Setting the speed-accuracy trade-off (SAT) is crucial for efficient decision making. Previous studies have reported that subjects adjust their SAT after individual decisions, usually choosing more conservatively after errors, but the neural correlates of this phenomenon are only partially known. Here, we show that neurons in PMd and M1 of monkeys performing a reach decision task support this mechanism by adequately modulating their firing rate as a function of the outcome of the previous decision.
Keywords: decision making, monkey, posterror slowing, premotor cortex, speed-accuracy trade-off
Abstract
Recent studies have shown that activity in sensorimotor structures varies depending on the speed-accuracy trade-off (SAT) context in which a decision is made. Here we tested the hypothesis that the same areas also reflect a more local adjustment of SAT established between individual trials, based on the outcome of the previous decision. Two monkeys performed a reaching decision task in which sensory evidence continuously evolves during the time course of a trial. In two SAT contexts, we compared neural activity in trials following a correct choice vs. those following an error. In dorsal premotor cortex (PMd), we found that 23% of cells exhibited significantly weaker baseline activity after error trials, and for ∼30% of these this effect persisted into the deliberation epoch. These cells also contributed to the process of combining sensory evidence with the growing urgency to commit to a choice. We also found that the activity of 22% of PMd cells was increased after error trials. These neurons appeared to carry less information about sensory evidence and time-dependent urgency. For most of these modulated cells, the effect was independent of whether the previous error was expected or unexpected. We found similar phenomena in primary motor cortex (M1), with 25% of cells decreasing and 34% increasing activity after error trials, but unlike PMd, these neurons showed less clear differences in their response properties. These findings suggest that PMd and M1 belong to a network of brain areas involved in SAT adjustments established using the recent history of reinforcement.
NEW & NOTEWORTHY Setting the speed-accuracy trade-off (SAT) is crucial for efficient decision making. Previous studies have reported that subjects adjust their SAT after individual decisions, usually choosing more conservatively after errors, but the neural correlates of this phenomenon are only partially known. Here, we show that neurons in PMd and M1 of monkeys performing a reach decision task support this mechanism by adequately modulating their firing rate as a function of the outcome of the previous decision.
when faced with a decision, an intelligent agent always balances the time to invest in the deliberation process with its desired probability of success. In most cases more time means more successful decisions, but taking more time also discounts the value of the outcome (Pachella 1974; Wickelgren 1977). Thus the ability to adjust the decision policy—known as the speed-accuracy tradeoff (SAT)—is necessary to produce flexible and adaptive behaviors that allow animals to maximize their rate of rewards (Balci et al. 2011).
The SAT is a well-studied mechanism demonstrated in several animal species as well as in humans (Chittka et al. 2009; Forstmann et al. 2010; Franks et al. 2003; Hanks et al. 2014; Heitz and Schall 2012; Thura and Cisek 2016), and it has been reported in many experimental tasks, including visual discrimination (Zhang and Rowe 2014), force perception (Rank and Di Luca 2015), flanker tasks (Uemura et al. 2013), and arm reaching tasks (Forstmann et al. 2008). At the single-cell level, neurons related to SAT adjustments have been observed in the oculomotor system when decisions were reported with saccades (Hanks et al. 2014; Heitz and Schall 2012) and in the arm motor system when monkeys expressed decisions with reaching movements (Thura and Cisek 2016). Importantly, in all of these tasks, SAT adjustments occurred between blocks of trials favoring either hasty or accurate decisions and hence on a global scale, at the level of an entire experimental session.
However, in addition to global adjustments, the optimal strategy can change in a more fine-grained way, from one trial to the next. One example of such local SAT adjustment is known as posterror slowing (PES) (Botvinick et al. 2001; Dutilh et al. 2012b; Holroyd et al. 2005; Kleiter and Schwarzenbacher 1989; Laming 1979a, 1979b; Purcell and Kiani 2016; Rabbitt and Rodgers 1977), whereby subjects become more conservative and slow down after committing errors. Despite being well documented, the neural basis of this mechanism remains incomplete (Danielmeier and Ullsperger 2011). In a recent study, Purcell and Kiani (2016) found cells in monkey lateral intraparietal area (LIP) that decreased their activity after errors. In so doing, these cells may be responsible for PES, providing a compelling instance of single-cell correlates of local adjustment in SAT in the eye movement system.
In the arm reach system, recent work from our lab has demonstrated that, in a dynamic decision making task, single cells in the dorsal premotor cortex (PMd) are related to global adjustments in SAT between blocks that encouraged hasty or conservative decisions (Thura and Cisek 2016). Specifically, although these cells reached the same level of activity at the time of commitment in both contexts, in situations that encouraged hastier decisions they increased their baseline activity as well as the gain of evidence-related activity during the deliberation period. These same cells had been shown to be involved in the decision process, tracking the evolving sensory evidence and signaling the choice commitment ∼280 ms before movement onset (Thura and Cisek 2014).
Similarly to the global (Hanks et al. 2014) and local (Purcell and Kiani 2016) single-cell correlates of SAT adjustments in LIP, we were interested in assessing whether SAT adjustments observed in our PMd cells (Thura and Cisek 2016) could also be observed locally as single-cell correlates of PES. On the basis of our previous results, we predicted that after error trials PMd cells should have lower baseline and deliberation period activity than after correct trials, leading to longer deliberation times and higher accuracy.
MATERIALS AND METHODS
Subjects and apparatus.
Two male macaque monkeys (Macaca mulatta; monkey S: 7 yr old, 8 kg; monkey Z: 5 yr old, 6 kg) were implanted, under anesthesia and aseptic conditions, with a titanium head fixation post and recording chambers. The local animal ethics committee approved surgery, testing procedure, and animal care. Monkeys sat head fixed in a custom primate chair and performed two planar reaching tasks with a vertically oriented cordless stylus whose position was recorded by a digitizing tablet (CalComp, 125 Hz). Their nonacting hand (monkey S: left hand for ∼2 yr then right hand; monkey Z: right hand for ∼1 yr then left hand) was restrained on an arm rest with Velcro bands. In some sessions, unconstrained eye movements were recorded with an infrared camera (ASL, 120 Hz). Stimuli and continuous cursor feedback were projected onto a mirror suspended between the monkeys' gaze and the tablet, creating the illusion that they were in the plane of the tablet. Neural activity was recorded from the hemisphere contralateral to the acting hand with one to four independently movable (NAN microdrive) microelectrodes (FHC), and data were acquired with the AlphaLab system (Alpha-Omega).
Behavioral tasks.
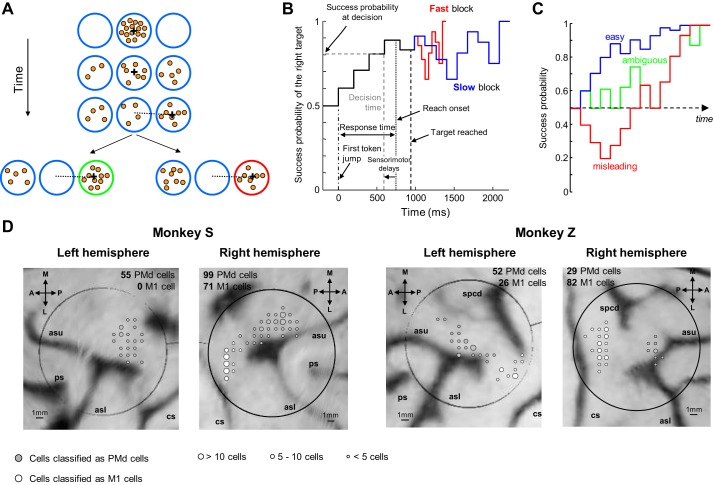
Monkeys were trained to perform the “tokens” task (Fig. 1A), in which they are presented with one central starting circle (1.75-cm radius) and two peripheral target circles (1.75-cm radius, arranged at 180° at 5 cm from the center). The monkey begins each trial by placing the cursor in the central circle, in which 15 small tokens are randomly arranged. The tokens then begin to jump, one by one every 200 ms (“predecision interval”), from the center to one of the two peripheral targets. The monkey's task is to move the cursor to the target that it believes will ultimately receive the majority of tokens. The monkey is allowed to make the decision as soon as it feels sufficiently confident and has 500 ms to bring the cursor into a target after leaving the center. When the monkey reaches a target, the remaining tokens move more quickly to their final targets (“postdecision interval,” which was either 50 ms or 150 ms in separate “fast” and “slow” blocks of trials, respectively). Once all tokens have jumped, visual feedback is provided to the monkey (the chosen target turns green for correct choices or red for error trials) and a drop of fruit juice is delivered for choosing the correct target. A 1,500-ms intertrial interval precedes the following trial.
Fig. 1.
Methods and recording sites. A: the “tokens” task. Each row illustrates a step in an example trial (see text for details). If monkey chooses the target that receives most of the tokens at the end of the trial, this target turns green and a drop of juice is delivered to the animal (bottom left). If he chooses the other target, this target turns red and no reward is delivered (bottom right). B: success probability profile of an example trial. The probability of success of choosing each target evolves over time (black trace, for the right target in this example). After the chosen target is reached (vertical black dashed line), the remaining tokens jump either every 150 ms (in slow blocks, blue trace) or every 50 ms (fast block, red trace) to their allocated target. C: example success probability profiles of “easy,” “ambiguous,” and “misleading” trials, classified a posteriori according to predefined criteria (see materials and methods). D: reconstructed images of the brain surface from anatomical MRI scans. Large circles illustrate the location of the recording chambers. Gray-scaled circles illustrate the location of the recording sites for cells recorded in the 2 blocks of trials (232 cells in PMd, 175 cells in M1), classified as PMd or M1 based on anatomical location. A, anterior; P, posterior; M, medial; L, lateral; ps, principal sulcus; spcd, superior precentral dimple; asu, arcuate sulcus upper limb; asl, arcuate sulcus lower limb; cs, central sulcus.
The monkeys were also trained to perform a delayed reach (DR) task (usually 30–48 trials per session). In this task, the monkey again begins by placing the cursor in the central circle containing the 15 tokens. Next, one of six peripheral targets is presented (1.75-cm radius, spaced at 60° intervals at 5 cm from the center), and after a variable delay (500 ± 100 ms) the 15 tokens simultaneously jump into that target. This “GO signal” instructs the monkey to move the handle to the target to receive a drop of juice. This task is used to determine cell tuning as well as the animal's mean reaction time, used as an estimate of the total delays attributable to sensory processing and response initiation.
Data set.
The last stage of monkeys' training in the tokens task involved providing animals with alternating blocks of slow and fast trials (∼100–150 trials in a block). Based on behavioral data (see Thura et al. 2014 for a detailed analysis of the same animals' behavior), we defined two periods during this last stage: first, when behavior was similar between the two blocks and second, when the monkeys began to behave differently in the two blocks, in terms of decision duration and success probability (SP). In this report, we analyze both behavioral and neurophysiological data collected during this last stage of training, whether or not the monkeys' behavior was similar in the two speed conditions.
Neural recordings.
Our standard procedures for single-unit recordings in the PMd and primary motor cortex (M1), signal processing, and data management have been described previously (Thura and Cisek 2014, 2016). During recording sessions, we focused on cells showing a change of activity in the tokens task, and monkeys were usually performing the task while we were searching for cells. When one or more task-related cells were isolated, we ran a block of 30–48 trials of the DR task to determine spatial tuning and select a preferred target (PT) for each cell (i.e., the target associated with the highest firing rate during 1 or more task epochs). Next, we ran blocks of tokens task trials using the PT of an isolated cell and the 180° opposite target (OT).
Behavioral data analysis.
Methods to analyze behavior in the tokens task have been described previously (Cisek et al. 2009; Thura et al. 2014). Briefly, the tokens task allows us to calculate, at each moment in time, the “success probability” (SP) associated with choosing each target. In particular, for a task with a total of 15 tokens, if there are NR tokens in the right target, NL in the left, and NC in the center, then the probability that the right target will be correct is given by
| (1) |
To characterize the SP profile for each trial, we calculated this quantity (with respect to the target ultimately chosen by the monkey) after each token jump. To estimate decision time, we detected the time of movement onset (response time, RT) and subtracted from it the mean reaction time calculated daily with the DR task (as a measure of sensory and motor delays). Knowing the decision time, we estimated the success probability at which the monkey committed to his choice in a given trial (Fig. 1B). Because we do not believe that monkeys could explicitly calculate Eq. 1, we also characterized the evidence available at each moment as the sum of the log-likelihood ratios (SumLogLR) of individual token jumps, such that
| (2) |
where p(ej|S) is the likelihood of a token even ej during trials in which the selected target is correct and p(ej|U) is its likelihood during trials in which the unselected target is correct.
Although each token jump and each trial was completely random, we could classify a posteriori some specific classes of trials embedded in the fully random sequence (e.g., “easy,” “ambiguous,” or “misleading” trials). A trial is classified as “easy” if SP exceeds 0.6 after two token jumps and 0.75 after five. A trial is ambiguous if SP is 0.5 after two jumps, between 0.4 and 0.65 after three, and then between 0.55 and 0.66 after five and seven jumps. A trial is misleading if SP is <0.4 after three token jumps (Fig. 1C).
Wilcoxon-Mann-Whitney (WMW) tests were used to compare RT and SP distributions.
Neural data analysis.
All neurophysiological data reported here were acquired from correct or error trials in which the monkeys completed the tokens task by choosing one of the two targets. The neural data set comprises all PMd or M1 cells recorded since the monkeys performed the tokens task when provided with alternating blocks of slow and fast trials (50–150 trials in a block). To be included in the different analyses described in the present report, neurons had to be recorded in both the slow and fast blocks of trials. The recording sites of neurons that met these criteria are depicted in Fig. 1D. All the analyses and methods used to summarize previous findings related to the specific effects of block on neural activity have been published previously (Thura and Cisek 2016).
To test the effect of previous trial outcome on next trial neural activity, we first assessed for each cell the effect of previous trial outcome on next trial baseline activity. We grouped trials as a function of previous trial outcome, either a correct choice or an error, and mean responses were statistically compared with a WMW test. The baseline was defined as the 400-ms period preceding the first token jump, and the instantaneous firing rate was assessed via a partial interspike interval method.
Population analyses consisted in averaging the mean response of neurons under investigation after grouping these responses as a function of the tested conditions. When analyzing data with respect to the start of the trial (first token jump), we always excluded all spikes occurring after our estimate of decision time (i.e., any activity associated with movement initiation and/or execution) to prevent averaging artifacts due to the very wide range of decision durations in the tokens task.
To investigate the effect of previous trial outcome on neural activity during deliberation, we computed for each cell its mean response in a 200-ms period extending from 600 to 800 ms after the first token jump in fast block trials and grouped those trials as a function of the previous trial outcome (correct choices or errors). To get rid of a potential confound due to sensory evidence, we only included trials for which SumLogLR during this time period was either −0.42 or +0.42. Indeed, the chosen time window assesses neural activity reflecting sensory evidence after three token jumps (token jump 1 at 0 ms, jump 2 at 200 ms, jump 3 at 400 ms, plus 200 ms of sensory delay). After three jumps, the distribution of potential token repartitions in the two targets appears as follows: three tokens in the cell's preferred target (in such situation, the SumLogLR = +1.26); two tokens in the cell's PT and one in the OT (SumLogLR = +0.42); one token in the cell's PT and two in the cell's OT (SumLogLR = −0.42); the three tokens in the cell's OT (SumLogLR = −1.26). We thus discarded trials in which the three tokens jumped to a single target, and we averaged the neural activity of the remaining trials to estimate the neural activity at “zero” evidence. In this analysis we also discarded all trials for which the decision duration was below 600 ms. We performed a bootstrapping analysis for each neuron to assess the confidence interval around the mean activity in each condition. To do so, we considered every cell that had more than five trials in each trial type (SumLogLR = +0.42 or −0.42), and we resampled the firing rate data of each cell 10,000 times in each tested condition (after error vs. after correct trial) to produce distributions of means and a distribution of the difference in the means. If zero lies outside the 2.5%–97.5% percentiles of the distribution of resampled differences, then the effect is considered significant at P < 0.05.
To assess the effect of previous trial outcome on activity aligned on movement onset, we further sorted trials according to monkeys' choice, either the cell's PT or the OT. In the tokens task, a cell's spatial preference is assessed by means of a receiver operating characteristic (ROC) analysis (Green and Swets 1966; Shadlen et al. 1996) performed on the mean activity for each target choice during the 200 ms preceding decision time with a criterion of 0.65.
To test the effect of the block condition on baseline activity, we defined an index of modulation computed as follows: (FRfast − FRslow)/(FRfast + FRslow), with FR being the mean firing rate of the cell during baseline (−400 ms to the first token jump).
To assess the effect of decision duration and block condition on neural activity during deliberation, we performed an analysis of covariance (ANCOVA) with decision duration and block as main independent factors and the mean neural activity of each cell computed in 200-ms bins from the first token jump as the independent variable. We further investigated the effect of block on activity related to each token jump with a bootstrap test that compared the neurons' responses (calculated in 200-ms bins, corresponding to each token jump) in the slow vs. fast blocks. To do so, we resampled for each bin the firing rate data of each cell 10,000 times in each condition (slow vs. fast blocks) to produce distributions of means and a distribution of the difference in the means. If zero lies outside the 2.5%–97.5% percentiles of the distribution of resampled differences, then the effect is considered significant at P < 0.05.
To evaluate how activity is influenced by the outcome of the previous two trials, we first separated baseline activity depending on whether the previous trial was an error or a correct trial. Then we separated baseline activity further, on the basis of whether the trial was preceded by two error trials, two correct trials, an error and a correct trial, or a correct and an error trial. For each category of trials we computed the average baseline neural activity using the same window as the previous analysis. We then normalized each of these values by dividing them by the average activity across all trials for each cell.
To test the possibility that effect of previous trial outcome was dependent on cells' PT or OT, we performed a two-way analysis of variance (ANOVA) on the activity of all cells whose baseline activity is affected by previous trial outcome, with previous trial outcome (correct vs. error) and monkey's choice (cell's PT vs. OT based on ROC analysis, see above) as independent variables.
The significance level of all statistical tests was set at 0.05, and highest levels of significance are reported when appropriate.
RESULTS
Summary of PMd and M1 “decision” cell properties in tokens task.
In two recent studies (Thura et al. 2014; Thura and Cisek 2016), we described the behavior and the neural mechanisms that underlie SAT adjustments while monkeys S and Z were performing the tokens task in the two speed conditions described above (fast and slow blocks).
Based on behavioral variables, we determined that animals voluntarily establish and adjust their SAT as a function of the timing parameters of the tokens task. In short, they made faster and less accurate decisions in the fast condition compared with the slow condition. We also found that their urgency to commit to a decision could be estimated from behavior, with longer decisions being made with less confidence, suggesting a growing urgency signal that pushes the system to commitment as time passes (Thura et al. 2014).
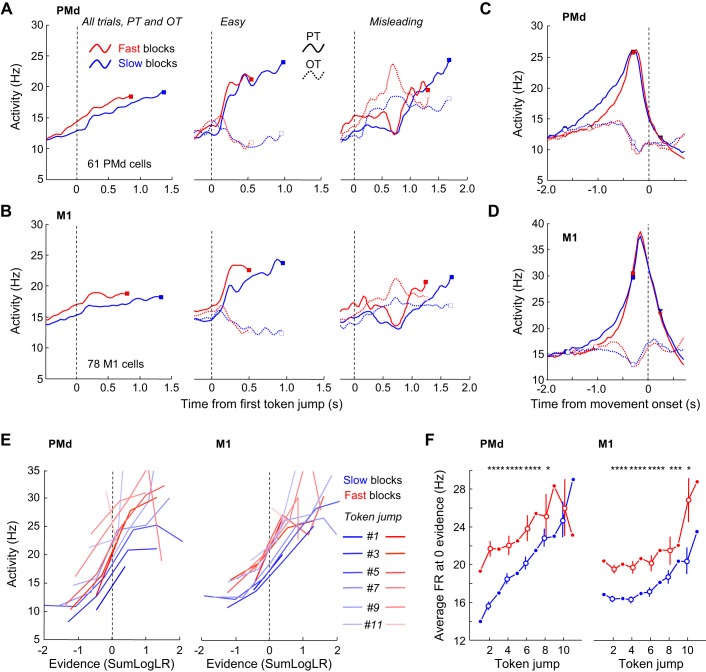
Next, we investigated the neural substrates of this mechanism in PMd and M1 (Thura and Cisek 2016). Figure 2 summarizes the main findings of that study. We first found that average activity patterns of PMd and M1 cells tuned before decision commitment (ROC > 0.65 during a 200-ms period preceding decision time) reflect the evolution of sensory evidence (see activity during easy and misleading trials in Fig. 2, A and B) and reach a peak about 280 ms (PMd) and 140 ms (M1) before movement onset (Fig. 2, C and D), regardless of the SAT condition in which the task was performed. We then observed that in the majority of these PMd and M1 cells activity was on average larger during fast blocks than during slow blocks, reflecting the larger urgency of the fast block type. This amplification of activity was observed during both the baseline and the deliberation periods (Fig. 2, A and B) but not at decision commitment (Fig. 2, C and D). Looking at the correlation between the modulated cell activity and sensory evidence in all trials as a function of time, we found that cells displayed a strong and positive correlation whose gain was amplified during fast blocks compared with slow blocks (Fig. 2E), suggesting the presence of growing urgency during deliberation until commitment. To estimate the shape of the urgency signal in the two blocks, we looked at the evolution of activity at the zero evidence point. We found that both PMd and M1 activity tended to grow over the course of the trials, regardless of block type (Fig. 2F). As expected, activity was much stronger in the fast condition compared with the slow condition, but this difference tended to vanish with elapsing time, especially in PMd.
Fig. 2.
Response patterns of PMd and M1 “decision” cells in the tokens task (from Thura and Cisek 2016). A, left: average activity of 61 “decision-related” PMd cells aligned on the 1st token jump in all trials during the fast blocks or slow blocks. In each block, activity is truncated 280 ms before movement onset (squares) and both error and correct trials are included. Center: average activity of the “decision-related” PMd cells during easy trials in which monkeys correctly chose the cells' preferred target (PT) or opposite target (OT) in the slow blocks or the fast block. To be included, cells had to be recorded during at least 5 trials per condition. Right: same as center for the “decision” PMd cells recorded in at least 5 misleading trials. B: same as A for a population of 78 M1 cells. C: average neural responses of 61 PMd decision-related cells aligned on movement onset when monkeys chose either the cells' PT (solid curves) or OT (dotted curves) in the slow (blue) or fast (red) blocks. D: same as C for a population of M1 cells. E: evolution of the relationship between neural firing and sensory evidence. Each line illustrates the relationship between the SumLogLR (see materials and methods) with respect to the PT and the mean neural activity averaged across 30 PMd (left) and 47 M1 (right) cells calculated 200 ms later in a 200-ms epoch, color coded from the darkest (1st token jump) to the lightest, in both the slow and the fast blocks. Only epochs preceding our estimate of decision time are included. F: evolution of the averaged activity of the PMd (n = 30, left) and M1 (n = 47, right) populations calculated for the condition when SumLogLR = 0 (the evidence is equal for each target) as a function of time in either the slow (blue) or the fast (red) blocks. For odd-numbered jumps, the firing rate at zero evidence was calculated by interpolation (filled circles). For even-numbered jumps, vertical bars represent the confidence intervals around the means calculated through a bootstrap procedure (see materials and methods). Asterisks indicate significant difference: *P < 0.05, ***P < 0.001, ****P < 0.0001.
Overall, these data provide neurophysiological support to the hypothesis that monkeys use an urgency signal to regulate the SAT of their decisions both within and between blocks of trials. We believe that this mechanism enables the maximization of the rate of reward globally, across a large time window. Given the monkeys' expertise as a result of regularly performing the task, this maximization of the global rate of reward was probably adjusted to the scale of an entire experimental session. However, do animals also adjust their policy more locally, between individual trials within a given SAT context, based on previous trial outcome?
Effect of trial outcome on monkeys' behavior.
To determine the effect of previous trial outcome on animals' behavior, we first compared the mean RT across all trials during which neurons described in the present report were recorded (monkey S, n = 27,857 trials; monkey Z, n = 31,722 trials).
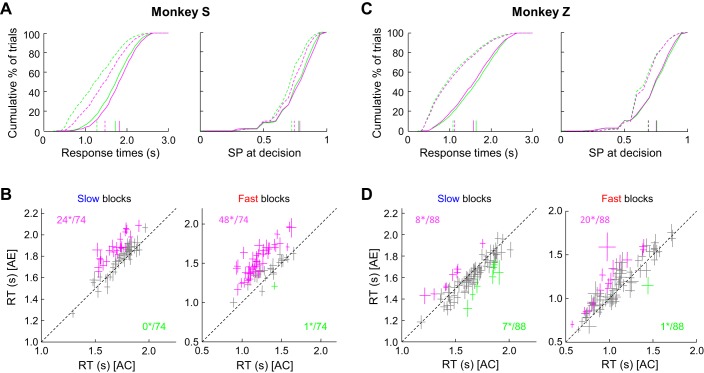
On average, monkey S made faster decisions in trials following a correct choice compared with trials following an error. This was observed in both block types, although the effect was larger in the fast blocks (−85 ms in slow blocks, −189 ms in fast blocks; WMW test, P < 0.0001) (Fig. 3A, left). These faster decisions after a correct choice were, on average, made at a slightly but significantly lower level of SP (0.78 vs. 0.79 in the slow blocks and 0.73 vs. 0.75 in the fast blocks; WMW test, P < 0.0001; Fig. 3A, right), as expected if the monkey traded off speed against accuracy during the task. The effect of trial outcome on the behavior of monkey S was often consistent between sessions (n = 74). In the slow blocks (Fig. 3B, left), monkey S often slowed down significantly more after error trials (24/74 sessions; WMW test, P < 0.05). It never significantly adopted the opposite policy in any of the 74 sessions tested. In the fast condition (Fig. 3B, right), this trend was even stronger, as it slowed down significantly more after error trials in 48 of 74 sessions and slowed down significantly after correct trials in only 1 session.
Fig. 3.
Effect of trial outcome on next trial response time and success probability. A: cumulative distributions of response time (RT) (left) and success probability (right) from trials executed by monkey S after either correct choices (green curves) or errors (magenta curves) in the slow (solid) and fast (dashed) blocks of trials in the token task. Colored vertical lines show the mean values in each group of trials. B: effect of previous trial outcome on RT calculated within each recording session. Each cross illustrates the mean (±SE) RT of trials following a correct choice [“after correct” (AC); x-axis] or an error [“after error” (AE); y-axis] for slow (left) or fast (right) block trials during which a PMd or a M1 neuron was recorded in monkey S (74 sessions). Colored crosses show cases where the difference between the 2 conditions is significant. Magenta crosses illustrate cases where RTs were significantly longer in trials following an error compared with trials following a correct choice. Green crosses illustrate the opposite effect. Numbers indicate how many of these effects were significant (*). C: same as A for monkey Z. D: same as B for monkey Z (88 sessions).
In monkey Z, we found the same results when analyzing the fast blocks, in which it made decisions on average 32 ms faster in trials following a correct choice compared with trials following an error (WMW test, P = 0.004; Fig. 3C, left). This effect was often consistent within recording sessions (n = 88), as we found that monkey Z slowed down significantly more after error trials compared with correct choices in 20/88 sessions and significantly more after correct trials compared with errors in only 1 session (WMW test, P < 0.05; Fig. 3D, right). However, in the slow blocks, the effect was reversed, with decisions made on average 52 ms faster after an error than in trials following a correct choice (WMW test, P < 0.0001; Fig. 3C, left). Within slow block sessions (Fig. 3D, left), we found that monkey Z slowed down significantly more after error trials than after correct choices in 8/88 sessions and more after correct trials than after errors in 7/88 sessions. In monkey Z, SP of decisions made in the fast conditions was unaffected by the previous trial outcome (0.69 in both conditions, Fig. 3C, right) whereas it was slightly higher in trials following a correct choice compared with trials following an error in the slow blocks (0.76 vs. 0.75, P = 0.04), consistent with the RT results if the monkey traded speed against accuracy in this task. As noted in discussion, such a behavioral phenomenon is observed in some subjects and referred to as “posterror speeding” (King et al. 2010; Notebaert et al. 2009; Purcell and Kiani 2016).
Effects of trial outcome on neural activity during baseline of next trial.
We then analyzed the activity of 235 PMd neurons (monkey S: n = 154) and 179 M1 neurons (monkey S: n = 71) recorded in both the fast and slow blocks of trials. Some of these cells have been analyzed previously (Thura and Cisek 2014, 2016). Here we first describe our analyses performed on PMd cells given our previous results demonstrating that PMd strongly reflects the deliberation process by tracking the evolution of sensory evidence, signaling the time at which animals commit to their choice and participating in the SAT adjustment between block conditions (Thura and Cisek 2014, 2016).
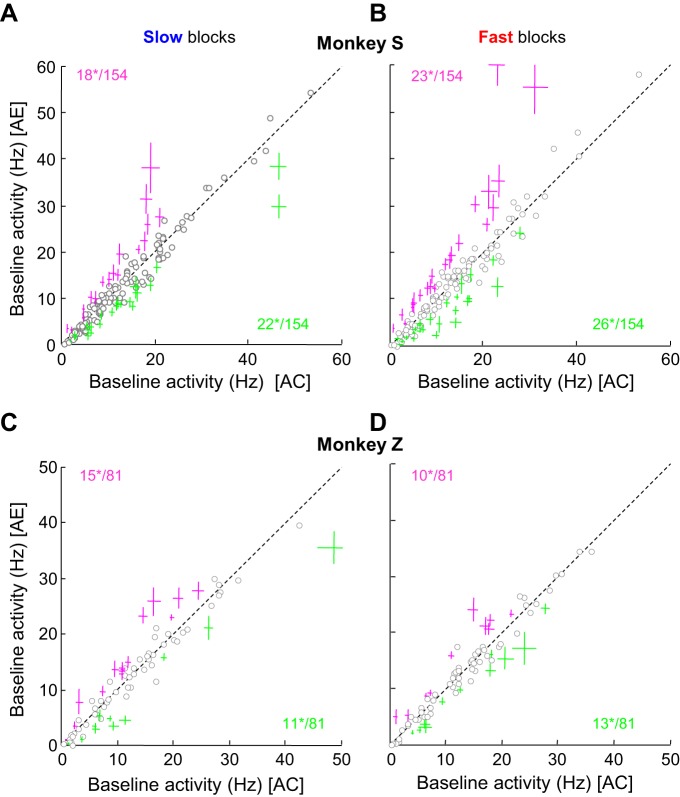
To determine the effect of the previous trial on PMd baseline activity, we measured the average activity of each cell during a 400-ms period preceding the first token jump and sorted trials according to the previous trial outcome, either a correct choice or an error. Overall, we found 106 cells (45%) significantly modulated by the previous trial outcome (WMW test, P < 0.05). We then separated the analysis by monkey (monkeys S and Z) and by block (slow and fast), which yielded the following results (Fig. 4): while monkey S performed the task in the slow blocks, we observed 22 of 154 cells (14%) whose baseline activity was significantly more active in trials following a correct decision compared with trials following an error (Fig. 4A). These cells are referred as “C cells” throughout this report. We also observed the opposite pattern, namely, higher baseline activity after error trials, for 18 of 154 cells (12%, Fig. 4A). These cells are called “E cells” throughout this report. When monkey S performed the task in the fast blocks, we found 26 C cells (17%) and 23 E cells (15%; Fig. 4B). In monkey Z, we observed a similar proportion of cells modulated by the previous trial outcome. In the slow block (Fig. 4C) we found 14% (11/81) C cells and 18% (15/81) E cells, whereas in the fast block (Fig. 4D) we observed 16% (13/81) C cells and 12% (10/81) E cells. Among the 33 PMd C cells and 33 E cells recorded in the two monkeys in the slow condition, 18 C cells (55%) and 14 E cells (42%) were also modulated in the fast blocks. Remarkably, these modulations were always consistent across the blocks for each individual cell, i.e., we never observed a cell that had higher baseline activity after errors in one block but higher activity after correct trials in the other.
Fig. 4.
Cell-by-cell analysis of the effect of trial outcome on PMd baseline activity. A: within-cell comparison of the mean neural baseline activity (from −400 ms to 1st token jump) of a population of 154 PMd cells recorded in monkey S in trials following a correct choice (x-axis) or an error (y-axis) in the slow block of trials. Each circle represents the mean response of a neuron. Green crosses illustrate the cells (mean ± SE) for which baseline activity between the 2 conditions is significantly greater after a correct choice than after an error. These cells are referred as C cells in this report. Magenta crosses show the cells with the opposite effect, i.e., a significantly greater baseline activity after an error than after a correct choice. We refer to these cells as E cells in this report. Numbers indicate how many cells reach significance (*), as assessed through a WMW test. B: same as A for the fast block in monkey S. C: same as A for a population of 81 PMd cells recorded during the slow block in monkey Z. D: same as B for monkey Z.
Control analyses.
Before investigating these outcome-sensitive cells further, we performed three control analyses to rule out the possibility that confounding factors could be responsible for the results described above.
First, we verified whether the pattern of activity of the C cells could be the result of a motor artifact, such as motor activity related to the behavior of licking the reward tube extending into the next trial. To this aim, we analyzed the effect that outcome two trials back had on the C cells' baseline activity (see materials and methods for details). In particular, we compared the baseline activity after a correct trial that was preceded by another correct trial with the baseline activity after a correct trial that was preceded by an error. If an effect was found, it could not be caused by licking behavior, which presumably would be the same in both groups of trials. Indeed, we found that the baseline activity (in slow blocks) of the 18 C cells modulated in both conditions was significantly higher in trials that were preceded by two consecutive correct choices than in trials preceded by an error and then a correct choice (WMW test, P < 0.01).
It is also possible that errors induced frustration movements (such as additional movements of the stylus, legs, etc.) that could explain modulations observed in PMd. However, the effects of previous trial outcome on baseline activity were measured when monkeys were required to hold their hand motionless in the central circle until the first token jump, ruling out the possibility that the reported effects are due to extra arm movements induced by previous trial outcome. Moreover, we compared how long it took the monkey to initiate a trial after errors or correct choices by measuring the duration between the end of the intertrial interval and the moment at which the monkey placed the cursor in the central circle to initiate the next trial. On average, this duration was very short, as monkeys very often entered the central circle before the end of the intertrial interval (initiation duration = 0 ms in this case). We reasoned that if errors induce any frustration movements, initiation durations should be significantly longer for trials following errors compared with trials following correct choices. While we sometimes found such a trend, the difference was significant in only 20% and 16% of the sessions during which C and E cells modulated in the fast blocks were recorded, respectively, and in only 22% of the sessions during which C and E cells modulated in the slow blocks were recorded. Overall, this suggests that the change of baseline activity following a correct or error choice is most likely not a motor artifact and instead reflects local changes in SAT policies.
Second, it has been shown previously that in the oculomotor system several different effects of previous trial direction take place (Fecteau and Munoz 2003). For instance, neurons in the superior colliculus were more active after two consecutive trials in which saccades occurred in the same direction (Everling et al. 1999), whereas neurons in the frontal eye fields were less active when a target appeared consecutively in the same location (Bichot and Schall 2002). To assess whether the direction chosen by the monkey in the previous trial could account for all or part of the postoutcome effects described in the present report and explain the difference between the PMd C and E cells, we analyzed cells' baseline activity on the basis of whether the previous trial was in the cell's preferred (PT) or opposite (OT) direction. If the effect of previous trial outcome was only apparent in the PT condition, it could be argued that these cells were tracking previous trial direction instead of outcome. An ANOVA yielded no significant effects of previous trial direction in either of the monkeys' C cells [F(1,39) = 0.55, P > 0.05] or E cells [F(1,33) = 1.06, P > 0.05], suggesting that the effects on baseline activity were not the result of previous trial direction.
Third, because monkeys' performance in the tokens task was usually very good, in both the slow and fast blocks of trials (SP = 0.78 vs. 0.73 for monkey S, 0.74 vs. 0.68 for monkey Z), we tested for the possibility that most of our posterror trials come from periods of reduced performance and the differences we report are actually confounded by factors such as general vigilance and attention. To eliminate this confound, we followed the approach proposed by previous studies (Dutilh et al. 2012a; Purcell and Kiani 2016) and defined a new category of trials, named postcorrect-preerror (PCPE) trials, whereby a trial following a correct choice also preceded an error. This criterion ensured that posterror and PCPE trials were sampled from periods of time with similar levels of performance and balanced the number of trials between the two categories. Despite this additional criterion included in the postcorrect trial category, we found the same qualitative effect of previous trial outcome on both behavior and neural data, suggesting that performance fluctuations were not a major factor in our data.
These three control analyses thus confirm that decision outcomes affect the neural activity during the following trial in a subset of PMd cells. This modulation could be part of the mechanism involved in PES. With the assumption that PES is a local adjustment of decision policy (Danielmeier and Ullsperger 2011), it is possible that some or all of the cells implicated in deciding and committing to the choice are also involved in this phenomenon. It is thus crucial to elucidate whether the C cells and the E cells carry some pertinent information related to the decision making process. As mentioned above, we previously described the features of the PMd cells involved in decision making and SAT adjustments during the tokens task (Thura and Cisek 2014, 2016). In particular, we demonstrated 1) how activity evolves during deliberation under the influence of task-relevant signals, including sensory evidence and urgency, and 2) how activity signals the time of decision commitment. In the following sections, we thus describe analyses aimed at better understanding the nature of the C and E cells as well as the function of the previous trial outcome modulation in the context of a decision making task.
Single-cell examples.
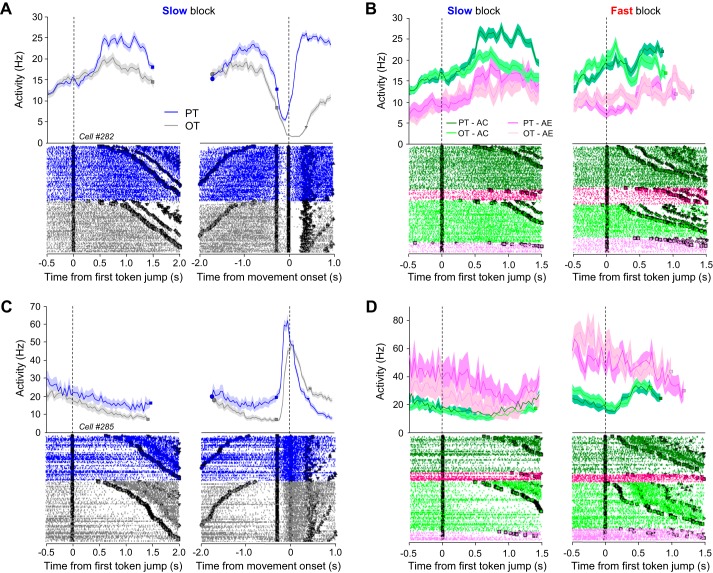
A PMd C cell is illustrated in Fig. 5, A and B. This cell increased its activity during deliberation, especially for its PT. Activity then dropped before the monkey committed to its choice and reported it by moving the cursor (Fig. 5A). When trials were sorted according to the previous trial outcome, baseline activity was higher in trials following a correct choice compared with error choices, which was true regardless of the block condition or the chosen target (Fig. 5B). Interestingly, the effect of trial outcome tended to persist during deliberation, when the tuning of the cell emerged (∼500 ms after the first token jump). At that time, the difference of activity between the previous trial outcome conditions diminished for the OT but remained strong for the PT. Figure 5, C and D, illustrate a PMd E cell. This cell's activity decreased during deliberation and also displayed slight tuning for a specific target. Right after commitment, its activity strongly increased and peaked around movement onset (Fig. 5C). When trials were sorted according to the previous trial outcome, baseline activity was higher in trials following an error choice compared with correct choices, which was true regardless of the block condition or the chosen target (Fig. 5D). Here, the effect of trial outcome tended to be sustained during the early deliberation period, irrespective of the target, and eventually vanished around commitment time.
Fig. 5.
Example of C and E cells in PMd. A: activity of a PMd C cell in the tokens task. Top: spike density functions computed in 30-ms bins. Bottom: rasters in which each row is a trial and each dot is a spike. Activity is aligned either on the 1st token jump (left) or on the movement onset (right) in trials for which monkey chose the cell's preferred (PT) or opposite (OT) target. Trials are sorted as a function of decision time. Circles illustrate 1st token jumps, squares show an estimate of monkey's decision times, diamonds represent movement onsets, and triangles show movement offsets. When aligned on 1st token jump, activity occurring after decision time is discarded in the spike density functions to avoid averaging artifacts. B: activity of the neuron shown in A, but here PT (dark colors)- and OT (light colors)-related trials are sorted according to the outcome of the preceding trial, either a correct choice (green) or an error (magenta). C: same as A for a PMd E cell. D: same as B for the PMd E cell shown in C.
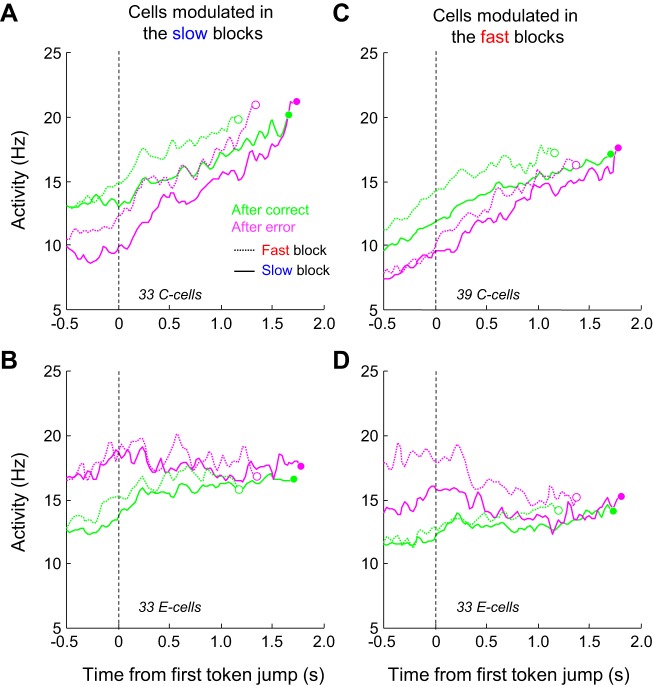
The average response of the 33 PMd C cells we found to be modulated in the slow blocks is shown in Fig. 6A. As expected, in the slow block baseline activity was stronger during trials following a correct choice compared with trials that followed an error (∼13 Hz vs. ∼9 Hz). As these cells were not necessarily modulated in the fast condition (see above), differences of activity between postcorrect and posterror trials in the fast blocks were reduced compared with those observed in the slow blocks (∼11 Hz vs. ∼13 Hz; Fig. 6A). Figure 6B illustrates the average response of the 33 PMd E cells modulated in the slow blocks but not necessarily in the fast blocks. For these cells, baseline activity was stronger during trials following an error compared with trials that followed a correct choice. The effect was present in both blocks, even if slightly less pronounced in the fast condition, as expected from this population. Figure 6, C and D, illustrate the average activity of the C and E cells modulated in the fast condition but not necessarily in the slow condition (39 C cells and 33 E cells). As expected, modulation of the baseline activity by the previous trial outcome was here more pronounced when monkeys performed the task in the fast block compared with the slow block for both populations.
Fig. 6.
Population activity of PMd C cells and E cells. A: average response of a population of PMd C cells in the tokens task. Cells (n = 33) are restricted to those showing significant modulations of previous trial outcome in the slow blocks, but not necessarily in the fast blocks. Activity is averaged across PT- and OT-related trials and is aligned on the 1st token jump and spikes occurring after the estimate of decision time (circles) are discarded. Trials are grouped according to the block type, with the slow block activity as solid curves and the fast block activity as dotted curves, and depending on the outcome of the previous trial, either a correct choice (green curves) or an error (magenta curves). B: same as A for the population of 33 PMd E cells significantly modulated by previous trial outcome in the slow blocks. C: same as A for the population of 39 PMd C cells significantly modulated by the previous trial outcome in the fast blocks, but not necessarily in the slow blocks. D: same as C for a population of 33 PMd E cells significantly modulated by the previous trial outcome in the fast blocks.
From these plots, two properties seemed to emerge and differentiate the C and E cells. First, the activity of C cells strongly built up during deliberation in all conditions (posterror vs. postcorrect, slow vs. fast blocks). By contrast, in all conditions, the activity of E cells appeared much more constant during deliberation. Second, the effect of previous trial outcome seemed to vanish more quickly during the deliberation period for the E cells than for the C cells.
Does previous trial outcome influence activity during deliberation?
As the propagation of the effect of previous trial outcome from baseline activity to the deliberation-related activity could be one of the neural correlates of animals' behavioral adjustment from trial to trial (i.e., by modulating the time at which the decision activity crosses the decision boundary, see below), we wanted to further investigate this question and assess the tendency of the effect of previous trial outcome on baseline activity to persist during the deliberation period in each of the C and E cells.
For the following investigations, we focused our analyses on both C and E cells modulated during the fast condition but not necessarily during the slow condition. We chose to study this particular population because 1) they represent the largest group of modulated cells (39 C cells and 33 E cells) and 2) at the behavioral level the effect of previous trial outcome was more pronounced and more consistent for each monkey in the fast block of trials.
To perform this analysis, we selected the neural activity of all “after correct” vs. “after error” trials performed in the fast blocks in a 200-ms period following the third token jump and averaged the instances when jump distributions in the two targets were either 2 vs. 1 or 1 vs. 2 (discarding trials in which the first 3 tokens all jumped into a single target). By averaging the activity in these two groups of trials, we generated an estimate of the neural activity at zero evidence after three token jumps (from 600 ms to 800 ms during deliberation, see materials and methods for details), eliminating the effect of sensory evidence on neural responses. This analysis shows that for many of the C cells (13/39, 33%), the effect of previous trial outcome still significantly influenced activity during deliberation, and always in the same direction (i.e., a baseline C cell never became an E cell during deliberation) (Fig. 7; permutation test, P < 0.05). For the population of E cells, however, the influence of previous trial outcome was weaker during deliberation, as suspected from Fig. 6B, as only 7 of 33 cells (21%) were significantly modulated by the previous trial outcome during deliberation in the fast blocks (Fig. 7). Here again, modulations during deliberation were almost always in the same direction as those observed during the baseline period. Only two E cells (of 33) became more active after correct trials during deliberation.
Fig. 7.
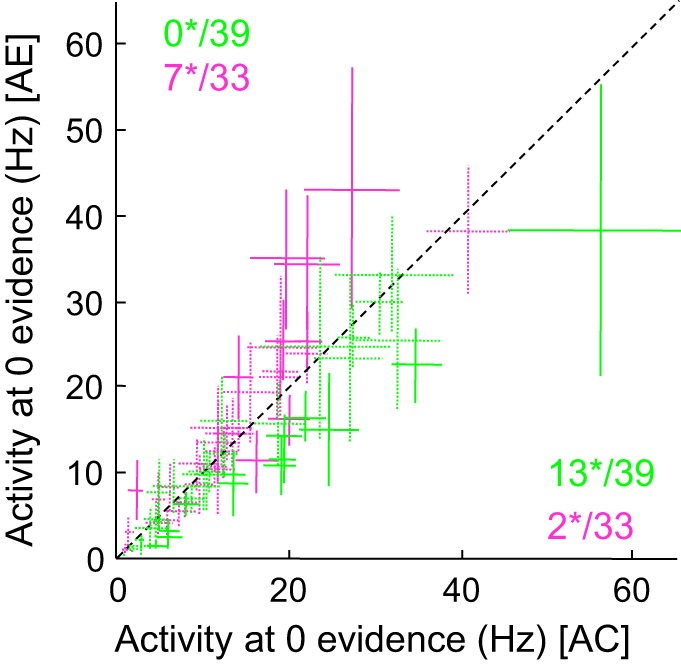
Effect of previous trial outcome on activity during deliberation. Each cross illustrates the mean (±confidence intervals around the means) activity of the 39 PMd C cells (green) and 33 PMd E cells (magenta) that were significantly modulated by previous trial outcome in the fast blocks but not necessarily in the slow blocks. These firing rates were collected in a 200-ms period extending from 600 to 800 ms after 1st token jump in fast block trials following a correct choice (x-axis) or an error (y-axis). The time window is chosen to assess the neural activity reflecting sensory evidence after 3 token jumps, plus a 200-ms delay. After 3 token jumps, the distribution of potential token jumps appears as follows: the 3 tokens jump in the cell's preferred target (in such situation, the SumLogLR = +1.26); 2 tokens jump in the cell's PT and 1 jumps in the OT (SumLogLR = +0.42); 1 token jumps in the cell's PT and 2 jump in the cell's OT (SumLogLR = −0.42); the 3 tokens jump in the cell's OT (SumLogLR = −1.26). To avoid any potential effect of sensory evidence on the analysis, we restricted the analysis to trials for which the SumLogLR was either −0.42 or +0.42. We averaged the neural activity of these 2 groups of trials in the 200-ms period following the 3rd token jump to get an estimate of the neural activity at zero evidence after 3 token jumps. In this analysis we also discarded all trials for which the decision duration was <600 ms. Numbers indicate how many cells reach significance (*) (solid lines), as assessed through a bootstrap procedure (see materials and methods).
Effect of previous trial outcome on C- and E-cell activity aligned on movement onset.
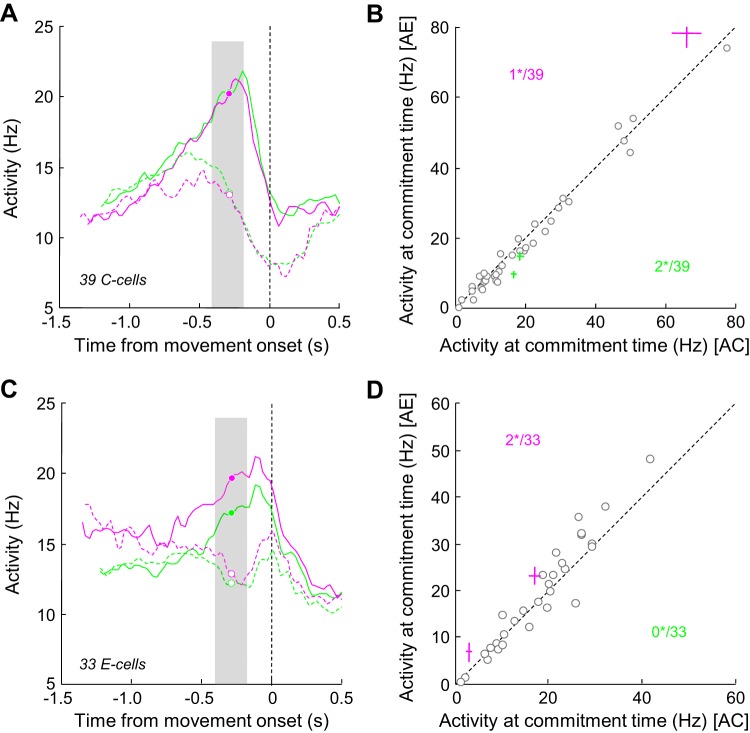
Another crucial feature of many decision making models, including the urgency-gating model (Cisek et al. 2009), relates to the decision-related activity pattern around time of movement initiation. More precisely, decision-related activity has been proposed to reach a critical level before movement initiation, regardless of the previous history of the trial, at which time the commitment to the choice is made (Hanes and Schall 1996; Thura and Cisek 2014). Here we investigate the extent to which the outcome-sensitive cells in PMd reflect this important feature.
Figure 8A illustrates the averaged response of the 39 PMd C cells described in Fig. 6C but here aligned on movement onset. Trials in the fast blocks are sorted according to the monkeys' choice, either PT or OT, and depending on the previous trial outcome. This analysis shows that PT-related activity reached a peak before movement, whereas OT-related activity was suppressed. Importantly, both the timing and the amplitude of this PT-related peak of activity were not dependent on the previous trial outcome. In a 200-ms period around our estimate of decision commitment (280 ms before movement; see Thura and Cisek 2014), we observe that only three cells (8%) were significantly modulated (WMW test, P < 0.05) by the previous trial outcome, showing either a stronger (2 of 3) or a weaker peak activity in trials following a correct choice (Fig. 8B). The averaged response of the PMd E cells aligned on movement onset reflects the same trend, with only 2 of 33 cells significantly influenced by the previous trial outcome around decision commitment (Fig. 8D). It is worth noting, however, that qualitatively 1) activity seems to reach its peak later compared with the C cell population, regardless of the previous trial conditions, and 2) there is a trend for activity to be stronger in trials following an error compared with trials following a correct choice around decision commitment (Fig. 8C), although this did not reach significance in more than 2 of 33 cells.
Fig. 8.
Effect of previous trial outcome on activity at the time of commitment. A: average response of the 39 PMd C cells aligned on movement onset. Trials are sorted according to monkeys' choice, either cells' preferred target (solid lines) or opposite target (dashed line), and previous trial outcome, a correct choice (green lines) or an error (magenta lines). Circles illustrate our estimate of commitment time, 280 ms before movement initiation (see Thura and Cisek 2014). B: comparison of mean neural activity of 39 PMd C cells recorded in a 200-ms window surrounding commitment time (gray area in A) during postcorrect (AC; x-axis) vs. posterror (AE; y-axis) trials. Each circle represents the mean response of a neuron. Colored crosses illustrate the mean (±SE) response of cells with a significant modulation of activity (green indicates stronger activity in postcorrect trials, magenta indicates stronger activity in posterror trials). Numbers indicate how many cells reach significance (*) C: same as A for a population of 33 E cells. D: same as B for the population of 33 E cells.
Effect of sensory evidence and urgency on C and E cell activity.
In a previous report, we described a population of PMd cells strongly involved in deliberation and decision commitment (Thura and Cisek 2014). These cells track the evolution of sensory evidence during deliberation, and they signal the time of commitment by reaching a peak of activity prior to movement onset. We also showed that these “decision” cells were modulated by a growing, motor-related signal that is adjusted between blocks to optimize the rate of reward of the monkey at the session level (Thura and Cisek 2016 and Fig. 2).
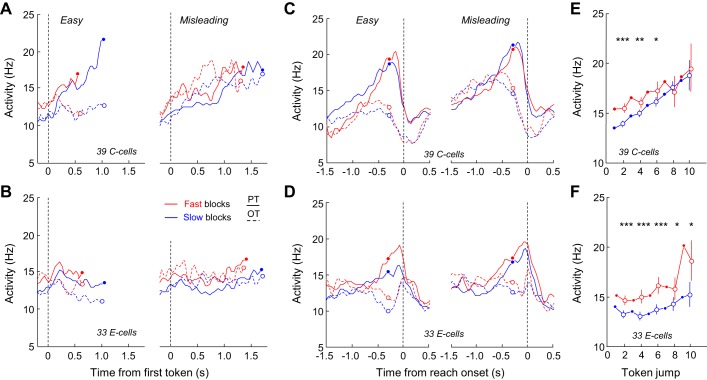
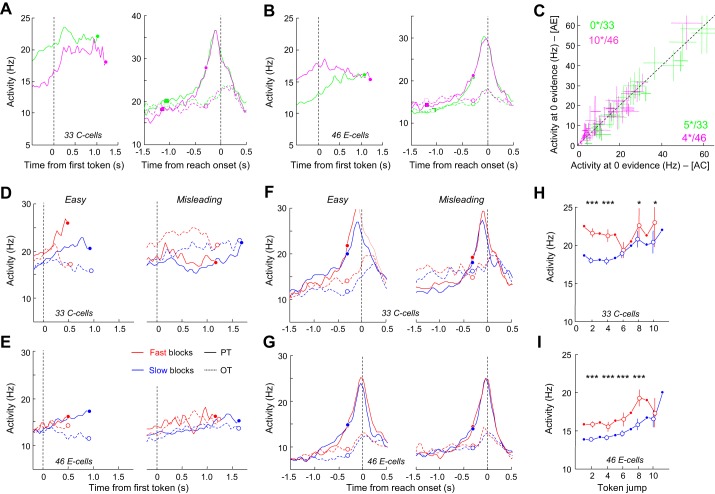
Here we submitted the PMd C and E cells to the same tests performed in these previous studies to evaluate the possibility that the two populations (the “decision” cells and the C and E cells) share common properties and belong to the same population. In PMd, for the 39 C cells and the 33 E cells modulated in the fast block, we found that 15 C cells (38%) and 11 E cells (33%) met the criterion (ROC > 0.65) to be classified as “decision” cells as in our previous work. We thus first looked at the activity of C and E cells by separating between trial types (easy and misleading) to evaluate whether their activity reflected or tracked the evolution of sensory evidence during the task. For the analysis, we pooled together data from both monkeys, and we only included cells for which at least five easy and five misleading trials were recorded. When aligned to the first token jump, C cells reflected the SP profiles of the trial types. Indeed, cells quickly discriminated the PT from the OT in easy trials, whereas discrimination was longer in misleading trials and cells' activity also reflected the switch of SP profile (at ∼1 s; Fig. 9A, right). By contrast, E cells seemed much less sensitive to the evolution of sensory evidence, especially in the fast condition (Fig. 9B). Another striking difference between the C and E cells relates to the effect of elapsing time and block condition. C cells showed a strong buildup during deliberation, and their activity was amplified in the fast blocks compared with the slow blocks. These properties were less marked in the E cell population, at least when activity was examined in the two special trial types. When aligned to movement onset, C cells' PT-related activity displayed a buildup, which reached a similar peak regardless of the trial history and block condition. Peak timing was also very consistent, occurring well before movement onset and close to the estimated time of commitment (280 ms before movement) (Fig. 9C). By contrast, peak activity of the E cells appeared much less consistent across conditions and occurred on average later, right before movement onset (Fig. 9D), as suspected from Fig. 8C.
Fig. 9.
Effect of sensory evidence, elapsing time and block condition on activity of PMd C and E cells. A, left: average activity of 39 PMd C cells aligned on 1st token jump in easy trials during which monkeys correctly chose the cells' preferred target (PT) or opposite target (OT) in the slow blocks or the fast block. To be included, cells had to be recorded during at least 5 trials per condition. In each block, activity is truncated 280 ms before movement onset (circles) and both error and correct trials are included. Right: same as left for the PMd C cells recorded in at least 5 misleading trials. B: same as A for the population of 33 E cells significantly modulated by previous trial outcome in the fast blocks. C: same as A with activity aligned on movement onset. D: same as B with activity aligned on movement onset. E: evolution of the averaged activity of the PMd C cell population calculated for the condition when SumLogLR = 0 (the evidence is equal for each target) as a function of time in either the slow (blue) or the fast (red) blocks. When data are shown for even-numbered jumps (open circles), vertical bars represent the confidence intervals around the means calculated through a bootstrap procedure (see materials and methods). Asterisks indicate significant difference: *P < 0.05, **P < 0.01, ***P < 0.001. F: same as E for 33 E cells.
The patterns of activity described above seem to suggest that C cells, but not E cells, are at least partly involved in decision formation and choice commitment, because they reflect the evolving sensory evidence and reach a critical and fixed level of activity before movement initiation. Moreover, the within-trial growth of activity as well as the effect of block condition on C cells indicate that the urgency signal could influence these cells by serving as a regulatory signal for the context-dependent SAT adjustments. Interestingly, when the neuronal activity at the zero evidence point is examined and plotted as a function of time in the two conditions (see materials and methods and Fig. 2F), C cell activity appears to linearly grow with time, independently of sensory evidence and regardless of the speed condition (ANCOVA, Time, F = 9.25, P = 0.002), and is slightly amplified in the fast condition, especially for the first six token jumps (bootstrap tests on token jumps 2, 4, and 6, P < 0.05; see materials and methods and Fig. 9), as predicted if cells are influenced by the context-dependent urgency signal (Fig. 9E). E cell activity also significantly grows with time (Fig. 9F, ANCOVA, Time, F = 8.8, P = 0.03), but in a less linear manner, as activity appears stable for the first five token jumps in both blocks. But the effect of block appears stronger in E cells compared with C cells, lasting for the entire period of deliberation (ANCOVA, Block, F = 6.32, P = 0.012, and bootstrap tests on the even token jumps, P < 0.05, Fig. 9F).
Do PMd C and E cells reflect whether the error was expected?
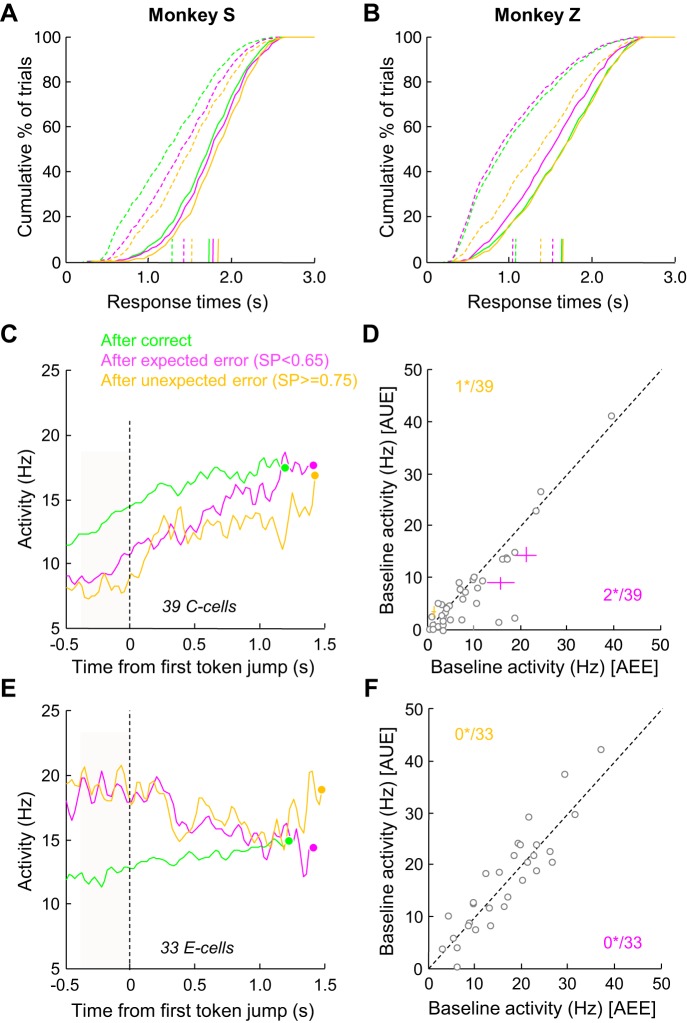
We investigated the possibility that the trial-to-trial SAT adjustment is dependent not only on the previous trial outcome, i.e., an error or a correct choice, but also on whether the error was expected or not. In the tokens task, some errors occur even when the evidence at decision time was strong (unexpected errors), while some errors occur simply because the subject makes a guess (expected errors). We thus classified trials according to the previous trial outcome, and among the posterror trials we further sorted trials according to the SP at decision time, defining either “expected” errors, when SP was <0.6, or “unexpected” (or “surprising”) errors, when SP was >0.75.
We observed that both monkeys made significantly slower decisions in trials following unexpected errors compared with trials following expected errors, regardless of the block condition (WMW test, P < 0.01 or less), but more pronounced in the fast conditions (Fig. 10, A and B). The effect of error likelihood on next trial decision duration was particularly strong in monkey Z, with a difference of decision duration reaching 346 ms between the two conditions in the fast blocks and 124 ms in the slow blocks. It is also interesting to note that in this slow condition monkey Z's RTs in trials following an unexpected error were very similar to those made in trials following a correct choice (1,634 vs. 1,650 ms), suggesting a different mechanism of trial-to-trial SAT adjustment between the blocks, as opposite results are observed in the fast condition (RTs in post-expected error and postcorrect trials are similar).
Fig. 10.
Effect of success probability on behavior and C- and E-cell response. A: cumulative distributions of response time of monkey S in trials following a correct choice, in trials following an expected error (SP < 0.65), and in trials following an unexpected error (SP > 0.75), either in the slow (solid curves) or fast (dashed curves) blocks. Vertical lines illustrate the mean of each distribution. B: same as A for monkey Z. C: average response of a population of 39 C cells modulated in the fast block, aligned on 1st token jump when monkeys chose either target. Trials are sorted according to the group defined in A: after a correct choice, after an expected error, after an unexpected error. D: comparison of mean neural activity of 39 PMd C cells recorded in a 400-ms window preceding 1st token jump during post-expected error trials (AEE; x-axis) vs. post-unexpected error trials (AUE; y-axis). Each circle represents the mean response of a neuron. Colored crosses illustrate the mean (±SE) response of cells with a significant modulation of activity (magenta indicates stronger activity in post-expected error trials). Numbers indicate how many cells reach significance (*) E and F: same as C and D for a population of 33 E cells modulated in the fast block.
Despite these strong effects of error likelihood on next trial RTs, we did not observe the corresponding neural correlates in the C and E cells in PMd (Fig. 10, C–F). Indeed, only three C cells (of 39) showed a significant modulation of baseline activity in trials following expected vs. unexpected errors, and none of the E cells showed such modulation.
Relation between neural activity and behavior.
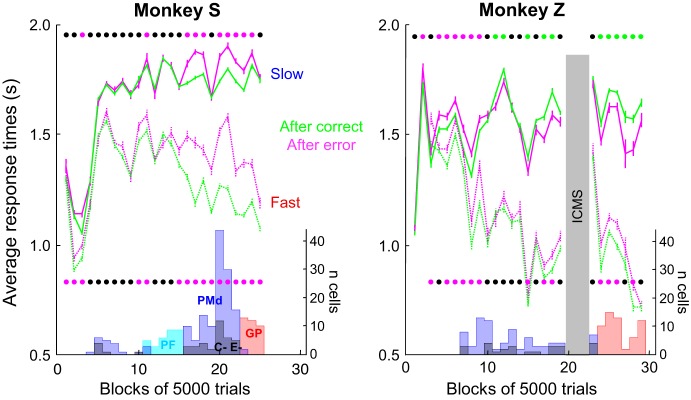
Finally, we investigated the degree of relationship between neural modulation and the behavioral SAT adjustments during the task. For monkey S, who displayed PES in both blocks, the dates when neurons were recorded coincided with the dates when the monkey displayed statistically significant PES. However, when monkey Z did not display PES during the slow block, we still at times detected C and E cells (Fig. 11). We found a similar discrepancy between neural modulations and behavior in the fast condition. More specifically, among the 39 C cells modulated during the fast blocks, 14 were recorded in sessions during which no significant PES was observed (mostly in monkey Z). Similarly, among the 33 E cells modulated in the fast blocks, 12 were recorded during sessions with no PES. We calculated the correlation between the difference of baseline activity in posterror vs. postcorrect trials and the difference of RTs in the same two conditions. Although we found a trend for a stronger modulation of neuronal activity in sessions during which monkeys displayed large PES, the effect was not significant, either for the C cells (Pearson linear correlation r = 0.27; P = 0.09) or for the E cells (r = −0.29, P = 0.1).
Fig. 11.
Monkeys' response times through practice and corresponding occurrence of recorded neurons. Each panel illustrates the evolution of monkey S (left) and monkey Z (right) mean (±SE) response times over the course of training in slow (solid lines) and fast (dotted lines) blocks. Within each block, trials are sorted according to the previous trial outcome, either a correct choice (green lines) or an error (magenta lines). Each bin represents the average response time calculated across 5,000 trials performed in either the fast or the slow blocks. Lines of dots show the results of statistical WMW tests performed on data within each bin to assess the significance of the effect of a correct choice vs. an error on the response time of the next trial in the slow (top line) and the fast (bottom line) blocks. Black dots show bins with no significant effect; green dots illustrate bins in which response times are significantly longer after a correct choice compared with an error. Magenta dots illustrate the opposite pattern. In monkey Z, bins of trials during which intracortical microstimulation (ICMS) sessions (data not shown) have been performed are excluded (gray vertical bar). At bottom of each panel, histograms illustrate the number of PMd (blue), PMd C and E (black), PFC (light blue), and globus pallidus (GP; red) cells recorded within each bin of 5,000 trials.
Effect of previous trial outcome on M1 activity.
We performed the same set of analyses (including the control analyses) described above for the population of 179 M1 cells (monkey S, n = 71). We found a substantial population of cells (105/179, 59%) whose baseline activity was significantly modulated by the previous trial outcome. As in PMd, some M1 cells' baseline activity increased in trials following a correct choice (C cells, monkey S: 10/71 and 12/71 in the slow and fast blocks, respectively, WMW, P < 0.05; monkey Z: 17/108 and 21/108) and other cells had the reverse pattern (E cells, monkey S: 13/71 and 16/71 in the slow and fast blocks, respectively; monkey Z: 24/108 and 31/108). As in PMd, of the cells that were modulated by the previous trial outcome in the two blocks (16 C cells and 19 E cells), all of them were modulated in the same direction, i.e., we never observed a cell for which the effect of previous trial outcome was reversed between the blocks. Figure 12, A and B, show the average response of the M1 C and E cells modulated in the fast blocks but not necessarily in the slow condition, aligned either on the first token jump, showing the effect of previous trial outcome on baseline activity, or on movement onset. The effect of previous trial outcome vanished quite quickly as soon as the trial started, especially for the E cells (see Fig. 12B, left). It is also interesting to note that, in contrast to PMd, the activity of M1 C cells does not tend to grow over the time course of deliberation. When aligned on movement onset, both C and E population activities are similar regardless of the previous trial outcome, showing a peak when monkeys chose the PT or a steady pattern when they chose the OT. It is worth noting that for both populations the PT-related peak of activity occurred later compared with what is observed in PMd for the C cells (see Fig. 8A).
Fig. 12.
C cells and E cells in motor cortex. A: average response of 33 M1 C cells modulated in the fast condition but not necessarily in the slow condition. Activity is aligned on 1st token jump (left, PT- and OT-related averaged activity) or on movement onset (right, PT- and OT-related activity separated; same conventions as in Fig. 8) when monkeys performed the tokens task in the fast condition. Trials are sorted according to the previous trial outcome, either a correct choice (green) or an error (magenta). Circles illustrate commitment time, squares show 1st token jump. B: same as A for a population of 46 E cells modulated in the fast block but not necessarily in the slow condition. C: mean (±confidence intervals) activity after 3 token jumps of the 33 M1 C cells (green) and 46 M1 E cells (magenta) that were significantly modulated by previous trial outcome in the fast blocks but not necessarily slow blocks. Same conventions as in Fig. 7. D: average response of the population of 33 M1 C cells recorded in easy (left) or misleading (right) trials in slow blocks and fast blocks aligned on 1st token jump. Same conventions as in Fig. 9. E: same as D for a population of 46 E cells. F: same as D, but here activity is aligned on movement onset. G: same as F for the population of 46 E cells. H: evolution of the averaged activity of the 33 M1 C cells population calculated for the condition when SumLogLR = 0 (the evidence is equal for each target) as a function of time in either the slow (blue) or the fast (red) blocks. Same conventions as in Fig. 9E. I: same as H for the population of M1 E cells. *P < 0.05, ***P < 0.001.
As suspected from Fig. 12, A and B, the effect of previous trial outcome extends to a certain degree during the deliberation process, but not as clearly as what we observed in PMd. Indeed, we found that in only 5/33 (15%) of the C cells and 10/46 (22%) of the E cells was the modulation of the previous trial outcome still significant and in the same direction from 600 to 800 ms after the first token jump. We also found that for four of the E cells the effect of previous trial outcome changed with higher activity in trials following a correct choice than following an error (Fig. 12C).
Together, these data suggest that the M1 C and E cells are likely to play less of a causal role in trial-to-trial adjustments of SAT than the PMd C and E cells. However, to better assess this possibility, we investigated the effect of sensory evidence and urgency on the M1 C- and E-cell activity.
Effect of sensory evidence and urgency on M1 C and E cells.
As for PMd, activity patterns of these cells, aligned on the first token jump in the easy or misleading trials, show that C cells reflected the evolving sensory evidence in the two trial types (note the early tuning of the C cells in easy trials compared with misleading trials; Fig. 12D). This effect of sensory evidence appeared less pronounced in E cells (Fig. 12E). In agreement with this observation, we found that about half of the M1 C cells (16/33, 48%), but fewer E cells (18/46, 39%), were significantly tuned before decision commitment, a criterion that allowed us to define cells as “decision related” in our previous work.
Compared with PMd C cells, both M1 C and E cell populations also appear to be strongly involved in movement initiation, as PT-related activity sharply grew before decision commitment and reached a peak shortly before movement onset. Although slightly higher in the fast blocks, this peak was not significantly influenced at the population level (in terms of both timing and magnitude) by the block condition in which trials were executed (Fig. 12, F and G).
Finally, we found a strong effect of the block condition on both C- and E-cell activity. Indeed, we found that the SAT condition in which the task was performed (slow or fast) significantly modulated the baseline activity in 19 of 33 C cells (57%) and in 30 of 46 E cells (65%). This result indicates that the urgency signal (which is hypothesized to be higher in the fast blocks compared with the slow blocks) could influence these cells, as observed in PMd. When the neuronal activity at the zero evidence point is examined and plotted as a function of time in the two conditions, C-cell activity appears to modestly grow with time, independently of sensory evidence and regardless of the speed condition (ANCOVA, Time, F = 0.79, P = 0.37), and is amplified in the fast condition, especially for the first five token jumps (Fig. 12H). In contrast to C cells, E-cell activity grows relatively weakly but significantly with time (ANCOVA, Time, F = 8.99, P = 0.003), but the effect of block appears stronger, lasting for almost the entire period of deliberation (ANCOVA, Block, F = 5.3, P = 0.02; Fig. 12I).
DISCUSSION
In this study, we were interested in exploring the neural basis of adjustments of SAT that were based on the result of the previous trial decision, either an error or a rewarded choice.
To this aim, we first examined that, at the behavioral level, monkeys engaged in a dynamic decision making task not only adjusted their SAT over extended periods of time, between blocks of trials encouraging either slow or fast decisions (Thura et al. 2014), but also more locally, from trial to trial, based on the result of the previous decision. Looking at decision duration and SP distributions, we observed such local adjustment, with monkeys making longer and more accurate decisions after error trials compared with trials following correct choices (Kleiter and Schwarzenbacher 1989; Laming 1979a, 1979b; Rabbitt and Rodgers 1977). Just like classic SAT adjustments, this phenomenon, named posterror slowing (PES), seems widespread across species and rather insensitive to constraints or instructions of laboratory tasks (Debener et al. 2005; Gehring and Fencsik 2001; Jentzsch and Dudschig 2009; King et al. 2010; Narayanan and Laubach 2008; Purcell and Kiani 2016). Both monkeys adopted this strategy in blocks of trials favoring fast decisions. In blocks encouraging slow and accurate decisions, however, one monkey tended to make faster decisions after error trials (Fig. 3). Although less often reported, such posterror speeding has been previously observed (King et al. 2010; Notebaert et al. 2009; Purcell and Kiani 2016). Taken together, these modifications in subjects' behavior induced by penalizing past events have been referred as “posterror adjustments” (Danielmeier and Ullsperger 2011).
In the present study, monkeys performed the task under two possible conditions in successive and alternating blocks of “slow” or “fast” trials, respectively encouraging either conservative and safe decisions or hasty and risky guesses. We previously demonstrated that animals' behavior significantly relies upon these SAT situations, as proper context-dependent SAT adjustments increase what matters the most for animals, the rate of reward (Thura et al. 2014). Here it appears that trial-to-trial adjustments of the SAT are idiosyncratic and less robust compared with the more global SAT adjustments between the blocks, yet it is also possible that each SAT context affected how animals treated the more local adjustments applied during successive decisions. In this view, two mechanisms for SAT adjustments could interact or even compete against each other during successive decisions: one global, set to optimize behavior across a large number of trials depending on timing parameters, and another more local, adjusted after every decision. One of our monkeys seemed to take advantage of the two mechanisms because it applied PES regardless of the block condition but also adjusted its behavior as a function of the block condition. The other animal's strategy was less clear because it displayed PES in the fast block but posterror speeding in the slow block. For this animal, it is possible that between the two mechanisms the more valuable (or penalizing) in terms of reward expectation—the global adjustment—dominated and suppressed the other. We can imagine for instance that the animal may have been “upset” because a lack of payoff caused a loss of time, leading to higher arousal that caused it to make hasty and impulsive decisions. However, even if true, arousal cannot be the only explanation because the probability of making errors also influenced this animal's behavior. Indeed, when expected, an error induced very fast decisions in the following trial, whereas unexpected errors induced slower decisions, similar to those made after correct choices (Fig. 10B). The reasons why one monkey tended to speed up after committing errors in the slow blocks are thus complex and not completely clear from our results.
We then explored the neural basis of SAT from trial to trial through posterror adjustment. We endeavored to mainly explain PES, as it is the major effect of previous trial outcome we report here and, contrary to posterror speeding, the significance of PES has been extensively studied and debated (Danielmeier and Ullsperger 2011). Several hypotheses have been proposed to explain the reason why one should slow down after making an error (Dutilh et al. 2012b; Laming 1979a, 1979b; Notebaert et al. 2009; Rabbitt and Rodgers 1977). Among these, one appealing possibility is that an error forces subjects to make the next decision with more caution by spending more time on the deliberation process in order to guarantee higher SP (Botvinick et al. 2001; Rabbitt and Rodgers 1977; Smith and Brewer 1995).
In most models of decision making where a decision variable starts from a given level and reaches a fixed decision threshold (Ratcliff et al. 2016), such elongated decisions can be implemented either by lowering the initial level of the decision variable or by increasing the threshold. At the neural level, it has been demonstrated that neurons of many areas involved in decision making reach a fixed level of activity at time of choice commitment (Hanes and Schall 1996; Roitman and Shadlen 2002; Thura and Cisek 2014), suggesting that for the same quality of sensory evidence any adjustment of decision time should be implemented by a change of the baseline activity of these “decision-related” cells. In the context of the present study, we predicted that after error trials, when making more conservative decisions, decision-related cells should have lower baseline and deliberation period activity.
We thus recorded activity of PMd and M1 neurons while monkeys were performing the tokens task. We demonstrated previously that these areas contain many neurons strongly involved in tracking the sensory information used by animals to guide their choices, signaling the time at which they commit to a choice and setting the global SAT regime in which the task is performed (Fig. 2; Thura and Cisek 2016). We reasoned that if previous trial outcome influences animals' behavior locally, from one decision to the next, these same areas should also show some signature of this phenomenon (Fecteau and Munoz 2003; Purcell and Kiani 2016). In agreement with this prediction, we found a group of cells (C cells) in both PMd and M1 that could in part underlie the neural circuits promoting PES. Indeed, these cells were significantly less active in trials following erroneous decisions compared with trials following correct choices (Fig. 5B, C cells). This decrease of activity was mostly visible during the intertrial interval (Fig. 6) but also extended for ∼30% of the cells during the period of deliberation (Fig. 7). Interestingly, however, at the time the decision was reported the activity of almost all C cells was similar regardless of the previous trial outcome (Fig. 8).
We proposed that if these C cells were part of the neural substrate of a local SAT adjustment during successive decisions, then, in addition to previous trial outcome information, they should also carry some pertinent information related to the tokens task. In agreement with this proposal, we found that C cells build up with urgency as the trial unfolds and they appear to follow the evolving sensory evidence provided to animals during every trial (Fig. 9). As a consequence, these data support our prediction that C cells are related to PES by reducing their baseline and their deliberation period activity after errors and increasing them after correct trials.
However, unexpectedly, we found another group of cells that were significantly modulated by the previous trial outcome. These neurons (termed E cells) were more active during their baseline period after error trials compared with trials following correct decisions (Fig. 5D). If modulated in both blocks these cells displayed the same perfect consistency in PMd and in M1 as C cells, and if modulated in the deliberation period most of them maintained their selectivity as well. However, unlike C cells, E cells appeared to be less related to sensory evidence (Fig. 9), and their activity did not build up as much as the trial unfolded (Fig. 6). This difference in the cells' waveforms may suggest that C and E cells are involved in PES in different ways. By starting at a lower baseline and having lower activity during the deliberation period after errors, C cells may take longer to reach the threshold for decision, thus leading to PES. On the other hand, E cells may withhold decisions from being taken, allowing the monkeys to slow down after errors. Another possibility is that both C- and E-cell activity contribute positively to commitment, and thus the posterror decrease of C-cell activity slows down the next trial while the posterror increase of E-cell activity speeds it up. In this scenario, it is the net effect at the population level that determines whether posterror slowing or speeding is observed, potentially explaining why error trials usually lead to slower RTs in the next trial but sometimes can have no effect (Fiehler et al. 2005) or lead to faster RTs (King et al. 2010; Notebaert et al. 2009; Purcell and Kiani 2016).
Although we also commonly found C and E cells in M1, these cell groups exhibited less of a difference in their properties (Fig. 12). The firing rate of M1 C cells displayed less buildup as the trial unfolded than PMd C cells, and they had more late peaks, suggesting they were more related to movements rather than decisions. Hence, these cells may be related to PES in a different way than PMd C cells, being more involved in the output behavior. Yet, it is worth mentioning another account for PES here. It has been proposed that PES might be a motor mechanism rather than a cognitive one. In this view, an error induces an increase of a selective suppression/inhibition of the motor programs engaged in the next trial (Ridderinkhof 2002). This hypothesis is supported by EEG data showing that PES correlates with the power of beta-band oscillations, with more beta oscillations following error trials, leading to PES (Marco-Pallares et al. 2008). Because M1 has been shown to be part of the neural circuits involved in inhibitory control (Bestmann and Duque 2016), the M1 neurons sensitive to the previous trial outcome shown in the present study strengthen a motor nature of PES. In agreement with a role of M1 in PES via motor inhibition, two other functional MRI studies and one transcranial magnetic stimulation study demonstrated reduced motor cortex activity after error trials (Amengual et al. 2013; Danielmeier et al. 2011; King et al. 2010).
Altogether, these data, including ours, suggest that PES results from multiple cognitive and motor mechanisms, which are not mutually exclusive and are in part implemented in PMd and M1 when decisions have to be reported via reaching movements. Several other studies have investigated the neural correlates of posterror adjustments through various recording techniques and behavioral paradigms. These studies have shown various cortical and subcortical brain regions whose activity correlates with PES (e.g., Cavanagh et al. 2014; Li et al. 2008; Narayanan and Laubach 2008), suggesting that a large network of brain areas may be involved in PES, potentially depending on the nature of the decisions or on the effector used to report them. For instance, in one of these studies (Narayanan and Laubach 2008), M1 neurons did not show any signature of PES, contradicting the present results. However, these neurons were recorded in rats performing a simple reaction time task, making the results difficult to compare with our data, in which monkeys can freely adjust their SAT.
More recently, Purcell and Kiani (2016) reported a set of cells recorded in monkey LIP while animals performed a decision task that is more comparable to the one used in our own study. Despite differences in effector and region studied, several similarities arise between Purcell and Kiani's study and ours. First, the majority of subjects in Purcell and Kiani exhibited clear PES, except for two (1 monkey and 1 human) that showed posterror speeding, similar to one of our monkeys, supporting the idea that PES is not a systematic behavior. Second, Purcell and Kiani described some LIP “decision-related” cells significantly modulated by the previous trial outcome. Similar to our decision-related cells, these PES-related LIP cells were sensitive to sensory evidence and reached a fixed threshold at time of commitment.
However, there are also some differences in the results reported in these two studies. First, while our study revealed two kinds of cells with different response dynamics, Purcell and Kiani's study reported only a single group of cells that respond to both posterror and postcorrect trials. The reason for this difference is unknown, although one possibility may be because they underlie different systems—the arm system vs. the oculomotor system. Another possibility may be that our cells are further downstream, in frontal rather than parietal cortex. Second, in the Purcell and Kiani report, the LIP cells' dynamic changes to error trials differ from those observed in our PMd/M1 cells: LIP neurons display a reduced urgency component and a reduced sensitivity to stimulus strength after error trials. In our study, neurons' response to previous trial outcome mainly occurs through baseline activity adjustments, with both populations increasing or decreasing their activity depending on the previous outcome. It is only when one monkey showed posterror speeding that Purcell and Kiani observed an increase of baseline activity in some of their LIP neurons (similarly to our E cells). Finally, in their study, when monkeys displayed different posterror adjustments, their cells behaved differently. Unlike Purcell and Kiani's results, we did not observe this difference when one of our monkeys did not display PES. However, the differences in behavior observed by Purcell and Kiani occurred between monkeys, rather than within a monkey and between blocks, such as in our study. Hence, it is possible that Purcell and Kiani were measuring from different cells in the monkey that did not display PES.
Hence, although both Purcell and Kiani's cells and our own are related to PES, different mechanisms appear to underlie the role of each set of cells. Thus further studies are needed to elucidate the relationship between the results of these studies.
Despite the robustness of the results in the present study, the functional significance of these neurons remains an issue that our data cannot fully explain. First, in terms of behavior, both monkeys displayed PES in a large proportion of trials, with the exception of monkey Z in the slow block. As noted above, such variability has been observed previously (King et al. 2010; Notebaert et al. 2009, Purcell and Kiani 2016). However, in our study, when monkey Z did not display PES during the slow block, we still at times detected C and E cells, suggesting that these cells cannot be by themselves sufficient to cause PES. For monkey S, who displayed PES in both blocks, the dates when neurons were recorded coincided with the dates when the monkey displayed statistically significant PES (Fig. 11). In monkey Z, we found no such coinciding dates, suggesting that even when these C and E cells were present, other factors must have also been influencing the monkey's decision, such as, for instance, its frustration with an error that may lead to arousal. Previous trial outcome is not necessarily the sole determinant of a decision, and hence it is reasonable that these cells are not, either. Instead, we believe that these cells are part of a circuit that keeps track of previous trial outcome and uses it, along with other factors, to generate a behavioral adjustment.
In addition, while the probability of making a correct choice significantly modulates monkeys' behavior in the next trial, the cells' activity was not significantly modulated by the expectedness of the previous trial outcome. This suggests that these cells may not be tracking the more cognitive aspect of the previous trial outcome that is used to adjust behavior in the next trial. In light of EEG research in humans (Taylor et al. 2007; Wang et al. 2005) and recent work on monkeys (Michelet et al. 2016), the prefrontal cortex areas, especially the anterior cingulate cortex, may serve the role of outcome trackers and error detectors. Instead, the C and E cells may serve to integrate the most basic outcome information with the consequent behavior.
Hence, the sum of our findings suggests that a subpopulation of cells in PMd and M1 is involved in PES, a local trial-to-trial adjustment in SAT. Although the functional role of these cells requires further studies to be more thoroughly elucidated, our results robustly suggest the existence of two groups of PES-related cells, with some of them also involved in the setting of a more global SAT adjustment mechanism occurring between fast and slow blocks of trials (Thura et al. 2014). The fact that both PMd and M1 C and E cells are strongly modulated by block condition agrees with this hypothesis. Altogether, these global and local SAT adjustments allow the monkeys to efficiently trade speed against accuracy during decision making and maximize their reward rate.
GRANTS
This research was supported by grants from the Canadian Institutes of Health Research (MOP-102662), the Canadian Foundation for Innovation, the Fonds de Recherche en Santé du Québec, the EJLB Foundation (to P. Cisek), and fellowships from the Fyssen Foundation and the Groupe de Recherche sur le Système Nerveux Central (to D. Thura).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.T., G.G., and P.C. conceived and designed research; D.T. performed experiments; D.T. and G.G. analyzed data; D.T., G.G., and P.C. interpreted results of experiments; D.T. and G.G. prepared figures; D.T., G.G., and P.C. drafted manuscript; D.T., G.G., and P.C. edited and revised manuscript; D.T., G.G., and P.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marie-Claude Labonté for technical support.
REFERENCES
- Amengual JL, Marco-Pallares J, Richter L, Oung S, Schweikard A, Mohammadi B, Rodriguez-Fornells A, Munte TF. Tracking post-error adaptation in the motor system by transcranial magnetic stimulation. Neuroscience 250: 342–351, 2013. [DOI] [PubMed] [Google Scholar]
- Balci F, Simen P, Niyogi R, Saxe A, Hughes JA, Holmes P, Cohen JD. Acquisition of decision making criteria: reward rate ultimately beats accuracy. Atten Percept Psychophys 73: 640–657, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Duque J. Transcranial magnetic stimulation: decomposing the processes underlying action preparation. Neuroscientist 22: 392–405, 2016. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Priming in macaque frontal cortex during popout visual search: feature-based facilitation and location-based inhibition of return. J Neurosci 22: 4675–4685, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev 108: 624–652, 2001. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Sanguinetti JL, Allen JJ, Sherman SJ, Frank MJ. The subthalamic nucleus contributes to post-error slowing. J Cogn Neurosci 26: 2637–2644, 2014. [DOI] [PubMed] [Google Scholar]
- Chittka L, Skorupski P, Raine NE. Speed-accuracy tradeoffs in animal decision making. Trends Ecol Evol 24: 400–407, 2009. [DOI] [PubMed] [Google Scholar]
- Cisek P, Puskas GA, El-Murr S. Decisions in changing conditions: the urgency-gating model. J Neurosci 29: 11560–11571, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. J Neurosci 31: 1780–1789, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Ullsperger M. Post-error adjustments. Front Psychol 2: 233, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci 25: 11730–11737, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilh G, van Ravenzwaaij D, Nieuwenhuis S, van der Maas HL, Forstmann BU, Wagenmakers EJ. How to measure post-error slowing: a confound and a simple solution. J Math Psychol 208–216, 2012a.
- Dutilh G, Vandekerckhove J, Forstmann BU, Keuleers E, Brysbaert M, Wagenmakers EJ. Testing theories of post-error slowing. Atten Percept Psychophys 74: 454–465, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci 19: 2740–2754, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Exploring the consequences of the previous trial. Nat Rev Neurosci 4: 435–443, 2003. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, von Cramon DY. Electrophysiological correlates of error correction. Psychophysiology 42: 72–82, 2005. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Anwander A, Schafer A, Neumann J, Brown S, Wagenmakers EJ, Bogacz R, Turner R. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci USA 107: 15916–15920, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmakers EJ. Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci USA 105: 17538–17542, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NR, Dornhaus A, Fitzsimmons JP, Stevens M. Speed versus accuracy in collective decision making. Proc Biol Sci 270: 2457–2463, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci 21: 9430–9437, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966. [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996. [DOI] [PubMed] [Google Scholar]
- Hanks T, Kiani R, Shadlen MN. A neural mechanism of speed-accuracy tradeoff in macaque area LIP. Elife 2014: e02260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz RP, Schall JD. Neural mechanisms of speed-accuracy tradeoff. Neuron 76: 616–628, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N, Coles MG, Cohen JD. A mechanism for error detection in speeded response time tasks. J Exp Psychol Gen 134: 163–191, 2005. [DOI] [PubMed] [Google Scholar]
- Jentzsch I, Dudschig C. Why do we slow down after an error? Mechanisms underlying the effects of posterror slowing. Q J Exp Psychol (Hove) 62: 209–218, 2009. [DOI] [PubMed] [Google Scholar]
- King JA, Korb FM, von Cramon DY, Ullsperger M. Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. J Neurosci 30: 12759–12769, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiter GD, Schwarzenbacher K. Beyond the answer: post-error processes. Cognition 3: 255–277, 1989. [DOI] [PubMed] [Google Scholar]
- Laming D. Autocorrelation of choice-reaction times. Acta Psychol (Amst) 43: 381–412, 1979a. [DOI] [PubMed] [Google Scholar]
- Laming D. Choice reaction performance following an error. Acta Psychol (Amst) 43: 199–224, 1979b. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci 20: 1021–1029, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Pallares J, Camara E, Munte TF, Rodriguez-Fornells A. Neural mechanisms underlying adaptive actions after slips. J Cogn Neurosci 20: 1595–1610, 2008. [DOI] [PubMed] [Google Scholar]
- Michelet T, Bioulac B, Langbour N, Goillandeau M, Guehl D, Burbaud P. Electrophysiological correlates of a versatile executive control system in the monkey anterior cingulate cortex. Cereb Cortex 26: 1684–1697, 2016. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J Neurophysiol 100: 520–525, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Opstal FV, Gevers W, Fias W, Verguts T. Post-error slowing: an orienting account. Cognition 111: 275–279, 2009. [DOI] [PubMed] [Google Scholar]
- Pachella RG. The interpretation of reaction time in information processing research. In: Human Information Processing: Tutorials in Performance and Cognition, edited by Kantowitz BH. Hillsdale, NJ: Erlbaum, 1974, p. 41–82. [Google Scholar]
- Purcell BA, Kiani R. Neural mechanisms of post-error adjustments of decision policy in parietal cortex. Neuron 89: 658–671, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P, Rodgers B. What does a man do after he makes an error? An analysis of response programming. Q J Exp Psychol 727–743, 1977.
- Rank M, Di Luca M. Speed/accuracy tradeoff in force perception. J Exp Psychol Hum Percept Perform 41: 738–746, 2015. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Smith PL, Brown SD, McKoon G. Diffusion decision model: current issues and history. Trends Cogn Sci 20: 260–281, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR. Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychol Res 66: 312–323, 2002. [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22: 9475–9489, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT, Movshon JA. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci 16: 1486–1510, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Brewer N. Slowness and age: speed-accuracy mechanisms. Psychol Aging 10: 238–247, 1995. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist 13: 160–172, 2007. [DOI] [PubMed] [Google Scholar]
- Thura D, Cisek P. Deliberation and commitment in the premotor and primary motor cortex during dynamic decision making. Neuron 81: 1401–1416, 2014. [DOI] [PubMed] [Google Scholar]
- Thura D, Cisek P. Modulation of premotor and primary motor cortical activity during volitional adjustments of speed-accuracy trade-offs. J Neurosci 36: 938–956, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thura D, Cos I, Trung J, Cisek P. Context-dependent urgency influences speed-accuracy trade-offs in decision-making and movement execution. J Neurosci 34: 16442–16454, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K, Oya T, Uchiyama Y. Effects of speed and accuracy strategy on choice step execution in response to the flanker interference task. Hum Mov Sci 32: 1393–1403, 2013. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci 25: 604–613, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren WA. Speed-accuracy trade-off and information processing dynamics. Acta Psychol 41: 67–85, 1977. [Google Scholar]
- Zhang J, Rowe JB. Dissociable mechanisms of speed-accuracy tradeoff during visual perceptual learning are revealed by a hierarchical drift-diffusion model. Front Neurosci 8: 69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]