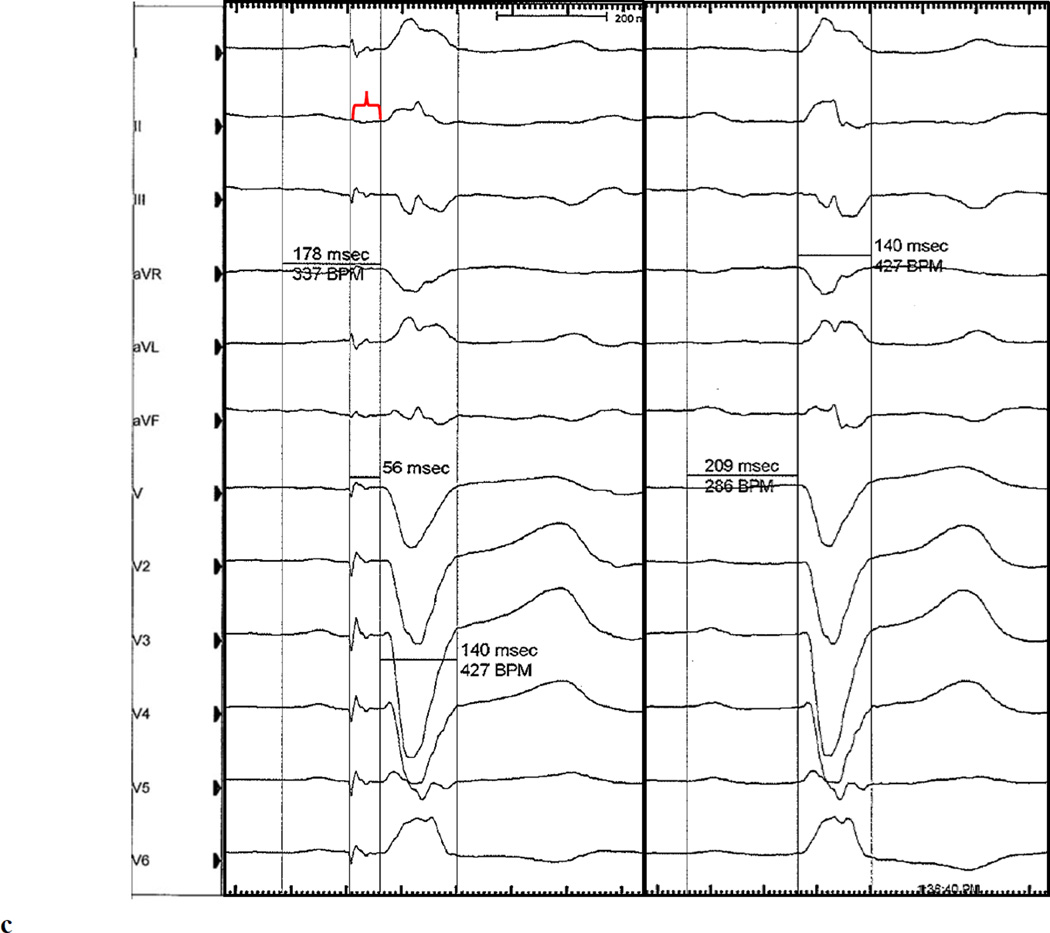
(a) Representative baseline 12-lead electrocardiogram (100mm/s sweep speed) demonstrating first degree AV block with a left bundle branch block. (b) His bundle electrogram at the final HB lead location demonstrated an His to anateroseptal RV interval of 160ms. No local A deflection is present, suggesting that the lead is positioned in the distal HB. An injury current (light blue arrow) is present within the H to anteroseptal RV interval because this electrogram was obtained shortly after implantiation of the active fixation (i.e. screw in) lead. (c) Representative electrocardiogram (100mm/s sweep speed) with a programmed HB lead output of 3V @ 0.4ms demonstrating a complex with a normal PR interval with persistent LBBB and a paced HV interval of 56ms, consistent with selective right fascicular capture (left pane). The isoelectric segment between the HB pacing spike and QRS onset (red brackets) demonstrates selective HB capture. A conducted QRS is displaced in the right pane for comparison.
The patient underwent implantation of a Medtronic Viva CRT-P C6TR01 pacemaker with a 5076 right atrial and 5076 right ventricular lead; a 3830 Select Secure lead was implanted into the His bundle (HB) in efforts to mitigate the deleterious effects of right ventricular pacing and LBBB. Sensing and pacing parameters were tested in both unipolar and bipolar configurations in 10 unique locations in the anatomical area of the HB. The anatomic location along the HB was determined based on the response to pacing. Capture of fibers predestined to become the right bundle (RB) and left bundle (LB) was possible only at the distal HB locations. Pacing distal to the final implant location led to ventricular capture. The HB electrogram at the final implant location demonstrated no visible atrial signal and a single HB deflection with a sensed His to anteroseptal RV interval of 160ms (Figure 1b). Atrial-HB sequential pacing from the final distal HB lead location was performed at an output of 3V (0.4ms). This resolved the 1st degree AV block with an unchanged QRS duration and morphology and a paced HV interval of 56ms (Figure 1c) suggesting selective capture of fibers predestined to become the RB. With the HB lead pacing output increased to 8V, intermittent selective capture of fibers predestined to become the RB and LB occurred as evidenced by intermittent resolution of both the 1st degree AV block and resolution of the LBBB with narrowing of the QRS to 88ms. Back-up right ventricular pacing was programmed in the event of loss of HB capture and the patient was discharged with the plan for short interval follow-up for device interrogation and non-invasive electrophysiology study to assess the nature of block and feasibility of long term HB pacing.
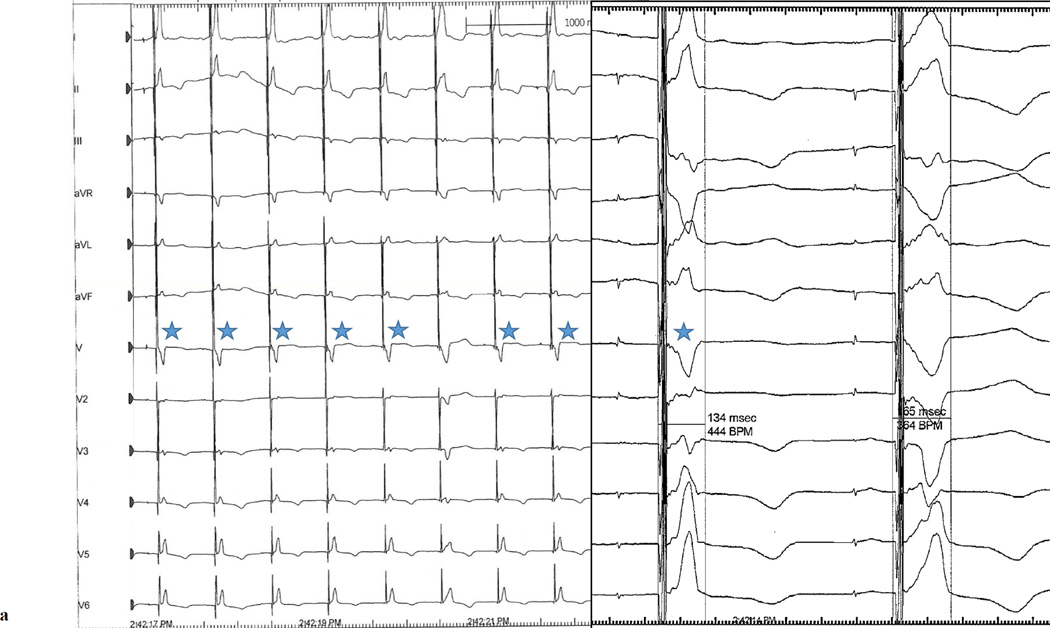
The patient returned to the electrophysiology laboratory 1 month after implantation for device interrogation with non-invasive EP study and continuous ECG monitoring (Mac-Lab v6.9.6, GE Healthcare). One month after implant, consistent RB recruitment with intermittent LB recruitment occurred only at the highest permanent programmable output (8.0V @ 1.2ms) and was only possible via the tip to can vector. Of note, this configuration provided non-selective HB capture as evidenced by a delta wave. The patient subsequently underwent pacing with each of the following parameters for 2 minutes: AAI at 90bpm, A-HB sequential pacing at 90bpm, AAI at 120bpm, and A-HB sequential pacing at 120bpm. The paced delay was programmed at 130ms for A-HB sequential pacing protocols to exclude the possibility of intrinsic AV conduction and HB pacing output was programmed at 8V @ 1.2ms. Table 1 depicts the frequency of RB recruitment, combined RB and LB recruitement, and intrinsic LBBB complexes, with the 4 pacing protocols. Notably, with A-HB sequential pacing at 90bpm, 66% of paced QRS complexes demonstrated evidence of recruitment of both RB and LB (Figure 2a). A-HB sequential pacing at 120bpm lead to alternating recruitement of RB only and both RB and LB, a consistent finding for the entirety of the 120 second maneuver (Figure 2b); after transition back to A-HB sequential pacing at 90bpm, the pattern of alternating recruitment immediately terminated and returned to the pattern that was previously observed at those same settings. Finally, temporary HB only pacing with a 15 beat drive train at 90bpm with a slightly higher output (8V @ 1.5ms) allowed for nearly 100% recruitment of both RB and LB across multiple successive drive trains.
Table 1.
Description of the 4 pacing protocols and resultant distribution of right bundle and left bundle recruitment.
| A-lead output |
HB lead output |
RB recruited | RB and LB recruited |
Intrinsic LBBB complexes |
(RB+LB recruited)/total paced complexes |
|
|---|---|---|---|---|---|---|
| A-HB 90 | 3.5 @ 0.4 | 8.0 @1.2 | 56 | 110 | 14* | 66% |
| AAI 90 | 3.5 @ 0.4 | N/A | 0 | 0 | 181† | N/A |
| A-HB 120 | 3.5 @ 0.4 | 8.0 @1.2 | 120 | 120 | 0 | 50% |
| AAI 120 | 3.5 @ 0.4 | N/A | 0 | 0 | 230‡ | N/A |
Due to 14 premature atrial contractions that occured at a coupling interval shorter than paced rate
One premature atrial contraction occured during the two minutes while pacing AAI 90
There were more atrial complexes than ventricular complexes due to 10 episodes of AV wenkebach.
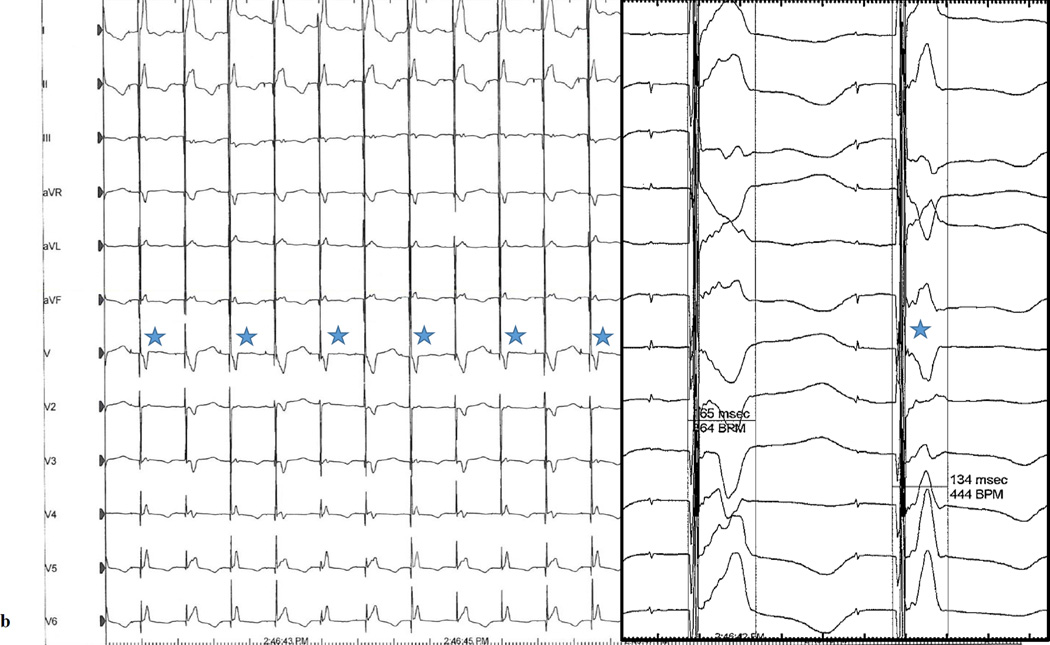
Figure 2.
A-HB sequential pacing with a programmed output of 8V @ 1.2ms at a rate of (a) 90bpm demonstrating frequent non-selective recruitment of both right and left bundles and (b) 120bpm demonstrating alternating non-selective recruitment of both right and left bundles and right bundle only. Blue stars denote complexes resulting from recruitment of the left and right bundles. Unmarked complexes are complexes resulting from right bundle recrutiment only. The left panels for both figures are at 25mm/s sweep speed; the right panels are at 100mm/s sweep speed.
Due to concerns with battery longevity, the HB lead was programmed off and the patient was reprogrammed to conventional DDDR pacing.
Discussion
We present a patient with a history of hypertension, coronary artery disease without evidence of infarction by MRI, 1st degree AV block, LBBB, and syncope who underwent implantation of a permanent pacemaker with a HB lead. The His to anteroseptal RV interval measured at implant (160ms) demonstrated evidence of significant concomitant conduction impairment of the RB. HB pacing at modest output led to recruitment of the RB only as evidenced by a normalized PR interval with preservation of the native LBBB. High output HB pacing allowed for additional recruitment of the LB and surrounding ventricular myocardium, resulting in a narrowed QRS. However, LB recruitment demonstrated rate dependency where increased pacing rates led to alternating recruitment of the RB only and both RB and LB. This case of physiologic LBBB due to acceleration dependent block at the level of the very distal HB illustrates a number of key concepts related to LBBB physiology and considerations for permanent HB pacing.
Multiple non-mutually exclusive mechanisms have been proposed to explain the ability for HB pacing to result in QRS narrowing among patients with bundle branch block due to intrahisian disease: (1) HB pacing distal to a site of purely anatomic block, (2) differential source-sink relationship during intrinsic HB conduction vs. direct HB stimulation, and (3) the virtual electrode polarization effect wherein electrical stimulation can render previously unexcitable tissue excitable. In our patient, although the final HB lead position was located in the distal HB (based on response to pacing along the length of the HB), it was unlikely to be beyond the level of block based on the very high capture threshold of the fascicles destined to become the LB. Prior reports have suggested that as the HB lead is positioned from proximal to distal relative to the level of anatomic block, an abrupt drop in pacing threshold can occur,1 often to values typically seen with ventricular pacing leads. Based on our findings, pacing distal to an anatomic block can be excluded as the mechanism for correction of LBBB in this patient. In this patient, very high output pacing (8V @ 1.5ms) from the HB lead was required to consistently recruit LB fibers. This finding is consistent with a differential source-sink relationship as the mechanism for HB pacing and LBBB correction in this patient where increased power is able to overcome refractoriness in a diseased distal HB. It is possible that virtual electrode polarization effect contributed to LB capture, allowing previously refractory tissue to conduct, although this mechanism is likely impossible to prove or disprove in this setting.
The rate and output dependence of LB recruitment demonstrates that the LBBB that appeared to be clinically persistent was in fact functional. The increased LB recruitment at lower rates demonstrates that the LBBB was rate dependent, albeit at non-physiologic levels of HB stimulation. A number of mechanisms for rate dependent block have been proposed, including (1) phase III block, (2) acceleration dependent block, (3) retrograde concealment, and (4) phase IV block.2 Notably, phase III block is a physiologic type of block that occurs in even normal conduction systems at high enough rates where action potentials encroach on each other. Acceleration dependent block occurs at lower (even normal) rates in diseased conduction tissue and is due to post-repolarization refractoriness leading to delayed recovery of excitability despite full repolarization.3 Phase IV block is thought to be due to failure to maintain a sufficiently negative membrane potential at longer cycle lengths. The presence of LBBB at a normal ventricular rate on the baseline ECG (Figure 1a) argues against phase III block as the explanatory mechanism as this should only occur at rates where action potentials are closely spaced. Although frequent concealed retrograde conduction into the LB is a possible mechanism, it is unlikely for a few reasons. First, the rate dependent nature of retrograde concealed conduction is such that it tends to persist briefly after a transition from a faster to slower rate2, and this was not observed when we transitioned pacing from A-HB 120bpm to A-HB 90bpm. Second, block due to retrograde concealed conduction is usually infrahisian2 and block at this level is frequently associated with inability to achieve HB pacing.1 Additionally, retrograde concealed conduction may be overcome at faster pacing rates, and the opposite was observed in this case. Although Phase IV block is thought to occur exclusively in a diseased conduction system (as is the case with our patient), it is a mechanism that occurs with pauses and lower heart rates (in contrast to our patient). After eliminating phase III & IV block and concealed retrograde conduction as plausible mechanisms, we posit that block in this patient is most consistent with postrepolarization refractoriness of predestined LB fibers4 at the level of the distal HB.
In summary, we present a case of a patient with LBBB due to postrepolarization refractoriness of LB predestined fibers in the distal HB that was defined with high output HB pacing. This case underscores a number of key observations. First, even clinically persistent LBBB can in fact be functional in the setting of HB pacing. Second, we demonstrate that postrepolarization refractoriness is a type of block that can be overcome with high energy output. Third, we demonstrate an example of how HB pacing can recruit the distal conduction system leading to QRS narrowing via a differential source-sink relationship during intrinsic HB conduction vs. direct HB stimulation, and that the efficacy of this mechanism can be rate related.
These observations have key implications for the field of HB pacing. With improved battery longevity and higher programmable pacemaker outputs, the type of block in our patient could be feasibly treated with HB pacing, allowing for cardiac resynchronization therapy without a left ventricular lead. Post repolarization refractoriness is hypothesized to be related to alterations in sodium/potassium homeostasis (during early Phase IV) that prevents depolarizations despite membrane potentials consistent with complete repolarization. Pacing approaches that may be less dependent on normal sodium/potassium homeostasis (e.g. anodal stimulation) require additional study as they may allow for successful HB pacing at lower outputs. Consistent with previous observations from Lustgarten and colleagues, the extent of fascicular capture (and QRS narrowing) in our patient was both output and pacing vector dependent.5 This underscores the importance of testing multiple pacing outputs during HB mapping and suggests that future development of HB pacing systems should incorporate the ability to pace using a variety of vectors. Finally, the rate dependency of predestined LB fiber capture in our patient demonstrates the importance of understanding the electrophysiologic mechanisms for block in patients undergoing HB pacing. We propose that all patients undergoing HB pacing (particularly those with a source-sink mechanism) should undergo threshold testing across the range of possible paced heart rates to assess for the presence of rate dependent block. For example, if a patient demonstrated Phase IV block with a source-sink mechanism for HB pacing, programming a higher lower rate limit could substantially improve capture threshold and battery longevity. Similarly, one might reduce an upper pacing rate or increase pacer output to reduce the risk of loss of capture at higher rates (e.g. during exertion) in a patient with post repolarization refractoriness and a source-sink mechanism.
Acknowledgments
Funding source: None
Footnotes
Conflicts of Interest: The authors have no conflicts relevant to this report.
References
- 1.Vijayaraman P, Naperkowski A, Ellenbogen KA, Dandamudi G. Electrophysiologic Insights Into Site of Atrioventricular Block: Lessons From Permanent His Bundle Pacing. JACC: Clinical Electrophysiology. 2015;1:571–581. doi: 10.1016/j.jacep.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Josephson ME. Josephson's Clinical Cardiac Electrophysiology. Philadelphia, PA: Wolters Kluwer; 2016. [Google Scholar]
- 3.El-Sherif N, Jalife J. Paroxysmal atrioventricular block: are phase 3 and phase 4 block mechanisms or misnomers? Heart Rhythm. 2009;6:1514–1521. doi: 10.1016/j.hrthm.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James TN, Sherf L. Fine structure of the His bundle. Circulation. 1971;44:9–28. doi: 10.1161/01.cir.44.1.9. [DOI] [PubMed] [Google Scholar]
- 5.Lustgarten DL, Crespo EM, Arkhipova-Jenkins I, Lobel R, Winget J, Koehler J, Liberman E, Sheldon T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart Rhythm. 2015;12:1548–1557. doi: 10.1016/j.hrthm.2015.03.048. [DOI] [PubMed] [Google Scholar]