Our manuscript describes a role of human Brodmann area 7 (BA7) in the integration of multiple visuomotor programs for reaching, grasping, and bimanual coordination. Our results are the first to suggest that right BA7 is critically involved in the coordination of reach-to-grasp movements of the two arms. The results complement previous reports of right-hemisphere lateralization for bimanual grasps.
Keywords: grasping, reaching, bimanual, coordination, TMS
Abstract
Skillful interaction with the world requires that the brain uses a multitude of sensorimotor programs and subroutines, such as for reaching, grasping, and the coordination of the two body halves. However, it is unclear how these programs operate together. Networks for reaching, grasping, and bimanual coordination might converge in common brain areas. For example, Brodmann area 7 (BA7) is known to activate in disparate tasks involving the three types of movements separately. Here, we asked whether BA7 plays a key role in integrating coordinated reach-to-grasp movements for both arms together. To test this, we applied transcranial magnetic stimulation (TMS) to disrupt BA7 activity in the left and right hemispheres, while human participants performed a bimanual size-perturbation grasping task using the index and middle fingers of both hands to grasp a rectangular object whose orientation (and thus grasp-relevant width dimension) might or might not change. We found that TMS of the right BA7 during object perturbation disrupted the bimanual grasp and transport/coordination components, and TMS over the left BA7 disrupted unimanual grasps. These results show that right BA7 is causally involved in the integration of reach-to-grasp movements of the two arms.
NEW & NOTEWORTHY Our manuscript describes a role of human Brodmann area 7 (BA7) in the integration of multiple visuomotor programs for reaching, grasping, and bimanual coordination. Our results are the first to suggest that right BA7 is critically involved in the coordination of reach-to-grasp movements of the two arms. The results complement previous reports of right-hemisphere lateralization for bimanual grasps.
consider a goalkeeper's attempt to intercept a free kick in a soccer match. The shot comes her way, her arms swing to propel the body, and then she dives. Her body and arms stretch and reach for the anticipated trajectory of the ball. Midflight, her hands meet the ball in a tight grip. It's a save.
The goalkeeper's catch highlights the brain's capability to compute object movements, such as the ball's trajectory, along with motor programs and subroutines for reaching, grasping, jumping, and so forth, and to integrate them seemingly effortlessly in sequence (Jeannerod 1984) and in parallel (Frietas et al. 2007). Of course, this applies not only in extreme situations of professional sports but also in many situations of daily life.
Relatively specialized networks in the posterior parietal cortex are thought to encode different goal-directed actions (Culham and Kanwisher 2001; Culham and Valyear 2006; Vingerhoets 2014). Reaching and grasping, for example, depend on separate parietofrontal networks: arm transport during reaching is controlled by a dorsomedial network that projects from the superior parietal occipital cortex (SPOC) to the dorsal premotor cortex (PMd) [Cavina-Pratesi et al. (2010); Davare et al. (2012); Desmurget et al. (1999); see Vesia and Crawford (2012) for a review], whereas grasping is predominantly controlled by a dorsolateral network that projects from the anterior intraparietal sulcus (aIPS) to the ventral premotor cortex (PMv) (Culham et al. 2003; Davare et al. 2010; Grafton 2010; Turella and Lingnau 2014). The neural underpinnings of unimanual movements are different from coordinated bimanual movements (Castiello et al. 1993; Donchin et al. 1998, 2002; Kermadi et al. 2000), with the latter involving interhemispheric interactions mediated by transcallosal projections, such as those connecting parietal, premotor, and primary motor cortices, respectively (Gooijers and Swinnen 2014; Walsh et al. 2008). Yet, it remains unclear how the distinct networks for reaching, grasping, and bimanual coordination operate together so as to integrate their various motor programs.
More recent evidence suggests that the dorsomedial and dorsolateral pathways interact in their activations for the purpose of integrating the transport with the grip component (De Sanctis et al. 2013; Fabbri et al. 2014; Fattori et al. 2010; Filimon 2010; Turella and Lingnau 2014; Vingerhoets 2014). It is possible that this integration may be attained through the networks converging in areas involved in more than one motor program. For example, the Brodmann area 7 (BA7) within the anterior-lateral superior parietal lobule (SPL) is involved in reaching (Filimon 2010; Turella and Lingnau 2014; Vesia and Crawford 2012), bimanual coordination (and has connectivity to reach-area PMd) (Gooijers et al. 2013; Kermadi and Rouiller 2000; Swinnen 2002; Walsh et al. 2008; Wilson et al. 2014), as well as unimanual grasping [where Fabbri et al. (2014) found overlap in activation of the anterior SPL for grasping and reaching; see also Glover et al. (2005); Marangon et al. (2011); Martin et al. (2011); Tunik et al. (2008); for reviews, see Castiello and Begliomini (2008); Turella and Lingnau (2014)]. Therefore, here, we hypothesized that BA7 might play a key role in the control of coordinated bimanual reach-to-grasp movements.
To test this hypothesis, we used a bimanual grasp task that requires temporally and spatially fine-tuned motor programs for reaching, grasping, and bimanual coordination (Le et al. 2014). This bimanual grasp task requires participants to reach to grasp an object with both hands, similar to a goalie catching a ball. We consider this bimanual task as a form of grasping, given that it has very similar computational requirements. It requires vision to guide the hand or hands to specific sets of grasp points on opposite sides of the object to achieve stable grasps, especially when the minimum number of contact points is used; that is, the search for grasp points requires the matching of numerous points against many other possible points (Blake 1992; Blake et al. 1993; Lederman and Wing 2003; Lukos et al. 2007; Vahrenkamp et al. 2011). Moreover, the bimanual grasp task shows similar trajectories and kinematics as the more common unimanual grasp task (Le and Niemeier 2013a; Tresilian and Stelmach 1997) and critically involves the grasp-related brain area aIPS (Le et al. 2014).
Here, we asked participants to grasp bimanually a rectangular object. During the movement, we changed the grasp-relevant size of the object, requiring the participant to reprogram the movement online, and at the same time, we stimulated the brain with magnetic pulses. In a previous study, we showed that the stimulation of the right aIPS disrupted the bimanual grip components during the maximum grip aperture (MGA) but not the reach component (Le et al. 2014). In contrast, here, we found that stimulation of anterior-lateral BA7 in the right hemisphere had biphasic disruption effects (and did not find disruption effects for stimulations of the left BA7 or stimulation of other control sites within the SPL). Specifically, stimulation of the right BA7 produced deficits in an earlier phase of control in the reach/transport components and bimanual coordination and later, the grip components of bimanual movements. In addition, stimulation of the left BA7 disrupted unimanual grasps of the contralateral right hand. We contend that the right BA7 in the SPL constitutes a critical hub to integrate sensorimotor programs for the coordination of reach-to-grasp movements of the two arms.
METHODS
Participants.
Eight healthy individuals participated in the experiment (mean = 26.4 yr, SD = 5.6, 3 women). All participants had normal or corrected-to-normal vision, were right handed (Oldfield 1971), and had no known risk factors for transcranial magnetic stimulation (TMS) (Keel et al. 2001). After the experiment, all participants completed a side-effects questionnaire (Machii et al. 2006), but no side effects were reported. All procedures were approved by the York University Human Participants Review Subcommittee and conformed to the ethical standards in the Declaration of Helsinki.
Procedure and apparatus.
Participants performed a size-perturbation grasping task (Glover et al. 2005; Le et al. 2014; Tunik et al. 2005) using the index and middle fingers of both hands to pick up an object at its left and right sides. We used MRI-guided TMS (Magstim Rapid2; Magstim, Morrisville, NC; see next section) to target putative processes in area BA7 [and medial transoccipital sulcus (mTOS) as a control site, given its lack of involvement in grasping and bimanual coordination; see below] in either hemisphere and measured the kinematics of grasp movements, recording from infrared, light-emitting diodes taped to the tips of the two index fingers (Optotrak 3020; NDI International, Waterloo, ON, Canada; sampling rate 250 Hz, accuracy 0.2 mm). We only recorded from the dominant, i.e., index finger (Glover et al. 2005), given that the middle-finger movements should be performed in a synergistic fashion (Zatsiorsky and Latash 2008).
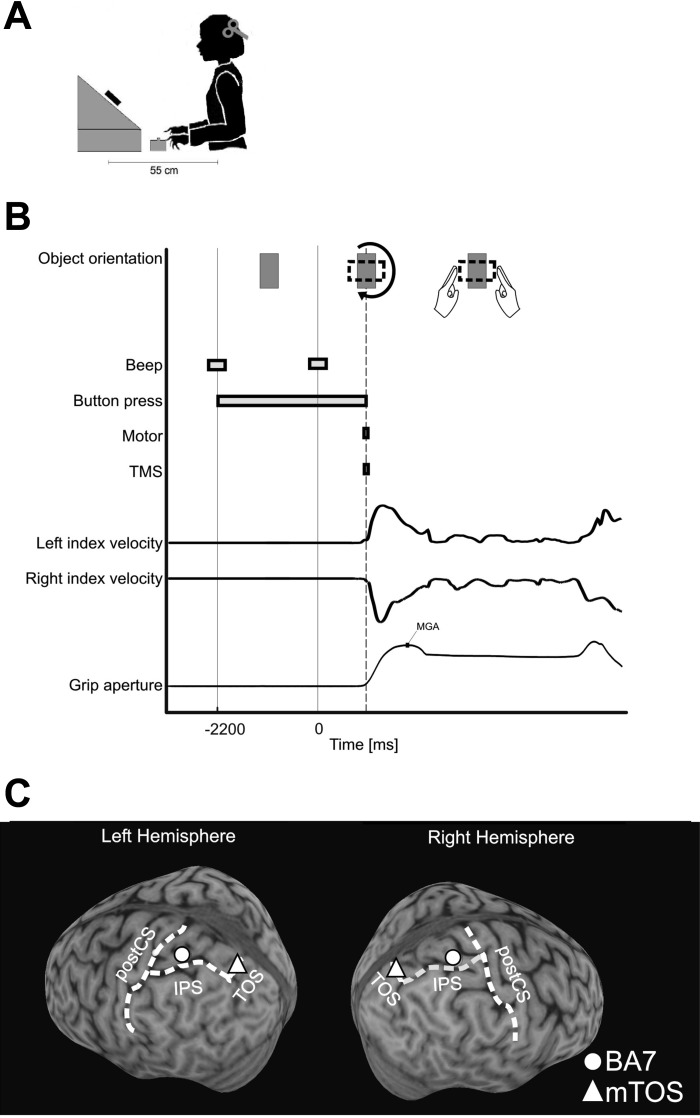
During the experiment, the participants had their head stabilized by a bite bar. Participants wore earplugs to reduce TMS noise. Participants' eyes fixated on a black dot on a white rectangular block (43 g; front side: 87 × 50 mm; thickness: 24 mm) that was mounted on the shaft of a motor (SureStep STP-MTR-17048; AutomationDirect, Atlanta, GA), situated 55 cm in front of the participants and 30 cm below eye level with the object's front side tilted backward (34° from horizon), orthogonal to the line of sight (Fig. 1A).
Fig. 1.
A: experiment setup. B: timeline of a single trial. C: location of stimulation sites BA7 and mTOS, as displayed in the left and right hemisphere of 1 subject. postCS, postcentral sulcus.
Trials began with a low-pitched beep that instructed participants to depress two buttons on a box, 14 cm in front of the body midline with their index fingers (40 cm below eye level; distance between buttons: 2 cm). This triggered the motor to rotate the object to its vertical start orientation, i.e., with the front, narrow edge parallel to the ground (Fig. 1B). Later (2,200 ms), a high-pitched beep sounded and cued participants to initiate movement. Release of the start buttons (movement onset) triggered both the TMS (see below) and motor. In the perturbation condition, the motor quickly rotated the object by ±90° or ±270° around the line of sight. In the no-perturbation condition, the object rotated by ±180°. This way, in both conditions, the object rotated 180°, on average; however, in the perturbation condition, the object's horizontal width increased, whereas in the no-perturbation condition, its width remained the same. Consequently, as the fingers approached the object, participants either had to adjust or not adjust their grasps. We adopted this specific size-perturbation task from small-to-large object width along the grasp-relevant object dimension because previous research has found that small-to-large perturbations plus TMS are more effective in disrupting unimanual (Glover et al. 2005; Tunik et al. 2005) and bimanual (Le et al. 2014) grasps (effective large-to-small perturbations may require later TMS timing) (Glover et al. 2005). We used equal numbers of perturbed and unperturbed trials, such that the two possible grasps were equally un-/predictable. (Although the object's orientation at the start should have biased motor planning, the rotations of the object at movement onset might have canceled motor plans.) Nevertheless, Le and colleagues (2014), with the use of the same perturbation protocol, have previously confirmed the TMS-by-Perturbation interaction reported for protocols using fewer perturbation trials (Glover et al. 2005; Tunik et al. 2005), whereas avoiding any confounds due to startle or surprise responses or due to systematic differences in average rotations.
TMS and localization of brain sites.
To localize the stimulation sites and monitor the TMS coil position, we used frameless stereotaxic neuronavigation (Brainsight; Rogue Research, Montréal, QC, Canada). Before testing in the behavioral sessions, three-dimensional (3D), structural T1-weighted MRIs were obtained for each participant to identify the parietal stimulation sites (BA7 and mTOS in both hemispheres) and the left and right primary motor-hand areas. We defined stimulation sites according to anatomical criteria. We stimulated the anterior-lateral section of the BA7 (Scheperjans et al. 2008) and specifically, the gyrus within the SPL that was ∼1.5 cm (in cortex distance) posterior to the postcentral sulcus and dorsal to the IPS, which was the same region that Glover and colleagues (2005) stimulated and found grasp-specific deficits. The average Talairach coordinates of the region that we stimulated were the following (with SDs in parentheses): left hemisphere = −26 (5.1), −57 (9.9), 55 (4.7); right hemisphere = 23 (5.1), −57 (11.1), 56 (4.8). Also note that this area within the BA7 is not the same brain area as the medial IPS (Seelke et al. 2012; Vingerhoets 2014). The BA7 stimulations should be confined within the anterior-lateral SPL and did not likely spread to other grasp areas, such as the aIPS, given that the locus of TMS stimulation has a spatial resolution of ∼0.5–1.0 cm (Brasil-Neto et al. 1992; Wilson et al. 1993), with an estimated penetration depth of ∼2 cm (Epstein et al. 1990; Rudiak and Marg 1994). Accordingly, the distance between BA7 and aIPS (mean = 1.77 cm, SD = 5.28 cm, in cortex distance) robustly controlled for the spatial specificity of TMS-induced effects. Next, we selected the mTOS as a control site and defined it as the part of the TOS that was 0.5 cm (in cortex distance) medial to its intersection with the IPS. The Talairach coordinates of the area that we stimulated were the following (with SDs in parentheses): left hemisphere = −12 (6.0), −78 (5.7), 32 (5.3); right hemisphere = 11 (3.4), −79 (3.8), 36 (3.7); cortex distance of mTOS from BA7 was 3.4 and 3.2 cm in the left and right hemispheres, respectively. mTOS is typically associated with reaching actions (Prado et al. 2005; Vesia and Crawford 2012; Vesia et al. 2010) (see Fig. 1C; note that mTOS is near but not identical to the SPOC; see discussion), but we did not expect to observe transport effects with stimulations of mTOS, given that our experimental paradigm was not designed to manipulate the reaching component specifically. The motor-hand area was defined as the segment of the precentral gyrus that had a knob-like structure (i.e., shaped like an omega or epsilon in the axial plane and like a hook in the sagittal plane) (Yousry et al. 1997) and that resulted in twitches of hand muscles when stimulated.
At the beginning of an experimental session, we determined the resting motor threshold of each hand. The threshold was defined as the lowest intensity that evoked 5 visible hand-muscle contractions in the contralateral index finger in a series of 10 stimuli when the participant kept the hand muscles relaxed in both hands. We then adjusted the intensity of the experimental stimulations to 110% of the individual resting motor threshold for each participant and hemisphere. The average stimulation intensity was 65.14% of machine output (SD = 10.72) for left and 65.63% for right (SD = 8.97) hemisphere [no significant difference was found between hemispheres, t(7) = 0.24, P = 0.82]. This is similar to previous TMS studies of posterior parietal (Buelte et al. 2008; Dambeck et al. 2006; Dessing et al. 2013; Lewald et al. 2002; Prime et al. 2008; Vesia et al. 2006, 2008). We administered double-pulse TMS (10 Hz) (Rice et al. 2006) immediately after button release using a Magstim Rapid2 TMS system and an air film-cooled, 70 mm figure-of-eight coil that was held tangential to the scalp surface with the handle pointing downward. The minimum time interval between two stimulations was 10 s.
Each stimulation site had its own block of 64 trials. Forty-eight trials were stimulation trials, and 16 were no-stimulation trials. One-half of the trials included perturbation trials, and the other one-half included no-perturbation trials. The order of the trials was randomized within each block, and the blocks were counterbalanced across participants. A fifth block of 96 trials served as a unimanual control condition [designed to be similar to Glover et al. (2005), where stimulations of the left BA7 disrupted unimanual grasps]. For this unimanual control block, participants used their thumb and index finger of their right hand to grasp the object. In one-half of the trials, we stimulated the left BA7, and the other one-half included no stimulation trials.
Data analysis.
We visually inspected the finger-movement data offline and excluded trials that had reaction times <50 ms or had artefacts/missing data in >10% of the total trajectory (total trial exclusion rate: 16.2%). For the remaining trials, we determined the onset of finger movements as well as movement end (the point at which the fingers reached the object) to occur when velocity exceeded/fell below 5% of the peak velocity (and this was calculated separately for each finger in each trial). To normalize the trajectories, we converted time into percent of movement time—defined as movement onset to end or from movement onset to the MGA (defined as the largest distance between 2 index fingers)—and then resampled 3D (x, y, z coordinates) finger positions with a Gaussian filter. With the use of the resulting trajectories, we calculated the grip aperture at each normalized time point.
We used the normalized grip aperture as a measure of grasping, as well as the variability of the grip aperture, by calculating the SD of the grip aperture. To measure transport and coordination, we calculated the SD of the trajectories of the two hands and of the averaged trajectories (average of the left and right hand) at each time point. As well, we calculated 3D velocities, which were computed based on non-normalized hand trajectories, from the Go signal (second beep) to object contact.
To examine these dependent variables, we compiled trials for individual participants and then performed group statistics using repeated-measures ANOVA for each stimulation site separately, due to differences in the number of subjects [left BA7, n = 8; right BA7, n = 6; left mTOS, n = 8; right mTOS, n = 8; BA7 unimanual, n = 7; stimulation sites had unequal numbers (n), due to corrupted data files for some blocks of trials]. For all dependent variables, at each time point, we conducted two [Perturbation (P): +, −; TMS: +, −]- or three (Perturbation, TMS, and Hand: Left, Right)-factorial repeated-measures ANOVAs, and we considered time periods as significant if P fell below 5% for 12 or more consecutive time points (Guthrie and Buchwald 1991). Across analyses for all dependent variables, we found that the power was 0.98, on average (SD = 0.015, range = 0.95–0.99). We ran the data analyses using the full data set for each stimulation site, as well as using the six participants from the right BA7 block (i.e., the same set of participants for each stimulation site), and found no differences in trends. Therefore, for brevity, we will report the results for the analyses that use the full data sets only.
RESULTS
The combination of factors TMS and Perturbation (TMS + P + and sometimes TMS + P −) has been previously reported to produce specific disruption effects for unimanual grasping (Glover et al. 2005; Rice et al. 2006; Tunik et al. 2005), as well as bimanual grasping (Le et al. 2014). Therefore, we expect that disruption effects here would also be reflected in the TMS × Perturbation interaction effects.
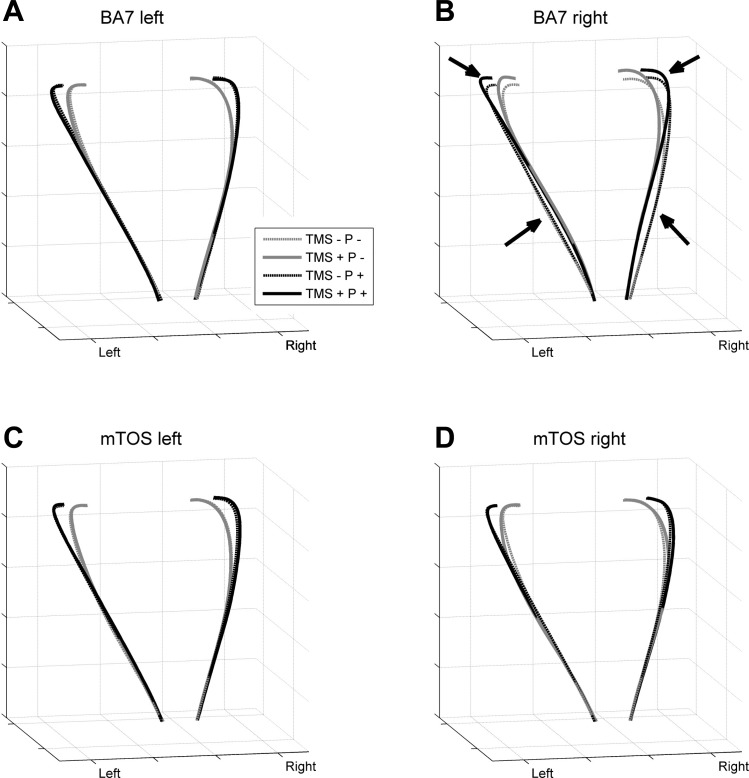
TMS of BA7 in the right hemisphere during size-perturbed grasps (TMS + P +) affected two phases of the bimanual grasp trajectories (see Fig. 2B). An early phase exhibited a narrower grip aperture, and a later phase showed a wider grip aperture relative to the other perturbation and TMS conditions. We did not observe similar trends of significant effects at the other stimulation sites. In subsequent sections, we will document this biphasic effect in more detail by investigating the underlying mechanisms by examining several variables of prehension, transport/coordination, and kinematics. That is, these subsequent variables were examined based on a nested approach of data analysis. Furthermore, we will examine unimanual grasping to look for equivalent effects in one-hand grasp.
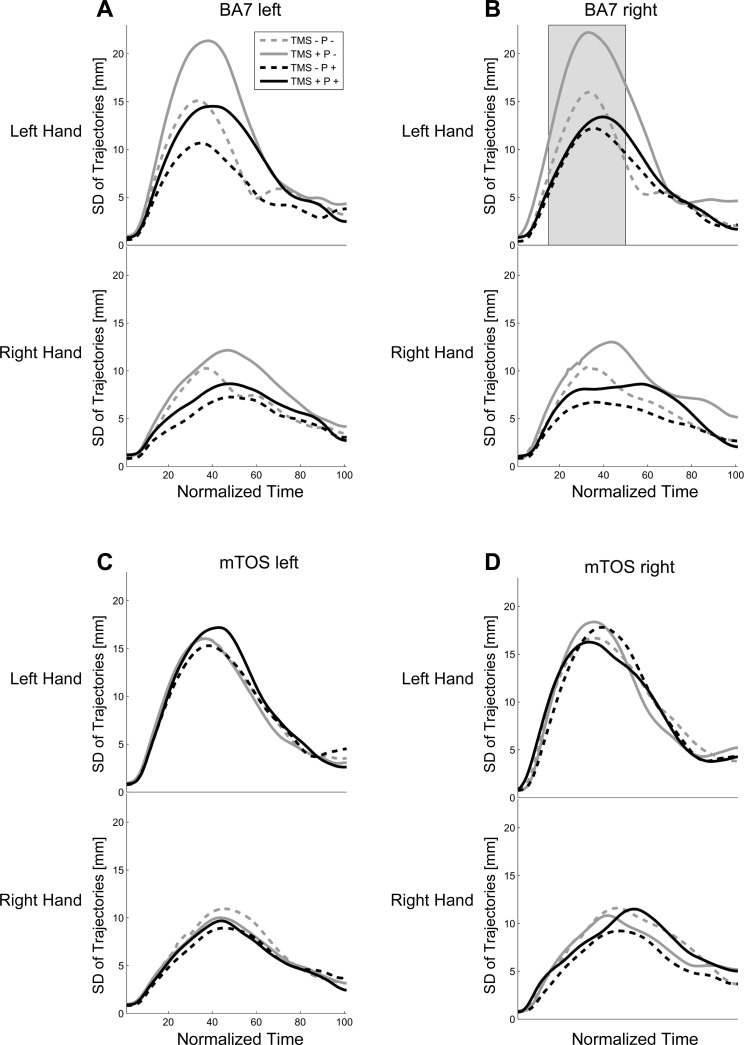
Fig. 2.
Trajectories for the left and right hands from movement onset to object contact (averaged across trials and then across participants). A: trajectories during the left BA7 experiment block. B: right BA7 block, with arrows highlighting effects due to TMS. C: left mTOS block. D: right mTOS block. TMS +, stimulation; TMS −, no stimulation; P +, perturbation; P −, no perturbation.
Measures of prehension.
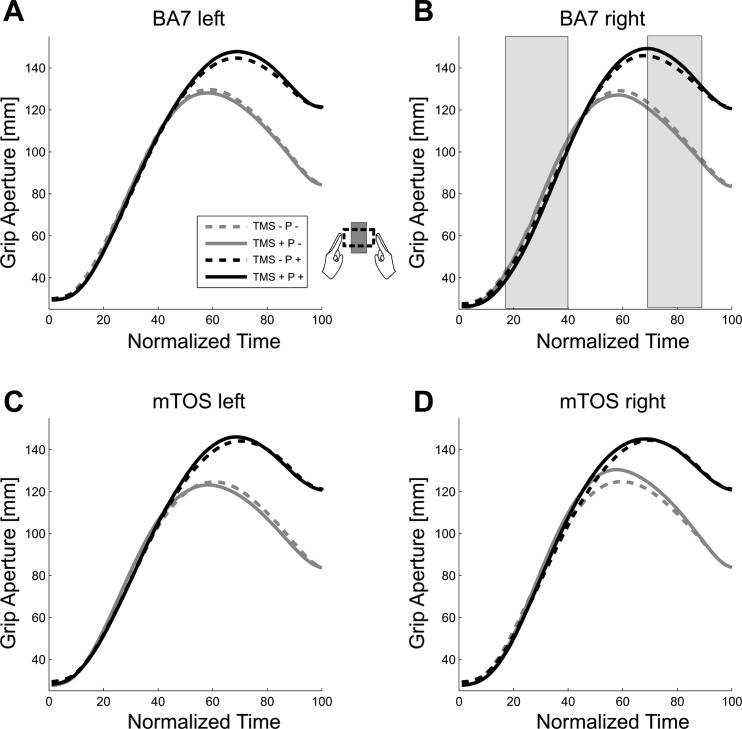
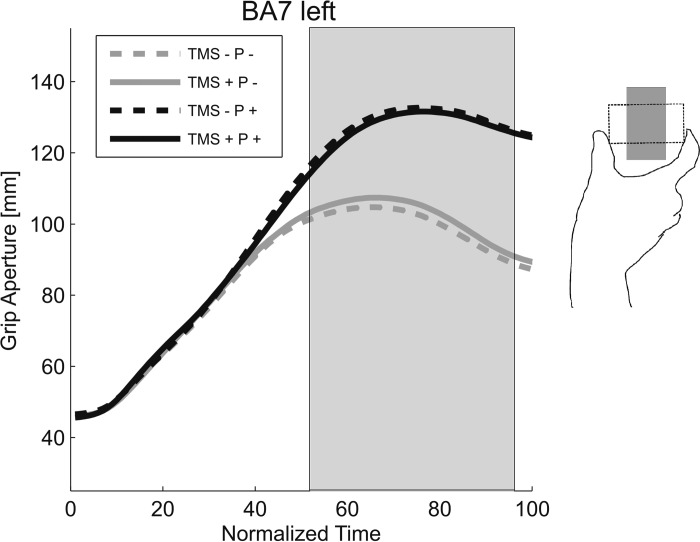
Figure 3 shows the grip apertures across normalized time for each of the four stimulation sites. In general, here, we observed increases in grip aperture over time, leading up to a peak of MGA at 60–80% of the total movement time, consistent with previous reports of unimanual and bimanual grasp trajectories (Jeannerod 1984; Le and Niemeier 2013a, b; Le et al. 2014; Tresilian and Stelmach 1997). Consistent with the observed biphasic effect for the right BA7 (Fig. 2B), a statistical analysis of grip aperture (Fig. 3) yielded TMS-by-Perturbation interactions for right BA7 at times before [17–40% of normalized movement time (NMT); TMS + P +: mean = 76.23 mm, SD = 19.80; other conditions averaged: mean = 77.37 mm, SD = 19.54; Table 1] and shortly after the MGA (69–89% NMT; TMS + P +: mean = 141.75 mm, SD = 5.66; all other conditions averaged: mean = 120.93 mm, SD = 7.30; see Table 1), with the interactions presenting with opposite trends (P values from 41% to 68% NMT in between the biphasic periods were not close to significance, peaking up to P = 0.93, with an average of P = 0.25). This possibly suggests that TMS disrupted more than one process in the right BA7. Post hoc analyses revealed that this biphasic interaction effect was primarily driven by differences between TMS + and TMS − in the Perturbation condition (10–31% NMT: P ≤ 0.05; 71–84% NMT: nonsignificant at P ≤ 0.1). No other stimulation site showed significant or trendwise interactions (F ≤ 5.56, P ≥ 0.51, or <12 consecutive significant time points).
Fig. 3.
Grip aperture across normalized time, averaged trials, and then across participants. A: grip apertures measured during the left BA7 experiment block. B: right BA7 block. Shaded bars overlaying this panel indicate significant perturbation × TMS interaction effects. C: left mTOS block. D: right mTOS block. See Fig. 2 for TMS/P descriptions.
Table 1.
Significant time periods for grip aperture: F and P values
| Stimulation Site | NMT*, % | F | P | ηp2 |
|---|---|---|---|---|
| BA7 left | ||||
| P† | 53–100 | ≥6.51 | ≤0.04 | ≥0.48 |
| BA7 right | ||||
| P | 53–100 | ≥5.96 | ≤0.04 | ≥0.54 |
| TMS‡ | 0–13 | ≥6.98 | ≤0.03 | ≥0.58 |
| TMS × P | 17–40, 69–89 | ≥6.88 | ≤0.03 | ≥0.58 |
| mTOS left | ||||
| P | 48–100 | ≥5.65 | <0.05 | ≥0.45 |
| mTOS right | ||||
| P | 56–100 | ≥6.82 | ≤0.04 | ≥0.49 |
| TMS | 49–68 | ≥6.22 | <0.05 | ≥0.47 |
NMT, normalized movement time;
P, Perturbation condition;
TMS, transcranial magnetic stimulation condition.
Main effects were of less interest: Perturbation effects simply reflected that grip apertures widened with increased object width across stimulation sites (P +: M = 136.28 mm, SD = 9.16 vs. P −: mean = 111.28 mm, SD = 15.43; averaged across 50–100% NMT; see Table 1). Main effects of TMS at BA7 and mTOS in the right hemisphere were due to grip apertures decreasing and increasing with TMS, respectively (BA7 right: mean = −1.59 mm, SD = 2.6, averaged across 0–13% NMT; mTOS right: mean = 4.15 mm, SD = 4.98, averaged across 49–68% NMT; Table 1). These effects will require future investigation to rule out artefacts, such as startle reflexes; that is, it is possible that some of these effects reflect TMS impacting the transport component of the task. Alternatively, however, they might be caused by the clicks of the coil or tactile stimulation of the scalp or other unspecific effects of the TMS. To sort out these confounds in the future, other experimental paradigms will be necessary (Vesia et al. 2013). Finally, to ensure that the TMS-by-Perturbation interaction was not an artefact of the normalization (i.e., aligning trajectories from movement onset to end), we used a different normalization strategy; we aligned trajectories from onset to the MGA, determined trial by trial as the largest distance between the two index fingers, and from MGA to movement end. Again, we found the same trends and effects, including the biphasic effect.
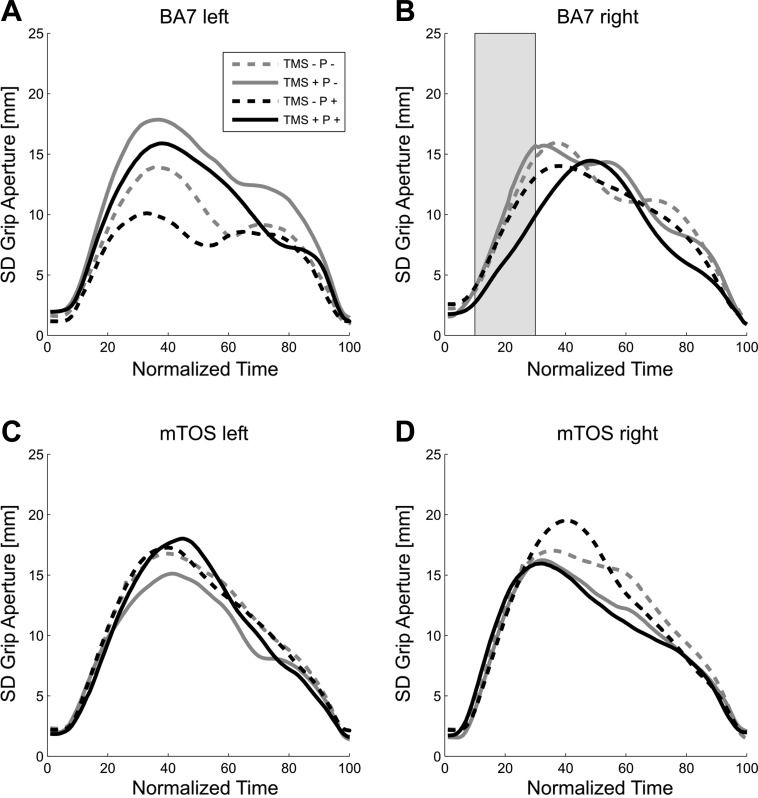
As for the later portion of the biphasic effect around the time of MGA (tMGA), equivalent effects have been reported previously for unimanual (Glover et al. 2005; Tunik et al. 2005) and bimanual (Le et al. 2014) grasping. The early phase of the effect, however, required further exploration. We hypothesized that TMS and perturbation together might have delayed the implementation of the prehension program so that early on, the fingers traveled along paths that largely maintained the fingers' distance at start. If so, then the grip aperture for TMS + P + should be less variable across trials compared with other conditions, during right BA7 stimulation, and specifically, during the early phase of the trajectory. This is what we observed: Fig. 4 shows the variability of grip aperture across normalized time, with variability peaking at between 30% and 50% NMT. As shown in Fig. 4B, TMS + P + of the right BA7 caused the average SD of grip aperture to increase later than in other conditions, specifically, during 10–30% of the NMT (mean = 6.20, SD = 1.75 vs. the other 3 conditions: mean = 9.53, SD = 2.93). That is, we found that BA7 in the right hemisphere had interaction effects that were significantly different from zero, from 10% to 30% NMT (t ≥ −7.65, P ≤ 0.047, −2.73 ≤ confidence interval ≤ −8.66). We did not find any interaction effects at time periods after 30% NMT. Post hoc analyses revealed that this effect, from 10% to 30% NMT, was due to differences between TMS + and TMS − in the Perturbation condition (11–32% NMT: P ≤ 0.05). Moreover, no other time periods for any other stimulation sites were significant (t ≤ 1.76, P ≥ 0.07). We also found main effects of Perturbation for BA7 left and right (27–49% and 83–95% NMT, respectively), as well as main effects of TMS for these two stimulation sites (12–33% and 70–84% NMT, respectively; see Table 2). All other analyses were not significant (F ≤ 3.84, P ≥ 0.10, or <12 consecutive P < 0.05); note that although Fig. 4A might suggest an interaction effect for BA7 left, this trend does not reach significance at any time (P > 0.07). When we aligned the trajectories from onset to the MGA and from MGA to movement end, we found similar trends and effects on grip aperture variability.
Fig. 4.
SD of grip aperture across normalized time. A: left BA7 block. B: right BA7 block. C: left mTOS block. D: right mTOS block. Same convention as Fig. 3.
Table 2.
Significant time periods for SD of grip aperture: F and P values
| Stimulation Site | NMT*, % | F | P | ηp2 |
|---|---|---|---|---|
| BA7 left | ||||
| P† | 27–49 | ≥6.24 | <0.05 | ≥0.47 |
| TMS‡ | 12–33 | ≥7.48 | ≤0.03 | ≥0.52 |
| BA7 right | ||||
| P | 83–95 | ≥6.41 | ≤0.04 | ≥0.56 |
| TMS | 70–84 | ≥8.96 | ≤0.02 | ≥0.64 |
| TMS × P | 10–30 | ≥7.17 | ≤0.04 | ≥0.59 |
NMT, normalized movement time;
P, Perturbation condition;
TMS, transcranial magnetic stimulation condition.
Measures of coordination and transport.
To investigate whether TMS disrupted coordinated bimanual transport, next, we examined the variability of each hand trajectory separately (see methods). Figure 5 shows the variability of the left- and right-hand positions across normalized time, where the SD for the curves peaked slightly before the MGA at 35–40% NMT. Continuous three-way ANOVAs revealed several main effects and interactions. Main effects of Perturbation for left BA7 and right BA7 (6–27% and 6–30% NMT, respectively; see Table 3) suggested that trajectories were more variable when the object was not perturbed (mean = 8.94, SD = 4.67 vs. perturbation: mean = 6.18, SD = 2.79 for 6–30% NMT). This could be related to the fact that object perturbation reduced the object's surface area for horizontal grasps; however, we observed no perturbation effects during stimulation over mTOS in both hemispheres. Alternatively, the effect might have been driven by TMS-by-Perturbation interactions (see below). A second main effect of Hand at all stimulation sites (∼13–58%; Table 3) was due to greater variability of left-hand trajectories (mean = 8.98, SD = 3.18) compared with right-hand trajectories (mean = 6.97, SD = 2.95 for 0–100% NMT). Crucially, we found that right BA7 showed influences of TMS (Fig. 5B and Table 3): a main effect of TMS (15–68% NMT) was likely driven by interactions with other factors, that is, a TMS × Perturbation interaction (7–25%, 37–48% NMT), as well as a TMS × Perturbation × Hand interaction (17–33% NMT). The interactions reflected that TMS was associated with increased variability, especially for the nonperturbed object and especially for the left hand (TMS +: P + mean = 10.23 vs. P − mean = 18.93 vs. TMS −: P + mean = 11.81 vs. P − mean = 15.23; averaged across 17–33% NMT: F ≥ 5.60, P < 0.05; right hand was not significant: F ≤ 2.46, P ≥ 0.06). Post hoc analyses confirmed this to be the case: post hoc analyses revealed that the increased variability from 17% to 33% NMT was due to differences between TMS + and TMS − in the P − condition for the left hand (18–49% NMT: P ≤ 0.05). This implies that TMS of right BA7 disrupted bimanual coordination, either due to a contralateral-hand effect and/or an effect of the nondominant hand. Interestingly, TMS of left BA7 yielded numerically similar SD curves for right BA7 stimulation (Fig. 5A), although the interaction did not reach significance (P > 0.06). The effect for right BA7 also coincided with the early part of the biphasic influence of TMS on grip aperture, although unlike grip aperture, here, the variability measure reflected a stronger TMS effect during the P − condition and not the P + condition. All other comparisons were not significant (F ≤ 0.38, P ≥ 0.05, or <12 consecutive P < 0.05). When we realigned trials according to MGA, the trends and effects were similar to the original analysis.
Fig. 5.
SD of hand trajectories across normalized time. A: left BA7 block. B: right BA7 block. C: left mTOS block. D: right mTOS block. Same convention as previous figures.
Table 3.
Significant time periods for SD of trajectories: F and P values
| Stimulation Site | NMT*, % | F | P | ηp2 |
|---|---|---|---|---|
| BA7 left | ||||
| P† | 6–27 | ≥17.24 | ≤0.006 | ≥0.71 |
| Hand | 13–48 | ≥6.16 | <0.05 | ≥0.47 |
| BA7 right | ||||
| P | 6–30 | ≥10.43 | ≤0.02 | ≥0.68 |
| TMS‡ | 15–68 | ≥7.81 | ≤0.03 | ≥0.61 |
| Hand | 18–49, 67–86 | ≥6.47 | ≤0.04 | ≥0.56 |
| TMS × P | 7–25, 37–48 | ≥6.17 | <0.05 | ≥0.55 |
| TMS × P × Hand | 17–33 | ≥7.61 | ≤0.03 | ≥0.60 |
| mTOS left | ||||
| Hand | 12–45 | ≥6.50 | ≤0.04 | ≥0.48 |
| mTOS right | ||||
| Hand | 10–51 | ≥6.59 | ≤0.04 | ≥0.48 |
NMT, normalized movement time;
P, Perturbation condition;
TMS, transcranial magnetic stimulation condition.
Kinetics of bimanual grasping.
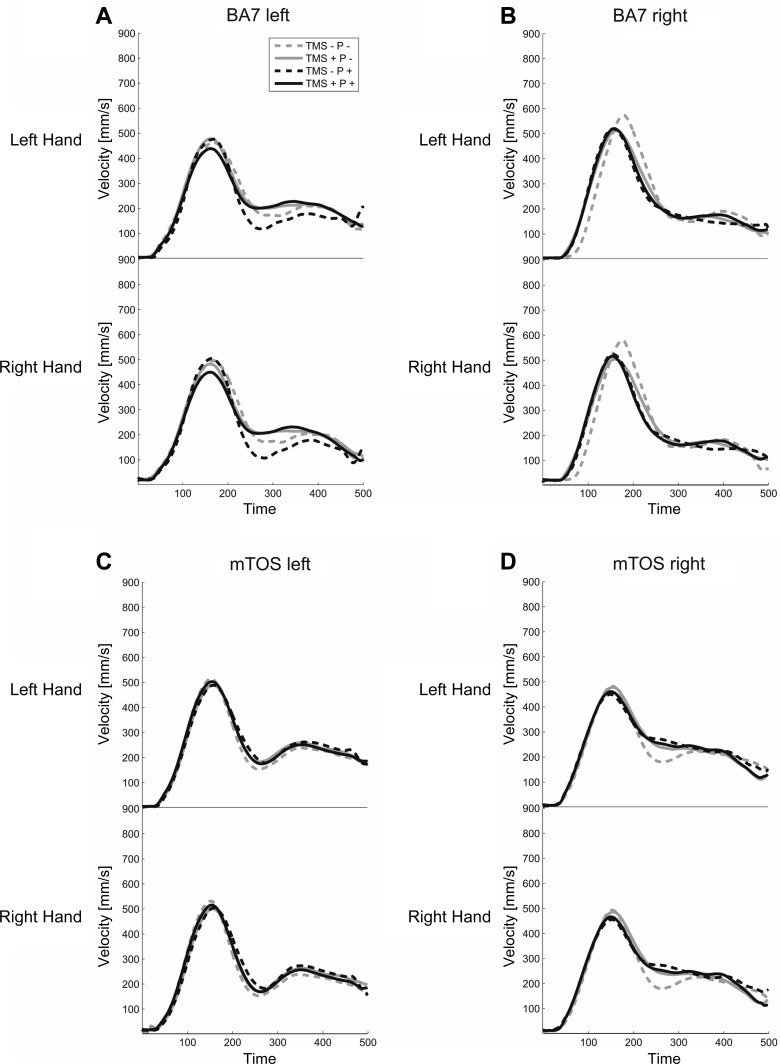
To investigate further whether TMS disrupted coordinated bimanual transport, we inspected velocity profiles, timing of MGA, and total movement time of bimanual grasping. The 3D velocity profiles for each hand separately (Fig. 6) revealed peak velocities between 30% and 40% of movement time, followed by a local minimum between 50% and 60% of movement time. The velocity profiles yielded no influences of perturbation or TMS (F ≤ 3.14, P ≥ 0.15). The left BA7 showed an early hand effect (1–90 time sample), where the right hand (mean = 54.85 mm/s, SD = 76.63) was faster than the left hand (mean = 39.07 mm/s, SD = 73.81), and the left mTOS showed a Perturbation × TMS effect (76–98 time sample), albeit very early and brief near movement onset (suggesting that it might be a spurious effect). There were no other Hand or interaction effects for all stimulation sites (F ≤ 2.97, P ≥ 0.14). The tMGA was delayed by Perturbation for all stimulation sites (P +: mean = 431.30 ms, SD = 178.57; P −: mean = 362.92 ms, SD = 143.65; F ≥ 30.73, P ≤ 0.001). There were no effects of TMS or interaction effects (F ≤ 1.67, P ≥ 0.23), except for the left BA7, where we observed a main effect of TMS [F(1,7) = 8.04, P = 0.025]. Finally, total movement time revealed a stimulation effect for left BA7, where TMS resulted in movement times that were slower for the right hand than for the left [F(1,4) = 44.59, P = 0.003]. There were no differences between the two hands, any influence of TMS on the hands, or any other effects, in general, for three of the four stimulation sites (F ≤ 5.90, P ≥ 0.07).
Fig. 6.
Velocity profiles of index-finger trajectories of the left and right hands across non-normalized time, from Go signal to object contact. A: velocity for left BA7 experiment block. B: right BA7 block. C: left mTOS block. D: right mTOS block. Same conventions as previous figures.
Unimanual control experiment.
We replicated the finding of Glover and colleagues (2005) that TMS of the BA7 in the left hemisphere disrupted right-hand unimanual grasping in the size-perturbation paradigm. Figure 7 shows the unimanual grip aperture across normalized time, with MGAs occurring between 60% and 80% of NMT. As expected, we observed a significant Perturbation × TMS interaction around the tMGA (52–94% NMT; TMS + P −: mean = 103.02 mm, SD = 12.17 vs. TMS − P −: mean = 100.45 mm, SD = 10.05; F ≥ 6.33, P < 0.05, ηp2 > 0.51; see Fig. 7), such that TMS was associated with an increase in aperture when the object size was not perturbed. The direction of this effect is consistent with what has been reported in a minority of individuals during aIPS stimulation (Tunik et al. 2005). The fact that it was more common here is possibly due to the object being placed somewhat too close to the body, making the larger width more difficult to grasp with one hand alone (see discussion). In addition to the interaction that we observed, Perturbation increased grip aperture (47–100% NMT; Fig. 7; F ≥ 6.43, P ≤ 0.04, ηp2 > 0.52; no other effects were observed; F ≤ 0.74, P ≥ 0.42). Once again, realignment relative to MGA produced similar results.
Fig. 7.
Grip aperture for the unimanual control condition: stimulation in BA7 left hemisphere.
DISCUSSION
The current study used a bimanual reach-to-grasp paradigm as a model to investigate how the brain coordinates different motor programs for reaching, grasping, and bimanual actions. Past research efforts have focused on unimanual grasping to characterize the different components for grasping, namely, the transport and grip components, as well as their independence (Cavina-Pratesi et al. 2010; Fattori et al. 2009; Gallivan et al. 2011; Monaco et al. 2011; Vesia et al. 2013; Vingerhoets 2014). For a grasp to be successful, the components must be intimately coordinated with each other, whether it be in time or space (Begliomini et al. 2014; Jeannerod et al. 1995; Marotta et al. 2003; Mason et al. 2001; Vingerhoets 2014). To create an even greater need for coordination, here, we used the bimanual grasping paradigm and investigated the contribution of anterior-lateral BA7 in the anterior-lateral SPL to the coordination of reach-to-grasp movements. We chose BA7, because it is believed to be involved in reaching (Vesia and Crawford 2012), unimanual grasping (Glover et al. 2005), as well as bimanual coordination (Walsh et al. 2008). With the use of TMS and a perturbation reach-to-grasp task, we found two phases of TMS-induced disruptions of arm transport, as well as grasping, that occurred during stimulation of BA7 right. During an early phase (17–40% NMT), stimulation and perturbation combined resulted in reduced grip apertures that coincided with reduced variability of grip aperture, as well as increased hand-transport variability for the left hand. During a later phase around the tMGA, stimulation plus perturbations resulted in enlarged grip apertures, thus affecting both hands, consistent with a previous report by Glover and colleagues (2005). This later effect might be due to an overcompensation of the smaller aperture earlier on and/or due to similar grasp recomputation, as observed with TMS of area aIPS (Tunik et al. 2005). Either way, the late effect observed here could reflect that BA7 influences motor areas at later times than the aIPS (Le et al. 2014) or through a chain of commands via aIPS. In addition, we did not observe the same significant trends for BA7 left, consistent with our previous research, showing that the right hemisphere is more specialized for bimanual grasping (Le and Niemeier 2013a, b; Le et al. 2014). Our findings indicate that BA7, in the right hemisphere, is critically involved in the early coordination of the transport and grip components of bimanual grasps, as well as the control of grasps themselves, similar to the role of aIPS in the right hemisphere (Le et al. 2014). Taken together, our findings indicate that the right BA7 has a special role in bimanual grasp control, whereby aIPS is predominantly involved in the grip component, and BA7 coordinates the grip with the transport component.
Before discussing the role of BA7 further, it seems prudent to address the question as to whether the paradigm used here actually tested bimanual grasping or rather multifinger pointing (Smeets and Brenner 1999). That is, the grasp movements of the two hands might resemble a reach-to-grasp action but actually reflect obstacle avoidance of the object's front as the fingers travel to the sides of the object (Smeets and Brenner 1999). Although this model is parsimonious in terms of describing the movement trajectories themselves, it is not sufficient to capture the computations that are required to prehense and lift objects of various physical properties, such as shape, weight, surface friction, and stability (Blake 1992). In the present study, participants did have to pick up the object. Furthermore, the perturbation paradigm applied here has been used in a number of TMS studies that have reported the involvement of the aIPS (Tunik et al. 2005), as well as the BA7 area that we targeted here (Glover et al. 2005), for unimanual grasping. The paradigm shows that perturbations of grasp-relevant object sizes yield changes around the tMGA. In line with this, we have previously shown that bimanual grasping involves the aIPS, as this is known for grasping with one hand; specifically, we found that TMS of aIPS disrupted grasp movements of both hands together rather than the reach movements of the contralateral hand alone (Le et al. 2014). Furthermore, since there is good evidence that the aIPS is associated with grasping more than reaching (Vesia et al. 2013), we argue that it is safe to say that bimanual grasping shares important features of grasping rather than mere multifinger pointing. Additional support comes from behavioral studies demonstrating a visual-field preference for bimanual grasping that is not shared by bimanual pointing (Le and Niemeier 2013a). Together, this suggests that our task involves mechanisms that should be considered as grasping.
As we now show, BA7 is involved in the integration of the components for grasps, consistent with previous findings about the function of this brain area. First, the SPL is known to be involved in the transport (Vesia and Crawford 2012), coordination (Walsh et al. 2008), and grasp (Glover et al. 2005) separately. Second, recent functional MRI findings have revealed that the anterior SPL (i.e., BA7) has overlapping and interacting representations for grip type and reach direction (Fabbri et al. 2014; Turella and Lingnau 2014). This makes BA7 a suitable candidate to combine information in general and especially, for the transport, grip, and bimanual coordination.
Other brain areas might also be involved in the coordination of the transport and grip components. Two possible candidates are the aIPS and PMv, which are associated with interaction activations for the two components (Cavina-Pratesi et al. 2010; Fabbri et al. 2014; Turella and Lingnau 2014). Although these two brain areas are well known to be involved in the visual analysis and grasp-type selection stages of grasping (Grafton 2010), it is conceivable that aIPS and PMv are involved in integrating the results of these stages with the transport, especially given that the grip is known to be temporally coupled with the transport (Begliomini et al. 2014; Verhagen et al. 2013). However, when we re-examined our data from a previous TMS-perturbation study on aIPS (Le et al. 2014), we did not find the expected TMS-by-Perturbation effects for the variability of bimanual grip aperture or hand transport (F < 1.36, P > 0.29; unpublished data). Nevertheless, it is plausible that aIPS (and PMv) is involved in the integration of transport and grip in a different way than BA7, and so it would be fruitful to investigate this further in future research.
Other possible candidates for combining transport and grip might be the SPOC, PMd, and caudal IPS. These areas are known for their involvement in reaching (Bernier and Grafton 2010; Cavina-Pratesi et al. 2010; Connolly et al. 2003; Gallivan et al. 2011, 2013; Prado et al. 2005; Vesia et al. 2010, 2013), as well as grasping [Davare et al. (2006); Fattori et al. (2004); Galletti et al. (2003); Grol et al. (2007); Koch et al. (2008), (2010); for review, see Turella and Lingnau (2014)].
The onus of the integration of the transport and grip, of course, likely does not fall on any given brain area but rather, a network of areas. For instance, reach signals from the SPOC-PMd circuit and grasp signals from the aIPS-PMv circuit might be sent to BA7 for integration. The integrated signals then might be sent from BA7 back to the SPOC-PMd circuit and the aIPS-PMv circuit for online corrections (Davare et al. 2011; Grafton 2010; Vesia et al. 2012). Alternatively, reach and grasp signal integration might begin at the aIPS-PMv and SPOC-PMd circuits and then become fully integrated at the BA7. (Future research will have to investigate further how BA7 is involved in the reach and grasp neural circuits.) This “integration network” might be lateralized to the left hemisphere for unimanual right-hand grasps. Consistent with this idea, previous research has found that unimanual grasping, in general, exhibits a left-hemisphere dominance, in particular, for some aspects of the grasp, such as grip force (Davare et al. 2007; Ehrsson et al. 2000; Gonzalez et al. 2006). This makes sense, given that unimanual grasping is often used for tasks associated with structures in the left hemisphere, including skilled fine-motor movements and handwriting (Knecht et al. 2000; Pujol et al. 1999; Serrien et al. 2006). Our data show that the stimulation of BA7 left during unimanual grasping resulted in disrupted grip apertures at around the tMGA. However, we did not find evidence of biphasic effects, suggesting that perhaps BA7 left is not directly involved in the integration of transport and grip or that we only observe biphasic effects when reaching needs to be integrated into bimanual coordination. This could suggest that bimanual grasping is qualitatively different from unimanual grasping or that bimanual grasping is quantitatively different, such as being more difficult or more susceptible to TMS. Alternatively, unimanual grasps might create smaller effects so that the early biphasic effect is easily overlooked. Future research will need to investigate this further, as well as investigate whether the stimulation of the left (or right) BA7 would result in disruptions of the contralateral hand only or if the left (or right) BA7 is critically involved in unimanual grasps for both hands.
These findings support the emerging idea of a right-lateralized integration network for bimanual grasping. First, we found disruptions in bimanual grasp movements during stimulation of BA7 right. Second, our previous TMS study on the aIPS also found disruptions during right-hemisphere stimulation (Le et al. 2014). Finally, our psychophysical studies confirm this, in that they show that bimanual grasp performance is better when objects are viewed in the left rather than the right visual field, regardless of position relative to the body (Le and Niemeier 2013a, b).
Given that bimanual reach to grasps is a type of bimanual movement, the right-hemisphere dominance makes sense: there is a need for synchronized bimanual coordination processes and comprehensive 3D representations of the body, and these processes are known to be associated with areas in the right hemisphere (Baas et al. 2011; Blanke et al. 2002; Duque et al. 2009; Mutha et al. 2012; van den Berg et al. 2010; Wenderoth et al. 2004). Indeed, stimulations of BA7 right produced early and late effects on transport and grip, suggesting that BA7, in the right hemisphere, plays a special role in coordinating reach-to-grasp movements and in particular, in situations where movements for the two arms need to be integrated. Nevertheless, our data indicate that BA7 is involved in both unimanual and bimanual grasps, even though the lateralization may be different, consistent with previous reports that unimanual and bimanual movements tend to share the same network of brain areas, albeit with different connection weights between regions (Walsh et al. 2008). Future research will need to investigate the potential influence of attention in unimanual vs. bimanual grasping and how this might affect the hemispheric specializations for the two tasks, given that attention is also right-hemisphere dominant (Le et al. 2015), although previously, we showed that attention is not likely to contribute to the right-hemisphere dominance for bimanual grasping (Le and Niemeier 2013a).
To conclude, here, we sought to determine the role of BA7 in the integration of different upper-limb motor programs. To study the integration of coordinated transport and grip, we used a bimanual reach-to-grasp task because of a heightened requirement for coordination of the two components. We found that stimulation of the right BA7, but not left BA7, results in disruptions of the grip aperture and transport together at an early phase, shortly after movement onset. These results suggest that BA7 is involved in the coordination of reach-to-grasp movements. Our findings offer a novel perspective on how the brain flexibly integrates various independent motor programs and can help further the research on rehabilitation programs for stroke patients and cognitive, neural prosthetics for paralyzed and amputated patients (Andersen et al. 2010).
GRANTS
Support for M. Niemeier, A. Le, and M. Vesia was provided by grants from the Natural Science and Engineering Research Council of Canada (NSERC), and support for J. D. Crawford was provided by the Canada Research Chair Program, as well as grants from the Canadian Institute for Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L., M.V., X.Y., and M.N. performed experiments; A.L. analyzed data; A.L., M.V., and M.N. interpreted results of experiments; A.L. prepared figures; A.L. and M.N. drafted manuscript; A.L., M.V., X.Y., J.D.C., and M.N. edited and revised manuscript; A.L., M.V., X.Y., J.D.C., and M.N. approved final version of manuscript.
REFERENCES
- Andersen RA, Hwang EJ, Mulliken GH. Cognitive neural prosthetics. Annu Rev Psychol 61: 169–190, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas U, de Haan B, Gras̈sli T, Karnath HO, Mueri R, Perrig WJ, Wurtz P, Gutbrod K. Personal neglect—a disorder of body representation? Neuropsychologia 49: 898–905, 2011. [DOI] [PubMed] [Google Scholar]
- Begliomini C, De Sanctis T, Marangon M, Tarantino V, Sartori L, Miotto D, Castiello U. An investigation of the neural circuits underlying reaching and reach-to-grasp movements: from planning to execution. Front Hum Neurosci 8: 1–14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PM, Grafton ST. Human posterior parietal cortex flexibly determines reference frames for reaching based on sensory context. Neuron 68: 776–788, 2010. [DOI] [PubMed] [Google Scholar]
- Blake A. Computational modelling of hand-eye coordination. Philos Trans R Soc Lond B Biol Sci 337: 351–360, 1992. [DOI] [PubMed] [Google Scholar]
- Blake A, Taylor M, Cox A. Grasping visual symmetry. Proceedings from the 4th International Conference on Computer Vision 1993, p. 724–733.
- Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature 419: 269–270, 2002. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol 9: 132–136, 1992. [PubMed] [Google Scholar]
- Buelte D, Meister IG, Staedtgen M, Dambeck N, Sparing R, Grefkes C, Boroojerdi B. The role of the anterior intraparietal sulcus in crossmodal processing of object features in humans: an rTMS study. Brain Res 1217: 110–118, 2008. [DOI] [PubMed] [Google Scholar]
- Castiello U, Begliomini C. The cortical control of visually guided grasping. Neuroscientist 14: 157–170, 2008. [DOI] [PubMed] [Google Scholar]
- Castiello U, Bennett KM, Stelmach GE. The bilateral reach to grasp movement. Behav Brain Res 56: 43–57, 1993. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Monaco S, Fattori P, Galletti C, McAdam TD, Quinlan DJ, Culham JC. Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. J Neurosci 30: 10306–10323, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a “parietal reach region” in the human brain. Exp Brain Res 153: 140–145, 2003. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res 153: 180–189, 2003. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol 11: 157–163, 2001. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol 16: 205–212, 2006. [DOI] [PubMed] [Google Scholar]
- Dambeck N, Sparing R, Meister IG, Wienemann M, Weidemann J, Topper R, Boroojerdi B. Interhemispheric imbalance during visuospatial attention investigated by unilateral and bilateral TMS over human parietal cortices. Brain Res 1072: 194–199, 2006. [DOI] [PubMed] [Google Scholar]
- Davare M, Andres M, Clerget E, Thonnard J-L, Olivier E. Temporal dissociation between hand shaping and grip force scaling in the anterior intraparietal area. J Neurosci 27: 3974–3980, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci 26: 2260–2268, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Kraskov A, Rothwell JC, Lemon RN. Interactions between areas of the cortical grasping network. Curr Opin Neurobiol 21: 565–570, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Rothwell JC, Lemon RN. Causal connectivity between the human anterior intraparietal area and premotor cortex during grasp. Curr Biol 20: 176–181, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Zenon A, Pourtois G, Desmurget M, Olivier E. Role of the medial part of the intraparietal sulcus in implementing movement direction. Cereb Cortex 22: 1382–1894, 2012. [DOI] [PubMed] [Google Scholar]
- De Sanctis T, Tarantino V, Straulino E, Begliomini C, Castiello U. Co-registering kinematics and evoked related potentials during visually guided reach-to-grasp movements. PLoS One 8: e65508, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci 2: 563–567, 1999. [DOI] [PubMed] [Google Scholar]
- Dessing JC, Vesia M, Crawford JD. The role of areas MT+/V5 and SPOC in spatial and temporal control of manual interception: an rTMS study. Front Behav Neurosci 7: 15, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Mitz AR, Bergman H, Vaadia E. Single-unit activity related to bimanual arm movements in the primary and supplementary motor cortices. J Neurophysiol 88: 3498–3517, 2002. [DOI] [PubMed] [Google Scholar]
- Donchin O, Oliveira SC, Vaadia E. Who tells one hand what the other is doing: the neurophysiology of bimanual movements. Neuron 23: 15–18, 1998. [DOI] [PubMed] [Google Scholar]
- Duque J, Davare M, Delaunay L. Monitoring coordination during bimanual movements: where is the mastermind? J Cogn Neurosci 22: 526–542, 2009. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Roland S, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol 83: 528–536, 2000. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Schwartzberg DG, Davey KR, Sudderth DB. Localizing the site of magnetic brain stimulation in humans. Neurology 40: 666–670, 1990. [DOI] [PubMed] [Google Scholar]
- Fabbri S, Strnad L, Caramazza A, Lingnau A. Overlapping representations for grip type and reach direction. Neuroimage 94: 138–146, 2014. [DOI] [PubMed] [Google Scholar]
- Fattori P, Breveglieri R. Evidence for both reaching and grasping activity in the medial parieto−occipital cortex of the macaque. Eur J Neurosci 20: 2457–2466, 2004. [DOI] [PubMed] [Google Scholar]
- Fattori P, Breveglieri R, Marzocchi N, Filippini D, Bosco A, Galletti C. Hand orientation during reach-to-grasp movements modulates neuronal activity in the medial posterior parietal area V6A. J Neurosci 29: 1928–1936, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori P, Raos V, Breveglieri R, Bosco A, Marzocchi N, Galleti C. The dorsomedial pathway is not just for reaching: grasping neurons in the medial parieto-occipital cortex of the macaque monkey. J Neurosci 30: 342–349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filimon F. Human cortical control of hand movements: parietofrontal networks for reaching, grasping, and pointing. Neuroscientist 16: 388–407, 2010. [DOI] [PubMed] [Google Scholar]
- Freitas PB Jr, Krishman V, Jaric S. Elaborate force coordination of precision grip could be generalized to bimanual grasping techniques. Neurosci Lett 412: 179–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti C, Kutz DF, Gamberini M, Breveglieri R, Fattori P. Role of the medial parieto-occipital cortex in the control of reaching and grasping movements. Exp Brain Res 153: 158–170, 2003. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Culham JC. Neuroimaging reveals enhanced activation in a reach-selective brain area for objects located within participants' typical hand workspace. Neuropsychologia 49: 3710–3721, 2011. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Flanagan JR, Culham JC. Where one hand meets the other: limb-specific and action-dependent movement plans decoded from preparatory signals in single human frontoparietal brain areas. J Neurosci 33: 1991–2008, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S, Miall RC, Rushworth MF. Parietal rTMS disrupts the initiation but not the execution of on-line adjustments to a perturbation of object size. J Cogn Neurosci 17: 124–136, 2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Ganel T, Goodale M. Hemispheric specialization for the visual control of action is independent of handedness. J Neurophysiol 95: 3496–3501, 2006. [DOI] [PubMed] [Google Scholar]
- Gooijers J, Caeyenberghs K, Sisti HM, Geurts M, Heitger MH, Leemans A, Swinnen SP. Diffusion tensor imaging metrics of the corpus callosum in relation to bimanual coordination: effect of task complexity and sensory feedback. Hum Brain Mapp 34: 241–252, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooijers J, Swinnen SP. Interactions between brain structure and behavior: the corpus callosum and bimanual coordination. Neurosci Biobehav Rev 43: 1–19, 2014. [DOI] [PubMed] [Google Scholar]
- Grafton ST. The cognitive neuroscience of prehension: recent developments. Exp Brain Res 204: 475–491, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grol MJ, Majdandzić J, Stephan KE, Verhagen L, Dijkerman HC, Bekkering H, Verstraten FA, Toni I. Parieto-frontal connectivity during visually guided grasping. J Neurosci 27: 11877–11887, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology 28: 240–244, 1991. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The timing of natural prehension movements. J Mot Behav 16: 235–254, 1984. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18: 314–320, 1995. [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 112: 720, 2001. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Rouiller M. Do bimanual motor actions involve the dorsal premotor (PMd), cingulate (CMA), and posterior parietal (PPC) cortices? Comparison with primary and supplementary motor cortical areas. Somatosens Mot Res 17: 255–271, 2000. [DOI] [PubMed] [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein E-B, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain 123: 2512–2518, 2000. [DOI] [PubMed] [Google Scholar]
- Koch G, Cercignani M, Pecchioli C, Versace V, Oliveri M, Caltagirone C, Rothwell J, Bozzali M. In vivo definition of parieto-motor connections involved in planning of grasping movements. Neuroimage 51: 300–312, 2010. [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, Rothwell JC. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J Neurosci 28: 5944–5953, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Niemeier M. A right hemisphere dominance for bimanual grasps. Exp Brain Res 224: 263–273, 2013a. [DOI] [PubMed] [Google Scholar]
- Le A, Niemeier M. Left visual field preference for a bimanual grasping task with ecologically valid object sizes. Exp Brain Res 230: 187–196, 2013b. [DOI] [PubMed] [Google Scholar]
- Le A, Stojanoski BB, Khan S, Keough M, Niemeier M. A toggle switch of visual awareness? Cortex 64: 169–178, 2015. [DOI] [PubMed] [Google Scholar]
- Le A, Vesia M, Yan X, Niemeier M, Crawford JD. The right anterior intraparietal sulcus is critical for bimanual grasping: a TMS study. Cereb Cortex 24: 2591–2603, 2014. [DOI] [PubMed] [Google Scholar]
- Lederman SJ, Wing AM. Perceptual judgement, grasp point selection and object symmetry. Exp Brain Res 152: 156–165, 2003. [DOI] [PubMed] [Google Scholar]
- Lewald J, Foltys H, Töpper R. Role of the posterior parietal cortex in spatial hearing. J Neurosci 22: RC207, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukos J, Ansuini C, Santello M. Choice of contact points during multidigit grasping: effect of predictability of object center of mass location. J Neurosci 27: 3894–3903, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol 117: 455–471, 2006. [DOI] [PubMed] [Google Scholar]
- Marangon M, Jacobs S, Frey SH. Evidence for context sensitivity of grasp representations in human parietal and premotor cortices. J Neurophysiol 105: 2536–2546, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta JJ, Medendorp WP, Crawford JD. Kinematic rules for upper and lower arm contributions to grasp orientation. J Neurophysiol 90: 3816–3827, 2003. [DOI] [PubMed] [Google Scholar]
- Martin K, Jacobs S, Frey SH. Handedness-dependent and -independent cerebral asymmetries in the anterior intraparietal sulcus and ventral premotor cortex during grasp planning. Neuroimage 57: 502–512, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CR, Gomez JE, Ebner TJ. Hand synergies during reach-to-grasp. J Neurophysiol 86: 2896–2910, 2001. [DOI] [PubMed] [Google Scholar]
- Monaco S, Cavina-Pratesi C, Sedda A, Fattori P, Galletti C, Culham JC. Functional magnetic resonance adaptation reveals the involvement of the dorsomedial stream in hand orientation for grasping. J Neurophysiol 106: 2248–2263, 2011. [DOI] [PubMed] [Google Scholar]
- Mutha PK, Haaland KY, Sainburg RL. The effects of brain lateralization on motor control and adaptation. J Mot Behav 44: 455–469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Prado J, Clavagnier S, Otzenberger H, Scheiber C, Kennedy H, Perenin MT. Two cortical systems for reaching in central and peripheral vision. Neuron 48: 849–858, 2005. [DOI] [PubMed] [Google Scholar]
- Prime SL, Vesia M, Crawford JD. Transcranial magnetic stimulation over posterior parietal cortex disrupts transsaccadic memory of multiple objects. J Neurosci 28: 6938–6949, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal lefthanded people studied by functional MRI. Neurology 52: 1038–1043, 1999. [DOI] [PubMed] [Google Scholar]
- Rice N, Tunik E, Grafton S. The anterior intraparietal sulcus mediates grasp execution, independent of requirement to update: new insights from transcranial magnetic stimulation. J Neurosci 26: 8176–8182, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiak D, Marg E. Finding the depth of magnetic brain stimulation: a re-evaluation. Electroencephalogr Clin Neurophysiol 93: 358–371, 1994. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18: 2141–2157, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AM, Padberg JJ, Disbrow E, Purnell SM, Recanzone G, Krubitzer L. Topographic maps within Brodmann's area 5 of macaque monkeys. Cereb Cortex 22: 1834–1850, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien D, Ivry R, Swinnen S. Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci 7: 160–167, 2006. [DOI] [PubMed] [Google Scholar]
- Smeets JB, Brenner E. A new view on grasping. Motor Control 3: 237–271, 1999. [DOI] [PubMed] [Google Scholar]
- Swinnen S. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci 3: 350–361, 2002. [DOI] [PubMed] [Google Scholar]
- Tresilian J, Stelmach G. Common organization for unimanual and bimanual reach-to-grasp tasks. Exp Brain Res 115: 283–299, 1997. [DOI] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST. Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nat Neurosci 8: 505–511, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Ortigue S, Adamovich SV, Grafton ST. Differential recruitment of anterior intraparietal sulcus and superior parietal lobule during visually guided grasping revealed by electrical neuroimaging. J Neurosci 28: 13615–13620, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turella L, Lingnau A. Neural correlates of grasping. Front Hum Neurosci 8: 686, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahrenkamp N, Przybylski M, Asfour T, Dillmann R. Bimanual grasp planning. 11th IEEE-RAS International Conference on Humanoid Robots, Oct. 26–28, 2011, p. 493–499. [Google Scholar]
- van den Berg FE, Swinnen SP, Wenderoth N. Hemispheric asymmetries of the premotor cortex are task specific as revealed by disruptive TMS during bimanual versus unimanual movements. Cereb Cortex 20: 2842–2851, 2010. [DOI] [PubMed] [Google Scholar]
- Verhagen L, Dijkerman HC, Medendorp WP, Toni I. Hierarchical organization of parietofrontal circuits during goal-directed action. J Neurosci 33: 6492–6503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesia M, Bolton DA, Mochizuki G, Staines WR. Human parietal and primary motor cortical interactions are selectively modulated during the transport and grip formation of goal-directed hand actions. Neuropsychologia 51: 410–417, 2013. [DOI] [PubMed] [Google Scholar]
- Vesia M, Crawford J. Specialization of reach function in human posterior parietal cortex. Exp Brain Res 221: 1–18, 2012. [DOI] [PubMed] [Google Scholar]
- Vesia M, Monteon JA, Sergio LE, Crawford JD. Hemispheric asymmetry in memory-guided pointing during single-pulse transcranial magnetic stimulation of human parietal cortex. J Neurophysiol 96: 3016–3027, 2006. [DOI] [PubMed] [Google Scholar]
- Vesia M, Prime SL, Yan X, Sergio LE, Crawford JD. Specificity of human parietal saccade and reach regions during transcranial magnetic stimulation. J Neurosci 30: 13053–13065, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesia M, Yan X, Henriques DY, Sergio LE, Crawford JD. Transcranial magnetic stimulation over human dorsal-lateral posterior parietal cortex disrupts integration of hand position signals into the reach plan. J Neurophysiol 100: 2005–2014, 2008. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G. Contribution of the posterior parietal cortex in reaching, grasping, and using objects and tools. Front Psychol 5: 151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RR, Small SL, Chen EE, Solodkin A. Network activation during bimanual movements in humans. Neuroimage 43: 540–553, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, van Hecke P, Swinnen SP. Parieto-premotor areas mediate directional interference during bimanual movements. Cereb Cortex 14: 1153–1163, 2004. [DOI] [PubMed] [Google Scholar]
- Wilson SA, Thickbroom GW, Mastaglia FL. Transcranial magneticstimulation mapping of the motor cortex in normal subjects. The representation of two intrinsic hand muscles. J Neurol Sci 118: 134–144, 1993. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Kurz MJ, Arpin DJ. Functional specialization within the supplementary motor area: a fNIRS study of bimanual coordination. Neuroimage 85: 445–450, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry T, Schmid U, Alkadhi H. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120: 141–157, 1997. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Latash ML. Multifinger prehension: an overview. J Mot Behav 40: 446–476, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]