This study increases our understanding of the mechanisms leading to vasopressin release under conditions of water restriction (acute dehydration). Specifically, these studies suggest that the aldose reductase-fructokinase pathways may be involved in vasopressin synthesis in the hypothalamus and secretion by the pituitary in response to acute dehydration. Nevertheless, mice undergoing water restriction remain capable of maintaining sufficient vasopressin (copeptin) levels to allow normal urinary concentration. Further studies of the aldose reductase-fructokinase system in vasopressin regulation appear indicated.
Keywords: vasopressin, dehydration, fructose, fructokinase, aldose reductase, uric acid
Abstract
Fructose stimulates vasopressin in humans and can be generated endogenously by activation of the polyol pathway with hyperosmolarity. We hypothesized that fructose metabolism in the hypothalamus might partly control vasopressin responses after acute dehydration. Wild-type and fructokinase-knockout mice were deprived of water for 24 h. The supraoptic nucleus was evaluated for vasopressin and markers of the aldose reductase-fructokinase pathway. The posterior pituitary vasopressin and serum copeptin levels were examined. Hypothalamic explants were evaluated for vasopressin secretion in response to exogenous fructose. Water restriction increased serum and urine osmolality and serum copeptin in both groups of mice, although the increase in copeptin in wild-type mice was larger than that in fructokinase-knockout mice. Water-restricted, wild-type mice showed an increase in vasopressin and aldose reductase mRNA, sorbitol, fructose and uric acid in the supraoptic nucleus. In contrast, fructokinase-knockout mice showed no change in vasopressin or aldose reductase mRNA, and no changes in sorbitol or uric acid, although fructose levels increased. With water restriction, vasopressin in the pituitary of wild-type mice was significantly less than that of fructokinase-knockout mice, indicating that fructokinase-driven vasopressin secretion overrode synthesis. Fructose increased vasopressin release in hypothalamic explants that was not observed in fructokinase-knockout mice. In situ hybridization documented fructokinase mRNA in the supraoptic nucleus, paraventricular nucleus and suprachiasmatic nucleus. Acute dehydration activates the aldose reductase-fructokinase pathway in the hypothalamus and partly drives the vasopressin response. Exogenous fructose increases vasopressin release in hypothalamic explants dependent on fructokinase. Nevertheless, circulating vasopressin is maintained and urinary concentrating is not impaired.
NEW & NOTEWORTHY This study increases our understanding of the mechanisms leading to vasopressin release under conditions of water restriction (acute dehydration). Specifically, these studies suggest that the aldose reductase-fructokinase pathways may be involved in vasopressin synthesis in the hypothalamus and secretion by the pituitary in response to acute dehydration. Nevertheless, mice undergoing water restriction remain capable of maintaining sufficient vasopressin (copeptin) levels to allow normal urinary concentration. Further studies of the aldose reductase-fructokinase system in vasopressin regulation appear indicated.
acute dehydration from water restriction stimulates vasopressin (VP) synthesis in the hypothalamus [the supraoptic nucleus (SON) and paraventricular nucleus (PVN)], as well as VP secretion from the posterior pituitary (PP) into the circulation. In the kidney, VP aids in urinary concentration (Bankir 2001; Burbach et al. 2001). One of the classical mechanisms for stimulation of VP secretion is from increased serum osmolality, such as from water loss, that results in the activation of osmo-sensitive, stretch-inactivated cation channels, a variant of transient receptor potential vallinoid 1 (TRPV1 channels). There are also other osmo-regulatory systems, not yet identified, that regulate VP synthesis and secretion in the hypothalamus (Bourque 2008; Verbalis 2007).
Here we hypothesize that one of the unidentified mediators of VP synthesis and release may be fructose. Previous studies have reported that the administration of fructose to humans stimulated VP release which was not observed with equimolar glucose (Wolf et al. 1992). Our laboratory has also reported that the rehydration of dehydrated rats with sugary beverages containing fructose results in a much more exuberant VP response, as noted by measuring serum copeptin (a stable VP derivative) in their blood (García-Arroyo et al. 2016).
While much of our exposure to fructose is from the diet, fructose can also be produced endogenously. To date only one mechanism for endogenous fructose has been reported, and that is by the polyol pathway, in which aldose reductase (AR) [for which the gene in the mouse is aldo-keto reductase family 1, member B3 (Akr1b3)] converts glucose to sorbitol, followed by the conversion of sorbitol by sorbitol dehydrogenase to fructose. This pathway is interesting as AR has osmolarity-sensitive elements and is induced by hyperosmolarity (Ko et al. 1997). Thus one might postulate that dehydration-induced osmolarity not only might lead to the activation of TRPV1-dependent channels in the hypothalamus, but potentially could result in the local induction of AR followed by the generation of fructose locally that could also stimulate VP synthesis and release.
Little is known about how fructose stimulates VP synthesis, but studies in humans suggest it is independent of its osmolarity (Wolf et al. 1992). In this regard, fructose has a metabolism that is distinct from glucose and is initiated by the rapid phosphorylation of fructose by fructokinase, also known as ketohexokinase (KHK) (Ishimoto et al. 2012). Fructokinase knockout (KO) mice have been shown to be protected against the metabolic and renal consequences of endogenous fructose, despite having higher circulating fructose levels (since it cannot metabolize the fructose through this pathway) (Lanaspa et al. 2013, 2014). Thus we decided to test the hypothesis that the increase in circulating VP (copeptin) levels following acute dehydration-mediated hyperosmolarity may be mediated in part by the generation of endogenous fructose and its metabolism by fructokinase in the hypothalamus. To test this hypothesis, we evaluated the effects of 24 h of water deprivation in wild-type (WT) and fructokinase KO (khk−/−) mice. We also assessed directly the effects of exogenously administered fructose on VP release using an in vitro explant model.
MATERIALS AND METHODS
Animals
Mice lacking fructokinase (both A and C isoforms) [KHK-KO mice (khk−/)] on a C57Bl6 background (Diggle et al. 2010) and their WT littermates were used in these studies. Approval for the protocol was provided by our local Institutional Animal Care and Use Committee for the University of Colorado.
In Vivo Acute Dehydration Experiments
Animal protocol.
Acute dehydration with water restriction (WR) (water deprivation for 24 h) results in robust changes in osmolality without significant morbidity and is an accepted approach to induce dehydration (Bekkevold et al. 2013). The mice were killed after 24-h water restriction by anesthesia and cardiac exsanguination. Urine and serum samples were collected directly from the bladder puncture and cardiac puncture, respectively.
Isolation of PP and SON.
Mouse brain was extracted out from the skull. PP was separated from the whole pituitary and snap frozen in liquid nitrogen. The brain was positioned with the ventral surface up, and the SON were microdissected using the optic chiasm and optic tracts as landmarks. The two SONs from each mouse were pooled together and snap frozen in liquid nitrogen.
Chemistry.
Serum and urine osmolality were measured using the Advance Micro Osmometer (model 3300, Advanced Instruments, Norwood, MA). Serum copeptin levels were measured using ELISA (enzyme-linked immunosorbent assay kit; Cloud-Clone, Houston, TX). SON samples were homogenized in a buffer containing 2 mM MgCl2, 1 mM EGTA, 1 mM DTT, and 0.5% (vol/vol) Triton X-100. Homogenates were centrifuged at 13,000 rpm for 10 min (4°C) using Eppendorf centrifuge 5415R (Eppendorf North America, Westbury, NY). Protein in the collected supernatant were then quantified using BCA protein assay kit (Thermo Scientific) and Beckman DU 640 spectrophotometer (Beckman Instruments, Fullerton, CA). For sorbitol determination, 5 μl of the supernatant were assayed using the sorbitol determination kit (K631-100, BioVision) as per manufacturer's instruction. For fructose determination, 5 μl of the supernatant were analyzed with the Bioassay Systems fructose determination kit (EFRU-100), and 5 μl of supernatant were used for uric acid determination with the Bioassay Systems determination kit (DIUA-250). Values were normalized to protein concentration in the lysate.
Quantitative RT-PCR studies for the mRNA of various target genes in SON.
Total RNA was extracted from the frozen SON punches using a sensitive RNA isolation kit (PicoPure, Life Technologies) and reverse transcribed to cDNA using VERSO cDNA kit (Thermo Scientific). Quantitative real-time PCR was performed using i-Cycler and iQ SYBR Green Supermix (Bio-Rad). The results were normalized to cyclophilin and β-actin expressions in the samples from the same mouse. See Table 1 for the primer sequence information of the four target genes.
Table 1.
Primer sequences of the target genes in real-time quantitative PCR
| Gene | Genbank No. | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| Aldose reductase (Akr1b3) | NM_009658.3 | TTATTCACTGGCCAACGGGG | CCATAGCCGTCCAAGTGTCC |
| Vasopressin (Avp) | NM_009732.1 | ATCTGCTGCAGCGACGAGAG | TGTACCAGCCTTAGCAGCAG |
| Apelin (Apln) | NM_013912.3 | TTGTGGAGTGCCACTGATGTT | CGAAGTTCTGGGCTTCACCA |
| TRPV1 (Trpv1) | NM_001001445.2 | GGCCGAGTTTCAGGGAGAAA | TATCTCGAGTGCTTGCGTCC |
Measurement of VP in the PP.
The PP was homogenized in 0.5-ml phosphate-buffered saline (PBS) buffer and was further diluted 10,000 times in PBS buffer (two-step dilution by 100 times dilution each step). VP concentration in the final dilution was measured using a sensitive ELISA kit from Enzo Life Sciences and was converted back to VP amount in nanograms per PP.
Statistical analysis.
Two-way ANOVA with post hoc Bonferroni multiple comparisons were used to assess acute dehydration and fructokinase effects on VP mRNA and other relevant physiological parameters. Values shown are means ± SE, and significance is defined as a P value < 0.05. Data graphics and statistical analysis were performed using Prism 7 (GraphPad Software, La Jolla, CA).
In Vitro VP Release Studies with Explants
Hypothalamo-neurohypophyseal explant preparation.
Explants of the hypothalamo-neurohypophyseal system (HNS) were used for hormone release studies. Explants from mice were prepared the same way as described previously from rats (Song et al. 2006). The HNS explants include the SON neurons, their axons, and axon terminals in the neural lobe, as well as organum vasculosum of the lamina terminalis and suprachiasmatic and arcuate nuclei. They do not include the PVN.
Hormone release from HNS explants.
Explants were positioned individually in perifusion chambers having a 500-μl volume and perifused at 2 ml/h, as described previously (Kapoor and Sladek 2000) with specially formulated F12 nutrient mixture containing a final glucose concentration of 1 mM. Following a 4–5 h equilibration period to allow hormone release to stabilize at basal level, explants were either maintained under control conditions or exposed to fructose (5 mM). Effluent was collected individually at 30-min intervals using a refrigerated fraction collector maintained at 4°C. VP concentration in the perifusate was determined using a sensitive ELISA kit from Enzo Life Sciences. VP release from HNS explants reflects changes in hormone release from nerve terminals in the neural lobe, because, although VP are released from dendrites in SON and from suprachiasmatic nucleus (SCN) (Earnest and Sladek 1987), the amount of VP released from neural lobe far exceeds these other sources (Gregg and Sladek 1984).
Statistical analysis.
Basal VP release was determined during the hour immediately preceding exposure to fructose. Hormone release in response to experimental manipulations is expressed as a percentage of this initial basal release for each explant. Two-way ANOVA with repeated measures, followed by post hoc simple main effects analysis, was performed to evaluate changes in hormone release by time and to compare responses between groups at each time points. The accumulated VP response on time [area under the curve (AUC)] for each explant was also calculated. One-way ANOVA was performed on AUC to evaluate group differences. Values shown for each group are means ± SE, and significance is defined as a P value < 0.05. Data graphics and statistical analysis were performed using Prism 7 (GraphPad Software, La Jolla, CA).
In Situ Hybridization
The brains of adult C57Bl6 mice were perfused with 10% (vol/vol) paraformaldehyde, dissected on ice, and stored at 4°C in PBS containing 30% (wt/vol) sucrose and 0.1% (vol/vol) diethylpyrocarbonate. Brains were mounted using Tissue-Tek OCT compound (Sakura) at −20°C, and coronal sections (14 μm) were prepared using a Cryocut 1800 cryostat. Tissue sections were collected on Gold Seal Micro slides, desiccated for 30 min under a vacuum, and stored at −80°C. RNA probes were generated under RNAse-free conditions, using the digoxigenin (DIG) nucleic acid detection kit from Boehringer Mannheim Biochemical (Indianapolis, IN), according to manufacturer's instructions. The T7 RNA polymerase (SP6 or T7) (Ambion) was used to generate probes (sense and anti-sense, respectively) from template cDNA plasmids containing the mouse khk (AI256253) mRNA sequence. Probe concentration was made using the Boehringer Genius System and DIG-labeled control RNA (Roche). Allele-specific oligonucleotide hybridization blots were performed as described (Coffee and Tolan 2010). RNA in situ hybridization (ISH) of brain slices was performed with both anti-sense probes to detect for gene expression, and sense probes as a negative control. Briefly, cryosections were rehydrated in decreasing concentrations of ethanol (100%, 95%, 70%, and 50%) and then in 2× SSC. The rehydrated sections were treated with proteinase K (10 μg/ml) in 0.1 M triethanolamine, pH 8.0, at 37°C for 30 min, then transferred to 28 mM acetic anhydride in the same buffer and incubated at 37°C for 10 min. After rinsing with 2× SSC, the sections were dehydrated in increasing concentrations of ethanol (50%, 75%, 90%, and 100%). DIG-labeled RNA probes (2 ng/μl) were incubated at 68°C for 10 min and cooled on ice before diluting (1:25) in hybridization buffer with a final concentrations of 1.4 mM Tris·HCl, pH 7.5, 42 mM NaCl, 28 μM EDTA, 53% (vol/vol) deionized formamide, 10% (vol/vol) dextran sulfate, and 1.3% (vol/vol) blocking reagent (Roche). This hybridization solution was sealed over the sections with DPX Mountant (Fluka) and incubated at 55°C for 18–36 h. Slides were unsealed and washed in 2× SSC at 55°C for 30 min, in 2× SSC, 50% (vol/vol) formamide at 55°C for 30 min, and finally twice in 2× SSC at 37°C. Slides were treated with RNase A (20 μg/ml in 0.5 M NaCl, 1 mM EDTA, 10 mM Tris·HCl, pH 7.5) at 37°C for 30 min, and washed with buffer only at 55°C for 30 min. Visualization using DIG-antibody (Roche, 1:1,000) was performed according to the manufacturer's instructions. Following color development, glycerol was applied to the tissue sections, and they were mounted with coverslips and sealed with nail polish. Dried slides were stored at 4°C in the dark, and pictures were taken on an Olympus 1X70 microscope using an attached Olympus camera (Model BH2-RFL-T3) and Picture Frame software.
Changes in Serum Copeptin Levels in a Chronic Model of Heat Stress and Recurrent Dehydration
Our laboratory previously published a study in which the effects of recurrent daily heat stress and water restriction was performed in WT and KHK-KO mice to assess the effects of fructokinase on the renal injury that occurs in this model (Roncal Jimenez et al. 2014). Serum from this study was available, and serum copeptin was assayed (Mouse Copeptin Elisa Kit, Cusabio Biotech Life Sciences, Waltham, MA).
RESULTS
Acute Dehydration Induced Hyperosmolality and an Increase in Plasma VP (Copeptin) Level
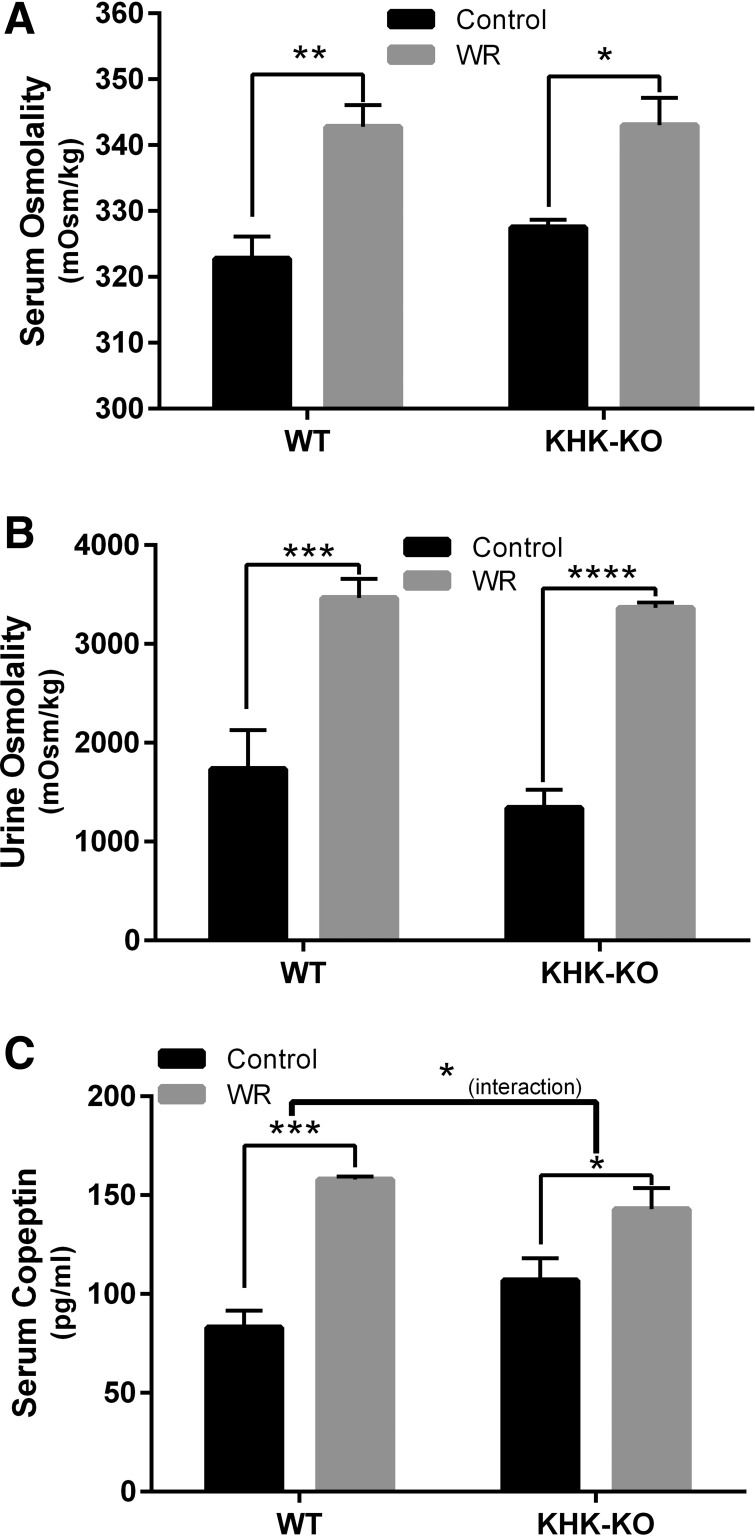
WT and KHK-KO mice were maintained under ad libitum conditions (control) or subjected to WR for 24 h using a standard protocol (Bekkevold et al. 2013). Acute WR was associated with an ∼20 mosmol/kgH2O increase in serum osmolality (Fig. 1A, P = 0.0019 and 0.0122 for WT and KHK-KO mice, respectively), and a doubling in urine osmolality (Fig. 1B, P = 0.0002 and <0.0001 for WT and KHK-KO mice, respectively) in both groups of mice. Serum copeptin, a stable marker of circulating VP, increased in both WT and KHK-KO mice (Fig. 1C, P = 0.0001 and 0.0278 for WT and KHK-KO mice, respectively). However, there was a significant difference in copeptin responses to acute WR between WT and KHK-KO mice [the interaction between WR and mice strains (WT or KHK-KO) was significant with P = 0.0478]. Specifically, the mean copeptin increase by WR in WT mice (90%) was greater than that in KHK-KO mice (34%). The copeptin level in control euhydrated WT mice appeared smaller than that in euhydrated KHK-KO mice, but the difference was not statistically significant.
Fig. 1.
Effects of acute dehydration on serum and urine osmolality and serum copeptin in wild-type (WT) or fructokinase A/C knockout (KHK-KO) mice. Shown are the effects of water restriction (WR) on serum osmolality (A), urine osmolality (B), and serum copeptin (C). C: although acute dehydration significantly increased serum copeptin in both WT and KHK-KO mice, the increase in WT mice was significantly greater than that in KHK-KO mice. The interaction between the effect of dehydration condition and the mouse strain was significant with P = 0.048. Control, ad libitum. Values shown are means ± SE; n = 5–6 animals/group. *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001.
Effects of Acute Dehydration in the SON
SONs were isolated from WT or KHK-KO mice under ad libitum conditions (control) or after 24 h of WR and analyzed for metabolites and gene expression of various target genes (Fig. 2).
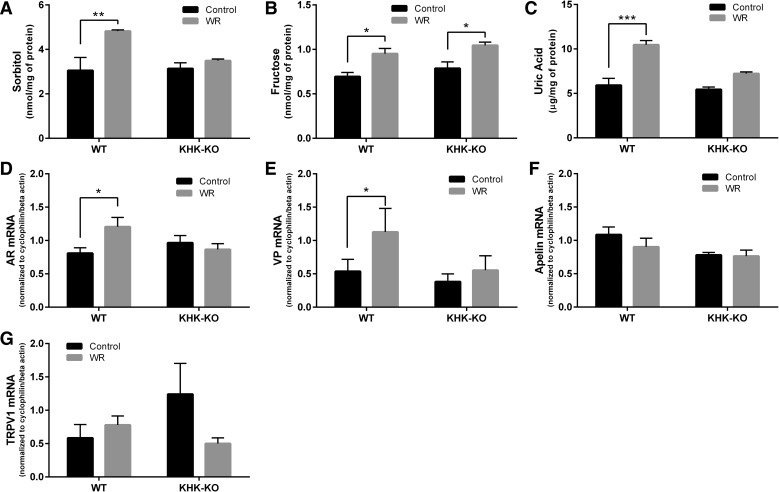
Fig. 2.
Effect of acute dehydration on supraoptic nucleus (SON) polyol and vasopressin pathways in wild-type (WT) or fructokinase A/C knockout (KHK-KO) mice. Shown is the effect of acute water restriction (WR) on SON sorbitol levels (A), fructose levels (B), uric acid (C), aldose reductase (AR) mRNA expression (D), vasopressin mRNA (E), apelin mRNA (F), and TRPV1 mRNA (G). mRNA of target genes was normalized to the mRNA of two housekeeping genes, cyclophilin/β-actin. C: the effect of mouse strain was significant (between WT and KHK-KO, P < 0.01), and there was a significant interaction (P = 0.022) between the mouse strains (WT and KHK-KO) and dehydration conditions (control and WR) for uric acid. E: the effect of mouse strain was significant (between WT and KHK-KO, P < 0.01) for VP mRNA. Control, ad libitum. Values shown are means ± SE; n = 3 animals/group (A–C) and 4–5 animals/group (D–G). *P < 0.05. **P < 0.01. ***P < 0.001.
Interestingly, WT mice showed significantly increased levels of sorbitol, fructose and uric acid in the SON following WR (Fig. 2, A–C, P = 0.0098, 0.0220, and 0.0003, respectively). This was accompanied by a significantly increased expression of both AR mRNA and VP mRNA in WR mice (Fig. 2, D and E; P = 0.0428 and 0.0109, respectively). No changes in mRNA expression with WR were noted for apelin, a peptide that has inhibitory actions to VP secretion (Azizi et al. 2008), or TRPV1 (Fig. 2, F and G).
In contrast, KHK-KO mice showed no significant activation of the polyol pathway (AR mRNA, Fig. 2D) or VP gene expression (Fig. 2E). There was no change in apelin or TRPV1 gene expression with WR in KHK-KO mice either (Fig. 2, F and G). Sorbitol and uric acid levels also did not change in KHK-KO mice (Fig. 2, A and C). Nevertheless, we still observed a significant increase in fructose levels in KHK-KO mice (Fig. 2B).
Acute Dehydration Induced Changes in Posterior Pituitary VP Content
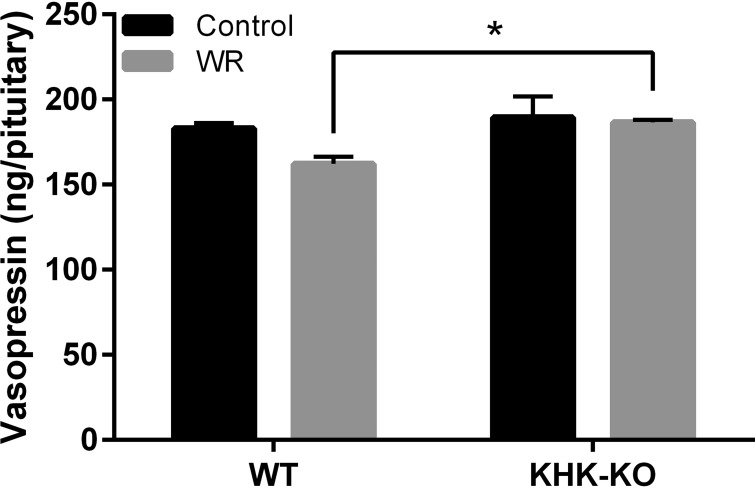
VP stores in the PP reflect the balance of recent synthesis (from the SON and PVN) and secretion to systemic circulation. Acute WR did not change VP content in the PP of KHK-KO mice, but had a tendency to decrease VP content in WT mice (Fig. 3, P = 0.0595). Moreover, VP content in WR WT mice was significantly lower than that in WR KHK-KO mice (Fig. 3, P = 0.0184). These results are consistent with the hypothesis that both VP synthesis in SON/PVN and pituitary VP secretion are partially dependent on fructokinase, and that the lesser secretion of VP in KHK-KO mice balanced with the decreased replacement from the SON and PVN, such that overall VP levels in the pituitary did not change (Fig. 3).
Fig. 3.
Effect of acute dehydration on vasopressin content in the posterior pituitaries of wild-type (WT) or fructokinase A/C knockout (KHK-KO) mice. Shown is the vasopressin storage in the posterior pituitaries of WT or KHK-KO mice under ad libitum condition or after 24-h water restriction (WR). WT mice showed a tendency for lower vasopressin levels in their pituitaries following WR, although this did not reach statistical significance at 0.05 level. However, there was significant difference between mouse strains (WT and KHK-KO, P < 0.01). Specifically, under WR condition, vasopressin content in WT mice was significantly lower than that in KHK-KO mice (P < 0.01). Control, ad libitum. Values shown are means ± SE; n = 4–5 per group. *P < 0.05.
Effects of Fructose on VP Release in Explants
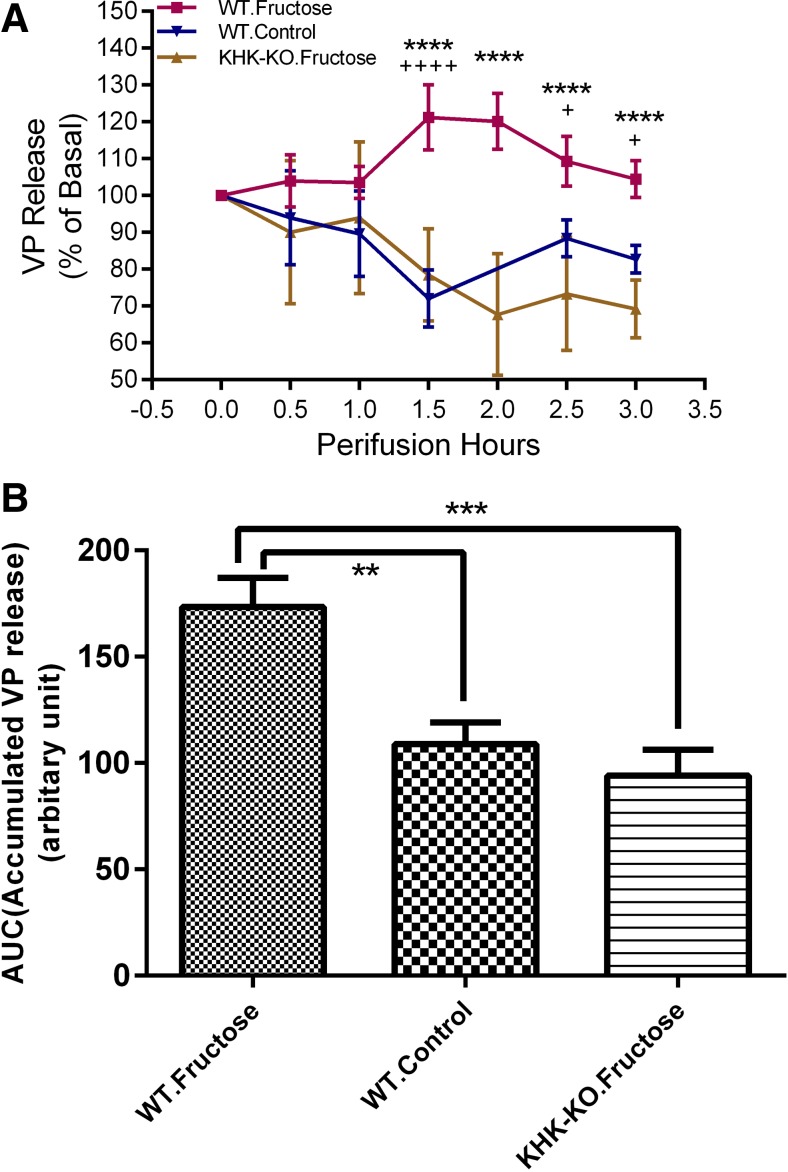
The effect of 5 mM fructose on VP release was evaluated in an in vitro explant model. The exposure to 5 mM fructose increased VP release in WT mice, but not in KHK-KO mice, compared with that in the WT mice without fructose treatment (Fig. 4A, Fgroup = 46.21, P < 0.0001). There was a significant interaction between group effect vs. time (Ftime = 5.416, P < 0.0001). Specifically, VP release in WT mice started to increase 1 h after fructose administration and lasted through the rest of the 3-h experimental period, compared with either control WT mice without fructose treatment or KHK-KO mice with fructose treatment. The accumulated VP release (AUC) over the 3-h period was also calculated. It was shown (in Fig. 4B) that mean AUCs for the three mouse groups are significantly different (F = 12.09, P = 0.0007). Specifically, the mean AUC for the WT mice with fructose treatment was greater than that for the WT control mice without fructose treatment (P < 0.01) or for the KHK-KO mice with fructose treatment (P < 0.001), but the mean AUCs for the latter two groups were not different.
Fig. 4.
Effect of fructose on vasopressin release. The HNS explants were perifused for 7 h. Fructose (5 mM) was included in the perifusing medium where applicable at time = 0. A: VP release (%basal, means ± SE) was plotted vs. perifusing time. +Significant difference between wild-type mice with fructose treatment (WT fructose) group vs. wild-type control mice without fructose treatment (WT control) group. *Significant difference between WT fructose vs. fructokinase A/C knockout mice with fructose treatment (KHK-KO fructose) group. B: the accumulated VP release (AUC) for each explant was calculated. Means ± SE of AUCs for each group were plotted. AUC of WT fructose group was bigger than that of either WT control group or KHK-KO fructose group. Basal VP release for the explants included in these studies was 129.84 ± 25.13, 113.53 ± 13.18 and 122.51 ± 24.84 pg/ml (means ± SE) for groups WT fructose, WT control and KHK-KO fructose, respectively. N = 6 per group. +P < 0.05. **P < 0.01. ***P < 0.001. **** or ++++P < 0.0001.
Expression of Fructokinase in the SON and PVN of the Hypothalamus
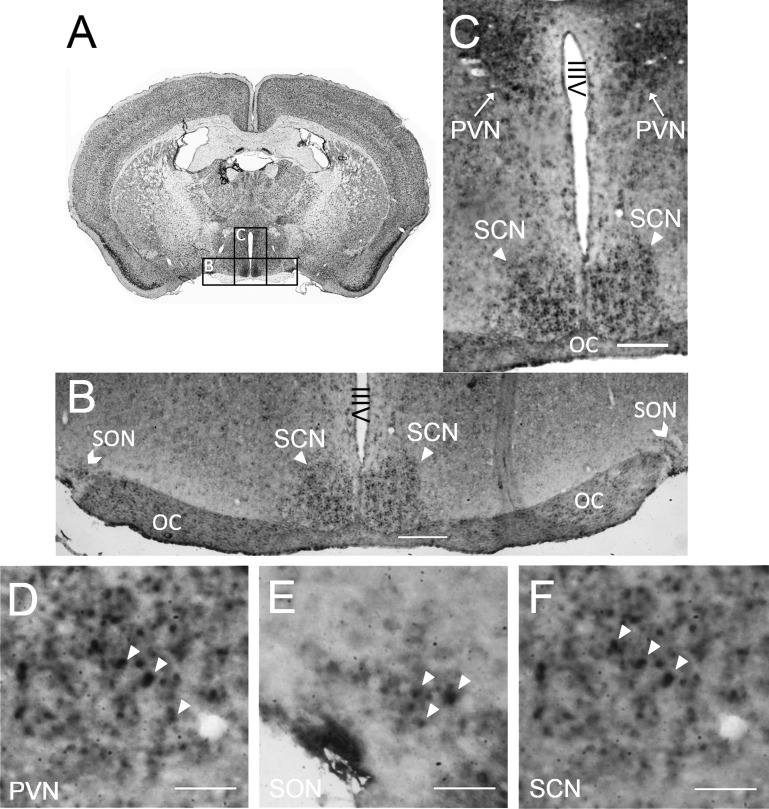
The differences between WT and KHK-KO mice in the dehydration-induced changes of hypothalamic parameters such as VP and AR suggest that fructose may be metabolized by fructokinase in the hypothalamus. To address this possibility, healthy C57Bl6 mice were killed and evaluated for the presence of KHK mRNA by ISH. KHK mRNA was localized in a subset of cells in the SON, PVN, and SCN of the hypothalamus (Fig. 5).
Fig. 5.
Localization of fructokinase (KHK) mRNA by in situ hybridization (ISH) in mouse brain. A: Nissl stained coronal section (http://brainmaps.org/) showing the relative location of B and C in the whole brain. B and C: ISH staining for khk expression (dark color) was shown in the hypothalamic regions of a coronal section of mouse brain that include the supraoptic nucleus (SON), the paraventricular nucleus (PVN) and the suprachiasmatic nucleus (SCN) (×4 magnification). Cells neighboring the PVN, SON and SCN were negative. D–F: higher magnification (×20) pictures showed positive ISH signals in a subset of cells of the PVN (D), SON (E) and SCN (F). Positive signals are shown by the white arrowheads. Scale bars in B and C = 150 μm; scale bars in all other panels = 50 μm. OC, optic chiasm; IIIV, third ventricle.
Serum Copeptin Levels in a Model of Recurrent Heat Stress and/or Dehydration
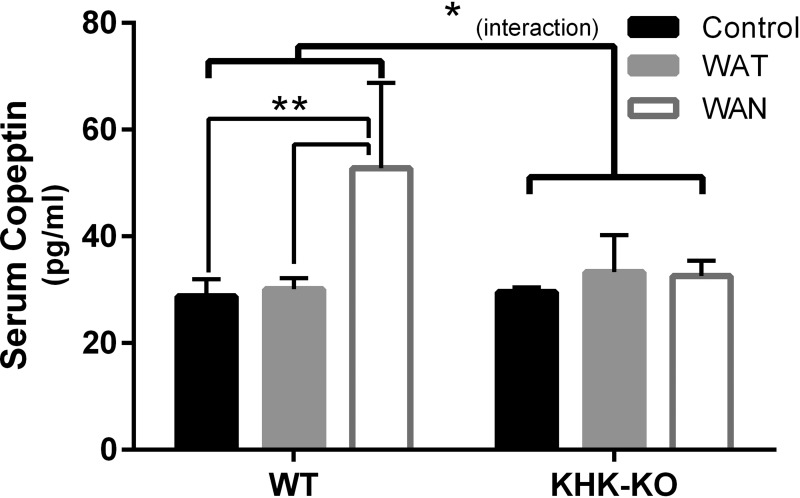
Serum samples were retrieved from a previously published study in which mice were subjected to recurrent heat stress and/or dehydration for 5 wk (Roncal Jimenez et al. 2014). In this study, WT mice were provided water ad libitum (control), water intake was provided throughout the day when the mice were exposed to recurrent heat stress (WAT), or water was restricted during 7 h period of intermittent heat stress but provided following this period (WAN). Similar studies were performed with KHK-KO mice. Results are shown in Fig. 6. Heat/dehydration significantly increased serum copeptin levels (F = 5.490, P = 0.0106), but the effects were different in WT or KHK-KO mice (interaction F = 4.501, P = 0.0214). Specifically, in WT mice, heat plus dehydration significantly increased serum copeptin compared with control or heat stress alone (control vs. WAN, P = 0.0019; WAT vs. WAN, P = 0.0021), while, in KHK-KO mice, serum copeptin did not change significantly (Fig. 6).
Fig. 6.
Serum copeptin levels in a model of recurrent heat stress and/or dehydration. Serum samples were retrieved from a previously published study in which mice were subjected to recurrent heat stress and/or dehydration for 5 wk (Roncal Jimenez et al. 2014). In this study, wild-type (WT) mice were provided water throughout the day (WT-WAT), or only after a 7-h period of intermittent heat stress (WT-WAN). Control mice were provided water ad libitum and were not subjected to heat stress. Similar studies were performed with fructokinase knockout (KHK-KO) mice that either had access to water throughout the day (KHK-WAT) or only after the period of heat stress (KHK-WAN). Serum copeptin was significantly increased in mice that were water-restricted during the period of intermittent heat stress (WAN) compared with mice exposed to heat stress with access to water during the heat stress period (WAT) or control mice. In contrast, the increase in serum copeptin was not observed in KHK-KO mice that were water restricted during the period of intermittent heat stress (KHK-WAN), and the levels were similar to KHK control mice or KHK-WAT mice. Control, ad libitum. Values are means ± SE; N = 5–6 per group. *P < 0.05. **P < 0.01.
DISCUSSION
We evaluated the role of fructokinase in stimulating vasopressin synthesis and secretion in response to acute water restriction. Our hypothesis was that hyperosmolality induced by water restriction might stimulate the expression of the osmolality-sensitive gene, aldose reductase, resulting in its increased activity and the endogenous generation of fructose. Since fructose is known to stimulate vasopressin (García-Arroyo et al. 2016; Wolf et al. 1992), we tested whether mice unable to metabolize fructose due to an absence of fructokinase might have a reduced vasopressin response. Our primary finding was that KHK-KO mice were able to increase serum copeptin levels in response to acute dehydration and maintain urinary concentration. However, there was evidence that the vasopressin axis was impaired, for there was no increase in vasopressin synthesis (mRNA) in the SON and a relatively smaller increase in circulating copeptin levels. We also showed in separate hypothalamic explant studies that fructose can directly stimulate vasopressin release in a fructokinase-dependent manner. These studies therefore suggest that the absence of fructokinase does not prevent the vasopressin response or urinary concentration in response to acute dehydration, but they do have subtle effects in modulating the vasopressin response.
While the absence of fructokinase did not prevent an increase in circulating levels of vasopressin (i.e., copeptin), it did affect vasopressin expression in the hypothalamus. Specifically, we found that water restriction in WT mice resulted in an increase in vasopressin mRNA expression in the SON (consistent with increased synthesis), whereas water-restricted mice lacking fructokinase showed similar increases in serum osmolality but no change in vasopressin mRNA in the SON. Since fructose is the only common sugar that is metabolized by fructokinase, these data provide strong evidence that dehydration-induced vasopressin synthesis and release is dependent in part on metabolism of endogenous fructose by fructokinase.
To further understand this pathway, we examined the presence of enzymes involved in the polyol pathway in the hypothalamus and found some evidence for their activation in the setting of acute water restriction. The polyol pathway involves the conversion of glucose to sorbitol by aldose reductase, followed by the conversion of sorbitol to fructose by sorbitol dehydrogenase. In turn, fructose can be metabolized by fructokinase, resulting in some uric acid generation (Van den Berghe 1986). Aldose reductase is known to be activated by an increase in serum osmolality in multiple different tissues (Ko et al. 1997) and has been shown by our group to be induced in the renal cortex with dehydration-associated hyperosmolality (Roncal Jimenez et al. 2014). Here we demonstrated higher aldose reductase expression in the SON of water-restricted WT mice, as well as an increase in sorbitol, fructose and uric acid in the SON consistent with activation of the polyol pathway. Of interest, Stewart et al. (2011) have reported that acute dehydration in mice induces an upregulation of a variety of genes, including aldose reductase (akr1b3). In their study, they also identified the presence of nuclear factor of activated T-cells 5 (ToneBP), sorbitol dehydrogenase and fructokinase, but expression for these genes did not significantly increase (defined as >1.5-fold) with dehydration (Stewart et al. 2011).
Studies in the KHK-KO mouse were a little more complicated. One might postulate that there would have been an upregulation of aldose reductase, as well as fructose and sorbitol, for the hyperosmolarity would have been expected to stimulate the aldose reductase pathway similarly to the WT mouse. However, there was no change in aldose reductase expression and no increase in sorbitol with water restriction. The absence of an increase in sorbitol in water-restricted KHK-KO mice suggests the presence of a positive feedback loop in which fructose metabolism by fructokinase stimulates aldose reductase. Consistent with this concept, we reported that mice lacking fructokinase failed to show a robust increase in sorbitol in the kidney following recurrent exposure to heat and water restriction, as opposed to WT mice (Roncal Jimenez et al. 2014). Furthermore, we also reported that KHK-KO mice fed a high glycemic diet did not show the expected increase in aldose reductase expression that was observed in similarly treated WT mice (Lanaspa et al. 2013). One potential explanation could be a feedback mechanism in which uric acid, generated during fructose metabolism, stimulates aldose reductase expression, as uric acid has been reported to stimulate aldose reductase activity in human umbilical vein endothelial cells (Zhang et al. 2014). The observation that uric acid levels in the SON were lower in the KHK-KO mice not only suggest that fructokinase metabolism was blocked, but would also be consistent with an interruption of a feedback loop to stimulate aldose reductase expression.
Another surprising finding was that fructose content increased in the hypothalamus of the KHK-KO mice following dehydration, given no change in aldose reductase expression or sorbitol levels. We do not have a specific explanation for this finding, although it might reflect differences in aldose reductase activity or differences in sorbitol dehydrogenase expression or activity. As mentioned, one study did not show a change in sorbitol dehydrogenase expression with dehydration in the mouse (Stewart et al. 2011). Unfortunately, we did not have more sample to specifically examine changes in expression of sorbitol dehydrogenase.
Definitive evidence for a role for hypothalamic fructokinase in mediating vasopressin secretion was provided by the hypothalamic explant studies. Here we were able to show that hypothalamic explants exposed to 5 mM fructose showed a robust increase in vasopressin release that was not observed in explants from KHK-KO mice. This finding documents that fructose can stimulate vasopressin secretion through a direct effect on the hypothalamus. The observation that the increase in vasopressin synthesis was completely abrogated in the KHK-KO mice demonstrates that the fructose must be metabolized by fructokinase to have its effect, and hence demonstrates functional fructokinase must be present in the SON in the vasopressin-producing neurons (consistent with our ISH studies). Finally, the observation that no effect was observed in the hypothalamic explants of KHK-KO mice also suggests that it is not the osmotic effect of fructose that is driving the response.
We also evaluated the vasopressin storage in posterior pituitaries. Vasopressin is synthesized in SON and PVN of the hypothalamus and is transported to posterior pituitary, from where it is released into systemic circulation upon excitation of the system. So the vasopressin content in the posterior pituitary reflects a balance between newly synthesized hormone and hormone being secreted into circulation. Acute dehydration only had a tendency to decrease vasopressin storage in WT mice. However, under acute dehydration, WT mice had significantly less vasopressin storage compared with KHK-KO mice, indicating that increased vasopressin release overrode synthesis, depending on fructokinase, under this condition. This is in line with our observation that plasma copeptin, the stable marker for vasopressin, showed a greater relative increase in WT mice compared with that in KHK-KO mice following acute water restriction.
An interesting question is whether the endogenous fructose pathway may be mediating vasopressin responses in the setting of chronic dehydration. Our laboratory previously published a study in which mice were exposed repeatedly to daily heat stress with water restriction during the day for a total period of 5 wk (Roncal Jimenez et al. 2014). The original aim was to determine whether heat stress and recurrent dehydration may lead to the induction of aldose reductase-fructokinase pathway in the kidney, and whether this would result in the generation of endogenous fructose in the kidney that might induce tubular injury and fibrosis through a fructokinase-dependent mechanism. Indeed, our laboratory found that recurrent heat stress and dehydration did induce the renal generation of fructose that led to a fructokinase-dependent renal injury (Roncal Jimenez et al. 2014). We have subsequently measured the serum copeptin in this study and observed a significant increase in serum copeptin in response to recurrent heat stress and dehydration in the WT but not the KHK-KO mice (Fig. 6), despite similar levels of serum osmolarity (Roncal Jimenez et al. 2014). We also noted a blunted rise in urine osmolarity in these mice as well, consistent with a mild urinary concentration defect (Roncal Jimenez et al. 2014). Thus it is likely that there exists an aldose reductase-fructokinase-dependent pathway for vasopressin synthesis and secretion in addition to the more classical osmolarity-TRPV1 pathways. Indeed, the observation that there is increased glucose utilization in the SON and PVN with chronic dehydration might be consistent with activation of aldose reductase and the metabolism of glucose to sorbitol and eventually fructose (Gross et al. 1985).
Our study has several limitations. First, we utilized mice that genetically lacked fructokinase, and, when using KO mice, one always has to consider off-target effects related to general metabolic differences. However, the explant studies strongly suggest the presence of an intact fructokinase system in the hypothalamus. Second, studies in mice may not necessarily translate to humans (Stewart et al. 2011). Finally, our data show significant but relatively modest findings, suggesting this is an ancillary pathway rather than a dominant one for vasopressin secretion under acute dehydration conditions. Having said this, the marked amplification of vasopressin increase in dehydrated animals administered fructose as their rehydration solution (García-Arroyo et al. 2016) suggests a synergy that could be important in conditions such as marathon-induced hyponatremia. Further studies are indicated to investigate these interactive pathways.
In summary, these studies provide the first evidence that fructokinase modulates vasopressin synthesis in the SON and secretion from the PP into circulation. Further studies on the interaction of the polyol-fructokinase pathway with vasopressin synthesis and secretion appear indicated.
GRANTS
This study was supported by Department of Defense grant W81XWH-14-1-0270; the Danone Research, Palaiseau, France; and the La Isla Foundation and Solidaridad. M. Kuwabara received a grant for studying abroad from Federation of National Public Service Personnel Mutual Aid Association in Japan. T. Jensen is supported by National Institutes of Health T32 Training Grant 5T32DK007446-34.
DISCLOSURES
C. A. Roncal-Jimenez, M. A. Lanaspa-Garcia, D. R. Tolan, L.-G. Sanchez-Lozada, and R. J. Johnson are members of Colorado Research Partners, LLC, which is developing inhibitors of fructose metabolism for the treatment of metabolic syndrome and kidney disease. R. J. Johnson is also on the Scientific Board of Amway.
AUTHOR CONTRIBUTIONS
Z.S., C.A.R.-J., M.A.L.-G., S.A.O., M.K., T.J., T.M., A.A.-H., T.I., D.R.T., and R.J.J. analyzed data; Z.S., C.A.R.-J., M.A.L.-G., M.K., T.J., T.I., and R.J.J. interpreted results of experiments; Z.S., C.A.R.-J., P.S.M., D.R.T., and R.J.J. drafted manuscript; Z.S., C.A.R.-J., M.A.L.-G., T.J., G.E.G., P.S.M., L.-G.S.-L., D.R.T., and R.J.J. edited and revised manuscript; Z.S., C.A.R.-J., L.-G.S.-L., D.R.T., and R.J.J. approved final version of manuscript; Z.S., C.A.R.-J., M.A.L.-G., S.A.O., T.M., A.A.-H., T.I., G.J., and R.J.J. performed experiments; Z.S., C.A.R.-J., M.A.L.-G., T.I., D.R.T., and R.J.J. prepared figures.
ACKNOWLEDGMENTS
This paper is considered a contribution by the University of Colorado Climate Change and Health consortium. We also thank the helpful comments of Dr. Zhiying You and Dr. Celia Sladek, University of Colorado.
REFERENCES
- Azizi M, Iturrioz X, Blanchard A, Peyrard S, De Mota N, Chartrel N, Vaudry H, Corvol P, Llorens-Cortes C. Reciprocal regulation of plasma apelin and vasopressin by osmotic stimuli. J Am Soc Nephrol 19: 1015–1024, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res 51: 372–390, 2001. [DOI] [PubMed] [Google Scholar]
- Bekkevold CM, Robertson KL, Reinhard MK, Battles AH, Rowland NE. Dehydration parameters and standards for laboratory mice. J Am Assoc Lab Anim Sci 52: 233–239, 2013. [PMC free article] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9: 519–531, 2008. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev 81: 1197–1267, 2001. [DOI] [PubMed] [Google Scholar]
- Coffee EM, Tolan DR. Mutations in the promoter region of the aldolase B gene that cause hereditary fructose intolerance. J Inherit Metab Dis 33: 715–725, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle CP, Shires M, McRae C, Crellin D, Fisher J, Carr IM, Markham AF, Hayward BE, Asipu A, Bonthron DT. Both isoforms of ketohexokinase are dispensable for normal growth and development. Physiol Genomics 42A: 235–243, 2010. [DOI] [PubMed] [Google Scholar]
- Earnest DJ, Sladek CD. Circadian vasopressin release from perifused rat suprachiasmatic explants in vitro: effects of acute stimulation. Brain Res 422: 398–402, 1987. [DOI] [PubMed] [Google Scholar]
- García-Arroyo FE, Cristobal M, Arellano-Buendia AS, Osorio H, Tapia E, Soto V, Madero M, Lanaspa M, Roncal-Jimenez CA, Bankir L, Johnson RJ, Sanchez-Lozada LG. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am J Physiol Regul Integr Comp Physiol 311: R57–R65, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg CM, Sladek CD. A compartmentalized, organ-cultured hypothalamo-neurohypophysial system for the study of vasopressin release. Neuroendocrinology 38: 397–402, 1984. [DOI] [PubMed] [Google Scholar]
- Gross PM, Kadekaro M, Sokoloff L, Holcomb HH, Saavedra JM. Alterations of local cerebral glucose utilization during chronic dehydration in rats. Brain Res 330: 329–336, 1985. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, Maclean PS, Jackman MR, Asipu A, Roncal-Jimenez CA, Kosugi T, Rivard CJ, Maruyama S, Rodriguez-Iturbe B, Sanchez-Lozada LG, Bonthron DT, Sautin YY, Johnson RJ. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci U S A 109: 4320–4325, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor JR, Sladek CD. Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J Neurosci 20: 8868–8875, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko BC, Ruepp B, Bohren KM, Gabbay KH, Chung SS. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J Biol Chem 272: 16431–16437, 1997. [DOI] [PubMed] [Google Scholar]
- Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, Tanabe K, Andres-Hernando A, Orlicky DJ, Finol E, Inaba S, Li N, Rivard CJ, Kosugi T, Sanchez-Lozada LG, Petrash JM, Sautin YY, Ejaz AA, Kitagawa W, Garcia GE, Bonthron DT, Asipu A, Diggle CP, Rodriguez-Iturbe B, Nakagawa T, Johnson RJ. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol 25: 2526–2538, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzicky P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES, Diggle CP, Asipu A, Petrash JM, Kosugi T, Maruyama S, Sanchez-Lozada LG, McManaman JL, Bonthron DT, Sautin YY, Johnson RJ. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun 4: 2434, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, Cicerchi C, Inaba S, Le M, Miyazaki M, Glaser J, Correa-Rotter R, Gonzalez MA, Aragon A, Wesseling C, Sanchez-Lozada LG, Johnson RJ. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 86: 294–302, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Vijayaraghavan S, Sladek CD. Simultaneous exposure to ATP and phenylephrine induces a sustained elevation in the intracellular calcium concentration in supraoptic neurons. Am J Physiol Regul Integr Comp Physiol 291: R37–R45, 2006. [DOI] [PubMed] [Google Scholar]
- Stewart L, Hindmarch CC, Qiu J, Tung YC, Yeo GS, Murphy D. Hypothalamic transcriptome plasticity in two rodent species reveals divergent differential gene expression but conserved pathways. J Neuroendocrinol 23: 177–185, 2011. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol 21: 1–32, 1986. [PubMed] [Google Scholar]
- Verbalis JG. How does the brain sense osmolality? J Am Soc Nephrol 18: 3056–3059, 2007. [DOI] [PubMed] [Google Scholar]
- Wolf JP, Nguyen NU, Dumoulin G, Berthelay S. Influence of hypertonic monosaccharide infusions on the release of plasma arginine vasopressin in normal humans. Horm Metab Res 24: 379–383, 1992. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hong Q, Huang Z, Xue P, Lv Y, Fu B, Chen X, Wu D. ALDR enhanced endothelial injury in hyperuricemia screened using SILAC. Cell Physiol Biochem 33: 479–490, 2014. [DOI] [PubMed] [Google Scholar]