Abstract
We recently reported that knockdown of delta-5-desaturase (a key enzyme that converts dihomo-γ-linolenic acid, DGLA, to the downstream ω-6 arachidonic acid) promotes formation of an anti-cancer byproduct 8-hydroxyoctanoic acid from cyclooxygenase (COX)-catalyzed DGLA peroxidation. 8-hydroxyoctanoic acid can exert its growth inhibitory effect on cancer cells (e.g. colon and pancreatic cancer) by serving as a histone deacetylase inhibitor. Since histone deacetylase inhibitors have been well-known to suppress cancer cell migration and invasion, we thus tested whether knockdown of delta-5-desaturase and DGLA treatment could also be used to inhibit cancer migration and invasion of colon cancer and pancreatic cancer cells. Wound healing assay, transwell assay and western blot were used to assess cell migration and invasion as well as the associated molecular mechanisms. Formation of threshold level of 8-hydroxyoctanoic acid was quantified from COX-catalyzed DGLA peroxidation in the cancer cells that overexpress COX-2 and their delta-5-desaturases were knocked down by shRNA transfection. Our results showed that knockdown of delta-5-desaturase along with DGLA supplement not only significantly inhibited cell migration, but also improved the efficacies of 5-flurouracil and gemcitabine, two frontline chemotherapy drugs currently used in the treatment of colon and pancreatic cancer, respectively. The molecular mechanism behind these observations is that 8-hydroxyoctanoic acid inhibits histone deacetylase, resulting in downregulation of cancer metastasis promotors, e.g., MMP-2 and MMP-9 as well as upregulation of cancer metastasis suppressor, e.g. E-cadherin. For the first time, we demonstrated that we could take the advantage of the common phenomenon of COX-2 overexpression in cancers to inhibit cancer cell migration and invasion. With the shifting paradigm of COX-2 cancer biology, our research outcome may provide us a novel cancer treatment strategy.
Abbreviations: AA, arachidonic acid; COX, Cyclooxygenase; DGLA, dihomo-γ-linolenic acid; D5D, delta-5-desaturase; D5D-KD, delta-5-desaturase knockdown; EMT, epithelial mesenchymal transition; HDAC, histone deacetylase; HDACi, histone deacetylase inhibitor; NC-sh, negative control shRNA transfected; PBS, phosphate buffered saline; 5-FU, 5-flurouracil; 8-HOA, 8-hydroxyoctanoic acid
Keywords: COX-catalyzed DGLA peroxidation, Delta-5-desaturase knockdown, ShRNA transfection, Cancer migration and invasion, 5-fluorouracil and gemcitabine, Inhibition of histone deacetylase
Graphical abstract
Highlights
-
•
High level of COX-2 could be used to inhibit cancer cell migration and invasion.
-
•
8-hydroxyoctanoic acid suppresses cancer migration and invasion via inhibiting HDAC.
-
•
D5D knockdown and DGLA improves efficacy of chemotherapy to inhibit cancer metastasis.
1. Introduction
It is reported that, rather than the primary tumors, cancer metastasis accounts for more than 90% of all cancer death, including colon and pancreatic cancer [1], [2], [3]. A majority of colon and pancreatic cancer patients with cancer metastasis showed a poor survival rates as cancer cells metastasize to important organs such as the liver or lungs. Various chemotherapy drugs have been used in clinical practice to treat colon and pancreatic cancer metastasis [4], [5]. However, cancer cells’ resistance to chemo-drugs has been the major obstacle for successful chemotherapy for colon and pancreatic cancer. For example, colon and pancreatic cancer cells have shown resistance to front-line chemo-drugs such as 5-flurouracil (5-FU) and gemcitabine, respectively [6], [7], [8], [9], [10]. Various complementary strategies such as ω-3 fatty acids have been studied in order to improve the efficacy and safety of chemotherapy [11], [12], [13]. Although ω-6s are more abundant fatty acids in our daily diet (e.g., traditional western diets of ω-6 vs. ω-3 ratio is between ~ 10:1 and 30:1), ω-6-based dietary strategies have not received much attention and have been challenging in cancer treatment as deleterious metabolites (e.g., PGE-2) can be formed from Cyclooxygenase (COX)-catalyzed arachidonic acid (AA, a downstream ɷ-6 fatty acid) peroxidation [14], [15], [16], [17], [18], [19].
COX is a bi-functional membrane bound enzyme with two isoforms: COX-1, the constitutive form; and COX-2, the inducible form that can be readily induced by stresses, growth factors, and pro-inflammatory signals. COX-2 overexpresses in various types of cancers, including colon and pancreatic cancer, and its expression is also positively correlated with cancer invasive potential [20], [21]. COX-2 can metabolize many polyunsaturated fatty acids, e.g. AA and its intermediate upstream dihomo-γ-linolenic acid (DGLA) to 2-series and 1-series prostaglandins (e.g., PGE-2 and PGE-1). Although PGE-1 has been reported to possess some beneficial effects on human health [22], [23], we found that, even at a high concentration (~10 µM), PGE-1 showed no anti-cancer effects on cancer cells [24], [25]. On the other hand, the deleterious metabolites (e.g. PGE-2) have been shown to play an important role in promoting cancer metastasis [26], [27], [28]. A variety of COX-2 inhibitors have thus been used to limit the generation of PGE-2 to inhibit cancer metastasis in many types of cancers, including colon and pancreatic cancer [29], [30], [31]. However, COX-2 inhibitors suffer from some safety issues in patients, e.g., increased risks of cardiovascular disease and gastrointestinal injury [32], [33].
Our lab has successfully used liquid chromatography/electron spin resonance/mass spectrometry along with spin trapping technique to characterize the common as well as exclusive free radicals generated from COX-catalyzed AA and DGLA peroxidation [34], [35]. The different structural moiety in DGLA (three C˭C double bond instead of four in AA) results in the formation of a novel free radical byproduct 8-hydroxyoctanoic acid (8-HOA) [24]. Recent studies from our lab showed that 8-HOA can serve as a histone deacetylase inhibitor (HDACi) to inhibit cancer growth in colon and pancreatic cancer cells (e.g. HCA-7 and BxPC-3, both overexpress COX-2) via arresting cell cycle in G1 and promoting cell apoptosis in a p53-dependent or independent manner [25], [36], [37]. Genetic knockdown of delta-5-desaturase (D5D, the key enzyme for converting DGLA to AA) was able to promote formation of 8-HOA from COX-catalyzed DGLA peroxidation which in turn suppressed cancer cell growth and enhance efficacies of many chemo-drugs for colon and pancreatic cancer treatment [36], [37].
Histone deacetylases (HDACs) are enzymes that can catalyze deacetylation of lysine residues of core histone [38], [39], [40]. Increased expression of HDACs have been found in several types of cancers, e.g. colon and pancreatic cancer, which can increase cell proliferation, migration and invasion [39], [40], [41]. For the first time, we demonstrated that 8-HOA, serving as a HDAC inhibitor, not only caused inhibition of cancer cell migration and invasion, but also improved efficacy of chemo-drugs in colon and pancreatic cancer cells. The outcome of our study is expected to provide guidance for developing a new diet care strategy to improve current chemotherapy by taking advantage of abundant dietary ω-6s and commonly overexpressed COX-2 in cancer.
2. Material and methods
2.1. Cell line and reagent
The human colon cancer cell line HCA-7 colony 29 (European collection of Cell Culture, Salisbury, UK) and pancreatic cancer cell line BxPC-3 (ATCC, Manassas, VA), both overexpressing COX-2, were used in this study for testing cell migration and invasion upon treatments (e.g. 8-HOA, DGLA and chemo-drugs). HCA-7 was grown in Dulbecco's Modified Eagle's medium, while BxPC-3 was grown in RPMI-1640 medium (Thermo Fisher Scientific, UT, USA), both supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, UT, USA). Cells were cultured in an incubator containing a 95% humidified atmosphere with 5% CO2 at 37 °C.
CelLytic™ lysis reagent, 8-HOA, 5-fluorouracil, D5D primary antibody (from rabbit) and β-actin primary antibody (from mouse) were obtained from Sigma-Aldrich (MO, USA). DGLA was purchased from Nu-Chek-Prep (MN, USA). Gemcitabine was purchased from Cayman Chemicals (MI, USA). Pierce ECL western blot substrates and Lipofectamine™ RNAiMAX transfection reagent were obtained from Thermo Fisher Scientific (MA, USA). X-ray film was purchased from Phoenix Research Products (NC, USA). COX-2 primary antibody (from rabbit) was acquired from Abcam (MA, USA). All other primary antibodies and secondary antibodies were obtained from Cell Signaling (MA, USA).
DNA oligos encoding D5D-targeted shRNA with sequence of TGCTGTAATCATCCAGGC-CAAGTCCAGTTTTGGCCACTGACTGACTGGACTTGCTGGATGATTA (top strand) and CCTGTAATCATCCAGCAAGTCCAGTCAGTCAGTCAGTGGCCAAAACTGGACTTGGCCTGGATGATTAC (bottom strand) were designed (using BLOCK-IT™ RNAi Designer, www.invitro gen.com/rnai) and obtained from Integrated DNA Technologies (IA, USA). pcDNA™ 6.2-GW/EmGFP-miR vector was purchased from Invitrogen (NY, USA).
2.2. ShRNA transfection
The DNA oligos encoding D5D-targeted shRNA were cloned into pcDNA™ 6.2-GW/EmGFP-miR vector and transformed into E. coli. The shRNA expressed vector was extracted and transfected into cells using X-tremeGENE HP DNA Transfection Reagent (Roche). Cells transfected with negative control shRNA (NC-sh) were used as controls.
2.3. Wound healing assay
Wound healing assay was used to assess cell migration of both cancer cell lines upon treatments (e.g. 8-HOA and DGLA). Negative control shRNA transfected (NC-sh) or delta-5-desatuarse knockdown (D5D-KD) HCA-7 and BxPC-3 cells were seeded 1×106 cells per well (6-well plate). After the cells reached 90% confluence, the cells were wounded by scratching with a sterile pipette tip and washed by phosphate buffered saline (PBS) subsequently to eliminate the impaired cells. The medium was changed to medium with 1% fetal bovine serum. The cells were subjected to different treatment (e.g. 8-HOA and DGLA) and observed for 48 h. The wound area was measured using Image-J software (NIH, Bethesda, MD, USA). The wound area percentage was calculated as the wound area from 24 h or 48 h vs. the wound area from 0 h in each group.
2.4. Transwell assay
Transwell migration assays were performed to assess cancer cell migration upon treatments (e.g. DGLA and chemo-drugs) in transwell chamber with the non-coated membrane (24-well insert, pore size: 8 mm, Corning, Life Sciences). 5×104 cells were plated in the top chamber and incubated overnight to allow the cells to attach. For invasion assays, 5×104 cells from both cell lines were plated in the top chamber with Matrigel-coated membrane. Medium without serum was added to the upper chamber, and the medium containing 10% fetal bovine serum was added in the lower chamber. After 48 h, the cells were fixed in 10% neutral buffered formalin solution for 30 min and stained with 0.05% crystal violet solution for 30 min, and the cells that invaded through the pores to the lower surface of the inserts were counted under an inverted microscope.
2.5. HDAC activity assay
HDAC activity assay was measured using HDAC activity assay kit (BioVision, Pal Alto, USA) according to manufacturer's instructions. Briefly, after cells treated with 8-HOA, nuclear proteins were extracted with NE-PER™ nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific, UT, USA). Nuclear extracts were incubated with HDAC substrate at 37 °C for 1 h and then lysine developer was added to the mixture and incubated for 30 min at 37 °C. The plate was read at 405 nm on a microplate reader. The HDAC activity in HCA-7 and BxPC-3 cells without 8-HOA treatment was set to 100% for controls.
2.6. Western blot
After cancer cells were subjected to different treatments (e.g. 8-HOA, ω-6s and chemo-drugs) for ~48 h, cells were collected and lysed using CelLytic™ lysis reagents. Cell lysates were quantified by protein assay (Bio-Rad) and loaded into each well of 10% or 15% SDS-PAGE gels After electrophoresis, proteins on gels were transferred to nitrocellulose membranes using BioRad Mini Trans-Blot® Cell. Membranes were blocked with 5% (w/v) non-fat milk and then incubated with primary antibodies (1:1000 dilution) overnight at 4 °C with continuous rocking. Membranes were washed 3 times and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000 dilution). After washed 3 times, the membranes were incubated in ECL western blot substrates for 1 min, and exposed to X-ray film. Luminescent signals were captured using a Mini-Medical Automatic Film Processor (Imageworks).
2.7. Gas chromatography/mass spectrometry detection of 8-HOA
Gas chromatography/mass spectrometry analysis was used to quantify 8-HOA (in its derivative of pentafluorobenzyl bromide) generated from D5D-KD and NC-sh transfected HCA-7 and BxPC-3 cells treated with DGLA as described elsewhere [36], [46]. Briefly, after DGLA treatment (48 h), the cells were scraped into ~1.0 mL medium and added with methanol containing internal standard (hexanoic acid) and 50 μL of 1.0 N HCl. The mixture was added with 3.0 mL dichloromethane and vortexed, centrifuged to extract 8-HOA, and the dichloromethane layer was collected. The extraction process was repeated again with another 3.0 mL dichloromethane. The dichloromethane layers were combined and evaporated to dryness using a vacuum evaporator and derivatized using diisopropylethylamine and pentafluorobenzyl bromide. After 20 min reaction at room temperature, the solvent was removed by vacuum evaporator and reconstitute with dichloromethane for gas chromatography/mass spectrometry analysis.
Gas chromatography/mass spectrometry analysis was carried out by injecting each sample into an Agilent 6890 A gas chromatograph. The temperature of gas chromatography oven is programmed from 60 to 300 °C at 25 °C/min. The injector and transfer line were kept at 280 °C. Quantitative analysis was performed by a mass selective detector with a source temperature of 230 °C and nebulizer pressure of 15 psi. The quantification of 8-HOA (in pentafluorobenzyl bromide derivative form) was calculated by comparing its base peak (m/z 181) with the base peak of internal standard (hexanoic acid- pentafluorobenzyl bromide derivative).
2.8. Statistic analysis
Statistic analysis was performed using Student's unpaired t-test (two-tailed) on all data. A statistically significant difference was considered with a p value <0.05.
3. Results
3.1. 8-HOA inhibits cancer cell migration
Colon cancer cell line HCA-7 was used to test whether direct treatment of 8-HOA could inhibit migration of cancer cells overexpressing COX-2. After treatment of 8-HOA (1.0 µM) for 48 h, cell migration was significantly inhibited with the wound area ~73.0% compared to control ~47.4% (Fig. 1A andB), consistent with increased expression of acetyl histone H3 (HDAC substrate) and decreased expressions of matrix metalloproteinase-2 (MMP-2) and MMP-9, two proteins involved in degradation of extracellular matrix (Fig. 1C). Our study indicated that 8-HOA could inhibit histone deacetylase, and then suppress breakdown of extracellular matrix and cancer cell migration.
Fig. 1.
8-HOA inhibited cell migration in HCA-7 cells. A) Wound healing assay of HCA-7 cells upon 8-HOA treatment (1.0 µM) for 48 h. The HCA-7 cells treated with vehicle were used as control; B) Quantification of wound area in control and 8-HOA treated HCA-7 cells; C) Western blot and protein expression level of acetyl histone H3, MMP-2 and MMP-9 in HCA-7 cells treated with vehicle and 8-HOA. Protein expression rate was normalized using β-actin as loading control HDAC activity was also tested in HCA-7 cells treated with 8-HOA (1.0 µM) and ~55% HDAC activity was inhibited (*: significant difference with p<0.05 from n≥3 using unpaired student t-test).
Pancreatic cancer cell line BxPC-3 was also used to assess the effect of 8-HOA (1.0 µM) on cancer cell migration. Upon treatment 8-HOA, cell migration of BxPC-3 was significantly suppressed (wound area ~81.1% at 48 h compared to control ~ 58.3%, Fig. 2A and B), associated with increased expression of acetyl histone H3 as well as decreased expressions of MMP-2 and MMP-9 (Fig. 2C), indicating that 8-HOA could also inhibit HDAC and cancer migration in BxPC-3 cells.
Fig. 2.
Cell migration of BxPC-3 cells was suppressed by 8-HOA. A) Wound healing assay of BxPC-3 cells upon 8-HOA treatment (1.0 µM) for 48 h. The BxPC-3 cells treated with vehicle were used as control; B) Quantification of wound area in control and 8-HOA treated BxPC-3 cells; C) Western blot and protein expression level of acetyl histone H3, MMP-2 and MMP-9 in BxPC-3 cells treated with vehicle and 8-HOA. Protein expression rate was normalized using β-actin as loading control. HDAC activity was also tested in BxPC-3 cells treated with 8-HOA (1.0 µM) and ~50% HDAC activity was inhibited (*: significant difference with p<0.05 from n≥3 using unpaired student t-test).
HDAC activity assay was also conducted to test the effect of 8-HOA on HDAC activity in HCA-7 and BxPC-3 cells. About 40–55% of HDAC activity was inhibited in HCA-7 and BxPC-3 cells upon treatment of 8-HOA (0.5–1.5 µM) compared to the cancer cells without 8-HOA treatment, suggesting that 8-HOA could also inhibit HDAC activity.
3.2. D5D-KD promotes 8-HOA formation from COX-catalyzed DGLA peroxidation
In previous studies, our strategy (i.e. D5D-KD and DGLA supplement) promotes formation of 8-HOA from COX-catalyzed DGLA peroxidation to the threshold level (above 0.5 µM) and thus inhibits cancer cell growth [36], [37]. When HCA-7 cells were transfected with shRNA to knock down D5D for DGLA metabolism manipulation, ~75% expression of D5D was inhibited in shRNA transfected HCA-7 cells compared to the cells transfected with NC-sh (Fig. 3A). 8-HOA (PFB-derivative form) generated from both D5D-KD and NC-sh HCA-7 cells treated by DGLA 48 h was measured by GC/MS [36], [37]. In D5D-KD HCA-7 cells, the endogenous 8-HOA maintained above the threshold level 0.5 µM [36], [37] during 48 h treatment due to continuous COX-catalyzed peroxidation (Fig. 3C). However, endogenous 8-HOA never reached 0.5 µM in NC-sh transfected HCA-7 cells upon DGLA treatment for 48 h.
Fig. 3.
D5D-KD promoted formation of 8-HOA in HCA-7 and BxPC-3 cells. A) Western blot and protein expression level of COX-2 and D5D in NC-sh transfected vs. D5D-KD HCA-7 cells. B) Western blot and protein expression level of COX-2 and D5D in NC-sh transfected vs. D5D-KD BxPC-3 cells. Protein expression rate was normalized using β-actin as loading control; C) GC/MS quantification of 8-HOA from NC-sh transfected or D5D-KD HCA-7 cells treated with 100 µM DGLA; D) GC/MS quantification of 8-HOA from NC-sh transfected or D5D-KD BxPC-3 cells treated with 100 µM DGLA. Data represent as mean±SD for n≥3 (*: significant difference with p<0.05 using unpaired student t-test).
Similarly, BxPC-3 cells were transfected with shRNA to knockdown D5D and about 70% D5D expression was inhibited (Fig. 3B), and the level of 8-HOA in D5D-KD BxPC-3 cells upon 48 h DGLA treatment was consistently high above 0.5 µM. However, similar to the profile of 8-HOA observed in NC-sh HCA-7 cells, the level of endogenous 8-HOA never accumulated above 0.5 µM from NC-sh BxPC-3 cells (Fig. 3D).
3.3. Formation of threshold level of 8-HOA is essential for suppressing cancer migration
We further tested whether the formation of threshold level of 8-HOA from D5D-KD and DGLA supplement is responsible for inhibiting cancer cell migration. Wound healing assay was used to test the effect of D5D-KD and DGLA on cell migration in HCA-7 cells. D5D-KD significantly inhibited cell migration in HCA-7 cells treated with DGLA (~wound area of 75.0% at 48 h compared to control 45.3%, Fig. 4A).
Fig. 4.
D5D-KD and DGLA supplement inhibited cell migration in HCA-7 and BxPC-3 cells. A) Wound healing assays of D5D-KD HCA-7 cells upon DGLA (100 µM, 48 h) treatment vs. controls (without DGLA); B) Wound healing assays of D5D-KD BxPC-3 cells upon DGLA (100 µM, 48 h) treatment vs. controls (without DGLA) *: significant difference with p<0.05 from n≥3 using unpaired student t-test.
BxPC-3 cells were used to assess whether the threshold level of 8-HOA formed from our strategy (e.g. D5D-KD and DGLA supplement) could inhibit pancreatic cancer migration. DGLA treatment significantly inhibited cell migration in D5D-KD BxPC-3 cells (~wound area of 83.4% at 48 h compared to 55.3% in control) (Fig. 4B).
3.4. D5D-KD enhances the efficacy of 5-FU treatment on colon cancer cells
5-FU, an irreversible thymidylate synthase inhibitor, has been used as a common chemotherapy drug for treating colon cancer [47], [48]. However, many colon cancer cell lines have shown resistance to 5-FU [9], [10]. When DGLA and 5-FU were co-incubated with D5D-KD HCA-7 cells, DGLA could significantly enhance efficacy of 5-FU on cell migration (transwell migration assay, ~36 cells for co-treatment vs. ~63 for 5-FU only, Fig. 5A) and cell invasion (number of cells ~43 for co-treatment 5-FU and DGLA vs. ~65 for 5-FU only, Fig. 5B).
Fig. 5.
Efficacy of 5-FU on cell migration and invasion in HCA-7 cells was enhanced by D5D-KD and DGLA treatment. A) Transwell migration assay of D5D-KD HCA-7 cells upon treatment of DGLA (100 µM), 5-FU (0.2 mM) alone or 5-FU+DGLA. The D5D-KD cells without fatty acid and drug treatment were used as controls; B) Transwell invasion assay of D5D-KD HCA-7 cells upon treatment of DGLA (100 µM), 5-FU (0.2 mM) alone or 5-FU+DGLA. The D5D-KD cells without fatty acid and drug treatment were used as controls; C) Western blot and protein expression level of acetyl histone H3, MMP-2, MMP-9, E-cadherin, vimentin and snail from D5D-KD HCA-7 cells treated with vehicle (control), DGLA (100 μM), 5-FU (0.2 mM) or 5-FU+DGLA. Protein expression rate was normalized using β-actin as loading control (*: significant difference vs. control with p<0.05; and #: significant difference vs. 5-FU group with p<0.05 from n≥3 using unpaired student t-test).
Increased expression of acetyl histone H3 as well as decreased expressions of MMP-2 and 9 were observed in D5D-KD HCA-7 cells treated with DGLA, confirming that 8-HOA could act as a histone deacetylase inhibitor (Fig. 5C). In order to investigate the mechanism of enhanced efficacy of 5-FU behind our strategy (i.e. D5D-KD and DGLA), D5D-KD HCA-7 cells were treated with 5-FU alone or a combination of 5-FU and DGLA. 5-FU alone could downregulate the expressions of MMP-9, mesenchymal marker vimentin and EMT-inducing transcription factor snail and upregulate the expression of epithelial marker E-cadherin, consistent with other observations [4], [49], [50], [51], [52]. Further decreased expressions of MMP-9, vimentin, snail and further increased expression of E-cadherin were observed when D5D-KD HCA-7 cells were co-treated with DGLA and 5-FU (Fig. 5C) compared to treatment 5-FU alone.
3.5. D5D-KD enhances the efficacy of gemcitabine treatment on pancreatic cancer cells
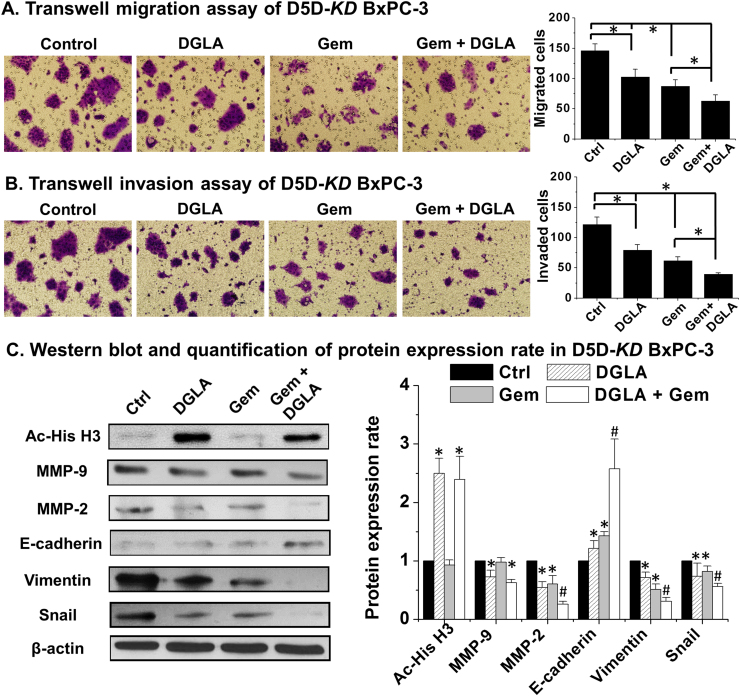
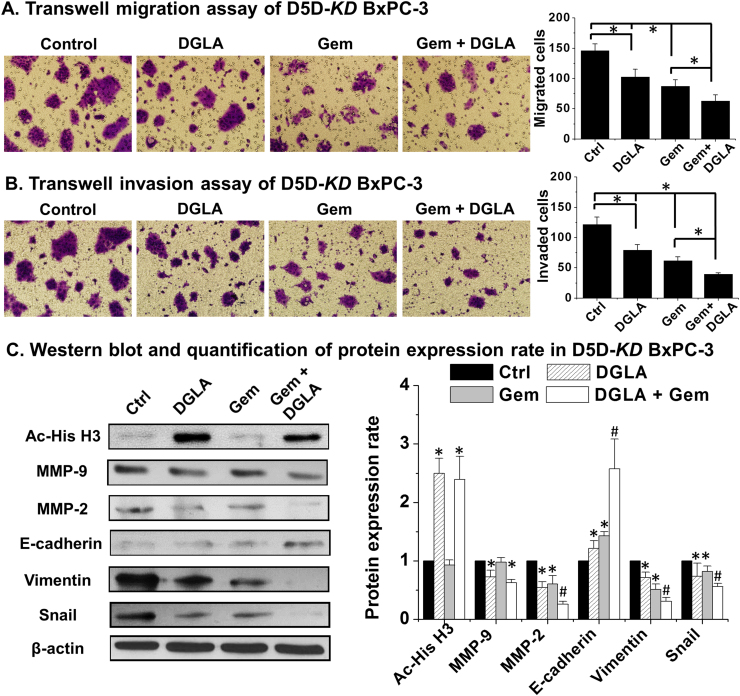
Gemcitabine, a nucleoside analog, has been used as a front-line chemo-drug for pancreatic cancer treatment [53]. Many pancreatic cancer cells are resistant to gemcitabine through various mechanisms [6], [7], [8]. We found that D5D-KD and DGLA treatment could improve the efficacy of gemcitabine on BxPC-3 cells as shown in transwell migration assays (~63 cells migrated for treatment DGLA plus gemcitabine vs. ~87 for gemcitabine only Fig. 6A). The transwell invasion assays also showed that co-treatment of DGLA with gemcitabine further inhibited cell invasion in D5D-KD BxPC-3 cells (~40 cells invaded for treatment DGLA plus gemcitabine vs. ~62 cells for gemcitabine alone Fig. 6B).
Fig. 6.
D5D-KD and DGLA treatment enhanced efficacy of gemcitabine on cell migration and invasion in BxPC-3 cells. A) Transwell migration assay of D5D-KD BxPC-3 cells upon treatment of DGLA (100 µM), gemcitabine (0.1 µM) alone or gemcitabine+DGLA. The D5D-KD cells without fatty acid and drug treatment were used as controls; B) Transwell invasion assay of D5D-KD BxPC-3 cells upon treatment of DGLA (100 µM), gemcitabine (0.1 µM) alone or gemcitabine+DGLA. The D5D-KD cells without fatty acid and drug treatment were used as controls; C) Western blot and protein expression level of acetyl histone H3, MMP-2, MMP-9, E-cadherin, vimentin and snail from D5D-KD BxPC-3 cells treated with vehicle (control), DGLA (100 μM), gemcitabine (0.1 μM) or gemcitabine+DGLA. Protein expression rate was normalized using β-actin as loading control (*: significant difference vs. control with p<0.05; and #: significant difference vs. gemcitabine group with p<0.05 from n≥3 using unpaired student t-test).
Gemcitabine has been shown to suppress cancer migration and invasion by altering MMP-2 expression and EMT protein levels [54], [55], [56], [57], [58]. We found that gemcitabine alone could decrease expression of MMP-2, vimentin and snail as well as increase expression of E-cadherin (Fig. 6C). When D5D-KD BxPC-3 cells were co-treated with DGLA and gemcitabine, further decreased expressions of MMP-2, vimentin and snail as well as further increased expression of E-cadherin compared to treatment gemcitabine alone (Fig. 6C).
4. Discussion
Our lab previously showed that direct treatment of 8-HOA as well as formation of 8-HOA from D5D-KD and COX-catalyzed DGLA peroxidation could inhibit cancer cell growth in colon and pancreatic cancer possibly acting as a histone deacetylase inhibitor (HDACi) [36], [37]. A variety of HDACis have been shown to suppress cancer cell migration and invasion [59], [60], [61]. We thus investigated whether the strategy of D5D-KD and DGLA treatment can also inhibit cancer cell migration and invasion.
The concentration of direct 8-HOA treatment is chosen based on our experimental result that 1.0 µM (Fig. 1A and Fig. 2A) is an effective concentration among a range of 0.1–10 µM, consistent with the concentration we have tested for inhibiting cancer cell growth as well as the endogenous concentration can be reached upon DGLA treatment [36], [37]. We also detected the levels of 8-HOA in D5D-KD HCA-7 and BxPC-3 cells treated with DGLA 48 h using GC/MS. A threshold level of endogenous 8-HOA (>0.5 µM) was found essential to exert anti-migratory effect on colon cancer cells and pancreatic cancer cells (Fig. 3C and D), consistent with the threshold level determined for inhibiting cancer cell growth in colon and pancreatic cancer cell [36], [37]. Our results clearly demonstrated that 8-HOA, either exogenous or endogenous, could inhibit cancer metastasis once a threshold level (>0.5 µM) was reached. We actually tested inhibitory effect of direct treatment of 8-HOA (range from 0.1 to 5.0 µM) on HDAC activity in HCA-7 and BxPC-3 cells. We found that 0.5–1.5 µM can significantly inhibit HDAC activity (40–55%). In addition, we also tested effect of D5D-KD and DGLA treatment (e.g., promoted formation of 8-HOA) on HDAC activity, which also inhibited ~50% activities for both HCA-7 and BxPC-3 cells.
Our strategy not only resulted in formation of a threshold level of 8-HOA, but also caused alterations in the levels of DGLA, AA and their metabolites PGE1 and PGE2 in D5D-KD HCA-7 and BxPC-3 cells treated with DGLA for 48 h, as measured by LC/MS (Supplement Tables 1 and 2). As the conversion from DGLA to AA was suppressed by D5D-KD, higher level of DGLA and formation of PGE1, and decreased level of AA and formation of PGE2, were detected in D5D-KD HCA-7 and BxPC-3. However, the direct treatment of concentration levels of PGE1 that could be generated in D5D-KD HCA-7 and BxPC-3 cells had no effect on cancer cell growth [24], [25] and migration in this study (data not show). Furthermore, DGLA treatment had no inhibitory effects on cell migration of the NC-sh HCA-7 cells (wound area ~46.2% at 48 h compared to wound area ~44.7% in control, Supplement Fig. 1A) and NC-sh BxPC-3 cells (~wound area of 57.9% vs. ~58.1% in control, Supplement Fig. 1B). These data strongly suggest that 8-HOA formed from COX-2 catalyzed DGLA peroxidation, rather than PGE1 or DGLA, is the anti-cancer metabolite.
In addition, we also did experiments to make sure that our strategy (i.e. D5D-KD and DGLA supplement) truly inhibited migration and invasion of living cancer cells. After treatment with DGLA, chemo-drugs and their combination for 48 h, D5D-KD HCA-7 and BxPC-3 cells were trypsinized, counted (5×104), and then seeded into inserts for transwell migration and invasion assays. Inhibition of migrated and invaded cancer cells showed the similar results as observed in Fig. 5, Fig. 6, indicating that our strategy is actually inhibiting migration and invasion of living cancer cells, instead of dead cells cannot migrate or invade. In this study we demonstrated that 8-HOA can inhibit cell migration and invasion possibly by serving as an HDACi to prevent histone deacetylation to generate relaxed chromatin, thereby allowing transcriptional activation of targeted genes (e.g. MMP-2 and MMP-9, Scheme 1) [38], [39], [40], [41], [42], [43], [44], [45], [59], [60], [61]. Our study also showed that D5D-KD and DGLA treatment could enhance efficacies of 5-FU to inhibit cell migration and invasion in HCA-7 cells by further inhibiting MMP-9, vimentin and snail as well as upregulating expression E-cadherin (Fig. 5C). On the other hand, the enhanced efficacy of gemcitabine on suppressing D5D-KD BxPC-3 cells’ migration and invasion upon DGLA treatment was likely associated with further increased expression of E-cadherin and decreased expression of MMP-2, vimentin and snail (Fig. 6C). Considering that the commonly low efficacy of chemotherapy for colon and pancreatic cancer patients, our strategy can be used to improve many frontline chemo-drugs to kill cancer.
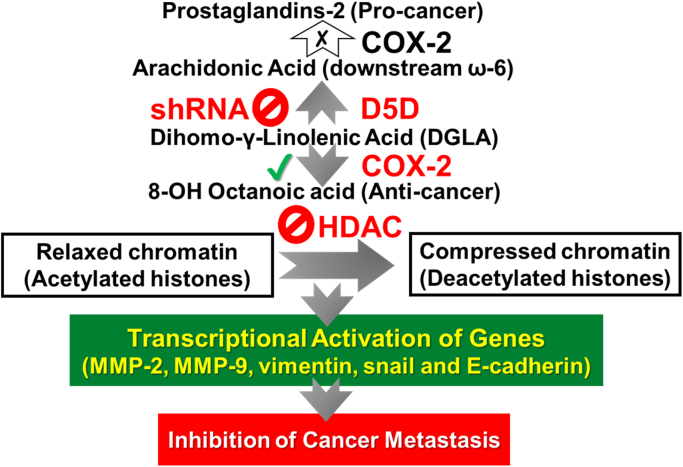
Scheme 1.
Proposed mechanism of our strategy to inhibit cancer metastasis.
Given that COX-2 is commonly overexpressed or can also be readily induced in cancerous condition in response of growth factors, tumors promotors and cytokines in many types of cancers, our strategy (i.e. D5D-KD and manipulated COX-2-catalyzed DGLA peroxidation) would be applied to treatments of all types of cancers. The strategy could also be used to inhibit growth, migration and invasion in the cancer cells with low or deficient COX-2 expression, as 8-HOA (in a paracrine manner) continuously produced from most nearby cancer cells that overexpress COX-2. We have observed that cell migration of wild-type PANC-1 cells (COX-2 deficient) was also inhibited upon 8-HOA treatment (data not show), demonstrating that, in paracrine meaner, 8-HOA can suppress migration of cancer cells regardless the COX-2 expression level.
In general, cancer patients have a much higher expression level of COX than the noncancerous population, and the key isoenzyme COX-2 is also readily induced by various agents, such as growth factors and tumor promoters. Thus, the prostaglandin from AA may continue to form at a deleterious (albeit reduced) level in spite of using COX inhibitors in cancer patients. D5D-KD not only prevents the buildup of AA to limit prostaglandin formation, but also promotes DGLA to generate 8-HOA that suppresses cancer growth and metastasis (Scheme 1). Therefore, the higher expression of COX-2 in cancer is actually a benefit in our strategy rather than a problem.
For the first time, we have demonstrated that high COX-2 expression could be used to improve formation of 8-HOA from DGLA peroxidation via D5D-KD for the inhibition of cancer cell migration and invasion, as well as for the improvement of efficacy of chemotherapy. We now can work on a more practical approach to downregulate D5D, e.g., to construct stable RNA nanoparticles harboring D5D siRNA to allow specific delivery of D5D siRNA to cancer cells and tumors. RNA nanoparticles have emerged recently as a new platform for targeted drug, siRNA, miRNA, anti-miRNA and immunomodulator delivery for cancer therapy [62], [63], [64]. The RNA nanoparticles can specifically target cancer with little to no accumulation in healthy tissues, highlighting the benefits of translating RNA nanoparticles for cancer therapy with enhanced targeting efficiency and reduced side effects [62], [63], [64]. The outcome of our work will reinforce our translational efforts to develop a novel ω−6 based dietary anti-cancer strategy in combination with modified COX-2-catalyzed DGLA peroxidation and D5D-mediated ω−6 conversion, and therefore improving the efficacies of chemotherapy.
Conflict of interest
The authors claim no conflict of interest.
Acknowledgements
This work was supported by NIC-1R15CA195499-01A (S Qian) and Sanford NDSU Seed Grant FAR0026507 (S Qian and K Miskimins).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.01.016.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Keleg S., Büchler P., Ludwig R., Büchler M.W., Friess H. Invasion and metastasis in pancreatic cancer. Mol. Cancer. 2003;2:14. doi: 10.1186/1476-4598-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao H., Xu E., Liu H., Wan L., Lai M. Epithelial-mesenchymal transition in colorectal cancer metastasis: a system review. Pathol. Res. Pract. 2015;211:557–569. doi: 10.1016/j.prp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Ortega A. A new role for GABA: inhibition of tumor cell migration. Trends Pharmacol. Sci. 2003;24:151–154. doi: 10.1016/S0165-6147(03)00052-X. [DOI] [PubMed] [Google Scholar]
- 4.Zhu P., Zhao N., Sheng D., Hou J., Hao C., Yang X., Zhu B., Zhang S., Han Z., Wei L., Zhang L. Inhibition of growth and metastasis of colon cancer by delivering 5-fluorouracil-loaded pluronic P85 copolymer micelles. Sci. Rep. 2016;6:20896. doi: 10.1038/srep20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quint K., Tonigold M., Di Fazio P., Montalbano R., Lingelbach S., Rückert F., Alinger B., Ocker M., Neureiter D. Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int. J. Oncol. 2012;41:2093–2102. doi: 10.3892/ijo.2012.1648. [DOI] [PubMed] [Google Scholar]
- 6.Andersson R., Aho U., Nilsson B.I., Peters G.J., Pastor-Anglada M., Rasch W., Sandvold M.L. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand. J. Gastroenterol. 2009;44:782–786. doi: 10.1080/00365520902745039. [DOI] [PubMed] [Google Scholar]
- 7.Zheng C., Jiao X., Jiang Y., Sun S. ERK1/2 activity contributes to gemcitabine resistance in pancreatic cancer cells. J. Int. Med. Res. 2013;41:300–306. doi: 10.1177/0300060512474128. [DOI] [PubMed] [Google Scholar]
- 8.Horiguchi S., Shiraha H., Nagahara T., Kataoka J., Iwamuro M., Matsubara M., Nishina S., Kato H., Takaki A., Nouso K., Tanaka T., Ichimura K., Yagi T., Yamamoto K. Loss of runt-related transcription factor 3 induces gemcitabine resistance in pancreatic cancer. Mol. Oncol. 2013;7:840–849. doi: 10.1016/j.molonc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang N., Yin Y., Xu S.J., Chen W.S. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551–1569. doi: 10.3390/molecules13081551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longley D.B., Johnston P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 11.Haqq J., Howells L.M., Garcea G., Dennison A.R. Targeting pancreatic cancer using a combination of gemcitabine with the omega-3 polyunsaturated fatty acid emulsion, Lipidem™. Mol. Nutr. Food Res. 2016;60:1437–1447. doi: 10.1002/mnfr.201500755. [DOI] [PubMed] [Google Scholar]
- 12.Hering J., Garrean S., Dekoj T.R., Razzak A., Saied A., Trevino J., Babcock T.A., Espat N.J. Inhibition of proliferation by omega-3 fatty acids in chemoresistant pancreatic cancer cells. Ann. Surg. Oncol. 2007;14:3620–3628. doi: 10.1245/s10434-007-9556-8. [DOI] [PubMed] [Google Scholar]
- 13.Funahashi H., Satake M., Hasan S., Sawai H., Newman R.A., Reber H.A., Hines O.J., Eibl G. Opposing effects of n-6 and n-3 polyunsaturated fatty acids on pancreatic cancer growth. Pancreas. 2008;36:353–362. doi: 10.1097/MPA.0b013e31815ccc44. [DOI] [PubMed] [Google Scholar]
- 14.Ito H., Duxbury M., Benoit E., Clancy T.E., Zinner M.J., Ashley S.W., Whang E.E. Prostaglandin E2 enhances pancreatic cancer invasiveness through an Ets-1-dependent induction of matrix metalloproteinase-2. Cancer Res. 2004;64:7439–7446. doi: 10.1158/0008-5472.CAN-04-1177. [DOI] [PubMed] [Google Scholar]
- 15.Dufour M., Faes S., Dormond-Meuwly A., Demartines N., Dormond O. PGE2-induced colon cancer growth is mediated by mTORC1. Biochem. Biophys. Res. Commun. 2014;451:587–591. doi: 10.1016/j.bbrc.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Reader J., Holt D., Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev. 2011;30:449–463. doi: 10.1007/s10555-011-9303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eibl G., Bruemmer D., Okada Y., Duffy J.P., Law R.E., Reber H.A., Hines O.J. PGE (2) is generated by specific COX-2 activity and increases VEGF production in COX-2-expressing human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2003;306:887–897. doi: 10.1016/s0006-291x(03)01079-9. [DOI] [PubMed] [Google Scholar]
- 18.Bu X., Zhao C., Dai X. Involvement of COX-2/PGE (2) pathway in the upregulation of MMP-9 expression in pancreatic cancer. Gastroenterol. Res. Pract. 2011 doi: 10.1155/2011/214269. (2011: 214269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi H., Li A., Dawson D.W., Hines O.J., Reber H.A., Eibl G. Cyclooxygenase-2 confers growth advantage to syngeneic pancreatic cancer cells. Pancreas. 2011;40:453–459. doi: 10.1097/MPA.0b013e31820b9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomozawa S., Nagawa H., Tsuno N., Hatano K., Osada T., Kitayama J., Sunami E., Nita M.E., Ishihara S., Yano H., Tsuruo T., Shibata Y., Muto T. Inhibition of haematogenous metastasis of colon cancer in mice by a selective COX-2 inhibitor, JTE-522. Br. J. Cancer. 1999;81:1274–1279. doi: 10.1038/sj.bjc.6694262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W.S., Wei S.J., Liu J.M., Hsiao M., Kou-Lin J., Yang W.K. Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int. J. Cancer. 2001;91:894–899. doi: 10.1002/1097-0215(200102)9999:9999<894::aid-ijc1146>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Tabolacci C., Lentini A., Provenzano B., Gismondi A., Rossi S., Beninati S. Similar antineoplastic effects of nimesulide, a selective COX-2 inhibitor, and prostaglandin E1 on B16-F10 murine melanoma cells. Melanoma Res. 2010;20:273–279. doi: 10.1097/CMR.0b013e328339d8ac. [DOI] [PubMed] [Google Scholar]
- 23.Sagar P.S., Das U.N. Cytotoxic action of cis-unsaturated fatty acids on human cervical carcinoma (HeLa) cells in vitro. Prostaglandins Leukot. Ess. Fat. Acids. 1995;53:287–299. doi: 10.1016/0952-3278(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y., Xu Y., Law B., Qian S.Y. The first characterization of free radicals formed from cellular COX-catalyzed peroxidation. Free Radic. Biol. Med. 2013;57:49–60. doi: 10.1016/j.freeradbiomed.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y., Qi J., Yang X., Wu E., Qian S.Y. Free radical derivatives formed from cyclooxygenase-catalyzed dihomo-γ-linolenic acid peroxidation can attenuate colon cancer cell growth and enhance 5-fluorouracil's cytotoxicity. Redox Biol. 2014;2:610–618. doi: 10.1016/j.redox.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutaud O., Sosa I.R., Amin T., Oram D., Adler D., Hwang H.S., Crews B., Milne G.L., Harris B.K., Hoeksema M.D., Knowllmann B.C., Lammers P.E., Marnett L.J., Massion P.P., Oates J.A. Inhibition of the biosynthesis of prostaglandin E2 by low dose aspirin: implications for adenocarcinoma metastasis. Cancer Prev. Res. (Phila) 2016;9:855–865. doi: 10.1158/1940-6207.CAPR-16-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregor J.I., Kilian M., Heukamp I., Kiewert C., Kristiansen G., Schimke I., Walz M.K., Jacobi C.A., Wenger F.A. Effects of selective COX-2 and 5-LOX inhibition on prostaglandin and leukotriene synthesis in ductal pancreatic cancer in Syrian hamster. Prostaglandins Leukot. Ess. Fat. Acids. 2005;73:89–97. doi: 10.1016/j.plefa.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Kirane A., Toombs J.E., Ostapoff K., Carbon J.G., Zaknoen S., Braunfeld J., Schwarz R.E., Burrows F.J., Brekken R.A. Apricoxib, a novel inhibitor of COX-2, markedly improves standard therapy response in molecularly defined models of pancreatic cancer. Clin. Cancer Res. 2012;18:5031–5042. doi: 10.1158/1078-0432.CCR-12-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao M., Kargman S., Lam E.C., Kelly C.R., Zheng Y., Luk P., Kwong E., Evans J.F., Wolfe M.M. Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic potential of colorectal carcinoma in mice. Cancer Res. 2003;63:586–592. [PubMed] [Google Scholar]
- 30.Kobayashi H., Uetake H., Higuchi T., Enomoto M., Sugihara K. JTE-522, a selective COX-2 inhibitor, inhibits growth of pulmonary metastases of colorectal cancer in rats. BMC Cancer. 2005;5:26. doi: 10.1186/1471-2407-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamauchi T., Watanabe M., Hasegawa H., Nishibori H., Ishii Y., Tatematsu H., Yamamoto K., Kubota T., Kitajima M. The potential for a selective cyclooxygenase-2 inhibitor in the prevention of liver metastasis in human colorectal cancer. Anticancer Res. 2003;23:245–249. [PubMed] [Google Scholar]
- 32.Hawkey C.J., Langman M.J.S. Non-steroidal anti-inflammatory drugs: overall risks and management. Complementary roles for COX-2 inhibitors and proton pump inhibitors. Gut. 2003;52:600–608. doi: 10.1136/gut.52.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenberg J.D., Fisher M.C., Kremer J., Chang H., Rosenstein E.D., Kishimoto M., Lee S., Yazici Y., Kavanaugh A., Abramson S.B. CORRONA investigators. The COX-2 inhibitor market withdrawals and prescribing patterns by rheumatologists in patients with gastrointestinal and cardiovascular risk. Clin. Exp. Rheumatol. 2009;27:395–401. [PubMed] [Google Scholar]
- 34.Yu Q., Purwaha P., Ni K., Sun C., Mallik S., Qian S.Y. Characterization of novel radicals from COX-catalyzed arachidonic acid peroxidation. Free Radic. Biol. Med. 2009;47:568–576. doi: 10.1016/j.freeradbiomed.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao Y., Gu Y., Purwaha P., Ni K., Law B., Mallik S., Qian S.Y. Characterization of free radicals formed from COX-catalyzed DGLA peroxidation. Free Radic. Biol. Med. 2011;50:1163–1170. doi: 10.1016/j.freeradbiomed.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y., Yang X., Zhao P., Yang Z., Yan C., Guo B., Qian S.Y. Knockdown of delta-5-desaturase promotes the anti-cancer activity of dihomo-γ-linolenic acid and enhances the efficacy of chemotherapy in colon cancer cells expressing COX-2. Free Radic. Biol. Med. 2016;96:67–77. doi: 10.1016/j.freeradbiomed.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X., Xu Y., Brooks A., Guo B., Miskimins K.W., Qian S.Y. Knockdown delta-5-desaturase promotes the formation of a novel free radical byproduct from COX-catalyzed ω-6 peroxidation to induce apoptosis and sensitize pancreatic cancer cells to chemotherapy drugs. Free Radic. Biol. Med. 2016;97:342–350. doi: 10.1016/j.freeradbiomed.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bi G., Jiang G. The molecular mechanism of HDAC inhibitors in anticancer effects. Cell Mol. Immunol. 2006;3:285–290. [PubMed] [Google Scholar]
- 39.Koutsounas I., Giaginis C., Theocharis S. Histone deacetylase inhibitors and pancreatic cancer: are there any promising clinical trials? World J. Gastroenterol. 2013;19:1173–1181. doi: 10.3748/wjg.v19.i8.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mariadason J.M. HDACs and HDAC inhibitors in colon cancer. Epigenetics. 2008;3:28–37. doi: 10.4161/epi.3.1.5736. [DOI] [PubMed] [Google Scholar]
- 41.Schneider G., Krämer O.H., Fritsche P., Schüler S., Schmid R.M., Saur D. Targeting histone deacetylases in pancreatic ductal adenocarcinoma. J. Cell Mol. Med. 2010;14:1255–1263. doi: 10.1111/j.1582-4934.2009.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X., Yang W., Pei F., Ma W., Wang Y. Downregulation of matrix metalloproteinases contributes to the inhibition of cell migration and invasion in HepG2 cells by sodium valproate. Oncol. Lett. 2015;10:531–535. doi: 10.3892/ol.2015.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z., Leng Y., Tsai L.K., Leeds P., Chuang D.M. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J. Cereb. Blood Flow Metab. 2011;31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitmaker E.J., Griff N.J., Grogan R.H., Sarkar R., Kebebew E., Duh Q.Y., Clark O.H., Shen W.T. Modulation of matrix metalloproteinase activity in human thyroid cancer cell lines using demethylating agents and histone deacetylase inhibitors. Surgery. 2011;149:504–511. doi: 10.1016/j.surg.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Lee J.C., Maa M.C., Yu H.S., Wang J.H., Yen C.K., Wang S.T., Chen Y.J., Liu Y., Jin Y.T., Leu T.H. Butyrate regulates the expression of c-Src and focal adhesion kinase and inhibits cell invasion of human colon cancer cells. Mol. Carcinog. 2005;43:207–214. doi: 10.1002/mc.20117. [DOI] [PubMed] [Google Scholar]
- 46.Quehenberger O., Armando A., Dumlao D., Stephens D.L., Dennis E.A. Lipidomics analysis of essential fatty acids in macrophages. Prostaglandins Leukot. Ess. Fat. Acids. 2008;79:123–129. doi: 10.1016/j.plefa.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas D.M., Zalcberg J.R. 5-fluorouracil: a pharmacological paradigm in the use of cytotoxics. Clin. Exp. Pharmacol. Physiol. 1998;25:887–895. doi: 10.1111/j.1440-1681.1998.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 48.Noordhuis P., Holwerda U., Van der Wilt C.L., Van Groeningen C.J., Smid K., Meijer S., Pinedo H.M., Peters G.J. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004;15:1025–1032. doi: 10.1093/annonc/mdh264. [DOI] [PubMed] [Google Scholar]
- 49.Shakibaei M., Kraehe P., Popper B., Shayan P., Goel A., Buhrmann C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer. 2015;15:250. doi: 10.1186/s12885-015-1291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng Z.R., Zhong W.H., Liu J., Xiao P.T. Effects of the combination of hyperbaric oxygen and 5-fluorouracil on proliferation and metastasis of human nasopharyngeal carcinoma CNE-2Z cells. Undersea Hyperb. Med. 2010;37:141–150. [PubMed] [Google Scholar]
- 51.Iovieno A., Lambiase A., Moretti C., Perrella E., Bonini S. Therapeutic effect of topical 5-fluorouracil in conjunctival squamous carcinoma is associated with changes in matrix metalloproteinases and tissue inhibitor of metalloproteinases expression. Cornea. 2009;28:821–824. doi: 10.1097/ICO.0b013e318190937d. [DOI] [PubMed] [Google Scholar]
- 52.Buhrmann C., Shayan P., Kraehe P., Popper B., Goel A., Shakibaei M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem. Pharmacol. 2015;98:51–68. doi: 10.1016/j.bcp.2015.08.105. [DOI] [PubMed] [Google Scholar]
- 53.de Sousa Cavalcante L., Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur. J. Pharmacol. 2014;741:8–16. doi: 10.1016/j.ejphar.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 54.Haq M., Shafii A., Zervos E.E., Rosemurgy A.S. Addition of matrix metalloproteinase inhibition to conventional cytotoxic therapy reduces tumor implantation and prolongs survival in a murine model of human pancreatic cancer. Cancer Res. 2000;60:3207–3211. [PubMed] [Google Scholar]
- 55.Banerjee J., Al-Wadei H.A., Al-Wadei M.H., Dagnon K., Schuller H.M. Differential modulation of nicotine-induced gemcitabine resistance by GABA receptor agonists in pancreatic cancer cell xenografts and in vitro. BMC Cancer. 2014;14:725. doi: 10.1186/1471-2407-14-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao Z.J., Guo Y.H., Zhao Z., Yao J.T., Xu R., Nan K.J. Gemcitabine inhibits the micrometastasis of non-small cell lung cancer by targeting the EpCAM-positive circulating tumor cells via the HGF/cMET pathway. Int. J. Oncol. 2014;45:651–658. doi: 10.3892/ijo.2014.2464. [DOI] [PubMed] [Google Scholar]
- 57.Quint K., Tonigold M., Di Fazio P., Montalbano R., Lingelbach S., Rückert F., Alinger B., Ocker M., Neureiter D. Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int. J. Oncol. 2012;41:2093–2102. doi: 10.3892/ijo.2012.1648. [DOI] [PubMed] [Google Scholar]
- 58.Yu Y., Wang J., Xia N., Li B., Jiang X. Maslinic acid potentiates the antitumor activities of gemcitabine in vitro and in vivo by inhibiting NF-κB-mediated survival signaling pathways in human gallbladder cancer cells. Oncol. Rep. 2015;33:1683–1690. doi: 10.3892/or.2015.3755. [DOI] [PubMed] [Google Scholar]
- 59.De U., Kundu S., Patra N., Ahn M.Y., Ahn J.H., Son J.Y., Yoon J.H., Moon H.R., Lee B.M., Kim H.S. A new histone deacetylase inhibitor, MHY219, inhibits the migration of human prostate cancer cells via HDAC1. Biomol. Ther. 2015;23:434–441. doi: 10.4062/biomolther.2015.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahn M.Y., Kang D.O., Na Y.J., Yoon S., Choi W.S., Kang K.W., Chung H.Y., Jung J.H., Min do S., Kim H.S. Histone deacetylase inhibitor, apicidin, inhibits human ovarian cancer cell migration via class II histone deacetylase 4 silencing. Cancer Lett. 2012;325:189–199. doi: 10.1016/j.canlet.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Glozak M.A., Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 62.Shu D., Shu Y., Haque F., Abdelmawla S., Guo P. Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics. Nat. Nanotechnol. 2011;6:658–667. doi: 10.1038/nnano.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui D., Zhang C., Liu B., Shu Y., Du T., Shu D., Wang K., Dai F., Liu Y., Li C., Pan F., Yang Y., Ni J., Li H., Brand-Saberi B., Guo P. Regression of gastric cancer by systemic injection of RNA nanoparticles carrying both ligand and siRNA. Sci. Rep. 2015;5:10726. doi: 10.1038/srep10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shu D., Li H., Shu Y., Xiong G., Carson W.E., 3rd, Haque F., Xu R., Guo P. Systemic delivery of anti-miRNA for suppression of triple negative breast cancer utilizing RNA nanotechnology. ACS Nano. 2015;9:9731–9740. doi: 10.1021/acsnano.5b02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material