Abstract
Objective
Recognition of potential for neurological recovery in patients who remain comatose after cardiac arrest is challenging and strains clinical decision making. Here, we utilize an approach that is based on physiological principles underlying recovery of consciousness and show correlation with clinical recovery after acute anoxic brain injury.
Methods
A cohort study of 54 patients admitted to an Intensive Care Unit after cardiac arrest who underwent standardized bedside behavioral testing (Coma Recovery Scale – Revised [CRS‐R]) during EEG monitoring. Blinded to all clinical variables, artifact‐free EEG segments were selected around maximally aroused states and analyzed using a multi‐taper method to assess frequency spectral content. EEG spectral features were assessed based on pre‐defined categories that are linked to anterior forebrain corticothalamic integrity. Clinical outcomes were determined at the time of hospital discharge, using Cerebral Performance Categories (CPC).
Results
Ten patients with ongoing seizures, myogenic artifacts or technical limitations obscuring recognition of underlying cortical dynamic activity were excluded from primary analysis. Of the 44 remaining patients with distinct EEG spectral features, 39 (88%) fit into our predefined categories. In these patients, spectral features corresponding to higher levels of anterior forebrain corticothalamic integrity correlated with higher levels of consciousness and favorable clinical outcome at the time of hospital discharge (P = 0.014).
Interpretation
Predicted transitions of neocortical dynamics that indicate functional integrity of anterior forebrain corticothalamic circuitry correlate with clinical outcomes in postcardiac‐arrest patients. Our results support a new biologically driven approach toward better understanding of neurological recovery after cardiac arrest.
Keywords: Cardiac arrest, coma, consciousness, corticothalamic integrity, EEG
Introduction
Approximately 420,000 people experience sudden cardiac arrest in the United States annually.1 Even though 80% of patients die before receiving hospital care; survival and functional outcomes have significantly improved because of advancements in emergency and acute medical care.2 However, withdrawal of care decisions continue to drive mortality rates after cardiac arrest, and accurate prognostication of neurological outcomes is evolving.3, 4
An important factor in clinical decision making in patients who do not readily wake up after cardiac arrest is time to recovery of consciousness.5 In fact, these patients have worse outcomes; up to 79% of patients who remain comatose after cardiac arrest have unfavorable long‐term neurological outcomes, including severe disability.6 An additional concern is the emerging evidence that some patients show signs of preserved consciousness even in the absence of purposeful motor responses detectable at the bedside.7, 8, 9, 10, 11 These considerations suggest that starting evaluation of brain networks that are known to support consciousness very early in the clinical course – even before consciousness is evident at the bedside – is important in order to better understand the recovery process and the potential for favorable neurological outcome in postcardiac arrest patients.
However, little is known about the specific cellular or network‐level mechanisms that are crucial to recovery of consciousness in post cardiac‐arrest patients. As such, tracking integrity of these brain circuits independently from other brain areas that are sensitive to anoxic or hypoxic conditions (such as motor areas, sensory areas, etc.) is an important aspect of understanding the recovery from anoxic brain injury.10
In this study, we aim to investigate neurological mechanisms of acute recovery of consciousness after cardiac arrest. As an initial approach, we apply a model that is based on physiological principles relevant to the recovery of consciousness in chronic severe brain injuries. Specifically, we utilize four pre‐defined EEG spectral patterns that represent increasing functional preservation of anterior forebrain corticothalamic circuit integrity.12, 13 We label these dynamic regimes as “A”, “B”, “C”, and “D” (and collectively refer to them as the ‘ABCD’ model); where “A” indicates complete loss of corticothalamic integrity; “D” indicates full recovery of corticothalamic integrity; while “B” and “C” represent interim regimes with distinct physiological foundations. We hypothesize that the emergence of these dynamic patterns seen in patients recovering after chronic severe brain injuries will also accurately index recovery of consciousness in the acute phases of severe anoxic brain injury, and as such, they will correlate with clinical outcome.
The approach taken here represents only the initial stages of possible quantitative analyses that can be aimed at further assessing anterior forebrain corticothalamic integrity in severe acute brain injuries. Future studies using additional imaging, neurophysiological or invasive measures will be needed to further elucidate the applicability our model in the context of recovery from acute, severe anoxic brain injury.
Methods
Study population
Fifty‐four patients (32 male, 22 female) who suffered cardiac arrest were enrolled as part of a prospective observational study assessing recovery of consciousness after cardiac arrest. All consecutive patients admitted with cardiac arrest to Columbia University Medical Center's (CUMC) Medical, Neurological, Cardiac, Cardiothoracic and Surgical Intensive Care Units (ICUs) between April 2013 and July 2014 were approached. The study was approved by the CUMC Institutional Review Board (IRB). Standard clinical care of post‐cardiac arrest patients at CUMC includes targeted temperature management protocol (hypothermia with target temperature of 33°C) and aggressive shivering control. None of the patients received stimulants during the observation period nor were placed on prophylactic anti‐epileptic medications. However, patients were treated for all neurological or medical conditions per standard of care as dictated by the clinical team. Patients were included irrespective of their age, sex, initial cardiac rhythm, location of the arrest, and whether or not they underwent therapeutic hypothermia protocol.
Theoretical foundation and underlying model
Our general theoretical framework emphasizes the fundamental role of circuit mechanisms in the recovery of consciousness after severe brain injuries. Two major circuits have been identified to show graded relationships with outcomes following coma, the anterior forebrain mesocircuit14, 15, 16 and the frontoparietal network17, 18, 19, 20, 21 (Fig. 1). The central thalamus is the key hub linking both networks.19, 22, 23 Excitatory output activity patterns of the central thalamus are predicted to influence neocortical dynamics that can be characterized based on identification of spectral features of the EEG in terms of frequency content, spatial distribution, and correlation structure.13, 24, 25 A model (the ‘ABCD’ model)12, 13 built on these predictions, in full form, defines specific regimes of expected EEG activity that shift with transitions of increasing functional reafferentation of the corticothalamic system and associated restoration of average neocortical neuronal membrane potentials.13, 24, 25 Furthermore, a coarse‐grained sequence of expected changes in spectral shape based on this model sets a simple framework for interpretation in the setting of structural brain injury (Fig. 2). The model postulates that the “A”‐type spectrum (delta [<4 Hz] frequencies only) represents complete functional corticothalamic deafferentation. In experimental studies of withdrawing all afferent input to the cortex from the thalamus or surrounding cortical areas EEG patterns demonstrate this spectral shape.26 The “B”‐type spectrum is characterized by theta (5–7 Hz) activity and in in vitro models is seen when a low level of afferent input to neocortical neurons result in spontaneous oscillations of Layer V pyramidal cells in this frequency range.27 EEG obtained from patients with severe structural brain injuries of varying etiologies demonstrate spectral features consistent with the underlying model for this proposed “B”‐type mechanism.25 A full evaluation of the consistency of this underlying physiological mechanism requires measurement of coherence patterns which also show a sharp peak of coherence only at the ~7 Hz proposed to reflect a broad diffusive coupling of the intrinsic oscillations.25 The “C”‐type spectrum (co‐localized theta and beta [~15–40 Hz]) arises in more fully reafferented corticothalamic systems. “C”‐type spectra are expected when a deafferented thalamus fires in burst mode and the afferent volley of synaptic activity is received by relatively intact neocortical regions (a pattern called ‘thalamo‐cortical dysrhythmia’).28 Full evaluation of “C” type dynamics requires assessment of the global EEG dynamics and isolation of the specific contribution and spatial localization of generators with linked theta and beta spectral components.13 The “D”‐type spectrum (alpha [8–12 Hz] and beta) represents normal tonic firing of the thalamus and normal resting cortical oscillations. A complete, multi‐channel quantified analysis was not possible with the EEG quality obtained in the ICU in this sample, therefore the ‘ABCD’ evaluations in this current analysis are restricted to assessment of the spectral shape of EEG activity over centroparietal electrodes. However, these limited EEG channels provide a reasonable initial assessment of the predicted dynamics under the ‘ABCD’ model because they are typically relativity free of muscle artifact and directly reflect activity over the posterior medial complex, a key element of the frontoparietal network.21
Figure 1.
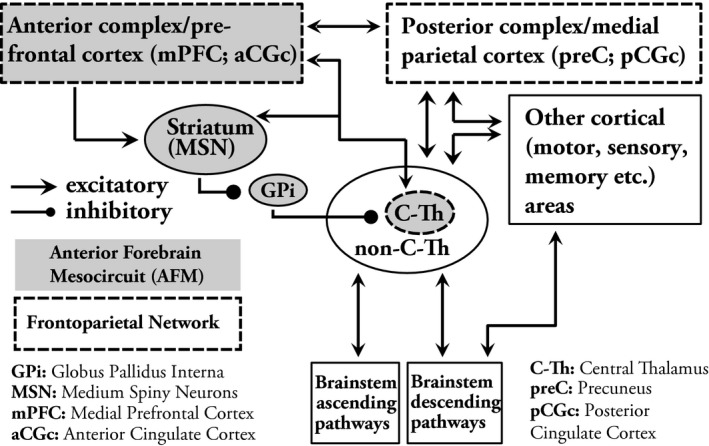
Conceptual framework of brain networks related to consciousness and their relation to other brain areas. The central thalamus (C‐Th) serves as the central hub for networks important in maintaining consciousness. According to the anterior forebrain mesocircuit (AFM) model, activity of the central thalamus is modulated by elements of basal ganglia (primarily inhibition from the globus pallidus interna (GPi), which is – in return ‐ inhibited by the medium spiny neurons (MSN) of the striatum), that are also influenced by alterations in afferent input from various cortical and subcortical areas. The central thalamus is also the main relay between and anterior and posterior complex of the frontoparietal network. The central thalamus has widespread projections to anterior forebrain, including the frontal cortex and striatum as well as to the posterior medial parietal areas. (Elements of the AFM are shaded with gray. Elements of the frontoparietal network are bracketed with dashed lines).
Figure 2.
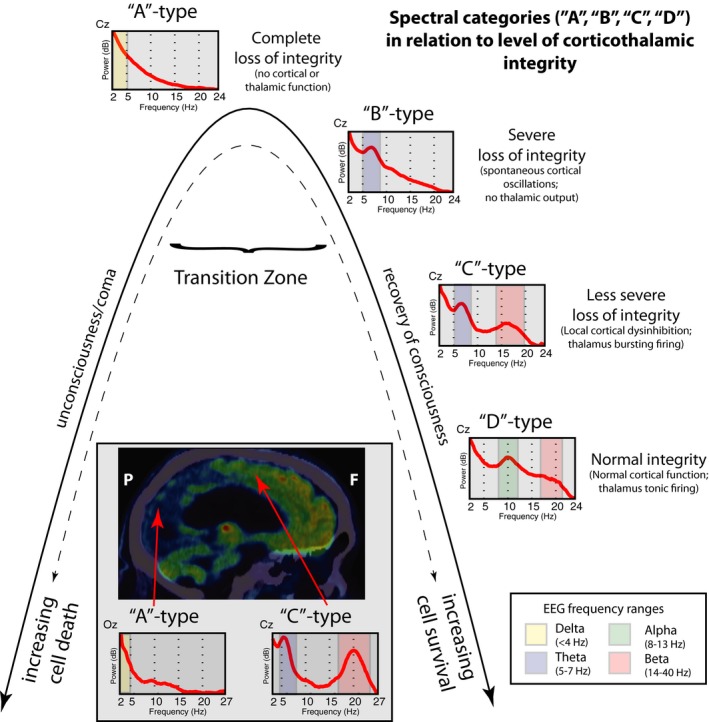
Conceptual framework of ‘ABCD’ spectral categories in relation to corticothalamic integrity. The “A”‐type spectrum (delta [<4 Hz] activity only) reflect global and profound downregulation of activity across the corticothalamic system and can be seen in severe structural injury or as the consequence of very deep anesthesia.45 In the context of global dysfunction after cardiac arrest here, the A‐type spectrum is ambiguous. It may represent the complete loss of corticothalamic integrity with no surviving inputs to the cortex from the thalamus or surrounding cortical areas26, or a functional loss that may be persist for some period of time when it is not possible to determine the potential of cellular survival based on assessments of neuronal activity (representing a “transition zone”).46 Based on currently unknown variables3, either overwhelming cell death occurs that leads to irreversible structural deafferentation (left side of the inverted U shape) or recovery of cellular or network functions may arise with increasing evidence for preserved corticothalamic integrity (right side of the inverted U shape). The “B”‐type spectrum, characterized by a single theta (5‐7 Hz) feature is proposed to occur when a low level of afferent input to neocortical neurons gives rise to spontaneous oscillations of Layer V pyramidal cells in this frequency range27, indicating an initial stage of recovering corticothalamic integrity.13, 25 The “C”‐type spectrum is expected to arise in the setting of incomplete recovery of corticothalamic integrity, specifically over cortical regions receiving input from deafferented sectors of the thalamus that exhibit burst mode firing and produce co‐localized theta and beta (~15–40 Hz) oscillations over its cortical projections zones.28 The “D”‐type spectrum is linked to normal, tonic firing mode of the thalamus, which gives rise to a co‐localized alpha (~8–12 Hz) and beta range over the assessed electrodes. The figure insert illustrates an example of co‐existence of “A” and “C” spectral shapes arising in a single subject 2.5 years following a cardiac arrest and therapeutic hypothermia (adopted and modified from Forgacs et al., 201610). In this patient, near‐total neuronal loss is suggested over the posterior aspect of the brain (including the occipital cortex, primary motor cortex and parts of the parietal cortex) as demonstrated on the18FDG‐PET image. Corresponding EEG recordings over ‘Oz’ electrode show an “A”‐type spectrum suggesting complete loss of corticothalamic integrity over the posterior areas. In contrast, relative structural and functional preservation of the anterior forebrain (including the frontal cortex and bilateral thalami) is evident on18FDG‐PET and also suggested by the presence of a “C”‐type spectrum over electrode ‘Cz’. Such sharp divergence between preservation of the anterior brain structures and thalamus with apparent loss of primary visual and sensory‐motor areas may represent a threshold phenomenon of cellular survival.10 This phenomenon may be a consequence of variable rescue of cells that may remain in the transition zone in the acute phases of injury with additional effect of therapeutic hypothermia that may also contributed to development of such striking dissociation in this patient.
Blinding of investigator performing EEG analyses
All EEG analyses and ‘ABCD’ spectral categorization were done by members of the investigational team who were blinded to all clinical variables. This included clinical details of the cardiac arrest, concurrent medications, clinical examination, behavioral level of consciousness at the time of the EEG recordings analyzed, and clinical outcome scores. This investigator did not have any direct patient contact and did not participate in collection of the data. A second, blinded ‘ABCD’ spectral categorization was also performed for verification purposes by an investigator who was not involved in the first analysis and had no prior experienced in ‘ABCD’ categorization or in visual interpretation of EEG.
EEG procedures and analysis
All patients underwent continuous EEG monitoring after cardiac arrest for at least 24 h after completion of therapeutic hypothermia protocol. Patients with concerning clinical EEG findings may have required longer monitoring at the discretion of the clinical team. Collodion‐pasted electrodes and standard clinical video‐EEG equipment were used for all recordings.29 For each patient on each day when EEG recordings were available, EEG segments were cut around times of maximally aroused state, e.g., around the time of standardized bedside examination. After visual assessment of artifacts (e.g., eye blinks, major movement artifacts or possible artifacts from other equipments present in the ICU), an average of 40–50 (range ~10–100), 10‐second long artifact‐free EEG segments were selected for further quantitative analysis. Power spectra was calculated for each channel using Laplacian montage and Thomson's multi‐taper method30 with five tapers and plotted between 2 and 24 Hz. Spectral features (i.e., shape of the spectra) were visually inspected and assigned to each of the pre‐defined categories according to the ‘ABCD’ model. All available channels were inspected, however, given the electrically noisy environment in the ICU frequently resulting in abundance of artifacts in frontal and temporal channels, midline and parasaggital centro‐parietal leads (Cz, C4, C3, Pz, P3, P4) were typically the most useful for determination of the spectral categories. Representative examples of each of the 4 categories of the ‘ABCD’ model are shown in Figure 3. Importantly, reproducibility of the ‘ABCD’ spectral categorization was verified by an independent visual inspection of a representative centroparietal channel by an investigator blinded to the results of the first categorization (see above). This recategorization demonstrated 89% concordance with the first analysis and did not affect the statistically significant differences in the results presented here.
Figure 3.
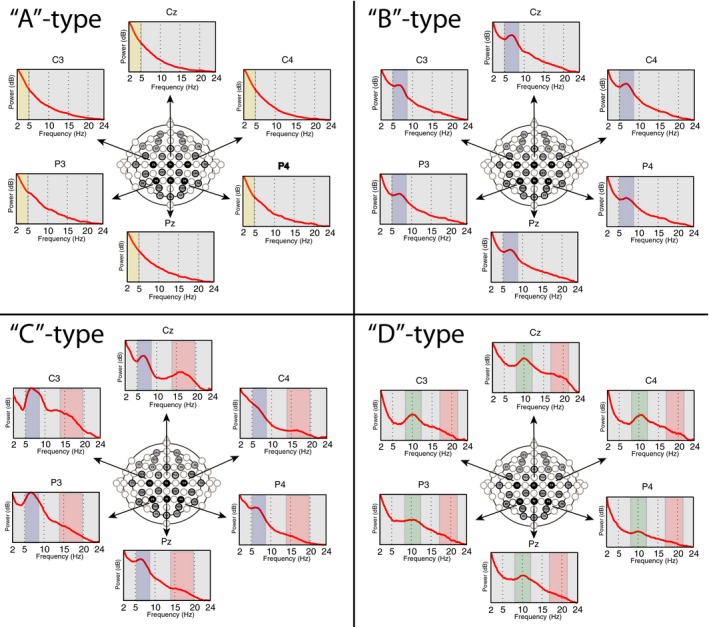
The ‘ABCD’ spectral categories. Representative examples of the EEG spectra of each of the pre‐defined (“A”,”B”,”C”,”D”) categories over the centro‐parietal areas in four patients enrolled into our study. (Colors represent same frequency features as in Fig. 2).
Analysis of behavioral level of consciousness
We used the Coma Recovery Scale – Revised (CRS‐R)31 to determine the level of consciousness of each patient. The CRS‐R ranges between 0 and 23 and composed of six subscales that address auditory, visual, motor, oromotor, communication, and arousal functions. Diagnostic categories within disorders of consciousness include coma, a state of unarousable unresponsiveness; vegetative state (VS), defined by periods of eye opening without recovery of awareness of the environment and minimally conscious state (MCS) which is characterized by the presence of reproducible, but inconsistent goal‐directed behaviors without functional communication channels. Patients emerging from minimally conscious state (EMCS) exhibit reliable and consistent demonstration of interactive communication or functional use of objects. To achieve the maximally aroused state possible, sedative medications were held prior to performing the CRS‐R examination, unless there were clinical contraindications. In addition, CRS‐R scores were typically obtained each day around noon to control for possible diurnal fluctuations of behavioral states.
Determination of clinical outcome
To determine clinical outcome, we recorded the Cerebral Performance Categories (CPC) scale32 at the time of hospital discharge. CPC is a widely used, 5‐point scale: 1 indicates good cerebral performance; 2 indicates moderate cerebral disability with independent activities of daily life; 3 indicates severe cerebral disability requiring daily support but preserved consciousness; 4 indicates coma or vegetative state, and 5 indicates brain death or death from other causes.
Statistical methods
Differences in clinical variables between sub‐cohorts of patients were determined using two‐tailed T‐test for continuous variables and Wilcoxon rank sum test for categorical variables. We used Chi square test to demonstrate statistical difference between EEG spectral categories and clinical outcome.
Results
Baseline characteristics of the patient cohort
The cohort included 54 postcardiac arrest patients; slightly more males (59%) than females (41%) with mean age of 60 years (range: 21–87, SD: 18). Initial cardiac rhythm was V‐fib/V‐tach in 14 patients, asystole in 10 patients, pulseless electrical activity in 22 patients; this information was not available for 8 patients. 44 patients underwent therapeutic hypothermia (for 1 patient this information was not available). The mean time to return of spontaneous circulation (ROSC) was 16 minutes (range: 1–90, SD: 15.1; data were available for 48 patients).
All patients enrolled had at least one CRS‐R examination during the observation period; however almost half of the patients (n = 25) had more than one CRS‐R scores obtained. Of these longitudinal patients, 12 had an examination on 2 days, 9 had on 3 days, and 4 had on 4 or more days. Of note, 2 patients did not have EEG recordings available for analysis on the same day when the CRS‐R examination was obtained.
Behavioral diagnoses based on the best observed CRS‐R on any day were as follows: 34 patients in coma or VS, 11 patients in MCS, and 9 patients in EMCS. Of the 25 longitudinal patients with multiple CRS‐R scores, 7 had improvement, 1 had worsening, and 17 had no change or fluctuations in the behavioral level.
Clinical outcomes (CPC) at the time of hospital discharge included CPC 1 in 2 patients, CPC 2 in 8 patients, CPC 3 in 5 patients, CPC 4 in 6 patients, and CPC 5 in 30 patients, while 3 subjects had unknown CPC scores. In 83.3% of the patients who deceased (25 of 30), death followed a withdrawal of care decision.
‘ABCD’ spectral categorization
Of the 44 patients with distinct EEG features in the cohort, 39 (88%) fit into our predefined categories (‘ABCD’ model) during at least one of the days of EEG recording. The distribution of the best available spectral categories among patients that fit into the ‘ABCD’ categories were the following: 10 patients had spectral features consistent with “A” type only, 16 patients had “B” type, 11 patients had “C” type and 2 patients had “D” type spectral features. Of the other 5 patients who had distinct spectral features that did not fit to the ‘ABCD’ categories, 4 patients had alpha frequency feature only and 1 patient who a beta frequency feature only. Of note, patients with ongoing seizure activity during all available EEG segments (n = 4) and those with overwhelming muscle artifact (n = 3) or technical difficulties limiting EEG analysis (n = 3) were identified and removed from the primary analysis (a total of 10 patients).
Relationship of ‘ABCD’ spectral categories and concurrent behavioral diagnosis
Figure 4 shows the relationship between EEG spectral categories and concurrent (same day) bedside behavioral diagnoses based on CRS‐R. For this analysis, in patients with more than one CRS‐R score available, EEG data collected on the same day as the best available CRS‐R score were used. Importantly, in the two patients with normal spectral features (“D” type), behavioral examinations were consistent with recovery of consciousness (EMCS). In addition, 18 patients with complete or severe loss of corticothalamic integrity (“A” or “B” type spectra) were in VS/Coma. However, there were some discrepant results. For example, 4 patients with “C” type spectra (suggesting relatively preserved corticothalamic integrity) were in VS/Coma or MCS. Importantly, 2 of these 4 patients eventually had good outcome at the time of hospital discharge (CPC 1 or 2). Therefore in these 2 patients, corticothalamic integrity markers predicted good outcome earlier than recovery of consciousness was detectable at the bedside. The other 2 such patients included one with no CPC score available; and another who died after withdrawal‐of‐care decision was made.
Figure 4.
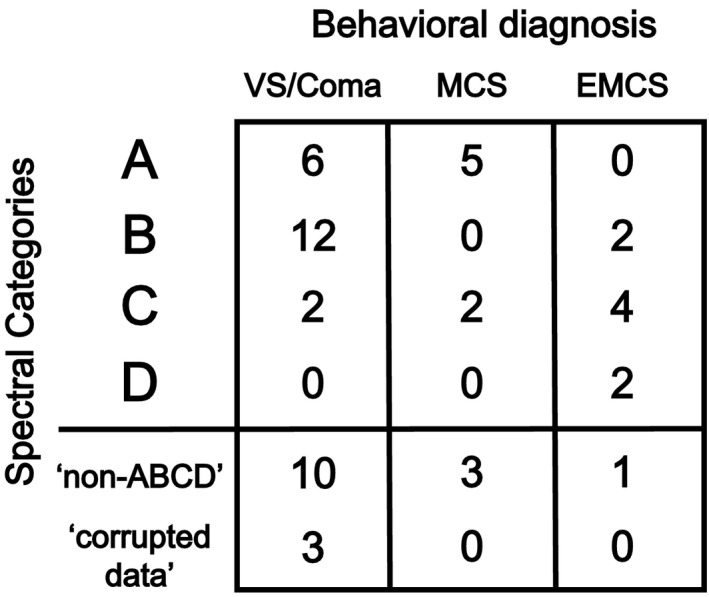
The ‘ABCD’ spectral categories in relation to behavioral diagnosis (n = 52; 2 patients had no CRS‐R and EEG collected on the same day). (CRS‐R, Coma Recovery Scale ‐Revised; VS, vegetative state, MCS, minimally conscious state; EMCS, emerged from minimally conscious state).
Relationship of ‘ABCD’ spectral categories after cardiac arrest and clinical outcome at hospital discharge
In the 36 patients who had EEG spectral features that fit into the ‘ABCD’ model and also had a CPC score available, we found that the level of corticothalamic integrity in the first days postcardiac arrest correlate with clinical outcome at the time of hospital discharge (P = 0.014) (Fig. 5).
Figure 5.
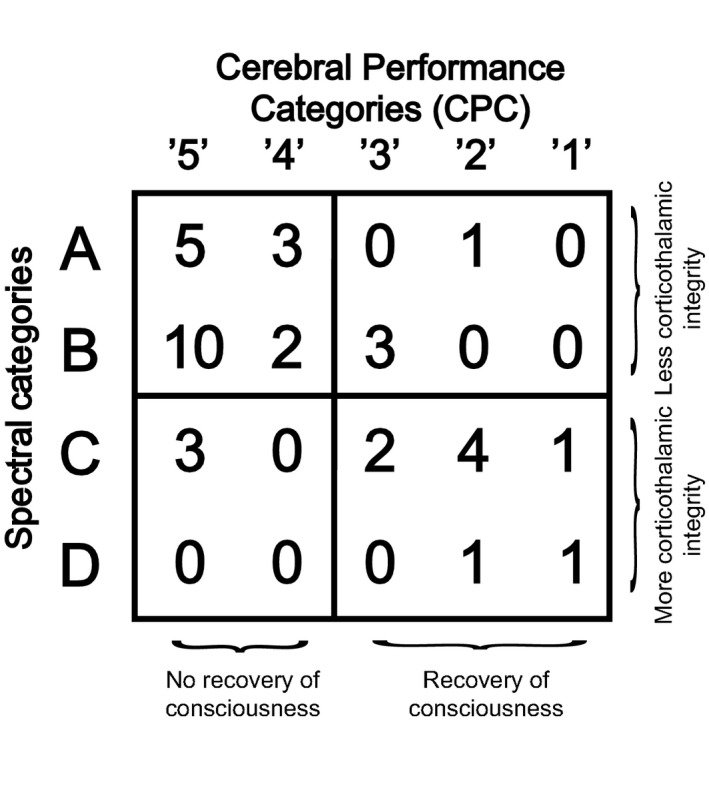
Contingency table showing ‘ABCD’ spectral categories in relation to clinical outcome (cerebral performance categories [CPC]) at hospital discharge. n = 36; P = 0.014 (Chi‐square test).
Importantly, we did not find significant differences in selected important clinical variables (age, ROSC, CRS‐R and CPC) between the ‘categorized’ subcohort used in this outcome analysis above and the rest of the patients in the cohort (the ‘non‐categorized’ subcohort) (Fig. 6). Of note, the differences in CRS‐R and CPC showed a tendency toward significance.
Figure 6.
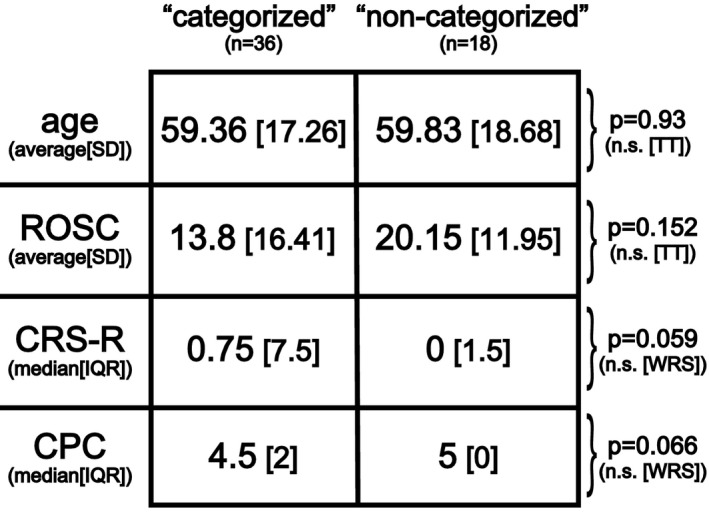
Comparison of patient sub‐cohorts based on baseline clinical variables. The ‘categorized’ subcohort included patients who had EEGs with spectral features that fit to at least one of the predefined ‘ABCD’ categories at any of the available days and had a CPC score available (n = 36). All other patients were included in the ‘non‐categorized’ sub‐cohort. There were no significant differences between the two sub‐cohorts based on these selected clinical variables. (ROSC, Return Of Spontaneous Circulation [in minutes]; CRS‐R, Coma Recovery Scale – Revised; CPC, Cerebral Performance Categories; SD, Standard Deviation; IQR, Inter Quartile Range; n.s., not significant; TT, 2‐tailed T‐test; WRS, Wilcoxon Rank Sum test).
Discussion
Here, we demonstrate that predefined EEG spectral categories proposed by the ‘ABCD’ model are applicable to recovery of consciousness in the context of acute severe anoxic brain injuries. Although the present analysis limited evaluation of the model to EEG dynamics within a small number of centroparietal channels, their spectral shape correlates with clinical outcome and accurately indicate concurrent levels of consciousness in patients after cardiac arrest.
We believe the approach taken here offers the first step toward developing new strategies that are motivated by physiological models to understand neurological recovery in the acute phases of anoxic brain injury. Our approach differs from current strategies that focus mainly on clinical features to assess prognosis after cardiac arrest, such as the neurological examination, somatosensory‐evoked potentials, and blood levels of neuron‐specific enolase, neuroimaging3, or visual analysis of the EEG33 early in the course of recovery. Many of these assessments correlate with the outcome; however, their accuracy may change with the advent of new therapies, as seen after the introduction of various targeted temperature management protocols.5, 34, 35 In contrast, the functional cellular and network‐level transitions captured in our ‘ABCD’ model set plausible but falsifiable predictions for changes across a range of relevant physiological variables present in anoxic brain injury that affects synaptic input to neocortical neurons. Overwhelming structural injuries or dysfunction at the cellular level as a result of hypoxia, e.g., temporary or permanent impairment in energy production (e.g. mitochondrial damage36) may limit recovery of neuronal firing as captured in “A”‐type spectra, even if the cells themselves may survive. Widespread but partial deafferentation across the corticothalamic system may produce the pathological neuronal activity patterns seen in “B”‐type spectra27, whereas some “C”‐type spectra may associate with only relatively focal injuries or functional alterations within the thalamus or overlying cortical lesions.28 These distinctions in underlying mechanism producing the ‘ABCD’ spectral features can be expected to link to variable recovery patterns after cardiac arrest. In some cases, further assessment of their regional variation may also reflect differences in neuronal cell type sensitivities to hypoxia, for example, the well‐known sensitivity of posterior parieto‐occipital regions (Fig. 2, insert). This type of pattern may also underlie a recently described isolation syndrome after severe anoxic brain injury.10
The ‘ABCD’ model utilized in this study derives from a combination of data obtained from both animal models and observations made of recovery patterns in patients with chronic disorders of consciousness resulting from severe brain injuries.25, 37, 38 Emerging models of brain networks that are important in maintaining consciousness emphasize the fundamental role of central thalamic activity and the functional integrity of its projections to cortical areas.13, 14, 18, 21, 22 Based on these models (Fig. 1), it is reasonable to expect specific spectral features related to corticothalamic activity over the midline frontal and centroparietal EEG channels, which are also tend to be less artifact corrupted in ICU recordings. However, it is also important to note that the ‘ABCD’ model in its full form is based on a strict assumption of increasing functional corticothalamic reafferentation starting with a fully disconnected corticothalamic unit (“A”‐type spectrum). This starting assumption places strong restrictions the possible interpretation of the observed peaks in the “B” or “C”‐type spectra as average neocortical membrane potentials increase.13 In addition, other models may also explain some of the spectral features seen in the single channel “C” and “D”‐type spectra39, however, the prediction that increasing corticothalamic integrity is associated with these alternative interpretations would preserve their association with level of recovery. In fact, other correlates of functional corticothalamic integrity, such as EEG organization during wakefulness and sleep9 and specific patterns of cerebral metabolism19, 40, 41 are also associated with the recovery of consciousness. In sum, while the presence of isolated spectral peaks at the observed frequencies does not alone provide strong evidence for the underlying physiological models, the strength and novelty of our approach is that it is based on a model that makes precise and testable predictions. Further studies using multi‐channel quantitative analyses or imaging methods can examine the specific predictions of each of the ‘ABCD’ spectral categories13 in the setting of acute severe anoxic brain injuries and validate or refute our results. In addition, studies that apply invasive brain monitoring techniques may provide the opportunity for more stringent tests of our model, including confirmation of the predicted relationships between intracellular membrane potentials of the neocortical neurons and the ‘ABCD’ classification.14 We believe, however, that the above considerations collectively provide not only a plausible conceptual framework for our approach and rational interpretation of our results, but an important proof‐of‐concept toward further investigation.
Notably, we also found different spectral regimes than our ‘ABCD’ categories in a small number of patients. In 4 patients, we observed an isolated alpha frequency feature that might be considered a variant of “B” type. In fact, an isolated alpha or theta feature (“alpha‐theta coma”) is a well‐known finding on EEG in the setting of severe anoxic brain injury and it is thought to emerge as a result of dysfunction of thalamocortical pathways.42, 43 In addition, coexistence of alpha‐theta patterns with burst suppression with frontocentral predominance in some instances44 representing intrinsic conditions of strongly hyperpolarized neocortical cells further support considering this feature as a variant of the “B”‐type spectra. Examination of the coherence structure of such isolated alpha frequency features would allow a further testing of their fulfilling “B” like dynamics.13, 24, 25 Additionally, a single beta feature seen in one patient may be a variant of the “C”‐type spectra. Ultimately, if further studies find similar or other intermediate spectral regimes consistently, it is possible that a more fine‐grained or different spectral classification will emerge to characterize the physiological states of the injured brain from anoxia.
There are several limitations of our study. First, this study involved EEG data that were limited by the constraints of standard clinical care; therefore the EEG was often removed before the patient regained consciousness limiting our ability to adequately sample the different behavioral states in these patients. Prospective studies with serial EEG recordings and longitudinal behavioral assessments throughout the progression of recovery of consciousness will be needed to further validate our results. Second, a large proportion of patients (25 of 30) who died following a withdrawal of care decision may have also affected our ability to collect sufficient amount of data in these patients, which may have altered our results. Third, the ‘ABCD’ categorization was not possible in a subset of patients because of ongoing processes that dominated the spectral power, such as ongoing seizures or overwhelming myogenic artifacts. Introduction of spectral analytical or artifact reduction methods that make optimal use of small amounts of data may increase the yield of finding correlates of underlying neuronal activity in these cases. Other limitations include our relatively small sample size, which did not allow sufficient power to investigate subcohorts of patients, such as comparing patients with or without utilization of therapeutic hypothermia protocol; different initial cardiac rhythms or effects of sedative medications on our measures.
Importantly, however, future studies may build on these findings as our approach is potentially generalizable to assess patients with alterations of consciousness in intensive care units across a wide range of etiologies and interventions. Our results are also potentially applicable to track neurobiological processes relevant to preservation of brain tissue and recognition of complications compromising cellular function in acute neurological care settings (e.g., increased intracranial pressure, vasospasm, loss of cerebral perfusion pressure etc.). In addition, our measures may be also used to track effects of neuroprotective or other specific restorative therapeutic interventions employed in very early stages of severe brain injuries.
In summary, this study brings a biologically motivated approach toward understanding of the recovery of consciousness in acute severe anoxic brain injury, using EEG recordings that are already routinely collected in these patients. However, further and extended studies are needed to verify and extend our findings and to provide a foundation for development of improved prognostication and therapeutic interventions in patients who remain unconscious after cardiac arrest.
Author Contributions
Study concept and design: Drs. Forgacs, Frey, Schiff, and Claassen; Data acquisition: Drs. Forgacs, Frey, Velazquez, Tompson, Brodie, Moitra, Rabbani, Park, Agarwal, Falo, Schiff, and Claassen; Data analysis and interpretation: Drs. Forgacs, Frey, Schiff, and Claassen: Manuscript preparation: Drs. Forgacs, Schiff, and Claassen; Critical revision of the manuscript for important intellectual content: Drs. Velazquez, Tompson, Brodie, Moitra, Rabbani, Park, Agarwal, Falo, Schiff, and Claassen.
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the James S McDonnell Foundation and the Charles A. Dana Foundation. This work was also supported in part by grant UL1 TR000043 and UL1 TR000457‐06 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program and by the Stavros Niarchos Foundation. We thank the nurses, attendings, fellows, and residents of the Columbia University ICUs for their overall support of this project. We also thank William H. Curley for his help with data analysis. Drs. Claassen and Schiff had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. doi:10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan PS, McNally B, Tang F, Kellermann A. Recent trends in survival from out‐of‐hospital cardiac arrest in the united states. Circulation 2014;130:1876–1882. doi:10.1161/CIRCULATIONAHA.114.009711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greer DM, Rosenthal ES, Wu O. Neuroprognostication of hypoxic‐ischaemic coma in the therapeutic hypothermia era. Nat Rev Neurol 2014;10:190–203. doi:10.1038/nrneurol.2014.36. [DOI] [PubMed] [Google Scholar]
- 4. Gold B, Puertas L, Davis SP, et al. Awakening after cardiac arrest and post resuscitation hypothermia: are we pulling the plug too early? Resuscitation 2014;85:211–214. doi:10.1016/j.resuscitation.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 5. Grossestreuer AV, Abella BS, Leary M, et al. Time to awakening and neurologic outcome in therapeutic hypothermia‐treated cardiac arrest patients. Resuscitation 2013;84:1741–1746. doi:10.1016/j.resuscitation.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 6. Kamps MJA, Horn J, Oddo M, et al. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta‐analysis of the current literature. Intensive Care Med 2013;39:1671–1682. doi:10.1007/s00134‐013‐3004‐y. [DOI] [PubMed] [Google Scholar]
- 7. Owen AM. Detecting awareness in the vegetative state. Science 2006;313:1402. doi:10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 8. Monti MM, Vanhaudenhuyse A, Coleman MR, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010;362:579–589. doi:10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- 9. Forgacs PB, Conte MM, Fridman EA, et al. Preservation of electroencephalographic organization in patients with impaired consciousness and imaging‐based evidence of command‐following. Ann Neurol 2014;76:869–879. doi:10.1002/ana.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forgacs PB, Fridman EA, Goldfine AM, Schiff ND. Isolation syndrome after cardiac arrest and therapeutic hypothermia. Front Neurosci. 2016;10:259. doi:10.3389/fnins.2016.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schiff ND. Cognitive Motor Dissociation Following Severe Brain Injuries. JAMA Neurol 2015;72:1413–1415. doi:10.1001/jamaneurol.2015.2899. [DOI] [PubMed] [Google Scholar]
- 12. Drover JD, Conte MM, Goldfine AM, et al. Are low frequency oscillations in the eeg of severely injured brains a marker for functional reserve of cortical neurons? In: Society for Neuroscience, Washington, DC, 2011:Program No. 675.07/AA9. [Google Scholar]
- 13. Schiff ND. Mesocircuit mechanisms underlying recovery of consciousness following severe brain injuries: model and predictions In: Monti MM, Sannita WG. (eds.) Brain function and responsiveness in disorders of consciousness. Switzerland; Springer International Publishing; 2016. [Google Scholar]
- 14. Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci 2010;33:1–9. doi:10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Lee HJ, Weitz AJ, et al. Frequency‐selective control of cortical and subcortical networks by central thalamus. eLife. 2015;4:e09215. doi:10.7554/eLife.09215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lutkenhoff ES, Chiang J, Tshibanda L, et al. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann Neurol 2015;78:68–76. doi:10.1002/ana.24423. [DOI] [PubMed] [Google Scholar]
- 17. Demertzi A, Soddu A, Laureys S. Consciousness supporting networks. Curr Opin Neurobiol 2013;23:239–244. doi:10.1016/j.conb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 18. Buckwalter JA, Parvizi J, Morecraft RJ, van Hoesen GW. Thalamic projections to the posteromedial cortex in the macaque. J Comp Neurol 2008;507:1709–1733. doi:10.1002/cne.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fridman EA, Beattie BJ, Broft A, et al. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc Natl Acad Sci USA 2014;111:6473–6478. doi:10.1073/pnas.1320969111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci USA 2001;98:676–682. doi:10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leon‐Carrion J, Leon‐Dominguez U, Pollonini L, et al. Synchronization between the anterior and posterior cortex determines consciousness level in patients with traumatic brain injury (TBI). Brain Res 2012;1476:22–30. doi:10.1016/j.brainres.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 22. Laureys S, Schiff ND. Coma and consciousness: Paradigms (re)framed by neuroimaging. NeuroImage 2012;61:478–491. doi:10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 23. Thibaut A, Di Perri C, Chatelle C, et al. Clinical Response to tDCS Depends on residual brain metabolism and grey matter integrity in patients with minimally conscious state. Brain Stimulat 2015;8:1116–1123. doi:10.1016/j.brs.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 24. Schiff ND, Nauvel T, Victor JD. Large‐scale brain dynamics in disorders of consciousness. Curr Opin Neurobiol 2014;25:7–14. doi:10.1016/j.conb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams ST, Conte MM, Goldfine AM, et al. Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severe brain injury. eLife. 2013;2:e01157. doi:10.7554/eLife.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Timofeev I, Grenier F, Bazhenov M, et al. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex 2000;10:1185–1199. doi:10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 27. Silva LR, Amitai Y, Connors BW. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science 1991;251:432–435. [DOI] [PubMed] [Google Scholar]
- 28. Llinás RR, Ribary U, Jeanmonod D, et al. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Claassen J, Hirsch LJ, Emerson RG, et al. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology 2001;57:1036–1042. [DOI] [PubMed] [Google Scholar]
- 30. Mitra P. Observed Brain Dynamics. Oxford; New York: Oxford University Press, 2008. [Google Scholar]
- 31. Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale‐revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85:2020–2029. [DOI] [PubMed] [Google Scholar]
- 32. Safar P. Resuscitation after Brain Ischemia In: Safar P, Grenvik A, eds. Brain failure and resuscitation. New York: Churchill Livingstone, 1981. [Google Scholar]
- 33. Westhall E, Rosén I, Rossetti AO, et al. Interrater variability of EEG interpretation in comatose cardiac arrest patients. Clin Neurophysiol 2015;126:2397–2404. doi:10.1016/j.clinph.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 34. Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta‐analysis.Part 2: Patients treated with therapeutic hypothermia. Resuscitation 2013;84:1324–1338. doi:10.1016/j.resuscitation.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 35. Dragancea I, Horn J, Kuiper M, et al. Neurological prognostication after cardiac arrest and targeted temperature management 33 C versus 36 C: Results from a randomised controlled clinical trial. Resuscitation 2015;93:164–170. doi:10.1016/j.resuscitation.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 36. Ching S, Purdon PL, Vijayan S, et al. A neurophysiological–metabolic model for burst suppression. Proc Natl Acad Sci 2012;109:3095–3100. doi:10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 2007;448:600–603. doi:10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 38. Chatelle C, Thibaut A, Gosseries O, et al. Changes in cerebral metabolism in patients with a minimally conscious state responding to zolpidem. Front Hum Neurosci 2014;8:917. doi:10.3389/fnhum.2014.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robinson PA, Rennie CJ, Wright JJ, et al. Prediction of electroencephalographic spectra from neurophysiology. Phys Rev E Stat Nonlin Soft Matter Phys 2001;63(2 Pt 1):21903. doi:10.1103/PhysRevE.63.021903. [DOI] [PubMed] [Google Scholar]
- 40. Stender J, Gosseries O, Bruno M‐A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet Lond Engl 2014;384:514–522. doi:10.1016/S0140‐6736(14)60042‐8. [DOI] [PubMed] [Google Scholar]
- 41. Stender J, Mortensen KN, Thibaut A, et al. The minimal energetic requirement of sustained awareness after brain injury. Curr Biol CB 2016;26:1494–1499. doi:10.1016/j.cub.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 42. Berkhoff M, Donati F, Bassetti C. Postanoxic alpha (theta) coma: a reappraisal of its prognostic significance. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 2000;111:297–304. [DOI] [PubMed] [Google Scholar]
- 43. Young GB, Blume WT, Campbell VM, et al. Alpha, theta and alpha‐theta coma: a clinical outcome study utilizing serial recordings. Electroencephalogr Clin Neurophysiol 1994;91:93–99. [DOI] [PubMed] [Google Scholar]
- 44. Janati A, Husain MM, Moore DB, Adametz JR. Suppression‐burst pattern associated with generalized epileptiform discharges and alpha‐theta pattern coma. Clin EEG Electroencephalogr 1986;17:82–88. [PubMed] [Google Scholar]
- 45. Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med 2010;363:2638–2650. doi:10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Becker DA, Schiff ND, Becker LB, et al. A major miss in prognostication after cardiac arrest: burst suppression and brain healing. Epilepsy Behav Case Rep. Accepted Manuscript, In Press (Available online 17 September 2016) doi:10.1016/j.ebcr.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


