Abstract
Objective
Liver transplantation is limited by ischemic injury which promotes endothelial cell and hepatocyte dysfunction and eventually organ failure. We sought to understand how endothelial state determines liver recover after hepatectomy and engraftment.
Design
Matrix-embedded endothelial cells (MEECs) with retained healthy phenotype or control acellular matrices were implanted in direct contact with the remaining median lobe of donor mice undergoing partial hepatectomy (70%), or in the interface between the remaining median lobe and an autograft or isograft from the left lobe in hepatectomized recipient mice. Hepatic vascular architecture, DNA fragmentation and apoptosis in the median lobe and grafts, serum markers of liver damage and phenotype of macrophage and lymphocyte subsets in the liver after engraftment were analyzed 7 days post-op.
Results
Healthy MEECs create a functional vascular splice in donor and recipient liver after 70% hepatectomy in mouse protecting these livers from ischemic injury, hepatic congestion and inflammation. Macrophages recruited adjacent to the vascular nodes into the implants switched to an anti-inflammatory and regenerative profile M2. MEECs improved liver function and the rate of liver regeneration and prevented apoptosis in donor liver lobes, autologous grafts, and allogeneic engraftment.
Conclusions
Implants with healthy endothelial cells rescue liver donor and recipient endothelium and parenchyma from ischemic injury after major hepatectomy and engraftment. This study highlights endothelial-hepatocyte crosstalk in hepatic repair and provides a promising new approach to improve regenerative medicine outcomes and liver transplantation.
Keywords: Liver, transplantation, immunology, regeneration
INTRODUCTION
Liver disease is one of the leading causes of death in the world.1 Hepatectomy and liver transplantation are the standard of care in patients with tumors of hepatic origin and end-stage liver disease.2 Yet, in 2014, only 40% of eligible patients received a liver transplant, which translates into a shortage of about 10,000 donors per year.3 During the same period, 23% of patients from the waiting list died and an additional 20% of patients were removed from that list as they became too sick to undergo surgery.3 Recent efforts have been devoted to generate hepatocyte-like cells and organ buds for transplantation.4–5 However those promising tools are still far from replacing liver transplantation in clinics. There is still much we do not know of the biology of liver injury and repair in these settings. Ischemic injury promotes a cascade of cellular responses that lead to inflammation, cell death, and ultimately hepatic and even multiorgan failure in recipients as well as donors.6–10 Further elucidation of the governing biology will help explain these events, provide potential means of avoiding them and perhaps even increase the number and size of successful donor grafts. Current therapeutic strategies focus on stimulating angiogenesis in grafts and reducing apoptosis and inflammation.11–12 Indeed, angiogenesis is an essential driving force for liver regeneration, the response to injury and organogenesis.13–14
Growing evidence suggests that liver sinusoidal endothelial cells (LSEC) are synergistic with hepatocyte proliferation and in establishing isograft tolerance.15–16 LSEC play critical protective roles controlling vascular tone, homeostasis, inflammation, and toxicant clearance.15 Preservation of a healthy LSEC phenotype is indispensable to minimization of liver injury and improvement of successful engraftment after hepatectomy and transplantation.17 Direct injection or transplantation of isolated healthy endothelial cells have been proposed to repair organ damage or replace deficient functions,18–19 but the immune reaction that they engender limits clinical utility.
Matrix-embedded endothelial cells (MEECs) places endothelial cells in a three-dimensional collagen-based scaffold that eliminates their immunogenicity in vitro and in vivo.20–21, stimulates Th2 lymphocyte and M2 macrophage phenotype, and exhibit a muted expression pattern of adhesion molecules and chemokines and a markedly decreased expression of major histocompatibility complex (MHC) class II molecules.22–23 We therefore proposed that healthy MEECs could boost the recovery of hepatocyte function by protecting host endothelium from inflammation and by promoting angiogenesis after hepatectomy and liver engraftment, and examined these effects in a murine model of hepatectomy and liver engraftment.
METHODS
Cell culture and seeding of MEECs or NIH/3T3 fibroblasts
Human umbilical vein endothelial cells (HUVECs) pooled from 3 donors or HUVECs constitutively expressing GFP were grown in endothelial growth medium supplemented with EGM-2 growth supplements (Lonza). NIH/3T3 fibroblasts (CRL-1658, American Type Culture Collection, ATCC, Manassas, VA) were grown in Dulbecco’s Modified Eagle Medium (DMEM) with 10% of fetal bovine serum. NIH/3T3 fibroblasts or HUVECs (passage 3–5) cultured on gelatin-coated tissue culture plates (0.1% gelatin type A, Sigma, St. Louis, MO) were then were seeded in 3D matrix. For cell-matrix seeding, compressed denatured collagen matrices (Gelfoam, Pfizer, New York, NY) were cut into 1 × 1 × 0.3 cm blocks and hydrated in culture medium at 37°C for 2h. Then 4.5 × 104 ECs or NIH/3T3 fibroblasts (suspended in 50 μL media) were seeded onto one surface of the hydrated matrix and allowed to attach for 1.5h. Subsequently, the matrix was turned over and additional 4.5 × 104 ECs were added to infiltrate from the second side. After an additional 1.5 h incubation period to enable cell attachment, each cell-seeded construct was carefully transferred to a separate 30 mL polypropylene tube containing 10 mL of culture medium. Matrices were cultured for 2 weeks, with media changed every 48h under standard culture conditions (37°C humidified environment with 5% CO2).
Animal model of 70% hepatectomy and liver engraftment
Male C57BL/6 mice (9–12 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). The animals were maintained in a temperature-controlled room (22°C) on a 12h light-dark cycle. After arrival, mice were continuously fed ad libitum until euthanasia. Partial hepatectomy was performed as previously described.24 An ischemic stump of ~7 mm was retained in the median lobe to allow immobilization of the different implants (acellular, MEECs or embedded NIH/3T3 fibroblasts) and to investigate the splicing and bridging of vessels to the irrigated part of the median lobe (supplementary figure 1). The right lobe was used to assess paracrine effects. For liver engraftment, excised mouse left lobes from a group of ten mice were excised and maintained in warm EGM-2 medium (37°C) until engraftment to the remaining median lobe and stump of a same or different group of ten mice in the presence or the absence of MEECs, or acellular matrices at the interface between recipient and donor liver (supplementary figure 1). Animals were sacrificed after one week. Mouse blood samples were collected by intracardiac puncture. Serum was separated by centrifugation at 3,000 × g for 10 min and was then transferred into polypropylene tubes and stored at −80°C until analysis. Liver restoration rate was calculated as liver weight/body weight × 100.
Whole-mount multiphoton imaging of macrophage presence and angiography in liver, gene expression analysis by Real-Time PCR, TUNEL assay, Western Blotting and “in vitro” studies
Statistical analysis
Data are expressed as mean standard error. Statistical analysis of the results was performed by one-way analysis of variance (ANOVA), the Newman-Keuls test, and the unpaired Student’s t test when appropriate. Differences were considered to be significant at a p value of 0.05 or less.
RESULTS
MEECs rescue the ischemic median lobe in mice undergoing 70% hepatectomy
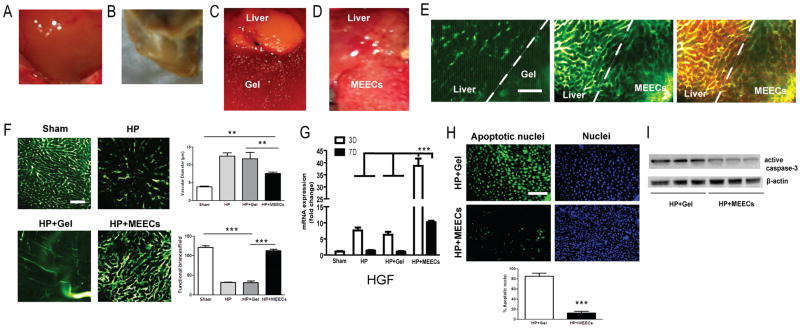
Partial hepatectomy (70%) consisted of excising most of healthy median lobe figure 1A and the whole left lobe. Acellular matrix or MEECs were implanted adjacent to the remaining ischemic portion of median liver lobe. Seven days later, the animals were sacrificed. At this time the macroscopic aspect of part of the residual median liver lobe from hepatectomized mice in the absence or the presence of acellular matrix indistinguishably displayed a phenotype of hepatic ischemia with a pale and stiff appearance typical in this animal model (figure 1B–C). Only 3 of 10 acellular implants were still attached to the liver at the time of sacrifice. In contrast, all implants with MEECs strongly attached to the ischemic part of the median liver lobe one week after implantation and the hepatic tissue macroscopically resembled normal liver (figure 1D). As this difference could be explained by a better blood perfusion of median lobes with MEECs, we analyzed the vascular structure at the interface between the injured liver and matrices by angiography. We observed that a new functional vascular network was created into the implant (supplementary figure 2) that anastomosed host livers (figure 1E). This network was not present in acellular matrices of denatured collagen (figure 1E). The newly formed vascular anastomoses were originated in part from the extension of hepatic vessels and in part from MEEK-generated angiogenesis as assessed by angiography after implanting ECs constitutively expressing GFP (supplementary figure 3). A small number of macrophages invaded the implant and were found adjacent to vessel ramifications (figure 1E) promoting vascular sprouting as recently reported.25 Vessel bypass between dysfunctional host vessels and implanted healthy MEECs allowed a reduction of blood congestion of the whole median lobe through the significant decrease of the vascular diameter (37%, p<0.01) and preservation of functional vessels (93%, p<0.0001) as compared to acellular matrices or the absence of implant (figure 1F). Since MEECs have been reported to attract endothelial progenitor cells (EPC)26 and EPC are major contributors of HGF levels after hepatectomy, we quantified hepatic expression of HGF in the ischemic lobe after 3 and 7 days of implantation of MEECs. HGF gene expression is up-regulated in ischemic liver lobes early after hepatectomy but drops to basal levels soon thereafter and has minimal if any impact on regeneration (figure 1G). Acellular matrices do not change this kinetics but MEECs drove HGF 5-fold higher than in ischemic livers attached to acellular implants and with those different levels maintained at day 7 (figure 1G). These increased levels of liver HGF were in part attributed to the increase of recruitment of bone marrow EPCs to the liver (supplementary figure 4).
Figure 1.
Beneficial effects of MEECs preventing liver damage in ischemic median lobe after 70% hepatectomy. C57BL/6 mice underwent 70% hepatectomy (excision of left lobe and half of median lobe). (A) Macroscopic aspect of a pre-op median lobe, 7 days post-op (B) or 7 days post-op with acellular denatured collagen implants (Gel) (C) or (D) MEECs. (E) Vascularity was analyzed in whole liver by angiography (intracardiac perfusion of FITC-dextran, MW 2×106 Da) using intravital multiphoton microscopy. Macrophages were also stained by intravenous injection of 70 kDa Texas red-dextran 2 hours before angiography and sacrifice. Representative images of the vascular network at the interface between the remaining median lobe and denatured collagen or MEECs are shown in green; macrophages are shown in red and intravascular merge of angiography and Texas red-dextran is shown in yellow. (F) Representative images of angiography and quantitative analysis of vascular diameter (congestion) and functional number of vessel branches in the hepatic median lobe of sham or hepatectomized mice (HP) in the presence or absence of acellular implants (HP+Gel) or MEECs (HP+MEECs) 7 days post-op. (G) Gene expression of hepatocyte growth factor (HGF) in ischemic median lobe after 3 or 7 days post-op assessed by Real-time PCR (H) To detect cell death, the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was used in median liver lobes from hepatectomized mice in contact with acellular implants or MEECs. Representative images of apoptotic nuclei are shown in green. Nuclei were stained with DAPI in blue. 200x magnification. Quantification of cell death is shown below. (I), Western blot corresponding to active caspase 3 was performed to assess apoptosis in median liver lobes from hepatectomized mice in contact with acellular implants or MEECs. Representative images of three samples of each group to detect active caspase 3 and the housekeeping β–actin are plotted. Scale bars, 100 μm. Data are represented as mean ± s.e.m. **P < 0.01, ***P < 0.001, analysis of variance (ANOVA) or t-student when appropriate.
We further investigated how MEECs attracted EPCs. MEECs (HUVECs in 3D) displayed much higher expression of the EPC attractants HGF and stromal cell-derived factor 1 (SDF-1) than HUVECs in standard 2D tissue-culture plates (supplementary figure 5A), and induced the expression of CXCR4 and CXCR7, two receptors involved in EPC migration- an induction obliterated in the presence of anti-HGF antibodies (supplementary figure 5B). To analyze cell damage and apoptosis induced by ischemia we stained median liver lobes using TUNEL assay and analyzed the activation of caspase 3. DNA fragmentation and damage was reduced by 85%, p<0.0001 (figure 1H) and apoptosis (i.e. active caspase 3 levels) dropped by 72%, p<0.01 (figure 1I) in livers implanted with MEECs as compared with livers receiving acellular implants. Therefore implants of healthy MEECs protect endothelium and parenchyma from death and loss of function in the ischemic lobe of liver donor after hepatectomy.
Beneficial effects of MEECs in vascular congestion, hepatic function, and liver regeneration after hepatectomy
To analyze the paracrine impact of implantation of healthy MEECs in the regenerating lobes we quantified vascular effects in right lobe 7 days post-op. Livers with or without acellular implant showed an identical increase of vascular diameter in comparison to sham livers (figure 2A). In contrast MEEC implants reduced vasodilation without altering angiogenesis in the growing organ expressed as number of new anastomoses (figure 2A). The same pattern was observed in the total number of macrophages in the right lobe, that is, an increase of the amount of macrophages after acellular implantation or without matrix and a drop in number of macrophages when MEECs were implanted (figure 2B). The recovery of the ischemic lobe by implants of MEECs resulted in an increase of 15% of total liver mass restoration as compared with livers with acellular matrices or without implants (figure 2C). This value of liver regeneration using MEECs implies complete recovery of original hepatic mass. As a result of the beneficial effects of MEECs, hepatic injury was reduced as seen in serum levels of ALT and AST (figure 2D).
Figure 2.
Vascular and immunomodulatory effects of MEECs in contact with ischemic median lobe improving liver regeneration and function. C57BL/6 mice underwent 70% hepatectomy (excision of left lobe and half of median lobe). (A) Representative images of angiography and quantitative analysis of vascular diameter (congestion) and angiogenesis (number of anastomoses) in the hepatic right lobe of sham or hepatectomized mice (HP) in the presence or absence of acellular implants (HP+Gel) or MEECs (HP+MEECs) 7 days post-op (B) Representative images and quantitative analysis of total number of macrophages and contacts with vessels in the hepatic right lobe analyzed by injection of 70 kDa Texas red-dextran 2 hours before sacrifice and angiography (intracardiac perfusion of FITC-dextran, MW 2×106 Da) using intravital multiphoton microscopy. Macrophages are shown in red and intravascular merge of angiography and Texas red-dextran is shown in yellow. (C) Liver restoration rate was assessed in sham or hepatectomized mice in the presence or absence of acellular implants or MEECs. Liver restoration rate was calculated as liver weight/body weight × 100. (D) Serum markers of liver damage Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) were quantified in hepatectomized mice in the presence of acellular implants or MEECs. Scale bars, 100 μm. Data are represented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, analysis of variance (ANOVA) or t-student when appropriate.
MEECs switch the phenotype of macrophages and T-helper lymphocytes from pro-inflammatory to anti-inflammatory and pro-regenerative
The reduction of the number of inflammatory cells using MEECs suggested that embedded ECs could have hepatic immunomodulatory effects on macrophage profile stimulating repair and reducing inflammation as reported.22 To identify the phenotype of macrophage subsets in livers after MEECs implantation we quantified the gene expression of M1 (inducible nitric oxide synthase: iNOS; cyclooxygenase 2: COX-2; interleukin 1β: IL1B) and M2 (arginase 1: Arg1; mannose receptor C type 1: MRC1; resistin-like alpha 1: Retn1a) genes by Real-Time PCR. Expression of genes corresponding to the pro-inflammatory macrophage profile M1 was progressively up-regulated from day 3 to day 7 post-hepatectomy in livers without matrix implants and those receiving acellular matrices - up-regulation that was significantly prevented by implants of MEECs (figure 3A). Expression of genes corresponding to the anti-inflammatory and pro-regenerative profile M2 was not significantly up-regulated in livers without MEECs and those receiving acellular matrices but was increased by implants of MEECs (figure 3B). It is documented that the switch from M1 to M2 in macrophages is mainly promoted by IL-4 and IL-10 released by Th2 cells27 and that Th2 subset is stimulated in T cells in contact with MEECs23. We found that hepatic abundance of Th1 genes progressively rose from day 3 to day 7 post-hepatectomy in ischemic lobe with or without acellular matrix but dropped to physiological levels in livers in contact with MEECs (figure 3C). Th2-derived cytokines were only up-regulated after hepatectomy when MEECs were implanted (figure 3D). We also investigated whether the beneficial effects of embedded endothelial cells on liver damage, regeneration and immunomodulation is specific for endothelial cell or can be promoted by other cell types, for example, fibroblasts. In contrast to MEECs, implants of embedded 3T3 fibroblasts allowed for cell invasion into host tissue promoting increased stiffness (supplementary figure 6A) and if anything reduced rather than improve hepatic damage (supplementary figure 6B) or regeneration (supplementary figure 6C) in the ischemic part of the median lobe. These implants also promoted a trend to a reduction in liver HGF expression that did not reach statistical significance (supplementary figure 6D). Moreover, again unlike endothelial cells, implants of 3T3 fibroblasts could not modify the profile of M1 macrophages (supplementary figure 6E) but did reduce significantly the subset of pro-regenerative M2 macrophages (supplementary figure 6G) as compared with livers implanted with acellular matrices. No differences were found in hepatic profiles of Th1 (supplementary figure 6G) or Th2 lymphocytes (supplementary figure 6H) between animals implanted with 3T3 fibroblasts or acellular matrices.
Figure 3.
Hepatic immunomodulation of gene expression profiles of macrophages and T helper lymphocytes 3 or 7 days after implantation of MEECs. Quantification of M1 (iNOS, COX-2 and IL1-β) (A) and M2 (Arg1, MRC1 and Retn1a) (B) gene expression profiles by Real-time PCR in sham or hepatectomized mice in the presence or absence of acellular implants (Gel) or MEECs. (C) Quantification of gene expression profiles of Th1 (INFγ and IL-2) and (D) Th2 (IL-4 and IL-10) by Real-time PCR in sham or hepatectomized mice in the presence or absence of acellular implants (Gel) or MEECs. Data are represented as mean of fold change ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, analysis of variance (ANOVA).
MEECs bridge vessels from recipient and donated autografts protecting from ischemic injury
Injury derived from ischemia occurs in various clinical settings, such as transplantation, hepatectomy for cancer resection, and hemorrhagic shock11. For that reason, we hypothesized that healthy MEECs could help re-vascularize liver grafts to rescue dysfunctional endothelium in transplantation. We implanted MEECs in the interface between median ischemic lobe after hepatectomy and a liver graft from the left lobe of the same mouse (figure 4A). Either median ischemic lobe or autograft displayed a pale color when acellular denatured collagen was implanted (figure 4B). In contrast, both remaining median lobe and autograft showed a normal liver color when MEECs were implanted in between (figure 4C). Analyzing the vascularity, we found that blood perfusion was very reduced or inexistent in median lobe and autograft in contact with acellular implants. In contrast implanted healthy MEECs bridged vessels between remaining median lobe and autograft (figure 4D) and promoted EPC recruitment into the injured lobe as shown by increased levels of HGF (supplementary figure 3A). Consequently, MEECs preserved vascular functionality in median lobe and reduced vessels diameter and congestion (figure 4E). That protection of MEECs against ischemia resulted in a drastic reduction of hepatic median lobe damage (supplementary figure 4A) and autograft cell injury (85% of reduction) (figure 4F) and apoptosis (figure 4G). Overall, mice receiving MEECs displayed significantly lower levels of serum transaminases indicating a reduction in hepatocyte damage (figure 4H).
Figure 4.
Beneficial effects of MEECs preventing liver damage after autologous engraftment. (A) Schematic representation of surgical implantation of MEECs or acellular implants in the interface between the ischemic median liver lobe and the donated graft from the left liver lobe. (B) Macroscopic aspect of median lobe and autologous grafts implanted with acellular denatured collagen or (C) MEECs 7 days post-op. (D) Vascularity was analyzed in the interface between median liver lobe and autologous graft by angiography using intravital multiphoton microscopy. Representative images of the vascular network at the interface between the remaining median lobe, acellular Denatured collagen or MEECs and the graft are shown in green. (E) Representative images of angiography and quantitative analysis of vascular diameter (congestion) and functional number of vessel branches in the hepatic median lobe of hepatectomized mice in the presence of acellular implants (HP+Gel) or MEECs (HP+MEECs) 7 days post-op. (F) To detect intragraft cell death, TUNEL assay was performed in autologous liver grafts in contact with acellular implants or MEECs. Representative images of apoptotic nuclei are shown in green. Nuclei were stained with DAPI in blue. 200x magnification. Quantification of cell death is shown below. (G) To assess apoptosis, Western blot corresponding to active caspase 3 was performed in autologous liver grafts from mice in contact with acellular implants or MEECs. Representative images of three samples of each group to detect active caspase 3 and the housekeeping β–actin are plotted. (H) Serum markers of liver damage Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) were quantified in hepatectomized mice in the presence of acellular implants or MEECs. Scale bars, 100 μm. Data are represented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, analysis of variance (ANOVA) or t-student when appropriate.
MEECs bridge vessels from recipient and donated isografts protecting from ischemic injury and immunomodulating a reduction of graft rejection
MEECs attenuate immune rejection in allo- and xenogeneic cell implants.21 For this reason, we now analyzed the effects of these implants in hepatic isografts. Median ischemic lobe and isograft displayed a pale color when acellular denatured collagen was implanted and that ischemic color was partially reverted when MEECs were used (figure 5A and B). Vascularity was significantly reduced or entirely obliterated in the median lobe and isograft in contact with acellular implants. In contrast, MEECs connected vessels between the median lobe and isograft (figure 5C) and stimulated EPC recruitment into the injured area as shown by enhanced levels of HGF (supplementary figure 3B). As a result, healthy MEECs protected the dysfunctional vascular network in median lobes and reduced congestion (figure 5D). These beneficial effects on ischemia were translated into a significant reduction of hepatic median lobe injury (supplementary figure 3B) and isograft cell death (79% of reduction) (figure 5E) and apoptosis (figure 5F). Although 50% of immunocompetent mice implanted with isografts died of acute tissue rejection within the first 24 hours, the other half that survived exhibited intragraft immunotolerance expressed as reduction of Th1 (INFγ and IL-2) and increase of Th2 (IL-4 and IL-10) cytokine expression (figure 5G). Those mice receiving isografts in the presence of MEECs implants showed improved levels of serum transaminases thus reducing hepatocyte damage (figure 5H).
Figure 5.
Beneficial effects of MEECs preventing liver damage after allogeneic engraftment. (A) Macroscopic aspect of median lobe and allogeneic grafts implanted with acellular denatured collagen (Gel) or (B) MEECs 7 days post-op. (C) Vascularity was analyzed in the interface between median liver lobe and allogeneic graft by angiography using intravital multiphoton microscopy. Representative images of the vascular network at the interface between the remaining median lobe, acellular Denatured collagen or MEECs and the graft are shown in green. (D) Representative images of angiography and quantitative analysis of vascular diameter (congestion) and functional number of vessel branches in the hepatic median lobe of hepatectomized mice in the presence of acellular implants (HP+Gel) or MEECs (HP+MEECs) 7 days post-op. (E) To detect intragraft cell death, TUNEL assay was performed in allogeneic liver grafts in contact with acellular implants or MEECs. Representative images of apoptotic nuclei are shown in green. Nuclei were stained with DAPI in blue. 200x magnification. Quantification of cell death is shown below. (F) To assess apoptosis, Western blot corresponding to active caspase 3 was performed in allogeneic liver grafts from mice in contact with acellular implants or MEECs. Representative images of three samples of each group to detect active caspase 3 and the housekeeping β–actin are plotted. (G) Intragraft gene expression profile of immunotolerance expressed as Th1 (INFγ and IL-2) and Th2 (IL-4 and IL-10) cytokine expression analyzed by Real-Time PCR (H) Serum markers of liver damage Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) were quantified in mice with isografts in the presence of acellular implants or MEECs. Scale bars, 100 μm. Data are represented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, analysis of variance (ANOVA) or t-student when appropriate.
DISCUSSION
Ischemic injury is a multifactorial process that affects graft function after liver transplantation. Although recent efforts have improved organ preservation and surgical outcomes28–29, there is still a need to understand the basic biology and provide further support of organ viability. Liver ischemia, apoptosis and endothelial dysfunction restrict the success of hepatectomy and liver transplantation. The recovery of blood perfusion in both the recipient and the graft, and protection from adverse inflammatory response are critical events for successful transplantation.2 M2 profile of macrophages is potentiated in response to partial hepatectomy or hepatic injury to regenerate the damaged tissue.30 However, M1/M2 balance in macrophages is flexible and the M1 inflammatory phenotype can perpetuate chronic hepatic inflammation and interfere with liver regeneration.31 This manuscript demonstrates that viability of liver sinusoidal endothelium determines the fate of an engrafted hepatic transplant. Implanted healthy matrix-embedded endothelial cells can rescue dysfunctional endothelium in an ischemic liver and stimulate the immune system to boost engraftment and regeneration.
Hepatic sinusoids are lined by a thin layer of functionally unique endothelial cells. LSECs display a high-capacity to clear colloids and soluble waste macromolecules from the circulation to protect hepatocytes, but as such are also the initial target of injury from circulating drugs and toxins and by ischemia-reperfusion injury.2 After toxic liver injury, partial hepatectomy or transplantation, damaged LSECs progressively become dysfunctional and may interfere with hepatocyte function and liver regeneration. LSEC progenitor cells arise from the liver and bone marrow (BM LSEC) to contribute to the regenerative response of hepatocytes. These mobilized BM LSEC progenitors engraft in the liver, proliferate and are the highest secretors of the mitogen hepatocyte growth factor (HGF).32–33 While mature LSECs express and secrete low levels of HGF, high levels of HGF are observed in liver endothelial progenitor cells and bone marrow-derived LSEC (BM LSEC) progenitors after liver injury.32–33 LSEC dysfunction or a failure of mobilization of BM LSEC translates into a defective secretion of HGF and an impaired hepatocyte proliferation.33 MEECs retain high capacity of attracting endothelial progenitors cells.26 Herein we describe that matrix embedding upregulates endothelial cell expression of two EPC-recruiting factors SDF-1 and HGF34, which especially in the case of HGF stimulated the expression of the receptors CXCR4 and CXCR7 in BM EPCs recruiting these latter cells to the implant area and injured liver.34 Once recruited, EPCs in turn secrete more HGF promoting a positive feedback (supplementary figure 7). The mobilization of BM LSEC progenitors by MEECs to the injured area stimulate angiogenesis, and this recovery of endothelial cells improves hepatocyte survival, function and liver regeneration. Indeed, the recruitment of BM LSEC is essential for hepatocyte proliferation and restoration of liver mass.35 MEECs also generate a functional vascular network that splice injured vessels protecting livers from ischemia and reducing apoptosis.
Controlled inflammation is important to the integration and vascularization of biomaterial scaffolds.36 MEECs achieve an energy state that minimizes stress, shields their immunogenic surface37 and maximizes the secretion of regulatory factors promoting the switch of Th1 to Th223 lymphocytes, and the subsequent switch of M1 to M2 macrophages to enhance repair. Indeed, some factors secreted by MEECs induce formation and differentiation of host splenocytes into Th2, but not Th1, cytokine-producing cells.23 We demonstrate here that one of these regulatory factors highly up-regulated in MEECs is HGF. A recent study has showed that HGF induces macrophages to switch to M2-profile and produce IL-10. 38 We also show how implantation of MEECs with injured livers or grafts stimulates the production of Th2 cytokines IL-4 and IL-10, and the reduction of the Th1 cytokines INFγ and IL-2. IL-4 is required for liver regeneration after partial hepatectomy as IL-4-deficient mice are associated with massive injury, higher morbidity and mortality and impaired liver regeneration.30 The promotion of Th2-derived cytokines in the Th1/th2 balance explains, in part, the faster and total recovery of liver mass after hepatectomy in mice implanted with healthy MEECs. The rescue of dysfunctional endothelium that MEECs promote in the ischemic lobe is an additional contribution to the protection and recovery of liver mass and reduction of apoptosis.
Embedded endothelial cells constructs can be stored for months, and then placed in challenging positions to regulate the local environment. We show that when placed in animals that underwent autologous and allogeneic liver grafts MEECs control the local and systemic immune response, promote the bridging between recipient liver and graft vessels and enhance regeneration. These therapeutic angiogenic, immunomodulatory and anti-apoptotic effects of MEECs overcome the current risks of stem cell-derived implants for transplantation as MEECs restore liver function minimizing any concomitant immune reaction. Long-term immunosuppression is required to avoid severe acute and chronic rejection and graft loss in transplanted patients.39 We show in immunocompetent mice how healthy MEECs can modulate the behavior of host dysfunctional endothelial cells and immune system to minimize isograft injury and rejection in the absence of any type of immunosuppression. Indeed MEECs reduce the impact of Th1 cytokines and increase Th2 cytokines in mice receiving isografts improving immunotolerance of implants and isografts. Such approach is in line with current strategies aiming to promote stable long-term immunological tolerance of the liver graft.39 MEEC rescuing dysfunctional endothelium and hepatocyte function after hepatectomy might present a novel treatment of ischemia and organ dysfunction in transplantation and suggest a pragmatic solution to the urgent global need for liver donations – maximizing efficiency of tissue recovery and reducing risks in donors. This embedded state shields the endothelial cells’ immunogenic surface and reduces the expression of MHC-II complex and inflammatory pathways independently of the origin of the endothelial cell.40 The immunomodulatory effects of MEECs together with our previous knowledge that any source of endothelial cells displays similar benefits when implanted after injury,41–42 suggest that the tissue engineering technology presented here might be also applied in a human setting. Matrix-embedding commercial primary endothelial cells or endothelial cells isolated from vessels harvested during an exploratory intervention may be re-implanted to bridge the gap between injured vessels, to stimulate angiogenesis, and to improve the success of the engraftment in transplanted patients. Possible challenges to take into account for this procedure are the source of these endothelial cells (species, tissue compatibility, artery or vein, etc.), the viability of the cells during maintenance without adequate CO2 incubators and the risk of contaminations if stored in non-sterile areas.
In conclusion, healthy MEECs rescue endothelium function in donor and grafts and also exert immunomodulatory effects to stimulate hepatic repair and regeneration and to reduce liver graft rejection. Since ischemic injury is a common trait in all of transplants and other clinical situations, our outcomes provide insight into potential beneficial use of MEECs in liver transplantation and in other ischemia-derived disorders.
Supplementary Material
Significance of this study.
What is already known about this subject?
Ischemic injury promotes endothelial dysfunction in recipient livers and grafts during liver transplantation.
Liver endothelial cell dysfunction or a failure of mobilization of endothelial progenitors impair liver regeneration.
Recovery of blood perfusion and hepatic mass is critical for recovery of liver function in patients undergoing hepatectomy and transplantation.
Immune reaction of T lymphocytes and macrophages can promote either inflammation or regeneration, immunotolerance or graft rejection.
What are the new findings?
Healthy matrix-embedded endothelial cells rescue dysfunctional endothelium from ischemic liver lobes, restoring blood perfusion and reducing apoptosis.
Healthy matrix-embedded endothelial cells switch the pro-inflammatory profile of Th1 and M1 cells to pro-regenerative Th2 and M2 after hepatectomy.
Healthy matrix-embedded endothelial cells bridge injured endothelia of recipient and graft livers and protect from inflammatory reaction and rejection after engraftment.
The recovery of endothelium functionality after matrix-embedded endothelial cells implantation improves liver regeneration and hepatocyte function after hepatectomy and engraftment.
How might it impact on clinical practice in the foreseeable future?
This investigation defines new strategies to improve the endothelial and hepatic function of remnant livers after major resection and liver grafts in living donor transplantation.
Healthy endothelial cells embedded in denatured collagen is a potential solution to the current urgent global need for liver donations - maximizing efficiency of tissue engraftment and recovery, and reducing risks in donors
Implantation of matrix-embedded endothelial cells represents a breakthrough in the treatment of ischemia and organ dysfunction in transplantation, opens new avenues to the management of surgery and intervention in urgent care and means a new hope to rescue ischemic organs and tissues.
Acknowledgments
This work was supported by a grant from the NIH (R01 GM 49039). P.M-L was supported by a post-doctoral fellowship from the Fundacion Alfonso Martin Escudero program 2012 and then by the Beatriu de Pinós Program, Modalitat-A awarded by AGAUR (fellowship number: 2013 BP_A 00051). M.B was supported by Proyecto Plan Nacional SAF2013-43302-R. We acknowledge support provided by the David H. Koch Institute for Integrative Cancer Research at the Massachusetts Institute of Technology for providing access to multiphoton microscopy used for this study.
Footnotes
Author contributions
P.M-L. and E.R.E. conceived of the idea, and P.M-L and M.B. carried out the work.
P.M-L. and E.R.E. co-wrote the paper, and M.B. edited.
Competing interests
The authors declare no competing financial interests.
Ethical approval.
The study was approved by the Animal Ethics Committee at Massachusetts Institute of Technology, MA, USA.
References
- 1.Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733–746. doi: 10.1016/j.cld.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Zhai Y, Petrowsky H, Hong JC, et al. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Organ Procurement and Transplantation Network. 2014 [online], http://optn.transplant.hrsa.gov/data/
- 4.Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 5.Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni S, Malagò M, Cronin DC., 2nd Living donor liver transplantation for pediatric and adult recipients. Nat Clin Pract Gastroenterol Hepatol. 2006;3:149–157. doi: 10.1038/ncpgasthep0437. [DOI] [PubMed] [Google Scholar]
- 7.Roll GR, Parekh JR, Parker WF, et al. Left hepatectomy versus right hepatectomy for living donor liver transplantation: shifting the risk from the donor to the recipient. Liver Transpl. 2013;19:472–481. doi: 10.1002/lt.23608. [DOI] [PubMed] [Google Scholar]
- 8.Nissing MH, Hayashi PH. Right hepatic lobe donation adversely affects donor life insurability up to one year after donation. Liver Transpl. 2005;11:843–847. doi: 10.1002/lt.20411. [DOI] [PubMed] [Google Scholar]
- 9.Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337–345. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lentsch AB, Kato A, Yoshidome, et al. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsung A, Klune JR, Zhang X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto K, Yoshitomi H, Rossant J, et al. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 15.DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123(5):1861–66. doi: 10.1172/JCI66025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumitran-Holgersson S, Ge X, Karrar A, et al. A novel mechanism of liver isograft rejection facilitated by antibodies to liver sinusoidal endothelial cells. Hepatology. 2004;40(5):1211–21. doi: 10.1002/hep.20434. [DOI] [PubMed] [Google Scholar]
- 17.Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59(5):1094–106. doi: 10.1016/j.jhep.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Follenzi A, Benten D, Novikoff P, et al. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J Clin Invest. 2008;118(3):935–45. doi: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Liu ZD, Wu N, et al. Transplanted endothelial progenitor cells ameliorate carbon tetrachloride-induced liver cirrhosis in rats. Liver Transpl. 2009;15(9):1092–100. doi: 10.1002/lt.21845. [DOI] [PubMed] [Google Scholar]
- 20.Methe H, Hess S, Edelman ER. The effect of three-dimensional matrix-embedding of endothelial cells on the humoral and cellular immune response. Semin Immunol. 2008;20:117–122. doi: 10.1016/j.smim.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Methe H, Groothuis A, Sayegh MH, et al. Matrix adherence of endothelial cells attenuates immune reactivity: induction of hyporesponsiveness in allo- and xenogeneic models. FASEB J. 2007;21:1515–1526. doi: 10.1096/fj.06-7051com. [DOI] [PubMed] [Google Scholar]
- 22.Methe H, Edelman ER. Cell-matrix contact prevents recognition and damage of endothelial cells in states of heightened immunity. Circulation. 2006;114:I233–I238. doi: 10.1161/CIRCULATIONAHA.105.000687. [DOI] [PubMed] [Google Scholar]
- 23.Methe H, Nanasato M, Spognardi AM, et al. T-helper 2 cells are essential for modulation of vascular repair by allogeneic endothelial cells. J Heart Lung Transplant. 2010;29:479–486. doi: 10.1016/j.healun.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 25.Melgar-Lesmes P, Edelman ER. Monocyte-endothelial cell interactions in the regulation of vascular sprouting and liver regeneration in mouse. J Hepatol. 2015 May 25; doi: 10.1016/j.jhep.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Indolfi L, Iaconetti C, Monteforte A, et al. Harnessing Cell: Materials Interactions to Develop Innovative Strategy for the Recruitment of Progenitor Cells. Society for Biomaterials. Annual meeting; 2014. Abstract 250. [Google Scholar]
- 27.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59(5):2034–42. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 28.Fondevila C, Hessheimer AJ, Maathuis MH, et al. Hypothermic oxygenated machine perfusion in porcine donation after circulatory determination of death liver transplant. Transplantation. 2012;94:22–29. doi: 10.1097/TP.0b013e31825774d7. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Cabús S, Fondevila C, Calatayud D, et al. Importance of the temporary portocaval shunt during adult living donor liver transplantation. Liver Transpl. 2013;19:174–183. doi: 10.1002/lt.23558. [DOI] [PubMed] [Google Scholar]
- 30.DeAngelis RA, Markiewski MM, Kourtzelis I, et al. A complement-IL-4 regulatory circuit controls liver regeneration. J Immunol. 2012;188(2):641–8. doi: 10.4049/jimmunol.1101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu R, Zhang Z, Wang FS. Liver fibrosis: mechanisms of immune-mediated liver injury. Cell Mol Immunol. 2012;9(4):296–301. doi: 10.1038/cmi.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maher JJ. Cell-specific expression of hepatocyte growth factor in liver: Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest. 1993;91(5):2244–52. doi: 10.1172/JCI116451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Wang X, Xie G, et al. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest. 2012;122(4):1567–73. doi: 10.1172/JCI58789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalakas E, Newsome PN, Harrison DJ, et al. Hematopoietic stem cell trafficking in liver injury. FASEB J. 2005;19(10):1225–31. doi: 10.1096/fj.04-2604rev. [DOI] [PubMed] [Google Scholar]
- 35.Harb R, Xie G, Lutzko C, et al. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology. 2009;137(2):704–12. doi: 10.1053/j.gastro.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–88. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Indolfi L, Baker AB, Edelman ER. The role of scaffold microarchitecture in engineering endothelial cell immunomodulation. Biomaterials. 2012;33(29):7019–27. doi: 10.1016/j.biomaterials.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proto JD, Tang Y, Lu A, et al. NF-κB inhibition reveals a novel role for HGF duringskeletal muscle repair. Cell Death Dis. 2015;6:e1730. doi: 10.1038/cddis.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams DH, Sanchez-Fueyo A, Samuel D. From immunosuppression to tolerance. J Hepatol. 2015;62(1S):S170–S185. doi: 10.1016/j.jhep.2015.02.042. [DOI] [PubMed] [Google Scholar]
- 40.Methe H, Edelman ER. Tissue engineering of endothelial cells and the immune response. Transplant Proc. 2006;38(10):3293–9. doi: 10.1016/j.transproceed.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nugent HM, Sjin RT, White D, et al. Adventitial endothelial implants reduce matrix metalloproteinase-2 expression and increase luminal diameter in porcine arteriovenous grafts. J Vasc Surg. 2007;46(3):548–556. doi: 10.1016/j.jvs.2007.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zani BG, Kojima K, Vacanti CA, et al. Tissue-engineered endothelial and epithelial implants differentially and synergistically regulate airway repair. Proc Natl Acad Sci U S A. 2008;105(19):7046–51. doi: 10.1073/pnas.0802463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.