It is largely unknown how fine motor control of acoustic parameters is achieved in vocal organs. Subtle manipulation of syringeal muscle function was used to test how active motor control influences acoustic parameters. Slowed activation kinetics of muscles reduced frequency modulation and, unexpectedly, caused a distinct decrease in sound amplitude and increase in phonation onset pressure. These results show that active control enhances the efficiency of energy conversion in the syrinx.
Keywords: glycosaminoglycan, heparan sulfate, neuromuscular control of acoustic attributes
Abstract
Neural control of complex vocal behaviors, such as birdsong and speech, requires integration of biomechanical nonlinearities through muscular output. Although control of airflow and tension of vibrating tissues are known functions of vocal muscles, it remains unclear how specific muscle characteristics contribute to specific acoustic parameters. To address this gap, we removed heparan sulfate chains using heparitinases to perturb neuromuscular transmission subtly in the syrinx of adult male zebra finches (Taeniopygia guttata). Infusion of heparitinases into ventral syringeal muscles altered their excitation threshold and reduced neuromuscular transmission changing their ability to modulate airflow. The changes in muscle activation dynamics caused a reduction in frequency modulation rates and elimination of many high-frequency syllables but did not alter the fundamental frequency of syllables. Sound amplitude was reduced and sound onset pressure was increased, suggesting a role of muscles in the induction of self-sustained oscillations under low-airflow conditions, thus enhancing vocal efficiency. These changes were reversed to preinfusion levels by 7 days after infusion. These results illustrate complex interactions between the control of airflow and tension and further define the importance of syringeal muscle in the control of a variety of acoustic song characteristics. In summary, the findings reported here show that altering neuromuscular transmission can lead to reversible changes to the acoustic structure of song. Understanding the full extent of muscle involvement in song production is critical in decoding the motor program for the production of complex vocal behavior, including our search for parallels between birdsong and human speech motor control.
NEW & NOTEWORTHY It is largely unknown how fine motor control of acoustic parameters is achieved in vocal organs. Subtle manipulation of syringeal muscle function was used to test how active motor control influences acoustic parameters. Slowed activation kinetics of muscles reduced frequency modulation and, unexpectedly, caused a distinct decrease in sound amplitude and increase in phonation onset pressure. These results show that active control enhances the efficiency of energy conversion in the syrinx.
to generate complex, learned vocal behaviors, such as human speech and birdsong, neural signals must be translated into muscle-mediated movements, and these interactions occur simultaneously and in coordinated fashion in multiple, interacting motor systems. Various nonlinear relationships and specializations in this cascade make it difficult to predict how neural signals are translated into biomechanical changes at the effector organs and, thus, how they influence the behavioral outcome. Although temporal aspects of sound production are fairly well-characterized, surprisingly little information is available on how the interplay between morphology and neuromuscular control mechanisms affects the acoustic structure of vocalizations.
Sound production involves precise coordination of motor systems that control respiration, the vocal organ, and upper vocal tract movements (e.g., Beckers 2013; Elemans 2014; Goller and Cooper 2004; Riede and Goller 2010; Suthers and Zollinger 2008). In songbirds, the vocal organ, the syrinx, contains at least six pairs of muscles (e.g., Düring et al. 2013), the activity of which controls the two independent sound generators. Syringeal muscles exhibit exceptionally fast contraction dynamics that facilitate rapid modulation of syringeal airflow (Elemans et al. 2008; Uchida et al. 2010). Temporal alignment between acoustic parameters (amplitude and frequency modulation) and activation patterns of syringeal muscles through electromyography (EMG) establishes correlative evidence for rapid, direct muscular control of song features. However, this evidence does not provide sufficient detail about precise functional relationships between muscle activation and acoustic features (Elemans 2014). For example, analysis of EMG activity of different species does not yield a uniform model for how sound frequency is controlled by syringeal muscles (Goller and Riede 2013). The fact that individual syringeal muscles can be divided into fascicles that attach to different syringeal components illustrates the potentially highly complex transduction of muscle shortening into acoustically relevant movements (Düring et al. 2013; Elemans 2014; Elemans et al. 2015).
Experimental tests of how syringeal muscles contribute to acoustic parameters involved unilateral and bilateral denervation of all muscles. These studies show, for example, that intact muscles are required for control of sound frequency (e.g., Okanoya and Yoneda 1995; Riede et al. 2010; Williams et al. 2004), but the complete elimination of muscle activation cannot provide specific information on how muscle activation dynamics contribute to acoustic features. To investigate how detailed aspects of song are affected by syringeal muscles, subtle experimental manipulation of muscle function is needed. One such approach illustrates the complexity of acoustic motor control by documenting how a single muscle contributes to the control of different acoustic parameters (Srivastava et al. 2015). We performed another experimental manipulation by removing heparan sulfate (HS) in the ventral syringeal muscles. Skeletal muscle contains HS, which is primarily found in the extracellular matrix (ECM) and at the neuromuscular junction. It is involved in synapse formation during development, aggregation of acetylcholine receptors, and the tethering of acetylcholine esterase (Anderson et al. 1984; Arikawa-Hirasawa et al. 2002; Burgess et al. 1999; Guatam et al. 1996; Torres and Inestrosa 1983). Heparitinase treatment of syringeal muscles resulted in slowed muscle activation, which caused predicted and unpredicted changes in various song features, including changes in amplitude and frequency modulation.
MATERIALS AND METHODS
Animals.
All procedures were carried out with institutional animal care and use committee approval. Adult male zebra finches from the breeding colony at the University of Utah were used in this study. Birds were housed in the aviary until experimental testing. During testing, birds were housed in 32- × 23- × 30-cm wire cages and kept on a 13:11-h light-dark cycle.
Air-sac pressure measurements and recordings.
Males were removed from the aviary, placed into individual cages, and isolated in custom-made sound-attenuating recording chambers (2 × 1 × 1 m; lined with foam). Although the acoustic properties of the chambers were not quantified, acoustic recording conditions of each bird were kept unchanged throughout the experiment to permit comparison of sound parameters. Several bouts of control song were recorded before any manipulation was begun. Then, an elastic belt was placed around the thorax, and a leash was connected to the belt using a Velcro tab. The leash was put through the top of the cage and attached to a counterbalanced tether arm to allow free movement around the cage. After the bird was accustomed to the tether, as indicated by singing, surgery was performed. Birds were deprived of food and water for 1 h before surgery and then anesthetized with isoflurane delivered in breathing air. A small hole was made in the abdominal wall to insert a flexible cannula into a thoracic air sac. The cannula was sutured to the rib cage, and the insertion site was sealed with tissue adhesive (Vetbond) to prevent air leakage. The other end of the cannula was connected to a pressure transducer (Fujikura FPM-02PG) mounted on the bird as a backpack fixed to the Velcro tab. Birds were allowed to recover until several bouts of control song with air-sac pressure were recorded. Song was recorded with an omnidirectional microphone (Audio-Technica AT3032) and amplified (Digital Preamp System II; Applied Research & Technology). Air-sac pressure was simultaneously recorded on a separate channel using amplified output (Hector Engineering) from the pressure transducer. Both channels were recorded with a Data Translation analog-to-digital board at 44.1-kHz sampling rate using Avisoft-RECORDER software. After sufficient control song was recorded, birds received injections of heparitinase enzymes.
Injection into syringeal muscles.
Several groups of birds received either bilateral injections of heparitinase enzymes or vehicle in the syrinx. This includes birds for the examination of song alone (n = 6), those for examining song and air-sac pressure patterns (n = 5), and birds in which the effect of enzyme treatment on syringeal muscle function was studied (n = 8; see below). All procedures were as follows, regardless of experimental group. Birds were deprived of food for 1 h before surgery and then anesthetized using isoflurane. The thoracic cavity was opened, and 10 μl of a mixture of heparitinase enzymes in vehicle or vehicle alone were injected bilaterally into the ventral muscles of the syrinx. Separate injections were made into the ventral syringeal and ventral tracheobronchial muscles on each side. The membrane of the interclavicular air sac was sealed with tissue adhesive, and the overlying skin was sutured closed. Birds were allowed to recover from anesthesia and then returned to their recording chambers. Song and air-sac pressure in the subset was recorded as described above. Birds usually began vocalizing within 2–6 h after surgery. Birds designated for the investigation of the treatment on song features (song alone as well as song and air-sac pressure) were recorded for up to 14 days after injection. For birds in which the effect of the infusion on muscle kinetics was tested, song was recorded for up to 24 h after injection, at which time the syringeal muscle kinetics were assessed.
Recording of muscle kinetics.
To assess how enzyme treatment affected muscle activation, the effect of oscillatory in situ muscle contractions on airflow was monitored. To do so, birds were anesthetized with isoflurane, and a custom-built flow probe was inserted into the trachea. This flow sensor was constructed with a miniature thermistor (Thermometrics BB05JA202), which was connected to a feedback circuit (Hector Engineering) that heated the thermistor to ∼60°C via output current. This feedback circuit provides sensitive detection of airflow in the trachea, as the convective cooling of the flow induces adjustments in the heating current. The voltage needed to drive this current is proportional to airflow. Syringeal muscles were stimulated directly with a pair of silver wires (A-M Systems) inserted into the ventral tracheobronchial muscle. Airflow modulation tests were conducted with stimulus currents that caused strong visible twitches in the ventral tracheobronchial muscle and airflow modulation depths that were not increased by further increases in current.
To detect the abductive activity of this muscle, the respiratory system of the bird was perfused through a cannula in the posterior thoracic air sac with a flow rate of ∼2 l/min. This rate was adjusted to ensure cessation of respiratory movements. Turning off the bird's respiratory drive with this method assured gas exchange and allowed us to test modulation of the constant air stream by the muscle without interference from fluctuations in airflow associated with the natural breathing rhythm. Stimulation pulses were delivered from an A-M Systems stimulator (model 2100) in trains consisting of 10 1-ms pulses delivered at different repetition rates, and between trains the muscle was allowed to recover for ≥2 min. Pulse frequency was 1st ramped up in discrete steps (10, 20, 25, 30, 40, and 50 Hz) and then ramped down (50, 40, 30, 25, 20, and 10 Hz). Airflow and pulse were recorded as separate channels using Avisoft-RECORDER software. The minimum current required to induce twitches was determined by visual observation of the syringeal muscle through the surgical microscope and was confirmed by minimal visible modulation of the recorded airflow.
Testing stimulation route.
To learn more about which aspect of neuromuscular control heparitinase treatment affected, we tested whether our electrodes inserted into the muscle directly stimulated muscle action potentials or indirectly by activating axon branches in the muscle. In three male zebra finches, we stimulated the muscle as described above and measured muscle activation via force production (Grass force transducer). The cranial end of the in situ ventral tracheobronchial muscle was hooked with an insect minutia pin and connected to the force transducer. The muscle was then stimulated with increasing current until twitches were recorded reliably (0.5–0.8 mA). We then injected 10 μl of a 5 μM tubocurarine chloride pentahydrate solution to block neuromuscular transmission partially and after 15 min stimulated again with the same current. We then increased the current 10-fold to ensure direct stimulation of muscle membranes and recorded twitches again for comparison with those evoked during low-current stimulation.
Song and air-sac pressure analyses.
At least 15 motifs from each bird were selected randomly from the recordings at each time point. Within these selected motifs, frequency modulation, fundamental frequency, sound onset pressure, and amplitude (decibel values re P0 = 2 × 10−5 Pa) were analyzed using Praat software (http://www.praat.org). Frequency modulation rate was examined (control: n = 6 syllables analyzed from 4 birds; enzyme: n = 8 syllables analyzed from 6 birds) by calculating the change in frequency over time from the highest to the lowest point in a frequency sweep. Sound amplitude was calculated by averaging rectified voltage over the entire motif (≥15 motifs analyzed for 4 birds in each treatment group). Subsyringeal air-sac pressure at the onset of phonation was measured by high-pass filtering a copy of the pressure trace for each bird to reveal the syringeal oscillations, which allowed accurate determination of the time when sound oscillations started and their frequency. The time stamp of this onset was then used to determine the relative pressure level at the onset of vibration (relative voltage). This was considered the start of phonation, and a power spectrum at this time point with window length of 10 ms was used to determine the fundamental frequency (control: n = 3 birds; enzyme: n = 4 birds).
To examine overall acoustic changes that may have occurred as the result of enzyme treatment, we collected 15 individual motifs from 6 birds from both vehicle and heparitinase-treated birds before and 24 h after injection. These motifs were analyzed and compared using Sound Analysis Pro (SAP) software, using specific scores for the percent similarity (longer time scale of approximately 50–70 ms) and accuracy (shorter time scale of approximately 5–10 ms) before and after the injection. To accomplish this, we compared motifs before treatment with other motifs before treatment and then motifs 24 h after treatment with the motifs from before treatment. Zebra finch song is highly stereotyped, and thus scores for both groups should be similar if no changes occur.
Statistics were calculated using Excel and SigmaPlot software and are presented as the mean ±1 SD or ±1 SE as indicated. Significance was assessed with paired or unpaired Student's t-tests or ANOVA where appropriate.
RESULTS
To examine changes induced by enzyme treatment, we first tested whether neuromuscular transmission was interrupted 24 h after enzyme injection. Stimulation of the tracheosyringeal nerve produced muscle twitches, indicating that neuromuscular transmission was not completely interrupted. To assess whether heparitinase caused a change in neuromuscular transmission, we tested whether electrodes inserted into the syringeal muscle stimulated twitches via activation of the axon branches in the muscle or via direct muscle membrane activation. Twitch amplitude after application of a partial curare block decreased >5-fold in all 3 birds. Increasing the stimulation current 10-fold restored full force production. This result indicates that the stimulation currents used activated axon branches, and observed changes therefore should indicate differences in neuromuscular transmission.
This is consistent with the change in threshold current with enzyme treatment. Whereas muscle contractions could be elicited with ≤1-mA stimulation current in vehicle-injected birds (0.5, 0.5, 0.5, 0.9, and 1 mA), in enzyme-injected birds the minimal current required to elicit visible muscle contraction was between 0.9 and 8 mA (0.9, 3, 3, and 8 mA).
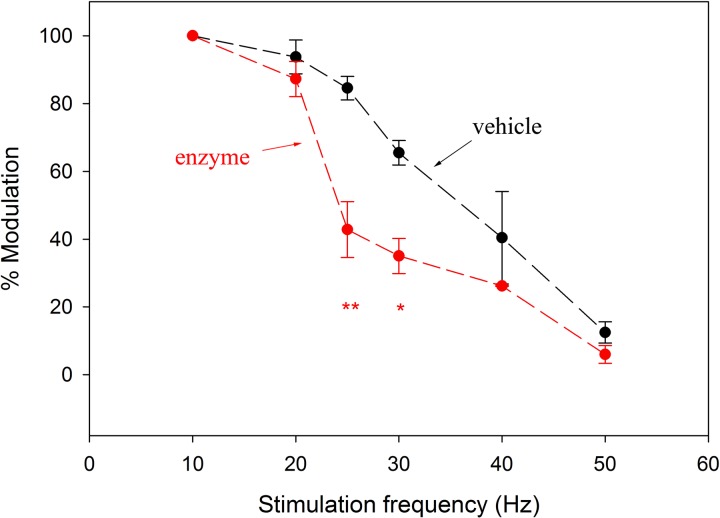
We next established how heparitinase treatment influenced the ability of syringeal muscles to modulate airflow. The muscles showed significantly slower contraction-relaxation cycles 24 h after enzyme injection. The ability to modulate airflow declined at lower stimulation frequencies in enzyme-treated birds compared with vehicle-injected birds (Fig. 1). Stimulation of enzymatically treated muscle fell <50% modulation depth at 25 Hz, whereas this reduction occurred >50 Hz in vehicle-treated muscles.
Fig. 1.
Enzyme treatment affects in situ muscle activation properties. Airflow modulation (mean percentage ±1 SD) by the ventral tracheobronchial muscle during increasing stimulation frequency is shown relative to that at 10-Hz stimulation (100%). Heparitinase-treated muscle shows significantly reduced modulation depth at lower stimulation frequencies than vehicle-treated controls (n = 4 for each condition; **P < 0.005, *P < 0.05).
This result also established that a postinjection period of 24 h is a reasonable time frame during which effects on song can be expected. Birds were therefore recorded continuously as soon as they had recovered from surgery.
Examples of subsyringeal air-sac pressure patterns and songs (oscillographic, spectrographic, and sound-amplitude representation) illustrate that enzyme treatment produced the desired effect of subtle disruption of song production (Fig. 2). Whereas pressure patterns remained mostly intact, some consistent changes to song occurred after enzyme treatment (Fig. 2). One of the most obvious and general changes at 24 h after injection was a reduction in sound amplitude of song. Treatment with the enzymes resulted in a significant decrease in mean song amplitude at 24 h after injection relative to that before injection (Fig. 3A). Vehicle treatment also resulted in a decrease in overall song amplitude of 1.7 ± 0.1 dB, which was most likely a result of the short recovery period after surgical procedures. However, enzyme treatment produced a significantly greater reduction of overall song amplitude (5.2 ± 0.94 dB) 24 h after injection into syringeal muscles (Fig. 3B).
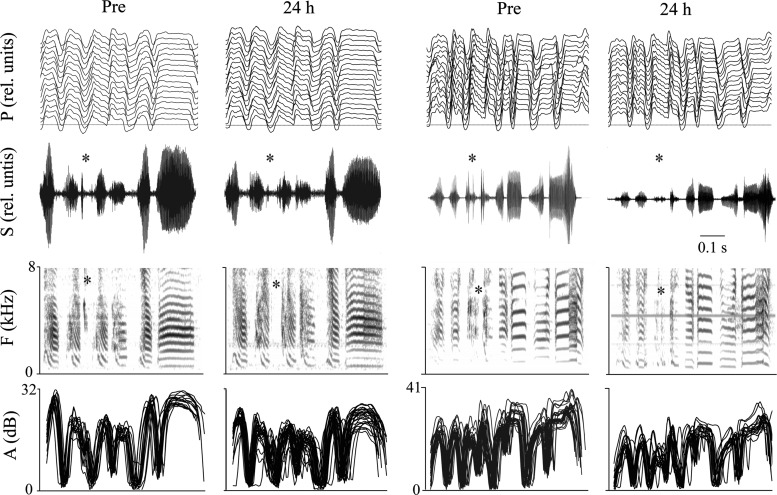
Fig. 2.
Enzyme treatment affects various aspects of song. Whereas the general pattern of air-sac pressure (P; multiple motifs overlaid; horizontal line on bottom trace shows ambient pressure) remains intact, several aspects of the sound (shown as oscillogram; S, spectrogram; F, frequency; and rectified amplitude, A) show effects of enzyme treatment. Examples are shown for 2 birds who received bilateral injections of heparitinase enzyme into the ventral syringeal muscles. The changes include a loss or reduction of high-frequency syllables (denoted by the asterisks), and the sound amplitude of song is visibly reduced 24 h after treatment. rel. units, Relative units.
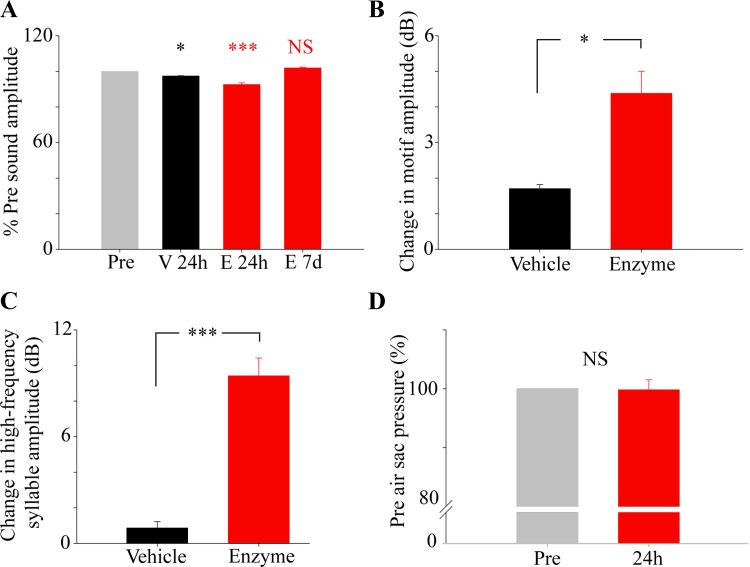
Fig. 3.
Sound amplitude is reduced, especially in high-frequency syllables, after heparitinase treatment, whereas the driving air-sac pressure remains unchanged. A: the percentage of preinjection amplitude of song at 24 h for heparitinase-treated birds and at 24 h for vehicle-treated birds (V) shows that both groups have a significant change in amplitude 24 h after treatment. However, there is a greater change in enzyme-treated birds (E) compared with pretreatment levels (vehicle mean = 98.6% ± 0.94; P < 0.05; enzyme mean = 92.6% ± 1.01; P < 0.001). B: the reduction in decibels of the song amplitude 24 h after injection of heparitinase or vehicle-treated birds. Both groups show a reduction, however, treated birds show a significantly (Student's t-test, P < 0.05) larger decrease in sound amplitude than the vehicle controls. C: high-frequency syllables show the greatest change in amplitude. Whereas vehicle-treated birds showed little loss of amplitude, amplitude was reduced by almost 10 dB (P < 0.001) in enzyme-treated birds. D: pressure remains unchanged at 24 h after injection compared with preinjection levels in heparitinase-treated birds, indicating that the loss of amplitude is not due to reduced air-sac pressure (data are presented as mean percentage ± SE, n = 4 for vehicle, n = 6 for enzyme threated; *P < 0.05; ***P < 0.001; NS, not significant).
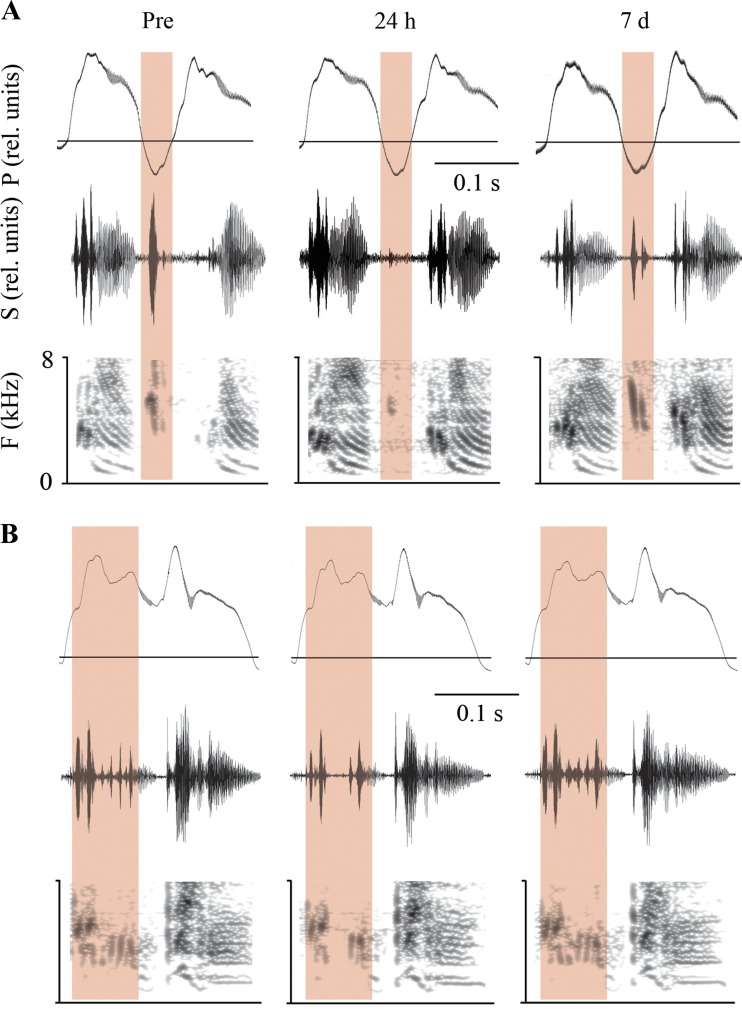
In comparison with the overall effect on song amplitude, particularly the amplitude of high-frequency notes was even further reduced 24 h after enzyme treatment. These syllables consist of tonal notes between 3.5 and 7 kHz, and heparitinase injection caused a mean decrease of 9.4 ± 1.0 dB. In contrast, birds who received vehicle showed a reduction of only 0.87 ± 0.35 dB (asterisk in Fig. 2; Fig. 3C; and Fig. 4 as indicated by the highlighted areas). This effect was temporary in enzyme-treated birds, as by 7 days after injection the amplitude had returned to preinfusion levels (Figs. 2 and 4). Loss of high-frequency syllables cannot be attributed to a substantial change in airflow as inferred from the largely intact air-sac pressure patterns (Figs. 3D and 4).
Fig. 4.
Heparitinase treatment leads to the loss of high-frequency syllables. Examples from 2 different heparitinase-treated birds (A and B) show the complete or partial loss of high-frequency syllables at 24 h after injection (the highlighted areas indicate the segment during which the high-frequency sounds are generated; P, air-sac pressure; S, oscillogram and spectrogram, bottom panels; F, frequency). To allow direct comparison, the spectrograms were calculated with the same dynamic range. The components of high-frequency syllables lost 24 h after the enzyme treatment have reappeared by 7 days (d) after injection, indicating this loss is temporary. This effect occurs in high-frequency syllables that are generated during inspiration (A) and expiration (B).
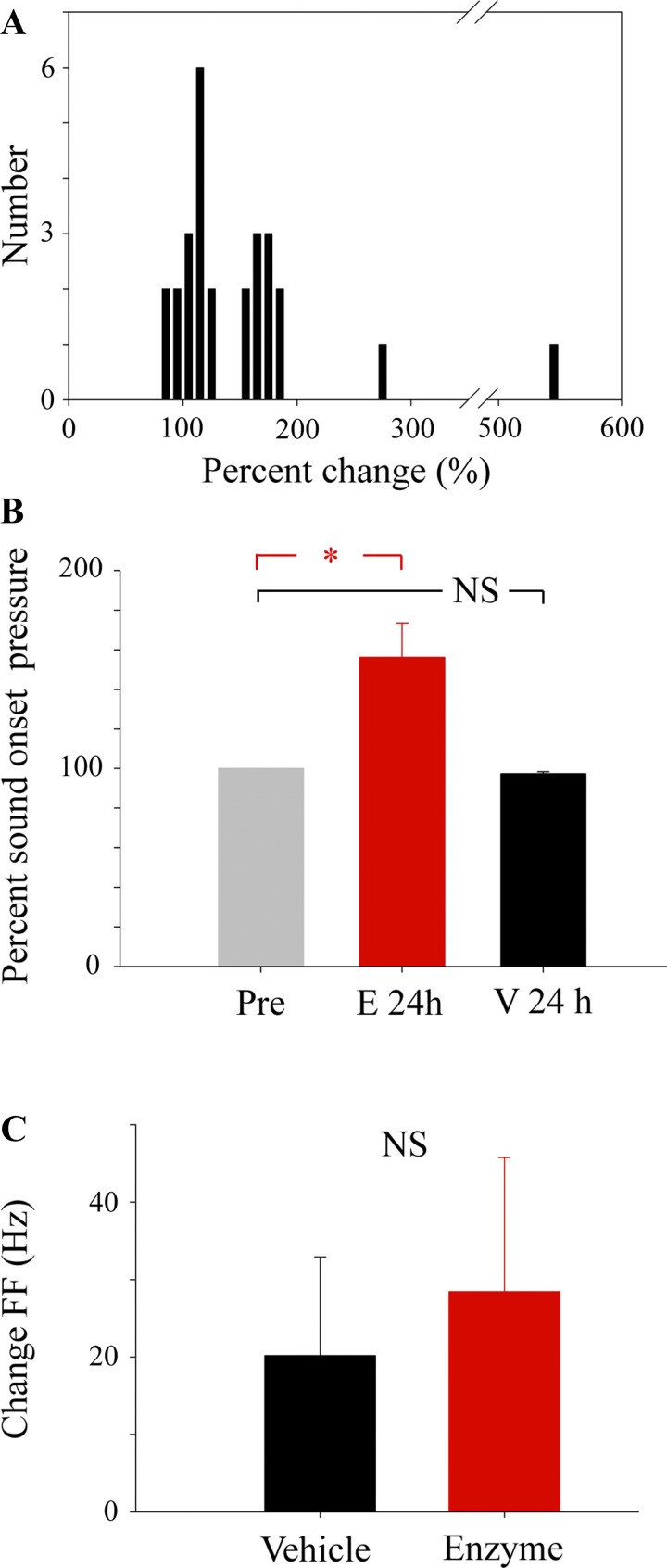
Pressure patterns in adult zebra finch song are as stereotyped as the generated song syllables. In these stereotyped pressure patterns, one can easily observe the pressure at which labial vibrations start (phonation onset pressure). This measure of phonation onset pressure is independent of the microphone recording and therefore not affected by background noise differences or low sound amplitude. The phonation onset pressure for individual syllables varied little during pretreatment song. In vehicle-treated birds, phonation onset pressure was not significantly different from pretreatment values (mean = 97 ± 0.99%). However, enzyme injection caused an increase of ≥5% in the phonation onset pressure 24 h after treatment in 22 of 26 syllables (Fig. 5A). The mean percentage change in phonation onset pressure was 156.1 ± 17.5% of pretreatment levels at the 24-h point (Fig. 5B). We were not able to sustain high-quality air-sac pressure recordings in all birds for 7 days after injection. However, for the 3 enzyme-treated birds in which pressure recordings were possible up to 7 days, phonation onset pressure had returned to preinjection levels for all 18 syllables (mean phonation onset pressure was 100.38 ± 0.89% of pretreatment).
Fig. 5.
Heparitinase treatment alters the air-sac pressure required to produce phonation (sound onset pressure) in a non-frequency-dependent manner. A: pressure at phonation onset is changed 24 h after heparitinase treatment. At least a 5% increase in phonation onset pressure was found in 22 of 26 measured syllables. B: in enzyme-treated birds, the average increase in phonation onset pressure was 56.1% greater (Student's t-test, *P < 0.05) than pretreatment levels (measured as relative voltages). No significant difference in phonation onset pressure was found in vehicle-treated birds. C: heparitinase treatment did not significantly alter the fundamental frequency (FF) of sound at 24 h after enzyme injection compared with vehicle controls when injected into the ventral syringeal and ventral tracheobronchial muscles.
Enzyme treatment did not affect the fundamental frequency of syllables. In both enzyme and control birds, no significant change in fundamental frequency of harmonic stack syllables was observed (Fig. 5). The mean observed changes of 20–50 Hz for fundamental frequencies ranging from 520 to 1,500 Hz fall within typical fluctuations and are not linked to enzyme treatment.
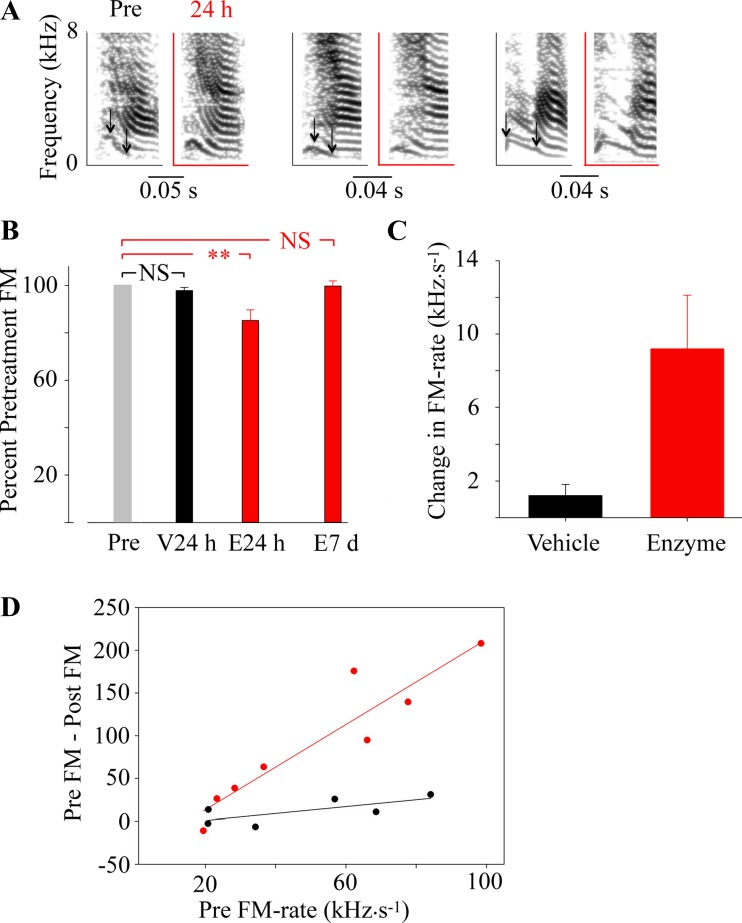
However, enzyme treatment did affect syllables containing frequency modulation (examples shown in Fig. 6A). The rate of frequency modulation (FM rate) decreased significantly to 85.2% ± 4.5 of the preinjection rate 24 h after enzyme injection, whereas no significant difference was seen in vehicle-treated birds. This change was temporary, and FM rates returned to pretreatment levels (99.7% ± 2.2) by 7 days after injection (Fig. 6B). An average change of 9.2 ± 2.9 kHz/s was observed in FM containing syllables 24 h after heparitinase treatment, whereas no significant change was detected in FM rate of syllables from birds who received vehicle treatment (Fig. 6C). The decrease in FM rate was positively related to the pretreatment FM rate (Fig. 6D), suggesting that enzyme treatment decreased FM rates of strongly modulated syllables more.
Fig. 6.
Frequency modulation (FM) is altered after heparitinase treatment in a syllable-specific pattern. A: 3 specific examples of syllables that were assessed for changes in FM by measuring the change in frequency over time measured through an area of greatest modulation, as marked by the black arrows. Recordings 24 h after enzyme treatment are indicated with red axes. B: enzyme treatment resulted in a significant decrease in FM rates 24 h after injection (E24 h; Student's t-test, P < 0.01), but this decrease was no longer present 7 days after injection (E7 d). FM rates were unchanged 24 h after vehicle injection (V24 h) relative to pretreatment levels. **P < 0.005. C: enzyme-treated birds showed a mean change in FM of 9.2 ± 2.9 kHz/s compared with pretreatment values (Student's t-test, P < 0.05). Vehicle treatment was not significantly different from pretreatment measurements (1.2 ± 0.6 kHz/s). Data are presented as means ± SE, n = 6 for vehicle, n = 8 for enzyme-treated birds. D: in birds treated with enzyme (red), the change in FM rate (Pre FM − Post FM) was larger with increasing pretreatment FM rate (linear regression, P = 0.0007; r2 = 0.87), whereas the regression was not significant for (P = 0.114; r2 = 0.5) in the animals treated with vehicle (black).
The comparison of song features within each group showed a significant decrease in song accuracy scores in both vehicle (pre to pre comparison mean = 83.3 ± 0.85 SE; 24 h to pre comparison mean = 79.4 ± 1.23 SE; P = 0.028) and enzyme-treated birds (pre to pre comparison mean = 87.7 ± 2.73 SE; 24 h to pre comparison mean = 76.2 ± 1.23 SE; P = 0.003). Although the decrease was greater in enzyme-treated birds, this analysis suggests that the surgical procedure may have influenced song production within the 1st 24 h. Percent similarity scores showed no significant change in either group (vehicle treatment: pre to pre comparison mean = 85.6 ± 1.98 SE; 24 h to pre comparison mean = 81.3 ± 3.33 SE; P = 0.28; enzyme treatment: pre to pre comparison mean = 82.2 ± 4.46 SE; 24 h to pre comparison mean = 76.6 ± 5.1 SE; P = 0.48). This analysis indicates that no large-scale changes occurred in song syllable structure, confirming that the manipulation only induced subtle changes.
DISCUSSION
The experimental results of this study show how changes in the activation of syringeal muscles critically affect acoustic features of zebra finch song. Syringeal muscles position the labia and thus control airflow through each sound generator of the bipartite syrinx and actively regulate their tension (e.g., Elemans et al. 2008; Goller and Suthers 1996a,b; Larsen and Goller 2002; Uchida et al. 2010; Vicario 1991). Unlike denervation studies (e.g., Daley and Goller 2004; Riede et al. 2010; Seller 1979), the more nuanced manipulation of muscle performance employed in this study revealed to what degree kinetic changes resulting from decreased muscle excitability affect specific acoustic parameters of song. The results provide evidence for predicted effects of this manipulation on sound production. However, they also illuminate an unexpected role of syringeal muscles in the control of sound amplitude and phonation onset pressure. Because muscles controlling the mammalian vocal organ, the larynx, are thought to serve similar functions (e.g., Titze 2000, 2006) to those of the syringeal muscles, the results presented here have more general implications for the production of complex vocal behavior (e.g., Riede and Goller 2010), including human speech, and illustrate some nuanced details of neuromuscular control of a complex motor skill.
Treatment of the syringeal muscles with HS degrading enzyme likely caused a change in neuromuscular transmission that manifested itself in different force production kinetics. Removal of HS led to a reduction in contraction/relaxation cycle frequency in syringeal muscles, as inferred from the in situ ability to modulate airflow. These effects allowed us to study the importance of the superfast characteristics of syringeal muscles (Elemans et al. 2008) for stereotyped song production.
Observed changes in muscle activation kinetics led to the prediction that rate-dependent aspects of phonation, for example modulation of amplitude and frequency, should be altered. Heparitinase treatment did change maximal FM rates as predicted from the reduced ability to modulate airflow at high rates, but there was no systematic change in the fundamental frequency of sounds. This result is somewhat surprising, because regulation of labial tension is thought to be under direct control of the ventral syringeal muscles into which enzyme was infused (Elemans et al. 2015; Goller and Riede 2013; Goller and Suthers 1996a; Riede et al. 2010; Srivastava et al. 2015; Vicario 1991). The induced changes in the activation kinetics of the syringeal muscles after heparitinase treatment must therefore not have interfered with this function. However, enzyme treatment caused the disappearance of most high-frequency tonal syllables, irrespective of whether they were produced on inhalation or exhalation (Goller and Daley 2001). The biomechanical basis for this change is not likely rooted in the previously discussed tension regulating function of ventral syringeal muscles (Goller and Riede 2013) but may stem from the fact that labial position has to be delicately and rapidly controlled to enable self-sustained high-frequency vibrations. High-frequency vibrations may arise from a dynamic oscillation regime that is different from that of lower-frequency harmonic stack syllables (Jensen et al. 2007; Sitt et al. 2008), and our results suggest that induction of these different regimes requires specialized neural control and the rapid muscle activation reduced after enzyme treatment. This interaction between the control of labial movement and labial tension highlights the complexity of the integration task that central neural control mechanisms must accomplish (Elemans et al. 2015; Srivastava et al. 2015).
An unexpected result of heparitinase treatment was the universal reduction in sound amplitude without concurrent change in the driving air-sac pressure. Whereas an overall correlation between muscle activation and sound amplitude has been demonstrated (Srivastava et al. 2015), the relationship found here indicates that muscle action delicately contributes to how energy from the passing airstream is converted into sound energy. Considering that only some dynamic aspects of muscle contraction were affected, the contribution of muscle to sound amplitude must be one in which they position the labia in a way that allows more efficient energy conversion. This interpretation is supported by the increased phonation threshold pressure. Phonation onset pressures in zebra finch vocalizations range from approximately 5 to 12 cmH2O (Riede et al. 2010), and the increase to 156% observed here would have elevated this range to 7.5–18 cmH2O. Presumably, rapid contraction of muscles is needed at the beginning of syllables to optimize the transfer of energy into vibrations, and the heparitinase treatment interfered with this process.
This dependence on the activation kinetics of vocal muscles has not been demonstrated previously and is particularly interesting because phonation threshold pressure did not change between the in situ intact and denervated syrinx as well as the excised syrinx when artificially phonated (Elemans et al. 2010). It therefore implies that syringeal muscles actively increase vocal efficiency and that this mechanism is dependent on activation kinetics or excitability of the muscles. Passive, induced phonation of vocal organs is frequently used to study various aspects of sound production (e.g., Elemans et al. 2015; Hottinger et al. 2007; Jiang et al. 2008; Titze 1992; Verdolini-Marston et al. 1990), but these results must be carefully interpreted with regard to energy conversion.
This study provides a method for subtle disruption of the specific roles of syringeal muscles in song production. Although the precise effects of the enzyme-mediated biochemical manipulation on neuromuscular transmission were not the focus here, future research can use this study as an essential prelude to examine how glycosaminoglycans contribute to muscle function, particularly to neuromuscular transmission kinetics found in avian and mammalian vocal muscles (e.g., Alipour and Titze 1999; Alipour et al. 2005; Hoh 2005).
The detailed involvement of heparan sulfate in neural control of muscle activation is still unclear. The previously proposed roles (Anderson et al. 1984; Arikawa-Hirasawa et al. 2002; Burgess et al. 1999; Guatam et al. 1996; Torres and Inestrosa 1983) may explain the observed effects on muscle activation kinetics and song observed in this study. Future work is needed to explore how neuromuscular transmission was altered and whether specific motor units corresponding to the two fiber types were excluded. Heparitinase treatment induced changes that most likely affected speed of muscle action indirectly by reducing activation and thus causing delayed force production.
Because the aim of this research was to manipulate muscle control of vocal behavior, the details of how the manipulation affects muscle kinetics were not investigated here. However, in future research, the mechanisms could be addressed with a more targeted application of heparitinase and direct twitch measurements of affected fiber bundles. Here, application of heparitinase to the syrinx was performed in a general manner, and neither the detailed penetration of the enzyme into injected muscles nor a dose response was investigated in detail. Rather, this approach used HS to alter overall contractile dynamics of whole ventral syringeal muscles and focused on the behavioral outcomes. Future studies will be aimed at observing in more detail how different dosage of the enzyme affects muscle characteristics and the cellular mechanisms of how enzymatic digestion of heparan sulfate proteoglycans affects muscle function.
The analysis of complex muscular control of acoustic features in songbirds, which is possible through the enzyme-mediated glycosaminoglycan removal/modification, most likely illuminates general mechanisms of neuromuscular control. The results of this study indicate that specific roles cannot be simply assigned to individual muscles but that the various muscles work in concert to give rise to specific behavioral features. A similar organization of motor control is likely shared by the human larynx during speech production but may also apply to complex motor control of other, nonvocal behaviors. The results emphasize a need for taking these complex interactive biomechanical mechanisms into account to facilitate a complete understanding of central control mechanisms.
GRANTS
This study was funded by the National Institutes of Health (Grants DC-006876 and HL-107152), and C. Mencio was partially supported by the Interdepartmental Program in Neuroscience Training Grant at the University of Utah.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.M. and F.G. planned, completed, discussed, and interpreted all experiments and their resulting data. B.K. participated in the planning of experiments and the discussion/interpretation of the resulting data.
ACKNOWLEDGMENTS
We thank Nataliia Verzhbitskiy for help in the laboratory and two anonymous referees whose suggestions decisively improved aspects of this manuscript.
REFERENCES
- Alipour F, Titze I. Active and passive characteristics of the canine cricothyroid muscles. J Voice 13: 1–10, 1999. [DOI] [PubMed] [Google Scholar]
- Alipour F, Titze I, Hunter E, Tayama N. Active and passive properties of canine abduction/adduction laryngeal muscles. J Voice 19: 350–359, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M, Klier F, Tanguay K. Acetylcholine receptor aggregation parallels the deposition of a basal lamina proteoglycan during development of the neuromuscular junction. J Cell Biol 99: 1769–1784, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat Neurosci 5: 119–123, 2002. [DOI] [PubMed] [Google Scholar]
- Beckers GJ. Peripheral mechansisms of vocalization in birds: a comparison with human speech. In: Birdsong Speech and Language, edited by Bolhuis JJ and Everaert M. Cambridge, MA: The MIT Press, 2013, p. 399–422. [Google Scholar]
- Burgess RW, Nguyen QT, Son Y, Lichtman JW, Sanes JR. Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron 23: 33–44, 1999. [DOI] [PubMed] [Google Scholar]
- Daley M, Goller F. Tracheal length changes during zebra finch song and their possible role in upper vocal tract filtering. J Neurobiol 59: 319–330, 2004. [DOI] [PubMed] [Google Scholar]
- Düring DN, Ziegler A, Thompson CK, Ziegler A, Faber C, Müller J, Scharff C, Elemans CP. The songbird syrinx morphome: a three-dimensional, high-resolution, interactive morphological map of the zebra finch vocal organ. BMC Biol 11: 1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemans CP. The singer and the song: the neuromechanics of avian sound production. Curr Opin Neurobiol 28: 171–178, 2014. [DOI] [PubMed] [Google Scholar]
- Elemans CP, Laje R, Mindlin GB, Goller F. Smooth operator: avoidance of subharmonic bifurcation through mechanical mechanisms simplifies song motor control in zebra finches. J Neurosci 30: 13246–13253, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemans CP, Mead AF, Rome LC, Goller F. Superfast vocal muscles control song production in songbirds. PLoS One 3: e2581, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemans CP, Rasmussen JH, Herbst CT, Düring DN, Zollinger SA, Brumm H, Srivastava K, Svane N, Ding M, Larsen ON, Sober SJ, Ŝvec JG. Universal mechanisms of sound production and control in birds and mammals. Nat Commun 6: 8978, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Cooper BG. Peripheral motor dynamics of song production in the zebra finch. Ann NY Acad Sci 1016: 130–152, 2004. [DOI] [PubMed] [Google Scholar]
- Goller F, Daley MA. Novel motor gestures for phonation during inspiration enhance the acoustic complexity of birdsong. Proc Biol Sci 268: 2301–2305, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Riede T. Integrative physiology of fundamental frequency control in birds. J Physiol (Paris) 107: 230–242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. J Neurophysiol 76: 287–300, 1996a. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in gating airflow and sound production in singing brown thrashers. J Neurophysiol 75: 867–876, 1996b. [DOI] [PubMed] [Google Scholar]
- Guatam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85: 525–535, 1996. [DOI] [PubMed] [Google Scholar]
- Hoh JF. Laryngeal muscle fibre types. Acta Physiol Scand 183: 113–149, 2005. [DOI] [PubMed] [Google Scholar]
- Hottinger DG, Tao C, Jiang JJ. Comparing phonation threshold flow and pressure by abducting excised larynges. Laryngoscope 117: 1695–1699, 2007. [DOI] [PubMed] [Google Scholar]
- Jensen KK, Cooper BG, Larsen ON, Goller F. Songbirds use pulse tone register in two voices to generate low-frequency sound. Proc Biol Sci 274: 2703–2710, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JJ, Regner MF, Tao C, Pauls S. Phonation threshold flow in elongated excised larynges. Ann Otol Rhinol Laryngol 117: 548–553, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen ON, Goller F. Direct observation of syringeal muscle function in songbirds and a parrot. J Exp Biol 205: 25–35, 2002. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Yoneda T. Effect of tracheosyringeal nerve section on sexually dimorphic distance calls in Bengalese finches (Lonchura striata var. domestica). Zoological Science 12: 801–805, 1995. [Google Scholar]
- Riede T, Fisher JH, Goller F. Sexual dimorphism of the zebra finch syrinx indicates adaptation for high fundamental frequencies in males. PLoS One 5: e11368, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Goller F. Peripheral mechanisms for vocal production in birds - differences and similarities to human speech and singing. Brain Lang 115: 69–80, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seller TJ. Unilateral nervous control of the syrinx in java sparrows (Padda oryzivora). J Comp Physiol 129: 281–288, 1979. [Google Scholar]
- Sitt JD, Amador A, Goller F, Mindlin GB. Dynamical origin of spectrally rich vocalizations in birdsong. Phys Rev E Stat Nonlin Soft Matter Phys 78: 011905, 2008. [DOI] [PubMed] [Google Scholar]
- Srivastava KH, Elemans CP, Sober SJ. Multifunctional and context-dependent control of vocal acoustics by individual muscles. J Neurosci 35: 14183–14194, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers RA, Zollinger SA. From brain to song: the vocal organ and vocal tract. In: Neuroscience of Birdsong, edited by Zeigler HP and Marler P. Cambridge, UK: Cambridge Univ. Press, 2008, p. 78–98. [Google Scholar]
- Titze IR. Phonation threshold pressure: a missing link in glottal aerodynamics. J Acoust Soc Am 91: 2926–2935, 1992. [DOI] [PubMed] [Google Scholar]
- Titze IR. Principles of Voice Production. Denver, CO: The National Center for Voice and Speech, 2000. [Google Scholar]
- Titze IR. The Myoelastic Aerodynamic Theory of Phonation. Denver, CO: The National Center for Voice and Speech, 2006. [Google Scholar]
- Torres JC, Inestrosa NC. Heparin solubilizes asymmetric acetylcholinesterase from rat neuromuscular junction. FEBS Lett 154: 265–268, 1983. [DOI] [PubMed] [Google Scholar]
- Uchida AM, Meyers RA, Cooper BG, Goller F. Fibre architecture and song activiation rates of syringeal muscles are not lateralized in the European starling. J Exp Biol 213: 1069–1078, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdolini-Marston K, Titze IR, Druker DG. Changes in phonation threshold pressure with induced conditions of hydration. J Voice 4: 142–151, 1990. [Google Scholar]
- Vicario DS. Contributions of syringeal muscles to respiration and vocalization in the zebra finch. J Neurobiol 22: 63–73, 1991. [DOI] [PubMed] [Google Scholar]
- Williams H, Crane LA, Hale TK, Esposito MA, Nottebohm F. Right-side dominance for song control in the zebra finch. J Neurobiol 23: 1006–1020, 2004. [DOI] [PubMed] [Google Scholar]