Abstract
Real‐Time Quaking‐Induced Conversion (RT‐QuIC) testing of human cerebrospinal fluid (CSF) is highly sensitive and specific in discriminating sporadic CJD patients from those without prion disease. Here, using CSF samples from 113 CJD and 64 non‐prion disease patients, we provide the first direct and concurrent comparison of our improved RT‐QuIC assay to our previous assay, which is similar to those commonly used internationally for CJD diagnosis. This extended comparison demonstrated a ~21% increase in diagnostic sensitivity, a 2‐day reduction in average detection time, and 100% specificity.
Creutzfeldt‐Jakob disease (CJD) is a human prion disease occurring with sporadic, genetic, or infectious etiologies. The agents responsible for these diseases are prions, which are altered forms of the ubiquitously and normally expressed prion protein (PrP). The most common form of prion disease in humans is sporadic CJD (sCJD), characterized by a spectrum of variable clinical signs evolving over a period of weeks to months. Early and accurate diagnosis has been a long standing difficulty, currently relying heavily on combinations of clinical features, specifically MRI and EEG changes, and the detection of surrogate markers such as the 14‐3‐3 protein in CSF.1 Such criteria can yield diagnoses of probable or possible sCJD, however, according to current international guidelines, definite diagnosis requires the detection of pathological prion protein (PrPCJD) in the central nervous system tissue. Because the acquisition of such tissue requires brain biopsies2 or autopsies, most definitive diagnoses are made postmortem.
The Real‐Time Quaking‐Induced Conversion (RT‐QuIC) and the amyloid seeding (ASA) assays exploit the ability of PrPCJD to seed the polymerization of protease sensitive recombinant PrP (rPrPsen) into amyloid fibrils detectable by thioflavin‐T (ThT) fluorescence.3, 4, 5 This test allows for rapid, sensitive, and quantitative detection of prion‐associated seeds in a variety of biological tissues and fluids.6, 7, 8, 9, 10, 11, 12, 13, 14
Recently the RT‐QuIC has been used for sensitive and specific detection of PrPCJD in human cerebrospinal fluid5, 15, 16, 17, 18, 19 which is much easier than brain tissue to obtain from live patients. Numerous laboratories have implemented and extensively assessed the use of RT‐QuIC analysis of CSF for intra vitam diagnosis of CJD using hamster or human rPrPsen with amino acids 23–231 as the substrate, no detergents, and 37 or 42°C incubations.13, 14, 15, 16, 17, 18 These assays require 2.5–5 days and miss 11–23% of CJD cases. We call our version of this generation of the assay PQ‐CSF, for previous RT‐QuIC for CSF.19 However, we recently described an improved, second generation RT‐QuIC for CSF (IQ‐CSF) assay which uses a truncated form of hamster rPrPSen (amino acids 90–231) as a substrate, 55°C incubations, and the addition of 0.002% sodium dodecyl sulfate (SDS). Our initial evaluation of the IQ‐CSF assay indicated enhanced fluorescence responses to sCJD seeds, greater analytical and diagnostic sensitivity, and markedly shorter testing times of ~4–24 h.19
For this study, we analyzed a new and larger panel of CJD patients and, for the first time, directly compared the performance of the PQ‐ and IQ‐CSF conditions when tested simultaneously on the same specimens. CSF samples were gathered at multiple locations in Italy including the University of Verona (Verona), Ospedale Maria Vittoria (Torino), and Istituto Superiore di Sanità (Rome). All of the 177 CSF samples, 113 CJD, and 64 non‐prion disease patients were tested blindly. Final diagnosis of the prion disease patients revealed 36 definite, 68 probable, three genetic CJD (including one E200K, and two V210I) cases, and one P102L Gerstmann‐Straussler‐Scheinker (GSS) syndrome case. Table S1 shows the demographic and clinical information for each prion disease patient, as well as 14‐3‐3 and tau measures when available. Direct comparative analysis of each sample using PQ‐ and IQ‐CSF RT‐QuIC conditions was done within the same experiment, using the same solutions and materials. Non‐prion disease control patients included 21 males and 43 females with an average age of 65 ± 18 years (mean ± SD). Eighteen patients had diagnoses of non‐neurological disease and 46 patients had non‐prion neurological diseases including: amyotrophic lateral sclerosis (n = 3), Alzheimer's disease (n = 3), chronic inflammatory demyelinating polyneuropathy (n = 1), cognitive decline (n = 13), dystonia (n = 1), frontotemporal dementia (n = 9), lewy body dementia (n = 1), mild cognitive impairment (n = 4), multiple system atrophy (n = 1), Parkinsonism (n = 1), and rapidly progressive dementia (n = 9).
Figure 1A shows the comparison of the average RT‐QuIC amplification kinetics for CJD and non‐prion disease CSF using PQ‐ or IQ‐CSF assays. Using PQ‐CSF and our previously established criteria for signal thresholds and time cutoffs6, 19, a majority of samples scored positive after 47 h and full analysis typically required at least 90 h (Fig. 1A and B). ThT fluorescence amplitudes averaged 26% of our maximum reading capacity with only 16% of the samples registering above 50% (Fig. 1C). The same samples tested on the same day by IQ‐CSF RT‐QuIC showed significantly stronger fluorescence readings (Fig. 1A and Table 1) and faster amplification kinetics (Fig 1B, P < 0.0001, Mann–Whitney test) with the majority of positives being detected within 10 h and assay concluded within 24 h (Fig. 1A and B). IQ‐CSF conditions averaged 85% of our maximum reading capacity with 84% of the readings being greater than 50% and 68% of all samples reading 100% (Fig. 1A and C). The median percent maximum ThT readings for PQ and IQ‐CSF conditions were 22 and 100%, respectively.
Figure 1.
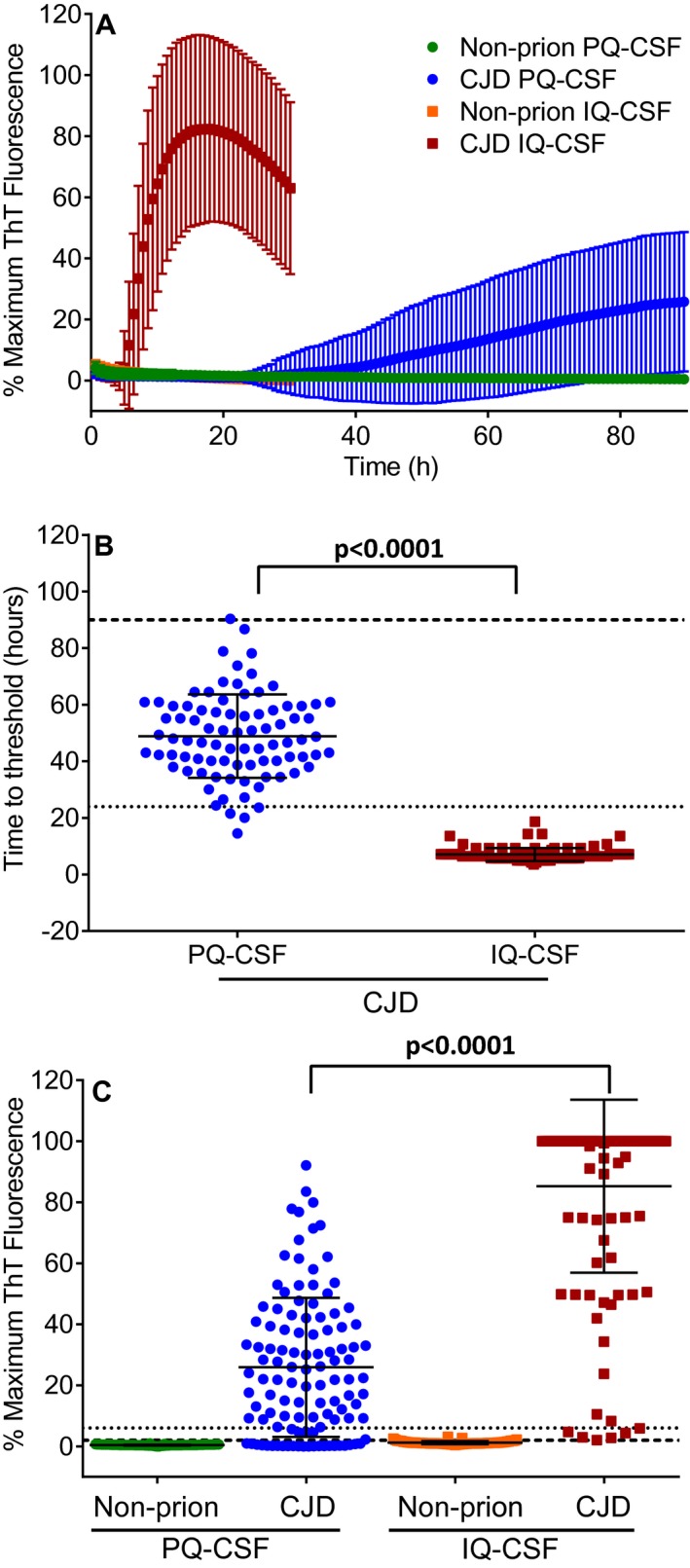
Faster and more sensitive detection of prion seeding activity in CSF from CJD patients by IQ‐CSF testing. (A) Mean ThT fluorescence of RT‐QuIC reactions seeded with CSF from non‐prion disease controls using PQ‐CSF (green) and IQ‐CSF (orange) or from CJD patients using PQ‐CSF (blue) and IQ‐CSF (red) are shown. Traces denote the mean ± SD of all biological replicates (n = 113 CJD and n = 64 non‐prion disease) from each category as indicated by the legend. Each biological replicate was in turn an average from four technical replicate wells. (B) Comparison of the time for each CJD sample to cross the designated positivity threshold for PQ‐CSF (blue circles, n = 82) and IQ‐CSF (red squares, n = 106). Samples that did not cross the threshold were excluded from this analysis and are not plotted. Each point is displayed as the mean of four technical replicates. Mean ± SD of the groups are displayed as the solid horizontal line and the vertical error bars, respectively. The large dashed line is the 90‐h time cutoff used for PQ‐CSF and the small dotted line is the 24‐h time cutoff used for IQ‐CSF. (C) End point values for PQ‐CSF or 24‐h maximum ThT fluorescence values for IQ‐CSF tests for each biological replicate displayed as the mean of four technical replicates. The average fluorescence value from all 113 CJD patients tested blinded using PQ‐CSF (blue circles) or IQ‐CSF (red squares) and 64 non‐prion disease patients tested blinded using PQ‐CSF (green circles) or IQ‐CSF (orange squares) conditions is shown for each individual sample with mean (horizontal line) and SD (vertical lines). Long and short dashed lines indicate the ThT fluorescence positivity thresholds calculated to be ~2 and ~6% for PQ‐CSF and IQ‐CSF, respectively [mean value at the time of assessment from all negative control samples plus 10 standard deviations6, 19]. P values were calculated using the Mann–Whitney test.
Table 1.
RT‐QuIC percent maximum ThT fluorescence following PQ‐ or IQ‐CSF analysis for 113 CJD CSF samples
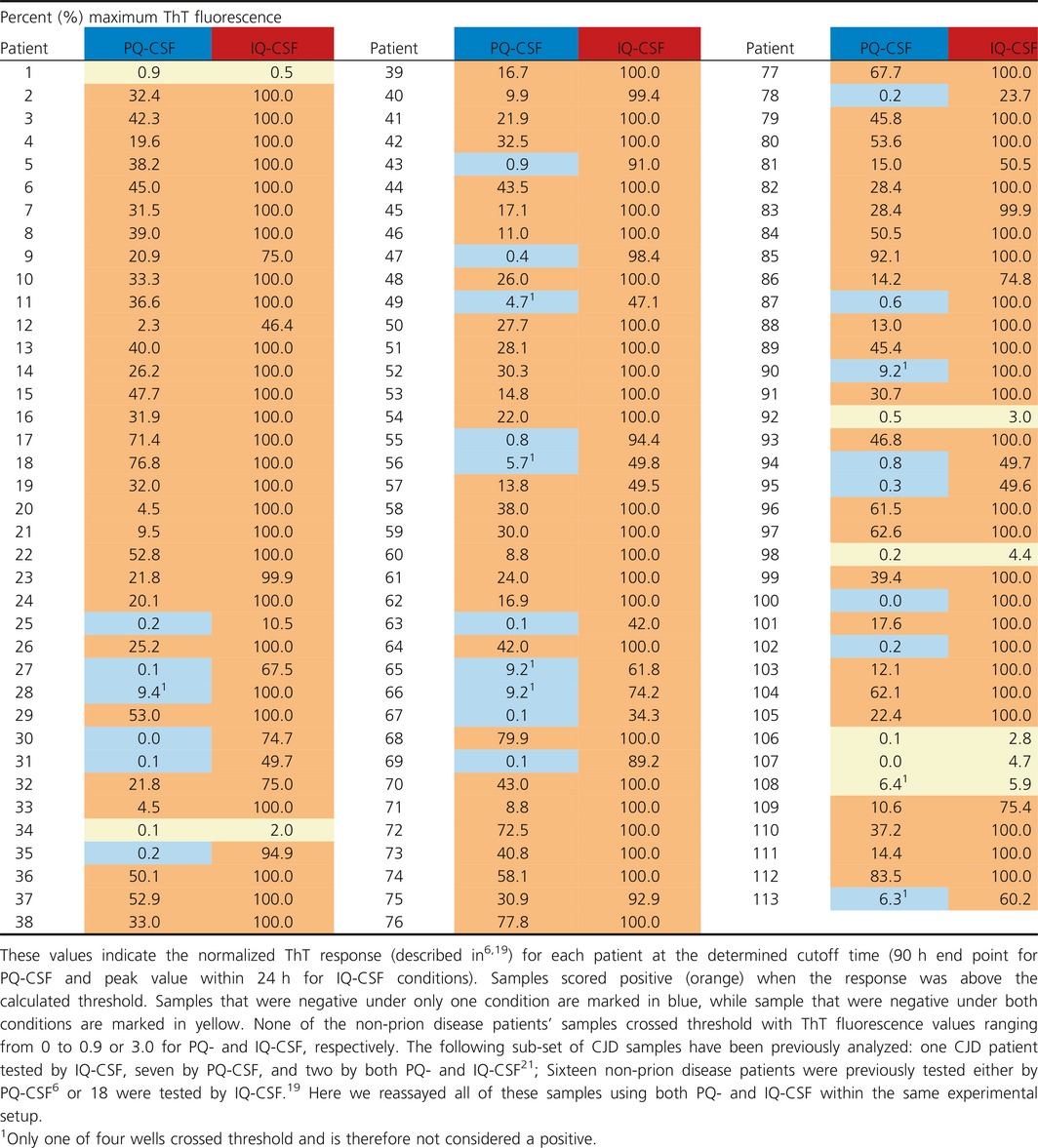
We calculated our ThT fluorescence threshold for a reaction to be considered positive based on the mean ThT value of all negative control samples at 90 h for PQ‐CSF, or 24 h for IQ‐CSF, plus 10 standard deviations6, 19, 20. This generated thresholds of ~2 and ~6% of saturating fluorescence for PQ‐CSF and IQ‐CSF, respectively. In addition, as has been common in previous studies6, 19, because of the rare occurrence of single wells exceeding the threshold in non‐prion disease controls within the cutoff time of the reaction, at least two of four replicate reactions had to each exceed the threshold for a sample to be considered overall positive. Since these cutoffs are based on the signal to noise ratio of the negative control samples, careful consideration was given to the designated cutoff value in order to maximize sensitivity without compromising specificity. Although the specificity for both protocols was 100% (CI: 93–100%), under PQ‐CSF conditions only 82 out of the 113 CJD samples tested positive (Table 1), leading to a 73% (95% CI: 63–80%) sensitivity. The same sample set analyzed by IQ‐CSF resulted in 106 positive specimens out of 113 tested, giving a 94% (CI: 87–97%) sensitivity. Sensitivities by IQ‐CSF for probable and definite CJD diagnoses were 97% and 92%, respectively. Furthermore, MM, MV, and VV polymorphisms at codon 129 had sensitivities by IQ‐CSF of 98, 96, and 90%, respectively. None of the non‐prion disease patients’ samples showed fluorescence that exceeded the designated threshold. Maximum ThT fluorescence values for non‐prion disease patients were under 0.9% and 3.0% of saturation for PQ‐ and IQ‐CSF, respectively. PQ‐CSF conditions produced an average maximum ThT fluorescence of 0.4 ± 0.2% (mean ± SD) with a median of 0.4% while IQ‐CSF conditions produced an average of 1.2 ± 0.5% (mean ± SD) with a median of 1.0%.
A comparison (Fig. 1B) of the time to threshold (hours) of the 82 positive PQ‐CSF versus the 106 positive IQ‐CSF samples further illustrates how much faster IQ‐CSF kinetics are compared to PQ‐CSF. Figure 1C shows the peak fluorescence values from the average of four replicate wells for each sample for PQ‐ and IQ‐CSF tests (Fig. 1C and Table 1). The baselines were subtracted from the data of the averaged individual samples and normalized to the saturating ThT fluorescence level of the plate reader (as described previously6, 19). These results illustrate that IQ‐CSF allowed significantly stronger discrimination between CJD‐positive and non‐prion disease control CSF samples. Although IQ‐CSF did not detect seeding activity in all of the 113 CJD patients (Table 1, see patients 1, 34, 92, 98, and 106–108), it was positive for all of those who were positive by PQ‐CSF as well as 24 additional patients who were negative by PQ‐CSF (Table 1, blue shading). When the sample codes were broken, we discovered that duplicate aliquots from five patients had been tested within the panels as replication controls. The duplicate aliquots from the same patients were consistently positive with IQ‐CSF but were not as consistent in the PQ‐CSF analyses (Table 1, patients: 62 and 90, 63 and 64, 66 and 70, 68 and 91, and 71 and 86). Additionally, all of the seven CJD patients who were negative by IQ‐CSF were also negative by PQ‐CSF (Table 1, yellow shading), suggesting that prion seeding activity in these samples was below the detection limit of both assays.
Overall, this faster and more sensitive IQ‐CSF assay provided a ~21% increase in diagnostic sensitivity and a ~42‐h reduction in average detection time for positive samples, while maintaining 100% diagnostic specificity. A much larger sample size tested across multiple laboratories will be required to fully validate these new IQ‐CSF conditions; however, our direct comparison confirms that IQ‐CSF allows for considerably faster and more sensitive detection of PrPCJD seeding activity in CSF samples from CJD patients. Therefore, with many diagnostic laboratories around the world already implementing PQ‐like RT‐QuIC analysis of CSF, we expect that the diagnostic criteria for sCJD will soon be revised to include direct detection of PrPCJD in CSF, or olfactory mucosal samples6, by assays such as RT‐QuIC. In clinical practice, IQ‐CSF testing, more so than PQ‐CSF testing, should have the ability to confirm CJD diagnoses or rapidly divert diagnoses to potentially treatable conditions, providing better outcomes for patients.
Conflict of Interest
None to declare.
Supporting information
Table S1. Patient Demographic information. Yellow shading indicates patients who tested negative by both PQ‐ and IQ‐CSF conditions.
Acknowledgments
This work was supported by the Intramural Research Program of the NIAID and in part by the Alliance Biosecure Foundation (FABS201502). The study was approved by the ethics committee at Istituto Superiore di Sanità (Italy), which is recognized by the Office for Human Research Protections of the U.S. Department of Health and Human Services. All the sampling of CSF was performed after informed consent was obtained from each patient or the patient's representative and informed consent for participation in research was obtained in accordance with the Declaration of Helsinki and the Additional Protocol to the Convention on Human Rights and Biomedicine, concerning Biomedical Research. The analyses of human specimens that were performed at the NIAID were performed under exemption numbers 11517 and 13253 for the use of encoded samples from the NIH Office of Human Subjects Research Protections. We thank Suzette Priola, Kentaro Masujin, and Eri Saijo for critical evaluation of the manuscript and Anita Mora for graphics assistance. We are thankful to Dr. Michael Coulthart for providing us with non‐prion disease cerebrospinal fluid samples.
Contributor Information
Bradley R. Groveman, Email: Bradley.groveman@nih.gov
Christina D. Orrú, Email: christina.orru@nih.gov
References
- 1. Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt‐Jakob disease. Brain 2009. ;132(Pt 10):2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown P, Brandel JP, Sato T, et al. Iatrogenic Creutzfeldt‐Jakob disease, final assessment. Emerg Infect Dis 2012;18:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colby DW, Zhang Q, Wang S, et al. Prion detection by an amyloid seeding assay. Proc Natl Acad Sci USA 2007;104:20914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilham JM, Orrú CD, Bessen RA, et al. Rapid end‐point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog 2010;2010(6):e1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H, Shirabe S, Yamada M, Mizusawa H, Kitamoto T, Klug G, McGlade A, Collins SJ, Nishida N. Ultrasensitive human prion detection in cerebrospinal fluid by real‐time quaking‐induced conversion. Narure Medicine 2011;2011(17):175–178. [DOI] [PubMed] [Google Scholar]
- 6. Orru CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt‐Jakob disease using nasal brushings. New Engl J Med 2014;2014(371):519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Orru CD, Favole A, Corona C, et al. Detection and discrimination of classical and atypical L‐type bovine spongiform encephalopathy by real‐time quaking‐induced conversion. J Clin Microbiol 2015;53:1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orru CD, Groveman BR, Raymond LD, et al. Bank vole prion protein as an apparently universal substrate for rt‐quic‐based detection and discrimination of prion strains. PLoS Pathog 2015;11:e1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orru CD, Hughson AG, Groveman BR, et al. Factors that improve rt‐quic detection of prion seeding activity. Viruses 2016;8:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zanusso G, Monaco S, Pocchiari M, Caughey B. Advanced tests for early and accurate diagnosis of Creutzfeldt‐Jakob disease. Nat Rev Neurol 2016;12:325–333. [DOI] [PubMed] [Google Scholar]
- 11. Henderson DM, Manca M, Haley NJ, et al. Rapid antemortem detection of CWD prions in deer saliva. PLoS ONE 2013;2013(8):e74377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haley NJ, Siepker C, Hoon‐Hanks LL, et al. Seeded amplification of chronic wasting disease prions in nasal brushings and recto‐anal mucosa associated lymphoid tissues from elk by real time quaking‐induced conversion. J Clin Microbiol 2016;54(4):1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sano K, Satoh K, Atarashi R, et al. Early detection of abnormal prion protein in genetic human prion diseases now possible using real‐time QUIC assay. PLoS ONE 2013;2013(8):e54915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masujin K, Orru CD, Miyazawa K, et al. Detection of atypical h‐type bovine spongiform encephalopathy and discrimination of bovine prion strains by real‐time quaking‐induced conversion. J Clin Microbiol 2016;54:676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGuire LI, Peden AH, Orru CD, et al. RT‐QuIC analysis of cerebrospinal fluid in sporadic Creutzfeldt‐Jakob disease. Ann Neurol 2012;2012(72):278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cramm M, Schmitz M, Karch A, et al. Stability and reproducibility underscore utility of rt‐quic for diagnosis of creutzfeldt‐jakob disease. Mol Neurobiol 2015;53(3):1896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cramm M, Schmitz M, Karch A, et al. Characteristic CSF prion seeding efficiency in humans with prion diseases. Mol Neurobiol 2015;51:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGuire LI, Poleggi A, Poggiolini I, et al. Cerebrospinal fluid real‐time quaking‐induced conversion is a robust and reliable test for sporadic creutzfeldt‐jakob disease: An international study. Ann Neurol 2016;80:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Orru CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT‐QuIC detection of human Creutzfeldt‐Jakob disease using cerebrospinal fluid. MBio. 2015;6(1):e02451–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denkers ND, Henderson DM, Mathiason CK, Hoover EA. Enhanced prion detection in biological samples by magnetic particle extraction and real‐time quaking‐induced conversion. J Gen Virol 2016;97:2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bongianni M, Orrù CD, Groveman BR, et al. Diagnosis of human prion disease using real‐time quaking‐induced conversion testing of olfactory mucosa and cerebrospinal fluid samples. JAMA Neurol 2017;74:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Demographic information. Yellow shading indicates patients who tested negative by both PQ‐ and IQ‐CSF conditions.


