Abstract
Maternal pregestational diabetes mellitus (PGDM) induces congenital heart defects (CHDs). The molecular mechanism underlying PGDM-induced CHDs is unknown. microRNAs (miRNAs), small non-coding RNAs, repress gene expression at the posttranscriptional level and play important roles in heart development. We performed a global miRNA profiling study to assist in revealing potential miRNAs modulated by PGDM and possible developmental pathways regulated by miRNAs during heart development. A total of 149 mapped miRNAs in the developing heart were significantly altered by PGDM. Bioinformatics analysis showed that the majority of the 2111 potential miRNA target genes were associated with cardiac development-related pathways including STAT3 and IGF-1 and transcription factors (Cited2, Zeb2, Mef2c, Smad4 and Ets1). Overexpression of the antioxidant enzyme, superoxide dismutase 1, reversed PGDM-altered miRNAs, suggesting that oxidative stress is responsible for dysregulation of miRNAs. Thus, our study provides the foundation for further investigation of a miRNA-dependent mechanism underlying PGDM-induced CHDs.
Keywords: Pregestational diabetes mellitus, Congenital heart defects, Diabetic embryopathy, miRNA profiling, Heart development, Oxidative stress
1. Introduction
Congenital heart defects (CHDs) are the most common structural birth defects, and the causes of CHDs are still largely unknown [20,40]. Pregestational maternal diabetes mellitus (PGDM) increases the risk of CHDs in the offspring five-fold, compared with the general population [32,44,56,59]. The major heart defects associated with PGDM are outflow tract (OFT) defects and ventricular septal defects (VSDs) [32,56]. PGDM-induced CHDs have become a significant public health concern because the number of women of reproductive age with diabetes is rising rapidly [10,26,44]. Previous studies have demonstrated that PGDM alters developmental pathways and gene expression essential for heart development [35,43,48,50,52], whereas the mechanism underlying PGDM-induced gene dysregulation is unclear.
MicroRNAs (miRNAs) are small, non-coding RNAs that negatively regulate gene expression at the post-transcription level [4]. By an imperfect sequence complementation, miRNAs recognize and bind to the 3′-untranslated regions (3′UTR) of target mRNAs, thereby inhibiting mRNA function through degradation, repression of translation, or both [27]. Approximately 50% of all mammalian protein-coding genes are post-transcriptionally controlled by miRNAs [24]. Currently, 2588 mature miRNAs have been described in humans, and 1915 mature miRNAs have been described in the mouse (miRbase release 21, June 2014, http://www.mirbase.org). Our recent studies have demonstrated that PGDM affects the expression of several miRNAs in the developing embryo [12,18,19], suggesting the importance of miRNA changes in PGDM-induced CHD formation.
miRNAs play important roles in many biological processes, including development, differentiation, proliferation and apoptosis [3]. Since the discovery of miRNAs, numerous studies have determined that miRNAs are differentially expressed in many diseases such as cancer, stroke, Alzheimer's disease, diabetes, and nephropathy [2,29]. In addition, there is ample evidence that miRNAs are associated with cardiovascular diseases, including cardiac hypertrophy, myocardial infarction, cardiac fibrosis, arrhythmia, heart failure and vascular disease [38,42]. However, although the function of miRNAs in heart development has been previously described [31], only a few studies have attempted to elucidate the role of miRNAs in the pathogenesis of CHD formation. A comprehensive miRNA profile in embryonic hearts from diabetic pregnancies has not been previously performed.
PGDM exerts harmful effects on the developing embryo because uncontrolled hyperglycemia resulting from PGDM in early pregnancy (2–8 weeks' gestation) can significantly influence development of the primary vital organs, including the heart [16,49,62]. We have developed a murine type 1 diabetes mellitus model that can successfully and consistently recapitulate the negative effects of human maternal diabetes on embryonic development, and have previously determined that embryonic day (E) 12.5 in the mouse corresponds approximately to the critical period of cardiogenesis in humans [48,52]. Hence, in the present study, we investigated the miRNA expression profiles of E12.5 hearts of embryos from nondiabetic and diabetic dams. We identified a set of potential miRNAs which may be associated with PGDM-induced CHDs. In addition, we explored possible target genes of those miRNAs modulated by PGDM.
2. Materials and methods
2.1. Mice and reagents
Wild-type (WT) C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The superoxide dismutase 1 transgenic (SOD1-Tg) mice in a C57BL/6J background were revived from frozen embryos by the Jackson Laboratory (Stock No.002298). Streptozotocin (STZ) from Sigma (St. Louis, MO) was dissolved in sterile 0.1 M citrate buffer (pH4.5).
2.2. Mouse models of diabetic embryopathy
All procedures for animal use were approved by the Institutional Animal Care and Use Committee of University of Maryland School of Medicine. The mouse model of PGDM-induced embryopathy was previously described [48,50,52]. Briefly, ten-week old WT female mice were intravenously injected daily with 75 mg/kg STZ for two days to induce diabetes. Diabetes was defined as a 12-h fasting blood glucose level of ≥16.7 mM. Male (WT or SOD1-Tg) and WT female mice were paired at 3:00 P.M., and day 0.5 (E0.5) of pregnancy was established at noon of the day when a vaginal plug was present. Embryonic hearts were harvested for miRNA microarray analysis on E12.5. The embryonic hearts from one litter were pooled as one sample.
2.3. Total RNA preparation and quality control
Total RNA was extracted from embryonic hearts using the miRNeasy Mini Kit (Qiagen, Valencia, CA). The RNA integrity level was measured for each RNA sample using the Agilent Bioanalyzer 2100 in order to obtain a RIN value (RIN = RNA integrity number). All samples were found to have RIN values higher than 7, indicating RNA of suitable quality for microarray analysis.
2.4. miRNA array profiling
The miRNA array was conducted at Exiqon Services, Denmark. Total RNA (750 ng) from both sample and reference was labeled with Hy3™ and Hy5™ fluorescent label, respectively. The Hy3™-labeled samples and a Hy5™-labeled reference RNA sample were mixed pair-wise and hybridized to the mercury LNA™ microRNA Array 7th Gen (Exiqon, Denmark), which contains capture probes targeting all murine miRNAs in the miRBASE 19.0. The hybridization was performed according to the miRCURY LNA™ microRNA Array Instruction manual using a Tecan HS4800™ hybridization station (Tecan, Austria). After hybridization the microarray slides were scanned and stored in an ozone free environment (ozone level below 2.0 ppb) in order to prevent potential bleaching of the fluorescent dyes. The miRCURY LNA™ microRNA Array slides were scanned using the Agilent G2565BA Microarray Scanner System (Agilent Technologies, Inc., USA) and the image analysis was carried out using the ImaGene® 9 (miRCURY LNA™ microRNA Array Analysis Software, Exiqon, Denmark). The quantified signals were background corrected (Normexp with offset value 10) and normalized using the global Lowess (LOcally WEighted Scatterplot Smoothing) regression algorithm.
2.5. miRNA expression profiling data analysis
Hierarchical clustering is used for grouping similar objects into “clusters”. Hierarchical clustering produces a tree (also called a dendogram) that shows the hierarchy of the clusters. This allows for exploratory analysis to determine the proximity of the samples inside an experimental based on similarity of features (miRNA expression levels). Principal components analysis (PCA) is a procedure for identifying a small number of uncorrelated variables, called “principal components,f̈rom a large dataset. The aim of PCA is to explain the maximum amount of variance with the fewest number of principal components. The hierarchical clustering and PCA analysis based on the entire miRNA expression dataset were used for comparing their similarity within and between the two experimental groups (nondiabetic and diabetic). To identify PGDM-regulated miRNAs between the two groups, a random variance t test was used to improve estimates of miRNA-specific variances without assuming that all miRNAs have the same variance [45]. The criteria for inclusion of a miRNA in the PGDM-regulated miRNA list were set to P < 0.05. A hierarchical clustering analysis (heat map) was used for visualizing the normalized expression values of the PGDM-regulated miRNAs
2.6. miRNA target gene prediction
A two-step bioinformatics approach was used to generate a high-confidence candidate miRNA target list. First, to increase the accuracy of in-silico miRNA target prediction, we generated a computationally based candidate miRNA target list by taking advantage of the following five in-silico computational miRNA target prediction tools: targetScan [1], picTar [23], RNA22 [34], PITA [22], and miRanda [6]. For each miRNA, one mRNA was considered its high-confidence predicted target gene if this regulation was predicted by at least two out of the above five prediction tools. Second, we identified miRNA-mRNA regulation relationships from public literature collected by the Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com/) software.
2.7. Functional annotation analysis of the predicted targets
The high-confidence miRNA target gene list was further annotated with the Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com/) platform. We queried IPA with this list aiming to map and generate putative biological processes/functions, networks and pathways based on the manually curated knowledge database of molecular interactions extracted from the public literature. Molecular and cellular functional analysis, physiological system development and functional analysis and canonical signaling pathways provided by IPA were used to identify the functions of the predicted target genes. The enrichment scores were defined as −log (p-value) from the Fisher's Exact Test. Each category is thought to be significantly enriched when the P-value < 0.01 (enrichment scores >2) [25]. The top 20 terms of functional and canonical signaling pathway analysis with the enrichment scores >2 were presented in the current study.
Gene ontology (GO) enrichment was evaluated by Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics tool (https://david.ncifcrf.gov). The DAVID Functional Annotation Tool application provided functional cluster of genes according to similar and redundant GO terms. GO Biological Process terms were used for this analysis. The top 20 GO terms based on the enrichment score (P < 0.01 from the Fisher's Exact Test, enrichment score >2) were presented in the current study.
2.8. Real-time qPCR (RT-qPCR)
Total RNA was isolated from embryos using an RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed using the qScript microRNA cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD). RT-qPCR for randomly selected miRNAs and their target genes were analyzed using Maxima SYBR Green/ROX qPCR Master Mix assay (Thermo Scientific, Rockford, IL). RT-qPCR was performed using the StepOnePlus system (Applied Biosystem, Grand Island, NY). The reaction conditions of RT-qPCR were 10 min at 95 °C for initial denaturation and DNA polymerase activation, followed by 40 cycles of PCR with 15 s at 95 °C for denaturation, 1 min at 60 °C for annealing and extension. The sequences of all primers were provided in Supplemental Table S5 in the online version at DOI: 10.1016/j.reprotox.2016.09.007.
2.9. Statistics
For miRNAs and target gene validation, data are presented as means ± standard errors (SE). Three embryos from three separate dams were used for analysis. One-way ANOVA was performed using the SigmaStat 3.5 software and a Tukey test was used to estimate statistical significance (P < 0.05).
3. Results
3.1. Distinct miRNA expression profiles in embryonic hearts from nondiabetic or diabetic dams
We compared the miRNA expression profiles in E12.5 hearts from embryos of diabetic mellitus (DM) versus nondiabetic (ND, control) dams using the miRCURY LNA™ microRNA Array, which contains 3100 capture probes covering human, mouse and rat microRNAs in miRBase v. 19.0. Because only 25–30% of hearts in the DM group eventually develop CHDs [48,52], miRNA expression in some of the DM samples may overlap with that in the ND group. To avoid this potential problem, embryonic hearts from the DM group were clustered together and separated from the ND group by sample clustering (Fig. 1A) and principal component analysis (PCA) (Fig. 1B). Based on the above analyses, miRNA expression in one sample of the ND group was overlapped with those in the DM group, and miRNA expression in two samples of the DM group overlapped with those in the ND group. Thus, these three samples were excluded from further analysis.
Fig. 1.

Sample clustering and miRNA expression in the developing heart. A. The unsupervised hierarchical clustering dendrogram of miRNA microarray data from the nondiabetic (ND) group and the diabetic (DM) group using centered correlation and average linkage. The dendrogram represents triplicates (DM-H3, H4, and H5) in the DM group and quadruplicates (ND-H1, H2, H4, and H5) in the ND group. B. Principal Component Analysis (PCA) showed that the first, second and third principal components were plotted on the X-, Y- and Z- axes. The ND group was clearly separated from the DM group, and the replicates within each group were tightly clustered. Balls represent the samples of the ND group, whereas blocks represent the samples of the DM group. C. Heat map (hierarchical clusters) analysis of 149 miRNAs that were significantly altered by maternal diabetes in embryonic hearts (adjusted P < 0.05). Samples are grouped by nondiabetic or diabetic conditions, and miRNAs arranged by the Pearson uncentered distance metric with average linkage. Each row of the heat map represents a miRNA, and each column represents an individual sample of the ND group or the DM group. The color legend at the right illustrates the relative miRNA expression levels: Dark for the highest expression, and light for the lowest expression, relative to the overall mean for each miRNA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A total of 167 unique miRNAs showed significantly different expression levels (P < 0.05). Of these, 149 could be mapped to miRNAs already identified in mice (Supplementary Table S1 in the online version at DOI: 10.1016/j.reprotox.2016.09.007). We performed a hierarchical clustering analysis of the 149 miRNAs to visualize their normalized expression values (Fig. 1C). Cluster analysis revealed a differential expression pattern of the 149 miRNAs in the developing heart of the ND group compared with the DM group (Fig. 1C). MiR-144-3p, miR-451a, miR-5625-5p, miR-142-3p and miR-582-3p were the top 5 up-regulated miRNAs, whereas miR-3962, miR-98-5p, miR-322-5p, miR-27a-3p and miR-3968 were the top 5 down-regulated miRNAs (Table 1). Analysis of sequence indicated that the top miRNAs (miR-144, miR-142, miR-451, miR-98, miR-322 and miR-27a) belong to a broadly conserved miRNA family among species shown in TargetScan (www.targetscan.org).
Table 1.
Top 5 most up-regulated or down-regulated miRNAs by PGDM in the developing embryonic heart.
| Name | Probe ID | Fold change | Parametric p-value | FDR |
|---|---|---|---|---|
| mmu-miR-144-3p | 29802 | 2.33 | 0.0201772 | 0.319 |
| mmu-miR-451a | 42866 | 2.23 | 0.011733 | 0.287 |
| mmu-miR-5625-5p | 168845 | 1.95 | 0.0101951 | 0.287 |
| mmu-miR-142-3p | 10947 | 1.89 | 0.0129889 | 0.287 |
| mmu-miR-582-3p | 168907 | 1.88 | 0.0355014 | 0.343 |
| mmu-miR-3968 | 168797 | 0.68 | 0.0153107 | 0.291 |
| mmu-miR-27a-3p | 46483 | 0.68 | 0.0412379 | 0.343 |
| mmu-miR-322-5p | 13150 | 0.67 | 0.0228096 | 0.319 |
| mmu-miR-98-5p | 11182 | 0.64 | 0.003152 | 0.278 |
| mmu-miR-3962 | 168859 | 0.62 | 0.0406957 | 0.343 |
Parametric p-value: from a random variance t-test. FDR: false discovery rate.
3.2. Validation for PGDM-regulated miRNA expression and the role of oxidative stress
To validate the data obtained by the miRNA microarray analysis, we verified 6 miRNAs by RT-qPCR. RT-qPCR confirmed the increase of three miRNAs (miR-142-3p, miR-144-3p, miR-448-3p) and the decrease another three miRNAs (miR-322-5p, miR-27a-3p, miR-181b-5p) in embryonic hearts exposed to maternal diabetes (Fig. 2).
Fig. 2.

Validation of PGDM-regulated miRNAs using quantitative PCR. miRNA levels in embryonic hearts (E12.5) of SOD1 overexpressing (SOD1-Tg) and wild type (WT) embryos from nondiabetic (ND) or diabetic mellitus (DM) dams are shown. Experiments were repeated three times (n = 3) using three hearts from three dams each group. * indicates significant differences (P < 0.05) compared with the other three groups.
Our previous studies have revealed that maternal diabetes-induced oxidative stress is a causal factor for diabetic embryopathy [49,62]. Overexpression of SOD1 ameliorated maternal diabetes-induced heart defects [48]. To test whether oxidative stress is responsible for the observed miRNA changes, SOD1-Tg male mice mated with nondiabetic and diabetic females to generate wild-type and SOD2 overexpressing embryos under nondiabetic and diabetic conditions. SOD1 overexpression reversed PGDM-increased expression of miR-142-3p, miR-144-3p, miR-448-3p and PGDM-decreased expression of miR-322-5p and miR-27a-3p in the developing heart (Fig. 2).
3.3. Functional annotation of target genes regulated by PGDM-modulated miRNAs
A two-step bioinformatic approach was used to identify high-confidence target genes. From the in-silico miRNA target prediction, 3516 predicted miRNA-mRNA interactions, between 27 miRNAs and 1905 mRNAs (Supplementary Table S2 in the online version at DOI: 10.1016/j.reprotox.2016.09.007), were obtained. Using IPA, 524 miRNA-mRNA interactions between 22 miRNAs and 464 mRNAs, were identified (Supplementary Table S3 in the online version at DOI: 10.1016/j.reprotox.2016.09.007). We combined the above two mRNA lists into one high-confidence miRNA target list, which was further used for downstream functional annotation analysis. In the high-confidence miRNA target list, 3960 miRNA-mRNA pairs between 32 miRNAs and 2111 mRNAs were revealed (Supplementary Table S4 in the online version at DOI: 10.1016/j.reprotox.2016.09.007).
Molecular and cellular functional analysis of the predicted target genes by IPA indicated that most of the genes participated in cell proliferation [enrichment score = 72.8, gene number = 873], cell death [enrichment score = 71.2, gene number = 816], apoptosis [enrichment score = 69.7, gene number = 687] and necrosis [enrichment score = 61.1, gene number =655] (Fig. 3A).
Fig. 3.

Functional annotation of putative miRNA target genes regulated by PGDM. A. The target genes were categorized based on molecular and cellular functions. The bars show the top 20 terms with enchrichment scores > 2. B. The target genes were categorized according to physiological system development and function. The bars show the top 20 terms with enchrichment scores > 2. Category names are presented on the y-axis. The x-axis indicates the enrichment gene numbers graphed in bars, and the enrichment scores were indicated by the top line and depicted by the yellow line in the graphs. The enrichment gene numbers were calculated from the number of genes regulated by PGDM-modulated miRNAs in the dataset of a given category. The enrichment scores are displayed as −log (p-value) in Fisher's Exact Tests. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Physiological system development and functional analysis of the predicted target genes by IPA suggested that most of the genes contributed to organismal death [enrichment score = 101.4, gene number = 695], quantity of cells [enrichment score = 51.4, gene number = 469], body cavity morphology [enrichment score = 51.0, gene number = 326], body trunk development [enrichment score = 52.7, gene number = 323] and cardiovascular system development [enrichment score = 40.0, gene number = 316] (Fig. 3B).
The IPA analysis also showed that our predicted miRNA target genes were intermediates of canonical signaling pathways involved in cardiac development (Fig. 4), including the STAT3 pathway [52.7% overlap, enrichment score = 20.4] and the IGF-1 signaling pathway [47.5% overlap, enrichment score = 21.9] (Fig. 5A and B). These two pathways appear to be dysregulated by maternal diabetes because most of the intermediates in these pathways are regulated by the miRNAs decreased by PGDM, such as miR-322-5p and miR-27a-3p, and the miRNAs increased by PGDM, such as miR-144, miR-142-3p (Fig. 5A and B).
Fig. 4.
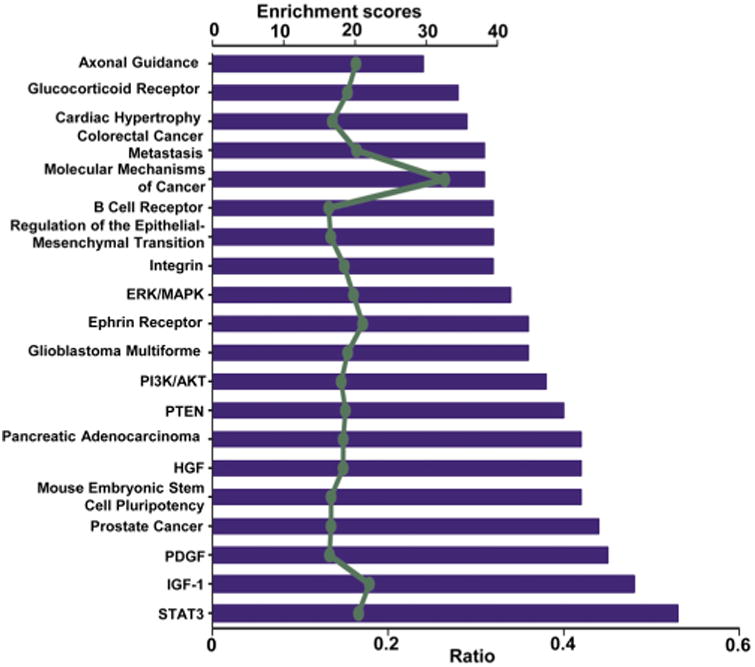
Canonical pathway annotation of miRNA target genes regulated by PGDM. The bar graph shows the top 20 terms with enchrichment scores > 2. Category names are presented on the y-axis. The x-axis indicates the enrichment ratio represented by the bars, and enrichment scores are depicted in the light green line. The enrichment ratio is calculated from the number of genes regulated by PGDM-modulated miRNAs in the dataset of a given category, divided by the total number of genes in the category. The enchrichment scores are displayed as −log (p-value) in Fisher's Exact Tests. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.

The gene network of STAT3 and IGF-1 signaling regulated by PGDM-modulated miRNAs. A. The molecular intermediates of the STAT3 signaling regulated by PGDM-modulated miRNAs. B. The molecular intermediates of the IGF1 signaling regulated by PGDM-regulated miRNAs. Pine means a gene is regulated by differentially expressed miRNAs. The relationship between miRNA target genes and PGDM-regulated miRNAs is shown in the table below the graph. Legend for network shapes and relationships are showed in the bottom right corner. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Transcription factors involved in cardiac development and disease were regulated by PGDM-modulated miRNAs
We performed a gene ontology (GO) annotation analysis on the high-confidence miRNA target gene list. The top 20 GO terms based on the enrichment score (P < 0.01 from the Fisher's Exact Test, enrichment score >2) are shown in Fig. 6. The majority of the miRNA target gene were categorized by regulation of transcription [17.9% overlap, enrichment score = 16.9] and transcription [14.5% overlap, enrichment score = 14.2]. Based on our findings, maternal diabetes appears to alter gene transcription and transcriptional regulation in the developing heart. Analysis of these genes showed that 284 of the 2111 target genes belong to transcription regulators which are located in the nucleus (Table 2). Furthermore, our data showed that most of these transcription factors are involved in hypertrophy, hypoplasia of the heart and apoptosis of cardiomyocytes (Table 2). Thus, altered expression of these transcription factors by PGDM-regulated miRNAs may play an important role in PGDM-induced CHDs.
Fig. 6.

Gene ontology annotation of miRNA target genes regulated by PGDM-regulated miRNAs, and validation of selected transcription factors. A. The bar graph shows the top 20 terms with enrichment scores > 2. Category names are presented on the y-axis. The x-axis indicates the enrichment ratios represented by the bars. The enrichment scores are depicted by the dots in the light green line. The enrichment ratios were calculated from the number of genes regulated by PGDM-regulated miRNAs in the dataset of a given category, divided by the total number of genes in the category. The enchrichment of ratio and scores corresponding to each gene ontology term were analyzed by the Database for Annotation, Visualization and Integrated Discovery (DAVID). The enchrichment scores are displayed as −log (p-value) in Fisher's Exact Tests. B. mRNA levels in embryonic hearts (E12.5) of SOD1 overexpressing (SOD1-TG) and wild type (WT) embryos from nondiabetic (ND) or diabetic mellitus (DM) dams. Experiments were repeated three times (n = 3) using three hearts from three dams each group. * indicates significant differences (P < 0.05) compared with the other three groups. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Functional annotation of transcription factors regulated by PGDM-modulated miRNAs in the developing heart identified by the Database for Annotation, Visualization and Integrated Discovery (DAVID).
| Diseases or Functions | p-Value | Molecules |
|---|---|---|
| hypertrophy of heart | 3.27E-09 | CREB1,CREM,EP300,EPAS1,FOXO3,GATA6,HDAC5,HIF1A,HMGA1,JUN,MEF2C, MEF2D,MNT,MTPN,NAB1,NFATC2,PBX1,PBX3,PPARGC1B, SMAD3,SMAD4,SMAD7,SP3,STAT3 |
| hypertrophy of heart cells | 5.20E-06 | CREM,EP300,EPAS1,FOXO3,GATA6,HDAC5,JUN,MNT,NFATC2,SMAD7,SP3,STAT3 |
| hypoplasia of heart ventricle | 9.19E-06 | FOXM1,MED1,MEF2C,NOTCH1,RBPJ,UBP1,ZFP36L1 |
| hypoplasia of trabeculae carne | 1.08E-05 | MED1,MEF2C,NOTCH1,RBPJ,UBP1,ZFP36L1 |
| apoptosis of cardiomyocytes | 3.19E-05 | BACH1,CBL,CREM,E2F1,E2F3,FOXO3,HIF1A,MTPN,PHB,PLRG1,RB1,STAT3 |
| ventricular hypertrophy | 5.39E-04 | CREM,EPAS1,HDAC5,HIF1A,HMGA1,PBX1,PBX3,SMAD7 |
| hypertrophy of cardiac muscle | 6.78E-04 | CREM,EP300,FOXO3,GATA6,HDAC5,MEF2C,MTPN,SMAD7,STAT3 |
| fibrosis of heart | 2.22E-03 | BCL6,CREB1,CREM,KLF15,MEF2D,SMAD2,SMAD3,STAT3,TSC22D1 |
| proliferation of heart cells | 3.27E-03 | FOXO1,GATA6,NFAT5,RB1,RBPJ,STAT3 |
| hypertrophy of cardiomyocytes | 3.98E-03 | CREM,EP300,FOXO3,GATA6,HDAC5,SMAD7,STAT3 |
| hypertrophy of right ventricle | 4.82E-03 | EPAS1,PBX1,PBX3 |
| proliferation of cardiomyocytes | 7.21E-03 | FOXO1,GATA6,NFAT5,RB1,RBPJ |
| hypertrophy of myocardium | 9.67E-03 | MTPN,STAT3 |
| hypoplasia of myocardium | 1.35E-02 | MED1,WT1 |
| autosomal dominant coronary artery disease type 1 | 1.38E-02 | MEF2A |
| permeability transition of ventricular myocytes | 1.38E-02 | E2F1 |
| dysfunction of heart | 2.41E-02 | CREM,E2F1,EP300,MEF2C |
| pressure overload hypertrophy | 2.75E-02 | PPARGC1B |
| regeneration of cardiomyocytes | 2.75E-02 | MTPN |
| dilation of heart ventricle | 3.36E-02 | EP300,KLF15,MEF2A |
| bleeding of pericardium | 4.10E-02 | WT1 |
| dilation of right ventricle | 4.10E-02 | MEF2A |
| hypertrophy of atrium | 4.10E-02 | CREM |
| perivascular fibrosis of artery | 4.10E-02 | ETS1 |
| hypertrophy of ventricular myocytes | 4.32E-02 | HDAC5,SMAD7 |
| dysfunction of heart ventricle | 4.65E-02 | CREM,E2F1 |
| enlargement of atrium | 6.73E-02 | CREM |
| stenosis of pulmonary artery | 6.73E-02 | CITED2 |
| ventricular extrasystole | 8.03E-02 | REST |
| oxidative stress response of heart | 1.06E-01 | PPARGC1B |
| hypertrophy of left ventricle | 1.08E-01 | HMGA1,PBX1,PBX3 |
| myocarditis | 1.10E-01 | BCL6,TBX21 |
| inflammation of heart | 1.15E-01 | BCL6,ZFP36 |
| dilation of left ventricle | 1.24E-01 | EP300,KLF15 |
| thrombosis of atrium | 1.30E-01 | E2F3 |
| stenosis of aortic valve | 1.33E-01 | KLF15,RUNX2 |
| proliferation of cardiac fibroblasts | 1.54E-01 | STAT3 |
| proliferation of ventricular myocytes | 1.54E-01 | FOXO1 |
| degeneration of cardiomyocytes | 1.89E-01 | ARNT |
| experimental autoimmune myocarditis | 2.00E-01 | TBX21 |
| fibrosis of left ventricle | 2.54E-01 | CREM |
| damage of heart | 2.73E-01 | ARNT,STAT3 |
| dysfunction of left ventricle | 2.74E-01 | CREM |
| cell death of ventricular myocytes | 3.69E-01 | E2F1 |
| cardiac output | 4.12E-01 | PPARGC1B |
| injury of heart | 4.44E-01 | STAT3 |
| congestive heart failure | 4.47E-01 | E2F3,PPARGC1B |
| coronary artery disease | 4.59E-01 | BPTF,FOS,MEF2A,PBX3,SMAD3 |
| ventricular tachycardia | 5.55E-01 | REST |
| arrhythmia | 1.00E00 | ARNTL,CREM,REST |
| atrial fibrillation | 1.00E00 | CREM |
| myocardial infarction | 1.00E00 | FOXO3,HIF1A |
P-value: from Fisher's Exact Test.
To investigate whether these transcription factors are truly regulated by PGDM, five of these transcription factors (Cited2, Zeb2, Mef2c, Smad4 and Ets1) were examined by RT-qPCR. PGDM significantly suppressed, whereas SOD1 overexpression restored, the expression of these five transcription factors (Fig. 6B). Target prediction (Supplementary Table S2 and S3 in the online version at DOI: 10.1016/j.reprotox.2016.09.007) revealed Cited2 as a target of miR-384-3p; ZEB2 as target of miR-205-5p, miR-488-3p and miR-142-3p; Mef2c as a target of miR-488-3p; Smad4 as a target of miR-144-3p and miR-544-3p; and EST1 as a target of miR-144-3p, miR-330-5p and miR-222-3p. The down-regulation of these five transcription factors was consistent with the up-regulation of those miRNAs (miR-142-3p, miR-144-3p, miR-384-3p, miR-205-5p, miR-488-3p, miR-544-3p, miR-330-3p and miR-222-3p) by PGDM (Supplementary Table S1 in the online version at DOI: 10.1016/j.reprotox.2016.09.007).
4. Discussion
The present study is among the first to demonstrate that PGDM-regulated miRNAs and their putative target genes modulate cardiac development-associated pathways and transcription factors, and, thus, play important roles in PGDM-induced CHDs. By employing miRNA microarray technology, we investigated the global miRNA changes in hearts of embryos from diabetic and nondiabetic dams. We detected a total of 149 mapped miRNAs that were differentially expressed in embryonic hearts exposed to PGDM, compared with controls. By integrating the results of various prediction programs, 3960 possible miRNA-mRNA interactions, between 32 miRNAs and 2111 mRNAs, were identified. Gene ontology and IPA analysis indicated that alterations of miRNAs and their target gene expression are involved in gene transcription and transcriptional regulation, as well as the STAT3 and IGF-1 pathways. Further analysis showed that these potential transcription factors participate in cardiac development and disease. IPA analysis also showed the dysregulation of cellular proliferation, apoptosis, and death in the fetal heart and the subsequent imbalance of development. We confirmed the changes of 6 of the miRNAs and 5 of transcription factors using RT-qPCR in embryonic hearts exposed to maternal diabetes. We further observed that transgenic mice that overexpress SOD1, which can ameliorate the negative effects of PGDM in vivo and high glucose in vitro on the developing embryo [15,18,28,48,50,51,55], diminished the changes in PGDM-regulated miRNAs and their target genes in response to diabetic conditions.
miRNAs play vital roles in cardiac development and cardiovascular disease [31,38,42]. The necessity of miRNAs for cardiac development has been revealed by Zhao and colleagues who found that deleting Dicer, the RNase essential for miRNA biosynthesis, in E8.5 cardiac progenitor cells causes pericardial edema, hypoplastic ventricular myocardium, and embryo lethality at E12.5 [66]. Postnatal deletion of Dicer in cardiomyocytes leads to dilated cardiomyopathy, heart failure and lethality [8]. Deletion of Dicer in the adult heart causes heart failure and death [11]. Our findings support the critical involvement of miRNAs in PGDM-induced CHD formation, and provide a list of miRNAs and their putative target gene for further studies.
Deletion of specific miRNAs causes cardiac malformations [5,9,31,39]. The roles for the miR-1/miR-133 family, the miR–17–92 cluster, MyomiRs (miR-208a, miR-208b and miR-499), the miR-143/145 family, miR-15, miR-138, and miR-126 in cardiac development have been elucidated in vivo and in vitro systems [5,9,31,39]. In our miRNA profiling, we did not find changes on these miRNAs, except miR-499. MiR-499 and its cluster miRNAs are encoded within the introns of three muscle-specific myosin genes [31,39,42]. miR-499 inhibits cardiomyocyte apoptosis and myocardial infarction, suggesting its therapeutic effects on cardiovascular diseases [54]. miR-499 was significantly down-regulated in embryonic hearts exposed to PGDM [54]. Our previous studies showed that PGDM induced apoptosis in the endocardial cushions of the OFT, AV canal and the epicardial lining of the ventricles of fetal heart [48,50,52]. Therefore, all above imply that overexpression of miR-499 may diminish apoptosis observed in PGDM-induced CHDs.
In the present study, we have employed an established mouse model of type 1 diabetes mellitus in pregnancy which induces CHDs that are similar to those observed in humans [48,50,52]. The defects observed in our model include VSDs and OFT defects [48,50,52]. Recently, Vijaya et al. used the STZ-induced diabetic mouse model to examine differential gene expression profiles in E13.5 and E15.5 hearts [47]. Using gene ontology analysis, they found that maternal diabetes alters the expression of most of genes involved in transcription [47], which is consistent with our findings that many of the potential gene targets of the PGDM-regulated miRNAs are involved in gene transcription. Another group examined changes in miRNA profiles in response to maternal obesity by sequencing miRNAs in the hearts of baboon fetuses at 165 days of gestation [33]. They identified 383 potential transcriptional regulators modulated by differential miRNA expression due to maternal obesity [33]. Taken together with the results of our current study, these data reveal a causal role for transcription network imbalance in diabetes or obesity-associated CHDs.
We found that maternal diabetes down-regulated the expression of Cited2 (Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain 2), ZEB2 (Zinc finger E-box binding homeobox 2), Mef2c (Myocyte enhancer factor 2C), Smad4, and ETS1 (E26 avian leukemia oncogene 1, 5′ domain) in the developing heart. Mef2c is involved in cardiac morphogenesis and vascular development. Mice with a mutation in the Mef2c gene are early embryonic lethal with severe cardiovascular defects, including failure of normal cardiac looping [7,30]. Deletion of Smad4 in cardiomyocytes [41,46,53], neural crest cells [21,37] and endothelial cells [36] causes severe cardiac and vascular defects. Disruption of the gene encoding Cited2 is embryonic lethal because of defects in the developing heart and neural tube [65]. Cited2-null embryos exhibit severe cardiovascular abnormalities, including pulmonic arterial stenosis and VSDs accompanied by high peak outflow velocities [65]. Mutations or deletions in the ZEB2 gene induce Mowat–Wilson syndrome [17]. Mowat-Wilson syndrome is a multiple congenital anomaly syndrome, including hirschsprung disease, congenital heart disease, and genitourinary anomalies and so on [17]. Deletion of ETS1, a gene residing in the Jacobsen syndrome critical region, causes VSDs and abnormal ventricular morphology in mice [64]. Therefore, Mef2c, Smad4, Cited2, ZEB2 and ETS1 may be important candidate genes that participate in the pathogenesis of PGDM-induced CHDs.
We have demonstrated that oxidative stress plays a causal role in PGDM-induced neural tube defects [12–14,16,49,58,60–63,67] and CHDs [48,50,52,57], and that overexpression of SOD1 in transgenic mice ameliorates PGDM-induced CHDs [48,50]. We previously reported that PGDM in vivo and high glucose in vitro decreased the expression of miR-322 in E8.5 embryos, and that overexpressing SOD1 restores miR-322 levels [19]. This suggests that the effects of PGDM on miRNA expression are mediated by hyperglycemia-induced oxidative stress. Indeed, SOD1 overexpression restores PGDM-altered miRNA expression and miRNA putative target gene expression. These data further confirm the oxidative stress hypothesis in diabetic embryopathy.
5. Conclusions
The current study identified a set of 149 differential miRNAs and 2111 predicted target genes in the developing heart exposed to PGDM that may regulate multiple cellular and developmental processes. Notably, most of these miRNA target genes are involved in gene transcription and transcriptional regulation. Furthermore, several cardiac transcription factors were confirmed to be regulated by PGDM-modulated miRNAs. Thus, the present work provides some important insights into the molecular mechanisms underlying PGDM -induced CHDs.
Supplementary Material
Acknowledgments
We are grateful to Dr. Julie Rosen, Offices of the Dean and Public Affairs & Communications at the University of Maryland School of Medicine for critical reading and editing. This work was supported by the National Institutes of Health. Grant number: R01DK083243, R01DK101972, R01HL131737 and R01DK103024.
Footnotes
Disclosures: None of the authors have any conflicts of interest, financial or otherwise.
References
- 1.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4 doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardekani AM, Naeini MM. The role of MicroRNAs in human diseases. Avicenna J Med Biotechnol. 2010;2:161–179. [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauersachs J, Thum T. Biogenesis and regulation of cardiovascular microRNAs. Circ Res. 2011;109:334–347. doi: 10.1161/CIRCRESAHA.110.228676. [DOI] [PubMed] [Google Scholar]
- 6.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi W, Drake CJ, Schwarz JJ. The transcription factor MEF2C-null mouse exhibits complex vascular malformations and reduced cardiac expression of angiopoietin 1 and VEGF. Dev Biol. 1999;211:255–267. doi: 10.1006/dbio.1999.9307. [DOI] [PubMed] [Google Scholar]
- 8.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ, Wang DZ. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, Cleves MA, Riehle-Colarusso TJ, Waller DK, Reece EA. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(237):e231–e239. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Costa Martins PA, Bourajjaj M, Gladka M, Kortland M, van Oort RJ, Pinto YM, Molkentin JD, De Windt LJ. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. 2008;118:1567–1576. doi: 10.1161/CIRCULATIONAHA.108.769984. [DOI] [PubMed] [Google Scholar]
- 12.Dong D, Fu N, Yang P. MiR-17 downregulation by high glucose stabilizes thioredoxin-Interacting protein and removes thioredoxin inhibition on ASK1 leading to apoptosis. Toxicol Sci. 2016;150:84–96. doi: 10.1093/toxsci/kfv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong D, Reece EA, Lin X, Wu Y, Arias Villela N, Yang P. New development of the yolk sac theory in diabetic embryopathy: molecular mechanism and link to structural birth defects. Am J Obstet Gynecol. 2016;214:192–202. doi: 10.1016/j.ajog.2015.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong D, Reece EA, Yang P. The nrf2 activator vinylsulfone reduces high glucose-Induced neural tube defects by suppressing cellular stress and apoptosis. Reprod Sci. 2016 doi: 10.1177/1933719115625846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong D, Yu J, Wu Y, Fu N, Villela NA, Yang P. Maternal diabetes triggers DNA damage and DNA damage response in neurulation stage embryos through oxidative stress. Biochem Biophys Res Commun. 2015;467:407–412. doi: 10.1016/j.bbrc.2015.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabbay-Benziv R, Reece EA, Wang F, Yang P. Birth defects in pregestational diabetes: defect range, glycemic threshold and pathogenesis. World J Diabetes. 2015;6:481–488. doi: 10.4239/wjd.v6.i3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garavelli L, Mainardi PC. Mowat-Wilson syndrome. Orphanet J Rare Dis. 2007;2:42. doi: 10.1186/1750-1172-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu H, Yu J, Dong D, Zhou Q, Wang JY, Fang S, Yang P. High glucose-repressed CITED2 expression through miR-200b triggers the unfolded protein response and endoplasmic reticulum stress. Diabetes. 2016;65:149–163. doi: 10.2337/db15-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu H, Yu J, Dong D, Zhou Q, Wang JY, Yang P. The miR-322-TRAF3 circuit mediates the pro-apoptotic effect of high glucose on neural stem cells. Toxicol Sci. 2015;144:186–196. doi: 10.1093/toxsci/kfu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL. American Heart Association Council on Cardiovascular Disease in the Y, Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 21.Jia Q, Mc Dill BW, Li SZ, Deng C, Chang CP, Chen F. Smad signaling in the neural crest regulates cardiac outflow tract remodeling through cell autonomous and non-cell autonomous effects. Dev Biol. 2007;311:172–184. doi: 10.1016/j.ydbio.2007.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 23.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 24.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 25.Kung LA, Tao SC, Qian J, Smith MG, Snyder M, Zhu H. Global analysis of the glycoproteome in Saccharomyces cerevisiae reveals new roles for protein glycosylation in eukaryotes. Mol Syst Biol. 2009;5(308) doi: 10.1038/msb.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31:899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III. Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Weng H, Reece EA, Yang P. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. Am J Obstet Gynecol. 2011;205(84):e81–e86. doi: 10.1016/j.ajog.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Kowdley KV. MicroRNAs in common human diseases. Genomics, proteomics & Bioinformatics. 2012;10:246–253. doi: 10.1016/j.gpb.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu N, Olson EN. MicroRNA regulatory networks in cardiovascular development. Dev Cell. 2010;18:510–525. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loffredo CA, Wilson PD, Ferencz C. Maternal diabetes: an independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology. 2001;64:98–106. doi: 10.1002/tera.1051. [DOI] [PubMed] [Google Scholar]
- 33.Maloyan A, Muralimanoharan S, Huffman S, Cox LA, Nathanielsz PW, Myatt L, Nijland MJ. Identification and comparative analyses of myocardial miRNAs involved in the fetal response to maternal obesity. Physiol Genomics. 2013;45:889–900. doi: 10.1152/physiolgenomics.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Morgan SC, Lee HY, Relaix F, Sandell LL, Levorse JM, Loeken MR. Cardiac outflow tract septation failure in Pax3-deficient embryos is due to p53-dependent regulation of migrating cardiac neural crest. Mech Dev. 2008;125:757–767. doi: 10.1016/j.mod.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moskowitz IP, Wang J, Peterson MA, Pu WT, Mackinnon AC, Oxburgh L, Chu GC, Sarkar M, Berul C, Smoot L, Robertson EJ, Schwartz R, Seidman JG, Seidman CE. Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development. [corrected] Proc Natl Acad Sci U S A. 2011;108:4006–4011. doi: 10.1073/pnas.1019025108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nie X, Deng CX, Wang Q, Jiao K. Disruption of Smad4 in neural crest cells leads to mid-gestation death with pharyngeal arch, craniofacial and cardiac defects. Dev Biol. 2008;316:417–430. doi: 10.1016/j.ydbio.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ono K, Kuwabara Y, Han J. MicroRNAs and cardiovascular diseases. FEBS J. 2011;278:1619–1633. doi: 10.1111/j.1742-4658.2011.08090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philippen LE, Dirkx E, da Costa-Martins PA, De Windt LJ. R.N.A. Non-coding, in control of gene regulatory programs in cardiac development and disease. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. American Heart Association Congenital Cardiac Defects Committee CoCDitY, Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- 41.Qi X, Yang G, Yang L, Lan Y, Weng T, Wang J, Wu Z, Xu J, Gao X, Yang X. Essential role of Smad4 in maintaining cardiomyocyte proliferation during murine embryonic heart development. Dev Biol. 2007;311:136–146. doi: 10.1016/j.ydbio.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 42.Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest. 2013;123:11–18. doi: 10.1172/JCI62876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roest PA, Molin DG, Schalkwijk CG, van Iperen L, Wentzel P, Eriksson UJ, Gittenberger-de Groot AC. Specific local cardiovascular changes of Nepsilon-(carboxymethyl)lysine, vascular endothelial growth factor, and Smad2 in the developing embryos coincide with maternal diabetes-induced congenital heart defects. Diabetes. 2009;58:1222–1228. doi: 10.2337/db07-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheffield JS, Butler-Koster EL, Casey BM, McIntire DD, Leveno KJ. Maternal diabetes mellitus and infant malformations. Obstet Gynecol. 2002;100:925–930. doi: 10.1016/s0029-7844(02)02242-1. [DOI] [PubMed] [Google Scholar]
- 45.Simon R, Lam A, Li MC, Ngan M, Menenzes S, Zhao Y. Analysis of gene expression data using BRB-ArrayTools. Cancer Inf. 2007;3:11–17. [PMC free article] [PubMed] [Google Scholar]
- 46.Song L, Yan W, Chen X, Deng CX, Wang Q, Jiao K. Myocardial smad4 is essential for cardiogenesis in mouse embryos. Circ Res. 2007;101:277–285. doi: 10.1161/CIRCRESAHA.107.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vijaya M, Manikandan J, Parakalan R, Dheen ST, Kumar SD, Tay SS. Differential gene expression profiles during embryonic heart development in diabetic mice pregnancy. Gene. 2013;516:218–227. doi: 10.1016/j.gene.2012.12.071. [DOI] [PubMed] [Google Scholar]
- 48.Wang F, Fisher SA, Zhong J, Wu Y, Yang P. Superoxide dismutase 1 in vivo ameliorates maternal diabetes-Induced apoptosis and heart defects through restoration of impaired wnt signaling. Circ Cardiovasc Genet. 2015 doi: 10.1161/CIRCGENETICS.115.001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang F, Reece EA, Yang P. Advances in revealing the molecular targets downstream of oxidative stress-induced proapoptotic kinase signaling in diabetic embryopathy. Am J Obstet Gynecol. 2015;213:125–134. doi: 10.1016/j.ajog.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang F, Reece EA, Yang P. Oxidative stress is responsible for maternal diabetes-impaired transforming growth factor beta signaling in the developing mouse heart. Am J Obstet Gynecol. 2015;212(650):e651–e611. doi: 10.1016/j.ajog.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang F, Reece EA, Yang P. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. Am J Obstet Gynecol. 2013;209(345):e341–e347. doi: 10.1016/j.ajog.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F, Wu Y, Quon MJ, Li X, Yang P. ASK1 mediates the teratogenicity of diabetes in the developing heart by inducing ER stress and inhibiting critical factors essential for cardiac development. Am J Physiol Endocrinol metab: Ajpendo. 2015;00121:02015. doi: 10.1152/ajpendo.00121.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Xu N, Feng X, Hou N, Zhang J, Cheng X, Chen Y, Zhang Y, Yang X. Targeted disruption of Smad4 in cardiomyocytes results in cardiac hypertrophy and heart failure. Circ Res. 2005;97:821–828. doi: 10.1161/01.RES.0000185833.42544.06. [DOI] [PubMed] [Google Scholar]
- 54.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 55.Weng H, Li X, Reece EA, Yang P. SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. Am J Obstet Gynecol. 2012;206(448):e441–e447. doi: 10.1016/j.ajog.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wren C, Birrell G, Hawthorne G. Cardiovascular malformations in infants of diabetic mothers. Heart. 2003;89:1217–1220. doi: 10.1136/heart.89.10.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y, Reece EA, Zhong J, Dong D, Shen WB, Harman CR, Yang P. Type 2 diabetes mellitus induces congenital heart defects in murine embryos by increasing oxidative stress, endoplasmic reticulum stress, and apoptosis. Am J Obstet Gynecol. 2016 doi: 10.1016/j.ajog.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y, Wang F, Reece EA, Yang P. Curcumin ameliorates high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. Am J Obstet Gynecol. 2015;212(802):e801–e808. doi: 10.1016/j.ajog.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J, Cummings EA, O'Connell C, Jangaard K. Fetal and neonatal outcomes of diabetic pregnancies. Obstet Gynecol. 2006;108:644–650. doi: 10.1097/01.AOG.0000231688.08263.47. [DOI] [PubMed] [Google Scholar]
- 60.Yang P, Cao Y, Li H. Hyperglycemia induces inducible nitric oxide synthase gene expression and consequent nitrosative stress via c-Jun N-terminal kinase activation. Am J Obstet Gynecol. 2010;203(185):e185–e111. doi: 10.1016/j.ajog.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang P, Li H. Epigallocatechin-3-gallate ameliorates hyperglycemia-induced embryonic vasculopathy and malformation by inhibition of Foxo3a activation. Am J Obstet Gynecol. 2010;203(75):e71–e76. doi: 10.1016/j.ajog.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang P, Reece EA, Wang F, Gabbay-Benziv R. Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. Am J Obstet Gynecol. 2015;212:569–579. doi: 10.1016/j.ajog.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198(130):e131–e137. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 64.Ye M, Coldren C, Liang X, Mattina T, Goldmuntz E, Benson DW, Ivy D, Perryman MB, Garrett-Sinha LA, Grossfeld P. Deletion of ETS-1, a gene in the Jacobsen syndrome critical region, causes ventricular septal defects and abnormal ventricular morphology in mice. Hum Mol Genet. 2010;19:648–656. doi: 10.1093/hmg/ddp532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin Z, Haynie J, Yang X, Han B, Kiatchoosakun S, Restivo J, Yuan S, Prabhakar NR, Herrup K, Conlon RA, Hoit BD, Watanabe M, Yang YC. The essential role of Cited2, a negative regulator for HIF-1alpha, in heart development and neurulation. Proc Natl Acad Sci U S A. 2002;99:10488–10493. doi: 10.1073/pnas.162371799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 67.Zhong J, Reece EA, Yang P. Punicalagin exerts protective effect against high glucose-induced cellular stress and neural tube defects. Biochem Biophys Res Commun. 2015;467:179–184. doi: 10.1016/j.bbrc.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


