These results are the first description of visual response properties of the most commonly studied marsupial model organism, the short-tailed opossum (Monodelphis domestica). Further, these results are the first to demonstrate experience-dependent plasticity in the visual system of a marsupial species. Thus the ability of cortical neurons to alter their properties based on the dynamics of the visual environment predates the emergence of eutherian mammals and was likely present in our earliest mammalian ancestors.
Keywords: primary visual cortex, neocortex, experience-dependent plasticity, comparative
Abstract
The functional organization of the primary visual area (V1) and the importance of sensory experience in its normal development have been well documented in eutherian mammals. However, very few studies have investigated the response properties of V1 neurons in another large class of mammals, or whether sensory experience plays a role in shaping their response properties. Thus we reared opossums (Monodelphis domestica) in normal and vertically striped cages until they reached adulthood. They were then anesthetized using urethane, and electrophysiological techniques were used to examine neuronal responses to different orientations, spatial and temporal frequencies, and contrast levels. For normal opossums, we observed responses to the temporal and spatial characteristics of the stimulus to be similar to those described in small, nocturnal, eutherian mammals such as rats and mice; neurons in V1 responded maximally to stimuli at 0.09 cycles per degree and 2.12 cycles per second. Unlike other eutherians, but similar to other marsupials investigated, only 40% of the neurons were orientation selective. In stripe-reared animals, neurons were significantly more likely to respond to vertical stimuli at a wider range of spatial frequencies, and were more sensitive to gratings at lower contrast values compared with normal animals. These results are the first to demonstrate experience-dependent plasticity in the visual system of a marsupial species. Thus the ability of cortical neurons to alter their properties based on the dynamics of the visual environment predates the emergence of eutherian mammals and was likely present in our earliest mammalian ancestors.
NEW & NOTEWORTHY These results are the first description of visual response properties of the most commonly studied marsupial model organism, the short-tailed opossum (Monodelphis domestica). Further, these results are the first to demonstrate experience-dependent plasticity in the visual system of a marsupial species. Thus the ability of cortical neurons to alter their properties based on the dynamics of the visual environment predates the emergence of eutherian mammals and was likely present in our earliest mammalian ancestors.
almost everything we know about visual cortex in mammals comes from work on a few animal models such as mice, rats, cats, ferrets, tree shrews, and monkeys (Chapman and Stryker 1993; Girman et al. 1999; Hubel and Wiesel 1959; LeVay et al. 1975; Niell and Stryker 2008; for reviews, see Espinosa and Stryker 2012; Veit et al. 2014). These models comprise an extremely small subset (∼0.1%) of more than 4,000 species of extant eutherian mammals, and an even smaller proportion of all mammals (0.07%), including extant marsupials and monotremes. Although few in number, the comparative studies that have been performed suggest a large degree of diversity of visual cortex organization (see Ibbotson and Mark 2003; Van Hooser 2007; Veit et al. 2014). However, the paucity of data on other species of eutherians, and especially other clades, makes it difficult to accurately infer the organization of visual cortex in our ancient ancestors and to directly extrapolate data on features of organization, such as neural response properties of primary visual cortex (V1), to the last common ancestor of both eutherian and metatherian mammals. For some time, our laboratory has been interested in how the neocortex in our early mammalian ancestors was organized, the features of the visual scene to which neurons in V1 were tuned, and how visual cortex and the visually mediated behavior it generates evolved in different lineages and diversified to meet environmental challenges. We are also interested in how this remarkable chunk of tissue came to adopt properties that allow the neurons to change their response properties over the course of a lifetime. This ability allows sensory neurons in the neocortex to be shaped by the statistics of the environment in which the brain develops. Often called experience-dependent plasticity, it ensures that salient features of the changing sensory environment can be detected. We, and others, have proposed that a distinguishing feature of humans is their ability to generate highly adaptive behaviors throughout a lifetime due to the capacity of their neocortex to be shaped by early experience (Kaas 2012; Krubitzer and Stolzenberg 2014).
This dynamic capacity of the brain did not evolve de novo in humans, or even in primates, but some form of this plasticity was present in the ancestor of eutherian mammals. This inference comes from studies in nonprimate mammals that demonstrate that retino-geniculo-cortical connections are highly impacted by sensory-driven activity early in development and that response properties of neurons in V1 can be shaped by early visual context (e.g., Blakemore and Mitchell 1973; Chapman and Stryker 1993; Hubel and Wiesel 1963; Kreile et al. 2011; Li et al. 2006; O'Hashi et al. 2007; Rochefort et al. 2011; Wiesel and Hubel 1963). Is this also true for our earliest ancestors? We appreciate that the neocortex is the hallmark of mammalian evolution, but could an equally important mammalian distinction be the capacity of the neocortex to be shaped by early sensory experience? This ability would allow mammals to generate adaptive behavior that matches the environment in which an individual develops, and generate a wider range of individual differences within a population upon which natural selection could act. Currently, there are no studies that have directly addressed this issue.
As ancestral forms can obviously not be studied directly, the next best alternative is to perform a comparative analysis of noneutherian mammals such as marsupials and monotremes. In the current investigation we reared the small, South American short-tailed opossum (Monodelphis domestica) in different visual environments (stripe-reared and standard) to address three questions. First, which features of a visual stimulus such as orientation, spatial and temporal frequency, and contrast, drive neurons in V1? Second, how do neural responses to these features compare with those in eutherian mammals in general, and with eutherian mammals that are similar in size and lifestyle to the short-tailed opossum? These data are essential for understanding what features of organization in primary visual cortex are shared between marsupial and eutherian mammals, likely due to inheritance from their last common ancestor that existed over 180 million years ago (Meredith et al. 2011; O'Leary et al. 2013; Woodburne et al. 2003). Third, to what extent are the properties of these neurons shaped by early visual context? By stripe-rearing opossums using similar methodologies to those previously used in studies of eutherian mammals, we could directly compare response properties of neurons in V1 to those of their standard reared counterparts, and to stripe-reared eutherian mammals. Visual response properties were assessed with single-unit electrophysiological recording techniques and recording sites were directly related to cortical architecture.
METHODS
Electrophysiological recordings were made in 13 adult short-tailed opossums (Monodelphis domestica; 8 male, 5 female) weighing 76–141 g. In these studies, we examined the neural response properties of V1 in both normal animals (n = 8) as well as animals reared from birth in a striped environment (n = 5). At all recording sites, tuning curves for neurons to different orientations, spatial frequencies (SF), temporal frequencies (TF), and contrast values were generated. Following electrophysiological mapping, animals were euthanized and the location of recording sites in neocortex was confirmed histologically. In two additional animals (1 male, 1 female) the eyes were extracted following perfusion, and axial length was measured. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Davis, and all experiments were performed under the National Institutes of Health's guidelines for the care of animals in research.
Rearing Conditions
Animals were reared in standard, clear polycarbonate rat cages (45 cm × 24 cm × 18 cm, Fig. 1A) with 2 to 5 cm of Biofresh comfort bedding (Absorption, Ferndale, WA), along with a plastic nesting cup and shredded paper towels. Cages were changed weekly. All animals were housed on a 14:10-h light/dark cycle, and food and water were available ad libitum (Purina Cat Chow, Nestle, St. Louis, MO).
Fig. 1.
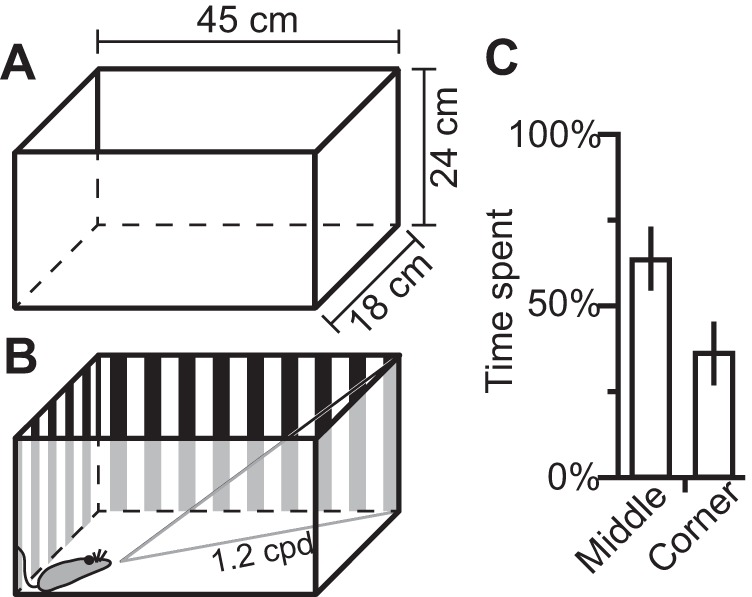
Illustrations of the size of normal (A) and stripe-reared animal's cages (B). The stripes were designed to be at a spatial frequency of 1.2 cpd when observed from the opposite corner of the cage. C: observations of 4 animals over a 4-h period showed that animals spend 36% of their active time in the corners of their cage looking outward.
Stripe-reared animals were raised in conditions identical to those described above, except that a vertical square wave grating surrounded the cage (Fig. 1B). Animals were born in the cages with high-contrast stripes affixed to the cage wall, and remained in these cages until electrophysiological mapping was performed. This grating had a spatial frequency of 1.2 cycles per degree (cpd) when viewed from the opposite corner of the cage. Observations of 4 animals across 3 days showed that opossums spent 36.2% of their nonactive time in the corners of the cage (Fig. 1C). Animal cages were always placed in the middle cage racks to limit variance in overall illumination. Although we cannot guarantee the animal's head remained parallel to the ground at all times, this stimulus was chosen to bias the animal's environment to high-contrast, vertical stimuli over a discrete range of spatial frequencies. As the maximum spatial frequency viewable to stripe-reared opossums is 1.2 cpd, these animals were disproportionately exposed to a subset of spatial frequencies ranging from very low (when opossums are close to the wall of the cage) to relatively high (1.2 cpd) compared with the animal's behavioral acuity (0.6 cpd; Dooley et al. 2012). If marsupials showed the same experience-dependent visual plasticity as previously studied in placental mammals, we expected neurons in V1 of stripe-reared animals to show increased responsivity to stimuli sharing these overrepresented spatial frequencies.
Electrophysiological Recordings
For all animals, electrophysiological recording experiments were performed once the animals reached adulthood (greater than 180 days of age). Anesthesia was induced using isoflurane, and a surgical plane of anesthesia was maintained using 30% urethane in propylene glycol (1.25 g/kg ip). A 2% lidocaine solution was injected subcutaneously at the midline of the scalp prior to the first incision, and around the ear canals, and animals were placed in a stereotaxic apparatus. Animals were given dexamethasone (1.0 mg/kg im). Respiration and body temperature were monitored and maintained throughout the experiment.
An incision was made at the midline of the scalp, and the temporal muscle was unilaterally retracted. A craniotomy was performed over the neocortex exposing the caudal pole of cortex, and the dura was retracted. A photograph of the exposed neocortex was taken as a reference map to relate the electrode penetrations to cortical vasculature (Nikon D5100 or Nikon D5200; Nikon, Melville, NY). The exposed cortex was then coated with silicon fluid (Dow Corning 200 Fluid, dimethylpolysiloxane; Dow Corning, Midland, MI). A screw was also inserted into the skull above the rostral portion of cortex contralateral to the craniotomy.
At this stage of the experiment, animals were removed from the stereotaxic apparatus. The skull screw was fixed to a metal bar using dental acrylic to ensure the brain and head remained stationary. Contralateral vibrissae were shaved so that they did not obscure the visual field. A custom-made eye ring was glued to the sclera of the contralateral eye to ensure that there were no eye movements throughout data collection. In many instances we would return to an earlier recording site to verify that visual receptive fields for neurons at that location had not shifted. Atropine was topically administered to the eye, and the eye was periodically rinsed with saline throughout the recording session to prevent desiccation.
For all recordings, tungsten electrodes were used (5 MΩ, 0.01-in. diameter, A-M Systems, Sequim, WA). Before experiments began, electrolysis was performed on the electrode until its impedance was between 0.8 and 1.2 MΩ. Electrodes were lowered into cortical layer 4 (∼400 μm below the pial surface) using a micromanipulator (Kopf Instruments, Tujunga, CA), and single- and multiunit activity were amplified and filtered (100-5,000 Hz; Model 1800 Microelectrode AC Amplifier; A-M Systems), monitored through a loudspeaker, sampled at 28 kHz, and viewed on a computer monitor (Spike2; CED, Cambridge, UK). Each electrode penetration was marked on a digital image of the cortex for later alignment with histologically processed tissue. Following completion of data collection, electrodes were coated with a fluorescent tracer (Fluoro-ruby, 10–15% concentration; ThermoFisher Scientific, Waltham, MA) and inserted into the cortex at strategic locations to align electrode penetrations with cortical field boundaries (see Seelke et al. 2012).
For each recording site, we first determined whether neurons were responsive to visual stimulation, and then defined the receptive field by stimulating the contralateral retina with full field flashes of light using an ophthalmoscope, as well as moving or flashing circles and bars of light presented on an opaque white paper covering a computer monitor 20 cm from the eye of the opossum (Fig. 2D). Preliminary data indicated that there was no difference in preferred spatial and temporal frequencies when varying the distance to the monitor or by placing corrective lenses in front of the eye. This is similar to studies in other small-eyed mammals (e.g., Balkema and Pinto 1982; Niell and Stryker 2008). At 20 cm, both the computer monitor and the opaque white paper subtended approximately 90° × 70° of visual space (Fig. 2E). The computer screen was positioned such that the horizontal meridian of the visual field bisected the computer screen, and the vertical meridian was aligned to the leading edge of the screen (Fig. 2, D and E). A grid (X = 0° to +90°, Y = −35° to +35°, in 5° increments) was printed on top of the white paper and was used to determine the extent of the receptive field to the nearest 5° in either dimension, so receptive fields were reported as squares or rectangles of visual space (for example: 5° × 5°, 10° × 10°, etc.).
Fig. 2.
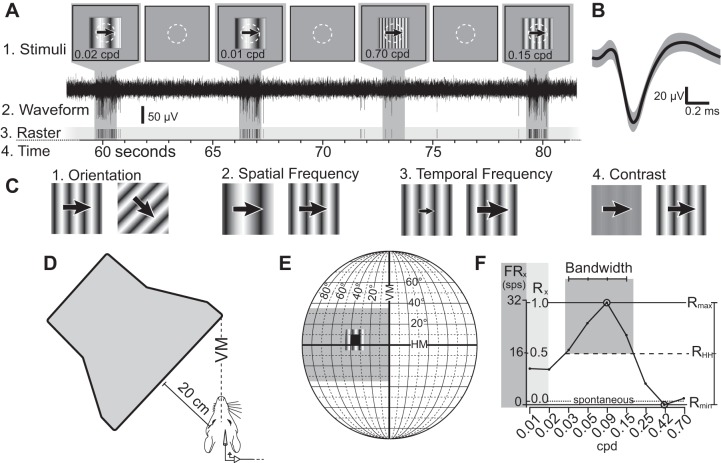
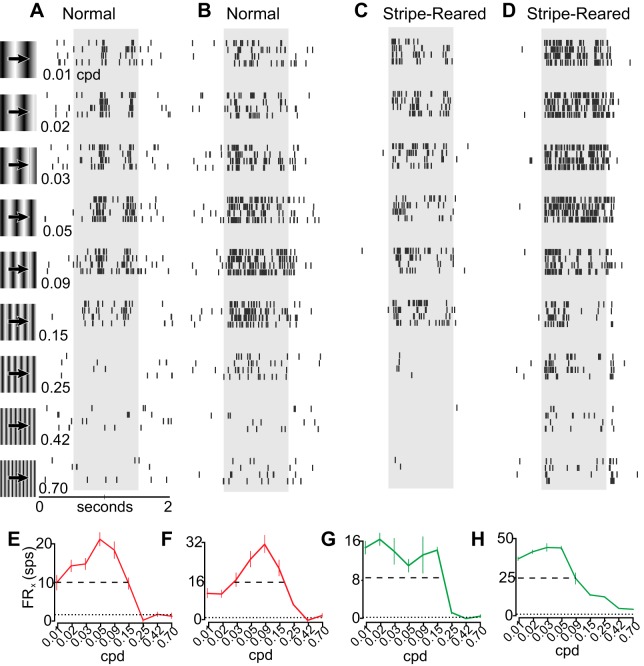
Presentation of stimuli during electrophysiological recording. Stimulus presentation (A1), waveform (A2), and raster plot (A3) over time (A4). A1: stimuli presented on the CRT, with neutral gray background and a 20° × 20° drifting sinusoidal stimulus. The neuron's receptive field is depicted by a white dotted circle. Between stimuli, only the neutral gray background was presented for 5 s. A2: electrophysiological waveform collected showing strong neuronal signal to background noise. A3: raster plot of single-unit action potentials collected during stimulus presentation. On this trial, the neuron did not respond above FRspon for the 0.70-cpd stimulus, was moderately responsive for the 0.01- and 0.02-cpd stimuli, and was highly responsive when presented with 0.15-cpd stimulus. B: average waveform for the putative unit shown in the raster in A, showing single unit isolation. Shaded region represents 1 SD from the mean. C: presented stimuli varied by orientation (C1), followed by spatial frequency (C2), temporal frequency (C3), and contrast (C4). D: top view of the animal and the monitor (not to scale). The computer monitor's leading edge was placed along the vertical meridian of the animal's visual field and covered 90° of the contralateral visual field. E: diagram of the visual field, showing the location of the monitor (gray box), an example of a receptive field spanning from 0° to 10° elevation and 30° to 40° azimuth (black box), and the 20° × 20° placement of the sinusoidal stimuli. F: example of a tuning curve of neuronal responses to different spatial frequencies. Y-axis shows responses in spikes per second (FRx, left, dark gray) and relative response rate (Rx, right, light gray). Rmax is defined as the stimuli to which neurons responded maximally (0.09 cpd in this example) and is set to equal 1. The spontaneous firing rate (dotted line) is the mean firing rate between stimulus presentations, and is the value where R = 0. In this example, Rmin < 0, as the response at 0.42 cpd is less than the spontaneous rate. RHH (dashed line) is the midpoint between Rmax and Rmin, whereas the bandwidth is the number of data points above RHH (4 in this example).
Stimulus Presentation
Following determination of the receptive fields for neurons in a given recording site, the paper was removed, exposing the cathode ray tube (CRT) monitor beneath it. Stimuli were generated in Matlab (2011a edition) using the Psychophysics Toolbox extensions (Brainard 1997; Pelli 1997), along with custom-written code. Each stimulus was presented on the CRT monitor in the location of the previously determined receptive field (NEC AccuSync 120-BK, 1,600 × 1,200, 75 Hz). Average screen luminance was measured to be 25 cd/m2. A photodiode attached to the computer monitor recorded the exact time of the initiation and termination of presented stimuli, allowing sub-millisecond precision in the measurement of neural response timing relative to the stimulus, using Spike2.
Presented stimuli consisted of drifting sinusoidal gratings that were repeated 4–8 times, with stimuli within a parameter randomly interleaved (see Fig. 2A). Surrounding the window containing the drifting sinusoidal grating was a neutral gray background with the same mean luminance as the stimuli (Fig. 2A). Between stimulus presentations, only the neutral gray background was displayed. Waveforms were initially categorized on-line in Spike2, using template matching (60% match) and an amplitude threshold varying from 25 to 50 μV, depending on the site. Once we identified putative single units (Fig. 2B), on-line classification of waveforms allowed immediate viewing of tuning curves to determine the preferred orientation and spatial frequency of neurons at the recording site. Waveforms were further filtered to 500–5,000 Hz. Spike-sorting was performed in Spike2 via template matching. Putative units were only assigned a template if their amplitude exceeded a signal-to-noise ratio of 2:1 (see Fig. 2A2 for a sample waveform and Fig. 2B for a template of a putative unit). Unit isolation was further confirmed using principal component analysis and investigation of interspike intervals (>2.0 ms) to ensure separation from background multiunit activity. Units that did not appear well isolated in this off-line analysis were not included in further quantitative analyses.
Stimulus presentation proceeded as follows: The response to different orientations of sinusoidal gratings was plotted in Spike2 to determine the orientation to which neurons were maximally responsive (Fig. 2C1). While varying orientation, a spatial frequency of 0.1 cpd, a temporal frequency of 2.1 cps, and 100% contrast were used because preliminary data collection indicated that at these parameters, the largest proportion of neurons in V1 respond above the spontaneous firing rate. This preferred orientation was then used while spatial frequency (Fig. 2C2), temporal frequency (Fig. 2C3), and contrast (Fig. 2C4) tuning curves were generated.
Episodic stimuli presented were drifting sinusoidal gratings (1.0- to 2.0-s duration, with 5.0 s between presentations; Fig. 2A). Eight different orientations were tested (0° to 315° at 45° intervals). Nine different spatial frequencies were tested (0.01 to 0.70 cpd) spanning six octaves of spatial frequencies, as well as nine different temporal frequencies (0.25–8.00 cps) spanning five octaves. Finally, eight contrast values (1 to 100%) were tested. Stimuli generated during spatial frequency, temporal frequency, and contrast testing were equidistant logarithmic values (for stimulus values, see Table 1).
Table 1.
Recording sites and neurons isolated in normal and stripe-reared animals
| Recording Sites | Neurons Isolated | |
|---|---|---|
| Normal 1 | 8 | 5 |
| Normal 2 | 24 | 4 |
| Normal 3 | 52 | 16 |
| Normal 4 | 37 | 15 |
| Normal 5 | 39 | 8 |
| Normal 6 | 36 | 12 |
| Normal 7 | 46 | 13 |
| Normal 8 | 26 | 5 |
| Total | 268 | 78 |
| Stripe-reared 1 | 26 | 18 |
| Stripe-reared 2 | 22 | 14 |
| Stripe-reared 3 | 34 | 18 |
| Stripe-reared 4 | 55 | 21 |
| Stripe-reared 5 | 49 | 26 |
| Total | 186 | 97 |
Data Analysis
The average spontaneous firing rate (FRspon; in spikes per second, sps) was determined for each unit by averaging the firing rate (in sps) throughout the period 1–5 s following stimulus presentation. The standard deviation of the spontaneous firing rate (SDspon) was determined by calculating the standard deviation of all poststimulus 4-s bins of a given stimulus presentation. For all stimuli, the responses for each type of stimulus were determined by averaging the spike rate (in sps) throughout the entire duration of stimulus presentation during all presentations of a given stimulus (FRx) and subtracting FRspon. These responses were then divided by the stimulus that generated a maximal response (FRmax) to give a relative response rate Rx:
As Rx is a ratio of firing rates, it is a unitless variable. This was done to allow comparisons of different neurons with different absolute firing rates. Thus, for each parameter, Rmax = 1, while all remaining values were less than 1. If Rx = 0, the firing rate for that stimulus is equal to the background firing rate. Negative values were possible but infrequent, and required the firing rate during stimulus presentation to be below the spontaneous firing rate (see 0.42 cpd in Fig. 2F for an example of a negative value). FRspon was also divided by FRmax to determine the ratio of spontaneous firing to that parameter's maximally responsive stimulus (Rspon). Similar to exclusion criteria of previous investigators, neurons were only included in the analysis if FRmax was greater than 4 sps and Rspon was less than 0.2 (e.g., Van Hooser et al. 2005). This ensures that only neurons with a strong driven response for a tested parameter and a high signal-to-noise ratio were included in subsequent analyses. The average values across all presented stimuli could then be plotted to generate a tuning curve. Rmax and Rmin are the relative response rates of the maximum and minimum responsive stimuli, respectively (See Fig. 2F).
The half-height (RHH) was then determined using the following equation:
RHH for the example tuning curve is shown in Fig. 2F. Importantly, RHH is only equal to 0.5 if Rmin = 0. In instances where Rmin > 0, RHH would be above 0.5. The bandwidth for each neuron (reported in octaves) was defined as the number of presented stimuli above half-height for each parameter multiplied by the number of octaves between each stimulus of that parameter. For each parameter tested, we also calculated the probability of neurons firing above RHH for each tested stimuli. This was expressed as the percentage of neurons included in the quantitative analysis that responded to stimuli above RHH over the total number of neurons included in the quantitative analysis. We considered neurons to be driven by a given stimulus if the firing rate during stimulus presentation was greater than two SDs above the spontaneous firing rate:
As with the probability of neurons firing above RHH, we calculated the percent of neurons driven by each presented stimulus.
We also calculated an orientation index and a direction index derived from circular variance for each unit's responses (OCV and DCV, respectively) based on equations from the literature that rely on vector summation of the response at each orientation (Naito et al. 2007; see Mazurek et al. 2014), where Rx is the response to angle x (in radians).
To allow thorough comparisons of selectivity with current and historical literature (e.g., Niell and Stryker 2008), we also calculated orientation and direction selectivity indexes (OSI and DSI, respectively) using the equations below.
Neurons were considered to potentially be orientation or direction selective if a Rayleigh test determined responses were nonuniformly distributed (P < 0.005). Given a nonuniform distribution, neurons were considered not orientation or direction selective if they had an OCV or DCV of less than 0.1. Neurons were considered moderately selective if the value was between 0.1 and 0.2, and neurons were considered highly selective if the value was above 0.2.
Contrast-response curves were fit to the Naka-Rushton equation (Naka and Rushton 1966) using custom-written Matlab code:
Contrast values (x) can vary from 0 to 1, and C50 and n are selected to best fit the data for that neuron. C50 represents the contrast at which the response is halfway between the spontaneous firing rate and the maximum (the contrast value at which the sigmoidal fit predicts the neuron would reach RHH). The exponent (n) reflects the slope of the rising phase of the function.
Statistical Analysis
Populations of preferred orientation of selective neurons were tested to see if they deviate from an equal distribution using Pearson's chi-squared test. Normal and stripe-reared populations were compared with each other using a 2 × 4 contingency table. Comparisons of the proportions of neurons above the FRspon and RHH were made using a Fisher's exact test. For each tested spatial frequency, temporal frequency and contrast percentage, the number of neurons that were and were not above FRspon and RHH were calculated for each group, and put into a 2 × 2 contingency table. Results were considered significant if P < 0.05 divided by the number of tests performed (the Bonferroni correction). All tests were two-tailed. C50 values generated from fits to the Naka-Rushton equation as well as receptive field sizes were compared using a Student's t-test. An unpaired Student's t-test was also used for determining differences in the bandwidth for tuning curves generated by neuron responses to different spatial and temporal frequency.
RESULTS
We recorded from 175 neurons across 13 opossums, including 8 normal and 5 stripe-reared animals (Table 1). Histological analysis confirmed that all isolated units were contained within V1 (Fig. 3). Recording sessions lasted between 5 and 15 h. Once a putative unit was isolated, it took approximately 20–30 min to determine the neural response to the different features of the stimulus described in the methods. Criteria for exclusion included a high spontaneous firing rate (Rspon > 0.2), a low maximum firing rate (FRmax < 4 sps), or poor unit isolation. Mean maximum firing rate and spontaneous firing rate did not significantly differ between rearing conditions.
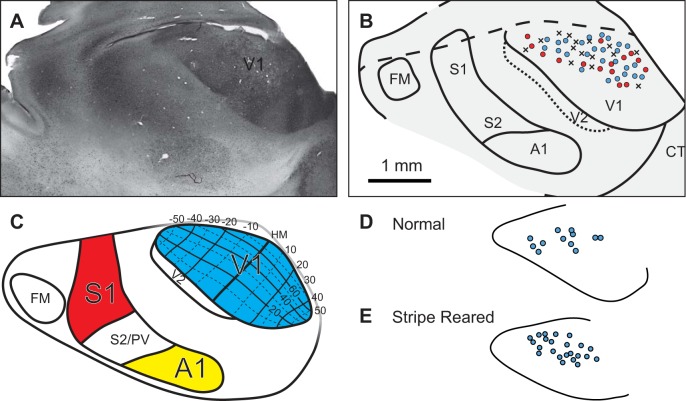
Fig. 3.
A: digital image of stripe-reared opossum's neocortex that has been flattened, sectioned tangentially and stained for parvalbumin, which clearly shows the boundaries of V1. B: reconstruction of the boundaries of primary sensory areas of the case shown in A, with the electrophysiological recording sites overlaid on V1. This reconstruction was made from the entire series of sections that were stained for parvalbumin. Recording sites at which neurons did not respond to visual stimulation are denoted with an X; red circles are sites in which neurons responded to visual stimulation, but the responses were not strong enough to be measured quantitatively. Light blue circles are sites in which single unit data were gathered. C: topographic organization of V1 in Monodelphis domestica [Adapted from Kahn et al. (2000) with permission. Copyright John Wiley and Sons]. Location within V1 of recording sites in which single-unit data were gathered in an example normal (D) and stripe-reared animal (E). FM = Frontal Myelinated area; CT = Caudotemporal area; PV = Parietal Ventral area.
Precise quantification of the size of receptive fields was not an objective of these experiments, and as described in methods, receptive fields were determined to the nearest 5° in azimuth and elevation. Thus the smallest receptive fields were recorded as 5° × 5°, which is comparable to the smallest receptive fields previously reported in V1 of the short-tailed opossum (Kahn et al. 2000). Receptive fields as large as 20° in either azimuth and elevation were also found, and thus some variability in receptive field size could still be captured. No receptive fields encountered were larger than the drifting sinusoidal window (20° × 20°). The mean receptive field size for normal animals (M = 109 deg2) did not differ significantly from stripe-reared animals (M = 121 deg2, P = 0.23). Generally, receptive fields of neurons in the representation of central vision were smaller than the receptive fields of neurons in the representation of peripheral vision.
Response Properties of Neurons in V1 in Normal Animals and Stripe-Reared Animals
Orientation and direction selectivity.
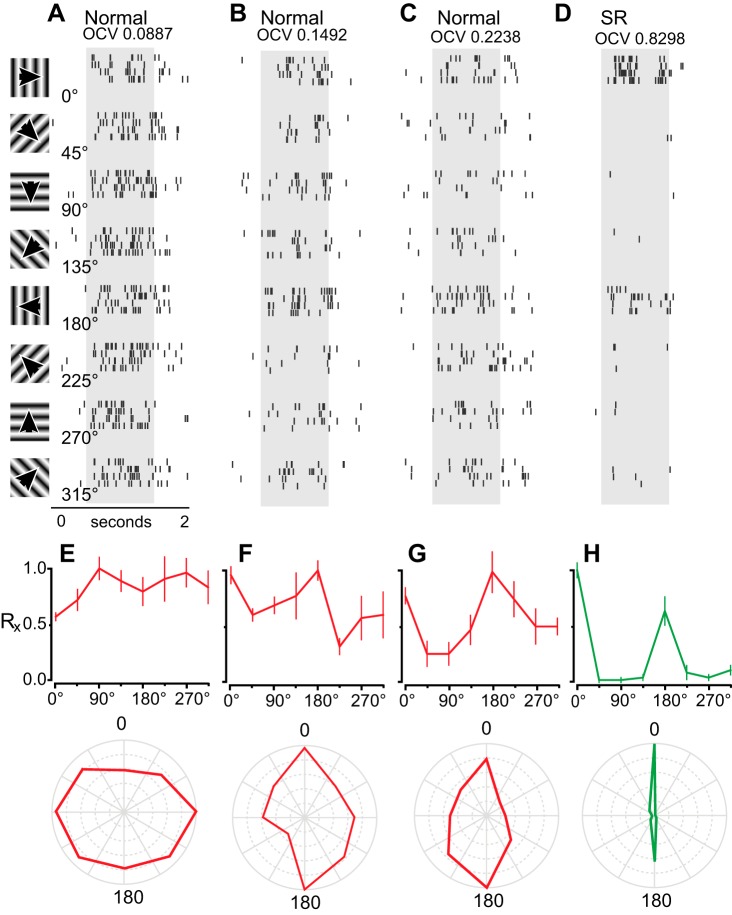
Using the equation for OCV detailed in methods, a neuron with a theoretically perfect OCV would have a value of 1, whereas a neuron that displays no orientation selectivity would have an OCV of 0. Raster plots, tuning curves, and polar plots are provided for normal animals that show neurons that are unselective (Fig. 4A), moderately selective (Fig. 4B), and highly selective (Fig. 4C).
Fig. 4.
Example cells displaying various degrees of orientation selectivity. Raster plots of neural responses in three normal animals (A–C), which display no selectivity (A), moderate selectivity (B), and high selectivity (C) for different orientations. Rasters span 2,000 ms, beginning 500 ms before stimulus onset (gray bar), and continue for 500 ms following stimulus presentation. D: neuron showing the highest degree of selectivity, recorded in a stripe-reared animal. Presented stimuli can be seen on the left, varying between 0° and 315° at 45° intervals. E–H: tuning curves and polar plots of Rx for the data presented in A–D. Top: Rx ± SE. Red lines represent neuronal responses from normal animals; green lines represent neurons from stripe-reared animals. Bottom: polar plot of data shown above, with Rx at 0° shown at the top, moving clockwise, spanning 360° degrees.
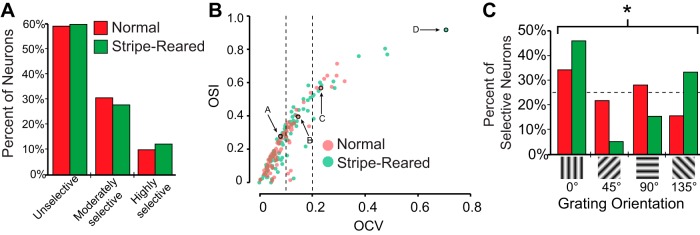
OCV values did not significantly differ in normal and stripe-reared groups, and except for one highly tuned neuron found in a stripe-reared case (Fig. 4D), 60% of neurons were unselective for orientation and resembled the response profile for the neuron depicted in Fig. 4A (see Fig. 5A for group response profiles). Among neurons that were orientation selective, the proportion of neurons in normal and stripe-reared animals that were moderately selective (31% and 28%, respectively) and highly selective (10% and 12%) for orientation were similar (Fig. 5A). OSI was also calculated to allow comparisons of the degree of orientation selectivity in Monodelphis with that of other species studied (Fig. 5B). Figure 5 shows a scatterplot of each neuron's OCV and OSI, and demonstrates that an OCV of 0.1 is approximately equivalent to an OSI of 0.33 and an OCV of 0.2 is approximately equivalent to an OSI of 0.5. Thus these thresholds for selectivity are consistent with previous thresholds used to determine orientation selectivity (e.g., Niell and Stryker 2008).
Fig. 5.
A: proportion of neurons classified as unselective (OCV ≤ 0.1), moderately selective (0.1 < OCV < 0.2), and highly selective (OCV ≥ 0.2) of normal (red) and stripe-reared (green) animals. B: scatterplot of OCV vs. OSI for normal (red) and stripe-reared (green) neurons. Data points encircled in black pointed out by arrows represent neurons depicted in Fig. 4. Neurons with higher OSI values generally had higher OCV values and were thus more selective. There were no significant differences in orientation selectivity for neurons in normal and stripe-reared animals. Dotted lines show thresholds for moderately and highly selective neurons. C: percent of moderately and highly selective neurons responsive to the four stimulus orientations presented for normal (red) and stripe-reared (green) animals. Stripe-reared animals showed an increase in the percentage of neurons responding to vertical gratings, which significantly differed from normal animals (*P < 0.05).
Among orientation-selective neurons (N = 32 for normal animals and N = 39 for stripe-reared animals), a 2 × 4 chi-square goodness-of-fit test revealed that for normal animals, the distribution of preferred grating orientation did not differ significantly from an expected equal distribution (25% for each grating orientation, dotted line in Fig. 5C). However, the distribution for stripe-reared animals did significantly differ from an equal distribution (Fig. 5C; P < 0.005). Thus V1 in stripe-reared animals showed an increase in the percent of neurons selective to a 0° grating orientation. Further, a 2 × 4 Fisher's exact test found the distributions of preferred grating orientation in neurons from normal and stripe-reared animals to significantly differ from each other (P < 0.05).
No neurons in either the normal or the stripe-reared groups showed a high DCV, leading us to conclude that V1 neurons in the short-tailed opossum are not direction-selective.
Spatial frequency.
Spatial frequencies tested varied between 0.01 cpd and 0.70 cpd (see Table 2); in preliminary studies the latter value produced a firing rate indistinguishable from the spontaneous firing rate in a majority of neurons and thus higher spatial frequencies were not tested. These spatial frequencies are also similar to those used in studies of V1 in mice (Niell and Stryker 2008), whose acuity is similar to that of opossums (see Dooley et al. 2012). Generally, Rx of neurons in normal animals was near 0.5 (50% of peak response) at the lowest spatial frequencies tested, followed by a peak response at between 0.05 and 0.15 cpd. At the highest spatial frequencies tested neuronal responses then dropped to FRspon (Fig. 6, A, B, E, F). Firing rates for some neurons showed phasic activity (Fig. 6A), whereas neurons at other sites had a more continuous rate of firing throughout stimulus presentation (Fig. 6B).
Table 2.
Presented stimuli
| Orientation, ° | Spatial Freq, cpd | Temporal Freq, cps | Contrast, % |
|---|---|---|---|
| 0 | 0.01 | 0.25 | 1 |
| 45 | 0.02 | 0.39 | 3 |
| 90 | 0.03 | 0.59 | 5 |
| 135 | 0.05 | 0.92 | 10 |
| 180 | 0.09 | 1.41 | 17 |
| 225 | 0.15 | 2.18 | 31 |
| 275 | 0.25 | 3.36 | 56 |
| 315 | 0.42 | 5.19 | 100 |
| 0.70 | 8.00 |
Fig. 6.
Examples of cells responding to different spatial frequencies in normal animals (A, B) and stripe-reared animals (C, D). Data are illustrated as in Fig. 4, with spatial frequency varying instead of orientation. Rasters are representative examples of a band-pass neuron found in a normal animal with phasic firing at preferred spatial frequencies (A), a band-pass neuron with continual firing at a range of spatial frequencies (B), a low-pass neuron found in a stripe-reared animal (C), and a low-pass neuron from a stripe-reared animal which continued to be driven at the highest spatial frequencies tested (D). E–H: tuning curves of the firing rate (FRx) from the neurons shown in A–D. Responses are shown as FRx ± SE. Data from normal animals are drawn in red and from stripe-reared animals in green. Dotted line illustrates FRspon; dashed line illustrates RHH.
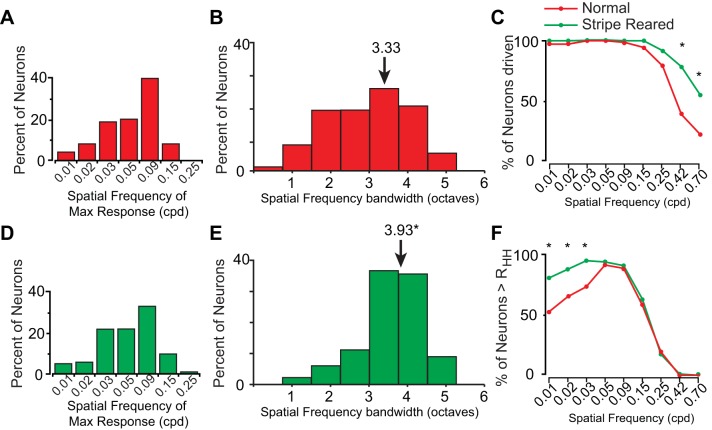
Forty percent of the neurons in normal animals had a maximum firing rate at 0.09 cpd and around 70% of the neurons had a maximum firing rate at 0.09 cpd or one of its two adjacent spatial frequencies (0.05 and 0.15 cpd; Fig. 7A). The bandwidth of spatial frequency tuning curves was a relatively regular distribution of 3 octaves (M = 3.33 octaves; Fig. 7B). The majority of neurons (>80%) had spatial frequency tuning curves that were broad with a bandwidth between 2 and 4 octaves.
Fig. 7.
Spatial frequency tuning curve summary for normal animals (A, B, red) and stripe-reared animals (D, E, green). A, D: histogram of the percentage of neurons with Rmax at a given spatial frequency. B, E: bandwidth (in octaves) of tuning curves. Arrows indicate means of each population. C: percentage of neurons firing 2 SDs above the FRspon of normal (light red) and stripe-reared (light green) animals at different spatial frequencies. Regions where both populations are firing above RHH are shown in brown. F: percentage of neurons firing above RHH at different spatial frequencies. Conventions as in C. *P < 0.0056. Neurons recorded in stripe-reared animals are more broadly tuned, both in terms of variability of peak response and bandwidth of tuning curves, and are significantly more likely to be firing above RHH at lower spatial frequencies as well as be driven by the highest spatial frequencies tested.
In stripe-reared animals, differences in neuronal responses to the presented spatial frequencies compared with normal animals were observed. Statistical analysis on the population of neurons examined revealed that a larger proportion of neurons in stripe-reared animals were driven by the two highest spatial frequencies compared with normal animals (Fisher's exact, P < 0.0056, Fig. 7C; for an example, see Fig. 6D). Further, stripe-reared animals had a significantly larger proportion of neurons firing above RHH at the lowest three spatial frequencies tested (P < 0.0056, Fig. 7F; for examples, see Fig. 6, C and D). In striped-reared animals, the spatial frequency at which neurons were most likely to have a maximum response was 0.09, and the overall distribution was similar to normal animals (Fig. 7D). The bandwidth of these tuning curves for neurons in stripe-reared animals was significantly broader compared with normal animals (P < 0.001), with neurons generally having sustained firing above RHH for a larger number of octaves compared with normal animals (M = 3.93 octaves; Fig. 7E).
Temporal frequency.
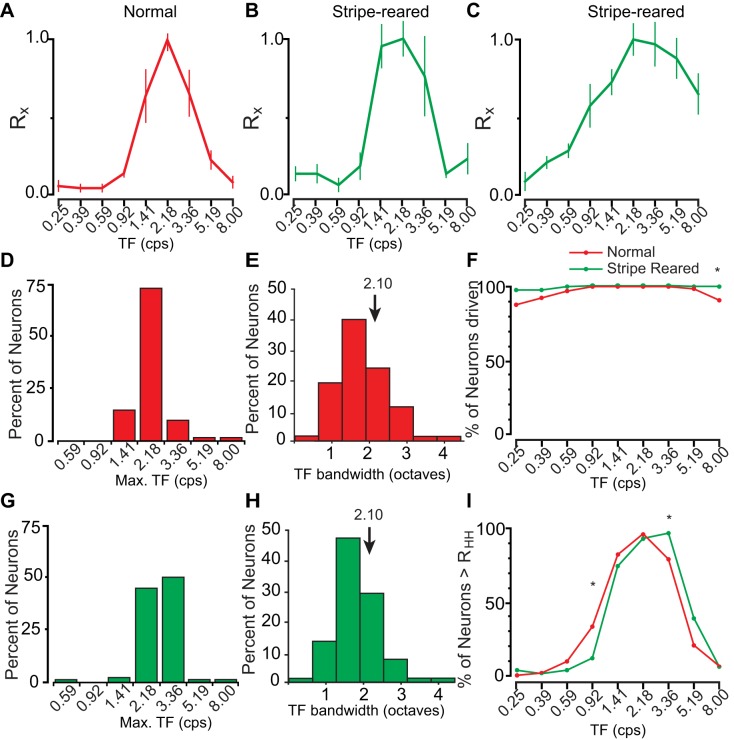
To sufficiently capture the preferred temporal frequencies of neuronal activity across the population, temporal frequency values to be tested were first determined in preliminary data collection. For normal animals, nearly all of the tuning curves resembled that of the example given (Fig. 8A). This includes a low firing rate at the slowest temporal frequencies (0.25 cps), followed by a steep climb to a peak at 2.18 cps, and an equally steep decline. The population of neurons was homogeneous, with Rmax for temporal frequencies being very tightly tuned (Fig. 8D). There was some variability in the width of this bandwidth tuning curve among neurons (Fig. 8E, M = 2.10); however, these responses were still remarkably consistent.
Fig. 8.
A: representative tuning curves of relative response rate (Rx) ± SE demonstrating the most commonly encountered band-pass tuning curve in normal animals (A). Visual stimulation of nearly all neurons generated a tuning curve similar to this representative neuron. B: a representative tuning curve of a V1 neuron from stripe-reared animals. C: a less commonly encountered tuning curve showing sustained firing at higher temporal frequencies. D, G: summary of the percent of neurons with a maximal response (Rmax) at different temporal frequencies from normal (D, red) and stripe-reared (G, green) animals. E, H: percent of neurons with a given bandwidth for normal (E, red) and stripe-reared (H, green) animals. F: percentage of neurons firing 2 SDs above the FRspon of normal (red) and stripe-reared (green) animals at different temporal frequencies. Regions where both populations are firing above RHH are shown in brown. I: percentage of neurons firing above RHH at different temporal frequencies. *P < 0.0056. A greater proportion of V1 neurons from stripe-reared animals are driven by the highest temporal frequencies tested, and there is a larger rightward shift in the percentage of neurons firing above half-height. Conventions are the same as in Fig. 7.
For stripe-reared animals, there were population-level differences compared with normal animals. Primarily, stripe-reared animals showed a rightward shift of the tuning curve, with about 50% of neurons maximally responsive at 3.38 cps, and 90% of neurons maximally responsive to either 2.18 or 3.36 cps. Consistent with this shift, neurons in normal animals were significantly more likely to fire above RHH at 0.92 cps, and stripe-reared animals were more likely to fire above half height at 3.36 cps (P < 0.0056; Fig. 8I). Also consistent with a rightward shift, there was no difference in the bandwidth of neuronal responses to temporal frequencies in normal and stripe-reared animals.
Contrast.
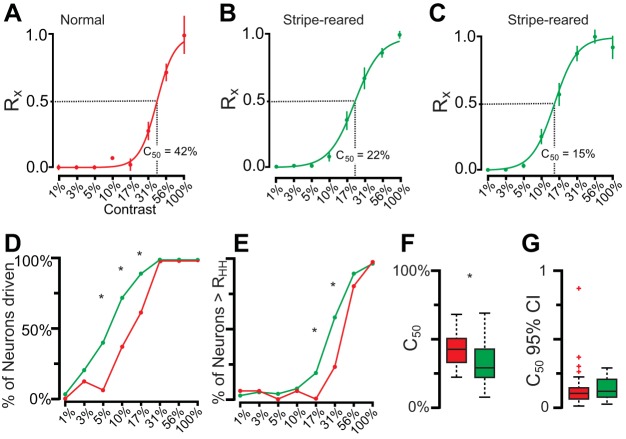
Contrast values were tested between 1% and 100% contrast, and a sigmoidal response function was observed for both normal and stripe-reared animals (Fig. 9, A–C). However, relative to normal animals, in stripe-reared animals the rising phase of the sigmoidal curve began at lower contrast values (Fig. 9, B and C). Generally, the maximum response was at 100% contrast; however, a subset of neurons (6% of normal animals, 16% of stripe-reared animals) showed a maximum response at 56% contrast. When this was the case, the response of neurons at 100% contrast was always comparable to the response at 56%, suggesting that for these neurons the response had saturated by 56% contrast.
Fig. 9.
A: a response in a normal animal for presentation of different stimulus contrasts (Rx) ± SE. This neuron begins responding at 10% contrast and continues to fire for all contrasts up to 100% contrast. The sigmoidal fit of this neuron's response is shown, along with the C50 (42%). B: a response for a typical neuron in a stripe-reared animal for presentations of different stimulus contrasts. Neurons in stripe-reared animals had a sigmoidal response similar to that seen in normal animals, but the profile was shifted to the left, with the rising phase beginning at 5% contrast rather than 10% contrast. C: more infrequently encountered sigmoidal response for neurons in stripe-reared animals. This neuron saturated at a much lower contrast value compared with the neurons shown in A and B. D: the percentage of neurons being driven at different contrasts for stripe-reared (green) and normal (red) animals. At all contrast levels tested, neurons from stripe-reared animals had a higher probability of being driven than normal animals. E: the percentage of neurons firing above RHH for different contrasts in normal and stripe-reared animals. F: box and whisker plot of C50 values of all neurons from normal (red) and stripe-reared (green) animals. G: the 95% confidence intervals of C50 for normal and stripe-reared animals. For F and G, box includes 25th through 75th percentiles, whiskers include all values outside of outliers (colored crosses). C50 values are significantly lower for stripe-reared animals. In D and E, *P < 0.00625; in F, *P < 0.05. Both the percentage of neurons being driven and firing above RHH at different contrast values, as well as the C50 values, support the observation that neurons in stripe-reared animals are responsive to lower contrast values, and are more likely to saturate at lower contrast levels.
The majority of neurons in normal animals were driven at contrast values of above 17%, whereas 5% contrast was sufficient to drive nearly 50% of neurons from stripe-reared animals (Fig. 9D), demonstrating that as a population the sigmoidal contrast response functions have been shifted to lower contrasts in stripe-reared animals. This shift of contrast response functions resulted in a significantly higher proportion of three intermediate contrast values (5%, 10%, and 17%; P < 0.0063) driving neuronal responses in stripe-reared animals compared with normal animals (Fig. 9D). This shift to lower contrast values was also observed for responses above RHH, with a statistically significant proportion of neurons responsive to contrast values of 17 and 31% firing above RHH in stripe-reared animals (P < 0.0063). When C50 values were fit to the data, the mean values were at significantly higher contrasts in normal animals (M = 41.2%) compared with stripe-reared animals (M = 32.4%; P < 0.05; see Fig. 9F for distributions). Thus neurons in stripe-reared animals were more responsive to lower contrast values and likewise more likely to saturate at higher contrast values compared with neurons in normal animals. The degree of sigmoidal fit was similar for both normal and stripe-reared tuning curves, with the C50 95% confidence intervals not statistically differing between groups (normal = 12.5, stripe-reared 14.3; P = 0.503; Fig. 9G).
Overall, these results demonstrate an effect of stripe-rearing on neural responses to preferred orientation, spatial frequency, temporal frequency, and contrast. First, there was a shift in the percentage of neurons responding to vertically oriented stimuli. Second, a greater proportion of V1 neurons in stripe-reared animals responded to the lowest and highest spatial frequencies, expanding the spatial frequency bandwidth of neural responses in these animals. Notably, this included continued responsivity above the spontaneous firing rate at the highest spatial frequencies tested for a greater percentage of neurons. Third, temporal frequency bandwidth was similar in both groups; however, there was a small but consistent shift toward a stronger response at higher temporal frequencies in stripe-reared animals compared with normal animals. Finally, in terms of contrast, neurons in stripe-reared animals began to respond at lower contrast percentages, and had significantly lower C50 values when fit to a sigmoid.
DISCUSSION
Marsupials compose one of three major clades of mammals and represent an important link to our common ancestor. Despite their important phylogenetic relationship to early mammals, there are only a few studies that have systematically examined and quantified response properties of neurons in visual cortex in marsupials. This type of data provides important information on the common features of neuronal function shared by all mammals, and insights into the processing capacity of the visual cortex of our earliest ancestors. The present study in the short-tailed opossum (Monodelphis domestica) addressed two important questions. First, are neurons in V1 of marsupials responsive to specific features of the visual stimulus, such as orientation and spatial frequency, that have been demonstrated to be effective in eliciting a response in V1 neurons in eutherian mammals? Second, can neural response properties in the opossum be shaped by early visual experience? We used single-unit electrophysiological recording techniques to determine the response properties of neurons in V1 in normal and stripe-reared Monodelphis and found that they are largely similar to those found in small-brained, nocturnal eutherian mammals such as rats and mice, although a smaller proportion of cells were orientation selective in Monodelphis. After stripe-rearing, we found that, as a population, neurons in V1 are tuned differently to several features of the visual stimulus. These changes in neuronal responses in stripe-reared animals are related to the high-contrast, vertical stripes these animals were disproportionately exposed to in their home cage. Thus we demonstrate that environmental context shapes the developing neocortex in marsupials, and speculate that this plasticity played an important role in the survival and subsequent evolutionary success of our early mammalian ancestors.
Response Properties of Neurons in V1 of Marsupials
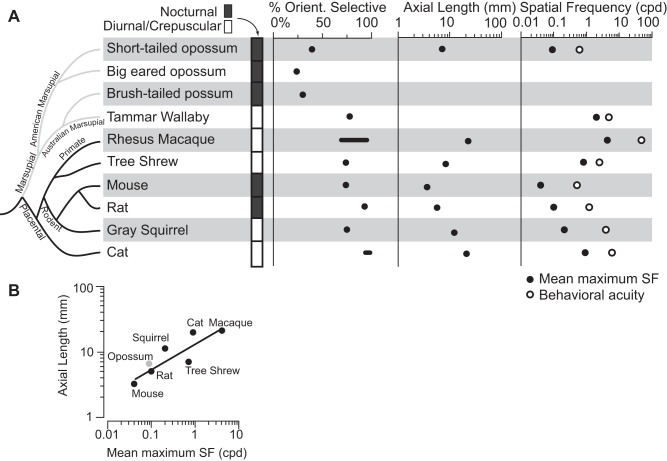
To date, single-unit recordings of V1 have been described in four marsupial species. These include the Virginia opossum (Didelphis virginiana; Christensen and Hill 1970), the big-eared opossum (Didelphis marsupialis aurita; Picanço-Diniz et al. 1983; Rocha-Miranda et al. 1973, 1976), the brush-tailed possum (Trichosurus vulpecula, Crewther et al. 1984), and the tammar wallaby (Macropus eugenii; Ibbotson and Mark 2003). Unlike other mammalian species investigated, the majority of neurons in V1 of three of these marsupial species do not display orientation selectivity, the exception being the tammar wallaby (Fig. 10A). In the present study, we found that about 40% of the neurons in V1 are orientation selective (a nonuniform distribution along with an OCV > 0.10). Among marsupials, the high percentage of orientation-selective neurons in V1 of tammar wallabies (78%) is partially explained by their crepuscular lifestyle and relatively large binocular overlap compared with their nocturnal relatives. Quantitative studies of the preferred spatial and temporal frequencies are only available for the tammar wallaby (Ibbotson and Mark 2003; Ibbotson et al. 2005). As in Monodelphis, V1 neurons in the tammar wallaby respond to a specific range of spatial and temporal frequencies, with a combination of low-pass and bandpass tuning curves. However, unlike some highly visual eutherian mammals, these neurons are not organized in discrete modules, such as pinwheels (Ibbotson and Mark 2003). Thus, whereas populations of neurons in V1 of marsupials possess (to differing degrees) the basic patterns of organization described across placental mammals, no marsupials investigated have the highly specialized, modular patterns described in carnivores, primates, and tree shrews (Bonhoeffer and Grinvald 1991; Bosking et al. 1997)
Fig. 10.
A: cladogram showing several mammalian species, the period of day in which they are active, and several features of a visual stimulus to which their neurons in V1 respond (left to right): active period, percentage of V1 neurons which are orientation selective, axial length of the eye, mean spatial frequency to which neurons are maximally responsive (left black dot), and behavioral visual acuity (right white dot). In the cladogram, marsupials are indicated with gray lines and eutherian mammals with black lines. Three of the four marsupials investigated have only a small percentage of orientation selective neurons in V1, with the exception of the diurnal (Tammar wallaby), which looks more like the tree shrew than other marsupials in most of the characteristics examined. For axial length of the eye and percentage of cells that are orientation selective, values are shown as either points (black dots) or a range of normal values (black line). Mean maximum spatial frequency (far right, black dot) is the average spatial frequency at which V1 neurons are maximally responsive for each species. White dots reflect the behavioral acuity measured in each species. These values were not available for Virginia opossums and Brush-tailed possums. B: data for the different species' axial length of the eye and spatial frequency of maximal neuronal response is plotted on the Y- and X-axis, respectively. Short-tailed opossums are plotted in gray and all other species (placental mammals) are plotted in black. When a trend line is fit to the data (solid line) short-tailed opossums remain consistent with the mammalian trend of eye size vs. acuity. Despite having a much lower proportion of orientation selective neurons in V1, the visual acuity of short-tailed opossums is similar to that with animals of a similar eye size, rather than being lower. Thus decreased orientation selectivity does not negatively impact short-tailed opossum's visual acuity measured in V1. Data for each species were obtained as follows: short-tailed opossum (present study, Dooley et al. 2012); big-eared opossum (Rocha-Miranda et al. 1973); brush-tailed opossum (Crewther et al. 1984); Tammar wallaby (Hemmi and Mark 1998; Ibbotson and Mark 2003); mouse (Niell and Stryker 2008; Park et al. 2013; Prusky et al. 2000); rat (Girmen et al. 1999; Mutti et al. 1998; Prusky et al. 2000); gray squirrel (Jacobs et al. 1982; Van Hooser et al. 2005); Rhesus macaque (De Valois et al. 1982; Foster et al. 1985; Kondo et al. 2006; Ringach et al. 2002; Sceniak et al. 2001); cat (Blake et al. 1974; Konrade et al. 2012; Maffei and Fiorentini 1973); tree shrew (Bosking et al. 1997; Phillips and McBrien 2004).
Together, these results suggest that although V1 in the common ancestor of marsupials and placental mammals contained orientation-selective neurons, the proportion of orientation-selective neurons in marsupials is relatively low (30%) compared with eutherian mammals (> 70%, see Van Hooser 2007). Orientation-selective neurons in nocturnal marsupials suggest that the ability to compute orientation was present in our common ancestors. Because converging evidence indicates that the common ancestor to eutherian and marsupial mammals was nocturnal (Heesy and Hall 2010; Kemp 2005), it is reasonable to hypothesize that these ancestors had a low percentage of orientation-selective neurons as well, compared with the high percentage of orientation-selective neurons found in many contemporary eutherian mammals. Thus the larger proportion of orientation-selective neurons in V1 of the tammar wallaby appears to be independently acquired from the large proportion of orientation-selective neurons observed in placental mammals, although more studies in a wider range of marsupials are necessary to confirm this hypothesis. We further hypothesize that the same selective pressures associated with a movement away from nocturnality and towards a crepuscular lifestyle (like that of the tammar wallaby), at least in part, led to this increase in orientation selectivity early in eutherian evolution. Orientation selectivity was then further enhanced with the acquisition of a diurnal lifestyle of some eutherians.
Response Properties of Neurons in V1 of Small Placental Mammals
In this discussion, we limit our comparisons to eutherian mammals whose body morphology, diel pattern, and lifestyle are similar to the short-tailed opossum. Not surprisingly, the most well-studied examples are the laboratory mouse (Mus musculus) and rat (Rattus norvegicus). Both the eye morphology and visual abilities of these animals (with visual acuities of 0.5 and 1.2 cpd, respectively) are more similar to short-tailed opossums than to more highly visual eutherian species. Neural responses to different spatial and temporal frequencies in short-tailed opossums fall between the values described in rats and mice. Likewise, the bandwidths of spatial and temporal frequencies of neurons in V1 in these two rodents are similar (between 2–3 octaves) to those reported here in opossums (Girmen et al. 1999; Niell and Stryker 2008). Despite these and other similarities between rats, mice, and opossums, there were notable differences, particularly the low proportion of orientation-selective neurons in V1 found in opossums (40% in opossums vs. >70% in rats and mice).
Despite these differences in organization, the behavioral visual acuity of short-tailed opossums is similar to other mammals of its size such as mice and rats; these rodents have eyes slightly smaller and larger than short-tailed opossums, respectively (Fig. 10A). When comparing the visual acuity of different species to their eye size (specifically axial length), it appears that short-tailed opossums follow the same general trends of mammalian vision (Dooley et al. 2012; Fig. 10A). Further, the mean spatial frequency to which V1 neurons are maximally responsive in short-tailed opossums is also between experimental values found in mice and rats (Fig. 10A). By plotting the axial length of the eye in a variety of mammals against the spatial frequency that produces a maximal response in neurons in V1, we can demonstrate that the short-tailed opossum conforms to the relationship observed in the placental mammals examined (Fig. 10B, solid line; R2 = 0.7446). Thus, despite differences in the percentage of V1 neurons that display orientation selectivity, opossums do not appear to have diminished visual acuity, both behaviorally and in terms of response properties of V1 neurons, compared with placental mammals with similarly sized eyes. However, primates such as macaques, and the closely related tree shew, do differ from the overall mammalian trend, showing higher than expected visual acuity given the size of their eyes. This is important, as it suggests that in at least for small animals, a V1 containing predominantly orientation-selective neurons is not necessary for the “expected” visual acuity. However, as an animal's body and eyes grow, visual acuity continues to increase. In this circumstance, a V1 with a preponderance of neurons that are orientation selective may take on an increased significance in animals with higher visual acuity.
Differences Between Normal and Stripe-Reared Opossums
By exposing developing short-tailed opossums to vertical stripes with a spatial frequency above their normal behavioral visual acuity, we hoped to shift neuronal response properties, inducing an overrepresentation of the rearing stimulus. Whereas the 40% of neurons that were orientation selective (OCV > 0.1, see Fig. 5B) in normal animals did not significantly differ from a uniform distribution across the four tested orientations, those of stripe-reared animals differed significantly from this distribution (Fig. 5C). Specifically, there was a preponderance of neurons selective for vertically orientated stimuli (0°) in stripe-reared animals, at the expense of neurons selective to the remaining three orientations. The distribution of orientation-selective neurons also differed significantly between stripe-reared and normal animals. Importantly, this was not accompanied by an increase in orientation-selective neurons, nor by a systematic shift in the mean OCV of stripe-reared opossums.
By rearing in a high spatial frequency environment, we also hoped to increase neuronal responsivity to high spatial frequency stimuli. In normal animals, the highest spatial frequency presented (0.70 cpd) was sufficient to drive a small percentage of V1 neurons (23%, red line, far right of Fig. 7C). However, compared with normal animals, neurons in V1 of stripe-reared animals showed an increased likelihood of responding to the highest two spatial frequencies presented (see 0.42 and 0.70 cpd in Fig. 7C), with 55% of neurons firing above baseline to the highest spatial frequency presented. This indicates that these higher spatial frequencies drive a larger percentage of V1 neurons following early and prolonged exposure to a high spatial frequency environment. It is important to note that stripe-rearing did not create neurons that responded to spatial frequencies that could never elicit a neural response in normal animals, but instead increased the proportion of neurons that responded to higher spatial frequencies. Further, when looking at the percentage of neurons above half height, there was no difference at higher spatial frequencies between these groups (Fig. 7F). Thus these responses at high spatial frequencies were above the baseline firing rate but still relatively weak compared with the preferred spatial frequency (see the lowest 3 spatial frequencies in Fig. 6, C and D).
Unexpectedly, in stripe-reared animals we also increased the neural response to low spatial frequency stimuli (see the first 3 data points of Fig. 7F). As a result, we observed an increase in the bandwidth of spatial frequency tuning curves of V1 neurons in stripe-reared animals. There was also an increased likelihood that neurons would fire at faster temporal frequencies in stripe-reared animals (Fig. 8, F and I). Finally, neurons from stripe-reared animals were consistently driven at lower contrast values compared with normal animals, and saturated at lower contrast values (Fig. 9).
Taken together, these results demonstrated an overall increase in responsivity in V1 neurons of stripe-reared animals to stimuli of different spatial frequencies, temporal frequencies, and contrast levels. Thus stripe-reared animals are capable of detecting and responding to a wider range of stimuli compared with normal opossums. However, because the bandwidth of responses of individual neurons is also increasing, the visual system may actually be poorer at differentiating between different stimuli. Future studies investigating the behavioral effects of stripe-rearing short-tailed opossums are needed to confirm and build upon these hypotheses.
Experience-Dependent Plasticity of V1 Response Properties
Previous studies on experience-dependent cortical plasticity have investigated the extent to which neural response properties develop in a deprived (e.g., dark rearing) or systematically altered environment (e.g., stripe-rearing, prism-rearing, etc.; Espinosa and Stryker 2012). These studies have almost exclusively reported changes in the proportion of neurons responsive to a given orientation. While stripe-rearing animals greatly skewed this orientation toward the presented stimuli (cats: Hirsch and Spinelli 1970; rats: O'Hashi et al. 2007; mice: Kreile et al. 2011; see Espinosa and Stryker 2012), dark-reared animals have neurons in V1 with a similar distribution of preferred visual orientations compared with controls (cat: Kalil 1978; rat: Fagiolini et al. 1994; ferret: White et al. 2001). In fact, dark-reared ferrets still have a pinwheel organization in V1 (White et al. 2001). Further, White and colleagues (2001) found that in ferrets, lid-suturing (which allows some visual input to the retina) actually resulted in less orientation selectivity compared with dark-rearing, presumably because of the aberrant visual input provided through the ferrets' eyelids. Conversely, by providing instructive signals that are heavily weighted toward a particular orientation in the stripe-rearing paradigm, competition between local circuit connectivity promotes an overrepresentation of the exposed orientation. Mechanistically, this result is possibly due to the instructive signal provided by the overrepresented orientations, and the lack of competition from competing orientations (Tieman and Hirsh 1982; Tieman et al. 1995). These studies have led to two competing hypotheses explaining these results: 1) stripe-rearing stunts the development of neurons responding to aberrant orientations (lowering the number of visually responsive neurons), or 2) stripe-rearing leads to the selective pruning of connections across development, shifting the orientation preference of V1 neurons toward the presented stimuli (Kreile et al. 2011).
Importantly, the majority of species that have been investigated are highly visual animals (e.g., cats and macaque), where often up to 99% of neurons are orientation selective (Maldonado et al. 1997; Schiller et al. 1976); even in animals that rely less on their visual system (e.g., mouse and rat), over 70% of neurons are orientation selective (Girman et al. 1999; Niell and Stryker 2008). As mentioned above, existing work on marsupials reveals a larger range in the percentage of orientation-selective neurons compared with placentals investigated (70% in the tammar wallaby to 30% in other marsupials; see above).
In the current study, because we did observe a shift in the preferred orientation of V1 neurons in stripe-reared animals without a change in the percentage of orientation-selective neurons, our results suggest that stripe-rearing shifts the preferred orientation of existing orientation-selective neurons, rather than eliminating neurons responsive to aberrant orientations or adding neurons responsive to overrepresented stimuli. As there are fewer orientation-selective neurons in V1, the overall percentage of V1 neurons showing this type of plasticity is modest compared with previous animals studied.
The changes to spatial, temporal, and contrast selectivity of neurons in V1 that we did observe after stripe-rearing are not reported in other species of stripe-reared mammals, often because these studies only investigate changes in orientation selectivity. However, one recent study describes differences in the percentage of neurons in V1 responding to different spatial and temporal frequencies in stripe-reared mice (Kreile et al. 2011). As this was an optical imaging study, only differences in neurons in layers 2/3 were reported. However, in more superficial portions of layers 2/3, there was a larger percentage of neurons in stripe-reared animals that were responsive to higher spatial frequencies, whereas in the deeper portions of layers 2/3, neurons were more responsive to higher temporal frequencies (Kreile et al. 2011). Mirroring the present investigation, these changes were orientation indifferent, and were present without any decrease at lower spatial or temporal frequencies. Thus, as reported in the current study, a larger fraction of neurons in these mice is driven by higher spatial and temporal frequencies. As with our results in opossums, this shift represents an increase in the fraction of neurons responding to the same stimuli, rather than a shift to neurons responding to completely novel stimuli.
Whereas the proposed mechanism explaining differences in neuronal response properties resulting from stripe-rearing is due to small anatomical differences, the observed significant reduction of C50 values in stripe-reared animals that we observe (Fig. 9F) suggests another alternative. Few studies have shown such a shift in contrast responses in V1; however, one recent study (Bhattacharyya et al. 2013) reports a significant decrease in C50 values in tree shrew primary visual cortex following stimulation of the basal forebrain. Thus the differences in contrast responses observed between stripe-reared and normal animals could be the result of differences in basal forebrain activity, which has been shown to modulate V1 neuron response properties. Mechanistically, how stripe-rearing would induce differences in modulatory inputs to V1 is unclear; however, this framework could prove to be a promising avenue for future studies of environmentally induced changes in response properties of neurons in V1.
Studying Vision in Laboratory vs. Wild-Caught Animals
It is important to remember that the visual environment of all laboratory animals is impoverished. Regardless of rearing condition, the visual statistics in the standard housing environment is dramatically different from that of the natural environment. Further, unlike laboratory conditions, in the wild, suboptimal visual acuity could make a difference in survival. This is particularly true for species such as the short-tailed opossums, which both hunt for food (small insects) and are subject to significant predation from birds, reptiles, and other mammals (Macrini 2004; VandeBerg and Williams-Blangero 2010). Although the present investigation describes differences between the visual environments of normal and stripe-reared laboratory animals, these differences are relatively minor (but better controlled) compared with the diversity of visual environments in the wild. Thus we would expect an even larger degree of differences between even the most “enriched” laboratory visual environments and wild-caught animals. Further, many commonly used laboratory animals, such as rats and mice, have been inbred for more than 100 years. Therefore, genetic diversity is lower than in wild populations. Whether this alters the distribution of response properties for a given feature of the visual stimulus is an empirical question that remains to be tested.
Conclusion
In summary, visual response properties of neurons in V1 of the short-tailed opossum are like those of other small-brained, nocturnal eutherian mammals; however, only about 40% of neurons in V1 are orientation selective. This percentage is consistent with results found previously in other nocturnal marsupials. The present investigation is the first one to examine the effects of stripe-rearing in any marsupial species. There was a shift in the preferred orientations of neurons between normal and stripe-reared opossums without an increase in the percentage of orientation-selective neurons. Further, we did observe a broadening in the spatial frequencies that neurons were responsive to, and a shift to faster temporal frequencies. Finally, V1 neurons of stripe-reared animals begin responding at a lower level of contrast compared with normally reared animals. These results suggest that the population of V1 neurons of stripe-reared mammals is more strongly driven by stimuli that resemble the vertical stripes in their home cage. Future behavioral studies are needed to confirm whether these functional differences are reflected in the visually mediated behavior of stripe-reared opossums.
GRANTS
This project was supported by funds to L. A. Krubitzer from the National Eye Institute (NEI) (R01-EY-022987) and to J. C. Dooley from the NEI (T32-EY-015387-05).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.C.D. and L.A.K. conceived and designed study; J.C.D., M.S.D., and L.A.K. performed experiments; J.C.D., M.S.D., and L.A.K. analyzed data; J.C.D., M.S.D., and L.A.K. interpreted results of experiments; J.C.D. and L.A.K. prepared figures; J.C.D. and L.A.K. drafted manuscript; J.C.D., M.S.D., and L.A.K. edited and revised manuscript critically for important intellectual content; L.A.K. supervised study; J.C.D., M.S.D., and L.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the software engineering module of the Vision Science Core Grant, particularly Drs. Jeffery Johnson and Dan Sperka, for hardware and programming assistance. We also thank Cindy Clayton, DVM and the rest of the animal care staff at the UC Davis Psychology Department Vivarium. We give additional thanks to Deepa Ramamurthy and Dr. Adam Goldring for critically reading the manuscript, Hoang Nguyen for surgical assistance, and Drs. Adele Seelke and Dylan Cooke for helpful comments during construction of the figures.
Glossary Abbreviations
- cpd
Cycles per degree
- cps
Cycles per second
- CRT
Cathode ray tube
- HM
Horizontal meridian
- sps
Spikes per second
- SF
Spatial frequency
- TF
Temporal frequency
- VM
Vertical meridian
Equation Variables
- C50
Contrast percentage where response is 50% of maximum
- DCV
Directional circular variance
- DSI
Directional selectivity index
- FR
Firing rate (in sps)
- FRmax
Maximum firing rate (within a parameter)
- FRmin
Minimum firing rate (within a parameter)
- FRspon
Spontaneous firing rate
- FRx
Firing rate for stimulusx
- RHH
Half-height
- OCV
Orientation circular variance
- OSI
Orientation selectivity index
- Rmax
Maximum relative response
- Rmin
Minimum relative response
- Rspon
Ratio of FRspon to FRmax
- Rx
Relative response rate for stimulusx
- SDspon
Standard deviation of spontaneous activity
REFERENCES
- Balkema GW Jr, Pinto LH. Electrophysiology of retinal ganglion cells in the mouse: a study of a normally pigmented mouse and a congenic hypopigmentation mutant, pearl. J Neurophysiol 48: 968–980, 1982. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Viet J, Kretz R, Bondar I, Rainer G. Basal forebrain activation controls contrast sensitivity in primary visual cortex. BMC Neurosci 14:5 5, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Cool SJ, Crawford ML. Visual resolution in the cat. Vision Res 14: 1211–1217, 1974. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Mitchell DE. Environmental modification of the visual cortex and the neural basis of learning and memory. Nature 241: 467–468, 1973. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature 353: 429–431, 1991. [DOI] [PubMed] [Google Scholar]
- Bosking WH, Zhang Y, Schofield B, Fitzpatrick D. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J Neurosci 17: 2112–2127, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci 13: 5251–5262, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JL, Hill RM. Receptive fields of single cells of a marsupial visual cortex of Didelphis virginiana. Experientia 26: 43–44, 1970. [DOI] [PubMed] [Google Scholar]
- Crewther DP, Crewther SG, Sanderson KJ. Primary visual cortex in the brushtailed possum: receptive field properties and corticocortical connections. Brain Behav Evol 24: 184–197, 1984. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Yund EW, Hepler N. The orientation and direction selectivity of cells in macaque visual cortex. Vision Res 22: 531–544, 1982. [DOI] [PubMed] [Google Scholar]
- Dooley JC, Nguyen HM, Seelke AM, Krubitzer L. Visual acuity in the short-tailed opossum (Monodelphis domestica). Neuroscience 223: 124–130, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron 75: 230–249, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: Dark rearing and monocular deprivation. Vision Res 34: 709–720, 1994. [DOI] [PubMed] [Google Scholar]
- Foster KH, Gaska JP, Nagler M, Pollen DA. Spatial and temporal frequency selectivity of neurones in visual cortical areas V1 and V2 of the macaque monkey. J Physiol 365: 331–363, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girman SV, Sauve Y, Lund RD. Receptive field properties of single neurons in rat primary visual cortex. J Neurophysiol 82: 301–311, 1999. [DOI] [PubMed] [Google Scholar]
- Hemmi JM, Mark RF. Visual acuity, contrast sensitivity and retinal magnification in a marsupial, the tammar wallaby (Macropus eugenii). J Comp Physiol A 183: 379–387, 1998. [DOI] [PubMed] [Google Scholar]
- Hirsch HVB, Spinelli DN. Visual experience modifies distribution of horizontally and vertically oriented receptive fields in cats. Science 168: 869–871, 1970. [DOI] [PubMed] [Google Scholar]
- Heesy CP, Hall MI. The nocturnal bottleneck and the evolution of mammalian vision. Brain Behav Evol 75: 195–203, 2010. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. J Physiol 148: 574–591, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Shape and arrangement of columns in cat's striate cortex. J Physiol 165: 559–568, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibbotson MR, Mark RF. Orientation and spatiotemporal tuning of cells in the primary visual cortex of an Australian marsupial, the wallaby Macropus eugenii. J Comp Physiol A 189: 115–123, 2003. [DOI] [PubMed] [Google Scholar]
- Ibbotson MR, Price NS, Crowder NA. On the division of cortical cells into simple and complex types: a comparative viewpoint. J Neurophysiol 93: 3699–3702, 2005. [DOI] [PubMed] [Google Scholar]
- Jacobs GH, Birch DG, Blakeslee B. Visual acuity and spatial contrast sensitivity in tree squirrels. Behav Processes 7: 367–375, 1982. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Evolution of columns, modules, and domains in the neocortex of primates. Proc Natl Acad Sci USA 109, Suppl 1: 10655–10660, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn DM, Huffman KJ, Krubitzer L. Organization and connections of V1 in Monodelphis domestica. J Comp Neurol 428: 337–354, 2000. [PubMed] [Google Scholar]
- Kalil R. Dark rearing in the cat: effects on visuomotor behavior and cell growth in the dorsal lateral geniculate nucleus. J Comp Neurol 178: 451–467, 1978. [DOI] [PubMed] [Google Scholar]
- Kemp TS. The Origin and Evolution of Mammals. London: Oxford Univ. Press, 2005. [Google Scholar]
- Kondo M, Ito Y, Miyata K, Kondo N, Ishikawa K, Terasaki H. Effect of axial length on laser spot size during photodynamic therapy: an experimental study in monkeys. Am J Ophthalmol 141: 214–215, 2006. [DOI] [PubMed] [Google Scholar]
- Konrade KA, Hoffman AR, Ramey KL, Goldenberg RB, Lehenbauer TW. Refractive states of eyes and associations between ametropia and age, breed, and axial globe length in domestic cats. Am J Vet Res 73: 279–284, 2012. [DOI] [PubMed] [Google Scholar]
- Kreile AK, Bonhoeffer T, Hubener M. Altered visual experience induces instructive changes of orientation preference in mouse visual cortex. J Neurosci 31: 13911–13920, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Stolzenberg DS. The evolutionary masquerade: genetic and epigenetic contributions to the neocortex. Curr Opin Neurobiol 24: 157–165, 2014. [DOI] [PubMed] [Google Scholar]
- LeVay S, Hubel DH, Wiesel TN. The pattern of ocular dominance columns in macaque visual cortex revealed by a reduced silver stain. J Comp Neurol 159: 559–576, 1975. [DOI] [PubMed] [Google Scholar]
- Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci 9: 676–681, 2006. [DOI] [PubMed] [Google Scholar]
- Macrini TE. Monodelphis domestica. Mammal Species 760: 1–8, 2004. [Google Scholar]
- Maffei L, Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res 13: 1255–1267, 1973. [DOI] [PubMed] [Google Scholar]
- Maldonado PE, Godecke I, Gray CM, Bonhoeffer T. Orientation selectivity in pinwheel centers in cat striate cortex. Science 276: 1551–1555, 1997. [DOI] [PubMed] [Google Scholar]
- Mazurek M, Kager M, Van Hooser SD. Robust quantification of orientation selectivity and direction selectivity. Front Neural Circuits 8: 92, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith RW, Janečka JE, Gatesy J, Ryder OA, Fisher CA, Teeling EC, Goodbla A, Eizirik E, Simão TLL, Stadler T, Rabosky DL, Honeycutt RL, Flynn JJ, Ingram CM, Steiner C, Williams TL, Robinson TJ, Burk-Herrick A, Westerman M, Ayoub NA, Springer MS, Murphy WJ. Impacts of the cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334: 521–524, 2011. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Zadnik K, and Murphy CJ. The effect of continuous light on refractive error and the ocular components of the rat. Exp Eye Res 67: 631–636, 1998. [DOI] [PubMed] [Google Scholar]
- Naito T, Sadakane O, Okamoto M, Sato H. Orientation tuning of surround suppression in lateral geniculate nucleus and primary visual cortex of cat. Neurosci 149: 962–975, 2007. [DOI] [PubMed] [Google Scholar]
- Naka KI, Rushton WA. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185: 536–555, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci 28: 7520–7536, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hashi K, Miyashita M, Tanaka S. Experience-dependent orientation plasticity in the visual cortex of rats chronically exposed to a single orientation. Neurosci Res 58: 86–90, 2007. [DOI] [PubMed] [Google Scholar]
- O'Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo ZX, Meng J, Ni X, Novacek MJ, Perini FA, Randall ZS, Rougier GW, Sargis EJ, Silcox MT, Simmons NB, Spaulding M, Velazco PM, Weksler M, Wible JR, Cirranello AL. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339: 662–667, 2013. [DOI] [PubMed] [Google Scholar]
- Park HN, Qazi Y, Tan C, Jabbar SB, Cao Y, Schmid G, Pardue MT. Assessment of axial length measurements in mouse eyes. Optom Vis Sci 89: 296–303, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Phillips JR, McBrien NA. Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera. Invest Ophthalmol Vis Sci 45: 758–763, 2004. [DOI] [PubMed] [Google Scholar]
- Picanco-Diniz CW, Silveira LC, Oswaldo-Cruz E. Electrophysiological determination of the refractive state of the eye of the opossum. Vision Res 23: 867–872, 1983. [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res 40: 2201–2209, 2000. [DOI] [PubMed] [Google Scholar]
- Ringach DL, Shapley RM, Hawken MJ. Orientation selectivity in macaque V1: diversity and laminar dependence. J Neurosci 22: 5639–5651, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Miranda CE, Bombardieri RA Jr, de Monasterio FM, Linden R. Receptive fields in the visual cortex of the opossum. Brain Res 63: 362–367, 1973. [DOI] [PubMed] [Google Scholar]
- Rocha-Miranda CE, Linden R, Volchan E, Lent R, Bombar-Dieri RA Jr. Receptive field properties of single units in the opossum striate cortex. Brain Res 104: 197–219, 1976. [DOI] [PubMed] [Google Scholar]
- Rochefort NL, Narushima M, Grienberger C, Marandi N, Hill DN, Konnerth A. Development of direction selectivity in mouse cortical neurons. Neuron 71: 425–432, 2011. [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Hawken MJ, Shapley R. Visual spatial characterization of macaque V1 neurons. J Neurophysiol 85: 1873–1887, 2001. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Finlay BL, Volman SF. Quantitative studies of single-cell properties in monkey striate cortex. II. Orientation specificity and ocular dominance. J Neurophysiol 39: 1320–1333, 1976. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Dooley JC, Krubitzer LA. The emergence of somatotopic maps of the body in S1 in rats: the correspondence between functional and anatomical representation. PLoS One. 7: e32322, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman SB, Hirsch HV. Exposure to lines of only one orientation modifies dendritic morphology of cells in the visual cortex of the cat. J Comp Neurol 211: 353–362, 1982. [DOI] [PubMed] [Google Scholar]
- Tieman SB, Zec N, Tieman DG. Dark-rearing fails to affect the basal dendritic fields of layer 3 pyramidal cells in the kitten's visual cortex. Brain Res Dev Brain Res 84: 39–45, 1995. [DOI] [PubMed] [Google Scholar]
- Van Hooser SD. Similarity and diversity in visual cortex: is there a unifying theory of cortical computation? Neuroscientist 13: 639–656, 2007. [DOI] [PubMed] [Google Scholar]
- Van Hooser SD, Heimel JA, Chung S, Nelson SB, Toth LJ. Orientation selectivity without orientation maps in visual cortex of a highly visual mammal. J Neurosci 25: 19–28, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeBerg JL, Williams-Blangero Sarah. The laboratory opossum. In: The UFAW Handbook on The Care and Management of Laboratory and Other Research Animals, edited by Hubrecht RC, Kirkwood J. The Universities Federation for Animal Welfare, 2010, p. 16. [Google Scholar]
- Veit J, Bhattacharya A, Kretz R, Rainer G. On the relation between receptive field structure and stimulus selectivity in the tree shrew primary visual cortex. Cerebral Cortex 24: 2761–2771, 2014. [DOI] [PubMed] [Google Scholar]
- White LE, Coppola DM, Fitzpatrick D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature 411: 1049–1052, 2001. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol 26: 1003–1007, 1963. [DOI] [PubMed] [Google Scholar]
- Woodburne MO, Rich TH, Springer MS. The evolution of tribospheny and the antiquity of mammalian clades. Mol Phylogenet Evolution 28: 360–385, 2003. [DOI] [PubMed] [Google Scholar]