Highlights
-
•
Case of infective endocarditis caused by a rare pathogen, Aggregatibacter aphrophilus is presented.
-
•
Aggregatibacter aphrophilus can’t be detected by common culture methods.
-
•
Br-PCR testing is a useful tool to identify pathogens of culture negative endocarditis.
-
•
Serum PR3-ANCA can be positive in Aggregatibacter aphrophilus endocarditis.
Abbreviations: IE, infective endocarditis; br-PCR, broad-range polymerase chain reaction; PR3-ANCA, serum proteinase 3 anti-neutrophil cytoplasmic antibody
Keywords: Case report, Aggregatibacter aphrophilus, Infective endocarditis, Culture-negative endocarditis, Broad-range polymerase chain reaction, Proteinase 3 anti-neutrophil cytoplasmic antibody
Abstract
Introduction
Aggregatibacter aphrophilus is a rare cause of infective endocarditis. This pathogen is difficult to identify with common culture methods, which can lead to incorrect diagnosis and treatment.
Presentation of case
A 72-year-old woman was admitted to a community hospital with a persistent high fever and deteriorating renal function. Based on negative blood culture and positive serum proteinase 3 anti-neutrophil cytoplasmic antibody (PR3-ANCA), acute renal failure associated with ANCA-rerated vasculitis was initially suspected. However, the patient developed heart failure soon afterward; echocardiography showed mitral insufficiency with mobile vegetation attached to the mitral valve, indicating infective endocarditis. After transfer to our hospital, the patient underwent mitral valve repair. Broad-range polymerase chain reaction (br-PCR) and sequencing identified Aggregatibacter aphrophilus in the excised vegetation. The patient had a good postoperative course, with recovery of renal function.
Conclusion
A rare disease, Aggregatibacter aphrophilus infective endocarditis was successfully treated with surgical repair and appropriate antibiotic therapy. To avoid misdiagnosis, br-PCR testing should be performed in patients with blood culture-negative endocarditis.
1. Introduction
For the successful treatment of infective endocarditis (IE), identification of the causative pathogens is crucial. However, conventional culture methods often fail to reveal the correct diagnosis in patients with IE because of prophylactic use of antibiotics before diagnosis or because the pathogen is difficult to culture. Among the organisms that cause IE, 3%–10% are reportedly culture-negative [1]. A molecular technique that combines broad-range polymerase chain reaction (br-PCR) amplification and direct sequencing is useful for detecting pathogens in patients with culture-negative endocarditis [2].
Aggregatibacter aphrophilus is one of the typical culture-negative species that can cause IE [1]. Due to the rarity of the organism and the difficulty of identification, the clinical features and outcomes of Aggregatibacter aphrophilus IE are not fully understood, leading to incorrect diagnoses. Here, we report a case of IE caused by Aggregatibacter aphrophilus that was detected with br-PCR and successfully treated with surgical repair and appropriate antibiotic therapy.
2. Presentation of case
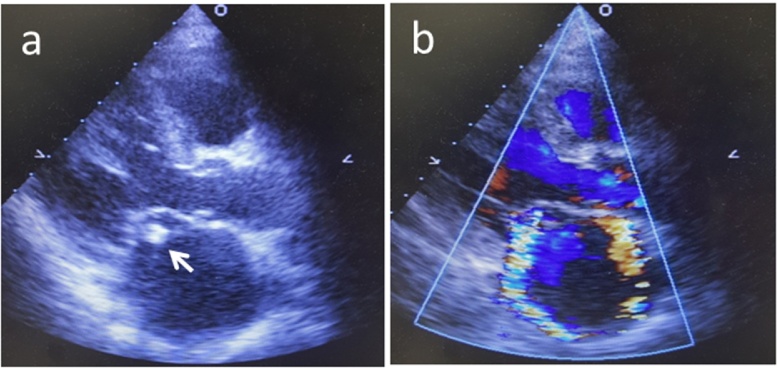
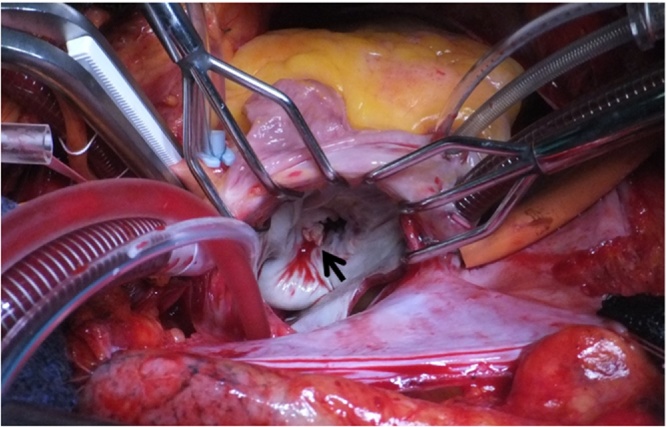
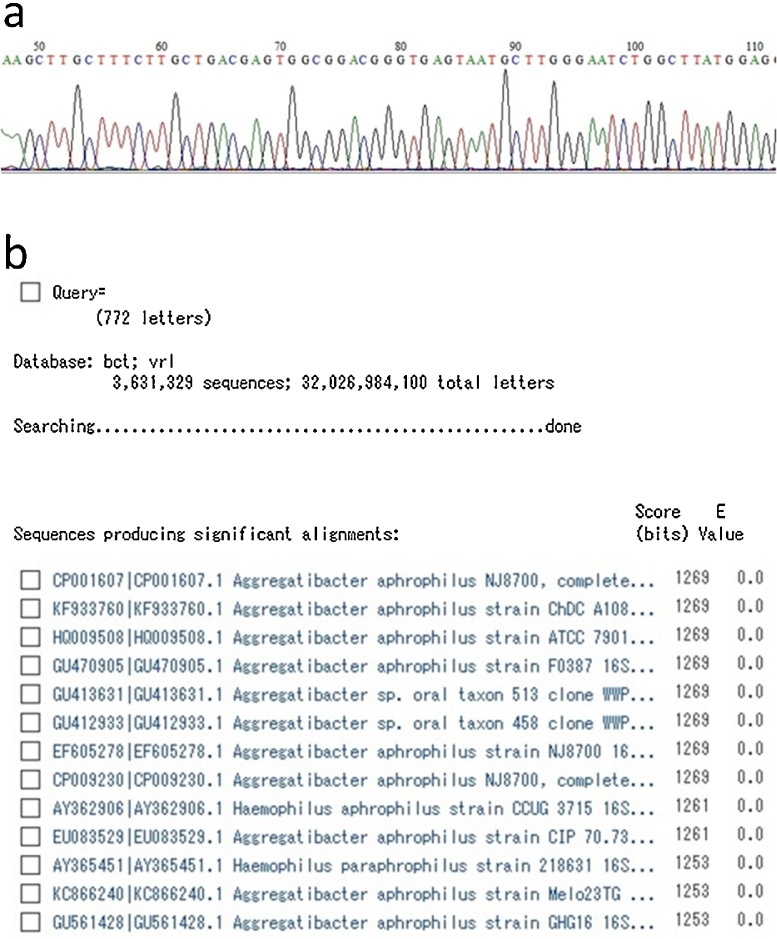
A 72-year-old woman was admitted to a community hospital with a persistent high fever. She also had hematuria with rapidly deteriorating renal function for which hemodialysis had been performed. Renal biopsy revealed crescentic glomerulonephritis and serum proteinase 3 anti-neutrophil cytoplasmic antibody (PR3-ANCA) testing was positive. ANCA-associated vasculitis was therefore suspected as the cause of renal dysfunction and oral prednisolone was initiated. However, the patent developed heart failure soon afterward. Echocardiography showed vegetation near the anterior commissure of the mitral valve as well as a perforation in part of the anterior leaflet, with severe regurgitation (Fig. 1). In addition, brain MRI revealed mycotic cerebral embolism with accompanying hemorrhage, although the patient did not display obvious neurological symptoms (Fig. 2). Laboratory testing revealed an increased peripheral leukocyte count of 19,800 cells/mm3 and an elevated C-reactive protein level of 10.8 mg/dL. At this point, IE appeared to be the probable diagnosis. However, no pathogen was detected in repeated blood cultures. Administration of broad-spectrum antibiotics (vancomycin hydrochloride and ceftriaxone sodium) was therefore started empirically. After the patient was referred to our hospital, mitral valve repair was performed. During surgery, a 1-cm diameter of spherical vegetation was found attached to the anterior commissure of the mitral leaflet (Fig. 3). The anterior commissure leaflet was resected, together with the vegetation, followed by patch repair with autologous pericardium and mitral annuloplasty using a 28-mm prosthetic ring (Physio II ring, Carpentier Edwards). Intraoperative transesophageal echocardiography showed no mitral regurgitation after the repair. Because cultures of the removed mitral valve and vegetation were negative, we performed br-PCR to identify microorganisms based on partial sequences of divergent regions of the 16S rRNA gene for bacteria, as follows (Fig. 4). Total DNA was purified from the tissue with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). We used 10F/800R primers to analyze a former part of 16S rRNA for bacteria and then performed 30 amplification cycles at 94 °C for 30 s, 55 °C for 60 s, and 72 °C for 60 s. This process amplified 772 bp DNA fragments. The PCR products were labeled with sequencing reagents and placed in the DNA sequencer. The nucleotide sequences of the PCR products were read and compared with the DNA Data Bank of Japan (http://www.ddbj.nig.ac.jp/), which revealed that the PCR products significantly matched the nucleotide sequences of Aggregatibacter aphrophilus [3]. Vancomycin administration was discontinued after identification of the pathogen; ceftriaxone was continued intravenously for 4 weeks after surgery to prevent recurrence of infection. The patient was transferred to the referring hospital without any complications to continue rehabilitation. Serum PR3-ANCA was negative at the time of transfer and the patient’s renal function had recovered. No sign of recurrence of endocarditis has been detected to date.
Fig. 1.
Echocardiography shows a vegetation (white arrow) attached to the anterior mitral leaflet (a) and severe mitral regurgitation (b).
Fig. 2.
MRI image shows subcortical infarction and hemorrhage in the right frontal lobe.
Fig. 3.
Intraoperative view of the mitral valve; a vegetation (black arrow) is located in the anterior mitral commissure.
Fig. 4.
The results of br-PCR. a, Part of the sequence of the PCR products obtained from the vegetation. b, Comparison between the sequence of the PCR products and that registered in the database, showing that they significantly matched the sequence of Aggregatibacter aphrophilus.
3. Discussion
Aggregatibacter aphrophilus was first described in 1940 by Khairat and colleagues [4] and is currently categorized as a member of the HACEK group of bacteria (Haemophilus species, Aggregatibacter species, Cardiobacterium hominis, Eikenella corrodens, and Kingella species) [5]. HACEK bacteria are commonly recognized as part of normal oral flora [6], [7], but are relatively rare as a cause of IE, accounting for only 1% to 3% of IE cases [7]. Aggregatibacter aphrophilus IE is especially rare, limited to a few case reports [8], [9]. HACEK bacteria are one cause of culture-negative endocarditis because the species are difficult to identify with normal blood culture methods [1]. Given these circumstances, the clinical features and outcomes of Aggregatibacter aphrophilus endocarditis have not been fully described.
Chambers and colleagues reported 77 cases of HACEK endocarditis; cerebral embolism occurred in 19 of these patients, including cerebral hemorrhage in eight. They concluded that the rate of cerebral complications was higher in HACEK endocarditis than in non-HACEK endocarditis [7]. The reasons for this difference are not clear. However, glomerulonephritis and positive rheumatoid factor are more frequently observed in HACEK endocarditis, suggesting that a more frequent microvascular/immunological response in these patients might contribute to the development of cerebral microbleeds and subsequent intracranial hemorrhage. Cerebral embolism accompanied by hemorrhage did occur in our patent.
PR3-ANCA are important diagnostic markers for small-vessel vasculitic syndromes (e.g. granulomatosis with polyangiitis, microscopic polyangiitis, eosinophilic granulomatosis and polyangiitis), which are commonly referred to as ANCA-associated vasculitis [10]. However, several infectious diseases, particularly IE, may have positive ANCA tests and mimic ANCA-associated vasculitis, leading to potential misdiagnosis and inappropriate treatment [11], [12]. To date, no report has described the pathogenicity of ANCA in IE. However, Ying et al. reported that ANCA reflects systemic inflammation rather than IE activity. In that report, white blood cell counts tended to be higher and LDH levels were higher in those with ANCA-positive IE than in those with ANCA-negative IE [13]. In another study, serum levels of rheumatoid factor and IgG were higher in patients with ANCA-positive IE than in those with ANCA-negative IE [11]. These reports suggest that ANCA-positive IE is likely an atypical immune response to infection. Similarly, the serum PR3-ANCA test was positive in our patient, but this did not indicate ANCA-associated vasculitis. It is rare that PR3-ANCA testing is positive in patients with culture-negative endocarditis [13]. To the best of our knowledge, there has been no previous report of positive PR3-ANCA in a patient with IE caused by Aggregatibacter aphrophilus. These rare circumstances may have made the correct diagnosis more difficult at the referring hospital.
Typically Aggregatibacter aphrophilus is part of the normal oral flora, frequently found in dental plaque and gingival scrapings [6], [7]. Dental procedures, tongue piercings, use of tongue scrapers, and recent upper respiratory tract infection are known causes of bacterial entry into the bloodstream [6], [9]. Our patient did not report any of these factors. However, she had poor dental condition, including tooth decay, which possibly induced transient bacteremia. We speculate that this is the most plausible source of infection in her case.
Our treatment method for Aggregatibacter aphrophilus endocarditis was similar to the treatment of endocarditis caused by other organisms. The patient received antibiotic treatment with ceftriaxone for a total of 8 weeks in accordance with antibiotic susceptibility and published guidelines [14].
Identification of the causative pathogen is very important for successful treatment of IE. However, blood cultures can sometimes be negative because of preceding antibiotic treatment or pathogens such as HACEK bacteria that are difficult to detect with common culture methods. The br-PCR method, which overcomes these problems, has recently been reported to be helpful in the diagnosis of IE and can improve antibiotic treatment, particularly in cases of culture-negative IE [2]. The method includes universal PCR for the 16S rRNA gene, with subsequent sequence analysis of the amplicons and comparison with the DNA database. Our patient’s repeated blood cultures were negative despite echocardiographic evidence of a mobile mass and valve destruction, which strongly suggested active IE. We therefore decided to identify the causative bacterium using the br-PCR method and successfully diagnosed IE caused by Aggregatibacter aphrophilus. In suspected cases of culture-negative IE, br-PCR tests should be immediately performed because correct identification of the causative pathogen helps determine an appropriate management plan and can lead to better outcomes. In this case, however, there was a time lag between the diagnosis of IE and the identification of the causative pathogen because an initial diagnosis of ANCA-associated vasculitis was made and steroid therapy was initiated at the community hospital. Fortunately, ceftriaxone, to which Aggregatibacter aphrophilus shows sensitivity, had already been administered empirically before surgery, which also contributed to a positive outcome.
4. Conclusion
In summary, Aggregatibacter aphrophilus endocarditis was successfully treated with excision of the vegetation followed by mitral valve repair and ceftriaxone infusion. Aggregatibacter aphrophilus endocarditis can cause positive PR3-ANCA tests and can mimic ANCA-associated vasculitis. Br-PCR analysis can help to diagnose IE and to identify causative pathogens such as Aggregatibacter aphrophilus, which are rare and difficult to detect with normal culture methods.
Conflict of interest
None.
Funding
None.
Ethical approval
The case report was approved by the ethics committee at Japan Red Cross Ise Hospital.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
KH and TT conceived of this case presentation and drafted the manuscript. MI, TF, YM and HT participated in the treatment of this case. All authors read and approved the final manuscript.
Guarantor
Koji Hirano have acceptful responsibility for this work and controlled the decision to publish.
References
- 1.Mylonakis E., Calderwood S.B. Infective endocarditis in adults. N. Engl. J. Med. 2001;345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 2.Marsch G., Orszag P., Mashaqi B., Kuehn C., Haverich A. Antibiotic therapy following polymerase chain reaction diagnosis of infective endocarditis: a single centre experience. Interact. Cardiovasc. Thorac. Surg. 2015;20:589–593. doi: 10.1093/icvts/ivv006. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Ministry of Health, Labour and Welfare . 17th edition. Yakuji Nippo Ltd.; 2016. The Japanese Pharmacopoeia; pp. 2503–2505. [Google Scholar]
- 4.Khairat O. Haemophilus aphrophilus endocarditis. Br. Med. J. 1971;1:728. doi: 10.1136/bmj.1.5751.728-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nørskov-Lauritsen N. Classification, identification, and clinical significance of haemophilus and aggregatibacter species with host specificity for humans. Clin. Microbiol. Rev. 2014;27:214–240. doi: 10.1128/CMR.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lockhart P.B., Brennan M.T., Thornhill M., Michalowicz B.S., Noll J., Bahrani-Mougeot F.K. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J. Am. Dent. Assoc. 2009;140:1238–1244. doi: 10.14219/jada.archive.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers S.T., Murdoch D., Morris A., Holland D., Pappas P., Almela M. HACEK infective endocarditis: characteristics and outcomes from a large, multinational cohort. PLoS One. 2013;8:e63181. doi: 10.1371/journal.pone.0063181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung G.W., Parkins M.D., Church D. Pyogenic ventriculitis complicating Aggregatibacter aphrophilus infective endocarditis: a case report and literature review. Can. J. Infect. Dis. Med. Microbiol. 2009;20:e107–e109. doi: 10.1155/2009/971735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel S.R., Patel N.H., Borah A., Saltzman H. Aggregatibacter aphrophilus pacemaker endocarditis: a case report. BMC Res. Notes. 2014;7:885. doi: 10.1186/1756-0500-7-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosch X., Guilabert A., Font J. Antineutrophil cytoplasmic antibodies. Lancet. 2006;368:404–418. doi: 10.1016/S0140-6736(06)69114-9. [DOI] [PubMed] [Google Scholar]
- 11.Mahr A., Batteux F., Tubiana S., Goulvestre C., Wolff M., Papo T. Brief report: prevalence of antineutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol. 2014;66:1672–1677. doi: 10.1002/art.38389. [DOI] [PubMed] [Google Scholar]
- 12.Chirinos J.A., Corrales-Medina V.F., Garcia S., Lichtstein D.M., Bisno A.L., Chakko S. Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin. Rheumatol. 2007;26:590–595. doi: 10.1007/s10067-005-0176-z. [DOI] [PubMed] [Google Scholar]
- 13.Ying C., Yao D., Ding H., Yang C. Infective endocarditis with antineutrophil cytoplasmic antibody: report of 13 cases and literature review. PLoS One. 2014;9:e89777. doi: 10.1371/journal.pone.0089777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baddour L.M., Wilson W.R., Bayer A.S., Fowler V.G., Bolger A.F., Levison M.E. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the committee on rheumatic fever, endocarditis, and kawasaki disease. Circulation. 2005;111:e394–e434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]