Abstract
BACKGROUND
Although safety of combination chemotherapy without primary tumor resection (PTR) in patients with stage 4 colon cancer has been established, questions remain regarding potential survival benefit with PTR. The purpose of this study is to compare mortality rates in colon cancer patients with unresectable metastases who have and have not received PTR.
METHODS
An observational cohort study was conducted of patients with unresectable metastatic colon cancer identified from the National Cancer Data Base(2003-2005). Multivariate Cox regression analysis, with and without Propensity Score Weighting (PSW), was performed to compare survival outcomes. Instrumental Variable (IV) analysis, using the annual hospital-level PTR rate as the instrument, was utilized to account for treatment selection bias. To account for survivor treatment bias, where patients might die soon after diagnosis from different reasons, a landmark method was utilized.
RESULTS
PTR was performed in 57% of the total cohort (8641/15154) and 73.8 % of those at landmark (4,972/6,735). PTR was associated with a significant reduction in mortality using Cox regression (HR, 0.45; 95%CI, 0.44-0.47) or PSW (HR, 0.46; 95%CI, 0. 44-0.49). However, IV analysis showed a much smaller effect,(RMR, 0.91; 95%CI, 0.87-0.96). While a smaller benefit was seen on landmark method using Cox regression (HR, 0.6; 95%CI, 0.55-0.64) or PSW (HR, 0.59; 95%CI, 0.54-0.64), IV analysis showed no survival benefit (RMR, 0.97; 95%CI, 0.87-1.06).
CONCLUSION
Among patients with unresectable metastatic colon cancer, following adjustment for confounder effects, PTR was not associated with improved survival when compared to systemic chemotherapy; therefore routine non-curative PTR is not recommended.
INTRODUCTION
Colorectal cancer is the second leading cause of cancer-related death in the United States,1 and approximately 20% of patients present with distant metastases at the time of diagnosis.2 In patients with unresectable metastatic disease, the primary treatment is systemic chemotherapy, and primary tumor resection (PTR) is indicated for the treatment of primary tumor-related complications. In the US, noncurative PTR in patients with metastatic disease is commonly performed and is the first treatment modality in most patients receiving surgical resection and chemotherapy.3 However, there is still significant controversy regarding the role of PTR and its potential impact on survival in stage IV patients.
PTR in the setting of unresectable metastatic disease is currently recommended for patients who present with tumor-related complications such as bleeding, obstruction, or perforation that may preclude the ability to administer systemic therapy. Systemic therapy can be well tolerated as initial therapy in asymptomatic patients with metastatic colorectal cancer, with only approximately 16% of patients developing subsequent primary tumor-related complications requiring intervention.4 However, recently there has been renewed interest in the potential associations between PTR and improved survival even among patients with asymptomatic intact primary tumors.5-9 Secondary analyses of patients enrolled in a multi-center randomized trial of systemic chemotherapy for metastatic colorectal cancer have shown improved survival associated with receipt of PTR prior to enrollment.10,11 Due to important, unaddressed potential biases in these retrospective analyses, the question of whether PTR improves oncologic outcomes remains unanswered.
With the potential to reduce unnecessary utilization of PTR in stage IV patients with asymptomatic intact primary tumors, the present study was conducted to compare the effectiveness of PTR among patients with colon cancer and unresectable metastases using nationwide hospital-based cancer registry data with modern analytical techniques aimed at control of sources of bias that threaten the validity of prior studies.
METHODS
Data Source and Patient Identification
The National Cancer Data Base (NCDB) was used to identify the study cohort. The NCDB is a joint program between the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society that serves as a surveillance tool to assess patterns of care for the cancer patient. The NCDB collects and reports patient data on over 70% of cancers diagnosed from over 1,500 CoC-accredited cancer programs in the United States.
Adult patients with stage 4 colon adenocarcinoma, diagnosed between 2003 and 2005 were included as the date of the definitive surgical procedure and patients’ comorbidity scores were not recorded in the NCDB for patients diagnosed prior to 2003, and complete survival data were only available for those diagnosed prior to 2006. Exclusions included patients receiving tumor-directed surgery within 24 hours of diagnosis (considered to have undergone non-elective resection) and those who underwent surgery for cancer of other sites (e.g. metastectomy). Cases were censored if death was not observed (patient was alive at the last follow-up or loss to follow-up).
Statistical Methods
Sequential analytic methods for bias adjustment were utilized to determine the rate of overall survival (OS) by PTR status. A multivariate Cox regression analysis stratified by receipt of systemic chemotherapy was performed initially. Propensity Score Weighting (PSW) and Instrumental Variable (IV) analyses, using the annual hospital-level PTR rate as the instrument, were performed to account for selection bias.
In studying diseases with high mortality, patients might die soon after diagnosis from different reasons, resulting in survivor treatment bias.12 Patients receiving PTR must survive from the date of diagnosis to the date of surgery to be included in the group, whereas no such requirement is made for patients in the non-PTR group. To overcome this issue, we employed the landmark method to correct for the bias inherent in the analysis of time-to-event outcomes. Specifically, the landmark cohort was limited to patients with at least one year of survival from diagnosis. The one-year landmark was selected because PTR would be expected to have little clinical impact for a patient who was unlikely to survive beyond 12 months. In general, patients who are expected to survive less than one year are considered poor operative candidates who would derive limited benefit from elective surgery. To ensure that our choice of landmark did not lead to additional bias, a priori-determined sensitivity analyses were perform using 3-, 6-, 9-, and 15- month landmarks.
Standard Risk-Adjustment Methods
Kaplan-Meier and multivariate Cox regression analyses were performed after stratification by receipt of systemic chemotherapy to compare survival outcomes with and without PTR. Multivariate Cox regression analyses adjusting for age, gender, race, year of diagnosis, tumor histology, tumor grade, tumor location, receipt of chemotherapy, insurance status, median income quartile, proportion without high school degree by residence ZIP code, population density of residence, facility location, facility type, and comorbidity score were performed. Missing data were labeled as unknown. Regression analysis was performed with and without PSW to estimate the treatment effect while accounting for covariates that predict treatment administration.13 A propensity model was developed to estimate an individual patient's probability of receiving PTR, denoted as p. All covariates that affect both treatment assignment and the outcome were included in the propensity score model.14 Cox regression models were then weighted by 1/p for patients who received PTR and 1/(1-p) for patients who did not to estimate the treatment effect on OS. To ensure stable weighting, we incorporated the proportion of patients receiving PTR and those without PTR into the weight.
Instrumental Variable Analysis
IV analysis is one established approach in observational studies to control for hidden variables.15 In determining the instrument, we considered the two key assumptions: (1) it is highly correlated with the treatment, and (2) it does not independently affect the outcome. The proportion of patients receiving PTR at the treating hospital was used as the instrument; therefore, patients treated at the same hospital, and those treated at different hospitals with similar PTR rates would share the same hospital-level PTR rate. IV models measure treatment effect on mortality at one time point on an absolute scale, estimated employing the two-stage-least squares regression. Linear regression was first employed to predict the treatment (PTR, yes vs no) as a function of instrument and other covariates. The obtained predicted treatment was then used to estimate the adjusted mortality difference in year 3 after diagnosis. The relative mortality rate (RMR) was calculated as 1+Δ/mnosurgery, where Δ is the instrument-adjusted absolute mortality difference and mnosurgery is the Kaplan-Meier mortality rate among patients who did not receive PTR. The obtained RMR was then compared with the hazard ratio (HR) derived from the multivariate Cox regression model. Instrument validation is described under the results section.
This paper is reported following the Strengthening the Reporting of Observational Studies in Epidemiology statement.16 All statistical analyses were performed using Stata MP Version 11.2 (College Station, TX) with a significance level of 0.05.
RESULTS
Patient Characteristics
A total of 15,154 patients met the inclusion criteria; a subgroup of 6,735 patients were eligible for one-year landmark analysis, (Appendix, figure). PTR was performed in 57% (n=8,641) of the total patient cohort and in 73.8% (n=4,972) of the landmark cohort. Thirty-day mortality was observed in 4.9% (n=755) of the entire cohort. Patients’ demographics, socioeconomic indicators, and type and location of the treating facility were similar between those who had PTR and those who did not in both the total cohort and the landmark cohort. In the landmark cohort, patients receiving PTR (vs no PTR) were more likely to have low histologic grade (71.3%, p<0.001). Baseline characteristics comparing patients based on 12-month OS are detailed in Table 1.
Table 1.
Baseline Characteristics of Stage IV Colon Cancer Patients by Primary Tumor Resection and Landmark Status (Abbreviations: NOS, not otherwise specified; NCI, National Cancer Institute)
| Variables | Without Landmark (N=15154) | With Landmark (N=6735) | ||||
|---|---|---|---|---|---|---|
| PTR No (n=6513) | PTR Yes (n=8641) | PTR No (n=1763) | PTR Yes (n=4972) | |||
| Age at diagnosis | P Value | P Value | ||||
| 18-49 | 699(10.7) | 1155(13.4) | 298 (16.9) | 810 (16.3) | ||
| 50-64 | 1888(29.0) | 2789(32.3) | <0.001 | 710 (40.3) | 1898 (38.2) | 0.26 |
| 65-74 | 1540(23.6) | 2219(25.7) | 427 (24.2) | 1269 (25.5) | ||
| 75-90 | 2386(36.6) | 2478(28.7) | 328 (18.6) | 995 (20) | ||
| Gender | ||||||
| Female | 3226(49.5) | 4243(49.1) | 0.601 | 806 (45.7) | 2371 (47.7) | 0.15 |
| Male | 3287(50.5) | 4398(50.9) | 957(54.3) | 2601 (52.3) | ||
| Race | ||||||
| White | 5060(77.7) | 7108(82.3) | 1329 (75.4) | 4073 (81.9) | ||
| African American | 1194(18.3) | 1201(13.9) | <0.001 | 342(19.4) | 696 (14) | <0.001 |
| Others | 259(4) | 332(3.8) | 92 (5.2) | 203 (4.1) | ||
| Year of diagnosis | ||||||
| 2003 | 2228(34.2) | 3055(35.4) | 548(31.1) | 1705 (34.3) | ||
| 2004 | 2142(32.9) | 2818(32.6) | 0.308 | 586 (33.2) | 1598 (32.1) | 0.04 |
| 2005 | 2143(32.9) | 2768(32) | 629(35.7) | 1669 (33.6) | ||
| Tumor histology | ||||||
| Non-mucinous adenocarcinoma | 5865(90.1) | 7474(86.5) | 1589 (90.1) | 4382 (88.1) | ||
| Signet-ring cell | 152(2.3) | 183(2.1) | <0.001 | 33(1.9) | 80 (1.6) | 0.01 |
| Mucinous | 496(7.6) | 984(11.4) | 141 (8) | 510 (10.3) | ||
| Tumor grade | ||||||
| Well/Moderately differentiated | 2396(36.8) | 5515(63.8) | 771(43.7) | 3543 (71.3) | ||
| Poorly/Undifferentiated | 1162(17.8) | 2800(32.4) | <0.001 | 208 (11.8) | 1239 (24.9) | 0.001 |
| Unknown | 2955(45.4) | 326(3.8) | 784(44.5) | 190 (3.8) | ||
| Tumor location | ||||||
| Right (C180, C182, C183, C184) | 2856(43.9) | 4812(55.7) | 667 (37.8) | 2460(49.5) | ||
| Left (C185, C186, C187, C199) | 2121(32.6) | 3496(40.5) | <0.001 | 701(39.8) | 2332 (46.9) | 0.001 |
| Other NOS (C188, C189) | 1536(23.6) | 333(3.9) | 395 (22.4) | 180 (3.6) | ||
| Use of Chemotherapy | ||||||
| No | 3503(53.8) | 3309(38.3) | <0.001 | 441(25) | 1143 (23) | 0.08 |
| Yes | 3010(46.2) | 5332(61.7) | 1322 (75) | 3829 (77) | ||
| Neoadjuvant | - | 302(6.1) | - | 199 (5.6) | ||
| Adjuvant | - | 4593(93.9) | - | 3332 (94.3) | ||
| Insurance status | ||||||
| Uninsured | 349(5.4) | 300(3.5) | 99 (5.6) | 182 (3.7) | ||
| Private | 584(9) | 1023(11.8) | 214 (12.1) | 722 (14.5) | ||
| Medicaid | 405(6.2) | 417(4.8) | <0.001 | 148 (8.4) | 258 (5.2) | 0.001 |
| Medicare | 3521(54.1) | 4270(49.4) | 663 (37.6) | 2040 (41) | ||
| Managed Care | 1441(22.1) | 2449(28.3) | 550 (31.2) | 1665 (33.5) | ||
| Unknown | 213(3.3) | 182(2.1) | 89 (5) | 105 (2.1) | ||
| Median income quartile | ||||||
| < $30,000 | 1066(16.4) | 1238(14.3) | 285 (16.2) | 696 (14) | ||
| $30,000 - $35,000 | 1181(18.1) | 1551(17.9) | 316 (17.9) | 853 (17.2) | ||
| $35,000 - $45,999 | 1740(26.7) | 2313(26.8) | 0.004 | 452 (25.6) | 1321 (26.6) | 0.10 |
| $46,000 + | 2184(33.5) | 3034(35.1) | 622 (35.3) | 1804 (36.3) | ||
| Unknown | 342(5.3) | 505(5..8) | 88 (5) | 298 (6) | ||
| Proportion without high school degree by ZIP code | ||||||
| < 14% | 1342(20.6) | 1467(17) | 374 (21.2) | 846 (17) | ||
| 14% - 19.9% | 1548(23.8) | 1921(22.2) | 442 (25.1) | 1090 (21.9) | ||
| 20% - 28.9% | 1392(21.4) | 1988(23) | <0.001 | 345 (19.6) | 1102 (22.2) | <0.001 |
| 29% + | 1889(29) | 2759(31.9) | 514 (29.2) | 1636 (32.9) | ||
| Unknown | 342(5.3) | 506(5.9) | 88(5) | 298(6) | ||
| Population density of residence | ||||||
| Metro area | 5223(80.2) | 6760(78.2) | 1450(82.2) | 3895(78.3) | ||
| Urban area | 810(12.4) | 1192(13.8) | 0.028 | 195(11.1) | 681(13.7) | 0.005 |
| Rural area | 115(1.8) | 155(1.8) | 21(1.2) | 85(1.7) | ||
| Unknown | 365(5.6) | 534(6.2) | 97(5.5) | 311(6.3) | ||
| Facility location | ||||||
| West | 900(13.8) | 1371(15.9) | 239(13.6) | 776(15.6) | ||
| Midwest | 1701(26.1) | 2235(25.9) | <0.001 | 442(25.1) | 1237(24.9) | <0.001 |
| Northeast | 1612(24.8) | 1793(20.7) | 468(26.5) | 1056(21.2) | ||
| South | 2300(35.3) | 3242(37.5) | 614(34.8) | 1903(38.3) | ||
| Facility type | ||||||
| Community Cancer Program | 1117(17.2) | 1601(18.5) | 227(12.9) | 900(18.1) | ||
| Comprehensive Community Cancer Program | 3112(47.8) | 4564(52.8) | <0.001 | 794(45) | 2522(50.7) | <0.001 |
| Academic/Research Program (includes NCI-designated comprehensive cancer centers) | 2167(33.3) | 2325(26.9) | 694(39.4) | 1463(29.4) | ||
| Unknown | 117(1.8) | 151(1.7) | 48(2.7) | 87(1.7) | ||
| Comorbidity score | ||||||
| 0 | 4879(74.9) | 6389(73.9) | 1501(85.1) | 3854(77.5) | ||
| 1 | 1167(17.9) | 1720(19.9) | 0.001 | 210(11.9) | 897(18) | <0.001 |
| 2 | 467(7.2) | 532(6.2) | 52(2.9) | 221(4.4) | ||
Within the landmark cohort, median follow-up time was 6.4 years (IQR 5.50-7.24). The median time to PTR was 13 days (IQR, 5-24). The median time to chemotherapy was 28 days (IQR, 16-47) among the no-PTR patients and 54 days (IQR, 38-74) among the PTR patients. Among patients who received chemotherapy in addition to PTR, 94.3% received the chemotherapy after surgery and only 5.6% received chemotherapy before surgery. Approximately one fourth of the patients did not receive any systemic chemotherapy and PTR was the only tumor-directed therapy used (n=1,143, 23%). The majority of surgical resections were performed within 30 days of diagnosis (n=4,115, 82.3%).
Treatment Effect
Standard Risk Adjustment and Propensity Score Analyses
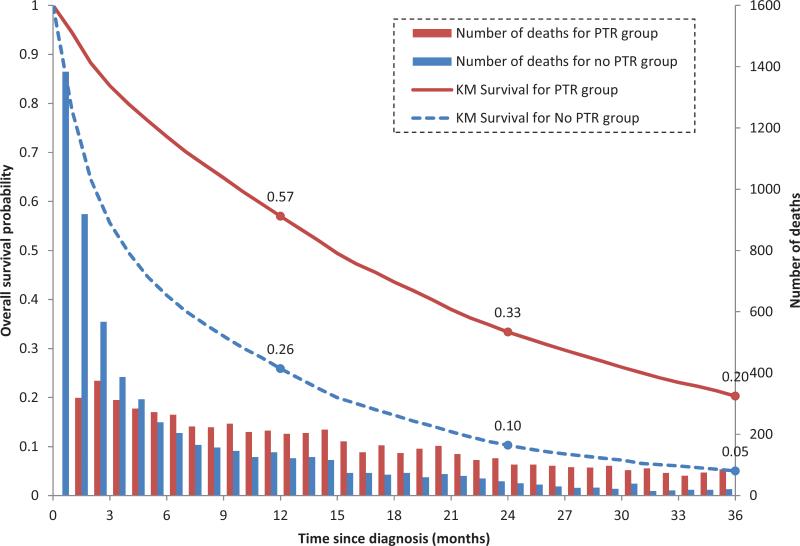
On unadjusted analysis PTR (vs. no PTR) was associated with a 10.87-month increase in median OS in the total patient cohort (14.78 vs 3.91 months) and a 10-month increase among those who received systemic chemotherapy (n=8,342; 20.34 vs 9.95 months). (Figure 1) PTR was associated with an approximately 55% relative reduction in mortality using multivariate Cox regression analysis (HR, 0.45; 95% CI, 0.43-0.47) and PSW (HR, 0.46; 95% CI, 0. 43-0.48). Performing both adjusted and unadjusted analyses and stratification to include only those who received chemotherapy did not significantly alter these results (Table 2).
Figure 1.
Overall survival probability and number of deaths by primary tumor resection (PTR) group.
Table 2.
Adjusted Mortality Data Associated with Primary Tumor Resection Among Stage IV Colon Cancer Patients, by Landmark Status (No-PTR (ref) vs PTR). (Abbreviations: PRT, primary tumor resection; IPTW, propensity score inverse probability of treatment weighting; CI, confidence interval)
| Risk-Adjustment Method | Total Cohort (N=15154) | Landmark Subgroup (N=6735) |
|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |
| Unadjusted Cox regression | 0.459(0.443, 0.475) | 0.630(0.593,0.669) |
| Adjusted Cox regression a | 0.453(0.435, 0.471) | 0.600(0.557,0.645) |
| Without chemotherapy | 0.466(0.439, 0.495) | 0.628(0.533,0.740) |
| With chemotherapy | 0.456(0.431, 0.483) | 0.582(0.536,0.632) |
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |
|---|---|---|
| Propensity score-unadjusted Cox model using IPTW | 0.519(0.493, 0.547) | 0.605(0.555, 0.659) |
| Propensity score-adjusted Cox model using IPTW a | 0.461(0.436, 0.488) | 0.591(0.544, 0.643) |
| Without chemotherapy | 0.478(0.443, 0.516) | 0.596(0.498, 0.713) |
| With chemotherapy | 0.453(0.423, 0.486) | 0.567(0.516, 0.623) |
| Adjusted Relative Mortality Rate (95% CI) | Adjusted Relative Mortality Rate (95% CI) | |
|---|---|---|
| Instrumental variable-adjusted a 3-year mortality | 0.913(0.865, 0.959) | 0.972(0.879, 1.063) |
| Without chemotherapy | 0.946(0.893, 0.999) | 1.019(0.820, 1.221) |
| With chemotherapy | 0.886(0.812, 0.962) | 0.948(0.844, 1.052) |
Adjusted for the following variables based on their availability in the NCDB and their clinical significance: age at diagnosis, gender, race, year of diagnosis, tumor histology, tumor grade, tumor location, chemotherapy, insurance status, median income quartile, proportion without high school degree by ZIP code, population density of residence by ZIP code, facility location, facility type, and comorbidity score
Instrumental Variable Analyses
The first-stage F-statistic derived from the two-stage-least squares regression was used to test the first assumption, that the instrument was highly correlated with treatment. The obtained F-statistic=1372.31 indicates that the proposed instrument is a sufficiently strong predictor of patients receiving PTR. As a general rule, an instrument with an F-statistic greater than 10 is thought to be strong. To evaluate the second assumption, that the instrument was unrelated to the characteristics of the patients, we analyzed the distribution of observed variables across levels of the instrument (Appendix). The mean hospital-level PTR rate within 30 days of diagnosis ranged from 0.42 to 0.99 across quintiles. The median OS across quintiles ranged from 23.3 months (IQR, 16.8-39.3) in quintile 1 (lowest PTR rate) to 26.7 months (IQR, 17.4-45.0) in quintile 5 (highest PTR rate). All measured risk factors across quintiles were balanced. Using IV analysis, the PTR-associated survival benefit was attenuated, with an adjusted absolute mortality difference of 0.08 at 3 years, (95% CI, 0.03-0.12). This corresponds to an adjusted RMR of 0.91 (95% CI, 0.86-0.95). Stratification by systemic chemotherapy further attenuated the observed benefit (Table 2). Finally, although the likelihood of receiving PTR at a given institution could have been influenced by patient referral, where patients treated at certain hospitals are more likely to undergo PTR (i.e. referral bias), the PTR associated survival benefit was similar across the different quintiles of PTR rate.
Landmark Analyses
When evaluating early mortality, we observed that the majority of deaths occurred within the first 90 days after diagnosis; this was especially true among the no-PTR group. (Figure 1) Patients who did not survive to the landmark time were less likely to receive any treatment modality (62% received no chemotherapy and 56% no resection vs 23% and 26%, respectively, in the landmark subgroup); thus, indicating survivor treatment bias. (Table 1) This significant difference was no longer observed when the landmark method was utilized.
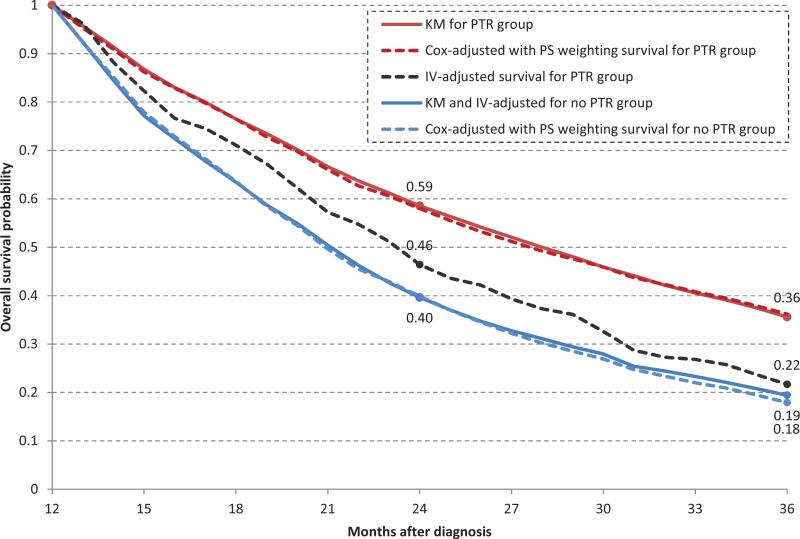
Using the one-year landmark, a survival benefit was still seen with PTR on standard multivariate Cox regression (HR, 0.60; 95% CI, 0.55-0.64) and PSW (HR, 0.59; 95% CI, 0.54-0.64). (Table 2) This benefit was abrogated when IV analysis was utilized (adjusted RMR 0.97, 95% CI 0.87-1.06). (Figure2)
Figure 2.
Kaplan-Meier (KM), instrumental variable (IV)-adjusted and Cox-adjusted (with inverse probability of treatment weighting (IPTW)) overall survival, with landmark method applied.
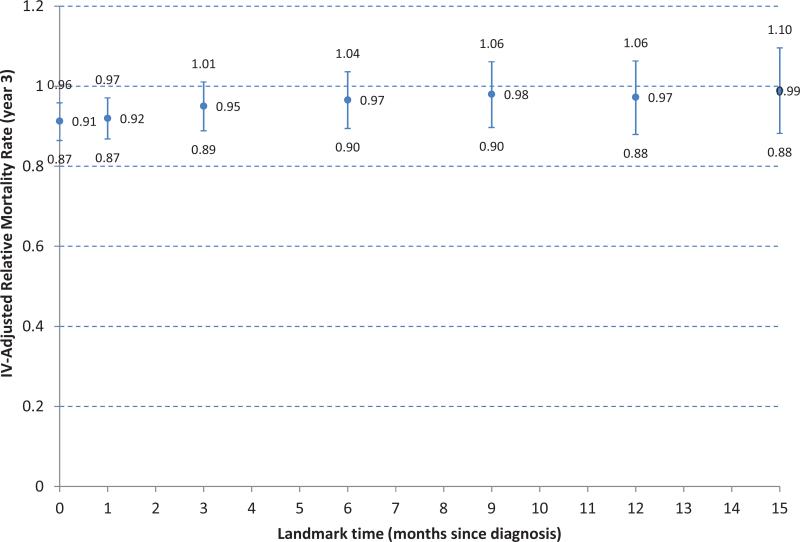
Sensitivity analysis was performed to evaluate the impact of landmark threshold on the IV-adjusted mortality three years after diagnosis, (Figure 3). The PTR-associated survival benefit became insignificant when the instrumental variable method was applied at the 3-month landmark (adjusted RMR of 0.95 [95% CI, 0.89-1.01]).
Figure3.
Sensitivity analysis of landmark time on instrumental variable-adjusted 3-year relative mortality rate.
DISCUSSION
The decision regarding resection of the primary tumor among patients who present with primary-intact stage 4 colon cancer is one that is commonly faced by patients and their doctors. Data regarding the survival impact of PTR in this setting are conflicting, and strong provider biases exist regarding the role of up-front PTR or selective resection when symptoms develop.17 Using standard statistical risk-adjustment methods which inadequately account for confounder effects, PTR appeared to be associated with a significant reduction in mortality. However, IV analysis to control for treatment selection and survivor time bias at the one-year landmark demonstrated that PTR was not associated with a survival benefit over systemic chemotherapy. Stratification by receipt of systemic chemotherapy did not alter the results. Thus, we demonstrate that the routine use of PTR in patients with stage 4 colon cancer and unresectable metastatic disease does not improve survival.
There is significant disagreement in the literature regarding PTR impact on survival. In the United States, PTR is performed in over 50% of patients with this pattern of advanced colorectal cancer and there are many providers who still advocate for its routine use.3 This may be in part due to the fact that much of the current literature suggests that palliative PTR may offer a significant survival benefit.5-7,10 In a secondary analysis of 294 patients with non-resectable colorectal cancer metastases who were enrolled in a phase III randomized controlled systemic chemotherapy trial, patients who underwent PTR prior to enrollment were observed to have higher rates of OS than patients who had not undergone PTR.11 This effect was independent of the site of the primary (colon vs rectum); however, the majority of these patients had single metastases confined to the liver. Similarly, in a meta-analysis7 using survival data from 21 retrospective studies with a total of 44,226 patients with metastatic colorectal cancer, PTR compared with chemotherapy alone was associated with a lower mortality. However, all but two of the studies in the analysis were single-center and the other two studies used administrative databases with limited specific information regarding how patients were selected for surgical intervention.
These studies are highly subject to treatment selection bias, and caution is required when inferring a causal relationship between PTR and improved survival. Patients with significant non-cancer comorbidities who are at greater risk for all-cause mortality are less likely to undergo PTR. Patients who undergo PTR have better performance status and are more likely to receive palliative systemic therapy than patients who do not undergo PTR.6 Patients with greater metastatic tumor burden are subject to greater cancer-related mortality but may be less likely to undergo PTR. Finally, failure to exclude patients who undergo resection of the both the primary tumor and metastases with curative intent can bias results in favor of PTR.
When estimating a survival benefit using observational data, where treatment assignment is not randomized or the indication for assignment is not well characterized, the analytic method can critically impact the results. Propensity score analysis can reduce the effect of selection bias by accounting for the conditional probability of treatment based on all known factors available for inclusion in the propensity model.; however, as with standard risk-adjustment methods, propensity score analyses are subject to the limitation of unmeasured or factors that contribute to treatment selection bias. IV analysis is an alternate analytic method for causal inference that can control for known and unknown confounders in observational studies and is particularly useful when randomized controlled trials are unavailable or cannot be performed.13,18 An additional major bias influencing survival outcome determination in diseases with high mortality is the length of survival itself and how it influences patients’ chances of being assigned to one treatment or another, so called survivor treatment bias.12 Patients with poor survival prognosis who would have died soon after diagnosis would not have had the opportunity to get surgical resection, thereby guaranteeing poorer survival for the nonsurgical group. One effective approach to overcome this problem is the landmark method.19 Previous studies have been limited by incomplete adjustment for both treatment selection and survivor time bias. In this study, we combined several techniques in order to account for both types of bias. Having done so, we could no longer demonstrate a survival benefit of PTR.
This study has several limitations. As with other observational studies using administrative databases, the intent of treatment was difficult to evaluate (e.g. patients receiving chemotherapy with the intent to undergo resection in the future versus those receiving chemotherapy purely with palliative intent). While the focus this study is on the effect of PTR in the palliative setting and excluded from analysis patients who underwent curative intent PTR with metastectomy, removing patients with good prognosis from the PTR group while leaving those who had poor response to chemotherapy could negatively impact survival in the PTR arm. We did note, however, that the time to systemic chemotherapy was nearly doubled among patients undergoing PTR and that one in four patients undergoing PTR never received systemic chemotherapy indicating the potential adverse impact of PTR on ability to receive chemotherapy. Similarly, resections performed to palliate symptoms could not be distinguished from those that were performed in asymptomatic patients. To address this issue, patients receiving tumor-directed surgery within 24 hours of diagnosis were excluded from the analysis (considered to have undergone non-elective resection). To ensure that our choice of 24- hours cutoff did not bias our results, additional sensitivity analyses were perform using 48- and 72 hours and showed similar results (data not shown). Furthermore, patients who were initially asymptomatic and developed symptoms over time could not be definitively identified. In fact it is likely that PTR is beneficial in initially asymptomatic patients who are at high risk for development of tumor related complications, such as those with long-segment circumferential tumors. If this group was represented by the 14% who underwent resection more than 30 days after diagnosis, then our findings are comparable to the 16% of such patients reported in literature.4 While the NCDB reports outcomes on over 70% of patients with new cancer diagnoses in the United States, outcomes for patients treated in non-CoC accredited hospitals could be different and it is possible that our results might not reflect treatment at non-CoC accredited hospitals. However, the PTR rate among our study cohort was similar to that reported in studies using different databases suggesting our findings can be generalized.3 Additionally, while IV analysis is a widely accepted technique for accounting for hidden bias, there remains potential for instrument-outcome confounding such as receipt of other treatments also associated with our instrument and the outcome.20 One example is if there was greater early mortality in high PTR use hospitals; however the impact of this potential confounder was minimized by landmark analysis as facility associated mortality tends to be associated with earlier deaths. Finally, survival has significantly improved in this patient population in the recent era and the potential for PTR after initial systemic chemotherapy should continue to be evaluated.
This study highlights, in contrast to what has been previously suggested from large registry studies, that in routine practice with unselected patients, PTR does not provide a survival advantage to patients who have colon cancer with unresectable metastases but may delay the time to initiation of systemic therapy. These results also demonstrate that the previously observed treatment-related benefits are subject to overestimation due to bias not accounted for using standard analytical methods. While there is potential for a subgroup of patients to benefit from PTR in addition to systemic chemotherapy, this study demonstrates in routine use, PTR does not improve survival. Ongoing randomized control trials (CIARO 4) will attempt to further the literature in this field, this report. as ongoing studies that may provide addition insight. I think you know which ones: cairo4 (Dutch) greccor8 (FFCD) and synchronous (German). And that we should encourage accrual to randomized investigation so that we can get to a definitive answer. This study provides a new perspective on the impact of PTR to guide decision-making in the real world.
Supplementary Material
Acknowledgements
This work was supported in part by National Institutes of Health/National Cancer Institute grants T32CA009599 (C.E.B.), K07-CA133187 (G.J.C.), The University of Texas MD Anderson Cancer Center Support Grant (CA16672) and the American Society of Colon and Rectal Surgeons Research Foundation Resident Initiation Grant (U.R.P.). The authors also acknowledge the expert assistance of Kathryn B. Carnes for scientific writing review.
Footnotes
Presented at the 2015 Gastrointestinal Cancers Symposium annual meeting, San Francisco, California
Presented at the 2015 Society of Surgical Oncology annual meeting, Houston, Texas
Winner of the 2015 Society of Surgical Oncology Annual Resident/Fellow Essay Award for the Best Clinical Research Paper
Disclaimers: The data used in this study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have neither verified nor are responsible for the analytic or statistical methodology employed or the conclusions drawn from these data.
None of the authors has any conflicts of interest to report.
REFERENCES
- 1.American Cancer Society [May 13, 2016];Cancer Statistics Center. 2016 Available from URL: https://cancerstatisticscenter.cancer.org/?_ga=1.75905685.183881736.1463405064#/
- 2.Van Cutsem E, Nordlinger B, Cervantes A, Group EGW. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21(Suppl 5):v93–97. doi: 10.1093/annonc/mdq222. [DOI] [PubMed] [Google Scholar]
- 3.Hu CY, Bailey CE, You YN, et al. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg. 2015;150(3):245–251. doi: 10.1001/jamasurg.2014.2253. [DOI] [PubMed] [Google Scholar]
- 4.McCahill LE, Yothers G, Sharif S, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10. J Clin Oncol. 2012;30(26):3223–3228. doi: 10.1200/JCO.2012.42.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed S, Leis A, Fields A, et al. Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: results from a large population-based cohort study. Cancer. 2014;120(5):683–691. doi: 10.1002/cncr.28464. [DOI] [PubMed] [Google Scholar]
- 6.Gresham G, Renouf DJ, Chan M, et al. Association Between Palliative Resection of the Primary Tumor and Overall Survival in a Population-Based Cohort of Metastatic Colorectal Cancer Patients. Annals of surgical oncology. 2014 doi: 10.1245/s10434-014-3797-0. [DOI] [PubMed] [Google Scholar]
- 7.Clancy C, Burke JP, Barry M, Kalady MF, Calvin Coffey J. A Meta-Analysis to Determine the Effect of Primary Tumor Resection for Stage IV Colorectal Cancer with Unresectable Metastases on Patient Survival. Annals of surgical oncology. 2014 doi: 10.1245/s10434-014-3805-4. [DOI] [PubMed] [Google Scholar]
- 8.Duraker N, Civelek Caynak Z, Hot S. The impact of primary tumor resection on overall survival in patients with colorectal carcinoma and unresectable distant metastases: a prospective cohort study. International journal of surgery. 2014;12(7):737–741. doi: 10.1016/j.ijsu.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara S, Hayama T, Yamada H, et al. Prognostic Impact of Primary Tumor Resection and Lymph Node Dissection in Stage IV Colorectal Cancer with Unresectable Metastasis: A Propensity Score Analysis in a Multicenter Retrospective Study. Annals of surgical oncology. 2014;21(9):2949–2955. doi: 10.1245/s10434-014-3719-1. [DOI] [PubMed] [Google Scholar]
- 10.Venderbosch S, de Wilt JH, Teerenstra S, et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Annals of surgical oncology. 2011;18(12):3252–3260. doi: 10.1245/s10434-011-1951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrand F, Malka D, Bourredjem A, et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Federation Francophone de Cancerologie Digestive 9601. European journal of cancer. 2013;49(1):90–97. doi: 10.1016/j.ejca.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Glesby MJ, Hoover DR. Survivor treatment selection bias in observational studies: examples from the AIDS literature. Annals of internal medicine. 1996;124(11):999–1005. doi: 10.7326/0003-4819-124-11-199606010-00008. [DOI] [PubMed] [Google Scholar]
- 13.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297(3):278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19(6):537–554. doi: 10.1002/pds.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. [December 12, 2015];The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Available from URL: http://www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_cohort.pdf.
- 17.Cirocchi R, Trastulli S, Abraha I, et al. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. The Cochrane database of systematic reviews. 2012;8:CD008997. doi: 10.1002/14651858.CD008997.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwashyna TJ, Kennedy EH. Instrumental variable analyses. Exploiting natural randomness to understand causal mechanisms. Annals of the American Thoracic Society. 2013;10(3):255–260. doi: 10.1513/AnnalsATS.201303-054FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 20.Garabedian LF, Chu P, Toh S, Zaslavsky AM, Soumerai SB. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Annals of internal medicine. 2014;161(2):131–138. doi: 10.7326/M13-1887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.