Abstract
Horizontal cells (HCs) are inhibitory interneurons of the vertebrate retina. Unlike typical neurons, HCs are chronically depolarized in the dark, leading to a constant influx of Ca2+. Therefore, mechanisms of Ca2+ homeostasis in HCs must differ from neurons elsewhere in the central nervous system, which undergo excitotoxicity when they are chronically depolarized or stressed with Ca2+. HCs are especially well characterized in teleost fish and have been used to unlock mysteries of the vertebrate retina for over one century. More recently, mammalian models of the retina have been increasingly informative for HC physiology. We draw from both teleost and mammalian models in this review, using a comparative approach to examine what is known about Ca2+ pathways in vertebrate HCs. We begin with a survey of Ca2+-permeable ion channels, exchangers, and pumps and summarize Ca2+ influx and efflux pathways, buffering, and intracellular stores. This includes evidence for Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and N-methyl-d-aspartate receptors and for voltage-gated Ca2+ channels. Special attention is given to interactions between ion channels, to differences among species, and in which subtypes of HCs these channels have been found. We then discuss a number of unresolved issues pertaining to Ca2+ dynamics in HCs, including a potential role for Ca2+ in feedback to photoreceptors, the role for Ca2+-induced Ca2+ release, and the properties and functions of Ca2+-based action potentials. This review aims to highlight the unique Ca2+ dynamics in HCs, as these are inextricably tied to retinal function.
Keywords: calcium, horizontal cell, teleost, ion channels, retina
horizontal cells (HCs) are inhibitory neurons that provide the first layer of neural processing in the vertebrate retina. They share a long history as model central neurons and have been studied for over one century (Ramón y Cajal 1909). Teleost HCs, in particular, are especially large, lending themselves well to electrophysiology, and were the first cells of the retina from which intracellular recordings were obtained (Dowling 1987; Svaetichin 1953). Their graded responses to light demonstrated, for the first time, that neurons could encode and relay information without using action potentials (APs). Today, the membrane properties and ionic currents of teleost HCs are well characterized (DeVries and Schwartz 1992; Dixon et al. 1996; Jonz and Barnes 2007; McMahon and Brown 1994; Shingai and Christensen 1986; Tachibana 1981, 1983), yet their Ca2+ dynamics are only beginning to be clarified. In neurons, Ca2+ plays a central role in vesicular neurotransmitter release (Wadel et al. 2007), synaptic plasticity (Zucker 1999), gene transcription (Lyons and West 2011), and second-messenger pathways (Clapham 2007). In addition, high levels of cytosolic Ca2+ are toxic to neurons (Choi 1992; Lipton 1999), and therefore, Ca2+ sequestration and efflux mechanisms must be present to maintain cell viability. Therefore, the understanding of the dynamics and effects of Ca2+ in a given neuron is often crucial to understanding its function.
In the outer retina, HCs provide inhibitory feedback to photoreceptors (PRs), modulating synaptic gain and mediating a center-surround antagonism that allows for edge detection (Baylor et al. 1971; Thoreson and Mangel 2012). Although there is no consensus as to how this feedback occurs, three mechanisms have been proposed in vertebrates: 1) HCs may release less GABA onto PRs during light stimulation (GABA disinhibition) (Hirano et al. 2005, 2016; Liu et al. 2013; Wu and Dowling 1980) by vesicular release in mammals and largely through an unconventional transporter mechanism in nonmammalian vertebrates (Schwartz 2002); 2) hemichannels at HC dendrites may alter PR membrane potential by an ephaptic mechanism (Byzov and Shura-Bura 1986; Fahrenfort et al. 2009; Gardner et al. 2015; Kamermans et al. 2001); and/or 3) HCs may release protons or regulate cleft acidity (Vroman et al. 2014) to reduce the conductance of PR voltage-gated Ca2+ channels (VGCCs) and shift their activation to more depolarized voltages (Barnes 2003; Hirasawa and Kaneko 2003; Vessey et al. 2005).
Ca2+ signaling in HCs would likely affect all three mechanisms. In rats, HC VGCCs have been shown to be necessary for vesicular GABA release and for feedback in a pH-dependent fashion (Liu et al. 2013), and GABA release from HCs in fish is probably partially Ca2+-dependent (Ayoub and Lam 1985; Lasater and Lam 1984). HC hemichannels are regulated by extracellular Ca2+ concentrations ([Ca2+]o) (DeVries and Schwartz 1992; Jonz and Barnes 2007). There is also evidence that intracellular Ca2+ concentration ([Ca2+]i) affects extracellular pH and may therefore affect pH at the HC-PR synapse (Kreitzer et al. 2012). In light of this evidence, a model for HC Ca2+ dynamics will be instrumental in understanding HC-to-PR feedback and neural processing in the outer retina in general.
This review will explore the experiments that have characterized Ca2+ pathways in teleost and mammalian HCs. Each section below begins by discussing what is known in teleost models and offers a comparison with mammals. First, the major Ca2+-permeable ion channels, pumps, and exchangers will be described, including influx and efflux pathways, as well as Ca2+ buffering, extrusion, and sequestration mechanisms. Subsequent sections will review what is known about Ca2+-based APs in teleost HCs. These have been reported in a variety of experimental preparations but largely remain a mystery. Finally, we will summarize the research thus far on Ca2+ homeostasis in HCs and highlight putative roles for Ca2+ in HC function. These details are summarized in Table 1, and a working model of Ca2+-permeable pathways and their regulation in teleost HCs is presented in Fig. 1.
Table 1.
Ca2+-permeable ion channels, pumps, and exchangers found in teleost HCs
| Ion Channel, Pump, or Exchanger | Species | Common Name | Subtype* | Reference |
|---|---|---|---|---|
| Ionotropic Glutamate Receptors | ||||
| NMDARs | Carassius auratus | Goldfish† | H1 | (Jiang et al. 2008; Shen et al. 2006; Wang et al. 2008) |
| Ictalurus punctatus | Catfish | Cone | (Linn and Christensen 1992; O'Dell and Christensen 1986, 1989) | |
| Perca fluviatus | European perch | Cone | (Schmidt 1997) | |
| Roccus americana | White perch | Cone | (Schmidt 1997) | |
| Ca2+-permeable AMPARs | Carassius auratus | Goldfish† | Cone | (Huang and Liang 2005) |
| H1 | (Huang et al. 2006; Sun et al. 2010) | |||
| Not provided | Carp | Cone | (Okada et al. 1999) | |
| Voltage-Gated Ca2+ Channels | ||||
| L-type | Carassius auratus | Goldfish | (Jonz and Barnes 2007; Tachibana 1981, 1983) | |
| Cyprinus carpio | Carp | (Hayashida and Yagi 2002b; Murakami and Takahashi 1987) | ||
| Roccus americana | White perch | All‡ | (Lasater 1986) | |
| Ictalurus punctatus | Channel catfish | Cone | (Dixon et al. 1993; Linn and Gafka 2001; Shingai and Christensen 1983, 1986; Takahashi et al. 1993) | |
| P/L-type | Roccus chrysops | White bass | All‡ | (Sullivan and Lasater 1992) |
| Cone | (Pfeiffer-Linn and Lasater 1996) | |||
| T-type | Roccus chrysops | White bass | All‡ | (Sullivan and Lasater 1992) |
| Store- and Extrusion-Related Channels | ||||
| Ryanodine receptors | Carassius auratus | Goldfish† | (Huang et al. 2004, 2006) | |
| H1 | (Lv et al. 2014) | |||
| Ictalurus punctatus | Channel catfish | Cone | (Linn and Gafka 2001; Linn and Christensen 1992) | |
| (Micci and Christensen 1998) | ||||
| Moroni chrysops | White bass | H2 | (Solessio and Lasater 2002) | |
| Inositol triphosphate receptors | Ictalurus punctatus | Channel catfish | (Micci and Christensen 1996) | |
| Ictalurus punctatus | Channel catfish | Cone | (Linn and Gafka 2001) | |
| Store-operated channels | Carassius auratus | Goldfish† | H1 | (Lv et al. 2014) |
| Na+/Ca2+ exchanger | Carassius auratus | Goldfish | (Hayashida et al. 1998; Yasui 1987) | |
| Cyprinus carpio | Carp | (Yasui 1987) | ||
| Ictalurus punctatus | Channel catfish | (Micci and Christensen 1998) | ||
| Plasma membrane Ca2+ pump | Carassius auratus | Goldfish | (Hayashida et al. 1998; Kreitzer et al. 2012) | |
| Ictalurus punctatus | Channel catfish | (Jacoby et al. 2012; Kreitzer et al. 2007; Micci and Christensen 1998) | ||
| Gap Junctions and Hemichannels | ||||
| Cx26 | Carassius carassius | Carp | (Janssen-Bienhold et al. 2001) | |
| Danio rerio | Zebrafish | (Kamermans et al. 2001) | ||
| Cx35/36 | Carassius auratus | Goldfish† | H1, H2 | (Liu et al. 2009) |
| Cx43 | Carassius carassius | Carp | (Dermietzel et al. 2000; Janssen-Bienhold et al. 1998) | |
| Cx52.6 | Danio rerio | Zebrafish | (Shields et al. 2007; Sun et al. 2012; Zoidl et al. 2004) | |
| Cx55.5 | Danio rerio | Zebrafish | (Dermietzel et al. 2000; Klaassen et al. 2011; Shields et al. 2007; Sun et al. 2012) | |
| Pannexin-1 | Danio rerio | Zebrafish | (Prochnow et al. 2009a, b) | |
| Uncharacterized | Carassius auratus | Goldfish | (Jonz and Barnes 2007) | |
| H1 | (Schmitz and Wolburg 1991) | |||
| Cone | (Shen et al. 2003) | |||
| Cyprinus carpio | Carp | All‡ | (Kaneko and Stuart 1984; Teranishi et al. 1983; Yamada and Ishikawa 1965) | |
| Danio rerio | Zebrafish | (McMahon 1994; McMahon and Brown 1994) | ||
| Devario danio | Giant danio | H1 | (McMahon and Mattson 1996) | |
| Ictalurus punctatus | Channel catfish | Cone | (DeVries and Schwartz 1989, 1992; Dixon et al. 1996; Sakuranaga and Naka 1985) | |
| Roccus americana | White perch | All‡ | (Lasater and Dowling 1985) | |
| Roccus chrysops | White bass | Cone | (Lasater 1987) | |
| Roccus chrysops x roccus saxitalis | Hybrid striped bass | (Sun et al. 2009; Zhang and McMahon 2000) |
Cx, connexin.
If blank, the associated references did not specify subtypes.
In these papers, the authors referred to Carassius auratus as carp instead of goldfish.
The ion channel, pump, or exchanger was found on all subtypes.
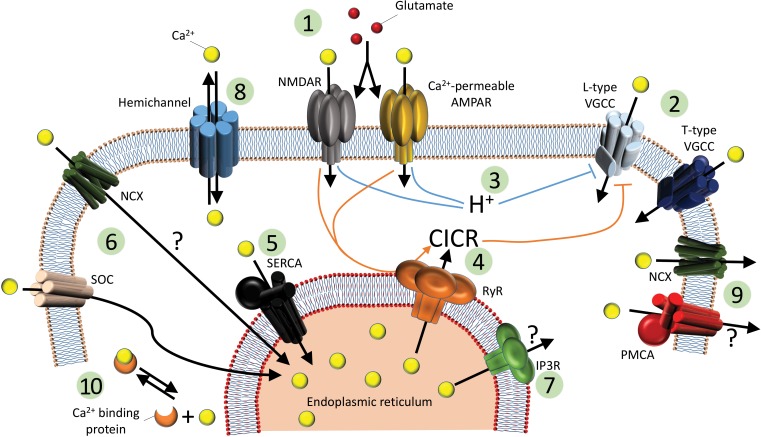
Fig. 1.
Ca2+ pathways and their regulation in teleost horizontal cells. 1: In darkness, glutamate (red circles) is released from photoreceptors and binds onto HC glutamate receptors, allowing Ca2+ influx through Ca2+-permeable AMPARs and NMDARs and leading to depolarization of the plasma membrane. 2: Depolarization also opens VGCCs, including L-type and (at least in bass) T-type VGCCs. 3: Glutamate receptors acidify the cytosol, downregulating L-type VGCCs. In contrast, group I and/or III metabotropic glutamate receptors may upregulate VGCCs (see text). 4: Additionally, glutamate triggers CICR from RyRs, further downregulating L-type VGCCs. 5 and 6: The SERCA pump then refills Ca2+ stores, along with SOCs and/or NCXs functioning in reverse. 7: IP3Rs have been found in catfish but do not contribute to CICR. 8: Gap junctions and hemichannels might allow Ca2+ flow between coupled cells or with the extracellular environment in some conditions. 9: Ca2+ influx from AMPARs, NMDARs, and VGCCs is extruded by the NCX and possibly to a lesser extent, the PMCA. 10: Cytosolic Ca2+ is strongly buffered by Ca2+-binding proteins. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; NMDAR, N-methyl-d-aspartate receptor; VGCC, voltage-gated Ca2+ channel; CICR, Ca2+-induced Ca2+ release; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase; SOC, store-operated channel; IP3R, inositol triphosphate receptor; NCX, Na+/Ca2+ exchanger; PMCA, plasma membrane Ca2+-ATPase.
Ca2+ INFLUX IN HCs
Ca2+ influx in teleost HCs: Ca2+-permeable and -impermeable iGluRs.
In the vertebrate outer retina, PRs are tonically depolarized in the dark by an Na+-dominant “dark current” (Stryer 1991; Yau 1994). As a result, glutamate is constantly released onto HCs and bipolar cells (BCs) (Dowling and Ripps 1973; Miller and Schwartz 1983). PRs, HCs, and off BCs are constantly depolarized to approximately −30 to −20 mV in the dark (Dowling 1987). For HCs, this triggers prolonged Ca2+ influx through both ionotropic glutamate receptors (iGluRs) and VGCCs (Linn and Christensen 1992; Tachibana 1983, 1985). HCs have been shown to respond to glutamate in a diversity of teleost species, including the following: crucian carp (Carassius carassius) (Lasater and Dowling 1982; Murakami et al. 1972), common goldfish (Carassius auratus) (Ishida et al. 1984; Tachibana 1985), channel catfish (Ictalurus punctatus) (O'Dell and Christensen 1989), European perch (Perca fluviatus), and white perch (Roccus americana) (Schmidt 1997).
It is important to understand which iGluRs exist on these cells, because iGluR subtypes have different biophysical properties and physiological functions, including Ca2+ permeability. N-Methyl-d-aspartate receptors (NMDARs) are permeable to Ca2+, whereas both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and kainate receptors (KARs) can be either Ca2+ permeable or Ca2+ impermeable. Notably, AMPARs lacking GluR2 subunits are Ca2+ permeable. Ca2+ permeability for AMPARs containing GluR2 subunits depends on editing at a single amino acid site (Hollmann and Heinemann 1994; Peng et al. 2006). Similarly, the Ca2+ permeability of KARs depends on RNA editing for at least some subunits (Köhler et al. 1993).
In all teleost species studied, HCs respond to a variety of iGluR agonists, including glutamate, kainate, quisqualate, AMPA, and NMDA (Huang et al. 2006; Ishida et al. 1984; Lasater and Dowling 1982; Linn and Christensen 1992; O'Dell and Christensen 1986; Okada et al. 1999; Schmidt 1997). Moreover, it was found that kainate, quisqualate, and glutamate competitively bind to the same receptor (Ishida and Neyton 1985) and that both kainate and quisqualate responses are inhibited by selective AMPAR antagonists (Linn and Christensen 1992). With the use of modulators of KAR desensitization (concanavalin A and cyclothiazide) in carp HCs, Shen et al. (1999) did not find evidence for KARs; instead, they concluded that the teleost iGluR on HCs was an AMPA-preferring receptor. Interestingly, Huang and Liang (2005) proposed that Ca2+-permeable and Ca2+-impermeable AMPARs coexist on HCs. When they coapplied AMPA with the selective Ca2+-permeable AMPAR blocker, joro spider toxin-3, they found a decrease in [Ca2+]i transients; in a similar manner, the selective Ca2+-impermeable AMPAR antagonist, pentobarbital (Yamakura et al. 1995), reduced AMPA-elicited responses. As further evidence for a Ca2+-permeable AMPAR, AMPA applied in situ elicits a rise in [Ca2+]i, even when coapplied with Co2+ to block VGCCs (Okada et al. 1999). Similarly, kainate-induced [Ca2+]i increases cannot be attributed to the Na+-Ca2+ exchange, since kainate elicits an increase in [Ca2+]i when extracellular Na+ is replaced with N-methyl-d-glucamine (Okada et al. 1999). Therefore, Ca2+-permeable AMPARs likely represent a major source of steady Ca2+ influx into HCs. Indeed, Sun et al. (2010) recently found that Ca2+-permeable AMPARs account for 75% of all iGluR currents.
Early investigators were unable to find NMDARs in fish HCs in a variety of species (Lasater and Dowling 1982; Lasater et al. 1984), and some even found a hyperpolarizing response to NMDA—and only at high concentrations (Ariel et al. 1984, 1986; Rowe and Ruddock 1982). After failing to find NMDARs in carp HCs, Okada et al. (1999) proposed that Ca2+-permeable AMPARs might be better suited to increase [Ca2+]i in response to graded potentials. AMPARs are not limited by a voltage threshold, whereas NMDARs would be blocked at most physiological voltages. The first evidence of an NMDAR eliciting a depolarizing response in teleost HCs came from the catfish retina (Linn and Christensen 1992; O'Dell and Christensen 1986). Thereafter, similar responses were shown in crucian carp (Jiang et al. 2008; Shen et al. 2006) and goldfish (Wang et al. 2008). Perhaps these later studies found greater success because they included coapplication of glycine (a necessary NMDAR coagonist) (VanDongen 2008) with NMDA or removal of extracellular Mg2+ (a voltage-dependent NMDAR blocker).
NMDARs may exist only on cone HCs. In most species, teleost HC subtypes are designated H1, H2, H3, and H4, in order of increasing dendritic field size, decreasing somatic size, and increasing distance from the outer retinal layers (Stell and Lightfoot 1975). These subtypes synapse with PRs selectively: H1, H2, and H3 synapse with cones, whereas H4 synapses with rods. All aforementioned studies that found NMDARs in HCs focused solely on cone HCs (predominantly H1), with one exception: a study on crucian carp looked at both rod and cone HCs but failed to find NMDARs in H4 (Shen et al. 2006).
Although the relative contribution of NMDARs and AMPARs to the [Ca2+]i response has not been clearly established, both likely contribute to Ca2+ influx in the dark in teleosts, as will be discussed in the next section. In contrast, there is no evidence for functional NMDARs in HCs of adult mammals (Kalloniatis et al. 2004; Massey and Miller 1987; Shen et al. 2004).
Ca2+ influx in teleost HCs: glutamate triggers an increase in [Ca2+]i through both VGCCs and iGluRs.
HC responses to glutamate are especially well characterized in Ca2+ imaging experiments, in which changes in [Ca2+]i are quantified over time. Initially, glutamate induces a large, transient peak in [Ca2+]i, which is then lowered to a sustained, elevated baseline for as long as glutamate is applied (Hayashida and Yagi 2002b). The transient peak is blocked by ryanodine and is likely due to Ca2+-induced Ca2+ release (CICR) through ryanodine-sensitive stores (Huang et al. 2004).
Several studies suggest that the sustained [Ca2+]i component is due to activation of more than one Ca2+-permeable pathway (Hayashida and Yagi 2002b; Hayashida et al. 1998; Linn and Christensen 1992). The sustained Ca2+ response to glutamate (Hayashida et al. 1998) or AMPA (Huang and Liang 2005) is greatly reduced by applying nifedipine, a selective blocker of L-type VGCCs in teleost HCs (Takahashi et al. 1993). This suggests that glutamate depolarizes HCs, thereby activating L-type VGCCs. Furthermore, in concurrent voltage-clamp and Ca2+ imaging experiments, glutamate only subtly increased sustained [Ca2+]i after a voltage step to −5 mV (Hayashida and Yagi 2002b), suggesting that once VGCCs are open by depolarization, iGluRs do not greatly increase [Ca2+]i. However, this sustained component of the glutamate-elicited Ca2+ response cannot be completely explained by VGCC conductance; for example, nifedipine application does not abolish the sustained response to glutamate (Hayashida et al. 1998).
There is also evidence for Ca2+ influx through iGluRs themselves. The blocking of VGCC conductance with the L-type blocker, nimodipine, does not abolish responses to kainate in catfish HCs (Linn and Christensen 1992). Furthermore, under voltage clamp at potentials high enough to ensure that VGCCs are deactivated, iGluR agonists, such as NMDA, glutamate, quisqualate, and kainate, increase [Ca2+]i to a new, elevated baseline (Hayashida et al. 1998; Linn and Gafka 2001; Linn and Christensen 1992). This suggests that both VGCC and iGluR conductances contribute to the sustained [Ca2+]i elevation during glutamate application.
Ca2+ influx in teleost HCs: most Ca2+ enters the cytosol through L-type VGCCs.
L-type VGCCs in HCs have been well studied with electrophysiological techniques in a number of species, including goldfish (Tachibana 1983), channel catfish (Shingai and Christensen 1983, 1986), white perch (Lasater 1986), and white bass (Roccus chrysops) (Sullivan and Lasater 1992). These studies reported an inward conductance that activated in a range between −45 and −30 mV and peaked between 0 and 25 mV.
Many reports found that L-type VGCCs contribute nearly 50% of the Ca2+ current elicited by glutamate application in situ (Hayashida and Yagi 2002b; Hayashida et al. 1998; Huang and Liang 2005), and therefore, they represent a major source of Ca2+ influx in teleost HCs. It should be mentioned here that unlike typical VGCCs that undergo strong, Ca2+-dependent inactivation, such inactivation is weaker in HCs (Tachibana 1983). Instead, there is evidence that VGCCs are downregulated by low intracellular pH, which can be induced by extracellular acidification (Jonz and Barnes 2007) or by intracellular acidification caused by glutamate application (Dixon et al. 1993; Takahashi et al. 1993). In addition, VGCCs are downregulated by CICR (Linn and Gafka 2001). The available evidence suggests that CICR can raise [Ca2+]i enough for substantial VGCC inactivation, but currents through VGCCs cannot.
VGCCs may also be enhanced by metabotropic glutamate receptors (mGluRs). In channel catfish, group I and III mGluR agonists expand the range of activation potentials into the physiological range, shifting the membrane potential at which peak VGCC currents can be obtained by −5 mV (Linn and Gafka 1999). Group I and III mGluR antagonists prevent this VGCC facilitation (Linn and Gafka 1999). This mGluR-mediated modulation of VGCC currents is probably widespread in vertebrate HCs, which express a range of mGluRs (Beraudi et al. 2007; Thoreson and Witkovsky 1999), although group I mGluRs may be absent in the goldfish (Peng et al. 1995).
Ca2+ influx in teleost HCs: other VGCC subtypes may contribute to Ca2+ influx in teleosts.
Several reports have found evidence for T-type VGCCs in teleost HCs but only in white bass. T-type channels are characterized by small, transient Ca2+ currents that activate approximately −65 mV and peak near −20 mV, a low permeability to Ba2+, and block by Ni2+ (Akopian et al. 1997; Perez-Reyes 2003). Sullivan and Lasater (1992) found a channel with most of these properties, although there were differences between this channel and archetypical VGCCs. Specifically, T-type VGCCs in white bass HCs are not blocked by millimolar concentrations of Ni2+. Whereas T-type channels generally increase excitability of the plasma membrane and can mediate oscillations in membrane potential (Perez-Reyes 2003), their role in white bass HCs is unknown.
Notably, there is evidence of T-type channels in HCs of the rabbit (as discussed below) and the African clawed frog (Xenopus laevis) (Akopian et al. 1997). In Xenopus, they cause voltage oscillations in the light-to-dark transition (Akopian et al. 1997), but the function of these oscillations is unclear. In addition to T-type channels, both rabbit and Xenopus HCs alone show Ca2+-dependent K+ currents (Akopian and Witkovsky 1994; Lohrke and Hofmann 1994). In Xenopus, Ca2+-dependent K+ currents activated close to the activation potential of T-type channels (Akopian and Witkovsky 1994), leading the authors to speculate that T-type channels may modulate outward K+ currents. One is left to wonder whether a similar situation occurs in white bass, the only other species known to have T-type channels in HCs (Sullivan and Lasater 1992).
Pfeiffer-Linn and Lasater (1996) argued that certain properties of high voltage-activated Ca2+ channels in white bass separate them from typical L-type channels. These channels are like L-type channels in their activation potential, single channel conductance, and sensitivity to Bay K8644, but they are antagonized by ω-agatoxin IVA (which is generally considered to be selective for P/Q-type VGCCs) instead of L-type antagonists, such as nifedipine and nimodipine. This aberrant channel, which Pfeiffer-Linn and Lasater (1996) named a P/L-type channel, has not been found in other teleost species.
Ca2+ influx in teleost HCs: gap junctions and hemichannels.
HCs of a given type are electrically coupled to each other by gap junctions, forming a syncytium throughout the inner nuclear layer (Kaneko and Stuart 1984; Sakuranaga and Naka 1985). These channels are permeable to ions and molecules up to ∼1 kDa (Contreras et al. 2004), including fluorescent dyes. As a result, injection of dyes (such as Procion and Lucifer yellow) into individual HCs has provided detailed illustrations of HC syncytia (Baldridge and Ball 1991; Kaneko 1971; Kaneko and Stuart 1984; Stewart 1978). Gap junctions allow for Ca2+ diffusion between cells (D'Andrea and Vittur 1996; Höfer et al. 2001; Nielsen et al. 2012; Saez et al. 1989). In other vertebrate cell types that form syncytia—including cardiac myocytes (Boyden et al. 2015); developing rat, cat, and ferret neurons in the neocortex and inner retina (Katz 1993; Kotak et al. 2007; Wong 1993; Yuste et al. 1992); and rat retinal glial cells (Newman and Zahs 1997)—Ca2+ propagates as waves. Ca2+ activity has been found to be synchronized in HCs, up to 1 mm apart (Murakami et al. 1995). Ca2+ waves, however, have not been identified in HC syncytia.
Much work needs to be done to clarify which connexins (Cx) form either gap junctions or hemichannels on HCs. There is immunohistochemical evidence for Cx35/36 in goldfish HCs (Liu et al. 2009) and Cx43 in carp (Janssen-Bienhold et al. 1998) and both immunohistochemical and in situ hybridization evidence for Cx52.6 and Cx55.5 in zebrafish (ZfCx52.6 and ZfCx55.5, respectively) (Dermietzel et al. 2000; Shields et al. 2007; Zoidl et al. 2004). Additionally, both pannexin-1 (Px1)- and ZfCx26-based hemichannels have been found in zebrafish HC dendrites, where they have been theorized to assist in feedback to cones (Kamermans et al. 2001; Prochnow et al. 2009b). Many retinal Cx and Px transcripts have recently been cloned (Zoidl et al. 2008), suggesting that more may be found in HCs in the near future.
Functionally, electrical coupling through gap junctions provides HCs with a large receptive field size (Kaneko and Stuart 1984), whereas uncoupling decreases this receptive field size and reduces center-surround antagonism (Shen et al. 2003). A number of neuromodulators have been found to adjust the extent of electrical coupling. In fish, interplexiform cells from the inner nuclear layer of the retina release dopamine onto HCs, activating D1 receptors and partially uncoupling them through a cAMP-dependent pathway (Baldridge and Ball 1991; DeVries and Schwartz 1989; Lasater and Dowling 1985; Mangel and Dowling 1987; McMahon and Brown 1994). Nitric oxide has been found to uncouple HCs through a cGMP-dependent mechanism (Daniels and Baldridge 2011; DeVries and Schwartz 1989; Lu and McMahon 1997), and external retinoic acid has been proposed to downregulate gap junctions by binding to them directly (Zhang and McMahon 2000). Low pH has also been shown to diminish teleost HC gap-junctional conductance (DeVries and Schwartz 1989; Jonz and Barnes 2007) and change connexon morphology (Schmitz and Wolburg 1991).
Ca2+ modulates both gap-junctional currents and coupling. [Ca2+]o is known to suppress the retinal hemichannel current (DeVries and Schwartz 1992; Jonz and Barnes 2007), but inward vs. outward hemichannel currents have a different IC50 for Ca2+. Whereas the outward hemichannel current (IC50 = 0.69 mM) is mostly blocked within the expected physiological range for [Ca2+]o (1–2 mM), inward currents (IC50 = 1.58 mM) are less sensitive to [Ca2+]o (Sun et al. 2012). The increase of [Ca2+]o has also been found to decrease the percentage of cells that are coupled in cultured giant danio (Devario aquipinnatus) HCs (McMahon and Mattson 1996). In contrast to the effects of [Ca2+]o, increases in [Ca2+]i have not been found to affect gap-junctional conductance (DeVries and Schwartz 1989).
Ca2+ influx in mammalian HCs: iGluRs.
Evidence from in situ hybridization, immunohistochemistry, and electrophysiological studies supports the presence of AMPARs in all mammalian species tested (Blanco and De La Villa 1999; Brandstätter et al. 1998; Hack et al. 2001; Hankins and Ikeda 1991; Haverkamp et al. 2000; Morigiwa and Vardi 1999; Qin and Pourcho 1999; Shen et al. 2004). As with teleosts, the case is less clear for NMDARs. An in situ hybridization study found mRNA for NR1 (an NMDAR subunit sufficient to make functional channels) throughout the inner nuclear layer of the rat retina and concluded that all cells in this layer likely express NMDARs (Brandstätter et al. 1994). By contrast, an immunohistochemical study did not find NR1 subunits in HCs; instead, BCs and amacrine cells accounted for all NR1 immunoreactivity in the inner nuclear layer (Fan et al. 2013). A similar controversy exists in cats. Whereas the outer edge of the cat inner nuclear layer showed immunoreactivity for another NMDAR subunit (NR2A), it was uncertain whether the labeling was for HCs or BCs (Hartveit et al. 1994). In adults of most other species, including the mouse, rat, rabbit, and monkey, there is no immunohistochemical evidence for NMDAR subunits in HCs at all (Brandstätter et al. 1998; Hartveit et al. 1994). Furthermore, all physiological reports have failed to find NMDARs in adult mammalian HCs (Kalloniatis et al. 2004; Massey and Miller 1987; Shen et al. 2004). Notably, these receptors may be present in the developing mammalian retina (Watanabe et al. 1994), which would suggest that any NR1 mRNA found in adults is a vestige of development (Brandstätter et al. 1998).
There has been more work to determine the particular iGluR subunits of the outer retina in mammals than in teleosts. There are four AMPAR subunits (GluR1-4). HCs labeled strongly with a GluR2/3 antibody in all mammals tested [cat (Morigiwa and Vardi 1999; Qin and Pourcho 2001), monkey (Haverkamp et al. 2000), mouse (Hack et al. 2001), rabbit (Pan and Massey 2007; Peng et al. 1995), rat (Hack et al. 2001), tree shrew (Peng et al. 1995)], and GluR2 labeling was found in mouse and rat (Hack et al. 2001). Similarly, GluR4 immunoreactivity has been found in all species tested (Hack et al. 2001; Haverkamp et al. 2000; Morigiwa and Vardi 1999; Pan and Massey 2007). GluR1 immunoreactivity is absent in most reports (Haverkamp et al. 2000; Morigiwa and Vardi 1999; Pan and Massey 2007; Peng et al. 1995; Qin and Pourcho 1999). Mammalian HC subtypes may differentially express iGluRs. In cat, GluR4 is present on A-type (but not B-type) HCs (Morigiwa and Vardi 1999). There is also one report on rabbits, supporting the existence of Ca2+-permeable AMPARs (Rivera et al. 2001). In this report, kainate and AMPA could elicit increases in [Ca2+]i even after VGCC currents were abolished. However, contributions from other sources (e.g., mGluRs or Na+-Ca2+ exchange) were not examined and could not be ruled out.
For the KAR subunits, an antibody for GluK2/3 (previously called GluR6/7) (Lerma and Marques 2013) labels HCs in cat (Morigiwa and Vardi 1999; Qin and Pourcho 2001), rat (Brandstätter et al. 1997; Peng et al. 1995), and primate (Haverkamp et al. 2001) retinas. However, Blanco and De La Villa (1999) did not find evidence for KARs in electrophysiological experiments. Thus far, the only support for functional KARs in mammalian HCs is in humans (Shen et al. 2004).
Ca2+ influx in mammalian HCs: VGCCs.
Electrophysiological reports in cat, rat, mouse, and rabbit HCs suggest that most VGCC current is through L-type channels (Liu et al. 2013, 2016; Lohrke and Hofmann 1994; Rivera et al. 2001; Schubert et al. 2006; Ueda et al. 1992), although B-type (axonless) HCs in cats probably have lower L-type channel densities (Ueda et al. 1992). In contrast to teleosts, there is growing support for N- and P/Q-type channels in mammals. Subunits for L-, N-, and P/Q-type VGCCs have been found in the outer plexiform layer in the rat retina (Witkovsky et al. 2006; Xu et al. 2002) and were later confirmed in HCs (Liu et al. 2013; Witkovsky et al. 2006). Additionally, blockers for N- and P/Q-type VGCCs reduce HC Ca2+ currents in mouse and rat (Liu et al. 2013, 2016; Schubert et al. 2006). These blockers were not tested in cat (Ueda et al. 1992) or rabbit (Lohrke and Hofmann 1994; Rivera et al. 2001), and so one cannot rule out N- and P/Q-type channels in these species. Lee and Brecha (2010) proposed that VGCCs may be involved in vesicular GABA release in mammals after finding several proteins involved in Ca2+-dependent neurotransmitter release in guinea pig HCs.
Most reports did not find T-type channels on mammalian HCs (Schubert et al. 2006; Ueda et al. 1992). However, one report in cultured rabbit HCs (Lohrke and Hofmann 1994) identified a transient Ca2+ current suggestive of T-type channels. The current was insensitive to nifedipine, had an activation potential at more negative potentials than the L-type current, and peaked at −30 to −10 mV.
Ca2+ influx in mammalian HCs: gap junctions and hemichannels.
In mouse and rabbit HCs, Cx50 and Cx57 are present (Cha et al. 2012; Hombach et al. 2004; Shelley et al. 2006). Notably, rabbits have different Cx expression in each HC subtype: A-type contains Cx50, whereas B-type contains Cx57 (Cha et al. 2012). Functionally, this may have effects on the receptive field size. A-Type HCs have a larger receptive field than B-type, which is presumed to be due to larger gap-junctional plaques in A-type HCs and a greater unitary conductance in Cx50 compared with Cx57 (Cha et al. 2012). It is unlikely that these Cxs are directly involved in feedback to cones, as neither Cx has been localized to the triad synapse (Cha et al. 2012), and knockout mice for Cx57 still exhibit negative feedback (Shelley et al. 2006). However, Px1 hemichannels remain candidates for mediating feedback in teleosts and mammals alike. They have been found in HCs in rat and mouse (Dvoriantchikova et al. 2006) and have been localized to HC axons and dendritic tips (Kranz et al. 2013).
Ca2+ EXTRUSION AND BUFFERING MECHANISMS
Ca2+ extrusion in teleost HCs.
Because high levels of cytosolic Ca2+ are toxic to neurons (Choi 1992; Lipton 1999), sustained Ca2+ influx through iGluRs and VGCCs must be balanced by sequestration and efflux mechanisms to maintain cell viability. Although cytosolic buffering mechanisms and sequestration into intracellular Ca2+ stores are known in HCs (see below), these stores must be finite and would be replete with Ca2+ over time. Therefore, Ca2+ must be extruded across the plasma membrane to limit [Ca2+]i. Two major Ca2+ extrusion mechanisms have been shown to exist in teleost HCs: 1) a plasma membrane Ca2+-ATPase (PMCA) and 2) an Na+/Ca2+ exchanger (NCX) (Kreitzer et al. 2007; Micci and Christensen 1998; Yasui 1987).
To demonstrate Na+/Ca2+ exchange, Hayashida et al. (1998) applied glutamate for >1 h so as to ensure that Ca2+ uptake and efflux reached equilibrium, both across the plasma membrane and in/out of intracellular Ca2+ stores. Changes in this new [Ca2+]i would have therefore reflected a change in Ca2+ homeostasis. When extracellular Na+ was removed or when Li+ (which cannot functionally replace Na+ in NCX activity) was applied, [Ca2+]i increased, suggesting the presence of NCX activity. Similarly, Na+/Ca2+ exchange was also inhibited by high [Ca2+]o, which reduced the driving force for Ca2+ and made NCX exchange energetically unfavorable.
The first report of PMCAs in teleost HCs came from Micci and Christensen (1998), who found that the nonspecific P-type ATPase inhibitor, vanadate, greatly increased [Ca2+]i in freshly dissociated cells. Vanadate can also inhibit Na+/K+-ATPases, which would increase intracellular Na+ concentration and therefore, the driving force for NCX activity. Yet [Ca2+]i only slightly increased when oubain, a selective Na+/K+-ATPase inhibitor, was applied (Micci and Christensen 1998). Vanadate, however, has been shown to inhibit refilling of certain intracellular Ca2+ stores (Watson et al. 2003), so a vanadate-elicited increase in [Ca2+]i may be caused by either sarco/endoplasmic reticulum Ca2+-ATPase or PMCA antagonism.
Further evidence for PMCAs on HCs comes from studies of proton efflux in skate, catfish, and goldfish. PMCAs exchange extracellular protons and intracellular Ca2+ at the cost of ATP, so that changes in extracellular pH may be related to PMCA activity (Di Leva et al. 2008). With the use of self-referencing proton electrodes, several studies found that the depolarization of isolated HCs with glutamate, kainate, high K+ solutions, or voltage clamp increased extracellular pH (Jacoby et al. 2012; Kreitzer et al. 2007, 2012; Molina et al. 2004). This extracellular alkalization was abolished with low Ca2+ solutions, Co2+, and nifedipine or with PMCA antagonists, such as carboxyeosin and lanthanum (Jacoby et al. 2012; Kreitzer et al. 2007, 2012). Furthermore, concurrent recordings showed that extracellular pH and [Ca2+]i varied synchronously, suggesting an exchange mechanism (Kreitzer et al. 2012). Collectively, these studies suggest that Ca2+ influx from VGCCs leads to Ca2+ extrusion and simultaneous proton influx through PMCAs. Along with immunohistochemical labeling in the outer plexiform layer and in dissociated cells of the skate (Molina et al. 2004), these findings strongly support PMCA activity in teleost and skate HCs.
It is unclear how physiologically important PMCAs are in HCs. In one report in goldfish and carp, orthovanadate was found to increase [Ca2+]i only marginally in HCs during prolonged glutamate application and had no effect in the absence of glutamate (Hayashida et al. 1998). This presents the possibility that the PMCA plays a minimal role in maintaining resting [Ca2+]i in HCs and that NCX dominates Ca2+ extrusion in these cells. This may be a strategy to reduce ATP expenditure. HCs are constantly taxed with Ca2+ influx, and so PMCA activity would be energetically costly.
Ca2+ buffering in teleost HCs.
Very few studies have analyzed buffering in HCs; however, the limited information available suggests that teleost HCs have some of the highest buffering capacities known of any cell type. Ca2+ buffering (β) is often defined as the ratio of entering Ca2+ ions that bind to cytosolic buffers compared with the Ca2+ ions that remain free in solution. As an example, β has been estimated to be 100 in rat gonadotrophs so that for every 101 Ca2+ ions that enter the cytosol, only 1 Ca2+ ion would remain free (Tse et al. 1994). A review of neuronal Ca2+ buffering lists typical β values between 20 and 200, with nearly all values <300 (Matthews and Dietrich 2015).
Surprisingly, the only study to examine Ca2+ buffering in teleost HCs measured β at 2,500 as a lower limit (Solessio and Lasater 2002). The authors also showed that this extraordinarily high buffering capacity slowed increases in [Ca2+]i. Although the VGCC current reaches a maximal value within milliseconds, the resulting increases in [Ca2+]i have time constants >8 s. Functionally, this extraordinarily high Ca2+ buffering capacity may be neuroprotective by reducing free [Ca2+]i during prolonged Ca2+ influx.
Ca2+ extrusion and buffering in mammalian HCs.
PMCA isoforms have been identified with immunohistochemistry in the mouse retina (Krizaj et al. 2002), in which PMCA1-3 colocalized with calbindin, a Ca2+-binding protein and HC marker. There was faint, diffuse immunolabeling for PMCA1 throughout HC somata and dendrites, in addition to weak immunolabeling for both PMCA2 and -3. PMCA2 and -3 were also found on HCs in the developing rat retina (Renteria et al. 2005).
Calbindin is found in a large number of mammalian species (Cuenca et al. 2002; Haverkamp and Wässle 2000; Kantor et al. 2016; Massey and Mills 1996; Pasteels et al. 1990). In addition, calretinin is found in a variety of mammalian species (Cuenca et al. 2002; Kantor et al. 2016; Pasteels et al. 1990), and parvalbumin stains HCs in dog (Hamano et al. 1990) and humans (Kantor et al. 2016). In teleosts, these Ca2+-binding proteins could not be labeled with immunohistochemistry (Hamano et al. 1990; Yazulla and Studholme 2002), despite physiological evidence for high Ca2+ buffering capacity (Solessio and Lasater 2002).
It has been suggested that high levels of calbindin are neuroprotective in HCs. In a rat model of retinal ischemia, most parvalbumin- and calretinin-positive retinal cells were shown to succumb to ischemia and reperfusion injury, whereas calbindin-positive HCs survived (Kim et al. 2010). Likewise, calbindin-positive cells in the ganglion cell layer were more resistant to ischemic insult than parvalbumin- and calretinin-positive cells (Kwon et al. 2005), and overexpression of calbindin reduced excitotoxic cell death elsewhere in the central nervous system (Fan et al. 2007; Yenari et al. 2001). Perhaps strong buffering with calbindin protects HCs against constant Ca2+ influx (Kim et al. 2010).
Ca2+ STORES IN HCs
Most studies on teleost Ca2+ stores have investigated ryanodine receptors.
Nonmitochondrial intracellular Ca2+ stores are often categorized by the involvement of either ryanodine receptors (RyRs) or inositol triphosphate receptors (IP3Rs). Numerous Ca2+ imaging experiments provide strong support for RyRs in HCs, which mediate CICR in these neurons (Huang et al. 2004; Linn and Gafka 2001; Lv et al. 2014; Solessio and Lasater 2002). In contrast, there are only two reports that suggest that IP3Rs exist in teleost HCs (both in catfish): specifically, 1) an immunohistochemical report (Micci and Christensen 1996) and 2) Ca2+ imaging experiments that showed a slow increase in [Ca2+]i with IP3 application (Linn and Gafka 2001). However, IP3Rs do not elicit CICR in these cells (Linn and Gafka 2001); furthermore, CICR does not occur when ryanodine or dantrolene is used to block RyRs in vitro (Lv et al. 2014; Micci and Christensen 1998). The role of IP3Rs in these cells is largely unexplored, and it is unknown if IP3Rs exist in HCs of other teleosts.
In catfish, Ca2+ stores were shown to be sensitive to caffeine, a RyR agonist that elicited CICR and presumably depleted Ca2+ from stores (Micci and Christensen 1998). If it was applied twice within a short period of time, then the second caffeine pulse induced a smaller CICR transient, which was comparably smaller than the first caffeine pulse. The authors interpreted this to mean that stores were refilling with Ca2+ during the interim and estimated that the refilling of stores occurred after 2 min. Refilling was shown to be dependent on extracellular Ca2+, even in the presence of nifedipine, to block Ca2+ influx through VGCCs. Interestingly, while extrusion mechanisms are blocked, HC Ca2+ stores can accumulate enough Ca2+ to double CICR amplitudes (Micci and Christensen 1998). This suggests that stores are not replete with Ca2+ but have an unexpectedly large capacity to accommodate additional Ca2+. Because HCs can be depolarized for hours at a time and undergo constant Ca2+ influx, large-capacity stores might buffer against otherwise excitotoxic increases in [Ca2+]i.
Interestingly, Micci and Christensen (1998) proposed that the NCX operates in reverse to refill stores. By inhibiting NCX activity with Li+, they found that subsequent caffeine application could not elicit CICR. Recently, additional evidence from bovine smooth muscle was presented to link NCX activity, in the reverse mode, to refilling of RyR-sensitive stores (Hirota et al. 2007); however, confirmation of this in teleost HCs awaits the use of selective NCX inhibitors.
Alternatively, it is possible that store-operated channels (SOCs) refill stores. In goldfish, it was shown that a SOC inhibitor (2-aminoethoxydiphenyl borate) prevented stores from refilling (Lv et al. 2014). Further research will need to clarify whether both mechanisms exist in both species and what their relative contributions are to refilling.
Functionally, CICR is known to occur immediately after glutamate application in HCs, followed by a sustained Ca2+ influx from VGCCs and iGluRs. Linn and Gafka (2001) found that Ca2+ from CICR is able to downregulate VGCCs. This downregulation was not seen when CICR was blocked (with ryanodine) or when stores were depleted (with caffeine). Conceivably, this would serve to decrease the sustained Ca2+ influx through VGCCs during glutamate application, preventing excitotoxic increases in [Ca2+]i.
Ca2+ stores in mammalian HCs.
In mouse HCs, CICR is also triggered by caffeine (Schubert et al. 2006), suggesting the presence of RyRs. Molecular and immunohistochemical studies corroborate the presence of RyRs on HCs. An antibody for the cardiac RyR isoform, RyR2, labels HC somata in cow (Shoshan-Barmatz et al. 2005) and rabbit (Shoshan-Barmatz et al. 2007). However, RyR (from total retinal fractions) showed unique variations from its cardiac or skeletal muscle counterparts. It was Ca2+ independent, showed different kinetics, and was inhibited by caffeine (Shoshan-Barmatz et al. 2005). The unique properties of this isoform have not been explored in other species, and it is unclear if it plays a role in HC physiology. In contrast to teleosts, we found no reports of IP3Rs in HCs of the mammalian retina.
Ca2+ APs IN HCs
Ca2+ APs in fish.
In teleosts (i.e., goldfish, carp, and catfish) and skates, isolated HCs are known to produce APs (Johnston and Lam 1981; Lasater et al. 1984; Murakami and Takahashi 1987; Tachibana 1981). There has been brief mention of similar regenerative membrane activity in HCs of white perch (Lasater 1986) and white bass (Sullivan and Lasater 1992). The physiological significance of APs in HCs is currently unknown.
Despite their all-or-nothing activity, these APs are quite unique. They persist in the absence of Na+ (Shingai and Christensen 1983) and are not abolished by Na+ channel blockers, such as tetrodotoxin (Tachibana 1981), although in some fish—such as skates (Malchow et al. 1990) and catfish (Shingai and Christensen 1983, 1986)—Na+ currents contribute to the rising phase of the AP. Instead, APs in HCs are primarily Ca2+ dependent. They are amplified with high [Ca2+]o and diminished with low [Ca2+]o (Tachibana 1981); blocked by Co2+, Mn2+, and Ni2+ (Johnston and Lam 1981); and enhanced and prolonged by VGCC-permeant ions, such as Ba2+ and Sr2+ (Tachibana 1981). The APs are also slow, lasting on the order of seconds to minutes (Dixon et al. 1993; Tachibana 1981).
APs have been described in HCs in a number of preparations, including the intact retina (Murakami and Takahashi 1987), cultured cells (Lasater et al. 1984; Tachibana 1981), and freshly dissociated cells (Shingai and Christensen 1983). Although all investigators could induce APs with depolarizing current pulses [e.g., Dixon et al. (1993); Lam and Ayoub (1983); Shingai and Christensen (1983)], some reports found them to occur spontaneously, especially with Ba2+ or K+ channel-blocker application (Murakami and Takahashi 1987; Tachibana 1981). Furthermore, both Ishida et al. (1984) and Lasater et al. (1984) initiated AP activity with glutamate application.
APs may be exploited to understand Ca2+ dynamics in HCs. There is evidence that APs coincide with extracellular acidification, possibly by activating the PMCA (Kreitzer et al. 2007, 2012). Conceivably, APs could change the [Ca2+] and pH of the cleft at the triad synapse, affecting HC feedback to PRs.
Ca2+ APs in other vertebrates.
APs occur in HCs of mammals. Ueda et al. (1992) found that A-type HCs of the cat showed spiking activity, whereas B-type cells did not. The authors demonstrated that APs were generated by VGCC current and that A-type HCs have a greater VGCC current density than B-type cells.
APs have also been recorded from HCs in eyecup preparations in other vertebrates. In the turtle (Pseudemys scripta elegans), HCs show spike-like depolarizations during the light-to-dark transition; furthermore, these spikes require extracellular Ca2+ and are suppressed by Co2+ (Akopian et al. 1991). In a similar eyecup preparation, Xenopus HCs were found to depolarize during the light-to-dark transition. This effect was enhanced by Ba2+ and Sr2+ and blocked by nifedipine (Akopian et al. 1997). This suggests that VGCCs may serve to repolarize HCs quickly to their dark membrane potential at light offset.
[Ca2+]i AND FEEDBACK
GABA release can occur through Ca2+-dependent or Ca2+-independent mechanisms.
Ca2+ may be involved in negative feedback from HCs to PRs through a GABAergic mechanism. Both GABA and the enzyme that synthesizes it (glutamic acid dehydrogenase) have been found in HCs from fish and mammals (Brandon 1985; Deniz et al. 2011; Guo et al. 2010; Lam et al. 1980; Mosinger and Yazulla 1987; Mosinger et al. 1986), suggesting that at least some HCs are GABAergic. For teleosts, in particular, it has been proposed that only H1 cells accumulate and release GABA (Ayoub and Lam 1987; Lam et al. 1980).
Mechanisms of GABA release likely differ between mammalian and nonmammalian species. In mammals, there is growing support for a Ca2+-dependent, vesicular release of GABA from HCs (Cueva et al. 2002; Haverkamp et al. 2000; Lee and Brecha 2010; Liu et al. 2013; Thoreson and Mangel 2012), as occurs elsewhere in the nervous system (Wadel et al. 2007). Several essential components for vesicular GABA release have been localized to HCs (Cueva et al. 2002; Guo et al. 2010; Lee and Brecha 2010), including SNARE proteins and synaptotagmin-2, a Ca2+ sensor for exocytosis (Lee and Brecha 2010). In retinal slices from rat, HC VGCCs open during depolarization, allowing [Ca2+]i to rise, which triggers vesicular GABA release (Liu et al. 2013).
In HCs of teleost and other nonmammalian species, only a small number of studies have found evidence for Ca2+-dependent release (Ayoub and Lam 1985; Lasater and Lam 1984). Instead, GABA release may occur through Ca2+-independent plasmalemmal GABA transporters operating in reverse [reviewed in Schwartz (2002)]. Both mechanisms (Ca2+-independent transporter and Ca2+-dependent vesicular release) might dominate in certain illumination conditions. Ayoub and Lam (1985) proposed a model in which mild depolarization would trigger Ca2+-dependent GABA release, whereas strong depolarization would rapidly exhaust the supply of GABA-containing vesicles while triggering transporter-dependent GABA release. Translated into light responses, this model suggests that GABA transport dominates in the dark, whereas Ca2+-dependent vesicular release dominates in dim lighting. There is no evidence for plasmalemmal GABA transporters in mammals (Feigenspan and Weiler 2004).
For mammals and other vertebrates alike, it is unclear whether PRs are the primary targets for GABA, since feedback has not been blocked by GABA antagonists in goldfish (Verweij et al. 1996), monkey (Verweij et al. 2003), or turtle (Thoreson and Burkhardt 1990). In the rat, a unifying mechanism for inhibitory feedback to PRs has been proposed in which GABA may modulate pH and/or ephaptic feedback (Liu et al. 2013). In this model, Ca2+-dependent GABA release would activate ionotropic GABA receptors on the HCs themselves. As GABA receptors are permeable to both Cl− and HCO3−, they could modulate cleft pH and shunt current into HCs (Liu et al. 2013). As further support, targeted deletion of HC vesicular GABA transporters in the mouse abolished inhibition to PRs (Hirano et al. 2016). Therefore, at least in rodents, Ca2+-dependent GABA release from HCs is important for negative feedback to PRs.
Ca2+-dependent synaptic plasticity may modulate feedback to PRs.
In teleost HCs, Ca2+ is necessary for rapid, synaptic plasticity at the PR synapse (Wagner and Djamgoz 1993). In light, HCs form spinules at their dendritic terminals in a matter of minutes. In darkness, these spinules retract. They have only been found in teleosts and are absent in mudpuppy, turtle, and mammals, including the dog, cat, rat, and monkey (Wagner 1980). Spinules may mediate feedback responses to PRs: as the number of spinules per synaptic ribbon increases, so does the strength of feedback-dependent, color-opponent responses in HCs (Kirsch et al. 1990; Weiler and Wagner 1984). As the retina is light adapted, improved feedback allows HCs to respond to increasingly higher frequencies of light stimuli (Lasater and Lam 1984; Prince et al. 1987), and this effect has been correlated with spinule formation (Djamgoz et al. 1988). These studies have led to the conclusion that spinules are candidates for the site of negative feedback from HCs to PRs (Wagner and Djamgoz 1993).
Spinule retraction is AMPAR dependent (Okada et al. 1999; Schmitz et al. 1995) and Ca2+ dependent (Okada et al. 1999; Schmitz et al. 1995; Weiler et al. 1995, 1996). There is also immunohistochemical evidence of heightened calmodulin levels in dendritic terminals in light, and calmodulin antagonists block spinule retraction in darkness (Schmitz et al. 1995; Weiler et al. 1995, 1996). Collectively, these studies suggest that Ca2+ enters Ca2+-permeable AMPARs during the light-to-dark transition and induces spinule retraction through a calmodulin-dependent pathway.
Ca2+ may also modulate synaptic plasticity through a separate pathway. In goldfish and carp, flickering light enhances monophasic, L-type HC voltage responses to red light (Hu et al. 2000). In other words, as light is repetitively flashed, L-type HCs hyperpolarize more over time (up to ∼10% more after 20 flashes of 500 ms duration). Expanding on this work, Huang et al. (2006) found that the injection of EGTA into HCs in situ led to a smaller increase in light-induced HC hyperpolarization. This increase did not occur if Ca2+-permeable AMPARs were selectively inhibited, if ryanodine was applied to block CICR, or if caffeine was preapplied to deplete Ca2+ stores. The authors concluded that [Ca2+]i entry through Ca2+-permeable AMPARs activates CICR, which in turn, increases the HC hyperpolarizing response in the retina. The exact mechanisms of Ca2+-mediated changes in voltage responses have not been proposed. Functionally, this would be expected to increase feedback onto PRs, enhancing the perception of light, during and shortly after flickering.
[Ca2+]i in HCs mediates positive feedback to PRs.
In addition to negative feedback, HCs can provide positive feedback to PRs. Such positive feedback has been found in multiple species, including the anole (Anolis segrei), tiger salamander (Ambystoma trigrinum), rabbit, and zebrafish (Jackman et al. 2011). The authors propose that positive feedback is spatially constrained so that it acts only upon glutamate-releasing PRs. This means that if a cone is already depolarized and releasing glutamate, HCs would further increase that cone's [Ca2+]i and facilitate more glutamate release. This would counteract negative feedback and preserve the initial PR signal.
Whereas negative feedback is voltage dependent, positive feedback is dependent on [Ca2+]i in HCs. Jackman et al. (2011) found that positive feedback can be elicited by glutamate release from PRs or by uncaging Ca2+ in HCs. Additionally, the Ca2+-permeable AMPAR antagonist, philanthotoxin-74, blocked positive feedback. From these findings, the authors conclude that Ca2+ must enter HCs directly through iGluRs. Once [Ca2+]i rises in HCs, it is unclear how it mediates positive feedback. One possibility is that PMCAs could pump protons out of the synaptic cleft, as PMCAs have been shown to cause extracellular alkalization in a Ca2+-dependent fashion (Jacoby et al. 2012; Kreitzer et al. 2012). However, Wang et al. (2014) found that the depolarization of HCs in vivo acidifies, instead of alkalizes, the cleft. They suggest that PMCAs may not be on HC dendritic tips and so may not regulate cleft pH or positive feedback.
CONCLUSION AND FUTURE DIRECTIONS
Teleost HCs present an extreme of Ca2+ homeostasis in neurons and are therefore informative models for Ca2+ regulation. Whereas ion concentrations are presumed to be relatively constant in typical neurons, since they are seldom depolarized for long (Wright 2004), this assumption may not be made for the tonically depolarized neurons of the outer retina (Hayashida and Yagi 2002a). The understanding of how HCs respond to elevated and sustained Ca2+ influx promises to provide insight into Ca2+ physiology. Moreover, it may provide insights into pathological conditions, such as excitotoxicity, where excessive Ca2+ influx is central to neuronal death (Choi 1992).
Indeed, HCs are reputedly resistant to excitotoxicity (McMahon and Ponomareva 1996; Mizuno et al. 2010). The high Ca2+ buffering capacity (Solessio and Lasater 2002) and large capacity of HC stores (Micci and Christensen 1998) may allow for neuroprotection against excitotoxicity, despite the prolonged depolarization that these cells endure. The understanding of these and other potentially neuroprotective methods of handling sustained Ca2+ influx may lead to novel approaches in treating and understanding diabetic retinopathy, strokes, and other conditions characterized by excitotoxicity.
Teleost HCs have provided many unique insights into retinal and cellular physiology, especially as it pertains to Ca2+. Research on HCs has contributed to many important findings, including mechanisms of neurotransmitter release (Schwartz 1987), refilling of Ca2+ stores (Micci and Christensen 1998), and synaptic plasticity (Okada et al. 1999). Many questions still remain for the future and will surely improve our understanding of Ca2+ physiology, including the nature of HC Ca2+ APs, the regulation and multifaceted roles of Ca2+ stores, and the role of NMDARs in HCs. Perhaps most importantly, the exploration of HC Ca2+ dynamics may clarify feedback mechanisms in the outer retina, so as to improve our understanding of neural processing in the retina.
GRANTS
Support for M. G. Jonz was provided by the Natural Sciences and Engineering Research Council (NSERC) of Canada (Grant 342303).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.W.C. prepared the figure; M.W.C. drafted the manuscript; M.G.J. and M.W.C. edited and revised the manuscript; M.W.C. and M.G.J. approved the final version of the manuscript.
REFERENCES
- Akopian A, Krizaj D, Witkovsky P. Both high-and low voltage-activated calcium currents contribute to the light-evoked responses of luminosity horizontal cells in the Xenopus retina. Brain Res 762: 121–130, 1997. [DOI] [PubMed] [Google Scholar]
- Akopian A, McReynolds J, Weiler R. Short-term potentiation of off-responses in turtle horizontal cells. Brain Res 546: 132–138, 1991. [DOI] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. Modulation of transient outward potassium current by GTP, calcium, and glutamate in horizontal cells of the Xenopus retina. J Neurophysiol 71: 1661–1671, 1994. [DOI] [PubMed] [Google Scholar]
- Ariel M, Lasater EM, Mangel SC, Dowling JE. On the sensitivity of H1 horizontal cells of the carp retina to glutamate, aspartate and their agonists. Brain Res 295: 179–183, 1984. [DOI] [PubMed] [Google Scholar]
- Ariel M, Mangel SC, Dowling JE. N-methyl d-aspartate acts as an antagonist of the photoreceptor transmitter in the carp retina. Brain Res 372: 143–148, 1986. [DOI] [PubMed] [Google Scholar]
- Ayoub GS, Lam DM. Accumulation of gamma-aminobutyric acid by horizontal cells isolated from the goldfish retina. Vision Res 27: 2027–2034, 1987. [DOI] [PubMed] [Google Scholar]
- Ayoub GS, Lam DM. The content and release of endogenous GABA in isolated horizontal cells of the goldfish retina. Vision Res 25: 1187–1193, 1985. [DOI] [PubMed] [Google Scholar]
- Baldridge WH, Ball AK. Background illumination reduces horizontal cell receptive-field size in both normal and 6-hydroxydopamine-lesioned goldfish retinas. Vis Neurosci 7: 441–450, 1991. [DOI] [PubMed] [Google Scholar]
- Barnes S. Center-surround antagonism mediated by proton signaling at the cone photoreceptor synapse. J Gen Physiol 122: 653–656, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fuortes MG, O'Bryan PM. Receptive fields of cones in the retina of the turtle. J Physiol 214: 265–294, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraudi A, Bruno V, Battaglia G, Biagioni F, Rampello L, Nicoletti F, Poli A. Pharmacological activation of mGlu2/3 metabotropic glutamate receptors protects retinal neurons against anoxic damage in the goldfish Carassius auratus. Exp Eye Res 84: 544–552, 2007. [DOI] [PubMed] [Google Scholar]
- Blanco R, De La Villa P. Ionotropic glutamate receptors in isolated horizontal cells of the rabbit retina. Eur J Neurosci 11: 867–873, 1999. [DOI] [PubMed] [Google Scholar]
- Boyden PA, Dun W, Stuyvers BD. What is a Ca(2+) wave? Is it like an electrical wave? Arrhythm Electrophysiol Rev 4: 35–39, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon C. Retinal GABA neurons: localization in vertebrate species using an antiserum to rabbit brain glutamate decarboxylase. Brain Res 344: 286–295, 1985. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Hartveit E, Sassoe-Pognetto M, Wässle H. Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur J Neurosci 6: 1100–1112, 1994. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Koulen P, Wässle H. Diversity of glutamate receptors in the mammalian retina. Vision Res 38: 1385–1397, 1998. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Koulen P, Wässle H. Selective synaptic distribution of kainate receptor subunits in the two plexiform layers of the rat retina. J Neurosci 17: 9298–9307, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzov AL, Shura-Bura TM. Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Res 26: 33–44, 1986. [DOI] [PubMed] [Google Scholar]
- Cha J, Kim HL, Pan F, Chun MH, Massey SC, Kim IB. Variety of horizontal cell gap junctions in the rabbit retina. Neurosci Lett 510: 99–103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol 23: 1261–1276, 1992. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell 131: 1047–1058, 2007. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Veliz LP, Bukauskas FF, Bennett MV, Saez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev 47: 290–303, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Deng P, Linberg KA, Lewis GP, Fisher SK, Kolb H. The neurons of the ground squirrel retina as revealed by immunostains for calcium binding proteins and neurotransmitters. J Neurocytol 31: 649–666, 2002. [DOI] [PubMed] [Google Scholar]
- Cueva JG, Haverkamp S, Reimer RJ, Edwards R, Wässle H, Brecha NC. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J Comp Neurol 445: 227–237, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea P, Vittur F. Gap junctions mediate intercellular calcium signalling in cultured articular chondrocytes. Cell Calcium 20: 389–397, 1996. [DOI] [PubMed] [Google Scholar]
- Daniels BA, Baldridge WH. The light-induced reduction of horizontal cell receptive field size in the goldfish retina involves nitric oxide. Vis Neurosci 28: 137–144, 2011. [DOI] [PubMed] [Google Scholar]
- Deniz S, Wersinger E, Schwab Y, Mura C, Erdelyi F, Szabó G, Rendon A, Sahel JA, Picaud S, Roux MJ. Mammalian retinal horizontal cells are unconventional GABAergic neurons. J Neurochem 116: 350–362, 2011. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Kremer M, Paputsoglu G, Stang A, Skerrett IM, Gomes D, Srinivas M, Janssen-Bienhold U, Weiler R, Nicholson BJ, Bruzzone R, Spray DC. Molecular and functional diversity of neural connexins in the retina. J Neurosci 20: 8331–8343, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol 445: 201–230, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Schwartz E. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol 414: 351–375, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva F, Domi T, Fedrizzi L, Lim D, Carafoli E. The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch Biochem Biophys 476: 65–74, 2008. [DOI] [PubMed] [Google Scholar]
- Dixon DB, Takahashi K, Bieda M, Copenhagen DR. Quinine, intracellular pH and modulation of hemi-gap junctions in catfish horizontal cells. Vision Res 36: 3925–3931, 1996. [DOI] [PubMed] [Google Scholar]
- Dixon DB, Takahashi K, Copenhagen DR. L-glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron 11: 267–277, 1993. [DOI] [PubMed] [Google Scholar]
- Djamgoz M, Downing J, Kirsch M, Prince D, Wagner HJ. Plasticity of cone horizontal cell functioning in cyprinid fish retina: effects of background illumination of moderate intensity. J Neurocytol 17: 701–710, 1988. [DOI] [PubMed] [Google Scholar]
- Dowling JE. The Retina: An Approachable Part of the Brain. Cambridge, MA: Harvard University Press, 1987. [Google Scholar]
- Dowling JE, Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature 242: 101–103, 1973. [DOI] [PubMed] [Google Scholar]
- Dvoriantchikova G, Ivanov D, Panchin Y, Shestopalov VI. Expression of pannexin family of proteins in the retina. FEBS Lett 580: 2178–2182, 2006. [DOI] [PubMed] [Google Scholar]
- Fahrenfort I, Steijaert M, Sjoerdsma T, Vickers E, Ripps H, van Asselt J, Endeman D, Klooster J, Numan R, ten Eikelder H, von Gersdorff H, Kamermans M. Hemichannel-mediated and pH-based feedback from horizontal cells to cones in the vertebrate retina. PLoS One 4: e6090, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Xing Y, Zhong Y, Chen C, Shen Y. Expression of NMDA receptor subunit 1 in the rat retina. Acta Histochem 115: 42–47, 2013. [DOI] [PubMed] [Google Scholar]
- Fan Y, Shi L, Gu Y, Zhao Y, Xie J, Qiao J, Yang GY, Wang Y, Lu CZ. Pretreatment with PTD-calbindin D 28k alleviates rat brain injury induced by ischemia and reperfusion. J Cereb Blood Flow Metab 27: 719–728, 2007. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Weiler R. Electrophysiological properties of mouse horizontal cell GABAA receptors. J Neurophysiol 92: 2789–2801, 2004. [DOI] [PubMed] [Google Scholar]
- Gardner CL, Jones JR, Baer SM, Crook SM. Drift-diffusion simulation of the ephaptic effect in the triad synapse of the retina. J Comput Neurosci 38: 129–142, 2015. [DOI] [PubMed] [Google Scholar]
- Guo C, Hirano AA, Stella SL, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD65, and the GABA vesicular transporter. J Comp Neurol 518: 1647–1669, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack I, Frech M, Dick O, Peichl L, Brandstätter JH. Heterogeneous distribution of AMPA glutamate receptor subunits at the photoreceptor synapses of rodent retina. Eur J Neurosci 13: 15–24, 2001. [PubMed] [Google Scholar]
- Hamano K, Kiyama H, Emson PC, Manabe R, Nakuchi M. Localization of two calcium binding proteins, calbindin (28 kD) and parvalbumin (12 kD), in the vertebrate retina. J Comp Neurol 302: 8, 1990. [DOI] [PubMed] [Google Scholar]
- Hankins M, Ikeda H. Non-NMDA type excitatory amino acid receptors mediate rod input to horizontal cells in the isolated rat retina. Vision Res 31: 609–617, 1991. [DOI] [PubMed] [Google Scholar]
- Hartveit E, Brandstätter JH, Sassoè-Pognetto M, Laurie DJ, Seeburg PH, Wässle H. Localization and developmental expression of the NMDA receptor subunit NR2A in the mammalian retina. J Comp Neurol 348: 570–582, 1994. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. Localization of kainate receptors at the cone pedicles of the primate retina. J Comp Neurol 436: 471–486, 2001. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wassle H. The cone pedicle, a complex synapse in the retina. Neuron 27: 85–95, 2000. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol 424: 1–23, 2000. [PubMed] [Google Scholar]
- Hayashida Y, Yagi T. Contribution of Ca2+ transporters to electrical response of a non-spiking retinal neuron. Neurocomputing 44–46: 6, 2002a. [Google Scholar]
- Hayashida Y, Yagi T. On the interaction between voltage-gated conductances and Ca2+ regulation mechanisms in retinal horizontal cells. J Neurophysiol 87: 11, 2002b. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Yagi T, Yasui S. Ca2+ regulation by the Na+-Ca2+ exchanger in retinal horizontal cells depolarized by L-glutamate. Neurosci Res 31: 189–199, 1998. [DOI] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Brecha NC. Cellular distribution and subcellular localization of molecular components of vesicular transmitter release in horizontal cells of rabbit retina. J Comp Neurol 488: 70–81, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano AA, Liu X, Boulter J, Grove J, Pérez de Sevilla Müller L, Barnes S, Brecha NC. Targeted deletion of vesicular GABA transporter from retinal horizontal cells eliminates feedback modulation of photoreceptor calcium channels. eNeuro 3: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol 122: 657–671, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S, Pertens E, Janssen LJ. The reverse mode of the Na+/Ca2+ exchanger provides a source of Ca2+ for store refilling following agonist-induced Ca2+ mobilization. Am J Physiol Lung Cell Mol Physiol 292: L438–L447, 2007. [DOI] [PubMed] [Google Scholar]
- Höfer T, Politi A, Heinrich R. Intercellular Ca2+ wave propagation through gap-junctional Ca2+ diffusion: a theoretical study. Biophys J 80: 75–87, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci 17: 31–108, 1994. [DOI] [PubMed] [Google Scholar]
- Hombach S, Janssen-Bienhold U, Söhl G, Schubert T, Büssow H, Ott T, Weiler R, Willecke K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur J Neurosci 19: 2633–2640, 2004. [DOI] [PubMed] [Google Scholar]
- Hu JF, Liu Y, Liang PJ. Stimulus pattern related plasticity of synapses between cones and horizontal cells in carp retina. Brain Res 857: 321–326, 2000. [DOI] [PubMed] [Google Scholar]
- Huang SY, Hu J, Gong H, Liang P. Postsynaptic calcium pathway contributes to synaptic plasticity between retinal cones and luminosity-type horizontal cells. Sheng Li Xue Bao 58: 407, 2006. [PubMed] [Google Scholar]
- Huang SY, Liang PJ. Ca2+-permeable and Ca2+-impermeable AMPA receptors coexist on horizontal cells. Neuroreport 16: 263–266, 2005. [DOI] [PubMed] [Google Scholar]
- Huang SY, Liu Y, Liang PJ. Role of Ca2+ store in AMPA-triggered Ca2+ dynamics in retinal horizontal cells. Neuroreport 15: 2311–2315, 2004. [DOI] [PubMed] [Google Scholar]
- Ishida AT, Kaneko A, Tachibana M. Responses of solitary retinal horizontal cells from Carassius auratus to L-glutamate and related amino acids. J Physiol 348: 16, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida AT, Neyton J. Quisqualate and L-glutamate inhibit retinal horizontal-cell responses to kainate. Proc Natl Acad Sci USA 82: 1837–1841, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Babai N, Chambers JJ, Thoreson WB, Kramer RH. A positive feedback synapse from retinal horizontal cells to cone photoreceptors. PLoS Biol 9: e1001057, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby J, Kreitzer MA, Alford S, Qian H, Tchernookova BK, Naylor ER, Malchow RP. Extracellular pH dynamics of retinal horizontal cells examined using electrochemical and fluorometric methods. J Neurophysiol 107: 868–879, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Bienhold U, Dermietzel R, Weiler R. Distribution of connexin43 immunoreactivity in the retinas of different vertebrates. J Comp Neurol 396: 310–321, 1998. [PubMed] [Google Scholar]
- Janssen-Bienhold U, Schultz K, Gellhaus A, Schmidt P, Ammermuller J, Weiler R. Identification and localization of connexin26 within the photoreceptor-horizontal cell synaptic complex. Vis Neurosci 18: 169–178, 2001. [DOI] [PubMed] [Google Scholar]
- Jiang XD, Wang XL, Sun Y, Gong HQ, Liang PJ. NMDA modulation of GABA transporter current in carp retinal horizontal cells. Brain Res 1240: 105–110, 2008. [DOI] [PubMed] [Google Scholar]
- Johnston D, Lam DM. Regenerative and passive membrane properties of isolated horizontal cells from a teleost retina. Nature 292: 451–454, 1981. [DOI] [PubMed] [Google Scholar]
- Jonz MG, Barnes S. Proton modulation of ion channels in isolated horizontal cells of the goldfish retina. J Physiol 581: 529–541, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalloniatis M, Sun D, Foster L, Haverkamp S, Wassle H. Localization of NMDA receptor subunits and mapping NMDA drive within the mammalian retina. Vis Neurosci 21: 587–597, 2004. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science 292: 13, 2001. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol 213: 95–105, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A, Stuart AE. Coupling between horizontal cells in the carp retina revealed by diffusion of Lucifer yellow. Neurosci Lett 47: 7, 1984. [DOI] [PubMed] [Google Scholar]
- Kantor O, Mezey S, Adeghate J, Naumann A, Nitschke R, Enzsoly A, Szabo A, Lukats A, Nemeth J, Somogyvari Z, Volgyi B. Calcium buffer proteins are specific markers of human retinal neurons. Cell Tissue Res 365: 29–50, 2016. [DOI] [PubMed] [Google Scholar]
- Katz LC. Coordinate activity in retinal and cortical development. Curr Opin Neurobiol 3: 93–99, 1993. [DOI] [PubMed] [Google Scholar]
- Kim SA, Jeon JH, Son MJ, Cha J, Chun MH, Kim IB. Changes in transcript and protein levels of calbindin D28k, calretinin and parvalbumin, and numbers of neuronal populations expressing these proteins in an ischemia model of rat retina. Anat Cell Biol 43: 218–229, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch M, Djamgoz MB, Wagner HJ. Correlation of spinule dynamics and plasticity of the horizontal cell spectral response in cyprinid fish retina: quantitative analysis. Cell Tissue Res 260: 123–130, 1990. [Google Scholar]
- Klaassen LJ, Sun Z, Steijaert MN, Bolte P, Fahrenfort I, Sjoerdsma T, Klooster J, Claassen Y, Shields CR, Ten Eikelder HM, Janssen-Bienhold U, Zoidl G, McMahon DG, Kamermans M. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biol 9: e1001107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron 10: 491–500, 1993. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sadahiro M, Fall CP. Developmental expression of endogenous oscillations and waves in the auditory cortex involves calcium, gap junctions, and GABA. Neuroscience 146: 1629–1639, 2007. [DOI] [PubMed] [Google Scholar]
- Kranz K, Dorgau B, Pottek M, Herrling R, Schultz K, Bolte P, Monyer H, Penuela S, Laird DW, Dedek K. Expression of Pannexin1 in the outer plexiform layer of the mouse retina and physiological impact of its knockout. J Comp Neurol 521: 1119–1135, 2013. [DOI] [PubMed] [Google Scholar]
- Kreitzer MA, Collis LP, Molina AJ, Smith PJ, Malchow RP. Modulation of extracellular proton fluxes from retinal horizontal cells of the catfish by depolarization and glutamate. J Gen Physiol 130: 169–182, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer MA, Jacoby J, Naylor E, Baker A, Grable T, Tran E, Booth SE, Qian H, Malchow RP. Distinctive patterns of alterations in proton efflux from goldfish retinal horizontal cells monitored with self-referencing H+-selective electrodes. Eur J Neurosci 36: 3040–3050, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Demarco SJ, Johnson J, Strehler EE, Copenhagen DR. Cell-specific expression of plasma membrane calcium ATPase isoforms in retinal neurons. J Comp Neurol 451: 1–21, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OJ, Kim JY, Kim SY, Jeon CJ. Alterations in the localization of calbindin D28K-, calretinin-, and parvalbumin-immunoreactive neurons of rabbit retinal ganglion cell layer from ischemia and reperfusion. Mol Cells 19: 382–390, 2005. [PubMed] [Google Scholar]
- Lam DM, Ayoub GS. Biochemical and biophysical studies of isolated horizontal cells from the teleost retina. Vision Res 23: 433–444, 1983. [DOI] [PubMed] [Google Scholar]
- Lam DM, Su Y, Chin C, Brandon C, Wu JY, Marc RE, Lasater E. GABA-ergic horizontal cells in the teleost retina. Brain Res Bull 5: 137–140, 1980. [Google Scholar]
- Lasater EM. Ionic currents of cultured horizontal cells isolated from white perch retina. J Neurophysiol 55: 499–513, 1986. [DOI] [PubMed] [Google Scholar]
- Lasater EM. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci USA 84: 7319–7323, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM, Dowling JE. Carp horizontal cells in culture respond selectively to L-glutamate and its agonists. Proc Natl Acad Sci USA 79: 936–940, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA 82: 3025–3029, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM, Dowling JE, Ripps H. Pharmacological properties of isolated horizontal and bipolar cells from the skate retina. J Neurosci 4: 1966–1975, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM, Lam DM. The identification and some functions of GABAergic neurons in the distal catfish retina. Vision Res 24: 497–506, 1984. [DOI] [PubMed] [Google Scholar]
- Lee H, Brecha NC. Immunocytochemical evidence for SNARE protein-dependent transmitter release from guinea pig horizontal cells. Eur J Neurosci 31: 1388–1401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Marques JM. Kainate receptors in health and disease. Neuron 80: 292–311, 2013. [DOI] [PubMed] [Google Scholar]
- Linn CL, Gafka AC. Activation of metabotropic glutamate receptors modulates the voltage-gated sustained calcium current in a teleost horizontal cell. J Neurophysiol 81: 425–434, 1999. [DOI] [PubMed] [Google Scholar]
- Linn CL, Gafka AC. Modulation of a voltage-gated calcium channel linked to activation of glutamate receptors and calcium-induced calcium release in the catfish retina. J Physiol 535: 47–63, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn CP, Christensen BN. Excitatory amino acid regulation of intracellular Ca2+ in isolated catfish cone horizontal cells measured under voltage- and concentration-clamp conditions. J Neurosci 12: 2156–2164, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev 79: 139, 1999. [DOI] [PubMed] [Google Scholar]
- Liu CR, Xu L, Zhong YM, Li RX, Yang XL. Expression of connexin 35/36 in retinal horizontal and bipolar cells of carp. Neuroscience 164: 1161–1169, 2009. [DOI] [PubMed] [Google Scholar]
- Liu X, Grove JC, Hirano AA, Brecha NC, Barnes S. Dopamine D1 receptor modulation of calcium channel currents in horizontal cells of mouse retina. J Neurophysiol 116: 686–697, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hirano AA, Sun X, Brecha NC, Barnes S. Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. J Physiol 591: 3309–3324, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrke S, Hofmann HD. Voltage-gated currents of rabbit A- and B-type horizontal cells in retinal monolayer cultures. Vis Neurosci 11: 369–378, 1994. [DOI] [PubMed] [Google Scholar]
- Lu C, McMahon DG. Modulation of hybrid bass retinal gap junctional channel gating by nitric oxide. J Physiol 499: 689–699, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv T, Gong HQ, Liang PJ. Caffeine-induced Ca2+ oscillations in type I horizontal cells of the carp retina and the contribution of the store-operated Ca2+ entry pathway. PLoS One 9: e100095, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Prog Neurobiol 94: 259–295, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow RP, Qian HH, Ripps H, Dowling JE. Structural and functional properties of two types of horizontal cell in the skate retina. J Gen Physiol 95: 177–198, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel SC, Dowling JE. The interplexiform-horizontal cell system of the fish retina: effects of dopamine, light stimulation and time in the dark. Proc R Soc Lond B Biol Sci 231: 91–121, 1987. [DOI] [PubMed] [Google Scholar]
- Massey SC, Miller RF. Excitatory amino acid receptors of rod- and cone-driven horizontal cells in the rabbit retina. J Neurophysiol 57: 645–659, 1987. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol 366: 15–33, 1996. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Dietrich D. Buffer mobility and the regulation of neuronal calcium domains. Front Cell Neurosci 9: 48, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG. Modulation of electrical synaptic transmission in zebrafish retinal horizontal cells. J Neurosci 14: 1722–1734, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Brown DR. Modulation of gap junction channel gating at zebrafish retinal electrical synapses. J Neurophysiol 72: 2257–2268, 1994. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Mattson MP. Horizontal cell electrical coupling in the giant danio: synaptic modulation by dopamine and synaptic maintenance by calcium. Brain Res 718: 89–96, 1996. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Ponomareva LV. Nitric oxide and cGMP modulate retinal glutamate receptors. J Neurophysiol 76: 2307–2315, 1996. [DOI] [PubMed] [Google Scholar]
- Micci MA, Christensen BN. Distribution of the inositol trisphosphate receptor in the catfish retina. Brain Res 720: 139–147, 1996. [DOI] [PubMed] [Google Scholar]
- Micci MA, Christensen BN. Na+/Ca2+ exchange in catfish retina horizontal cells: regulation of intracellular Ca2+ store function. Am J Physiol Cell Physiol 274: C1625–C1633, 1998. [DOI] [PubMed] [Google Scholar]
- Miller AM, Schwartz EA. Evidence for the identification of synaptic transmitters released by photoreceptors of the toad retina. J Physiol 334: 325–349, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno F, Barabas P, Krizaj D, Akopian A. Glutamate-induced internalization of Cav1.3 L-type Ca2+ channels protects retinal neurons against excitotoxicity. J Physiol 588: 953–966, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina AJ, Verzi MP, Birnbaum AD, Yamoah EN, Hammar K, Smith PJ, Malchow RP. Neurotransmitter modulation of extracellular H+ fluxes from isolated retinal horizontal cells of the skate. J Physiol 560: 639–657, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigiwa K, Vardi N. Differential expression of ionotropic glutamate receptor subunits in the outer retina. J Comp Neurol 405: 173–184, 1999. [DOI] [PubMed] [Google Scholar]
- Mosinger J, Yazulla S. Double-label analysis of GAD-and GABA-like immunoreactivity in the rabbit retina. Vision Res 27: 23–30, 1987. [DOI] [PubMed] [Google Scholar]
- Mosinger JL, Yazulla S, Studholme KM. GABA-like immunoreactivity in the vertebrate retina: a species comparison. Exp Eye Res 42: 631–644, 1986. [DOI] [PubMed] [Google Scholar]
- Murakami M, Miyachi EI, Takahashi KI. Modulation of gap junctions between horizontal cells by second messengers. Prog Retin Eye Res 14: 197–221, 1995. [Google Scholar]
- Murakami M, Otsu K, Otsuka T. Effects of chemicals on receptors and horizontal cells in the retina. J Physiol 227: 899–913, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Takahashi KI. Calcium action potential and its use for measurement of reversal potentials of horizontal cell responses in carp retina. J Physiol 386: 16, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science 275: 844–847, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. Gap junctions. Compr Physiol 2: 1981–2035, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell T, Christensen BN. N-methyl-d-aspartate receptors coexist with kainate and quisqualate receptors on single isolated catfish horizontal cells. Brain Res 381: 359–362, 1986. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Christensen BN. Horizontal cells isolated from catfish retina contain two types of excitatory amino acid receptors. J Neurophysiol 61: 1097–1109, 1989. [DOI] [PubMed] [Google Scholar]
- Okada T, Schultz K, Geurtz W, Hatt H, Weiler R. AMPA-preferring receptors with high Ca2+ permeability mediate dendritic plasticity of retinal horizontal cells. Eur J Neurosci 11: 1085–1095, 1999. [DOI] [PubMed] [Google Scholar]
- Pan F, Massey SC. Rod and cone input to horizontal cells in the rabbit retina. J Comp Neurol 500: 815–831, 2007. [DOI] [PubMed] [Google Scholar]
- Pasteels B, Rogers J, Blachier F, Pochet R. Calbindin and calretinin localization in retina from different species. Vis Neurosci 5: 1–16, 1990. [DOI] [PubMed] [Google Scholar]
- Peng PL, Zhong X, Tu W, Soundarapandian MM, Molner P, Zhu D, Lau L, Liu S, Liu F, Lu Y. ADAR2-dependent RNA editing of AMPA receptor subunit GluR2 determines vulnerability of neurons in forebrain ischemia. Neuron 49: 719–733, 2006. [DOI] [PubMed] [Google Scholar]
- Peng YW, Blackstone C, Huganir R, Yau KW. Distribution of glutamate receptor subtypes in the vertebrate retina. Neuroscience 66: 483–497, 1995. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 83: 117–161, 2003. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Linn CL, Lasater EM. Whole cell and single-channel properties of a unique voltage-activated sustained calcium current identified in teleost retinal horizontal cells. J Neurophysiol 75: 609–619, 1996. [DOI] [PubMed] [Google Scholar]
- Prince DJ, Djamgoz M, Karten H. GABA transaminase in cyprinid fish retina: localization and effects of inhibitors on temporal characteristics of S-potentials. Neurochem Int 11: 23–30, 1987. [DOI] [PubMed] [Google Scholar]
- Prochnow N, Hoffmann S, Dermietzel R, Zoidl G. Replacement of a single cysteine in the fourth transmembrane region of zebrafish pannexin 1 alters hemichannel gating behavior. Exp Brain Res 199: 255–264, 2009a. [DOI] [PubMed] [Google Scholar]
- Prochnow N, Hoffmann S, Vroman R, Klooster J, Bunse S, Kamermans M, Dermietzel R, Zoidl G. Pannexin1 in the outer retina of the zebrafish, Danio rerio. Neuroscience 162: 1039–1054, 2009b. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. Immunocytochemical localization of kainate-selective glutamate receptor subunits GluR5, GluR6, and GluR7 in the cat retina. Brain Res 890: 211–221, 2001. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. Localization of AMPA-selective glutamate receptor subunits in the cat retina: a light- and electron-microscopic study. Vis Neurosci 16: 169–177, 1999. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du système nerveux de l'homme & des vertébrés. Paris: Consejo Superior de Investigaciones Científicas. Instituto Ramón y Cajal, 1909. [Google Scholar]
- Renteria RC, Strehler EE, Copenhagen DR, Krizaj D. Ontogeny of plasma membrane Ca2+ ATPase isoforms in the neural retina of the postnatal rat. Vis Neurosci 22: 263–274, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera L, Blanco R, de la Villa P. Calcium-permeable glutamate receptors in horizontal cells of the mammalian retina. Vis Neurosci 18: 995–1002, 2001. [PubMed] [Google Scholar]
- Rowe JS, Ruddock KH. Hyperpolarization of retinal horizontal cells by excitatory amino acid neurotransmitter antagonists. Neurosci Lett 30: 251–256, 1982. [DOI] [PubMed] [Google Scholar]
- Saez JC, Connor JA, Spray DC, Bennett MV. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci USA 86: 2708–2712, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuranaga M, Naka K. Signal transmission in the catfish retina. I. Transmission in the outer retina. J Neurophysiol 53: 373–389, 1985. [DOI] [PubMed] [Google Scholar]
- Schmidt KF. Properties of glutamate-gated ion channels in horizontal cells of the perch retina. Vision Res 37: 2023–2028, 1997. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Kohler K, Zrenner E. Evidence for calcium/calmodulin dependence of spinule retraction in retinal horizontal cells. Vis Neurosci 12: 413–424, 1995. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Wolburg H. Gap junction morphology of retinal horizontal cells is sensitive to pH alterations in vitro. Cell Tissue Res 263: 303–310, 1991. [DOI] [PubMed] [Google Scholar]
- Schubert T, Weiler R, Feigenspan A. Intracellular calcium is regulated by different pathways in horizontal cells of the mouse retina. J Neurophysiol 96: 1278–1292, 2006. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science 238: 350–355, 1987. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Transport-mediated synapses in the retina. Physiol Rev 82: 875–891, 2002. [DOI] [PubMed] [Google Scholar]
- Shelley J, Dedek K, Schubert T, Feigenspan A, Schultz K, Hombach S, Willecke K, Weiler R. Horizontal cell receptive fields are reduced in connexin57-deficient mice. Eur J Neurosci 23: 3176–3186, 2006. [DOI] [PubMed] [Google Scholar]
- Shen W, Finnegan SG, Slaughter MM. Glutamate receptor subtypes in human retinal horizontal cells. Vis Neurosci 21: 89–95, 2004. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lu T, Yang XL. Modulation of desensitization at glutamate receptors in isolated crucian carp horizontal cells by concanavalin A, cyclothiazide, aniracetam and PEPA. Neuroscience 89: 979–990, 1999. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhang AJ, Yang XL. Uncoupling of horizontal cells alters the receptive fields of retinal bipolar cells. Neuroreport 14: 2159–2162, 2003. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhang M, Jin Y, Yang XL. Functional N-methyl-d-aspartate receptors are expressed in cone-driven horizontal cells in carp retina. Neurosignals 15: 174–179, 2006. [DOI] [PubMed] [Google Scholar]
- Shields CR, Klooster J, Claassen Y, Ul-Hussain M, Zoidl G, Dermietzel R, Kamermans M. Retinal horizontal cell-specific promoter activity and protein expression of zebrafish connexin 52.6 and connexin 555. J Comp Neurol 501: 765–779, 2007. [DOI] [PubMed] [Google Scholar]
- Shingai R, Christensen BN. Excitable properties and voltage-sensitive ion conductances of horizontal cells isolated from catfish (Ictalurus punctatus) retina. J Neurophysiol 56: 32–49, 1986. [DOI] [PubMed] [Google Scholar]
- Shingai R, Christensen BN. Sodium and calcium currents measured in isolated catfish horizontal cells under voltage clamp. Neuroscience 10: 893–897, 1983. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Orr I, Martin C, Vardi N. Novel ryanodine-binding properties in mammalian retina. Int J Biochem Cell Biol 37: 1681–1695, 2005. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Zakar M, Shmuelivich F, Nahon E, Vardi N. Retina expresses a novel variant of the ryanodine receptor. Eur J Neurosci 26: 3113–3125, 2007. [DOI] [PubMed] [Google Scholar]
- Solessio E, Lasater EM. Calcium-induced calcium release and calcium buffering in retinal horizontal cells. Vis Neurosci 19: 713–725, 2002. [DOI] [PubMed] [Google Scholar]
- Stell WK, Lightfoot DO. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol 159: 473–502, 1975. [DOI] [PubMed] [Google Scholar]
- Stewart WW. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell 14: 741–759, 1978. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem 266: 10711–10714, 1991. [PubMed] [Google Scholar]
- Sullivan JM, Lasater EM. Sustained and transient calcium currents in horizontal cells of the white bass retina. J Gen Physiol 99: 85–107, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jiang XD, Liu X, Gong HQ, Liang PJ. Synaptic contribution of Ca2+-permeable and Ca2+-impermeable AMPA receptors on isolated carp retinal horizontal cells and their modulation by Zn2+. Brain Res 1317: 60–68, 2010. [DOI] [PubMed] [Google Scholar]
- Sun Z, Risner ML, van Asselt JB, Zhang DQ, Kamermans M, McMahon DG. Physiological and molecular characterization of connexin hemichannels in zebrafish retinal horizontal cells. J Neurophysiol 107: 2624–2632, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Zhang DQ, McMahon DG. Zinc modulation of hemi-gap-junction channel currents in retinal horizontal cells. J Neurophysiol 101: 1774–1780, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaetichin G. The cone action potential. Acta Physiol Scand 29: 565–599, 1953. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Ionic currents of solitary horizontal cells isolated from goldfish retina. J Physiol 345: 329–351, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Membrane properties of solitary horizontal cells isolated from goldfish retina. J Physiol 321: 141–161, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Permeability changes induced by L-glutamate in solitary retinal horizontal cells isolated from Carassius auratus. J Physiol 358: 153–167, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Dixon DB, Copenhagen DR. Modulation of a sustained calcium current by intracellular pH in horizontal cells of fish retina. J Gen Physiol 101: 695–714, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi T, Negishi K, Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature 301: 4, 1983. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Burkhardt DA. Effects of synaptic blocking agents on the depolarizing responses of turtle cones evoked by surround illumination. Vis Neurosci 5: 571–583, 1990. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res 31: 407–441, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Witkovsky P. Glutamate receptors and circuits in the vertebrate retina. Prog Retin Eye Res 18: 765–810, 1999. [DOI] [PubMed] [Google Scholar]
- Tse A, Tse FW, Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol 477: 511–525, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kaneko A, Kaneda M. Voltage-dependent ionic currents in solitary horizontal cells isolated from cat retina. J Neurophysiol 68: 1143–1150, 1992. [DOI] [PubMed] [Google Scholar]
- VanDongen AM. Biology of the NMDA Receptor. Boca Raton, FL: CRC, 2008. [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci 23: 10249–10257, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res 36: 11, 1996. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci 25: 4108–4117, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman R, Klaassen LJ, Howlett MH, Cenedese V, Klooster J, Sjoerdsma T, Kamermans M. Extracellular ATP hydrolysis inhibits synaptic transmission by increasing pH buffering in the synaptic cleft. PLoS Biol 12: e1001864, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadel K, Neher E, Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron 53: 563–575, 2007. [DOI] [PubMed] [Google Scholar]
- Wagner HJ. Light-dependent plasticity of the morphology of horizontal cell terminals in cone pedicles of fish retinas. J Neurocytol 9: 573–590, 1980. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Djamgoz MB. Spinules: a case for retinal synaptic plasticity. Trends Neurosci 16: 201–206, 1993. [DOI] [PubMed] [Google Scholar]
- Wang TM, Holzhausen LC, Kramer RH. Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina. Nat Neurosci 17: 262–268, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Jiang XD, Liang PJ. Intracellular calcium concentration changes initiated by N-methyl-d-aspartic acid receptors in retinal horizontal cells. Neuroreport 19: 675–678, 2008. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Differential distributions of the NMDA receptor channel subunit mRNAs in the mouse retina. Brain Res 634: 328–332, 1994. [DOI] [PubMed] [Google Scholar]
- Watson WD, Facchina SL, Grimaldi M, Verma A. Sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitors identify a novel calcium pool in the central nervous system. J Neurochem 87: 30–43, 2003. [DOI] [PubMed] [Google Scholar]
- Weiler R, Schultz K, Janssen-Bienhold U. Ca2+-dependency of spinule plasticity at dendrites of retinal horizontal cells and its possible implication for the functional role of spinules. Vision Res 36: 3891–3900, 1996. [DOI] [PubMed] [Google Scholar]
- Weiler R, Schultz K, Janssen-Bienhold U. Retraction of spinule-type neurites from carp retinal horizontal cell dendrites during dark adaptation involves the activation of Ca2+/calmodulin-dependent protein kinase II. Eur J Neurosci 7: 1914–1919, 1995. [DOI] [PubMed] [Google Scholar]
- Weiler R, Wagner HJ. Light-dependent change of cone-horizontal cell interactions in carp retina. Brain Res 298: 1–9, 1984. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Shen C, McRory J. Differential distribution of voltage-gated calcium channels in dopaminergic neurons of the rat retina. J Comp Neurol 497: 384–396, 2006. [DOI] [PubMed] [Google Scholar]
- Wong RO. The role of spatio-temporal firing patterns in neuronal development of sensory systems. Curr Opin Neurobiol 3: 595–601, 1993. [DOI] [PubMed] [Google Scholar]
- Wright SH. Generation of resting membrane potential. Adv Physiol Educ 28: 139–142, 2004. [DOI] [PubMed] [Google Scholar]
- Wu SM, Dowling JE. Effects of GABA and glycine on the distal cells of the cyprinid retina. Brain Res 199: 401–414, 1980. [DOI] [PubMed] [Google Scholar]
- Xu HP, Zhao JW, Yang XL. Expression of voltage-dependent calcium channel subunits in the rat retina. Neurosci Lett 329: 297–300, 2002. [DOI] [PubMed] [Google Scholar]
- Yamada E, Ishikawa T. The fine structure of the horizontal cells in some vertebrate retinae. Cold Spring Harb Symp Quant Biol 30: 383–392. 1965. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Sakimura K, Masayoshi M, Shimoji K. The sensitivity of AMPA-selective glutamate receptor channels to pentobarbital is determined by a single amino acid residue of the α2 subunit. FEBS Lett 374: 412–414, 1995. [DOI] [PubMed] [Google Scholar]
- Yasui S. Ca and Na homeostasis in horizontal cells of the cyprinid fish retina: evidence for Na-Ca exchanger and Na-K pump. Neurosci Res Suppl 6: S133–S146, 1987. [DOI] [PubMed] [Google Scholar]
- Yau KW. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 35: 9–32, 1994. [PubMed] [Google Scholar]
- Yazulla S, Studholme KM. Neurochemical anatomy of the zebrafish retina as determined by immunocytochemistry. In: Chemical Anatomy of the Zebrafish Retina, edited by Yazulla S, Studholme KM, Marc RE, Cameron D. New York: Springer, 2002, p. 3–44. [Google Scholar]
- Yenari MA, Minami M, Sun GH, Meier TJ, Kunis DM, McLaughlin JR, Ho DY, Sapolsky RM, Steinberg GK. Calbindin d28k overexpression protects striatal neurons from transient focal cerebral ischemia. Stroke 32: 1028–1035, 2001. [DOI] [PubMed] [Google Scholar]
- Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science 257: 665–669, 1992. [DOI] [PubMed] [Google Scholar]
- Zhang DQ, McMahon DG. Direct gating by retinoic acid of retinal electrical synapses. Proc Natl Acad Sci USA 97: 14754–14759, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoidl G, Bruzzone R, Weickert S, Kremer M, Zoidl C, Mitropoulou G, Srinivas M, Spray DC, Dermietzel R. Molecular cloning and functional expression of zfCx52.6: a novel connexin with hemichannel-forming properties expressed in horizontal cells of the zebrafish retina. J Biol Chem 279: 2913–2921, 2004. [DOI] [PubMed] [Google Scholar]
- Zoidl G, Kremer M, Zoidl C, Bunse S, Dermietzel R. Molecular diversity of connexin and pannexin genes in the retina of the zebrafish Danio rerio. Cell Commun Adhes 15: 169–183, 2008. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol 9: 305–313, 1999. [DOI] [PubMed] [Google Scholar]