Abstract
Oxidative damage occurs in multiple sclerosis, but is difficult to identify antemortem and remains an unknown contributor to disease progression. Carbonylation is a quantitative measure of protein oxidation. Cerebrospinal fluid samples from multiple sclerosis patients showed elevated carbonylated protein levels compared to controls. In experimental autoimmune encephalomyelitis, carbonylated protein levels in cerebrospinal fluid correlated tightly with those found in inflamed spinal cord tissues. Furthermore, concentrations in cerebrospinal fluid and spinal cord responded in parallel to an antioxidant intervention that also attenuated disease symptoms. These data suggest that carbonylated cerebrospinal fluid proteins could be a quantitative, sensitive, and disease‐relevant biomarker in multiple sclerosis.
Introduction
Oxidative damage occurs in both the gray and white matter of multiple sclerosis (MS) patients.1, 2, 3, 4, 5, 6, 7, 8 Tissue myeloid cells, both infiltrating monocytes and resident microglia, generate reactive oxygen species (ROS) in this disease setting,1, 2, 3 and confirmed central nervous system (CNS) targets of oxidation include the lipids, nucleic acids, and proteins of both glia and neurons.1, 2, 3, 4, 5, 6, 7, 8 While such oxidative changes generally increase with disease duration and clinical measures of disability,1, 2, 3, 4, 5, 6, 7, 8 a convenient way to measure them within the CNS of living patients has remained elusive. This greatly hampers investigation of potential antioxidant interventions, particularly among patients with secondary‐progressive MS (SPMS) where oxidative damage is most apparent and where approved therapies are lacking.
While ROS are short‐lived molecules, the proteins they modify are more stable and are amenable to measurement as a surrogate of oxidative injury. Carbonylation is the most frequent form of irreversible protein oxidation, and carbonylated proteins are easily quantified in biological samples.9 Proteins modified in this manner are well known to accumulate in the brains of MS patients.4 Still, only a handful of studies have measured carbonylated proteins in human cerebrospinal fluid (CSF),10, 11 and only one suggests that these proteins might serve as a biomarker of oxidative injury within the CNS.11 This study investigated this question using samples from humans with MS and mice with experimental autoimmune encephalomyelitis (EAE). The data show that carbonylated proteins accumulate in the CSF of MS patients compared to controls, and that levels correlate directly with both disease duration and expanded disability status scale (EDSS) scores. In EAE, CSF carbonylated protein levels correlate closely with those measured directly in inflamed spinal cord tissues, and both CSF and tissue levels respond in parallel to an intervention that inhibits CNS ROS production and attenuates disease symptoms. Together, these findings suggest that quantification of carbonylated proteins in CSF could be a convenient biomarker of ongoing oxidative injury in the CNS of SPMS patients.
Materials and Methods
Human CSF samples
All CSF samples were obtained from patients who had a lumbar puncture for clinical purposes. Collection of an additional specimen for research use was done so with written informed consent. The Institutional Review Board of the University of Michigan Medical School approved the acquisition and storage of these samples (HUM00066792). Specimens were collected on ice, centrifuged at 2000g to remove cellular contents, and stored at −80°C until use.
Animals
Female C57BL/6 mice (8–10 weeks old) were purchased from Harlan Laboratories (Indianapolis, IN). The University of Michigan Committee on the Use and Care of Animals approved all the procedures herein (PRO00006745), and experiments were conducted in accordance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals. Subcutaneous immunization of mice with 100 μg of myelin oligodendrocyte glycoprotein (MOG)35‐55 peptide (Biosynthesis, Lewisville, TX) emulsified in complete Freund's adjuvant (Difco, Detroit, MI) containing an additional 4 mg/mL heat killed Mycobacterium tuberculosis, H37Ra (Difco) induced EAE. All mice also received 300 ng of pertussis toxin (List Biological Laboratories, Campbell, CA) via intraperitoneal (i.p.) injection on the day of immunization and 48 h later. Animals were then given 25 mg/kg of telmisartan (Sigma‐Aldrich, St. Louis, MO) or a vehicle control twice daily via i.p. injection in 200 μL of 0.9% sodium chloride solution, pH 9.0, beginning on day 10. Thereafter, each animal was scored daily for clinical signs of EAE using the following scale: 0 = no signs of disease; 1 = flaccid tail or hind limb weakness; 2 = flaccid tail, hind limb weakness, and loss of righting reflex; 3 = partial hind limb paralysis; 4 = complete hind limb paralysis; 5 = moribund or dead. At day 20 postimmunization, some mice were killed and CSF collected using a pulled glass capillary pipette via cisternal puncture. Animals then underwent transcardiac perfusion with chilled phosphate‐buffered saline, and spinal cords were dissected for biochemical analyses.
Measurement of carbonyl groups
Lumbar spinal cords were homogenized in 10 mmol/L EDTA, pH 7.5, and kept at −80°C until use. Samples were treated with 1% streptomycin sulfate in 50 mmol/L potassium phosphate buffer, pH 7.2, for 15 min and centrifuged at 6000g to remove nucleic acids. The protein content of each sample was measured using the Pierce Coomassie Protein Assay Reagent (Thermo Fisher Scientific, Rockford, IL) and adjusted to 1 mg/mL. Protein carbonylation was measured in each experimental sample using the Protein Carbonyl Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI). Two hundred microliters of each mouse spinal cord homogenate and human CSF sample, and 5 μL of each mouse CSF sample, were incubated with 800 μL of 2,4‐dinitrophenylhydrazine in 2.5 mol/L HCl for 1 h at room temperature. Controls for each specimen were treated with 2.5 mol/L HCl alone. Samples were sequentially precipitated with 20% trichloroacetic acid (TCA), 10% TCA, and a 1:1 mixture of ethanol/ethyl acetate. Pellets were suspended in guanidine hydrochloride and the absorbance of each sample and its respective control read at 360 nm. A portion of each sample was retained to remeasure its total protein content as described above. The amount of carbonyl groups present in each sample was calculated using a molar extinction coefficient of 22,000 cm−1, its total protein concentration, and its corrected absorbance (sample minus control).
Measurement of tissue superoxide‐generating activity
The capacity of each mouse spinal cord to generate ROS was measured directly ex vivo using the well‐established cytochrome c reduction assay, as described.12 Results are presented as nmol O2 ‐ produced/mg tissue protein.
Statistical comparisons
The Prism 6.0 software package (GraphPad Software, La Jolla, CA) was used for all statistical analyses. Student's t‐tests were applied when comparing a single parameter under two experimental conditions, a one‐way analysis of variance (ANOVA) with a post hoc Bonferroni's multiple comparison test was used to investigate the significance of a single parameter's change across multiple groups, while a two‐way ANOVA with a post hoc Bonferroni's multiple comparison test was used to compare experimental findings between two groups over time. Correlations were determined using Spearman nonparametric tests and two‐tailed P‐values. In all cases, differences at a P < 0.05 level were considered significant.
Results
Carbonylated proteins accumulate directly in the brains of patients with SPMS,4 and proteins modified in this way are considered both a sensitive and reliable indicator of ongoing ROS‐mediated tissue damage.13 Levels of carbonylated proteins were measured in CSF samples obtained from MS patients (n = 73) and noninflammatory neurologic disease (NIND) controls (n = 17). Mean concentrations were elevated across the spectrum of MS subtypes, rising steadily from patients with clinically isolated syndromes (CIS) through relapsing‐remitting disease and into SPMS (Fig. 1A). Primary progressive MS was not assessed due to a lack of available samples. Among the 73 MS samples, CSF carbonylated protein concentrations correlated directly with both disease duration and EDSS score, but not with age (Fig. 1B–D).
Figure 1.
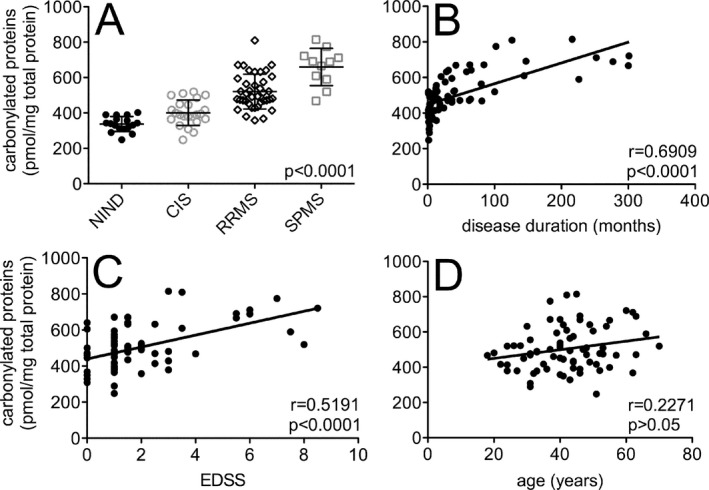
Carbonylated proteins are increased in the CSF of patients with MS compared to non‐MS controls, and levels in MS patients correlate directly with disease duration and disability scores. (A) Protein carbonyls were quantified in CSF samples collected from patients with various noninflammatory neurologic diseases (NIND, n = 17), clinically isolated syndromes (CIS, n = 23), relapsing‐remitting MS (RRMS, n = 39) or secondary‐progressive MS (SPMS, n = 11). Mean concentrations in each group were significantly different from one another (P < 0.0001). (B–D) When CIS, RRMS, and SPMS patient samples were grouped together (n = 73), CSF carbonylated protein levels correlated directly with disease duration and EDSS score, but not with age. Correlation coefficients (r) and two‐tailed P ‐values are shown. CSF, Cerebrospinal fluid; MS, multiple sclerosis; EDSS, expanded disability status scale.
The angiotensin receptor blocking (ARB) drug, telmisartan, was recently shown to be neuroprotective in mice with lethal viral encephalitis by exerting antioxidant activity directly within the CNS.12 To determine whether this same intervention could modulate CNS protein carbonylation during autoimmune demyelination, mice with active EAE were treated twice daily with telmisartan starting just prior to symptom onset. Similar to what has been reported with other ARBs in EAE,14, 15, 16 this regimen suppressed clinical disease severity (Fig. 2A). Furthermore, 10 days of drug administration also inhibited ROS production directly in the spinal cord (Fig. 2B), and it significantly reduced levels of carbonylated moieties present in both the spinal cord (Fig. 2C) and CSF compartments (Fig. 2D). A remarkable correlation was observed between levels of carbonyl groups in CSF and spinal cord among individual animals (Fig. 2E).
Figure 2.
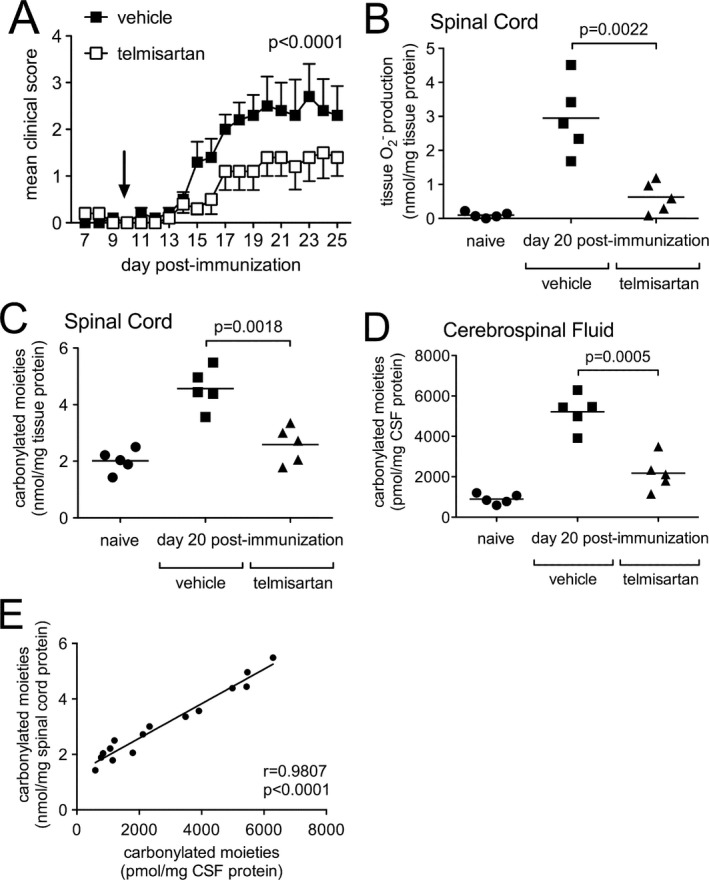
Systemic administration of telmisartan suppresses clinical disease severity, tissue ROS production, and protein carbonylation in the central nervous system of mice with EAE. (A) Mice with active immunization EAE were treated twice daily with telmisartan (n = 10) or a vehicle control (n = 10) beginning 10 days following disease induction (arrow). Clinical disease scores were significantly different between the two groups (P < 0.0001). (B) Telmisartan potently suppressed EAE‐induced tissue ROS production compared to mice given a vehicle control (P = 0.0022). (C, D) Telmisartan simultaneously inhibited the formation of carbonylated moieties directly in spinal cord tissue (P = 0.0018) and CSF (P = 0.0005) compared to a vehicle control. (E) Levels of carbonylated moieties in CSF and spinal cord were tightly correlated with each other in individual animals (P < 0.0001). CSF, cerebrospinal fluid.
Discussion
Recent microscopic studies of SPMS tissues have fostered a growing consensus that processes driving this phase of disease must account for a relative shift away from focal areas of inflammatory demyelination and toward ones causing more widespread degeneration of chronically demyelinated axons, cortical demyelination, and global CNS tissue volume loss. These shared pathologic features are poorly reproduced in current animal models, and the lack of a validated experimental system has clouded investigation of disease mechanisms and impeded the development of therapies to slow SPMS progression. Clinical and radiological heterogeneity is another obstacle that has hampered the development of treatments for this disorder. Indeed, without both good mechanistic targets and validated study endpoints, clinical trials in SPMS have lagged far behind the remarkable progress made for patients with relapsing‐remitting disease. A quantitative, reliable, and disease‐relevant biomarker for SPMS is sorely needed.
Oxidative damage is found in MS tissues at autopsy,1, 2, 3, 4, 5, 6, 7, 8 but this process escapes detection using conventional laboratory and CNS imaging techniques. It also remains unproven as a pathogenic mechanism that actually drives MS progression. The data presented here show that increased carbonylation of CSF proteins is a consistent finding in MS patients, and that proteins modified in this way progressively accrue as the disease course lengthens and disability accumulates, and not simply as patients get older. Furthermore, CSF protein carbonylation accurately reflects oxidative events occurring within the inflamed spinal cords of mice with EAE. This suggests either tissue ROS can reach the CSF directly, or there is a dynamic exchange of these modified proteins between the CNS parenchyma and the CSF compartment. The fact that protein carbonyl levels respond to an intervention that both reduces tissue ROS production and attenuates disease symptoms demonstrates that a shift between their production and removal can be accomplished in EAE. Reduced proteosomal degradation contributes to the accumulation of tissue carbonylated proteins during chronic EAE,17 but these protein modifications also clearly happen faster in diseased versus control mice.
Because CSF proteins rapidly efflux from the CNS via macrovesicular transport across arachnoid granulations,18 elevated protein carbonyl content in this compartment must reflect an active, ongoing protein oxidation process. This raises some hope that antioxidant approaches could become a viable therapeutic strategy in SPMS. Having a convenient tool to quantify such biochemical events within the CNS should greatly accelerate the drug discovery process.
Author Contributions
D.N.I. conceived and designed the study. He oversaw collection of the human CSF samples, supervised the performance of the assays, analyzed the data, prepared the figures, and wrote the paper.
Conflict of Interest
Nothing to report.
Acknowledgment
The technical expertise of Pennelope Blakely who assisted with the animal experiments is gratefully acknowledged.
References
- 1. Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. [DOI] [PubMed] [Google Scholar]
- 2. Lassmann H. Mechanisms of white matter damage in multiple sclerosis. Glia 2014;62:1816–1830. [DOI] [PubMed] [Google Scholar]
- 3. Fischer MT, Sharma R, Lim JL, et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 2012;135:886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bizzozero OA, DeJesus G, Callahan K, Pastuszyn A. Elevated protein carbonylation in the white matter and grey matter of patients with multiple sclerosis. J Neurosci Res 2005;81:687–695. [DOI] [PubMed] [Google Scholar]
- 5. van Horssen J, Schreibelt G, Drexhage J, et al. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med 2008;45:1729–1737. [DOI] [PubMed] [Google Scholar]
- 6. Haider L, Fischer MT, Frischer JM, et al. Oxidative damage in multiple sclerosis. Brain 2011;134:1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fischer MT, Wimmer I, Hoftberger R, et al. Disease‐specific molecular events in cortical multiple sclerosis lesions. Brain 2013;136:1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kharel P, McDonough J, Basu S. Evidence of extensive RNA oxidation in normal appearing cortex of multiple sclerosis brain. Neurochem Int 2016;92:43–48. [DOI] [PubMed] [Google Scholar]
- 9. Rogowska‐Wrzesinska A, Wojdyla K, Nedic O, et al. Analysis of protein carbonylation – pitfalls and promise in commonly used methods. Free Radic Res. 2014;48:1145–1162. [DOI] [PubMed] [Google Scholar]
- 10. Korolainen MA, Pirttila T. Cerebrospinal fluid, serum and plasma protein oxidation in Alzheimer's disease. Acta Neurol Scand 2009;119:32–38. [DOI] [PubMed] [Google Scholar]
- 11. Rommer PS, Greilberger J, Salhofer‐Polanyi S, et al. Elevated levels of carbonyl proteins in cerebrospinal fluid of patients with neurodegenerative diseases. Tohoku J Exp Med 2014;234:313–317. [DOI] [PubMed] [Google Scholar]
- 12. Blakely PK, Huber AK, Irani DN. Type‐1 angiotensin receptor signaling in central nervous system myeloid cells is pathogenic during fatal alphavirus encephalitis in mice. J Neuroinflammation 2016;13:196. doi:10.1186/s12974‐016‐0683‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berlett BS, Stadtman ER. Protein oxidation in aging, disease and oxidative stress. J Biol Chem 1997;272:20313–20316. [DOI] [PubMed] [Google Scholar]
- 14. Platten M, Youssef S, Hur EM, et al. Blocking angiotensin‐converting enzyme induces potent regulatory T cells and modulates Th1‐ and Th17‐mediated autoimmunity. Proc Natl Acad Sci USA 2009;106:14948–14953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stegbauer J, Lee DH, Seubert S, et al. Role of the renin‐angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci USA 2009;106:14942–14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lanz TV, Ding Z, Ho PP, et al. Angiotensin II sustains brain inflammation in mice via TGF‐beta. J. Clin. Invest. 2010;120:2782–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng J, Bizzozero OA. Reduced proteosomal activity contributes to the accumulation of carbonylated proteins in chronic experimental autoimmune encephalomyelitis. J Neurochem 2010;115:1556–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irani DN. Properties and composition of normal cerebrospinal fluid In: Irani DN, ed. Cerebrospinal fluid in clinical practice. Philadelphia, PA: Saunders/Elsevier, 2009:69–89. [Google Scholar]


