Abstract
Background
Costimulatory blockade of T lymphocytes with the CTLA4-Ig fusion protein abatacept could be an effective treatment for the immune mediated neuroinflammatory disease relapsing-remitting multiple sclerosis (RRMS).
Objective
To evaluate efficacy and safety of abatacept in RRMS.
Methods
ACCLAIM (A Cooperative Clinical Study of Abatacept in Multiple Sclerosis) was a phase II, randomized, double-blind, placebo-controlled, multi-center trial. Sixty-five of 123 planned participants with RRMS were randomized to monthly intravenous infusions of abatacept or placebo for 24 weeks in a 2:1 ratio, switched to the opposite treatment at 28 weeks, and received their final dose of study medication at 52 weeks. Enrollment was closed early due to slow accrual. The primary endpoint was the mean number of new gadolinium-enhancing (Gd+) lesions obtained on magnetic resonance imaging (MRI) scans performed every 4 weeks.
Results
No statistically significant differences were observed in mean number of new Gd+ MRI lesions between the abatacept and placebo groups. No statistically significant differences were observed in other MRI and clinical parameters of RRMS disease activity. Abatacept was well tolerated.
Conclusion
The ACCLAIM study did not demonstrate efficacy of abatacept in reducing the number of new Gd+ MRI lesions, or clinical measures of disease activity in RRMS.
MeSH Keywords: Abatacept; Autoimmune Diseases, Clinical Trial; Costimulatory and Inhibitory T-Cell Receptors, Gadolinium; Intervention Study; Magnetic Resonance Imaging; Multiple Sclerosis, Relapsing-Remitting
Introduction
Relapsing-remitting multiple sclerosis (RRMS) is an immune mediated neuroinflammatory disease. New therapies reduce the frequency of relapses in patients with RRMS [1–3], however efficacy is difficult to sustain over time [4–6] and side effects are common. Abatacept (Orencia, Bristol-Myers Squibb Company, New York, NY) is a CTLA4-Ig fusion protein that targets the adaptive arm of the immune system by blocking the CD28-B7 costimulatory pathway, which is central to activation of T lymphocytes [7–10]. Abatacept is approved for treatment of rheumatoid arthritis and juvenile idiopathic arthritis, has a favorable safety profile [11–13], and has been shown to slow the decline of C-peptide in type 1 diabetes, associated with a reduction in the CD4 central memory T cell subset [14, 15].
Since T lymphocytes have been implicated in pathogenesis of RRMS, treatment with abatacept has the potential to reduce immune-mediated disease activity. The scientific rationale for abatacept in RRMS is further supported by the benefit of costimulatory blockade in murine models of experimental autoimmune encephalomyelitis [16]. Moreover, in an open label Phase I trial in RRMS, CTLA4-Ig was well tolerated and reduced T cell proliferation and γ-interferon production in vitro by peripheral blood mononuclear cells in response to myelin peptide stimulation in vitro [17]. A phase II trial of abatacept in RRMS (NCT00035529) was terminated prematurely due to safety events, however the results of this trial were inconclusive due to imbalance in disease activity between treatment groups at baseline [18]. Therefore, we undertook a second phase II trial, known as ACCLAIM (A Cooperative Clinical Study of Abatacept in Multiple Sclerosis), to further investigate the clinical efficacy and tolerability of abatacept therapy for RRMS.
Methods
Patients
Male and female participants age 18–65 with clinically definite multiple sclerosis according to the McDonald criteria [19, 20] were eligible for enrollment in the study. Participants met the following additional eligibility criteria: a) RRMS; b) Kurtzke Expanded Disability Status Scale (EDSS) scores between 0 and 5; c) Active disease defined as at least one documented clinical exacerbation or at least one gadolinium-enhancing (Gd+) magnetic resonance imaging (MRI) lesion within one year prior to study entry. Participants were excluded if they had a normal baseline MRI or a diagnosis of primary or secondary progressive MS. Sixty-five participants were enrolled between 2010 and 2013, with a range of 1 to 9 participants enrolled at each site. Due to slow enrollment, the study was closed prior to reaching the planned target of 123 participants.
Standard Protocol Approvals, Registrations, and Patient Consents
The study, registered as NCT01116427, was conducted by the Immune Tolerance Network at 19 clinical sites in the United States and Canada. The study received institutional review or ethics board approval at each site, and was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. Participants signed informed consent and were not compensated except for travel expense reimbursement.
Study Design and Procedures
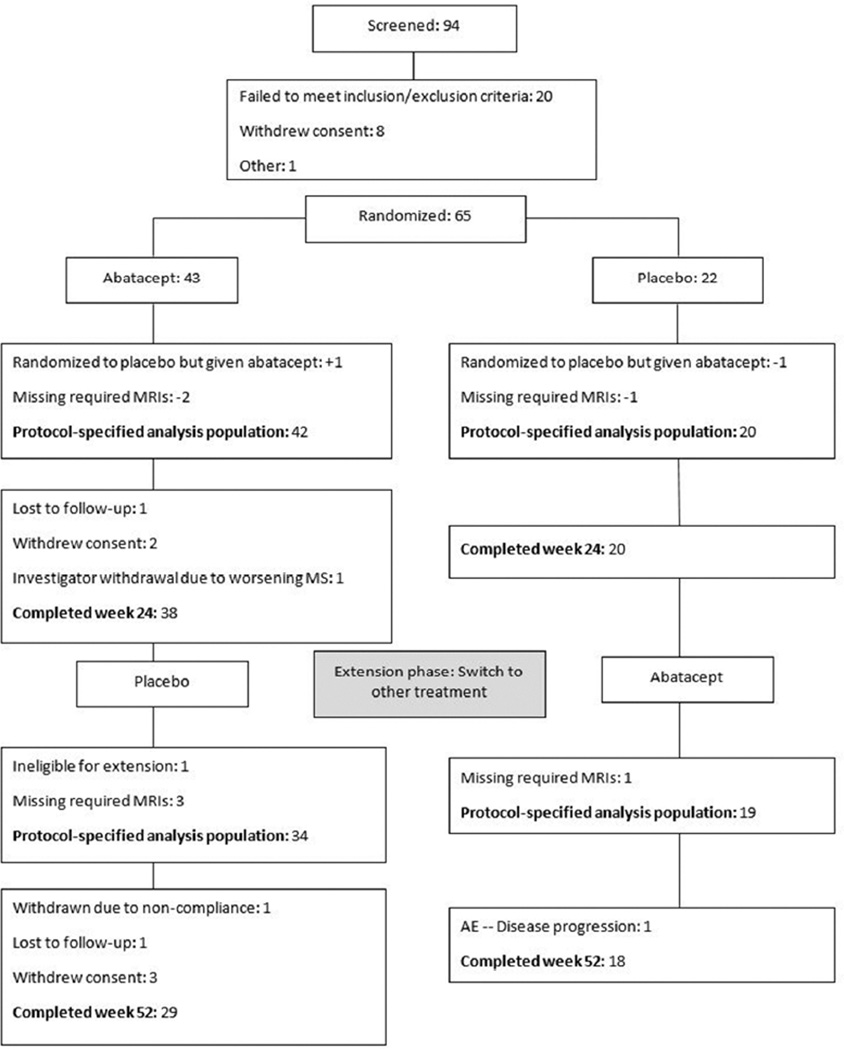
ACCLAIM was a phase II, double-blind, placebo-controlled study of abatacept in RRMS (Figure 1). Enrolled participants were randomly assigned to either intravenous abatacept or placebo treatment in a 2:1 ratio favoring abatacept. Randomization was stratified according to presence or absence of subclinical activity defined as a Gd+ lesion on at least one MRI in the year prior to screening. Concomitant therapy with other multiple sclerosis disease modifying agents was prohibited. The abatacept dose was adjusted for weight according to approved dosing for adult rheumatoid arthritis (less than 60 kg, 500 mg; 60–100 kg, 750 mg; greater than 100 kg, 1 gram). Study medication was administered at weeks 0, 2, and 4, and then every 4 weeks through week 24, designated the Core Phase.
Figure 1.
Flow diagram and disposition of the treatment groups
During the Extension Phase (weeks 28–52), participants in the Core Phase placebo group received treatment with abatacept at weeks 28, 30, and 32, and then every 4 weeks through week 52 (P→A). Participants in the Core Phase abatacept group received treatment with placebo according to the same treatment schedule (A→P).
Study Evaluations
Gd-enhanced and unenhanced MRI scans were obtained at 5 weeks and at 1 week prior to initiation of study treatment. MRI scans then were obtained every 4 weeks from week 4 to week 24. Following the treatment switch at week 28, MRIs were obtained at weeks 36, 52, and 64. EDSS scores and Multiple Sclerosis Functional Composite (MSFC) scores were assessed 5 weeks and 1 week prior to initiation of study treatment, and at weeks 8, 16, 24, 30, 36, 44, 52, and 64. Subjects were assessed for clinical exacerbations and adverse events at each study visit and at unscheduled visits when indicated.
Primary Endpoint and Secondary Endpoints
The primary endpoint was the mean number of new Gd+ MRI lesions obtained on scans performed every 4 weeks, averaged over the interval from weeks 8 to 24. Gd+ MRI lesions were included in the count of new lesions only if they did not appear as Gd+ lesions on the immediate prior MRI.
Secondary MRI endpoints included the total number of new Gd+ MRI lesions over 4 week scans, the change in lesion volume on T2-weighted MRI scans, and the percent change in brain parenchymal fraction. Clinical endpoints included progression on the EDSS scale, annualized relapse rate, and change from baseline in the MSFC score [21]. Adverse events were also assessed as a secondary endpoint. Analysis of no evidence of disease activity (NEDA) during the Core Phase was undertaken post-hoc. Subjects achieved NEDA if from week 8 and before week 28 they had absence of the following: 1) EDSS progression; 2) clinical exacerbations; 3) new Gd+ lesions on MRIs performed every 4 weeks.
Progression on the EDSS scale was defined as an increase of at least 1.0 point compared to baseline if baseline was greater than 1.0, or 1.5 points if baseline was less than or equal to 1.0, that persisted for a minimum of 12 weeks. A relapse was defined as the occurrence of new, worsening, or recurring symptoms of neurologic dysfunction lasting more than 24 hours and associated with an increase of at least 1.0 point on EDSS compared to the last scheduled EDSS assessment, following a period of symptomatic stability of at least 29 days in the absence of a febrile illness or steroid withdrawal. If the last scheduled EDSS was less than or equal to 1.0, then an exacerbation or relapse required an increase of at least 1.5 points.
Adverse events related to multiple sclerosis disease included protocol-defined clinical relapses and also RRMS disease signs and symptoms not meeting the protocol definition of relapse. Attribution of disease signs and symptoms not meeting the definition of relapse was made by an independent safety event review committee of three blinded multiple sclerosis experts who were not otherwise involved in the ACCLAIM study.
Statistical Analysis
The protocol-specified analysis population comprised participants who had MRIs at both 5 weeks and 1 week prior to study treatment, underwent random assignment, and had at least 3 of the MRIs from weeks 8 to 24. The protocol specified a rank-based analysis based on change scores. For each participant, the number of new Gd+ MRI lesions was averaged over 4-week intervals from weeks 8 to 24, the number of new lesions from weeks −5 to −1 prior to initiation of study treatment was subtracted, and the change was converted to a rank. Analysis of covariance (ANCOVA) was applied with terms for treatment and the presence or absence of subclinical disease activity prior to screening. Sensitivity analyses included rank ANCOVA with average of new lesions as the dependent variable and number of new lesions from weeks 5 to 1 prior to treatment as a covariate, both converted to ranks [22]. Additionally, the number of new lesions averaged over 8-week intervals from weeks 8 to 24, and the number of new lesions summed over 4-week intervals from weeks 4 to 24 were compared using a Wilcoxon test without covariate adjustment. For the Core phase, a post hoc sensitivity analysis was conducted in which outlier participants with the highest number of Gd+ MRI lesions at baseline were excluded from the analysis.
The Wilcoxon test was also applied to the change in T2 lesion volume and percent change in brain volume from week −1 to week 24. A Z-score was derived for each of the MSFC component scores by subtracting its overall mean and dividing by its overall standard deviation at week -1. The combined score was taken as the average of the three component Z-scores, and each change from baseline was analyzed using a Wilcoxon test. Fisher’s exact test was applied for analyses of EDSS progression, relapses, and NEDA. The annualized relapse rate in each treatment group was derived as the total number of relapses divided by the total number of days during which participants in that group were under observation, multiplied by 365.25. A between-group comparison was performed using a Poisson regression model [23] with a term for treatment; the natural logarithm of the observation time contributed by any participant was included as an offset.
The same methods were applied to the Extension Phase data. The week 24 evaluation was taken as the baseline for the change in T2 lesion volume, the percent change in brain volume, the MSFC component and overall scores, and EDSS progression, while the number of new lesions from week 20 to 24 was used as the baseline for the number of new Gd+ lesions. Separate analyses were performed within each treatment sequence to assess the effect of changing treatments on the MRI results. The average number of new lesions over weeks 36 and 52 was compared to that from weeks 8 to 24 using a Wilcoxon signed-rank test.
Datasets for the analyses are available through TrialShare, a public website managed by the Immune Tolerance Network (www.itntrialshare.org).
Results
Forty-three and 22 participants were randomized to the abatacept and placebo groups, respectively. The CONSORT Figure 1 shows the enrollment, treatment allocation, analysis groups, and number of participants who completed each phase of the study. Two abatacept and one placebo participants did not receive the required number of MRIs to be eligible for the primary analysis. One participant was inadvertently started on the wrong treatment. This participant continued in the study and was included in the analysis according to his or her actual treatment. The Core Phase analysis population therefore consisted of 42 and 20 participants in the abatacept and placebo groups, respectively. There was little attrition during the Core Phase, with 38/42 abatacept group participants and 20/20 placebo group participants completing the Core Phase. Ninety-eight percent of planned infusions of study medication were administered in the Core Phase.
Baseline participant demographics and clinical characteristics
Table 1 shows the baseline characteristics of the participants. Age, gender, race, and time from RRMS diagnosis were similar between the two groups, which were also similar in proportion with clinical exacerbations in the prior year and in baseline EDSS scores. The majority of participants in both groups lacked Gd+ MRI lesions at baseline. Although participants were stratified for the presence or absence of MRI lesions in the year prior to baseline, a greater number of Gd+ MRI lesion counts was noted in the placebo group at baseline (range 0–48), compared to the abatacept group (range 0–7). Most of the difference in baseline Gd+ MRI lesions between the two groups was accounted for by two outlier participants in the placebo group who had 14 and 48 Gd+ MRI lesions at baseline.
Table 1.
Baseline Demographics and Clinical Characteristics
| Abatacept n=42 |
Placebo n=20 |
|
|---|---|---|
| Age (years) | ||
| Mean (SD) | 40.4 (9.81) | 42.9 (11.23) |
| Median | 40.5 | 45.5 |
| Min, Max | 23, 59 | 24, 57 |
| Sex | ||
| Male | 12 (28.6) | 2 (10.0) |
| Female | 30 (71.4) | 18 (90.0) |
| Race | ||
| White | 33 (78.6) | 15 (75.0) |
| Black, African American | 7 (16.7) | 5 (25.0) |
| Other | 2 (4.8) | 0 (0) |
| Time since RRMS diagnosis (years) | ||
| Mean (SD) | 5.9 (7.36) | 6.9 (6.66) |
| Median | 3.5 | 4.5 |
| Min, Max | 0, 38 | 0, 25 |
| Baseline EDSS Scorea | ||
| Mean (SD) | 1.9 (1.34) | 2.4 (1.17) |
| Median | 2 | 2 |
| Min, Max | 0, 5 | 0, 5 |
| ≥ 1 clinical exacerbation prior year | ||
| n (%) | 40 (95.2) | 18 (90.0) |
| ≥ 1 Gd+ MRI lesion prior year | ||
| n (%) | 20 (47.6) | 11 (55.0) |
| Baseline Gd+ MRI lesionsb | ||
| Mean (SD) | 0.6 (1.38) | 3.8 (10.96) |
| Median | 0 | 0 |
| Min, Max | 0, 7 | 0, 48 |
| Baseline T2 MRI lesion volumeb | ||
| Mean (SD) | 6.41 (7.878) | 8.93 (8.706) |
| Median | 3.25 | 5.74 |
| Min, Max | 0.1, 42.2 | 0.6, 32.1 |
Abbreviations: SD = standard deviation; Min = minimum; Max = maximum; RRMS = relapsing-remitting multiple sclerosis; EDSS = Kurtzke Expanded Disability Status Scale; Gd+ = gadolinium-enhancing; MRI = magnetic resonance imaging
Baseline EDSS for the Core phase was derived as the lowest EDSS score observed at five weeks or one week prior to initiation of study medication.
Baseline MRI evaluations for the Core phase were derived as the value obtained one week prior to the initiation of study medication.
MRI and clinical outcomes during the Core Phase
There was no significant difference between the abatacept and placebo groups in the mean number of new Gd+ lesions, which was the primary endpoint of the study (Table 2). The number of new Gd+ lesions averaged over 4 week intervals was greater in the placebo group (range = 0–16) compared to the abatacept group (range = 0–5), but this difference did not reach statistical significance (p = 0.87). The median values were similarly low in both groups, with a median of 0.2 new inflammatory lesions in the placebo group and 0.3 new inflammatory lesions in the abatacept group. None of the primary endpoint sensitivity analyses resulted in significant between-group differences (described in Methods, data not shown), including a post hoc analysis in which outlier participants with the highest number of Gd+ MRI lesions at baseline were excluded. Significant differences were not observed in any of the secondary MRI and clinical endpoints at 24 weeks (Table 2). In the Core Phase NEDA analysis, no significant difference in any of the NEDA components or in the overall NEDA was observed between groups (Table 2). Although few participants had a documented MS relapse or EDSS progression during the Core Phase, NEDA analysis showed that over half of the participants had at least one new Gd+ lesion on MRIs performed every 4 weeks, a higher MRI frequency than usual for NEDA analysis.
Table 2.
MRI and Clinical Endpoints – Core Phase
| Abatacept n=42 |
Placebo n=20 |
p value | |
|---|---|---|---|
| Average new Gd+ lesions | |||
| Mean (SD) | 0.43 (0.94) | 1.66 (3.63) | |
| Median | 0.2 | 0.3 | 0.87 |
| Min, Max | 0, 5 | 0, 16 | |
| Lesion volume change | |||
| Mean (SD) | −0.05 (0.42) | −0.18 (1.27) | |
| Median | 0.0 | −0.01 | 0.93 |
| Min, Max | −1.2, 0.9 | −3.6, 2.6 | |
| Percent brain volume change | |||
| Mean (SD) | −0.09 (0.54) | −0.25 (0.53) | |
| Median | −0.2 | −0.1 | 0.68 |
| Min, Max | −1.3, 1.5 | −1.4, 0.4 | |
| MSFC score change | |||
| Mean (SD) | 0.102 (0.297) | −0.036 (0.401) | |
| Median | 0.14 | 0.05 | 0.13 |
| Min, Max | −0.77, 0.57 | −0.97, 0.67 | |
| EDSS progression | |||
| n (%) | 5 (11.9) | 1 (5.0) | 0.65 |
| Annualized relapse rate | |||
| Rate (SE) | 0.13 (0.08) | 0.09 (0.09) | 0.73 |
| Subjects with MS relapse | |||
| n (%) | 2 (4.8) | 1 (5.0) | >0.99 |
| No Evidence of Disease Activity (NEDA) | |||
| n (%) | |||
| No EDSS Progression | 37 (88.1%) | 19 (95.0%) | 0.65 |
| No Clinical Exacerbations | 40 (95.2%) | 19 (95.0%) | >0.99 |
| No new Gd+ MRI lesions | 20 (47.6%) | 8 (40.0%) | 0.60 |
| No evidence of disease activity | 16 (38.1%) | 6 (30.0%) | 0.58 |
Abbreviations: Gd+ = gadolinium-enhancing; SD = standard deviation; SE = standard error derived from Poisson model (see text); min = minimum; max = maximum; MSFC = Multiple Sclerosis Functional Composite; EDSS = Kurtzke Expanded Disability Status Scale; MS = multiple sclerosis
MRI and clinical outcomes during the Extension Phase
In the Extension Phase following the treatment switch, the A→P group had a greater number of new Gd+ MRI lesions (range = 0–14) compared to the P→A group (range = 0–3), but this difference did not reach statistical significance (Table 3). The median number of new Gd+ lesions in the Extension Phase was 0 in both groups, indicating continuing low number of new Gd+ lesions overall. During the Extension Phase, the placebo participant with the highest number of new Gd+ lesions during the Core Phase withdrew from the study prior to undergoing an MRI in the Extension Phase, and could not be included in the Extension Phase efficacy analysis. Other radiographic and clinical endpoints were not significantly different between the two groups in the Extension Phase (Table 3). Within the A→P treatment group, no significant difference was observed between the Core and Extension Phases in number of new Gd+ MRI lesions (p = 0.09, data not shown). A similar result was observed in the P→A group (p = 0.11, data not shown).
Table 3.
MRI and Clinical Endpoints – Extension Phase
| Abatacept to Placebo n=34 |
Placebo to Abatacept n=19 |
p value | |
|---|---|---|---|
| Average new Gd+ lesions | |||
| Mean (SD) | 1.25 (2.71) | 0.61 (0.95) | |
| Median | 0.0 | 0.0 | 0.60 |
| Min, Max | 0, 14 | 0, 3 | |
| Lesion volume change | |||
| Mean (SD) | 0.47 (0.99) | 0.07 (1.19) | |
| Median | 0.1 | −0.1 | 0.07 |
| Min, Max | −0.7, 3.6 | −2.7, 3.4 | |
| Percent brain volume change | |||
| Mean (SD) | −0.28 (0.42) | −0.31 (0.35) | |
| Median | −0.2 | −0.2 | 0.88 |
| Min, Max | −1.0, 0.3 | −1.2, 0.1 | |
| MSFC score change | |||
| Mean (SD) | 0.060 (0.206) | −0.006 (0.459) | |
| Median | 0.07 | 0.04 | 0.67 |
| Min, Max | −0.31, 0.62 | −1.29, 0.96 | |
| EDSS progression | |||
| n (%) | 1 (2.9) | 2 (10.5) | 0.29 |
| Annualized relapse rate | |||
| Rate (SE) | 0.40 (0.16) | 0.12 (0.12) | 0.19 |
| Subjects with MS relapse | |||
| n (%) | 6 (17.6) | 1 (5.3) | 0.40 |
Abbreviations: Gd+ = gadolinium-enhancing; SD = standard deviation; SE = standard error derived from Poisson model (see text); min = minimum; max = maximum; MSFC = Multiple Sclerosis Functional Composite; EDSS = Kurtzke Expanded Disability Status Scale; MS = multiple sclerosis
Adverse Events
Abatacept was well tolerated by participants in this study. A summary of adverse events is shown in Table 4. The proportion of participants with adverse events, serious adverse events, infections, and adverse events related to multiple sclerosis disease activity did not significantly differ between the two groups in either phase of the study. A significantly higher rate of adverse events related to study medication was reported in the abatacept group during the Core Phase, but this difference was largely due to events attributed as possibly related to study medication in two participants at a single site. A significantly higher rate of Grade 3 adverse events was observed in the Extension Phase in the A→P group, but there was no significant difference in the proportion of participants with Grade 3 adverse events. Seven of the ten events occurred in two participants, and there was no specific pattern observed among the ten events, which included MS relapse, pain in extremity, visual impairment, fatigue, abdominal tenderness, contusion, flu, and decreased neutrophil count. No safety concerns were observed at the week 64 time point (data not shown).
Table 4.
Adverse Events
| By Subjecta | By Eventb, c | |||||
|---|---|---|---|---|---|---|
| Core Phase | ||||||
| Abatacept n=44 n (%) |
Placebo n=21 n (%) |
p value |
Abatacept n=44 n (Rate) |
Placebo n=21 n (Rate) |
p value | |
| All AEs | 34 (77.3) | 17 (81.0) | >0.99 | 125 (5.37) | 54 (4.84) | 0.52 |
| Serious AEs | 2 (4.5) | 0 (0) | >0.99 | 4 (0.17) | 0 (0) | 0.077 |
| Grade 3 | 4 (9.1) | 2 (9.5) | >0.99 | 11 (0.47) | 3 (0.27) | 0.36 |
| Grade 2 | 33 (75.0) | 17 (81.0) | 0.76 | 114 (4.90) | 51 (4.57) | 0.68 |
| Related to: | ||||||
| Study Drug | 17 (38.6) | 5 (23.8) | 0.28 | 41 (1.76) | 6 (0.54) | 0.002 |
| MS | 16 (36.4) | 7 (33.3) | >0.99 | 40 (1.72) | 13 (1.17) | 0.21 |
| AE Infections | 15 (34.1) | 7 (33.3) | >0.99 | 22 (0.95) | 15 (1.34) | 0.30 |
| Extension Phase | ||||||
| Abatacept to Placebo n=37 n (%) |
Placebo to Abatacept n=19 n (%) |
p value |
Abatacept to Placebo n=37 n (Rate) |
Placebo to Abatacept n=19 n (Rate) |
p value | |
| All AEs | 21 (56.8) | 15 (78.9) | 0.14 | 65 (4.13) | 28 (3.22) | 0.26 |
| Serious AEs | 2 (5.4) | 0 (0) | 0.54 | 2. (0.13) | 0 (0) | 0.19 |
| Grade 3 | 5 (13.5) | 0 (0) | 0.16 | 10 (0.64) | 0 (0) | 0.003 |
| Grade 2 | 21 (56.8) | 15 (78.9) | 0.14 | 55 (3.50) | 28 (3.22) | 0.72 |
| Related to: | ||||||
| Study Drug | 8 (21.6) | 3 (15.8) | 0.73 | 23 (1.46) | 6 (0.69) | 0.080 |
| MS | 13 (35.1) | 6 (31.6) | >0.99 | 20 (1.27) | 8 (0.92) | 0.43 |
| AE Infections | 6 (16.2) | 5 (26.3) | 0.48 | 7 (0.45) | 5 (0.58) | 0.66 |
Abbreviations: AE = adverse event; MS = multiple sclerosis
The subject-level p-value comes from a Fisher’s exact test that compared the proportion of subjects experiencing the event between the two treatment groups.
The event-level p-value comes from a Poisson regression comparing the person-year adjusted event rates between the two treatment groups.
Event incidence rates are per person-years of exposure. (IR/P-Y) = event count / person-years of exposure. Person- years of exposure is calculated as the total follow-up time across all subjects in each treatment group for the study phase.
Discussion
In this phase II study comparing the efficacy and safety of abatacept versus placebo in RRMS, abatacept did not significantly reduce new Gd+ MRI lesions in the Core Phase nor the Extension Phase of the study. There were no significant differences in any of the other MRI or clinical endpoints. The primary outcome was the number of new Gd+ lesions, and the protocol specified a rank-based analysis, ensuring that the outliers did not skew the analysis. Although we considered applying statistical methods based on Poisson or negative binomial distributions [23] and zero-inflated alternatives [24], a fully parametric approach was difficult to justify due to low numbers of new Gd+ lesions in the majority of participants.
MRI outliers and infrequent relapses are problematic issues for clinical trials in RRMS, and alternative endpoints such as No Evidence of Disease Activity (NEDA) are potentially more sensitive and clinically relevant outcome measures [6, 25]. An analysis of NEDA during the Core Phase was undertaken according to a highly sensitive definition based on EDSS progression, clinical exacerbations, and new Gd+ lesions on MRIs performed every 4 weeks. The majority of participants in the study had disease activity according to this NEDA definition: however, no difference was observed in any of the NEDA components between the abatacept group and the placebo group.
The ACCLAIM study had some limitations. The original study design specified 123 participants, a sample size chosen to demonstrate a treatment effect of 50% reduction of new Gd+ MRI lesions. Due to slow enrollment related to the placebo control, the investigators decided to close the study earlier than planned at an enrollment of 65, a sample size which was too small to demonstrate efficacy at the 50% level. A treatment effect greater than 50% is a realistic goal in RRMS clinical trials [1, 3], but was not achieved in this study. Overall low numbers of new Gd+ MRI lesions in the study population reduced the chances of demonstrating a treatment effect for abatacept. In the setting of several available FDA-approved disease-modifying medications for RRMS in the United States and Canada, patients with highly active RRMS might be less likely to be enrolled in placebo-controlled trials, including those conducted according to strict ethical guidelines such as ACCLAIM [26]. Although the current study does not support efficacy of abatacept for RRMS, it does not exclude the possibility that an abatacept treatment effect might be observed in a larger study of subjects with higher disease activity.
Supplementary Material
Acknowledgments
The authors are grateful to the study participants, and thank the ACCLAIM clinical site investigators and study coordinators. The authors thank F. Lublin (Mt. Sinai), E. Waubant (UCSF), and J. Bowen (Swedish Medical Center) for participating in the Safety Event Review Committee. The authors thank their colleagues at the Immune Tolerance Network, and their collaborators who contribute in many capacities to Immune Tolerance Network projects and perspectives.
SJK reports grant support from Novartis Pharmaceuticals.
MSF reports honoraria or consulting fees from Actelion, BayerHealthcare, BiogenIdec, Chugai, EMD Canada, Genzyme, Merck Serono, Novartis, Hoffman La-Roche, Sanofi-Aventis, Teva Canada Innovation, advisory board or board of directors membership for Actelion, BayerHealthcare, BiogenIdec, Hoffman La-Roche, Merck Serono, Novartis, Opexa, Sanofi-Aventis, and participation in a company sponsored speakers bureau for Genzyme.
DLA reports ownership interest in NeuroRx, which performed MRI analysis for the trial, and personal fees from Acorda, Biogen Idec, Genzyme, Hoffman-LaRoche, Innate Immunotherapeutics, MedImmune, Mitsubishi Pharma, Novartis, Receptos, Sanofi Aventis, and Teva.
Funding
Conducted by the Immune Tolerance Network. Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number UM1AI109565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study was also funded by the National Institute of Allergy and Infectious Diseases and National Institute of Neurological Disorders and Stroke (N01 AI15416, 1UM2 AI117870-01, and HHSN272200800029C). Study medication and financial support also provided by Bristol-Myers Squibb.
Co-investigators of the ACCLAIM Study Group
Mariko Kita, Syed Rizvi, Walter Royal III, Joanna Cooper, Silva Markovic-Plese, Leslie Weiner, Alireza Minagar, Marvin Zelkowitz, Samuel Hunter, Stanley Cohan, Clyde Markowitz, Adam F. Carpenter, Robert Tillett Jr., Mark Agius, Daniel S. Bandari, Roberto Bomprezzi, Susan A. Gauthier, Sandra Cook, Suraj Muley, Darin Okuda, Rohit Bakshi, Charles R.G. Guttmann.
Footnotes
Declaration of Conflicting Interests
JR, LD, MB, KR, PT, WG, PHS, and DES declare no conflicts of interest.
Contributor Information
Samia J. Khoury, Brigham and Women’s Hospital, Partners Multiple Sclerosis Center, Boston, USA; and American University of Beirut, Abu Haidar Neuroscience Institute, Beirut, Lebanon
James Rochon, Rho, Inc., Chapel Hill, USA.
Linna Ding, National Institute of Allergy and Infectious Diseases, Division of Allergy, Immunology, and Transplantation, Rockville, USA.
Margie Byron, Rho, Inc., Chapel Hill, USA.
Kristin Ryker, Immune Tolerance Network, San Francisco, USA.
Patti Tosta, Immune Tolerance Network, San Francisco, USA.
Wendy Gao, National Institute of Allergy and Infectious Diseases, Division of Allergy, Immunology, and Transplantation, Rockville, USA.
Mark S. Freedman, University of Ottawa, ON, Canada
Douglas L. Arnold, NeuroRx Research and McGill University, Montreal, Canada
Peter H. Sayre, Immune Tolerance Network, San Francisco, USA
Dawn E. Smilek, Immune Tolerance Network, San Francisco, USA
References
- 1.Polman CH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JA, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 3.Fox RJ, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–1097. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 4.Shirani A, et al. Association between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2012;308(3):247–256. doi: 10.1001/jama.2012.7625. [DOI] [PubMed] [Google Scholar]
- 5.Rotstein DL, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015;72(2):152–158. doi: 10.1001/jamaneurol.2014.3537. [DOI] [PubMed] [Google Scholar]
- 6.Imitola J, Racke MK. Is no evidence of disease activity a realistic goal for patients with multiple sclerosis? JAMA Neurol. 2015;72(2):145–147. doi: 10.1001/jamaneurol.2014.3860. [DOI] [PubMed] [Google Scholar]
- 7.Linsley PS, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257(5071):792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 8.Judge TA, et al. The in vivo mechanism of action of CTLA4Ig. J Immunol. 1996;156(6):2294–2299. [PMC free article] [PubMed] [Google Scholar]
- 9.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding FA, et al. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 11.Kremer JM, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 12.Ruperto N, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372(9636):383–391. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 13.Khraishi M, Russell A, Olszynski WP. Safety profile of abatacept in rheumatoid arthritis: a review. Clin Ther. 2010;32(11):1855–1870. doi: 10.1016/j.clinthera.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Orban T, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orban T, et al. Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes. 2014;63(10):3449–3457. doi: 10.2337/db14-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury SJ, et al. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155(10):4521–4524. [PubMed] [Google Scholar]
- 17.Viglietta V, et al. CTLA4Ig treatment in patients with multiple sclerosis: an open-label, phase 1 clinical trial. Neurology. 2008;71(12):917–924. doi: 10.1212/01.wnl.0000325915.00112.61. [DOI] [PubMed] [Google Scholar]
- 18.SYNOPSIS. Clinical Study Report IM101200. [Accessed March 30, 2016]; Available at http://ctr.bms.com/pdf/IM101200.pdf. [Google Scholar]
- 19.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005;58(6):840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer JS, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5(4):244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 22.Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38(3):715–724. [PubMed] [Google Scholar]
- 23.McCullagh P N.J.G.L.M. Monographs on statistics and applied probability. 2nd. London; New York: Chapman and Hall; 1989. [Google Scholar]
- 24.Lambert D. Zero-inflated Poisson regression with an application to defects in manufacturing. Technometrics. 1992;34:1–14. [Google Scholar]
- 25.Bevan CJ, Cree BA. Disease activity free status: a new end point for a new era in multiple sclerosis clinical research? JAMA Neurol. 2014;71(3):269–270. doi: 10.1001/jamaneurol.2013.5486. [DOI] [PubMed] [Google Scholar]
- 26.Polman CH, et al. Ethics of placebo-controlled clinical trials in multiple sclerosis: a reassessment. Neurology. 2008;70(13 Pt 2):1134–1140. doi: 10.1212/01.wnl.0000306410.84794.4d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.