A central question relevant to the prevention of falls is: How does the robust control of walking and balance break down during a fall? Previous work has focused on muscle coordination during successful balance recoveries or the kinematics and kinetics of falls. Here, for the first time, we identified differences in the spatial and temporal coordination of muscles among older adults who fell and those who recovered from an unexpected slip.
Keywords: balance, falls, muscle coordination, muscle synergy, neuromechanics
Abstract
How does the robust control of walking and balance break down during a fall? Here, as a first step in identifying the neuromuscular determinants of falls, we tested the hypothesis that falls and recoveries are characterized by differences in neuromuscular responses. Using muscle synergy analysis, conventional onset latencies, and peak activity, we identified differences in muscle coordination between older adults who fell and those who recovered from a laboratory-induced slip. We found that subjects who fell recruited fewer muscle synergies than those who recovered, suggesting a smaller motor repertoire. During slip trials, compared with subjects who recovered, subjects who fell had delayed knee flexor and extensor onset times in the leading/slip leg, as well as different muscle synergy structure involving those muscles. Therefore, the ability to coordinate muscle activity around the knee in a timely manner may be critical to avoiding falls from slips. Unique to subjects who fell during slip trials were greater bilateral (interlimb) muscle activation and the recruitment of a muscle synergy with excessive coactivation. These differences in muscle coordination between subjects who fell and those who recovered could not be explained by differences in gait-related variables at slip onset (i.e., initial motion state) or variations in slip difficulty, suggesting that differences in muscle coordination may reflect differences in neural control of movement rather than biomechanical constraints imposed by perturbation or initial walking mechanics. These results are the first step in determining the causation of falls from the perspective of muscle coordination. They suggest that there may be a neuromuscular basis for falls that could provide new insights into treatment and prevention. Further research comparing the muscle coordination and mechanics of falls and recoveries within subjects is necessary to establish the neuromuscular causation of falls.
NEW & NOTEWORTHY A central question relevant to the prevention of falls is: How does the robust control of walking and balance break down during a fall? Previous work has focused on muscle coordination during successful balance recoveries or the kinematics and kinetics of falls. Here, for the first time, we identified differences in the spatial and temporal coordination of muscles among older adults who fell and those who recovered from an unexpected slip.
disruptions to balance occur on a regular basis during everyday activities. Whether we are bumped while walking along a busy city street or are traversing an icy sidewalk, there are countless situations that can result in a fall. Fortunately, our neuromuscular system is quite adept at responding to these situations, such that young adults actually fall <6% of the time such destabilizing events occur (Heijnen and Rietdyk 2016). However, as we age the function of our neuromuscular system declines (Delbono 2003) and the rate of falls per slip/trip increases to 17–50% (Shigematsu et al. 2008). These falls, which can even occur among unimpaired older adults, result in injury (Fingerhut and Warner 1997), decreased mobility and physical activity (Tinetti and Williams 1998), as well as increased health care costs (Stevens et al. 2006) that are expected to rise to 100 billion dollars by the year 2030 (Houry et al. 2016). Therefore, a central question relevant to the prevention of falls is: What distinguishes those situations when we recover our balance from those when we do not? Specifically, it remains unclear how the robust neuromuscular control of walking and balance breaks down during a fall.
Our gap in knowledge regarding neuromuscular activity during falls may be attributable to the focus placed on corrective actions during successful balance recoveries (Cham and Redfern 2001; Dietz et al. 1986; Eng et al. 1994; Marigold et al. 2003; Nashner 1980; Tang et al. 1998). These studies have provided important insight into the phase dependence (Dietz et al. 1986; Eng et al. 1994; Nashner 1980; Tang and Woollacott 1999), motor circuits (Dietz et al. 1986; Eng et al. 1994; Lam et al. 2003; Schillings et al. 2000), and sequencing of appropriate motor actions within (Cham and Redfern 2001; Ferber et al. 2002; Tang et al. 1998) and between (Eng et al. 1994; Marigold et al. 2003; Moyer et al. 2009; Tang et al. 1998) legs during successful balance recoveries. However, given the absence of falls in these studies it remains unclear if or how the features of successful balance responses break down during a fall. For example, do the timing and magnitude of knee muscle activity, which have been implicated in successful recoveries (Chambers and Cham 2007; Ferber et al. 2002), deteriorate during falls?
Only a small number of studies have used experimental paradigms that feature mechanical perturbations of sufficient magnitude to reproduce falls in a laboratory setting (Cham and Redfern 2001; Lockhart et al. 2002; Pavol et al. 1999; Weerdesteyn et al. 2012). These studies have focused on mechanical causes of falls (i.e., inefficient vertical support, loss of stability) (Lockhart et al. 2003; Pai et al. 2006; Pavol et al. 2001), overlooking their neuromuscular basis. The distinction between mechanical and neuromuscular causes of falls may be critical to mitigating their incidence given the abundance of neuromuscular solutions for the same mechanical outcome (Latash 2012; Ting et al. 2015). Thus the neuromuscular basis of falls may provide more personalized approaches to balance rehabilitation. A logical first step in testing this proposition is to determine how the neuromuscular control of walking and balance breaks down when a fall is actually occurring, that is, in the “failed state.”
Muscle synergy analysis is a useful framework for visualizing, characterizing, and interpreting differences in neuromuscular coordination among multiple muscles (Lacquaniti et al. 2012; Ting et al. 2015; Tresch and Jarc 2009). Muscle synergies are groups of coactive muscles with a fixed spatial structure and time-varying temporal components that can be extracted from electromyographic (EMG) signals with a variety of decomposition techniques (Tresch et al. 2006). Muscle synergies are organized around producing the biomechanical functions (e.g., propulsion, stability, body weight support) required to perform motor behaviors (e.g., walking) (Lee 1984; Neptune et al. 2009; Ting et al. 2015) across a variety of contexts (Chvatal and Ting 2013; d'Avella and Bizzi 2005). Owing to this link between muscle synergies and biomechanical output, a decrease in muscle synergy number has been interpreted as a reduction in the complexity of neuromuscular control (Clark et al. 2010; Dominici et al. 2011; Roemmich et al. 2014). It is generally believed, although not universally accepted (Kutch et al. 2008; Tresch and Jarc 2009), that muscle synergies reflect underlying neural mechanisms for coordinating movement and may be expressed via motor circuits in the cortex (Asavasopon et al. 2014; Overduin et al. 2012), brain stem (Chvatal et al. 2013; Roh et al. 2011), and/or spinal cord (Hart 2004; Tresch et al. 1999). To date, muscle synergy analysis of locomotor balance control has been limited to successfully maintaining balance while traversing challenging terrain (Martino et al. 2015; Sawers et al. 2015) and recovering from modest mechanical perturbations (Chvatal and Ting 2012; Oliveira et al. 2012). Modifications to the temporal activation of muscle synergies appear to govern the successful performance of these activities (Chvatal and Ting 2012; Martino et al. 2015; Oliveira et al. 2012), with variations in spatial coordination and muscle synergy number limited to differences in skill level (Sawers et al. 2015). Additionally, it has been suggested, but not tested, that successful balance responses are mediated by greater independent control of each leg (Chvatal and Ting 2012; Martino et al. 2015; Oliveira et al. 2012). More independent leg coordination may ensure that the biomechanical functions necessary to preserve stability and body weight support are achieved when the motor function of one of the legs is interrupted. However, as with the activation of individual muscles, it remains unclear if or how features of multimuscle coordination, such as the number of muscle synergies or coordination within and between legs, differ during falls vs. recoveries.
The purpose of this study was therefore to examine differences in muscle coordination between individuals who fell and those who recovered with muscle synergy analysis as well as conventional muscle onset latencies and peak activity. We hypothesized that falls and recoveries are characterized by differences in neuromuscular control. Specifically, during an unexpected mechanically induced slip while walking we compared 1) the number and composition of muscle synergies and 2) the onset latencies and peak activity between a group of older adults who fell and a group who recovered. We predicted that subjects who fell would 1) use fewer muscle synergies, suggesting a reduction in the complexity of neuromuscular control, 2) use muscle synergies different from those who recovered, implying differences in the execution of the neuromuscular response, and 3) have longer onset latencies, signifying a delay in the neuromuscular response.
METHODS
Participant Recruitment
Twenty-eight community-living older adults, 15 who fell and 13 who recovered their balance after an unexpected mechanically induced slip to their right leg, were selected from a set of >100 participants described in a previous study (Pai et al. 2014), all of whom had EMG recorded. Our sample was selected on the basis of three criteria: 1) slip type, 2) quality of EMG signals, and 3) clear designation of fall or recovery. For this study only participants who experienced a “split” slip with the slipping and trailing feet traveling apart were included. “Feet-forward” slips with both feet moving forward (Yang et al. 2012) were excluded from the present study. Participants whose EMG included artifacts due to motion or other sources were excluded, as were those individuals whose recovery was assisted by the harness worn by all participants (i.e., “harness-assisted recovery”). All protocols were approved by the Institutional Review Board at the University of Illinois at Chicago, and stated in the consent form, and all participants gave informed consent to participate.
Experimental Procedures
Subjects were instructed to walk along a 7-m walkway at a self-selected speed. An unexpected slip occurred after several unperturbed walking trials. Slips during walking were induced with a pair of side-by-side, low-friction, movable platforms embedded near the middle of the walkway (Yang and Pai 2007) that were firmly locked in place during regular walking trials. Slip onset occurred when the movable platforms were unlocked by a computer-controlled release mechanism after force plates (AMTI, Newton, MA) installed beneath them detected the ratio of the horizontal to vertical ground reaction force exceeding a preset threshold, comparable to a low coefficient of friction of 0.02 (Bhatt et al. 2005). During a slip trial the platforms were allowed to slide freely for up to 90 cm in the forward direction after their release (Pai et al. 2014). A full-body safety harness and shock-absorbing suspension ropes connected through a load cell to an overhead trolley on a track over the walkway were worn by all subjects to protect them from any harmful body impact with the floor surface.
Subjects were informed that they “may or may not be slipped” at any time and that if a slip occurred they should “try to recover” and “continue to walk.” None of them was told when, where, or how they might slip. All slips were unannounced in order to mimic real-life slips. The present study only included that first novel slip trial, to reveal their original unadapted motor behavior and avoid predictive feedforward modifications that might be present in subsequent trials. Importantly, all nonslip trials were completed before the first unannounced slip trial, preventing exposure to a fall from affecting nonslip walking muscle patterns.
Data Collection and Processing
Marker coordinate data were collected at 120 Hz with an eight-camera motion capture system (Motion Analysis, Santa Rosa, CA) and a 24-marker set (Bhatt et al. 2012). Marker trajectories were low-pass filtered at marker-specific cutoff frequencies (ranging from 4.5 to 9 Hz) with fourth-order, zero-lag Butterworth filters (Winter 2009). Whole body center of mass (CoM) position and velocity were computed from the filtered marker coordinate data with known sex-dependent segmental parameters for a 13-segment whole body model (de Leva 1996; Pavol and Pai 2002).
Force data and surface EMG activity from four muscles on each leg—the tibialis anterior (TA), medial gastrocnemius (MGAS), vastus lateralis (VLAT), and biceps femoris long head (BFLH)—were recorded at 600 Hz, adequate for capturing the majority of the EMG signal of interest (20–200 Hz) (Winter 2009). Raw unrectified EMG signals from silver-silver chloride electrodes with built-in preamplifiers (gain 35×) (E Q Inc, Chalfont, PA) were filtered with a built-in fourth-order low-pass Bessel filter with a 300-Hz cutoff frequency and amplified (prefilter gain = 1×, postfilter gain = 100×) with a CyberAmp 380 amplifier (Axon Instruments, Union City, CA). With custom MATLAB (MathWorks, Natick, MA) routines, EMG signals were digitally band-pass filtered at 10–200 Hz after data collection and then full-wave rectified and low pass-filtered with a second-order dual-pass Butterworth filter with a 50-Hz cutoff frequency (Grasso et al. 1998). Force plate, load cell, and platform trigger-release onset signals were synchronized with EMG and marker coordinate data. With custom MATLAB (MathWorks) routines, ground reaction and load cell force data were low-pass filtered with a fourth-order Butterworth filter with 25- and 6-Hz cutoff frequencies, respectively (Yang and Pai 2011). Filtered vertical ground reaction force data were used to identify gait events (touchdown and toe-off), while filtered force data from the load cell were used to classify slip outcomes.
Subject-specific EMG data matrices to be used in muscle synergy extraction were generated for each walking condition. First, the EMG data were downsampled by averaging the data in 35-ms time bins (Chvatal and Ting 2012). Next, EMG data from each trial were concatenated, not averaged (Oliveira et al. 2014), to create matrices that were 8 (number of muscles) × n (number of time bins) in size. For each subject three nonslip trials (average of 6–8 gait cycles excluding gait initiation and termination) and one slip trial were used. No time normalization procedures were performed on the EMG data. Therefore, the size of the data matrices (i.e., the length of n) varied between participants. The EMG data matrices for slip trials only included EMG occurring between the right heel strike immediately preceding the slip and the time of the fall or the end of the recovery. No additional walking data before the slip or after the fall/recovery were included. A single trial is not ideal for muscle synergy analysis (Oliveira et al. 2014; Shuman et al. 2016); it was warranted in the present study to avoid predictive feedforward modifications in neuromuscular control during subsequent slip trials that would obscure interpretation of the results. The EMG matrices were then normalized to the maximum activation during nonslip walking trials, and each row (i.e., muscle vector) was scaled to have unit variance to ensure that equal weighting was applied to each muscle in the subsequent extraction process. This unit variance was removed after muscle synergy extraction to restore the original scaling and enable comparison of muscle synergies (Torres-Oviedo and Ting 2007).
Data Analysis
Slip outcome: fall vs. recovery.
Each slip trial was classified as a fall or a recovery on the basis of a force criterion from the load cell inline with the overhead safety harness system. The outcome of a slip was classified as a fall if the peak force in the load cell during the slip trial exceeded 30% of the subject's body weight (Yang and Pai 2011). The time point when the load cell force exceeded 30% of body weight was chosen as the end of the fall period (solid black line in Fig. 1A). Falls were confirmed by a loss of vertical hip height (Pai et al. 2006), attenuation of forward (positive) CoM velocity, and visual inspection of recorded videos. The outcome of a slip was classified as a recovery if the moving average of force on the harness did not exceed 4.5% of body weight over any 1-s period after slip onset (Pavol and Pai 2002). Given the differences between falls and recoveries, and owing to the lack of a force-based criterion similar to that used for defining the end of fall trials, the end of recovery trials was defined by a different criterion: the attenuation of a loss of forward velocity (i.e., local minimum in anterior-posterior CoM velocity after slip onset) (solid black line in Fig. 1B). While this results in the slip times for falls and recoveries being based on different termination criteria, we believe that they reflect a fair assessment of each slip outcome. With these criteria, the time window for a fall was defined as slip onset to harness loading threshold (i.e., >30% body weight), while the time window for a recovery was defined as slip onset to velocity attenuation (Fig. 1).
Fig. 1.
Example electromyography (EMG) and whole body responses during slip trials for 1 subject who fell (A; red color) and 1 who recovered (B; gray color). Note the difference in scales on the y-axis between some plots as well as the offset of the left EMG traces for visualization purposes (scaling was maintained between right and left signals). EMG recordings of the left (light color) and right (dark color) muscles during a slip trial illustrate increased coactivation in the subject who fell. Whole body responses were used to classify slip outcomes as falls or recoveries and establish their time course. The harness load cell recording was the primary indicator of fall vs. recovery. If the force recorded by the load cell after slip onset exceeded 30% of the subject's body weight, the trial was considered a fall. The time point when the load cell force exceeded 30% of body weight was selected as the “time of the fall” (second solid black line in example fall subject). Falls were confirmed by a loss of vertical hip height and visual inspection of recorded videos. Slip trials were classified as recoveries if the moving average of force on the harness did not exceed 4.5% of body weight over any 1-s period after slip onset. The attenuation of a loss of forward velocity (local minimum in anterior-posterior CoM velocity after slip onset) was defined as the end of the recovery (second solid black line in example recovery subject). Note the 2 consecutive left heel strikes in A suggesting repeated, yet unsuccessful, attempts to reestablish the posterior base of support and avoid a backward loss of balance.
Gait- and slip-related variables.
On the basis of previous reports of their potential influence on slip outcome, three gait- and three slip-related variables were calculated. Gait speed was calculated as the average velocity of the CoM across three unperturbed nonslip walking trials. Shank angle at slip onset was calculated as the angle between the shank and the ground at heel strike immediately before slip onset and was defined by the ankle and knee marker positions in the sagittal plane (Brady et al. 2000). Whole body stability was calculated at the instant of heel strike immediately before slip onset as the shortest perpendicular distance from the CoM motion state (i.e., its horizontal position and velocity relative to the base of support) (Eq. 1) to previously published threshold values for backward loss of balance under slip conditions (Pai and Patton 1997). Negative stability values indicate an unstable state, while positive stability values indicate a stable state against a loss of balance.
| (1) |
Peak slip velocity was identified as the first peak in the horizontal velocity of the right (slipping) heel marker after its touchdown (Lockhart et al. 2003; Strandberg 1983). Slip distance was calculated at the distance traveled by the heel marker on the slipping foot (right) along the floor from heel strike to a stable zero velocity (Grönqvist et al. 2001). The gait- and slip-related variables were compared between the group of subjects who fell and the group who recovered with one-sided independent t-tests (σ = 0.05).
Individual muscle onset latencies and peak activity.
EMG onset latencies and peak activity were calculated from full-wave rectified, low-pass filtered (50 Hz) EMG signals (Chambers and Cham 2007). Onset latency was identified as the time between slip onset and the end of a recovery or fall when the slip EMG signal exceeded 2 standard deviations (SDs) of the ensemble average of the nonslip walking signal for a minimum of 30 ms (Marigold et al. 2003; O'Connell et al. 2016). EMG peak activity during slip trials was identified as the local maximum of the slip EMG signal normalized to the maximum activation during nonslip walking trials between slip onset and fall or recovery. Onset latencies and peak activity were calculated with custom MATLAB (MathWorks) routines and verified by hand. To test our prediction that subjects who fell would have delayed and reduced activation patterns, differences in muscle onset latencies and peak activity between subjects who fell and those who recovered during slip trials were tested with separate multivariate (M)ANOVAs (σ = 0.05) and Cohen's d mean difference effect sizes adjusted for unequal sample sizes.
Muscle synergy extraction and analysis.
Muscle synergies were extracted from the subject- and condition-specific bilateral EMG data matrices with nonnegative matrix factorization (NNMF) (Lee and Seung 1999), a decomposition algorithm used extensively in muscle synergy analysis (Tresch et al. 2006). NNMF extracts muscle synergies with the assumption that the recorded muscle activity, M, is the product of a small number of spatial components, Wi, that are each activated by a temporal recruitment coefficient, ci. According to this model, a particular muscle activation pattern M would be characterized by
| (2) |
where Wi indicates the relative contributions of each muscle in muscle synergy i. An important feature of this model is that the spatial components (Wi) are considered fixed time-invariant patterns while the temporal activation coefficients (ci) vary over time (Torres-Oviedo and Ting 2007).
Beginning with random initial estimates of W and c, NNMF was performed as an iterative optimization process to minimize the sum of squared error between the muscle activation patterns M reconstructed by W × c and the original muscle signals. To determine the number of muscle synergies needed to minimize the sum of the squared error in slip and nonslip conditions we extracted 1–8 muscle synergies. The goodness of fit of the original EMG data compared with the reconstructed EMG for each number of muscle synergies was quantified by the variance accounted for (VAF). To ensure consistency in selecting the number of muscle synergies within each condition, 95% confidence intervals (CIs) were calculated for the VAF of the reconstructed EMG at each muscle synergy number (1–8) (Cheung et al. 2009). This was accomplished by implementing a bootstrapping procedure in which the EMG data sets were resampled 500 times with replacements and the VAF of the reconstructed EMG was recalculated after each resampling. Ninety-five percent CIs were then constructed from the bootstrapped VAF values at each muscle synergy number, and the number of muscle synergies was selected as the minimum number at which the lower bound of the 95% CI exceeded 90% VAF (Cheung et al. 2009). To test our prediction that subjects who fell would use fewer muscle synergies, the number of muscle synergies extracted from slip and nonslip trial EMG were compared between slip outcomes with one-sided independent t-tests (σ = 0.05) and Cohen's d mean difference effect sizes adjusted for unequal sample sizes.
As a complement to the number of muscle synergies, the dynamic motor control index during slip trials (slip-DMC) (Steele et al. 2015) was calculated as a metric of muscle activity complexity for slip responses (Eq. 3). In contrast to its initial application, characterizing differences in the complexity of muscle activity among children with cerebral palsy with respect to unimpaired children, here it was used to examine differences in the complexity of muscle activity between slip outcomes with respect to nonslip responses. For each subject's slip trial, slip-DMC was calculated as
| (3) |
where VAF1 is the variance accounted for when limiting the decomposition of EMG data to a single muscle synergy from a single participant during a slip trial while (1 − VAF1)AVG and (1 − VAF1)SD are the average and SD of the variance unaccounted for when limiting the decomposition of EMG data to a single muscle synergy from the nonslip trials of all 28 subjects. This formulation presents the slip-DMC score as a z score, where 100 equals the average slip-DMC of nonslip walking trials and every 10-point change is equal to 1 SD. Therefore, a slip-DMC score < 100 denotes a simplified muscle activation pattern during a slip response, where a single synergy describes a larger percentage of the variance in muscle activity compared with nonslip trials (Steele et al. 2015). The presentation of slip-DMC as a z score facilitates comparison to other populations or conditions in future studies. While alternative approaches to the calculation of slip-DMC were explored, such as excluding the nonslip data of the subject for whom slip-DMC was being calculated to avoid potential correlations in the data, these modifications had no significant impact on the outcome and deviated from the methods for calculating a z score. Therefore, we elected to retain and use the original formulation for calculating slip-DMC. To test our prediction that the group of subjects who fell would have less complex neuromuscular control during slip trials with respect to nonslip trials, differences in slip-DMC scores between subjects who fell and those who recovered were compared with a one-sided independent t-test (σ = 0.05). Cohen's d mean difference effect size adjusted for unequal sample sizes was also calculated.
Intra- and interlimb muscle coordination patterns were identified for each subject during slip and nonslip trials by classifying muscle contributions within each muscle synergy as significantly or not significantly active. Significantly active muscles were computed by establishing 95% CIs for the contribution, i.e., the values of the elements wij, to each muscle i in each muscle synergy j extracted from 500 bootstrapped versions of the subject-specific EMG matrices. In the case of the slip trials, because we only had a single trial, the CIs may have been overly conservative. Significantly active muscles were considered those whose CI did not include 0 (Hayes et al. 2014) (see Fig. 5A). Muscle synergies were classified as intralimb if they contained contributions from either significantly active right or left leg muscles. Interlimb muscle synergies were those that received contributions from right and left significantly active muscles. To test our prediction that subjects who fell would use different muscle synergies, and specifically that they would use a larger proportion of interlimb muscle synergies, differences in the percentage of interlimb muscle synergies during slip and nonslip trials were compared between slip outcomes with one-sided independent t-tests (σ = 0.05) and Cohen's d mean difference effect sizes adjusted for unequal sample sizes.
Fig. 5.
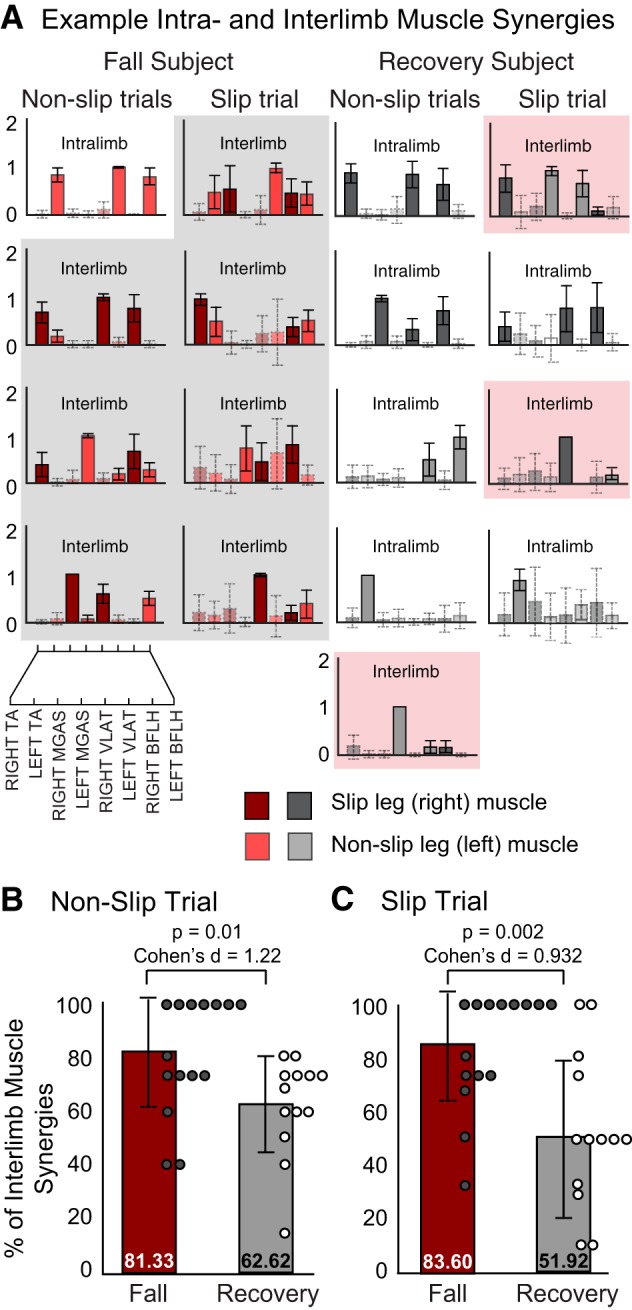
Example intra- and interlimb muscle synergies during slip and nonslip trials. A: example fall and recovery muscle synergies with 95% confidence intervals (CIs). Muscle contributions with 95% CI that did not include 0 were considered significantly active muscles (filled, solid borders). Those that included 0 were considered not significantly active (transparent, dashed borders). Muscle synergies were classified as intralimb if they contained contributions from either significantly active right or left leg muscles. Interlimb muscle synergies were those that received contributions from right and left significantly active muscles. B: subjects who fell during the slip trial (red bar) used significantly larger % of interlimb muscle synergies during nonslip trials than subjects who recovered (gray bar). Average % of interlimb muscle synergies per subject is denoted by circles (filled circles, fall; open circles, recovery). C: subjects who fell during the slip trial (red bar) used significantly larger % of interlimb muscle synergies during the slip trial than subjects who recovered (gray bar). TA, tibialis anterior; MGAS, medial gastrocnemius; VLAT, vastus lateralis; BFLH, biceps femoris long head.
To facilitate the comparison of muscle coordination patterns between slip outcomes, muscle synergies extracted from subjects who fell and those who recovered were pooled across subjects and grouped with a hierarchical cluster analysis. MATLAB statistics-toolbox functions pdist (Minkowski option; P = 3), linkage (ward option), and cluster were applied to the pooled muscle synergy matrices for falls and recoveries. pdist computes the Euclidean distance between muscle synergy vectors; linkage determines the proximity (similarity) of the muscle synergy vectors to each other; and cluster groups similar muscle synergy vectors according to a proximity threshold. The number of clusters for each slip outcome (i.e., fall and recovery) was determined by identifying the minimum number of clusters that partitioned the muscle synergies such that no cluster contained more than one muscle synergy from the same subject (Cheung et al. 2005). To identify fall and recovery specific patterns the correlation coefficient (r) was calculated between average muscle synergy vectors from the fall and recovery clusters. A pair of muscle synergies across slip outcomes were considered similar if they had r > 0.834. This cutoff point was selected because it corresponds to the critical value of r2 for eight muscles (6 degrees of freedom) that represents statistically significant similarity (r2 = 0.834; P = 0.01) (Portney and Watkins 2013). The same cluster and correlation analyses were performed on muscle synergies extracted from nonslip trials. The assumption of normality in the data was confirmed with a Shapiro-Wilk test (P > 0.05). All statistical analyses were conducted with SPSS (version 23; SPSS, Chicago, IL).
RESULTS
Participants
There was no statistically significant difference between subjects who fell and those who recovered in age (mean ± 1 SD) [fall: 71 ± 2 yr, recovery: 72 ± 5 yr; t(26) = −1.02, P = 0.32], height [fall: 1.66 ± 0.07 m, recovery: 1.73 ± 0.10 m; t(26) = −2.08, P = 0.05], or weight [fall: 78.1 ± 9.45 kg, recovery 80.1 ± 10.14 kg; t(26) = −0.32, P = 0.75]. Thirteen of the 15 participants who fell and 5 of the 13 participants who recovered were women, consistent with previous research that women are more likely than men to fall after a perturbation (Pavol et al. 1999).
Initial Motion State and Most Slip-Related Variables Did Not Differ Between Subjects Who Fell and Those Who Recovered During Slip Trials
There was no statistically significant difference between the fall and recovery groups in slip duration [t(26) = 0.88, P = 0.19] or peak slip velocity [t(26) = 0.7, P = 0.24]. However, slip distance was significantly greater during falls than during recoveries [t(26) = 3.17, P < 0.01] (Table 1). Among gait-related variables there was no statistically significant difference between the fall and recovery groups in whole body stability [t(26) = 0.81, P = 0.21], walking speed [t(26) = 1.95, P = 0.05], or shank angle at slip onset [t(26) = 0.5, P = 0.31] (Table 1).
Table 1.
Slip- and gait-related variables
| Slip-Related Variables |
Gait-Related Variables at Slip Onset |
|||||
|---|---|---|---|---|---|---|
| Cohort | Peak slip velocity, m/s | Slip duration, s | Slip distance, m | Shank angle, ° | Dynamic stability | Walking speed, m/s |
| Fall (n = 15) | 2.00 (0.58) | 0.82 (0.34) | 0.78 (0.10)* | 74 (5.6) | −0.18 (0.05) | 0.89 (0.17) |
| Recover (n = 13) | 1.84 (0.59) | 0.94 (0.35) | 0.61 (0.14) | 73 (4.0) | −0.16 (0.03) | 1.00 (0.13) |
Values are means (SD). Slip duration, time between slip onset and fall or recovery.
P ≤ 0.01.
EMG Onset Latencies But Not Magnitudes Differed Between Subjects Who Fell and Those Who Recovered During Slip Trials
Consistent with our expectation, muscle onset latency was longer among subjects who fell compared with those who recovered during slip trials [F(8,19) = 3.08, P = 0.021; Wilk's λ = 0.436]. Fallers had significantly longer onset latencies for two muscles in the leading/slip leg: BFLH [F(1,26) = 8.16; P = 0.008; Cohen's d = 1.09] and VLAT [F(1,26) = 10.9; P = 0.003; Cohen's d = 1.24] (Table 2). No differences were found in peak muscle activity between slip outcomes [F(8, 19) = 0.288, P = 0.96; Wilk's λ = 0.892] (Table 3).
Table 2.
Mean EMG onset latency in response to unexpected slip
| TA |
MGAS |
VLAT |
BFLH |
|||||
|---|---|---|---|---|---|---|---|---|
| Cohort | Right (S) | Left (NS) | Right (S) | Left (NS) | Right (S) | Left (NS) | Right (S) | Left (NS) |
| Fallers (n = 15) | 173 (48.5) | 162 (28.3) | 234 (54.8) | 215 (40.2) | 239 (43.0)* | 165 (29.6) | 170 (61.3)* | 150 (20.1) |
| Nonfallers (n = 13) | 151 (29.8) | 157 (35.0) | 232 (45.2) | 198 (46.7) | 186 (40.7) | 154 (26.5) | 120 (16.1) | 155 (40.5) |
Values (in ms) are means (SD). TA, tibialis anterior; MGAS, medial gastrocnemius; VLAT, vastus lateralis; BFLH, biceps femoris long head; S, slip leg; NS, nonslip leg.
P ≤ 0.01.
Table 3.
Mean EMG peak magnitude in response to unexpected slip
| TA |
MGAS |
VLAT |
BFLH |
|||||
|---|---|---|---|---|---|---|---|---|
| Cohort | Right (S) | Left (NS) | Right (S) | Left (NS) | Right (S) | Left (NS) | Right (S) | Left (NS) |
| Fallers (n = 15) | 2.30 (0.64) | 2.86 (1.27) | 2.32 (0.96) | 1.64 (0.96) | 2.09 (1.01) | 4.75 (2.42) | 3.45 (1.91) | 3.75 (2.21) |
| Nonfallers (n = 13) | 2.28 (0.77) | 2.58 (0.95) | 2.32 (0.97) | 1.87 (1.10) | 2.36 (0.89) | 3.56 (1.22) | 3.87 (2.75) | 3.22 (2.11) |
Values are means (SD). TA, tibialis anterior; MGAS, medial gastrocnemius; VLAT, vastus lateralis; BFLH, biceps femoris long head; S, slip leg; NS, nonslip leg.
Individuals Who Fell During Slip Trials Had a Smaller Repertoire of Muscle Synergies than Individuals Who Recovered
During slip trials, subjects who fell used fewer muscle synergies than those who recovered [fall: 3.7 ± 0.9, range: 3–6; recovery: 4.7 ± 1.0, range: 4–6; t(26) = 2.83, P < 0.01]. Furthermore, Cohen's effect size value (d = 1.06) suggested a large practical significance in muscle synergy number between slip outcomes (Fig. 2). The number of muscle synergies was also significantly smaller during nonslip trials for subjects who fell compared with those who recovered during slip trials [fall: 4.4 ± 0.5, range: 4–5; recover: 5.0 ± 0.8, range: 4–6; t(26) = 2.17, P = 0.02]. Again, Cohen's effect size value (d = 0.915) suggested a large practical significance in muscle synergy number between groups during nonslip trials. Finally, during slip trials slip-DMC scores were significantly lower among subjects who fell compared with those who recovered [fall: 66.9 ± 16.8, recovery: 84.8 ± 14.4; t(26) = 2.99, P < 0.001]. Cohen's effect size value (d = 1.13) suggested that there was a large practical significance to the difference in slip-DMC scores between slip outcomes.
Fig. 2.

Number of muscle synergies during slip and nonslip trials for each slip outcome as well as slip-DMC (dynamic motor control) index. A: the number of muscle synergies (means ± SD) during nonslip trials was significantly greater among subjects who recovered (gray bar) compared with those who fell (red bar). Circles denote the average number of muscle synergies per subject (filled circles, fall group; open circles, recovery group). B, left: the number of muscle synergies (means ± SD) during slip trials was significantly greater among subjects who recovered compared with those who fell. Right: Slip-DMC scores were significantly greater for subjects who recovered compared with those who fell. Note that the values of data points > 100 are labeled.
Nonslip Trial Muscle Synergies Had Similar Spatial Structure and Temporal Activation Between Groups
Cluster analysis of the nonslip muscle synergies for each group identified repertoires of six and seven muscle synergies among the fall and recovery groups, respectively (Fig. 3). Correlation analysis between the fall and recovery group cluster average muscle synergies revealed a pool of six muscle synergies (W1–6 Walk) that were similar (r > 0.834) between the two groups. W1 and W4 were composed of ipsilateral TA, VLAT, and BFLH activity in the right and left legs, respectively. W3 and W5 consisted mainly of MGAS and contralateral BFLH activity from the right and left leg, respectively, W2 consisted of additional left TA activity, while W6 was composed of bilateral muscles, but mainly the left BFLH. The lone nonslip muscle synergy specific to the recovery group (W7) was composed mainly of right TA and BFLH activity. Minimal differences were observed in the temporal activation of the muscle synergies between the fall and recovery groups during nonslip trials (Fig. 3).
Fig. 3.
Nonslip walk cluster averaged muscle synergies (W Walk). Cluster analysis of nonslip muscle synergies identified repertoires of 6 and 7 muscle synergies in the fall (red bars) and recovery (gray bars) groups, respectively. Six of these muscle synergies had similar spatial structure (r > 0.834) between the 2 groups (W1–6 Walk). Error bars are 1 SD, darker shade bars are right/slip leg muscles, and lighter shade bars are left/nonslip leg muscles. The % values adjacent to each muscle synergy indicate % of subjects in each group who were found to recruit that muscle synergy. The average (mean ± 1 SD) temporal activation coefficients (fall group: red, dashed line; recovery group: black, solid line) for the 6 common muscle synergies were also similar between the fall (red) and recovery (black) groups. TA, tibialis anterior; MGAS, medial gastrocnemius; VLAT, vastus lateralis; BFLH, biceps femoris long head.
Differences in Muscle Synergy Spatial Structure and Temporal Activation Were Observed Between Subjects Who Fell and Those Who Recovered
Cluster analysis of the slip trial muscle synergies for each group identified repertoires of 7 and 11 muscle synergies in the fall and recovery groups, respectively (Fig. 4). Correlation analysis between the fall and recovery cluster average muscle synergies revealed a pool of five muscle synergies (W1–5) that were similar (r > 0.834) between the two groups (i.e., outcome independent). Among the two muscle synergies exclusive to the fall group, 80% of the fallers recruited an “all-on” muscle synergy (W6 Fall) characterized by extensive coactivity across all (bilateral) muscles. The second fall-only muscle synergy was used by half of the subjects who fell (53%) and consisted primarily of unilateral nonslip leg BFLH activity (W7 Fall), along with small contributions from many of the other muscles. The six recovery-specific muscle synergies (W6–11 Recovery) consisted of patterns composed of ankle and knee muscles. Several differences were observed in the temporal activation of muscle synergies that were common to falls and recoveries. Subjects who fell and recruited muscle synergy W1 did so with greater activation magnitude than subjects who recovered. Additionally, subjects who recovered and recruited muscle synergy W4 tended to do so with a shorter latency after slip onset than subjects who fell. Finally, W5 was recruited with greater activation magnitude among subjects who recovered compared with those who fell. No other differences were observed in the temporal activation of the remaining muscle synergies (W2, W3) common to both subjects who fell and those who recovered (Fig. 4).
Fig. 4.
Slip fall and recovery cluster averaged muscle synergies (W Fall, W Recovery). Cluster analysis of slip trial muscle synergies identified repertoires of 7 and 11 muscle synergies in the fall (red bars) and recovery (gray bars) groups, respectively. Five of these muscle synergies had similar spatial structure (r > 0.834) between the 2 groups (W1–5 Slip). Error bars are 1 SD, darker shade bars are right/slip leg muscles, and lighter shade bars are left/nonslip leg muscles. The % values adjacent to each muscle synergy indicate % of subjects in each group who were found to recruit that muscle synergy. The average (mean ± 1 SD) temporal activation coefficients (fall group: red, dashed line; recovery group: black, solid line) for the 5 common muscle synergies differed for W1 (larger magnitude in fall group) and W4 (earlier onset in recovery group) but were similar for the remaining 3. TA, tibialis anterior; MGAS, medial gastrocnemius; VLAT, vastus lateralis; BFLH, biceps femoris long head.
Subjects Who Fell Used a Larger Proportion of Interlimb Muscle Synergies than Subjects Who Recovered
Confirming our prediction that subjects who fell would use a larger proportion of interlimb muscle synergies, the percentage of interlimb muscle synergies was higher among subjects who fell during slip trials [fall: 83.60 ± 21.36, recover: 51.92 ± 30.64; t(26) = 3.21, P = 0.002] as well as during nonslip trials [fall: 81.33 ± 21.50, recover: 62.62 ± 18.28; t(26) = 2.46, P = 0.01] compared with subjects who recovered. Cohen's effect size values (slip trial: d = 0.932, nonslip trials: d = 1.216) indicated that there was a large practical significance in the proportion of inter- vs. intralimb muscle synergies between groups during slip and nonslip trials.
DISCUSSION
The present study is the first step to understanding the causation of falls from the perspective of muscle coordination among community-living older adults who experienced a severe lifelike slip in a laboratory setting. The differences in muscle coordination between subjects who fell and those who recovered could not be explained by gait-related variables (i.e., initial motion state) at slip onset or variations in slip difficulty. Therefore, they may reflect differences in the neural control of movement but may also be due to differences in movement mechanics during the slip. The differences observed in muscle coordination between subjects who fell and those who recovered were characterized by less complex neuromuscular control (i.e., fewer muscle synergies during slip and nonslip walking as well as lower slip-DMC relative to nonslip walking), delayed temporal coordination of knee flexors and extensors as well as excessive muscle coactivation during slip trials, and increased interlimb muscle coordination during both slip and nonslip trials. These findings shed new light on the potential causation of falls from the perspective of neuromuscular control, and may inform novel and focused approaches to balance rehabilitation with further study. Additional research comparing the muscle coordination and mechanics of falls and recoveries within the same subject(s) is necessary to establish whether these results reflect neuromuscular determinants of falls.
Differences in Slip Outcome and Muscle Coordination Could Not Be Accounted For By Differences in Slip Difficulty or Initial Motion State
The differences in muscle coordination between subjects who fell and those who recovered may reflect differences in neuromuscular control rather than mechanical constraints imposed by the perturbation or walking mechanics at slip onset. This assertion is based on two pieces of evidence. First, both groups' slip velocity and duration were similar and sufficient to cause a hazardous slip (Chambers and Cham 2007) or a fall (Perkins and Wilson 1983) (Table 1). While slip velocity was lower among individuals who fell in this study compared with previously reported values (Brady et al. 2000), individuals in this study were markedly older (71.5 yr vs. 26.6 yr), potentially lowering the threshold for a fall. Additionally, that previous study (Brady et al. 2000) did not report slip type (i.e., “split slip vs. feet-forward slip”). Although slip distance was greater among individuals who fell, values in both groups exceeded those that previously predicted falls after a slip (Brady et al. 2000). Thus it would appear that differences in slip outcome and associated muscle coordination patterns cannot be attributed to perturbations that were insufficiently challenging and unlikely to result in a fall. Second, before slip onset, gait-related variables thought to dictate slip outcome did not differ between groups. Shank angle (Brady et al. 2000), dynamic stability (Bhatt et al. 2011) at slip onset, and walking speed (Bhatt et al. 2005) have all been reported as determinants of slip outcome. Here there were no differences in any of these variables between individuals who fell and those who recovered (Table 1). Therefore, in this study differences in muscle coordination between falls and recoveries cannot be attributed to differences in the motion state at slip onset. Owing to the similarity in slip type (i.e., split slips), slip difficulty, and gait pattern at slip onset, our results suggest that the falls observed in this study may have arisen from differences in neuromuscular control rather than mechanical constraints imposed by locomotor or perturbation mechanics. However, additional consideration of the kinematics and kinetics during slip responses in future studies is required to further clarify the mechanical vs. neuromuscular basis for these differences in muscle coordination.
A Larger Repertoire of Muscle Synergies May Be Required to Recover from a Fall
While studies of sensorimotor impairment (Clark et al. 2010; Fox et al. 2013; Rodriguez et al. 2013; Steele et al. 2015) and long-term training (Sawers et al. 2015) have related increases and decreases in neuromuscular complexity to deficits and expertise in walking and balance performance, respectively, ours is the first demonstration that falls in response to a slip are characterized by fewer muscle synergies and thus lower complexity of neuromuscular control. Prior studies have used muscle synergy analysis to examine differences in the complexity of neuromuscular control between perturbed and nonperturbed gait (Chvatal and Ting 2012; Oliveira et al. 2012). However, because the perturbations in those studies were not designed to evoke falls they were unable to answer a fundamental question: Is there a loss of neuromuscular complexity during falls? As reflected in the number of muscle synergies per subject (Fig. 2) as well as the size of the “group motor repertoires” (Fig. 3 and Fig. 4), the group of individuals who fell during slip trials had a smaller repertoire of muscle synergies than those individuals who recovered. Collectively, these differences in muscle synergy number suggest less complexity in neuromuscular control during falls compared with recoveries. This interpretation is strengthened by lower slip-DMC scores, a measure of muscle activity complexity (Steele et al. 2015), among individuals who fell during the slip trial (Fig. 2). Subjects who fell had an average slip-DMC score that was >30 points or 3 SDs below 100. In contrast, slip-DMC was only 15 points or 1.5 SDs below 100 among individuals who recovered during the slip trial. Hence, while both groups had reductions in the complexity of neuromuscular control during slip trials compared with regular nonslip trials, this reduction was substantially greater among individuals who fell. Therefore, one way in which the neuromuscular control of walking and balance may fail in older adults during a fall could be a reduction in the complexity of neuromuscular control used in the motor response. Further research that tests the complexity of neuromuscular control during falls and recoveries within the same subject(s), while accounting for potential differences in mechanics during falls and recoveries, is necessary to confirm these results and infer neuromuscular causation.
The mechanism(s) through which differences in muscle synergy number may act to mediate slip outcome is unclear. In the context of this study the proposed functional consequence of a smaller repertoire of muscle synergies is apparent, fall vs. recovery, but how might a greater number of muscle synergies prevent a fall? One possibility is that a larger motor repertoire allowed individuals who recovered to produce biomechanical functions critical to maintaining balance that those who fell could not produce. An alternative explanation is that additional muscle synergies may enable individuals to decompose the necessary biomechanical functions into smaller, more specific subfunctions. Therefore, subjects who recovered may have used multiple muscle synergies for the same biomechanical function that individuals who fell executed with a single muscle synergy (Sawers et al. 2015). Such exactness may instill greater robustness in the neuromuscular response. To test these hypotheses, additional experiments linking muscle synergies to biomechanical output of falls and recoveries during slips and trips is required.
The variation in muscle synergies observed across subjects in the recovery group during slip trials (Fig. 4) does not suggest the absence of dimensionality reduction but rather that there are substantial interindividual differences in how muscles can be coordinated to maintain balance after a slip. While interindividual differences are frequently acknowledged, they are rarely quantified. Here we demonstrated that motor abundance (Latash 2012; Ting et al. 2015) may extend to the control of balance recovery. These interindividual differences and variation in motor responses suggest that a “one size fits all” approach to rehabilitation may not be sufficient.
It is possible that the limited number of muscles recorded in this study may have led to an underestimation of the number of muscle synergies recruited during walking and slip responses. However, the muscles included in the present analysis can be considered among the dominant muscles involved in a slip response (Cham and Redfern 2001; Dietz et al. 1986; Eng et al. 1994; Marigold et al. 2003; Nashner 1980; Tang et al. 1998). This may help offset the effect of a limited number of muscles in the muscle synergy analysis (Steele et al. 2013). Owing to the limited number of muscles, the goal of this study was not to determine the full dimensionality of the neuromuscular response but rather to characterize how a select number of muscles that had been recorded previously were coordinated during falls and recoveries. The present study is the first examination of muscle coordination during a fall. Therefore, despite the limited number of muscles included in this study, these results provide initial and promising insight into how differences in the complexity of neuromuscular control may contribute to slip outcomes. Further research with a larger number of bilateral muscles is necessary to confirm these results and establish the full dimensionality of neuromuscular control during falls vs. that of recoveries.
Muscle Coordination About the Knee May Dictate Slip Outcome
Several differences in muscle coordination between subjects who fell and those who recovered suggest that the ability to coordinate muscle activity around the knee in a timely manner may dictate slip outcome. First, onset latency was found to differ between individuals who fell and those who recovered during slip trials. Specifically, the BFLH and VLAT of the leading/slip leg had significantly shorter onset latencies in subjects who recovered compared with those who fell, as much as 50 ms after the slip onset (Table 2). While previous studies have emphasized the importance of mechanical (Ferber et al. 2002; Han and Yang 2015; Iqbal and Pai 2000) and muscular (Chambers and Cham 2007; Ding and Yang 2016; Lockhart and Kim 2006; Parijat et al. 2015; Tang et al. 1998) contributions from the knee in maintaining balance after a slip, ours is the first to demonstrate a difference in timing of these muscles between slip outcomes. The late knee flexor activity in subjects who fell may have delayed the necessary knee flexion moment (Cham and Redfern 2001; Ferber et al. 2002) required to limit knee extension and return the slipping foot back toward the body (Cham and Redfern 2001; Lockhart and Kim 2006; Pai et al. 2006) such that a sufficient base of support exists upon which knee extension can be applied to prevent limb collapse. In contrast to onset latency, the magnitude of muscle activation during slip trials, which increases with slip severity (Chambers and Cham 2007), did not differ between individuals who fell and those who recovered in the present study (Table 3). Further supporting the role of slipping/leading leg knee muscle activity, subjects who recovered during slip trials recruited the predominantly slip-leg knee flexor muscle synergy W4 Recover much earlier than subjects who fell (W4 Fall) (Fig. 4). Additionally, the magnitude of the temporal recruitment of W4 Recover and W4 Fall was similar between slip outcomes, again suggesting the potential importance of timing over magnitude. Therefore, our results suggest that another way in which the neuromuscular control of walking and balance may potentially break down in older adults during a fall is a delay in the activation of muscles about the slipping/leading leg knee. Future analysis of slip mechanics is required to definitively propose that the observed differences in muscle coordination about the knee are governed by neural rather than mechanical features.
In addition to timing, the ability to reorganize one's motor repertoire in the face of a perturbation may be critical to balance recovery. Less than a third of the subjects who fell (27%) recruited what was a slip-leg knee flexor dominant muscle synergy, W4 Fall, compared with nearly three-quarters of the subjects who recovered, W4 Recover (71%). In contrast, W2, which contained substantial slip-leg knee flexor and extensor contributions, was recruited by the opposite proportion of subjects, 67% of those who fell, W2 Fall, and 31% of those that did not, W2 Recover. The temporal activation of W2 Fall and W2 Recover is consistent with knee muscle activity that would be expected during terminal swing through initial stance of unperturbed walking (i.e., it was on before and during heel strike/slip onset). This suggests that rather than recruiting slip-specific knee flexor muscle activity, the majority of subjects who fell may have attempted to utilize rhythmic knee muscle activity typical of unperturbed walking. To this point, the spatial and temporal components of W2 Fall and W2 Recover during slip trials are remarkably similar to those of W5 Walk during nonslip trials. Therefore, an inability to reorganize one's motor repertoire in the face of an unannounced and abrupt perturbation is another difference in the neuromuscular control between fall and recovery slip outcomes that may represent a breakdown in the neuromuscular control of walking and balance during falls among older adults.
Owing to the limited number of muscles that were recorded in this study, we cannot speak to the role of more proximal muscles around the hip or trunk that have been implicated in previous work (Cham and Redfern 2001; Ferber et al. 2002; Tang et al. 1998). As a result, we cannot definitively state that control of the knee supersedes that of the hip as a determinant of slip outcome. Further research to replicate these findings and examine the contributions of hip and trunk muscles in falls vs. recoveries remains necessary. Nonetheless, these results provide promising initial insight into the importance of the knee in maintaining balance after a slip.
Recruitment of Intra- vs. Interlimb Muscle Coordination Patterns May Facilitate Recoveries from a Severe Slip
Maintaining greater independent leg control may have the advantage of providing more flexible and context-specific responses, ensuring that each leg generates appropriate output to avoid a fall. While bilateral coordination between lower limb muscles is considered an essential feature of locomotion and balance control (Dietz et al. 1994; Ivanenko 2006; Moyer et al. 2009; Olree and Vaughan 1995), we found that the percentage of interlimb muscle coordination patterns during slip and nonslip trials was lower among subjects who recovered (Fig. 5). This suggests that greater independent leg control, expressed through a larger proportion of intralimb muscle coordination patterns, may be critical to preserving balance in response to a severe slip. The neural vs. mechanical basis for this interpretation remains to be established; however, it is consistent with earlier work suggesting that the perturbed and nonperturbed legs are under independent control (Oliveira et al. 2012) during successful balance responses. This may be of particular value when considering the myriad of ways our balance can be perturbed. While the biomechanical function of each leg must certainly be coupled to preserve balance, our findings suggest that such coupling may not occur through the coordination of muscles between legs. These results suggest that in addition to traditional reduction and visualization of EMG data, muscle synergy analysis may be suitable for examining differences in inter- vs. intralimb muscle coordination. However, further research using a larger set of muscles, as well as alternative analysis techniques such as intermuscular coherence (De Marchis et al. 2015; Farmer et al. 1993), correlation analysis (Courtine et al. 2005), and clustering methods (Krouchev et al. 2006), is required to confirm and validate this approach.
Does Startle Reflex Activity Disrupt Balance?
Among the muscle coordination patterns that were unique to subjects who fell during slip trials, perhaps the most striking was the “all-on” muscle synergy, W6 Fall (Fig. 4). One interpretation of this distinct motor pattern is that it may represent activity consistent with the startle reflex, an involuntary motor reaction to unexpected sensory input (Nonnekes et al. 2015). While the present data lack bilateral sternocleomastoid activity required to verify the presence of a startle reflex (Brown et al. 1991b), several features of the “all-on” muscle synergy support this interpretation. First, startle reflex activity has been linked to first trial responses during unexpected balance perturbations (Blouin et al. 2006; Oude Nijhuis et al. 2010; Siegmund et al. 2008). In the present study all slip trials consisted of the first, unannounced slip to which subjects were exposed. Second, while originally considered a flexor response (Davis 1984; Landis and Hunt 1939), during locomotion responses to startling stimuli have been observed in both flexors and extensors of the lower extremities, often in coactivation, at elevated or extreme levels (Nieuwenhuijzen et al. 2000). Thus the distributed neuromuscular response captured by the “all-on” muscle synergy that was prominent among subjects who fell may reflect motor output consistent with a startle response. Despite its thorough characterization, the functional contribution(s) of the startle reflex to balance remain unclear. While some have argued that the startle reflex is protective (Brown et al. 1991b; Nieuwenhuijzen et al. 2000) and aimed at maintaining maximum stability (Brown et al. 1991a), there is little evidence to support its role as protective or disruptive (Sanders et al. 2015). Here the startlelike motor output of the “all-on” muscle synergy was only found in subjects who fell during slip trials. This suggests that startle reflex activity may be disruptive to balance, possibly by limiting the efficacy of the motor response through increases in joint stiffness. Alternatively, this “all-on” muscle synergy may protect not against a fall but against a possible impact with the ground during a fall. Regardless, while appealing, without further study it remains speculative to associate startle reflex activity with the “all-on” muscle synergy and balance disruption. However, if startle reflex activity is prevalent or exaggerated among individuals with balance deficits it could provide novel insight into neuromuscular mechanisms of falls. Given the ease with which the startle reflex can be habituated with repeated exposure to startling stimuli (Brown et al. 1991b), this may represent a promising avenue for balance rehabilitation.
Our data may have been biased by the criteria for defining the end of the fall period. Selecting the 30% body weight criterion to define the end of the “fall period” may have permitted data corresponding to either a fall or preparing for an impact to be included in the analysis. Ideally, the analysis would only include EMG data within a “reversible window” (i.e., the time before a fall is unavoidable and subjects are still attempting to recover their balance). However, such a window is not well defined in the literature but would likely be beneficial in understanding the neuromuscular basis of falls, as well as in designing fall prevention interventions. Future efforts will be directed toward a rigorous definition of such a “reversible window.” In lieu of a more established and validated cutoff point, the 30% body weight criterion provides a reasonable point of termination for the analysis of falls in our present characterization of the differences in muscle coordination between falls and recoveries.
Conclusions
As a first step in determining how the neuromuscular control of walking and balance breaks down during a fall, we sought to identify differences in muscle coordination between subjects who fell and those who recovered from an unexpected slip. Our results suggest that falls may arise from differences in neuromuscular control of walking and balance that are characterized by lower complexity of neuromuscular control during slip and nonslip trials, delayed temporal coordination of muscle activity about the knee and excessive coactivation during slip responses, as well as greater interlimb muscle coordination during slip and nonslip conditions. If these differences are found to coincide with similar slip mechanics, and are robust across 1) different slip types (i.e., feet forward vs. split), 2) a variety of perturbations (i.e., slip vs. trip), 3) different mechanical causes of a fall (i.e., loss of stability vs. limb collapse), and 4) subjects who initially fall and then learn to recover with training, they may provide needed insight into neuromuscular mechanisms of falls to inform novel and focused approaches to balance rehabilitation.
GRANTS
Research reported in this publication was supported by National Institutes of Health Grants K12-HD-073945, R01-AG-029616, and R01-AG-044364.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.S. analyzed data; A.S., Y.-C.C.P., and L.H.T. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S., Y.-C.C.P., T.B., and L.H.T. edited and revised manuscript; Y.-C.C.P., T.B., and L.H.T. approved final version of manuscript; T.B. performed experiments.
ACKNOWLEDGMENTS
The authors thank Anna Lee and Shuaijie Wang for assistance with data processing and Jessica Allen for helpful feedback on the manuscript.
REFERENCES
- Asavasopon S, Rana M, Kirages DJ, Yani MS, Fisher BE, Hwang DH, Lohman EB, Berk LS, Kutch JJ. Cortical activation associated with muscle synergies of the human male pelvic floor. J Neurosci 34: 13811–13818, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt T, Espy D, Yang F, Pai YC. Dynamic gait stability, clinical correlates, and prognosis of falls among community-dwelling older adults. Arch Phys Med Rehabil 92: 799–805, 2011. [DOI] [PubMed] [Google Scholar]
- Bhatt T, Wening JD, Pai YC. Influence of gait speed on stability: recovery from anterior slips and compensatory stepping. Gait Posture 21: 146–156, 2005. [DOI] [PubMed] [Google Scholar]
- Bhatt T, Yang F, Pai YC. Learning to resist gait-slip falls: long-term retention in community-dwelling older adults. Arch Phys Med Rehabil 93: 557–564, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin JS, Inglis JT, Siegmund GP. Startle responses elicited by whiplash perturbations. J Physiol 573: 857–867, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RA, Pavol MJ, Owings TM, Grabiner MD. Foot displacement but not velocity predicts the outcome of a slip induced in young subjects while walking. J Biomech 33: 803–808, 2000. [DOI] [PubMed] [Google Scholar]
- Brown P, Day BL, Rothwell JC, Thompson PD, Marsden CD. The effect of posture on the normal and pathological auditory startle reflex. J Neurol Neurosurg Psychiatry 54: 892–897, 1991a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain 114: 1891–1902, 1991b. [DOI] [PubMed] [Google Scholar]
- Cham R, Redfern MS. Lower extremity corrective reactions to slip events. J Biomech 34: 1439–1445, 2001. [DOI] [PubMed] [Google Scholar]
- Chambers AJ, Cham R. Slip-related muscle activation patterns in the stance leg during walking. Gait Posture 25: 565–572, 2007. [DOI] [PubMed] [Google Scholar]
- Cheung VC, d'Avella A, Bizzi E. Adjustments of motor pattern for load compensation via modulated activations of muscle synergies during natural behaviors. J Neurophysiol 101: 1235–1257, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, d'Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci 25: 6419–6434, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Macpherson JM, Torres-Oviedo G, Ting LH. Absence of postural muscle synergies for balance after spinal cord transection. J Neurophysiol 110: 1301–1310, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci 32: 12237–12250, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Common muscle synergies for balance and walking. Front Comput Neurosci 7: 48, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 103: 844–857, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Papaxanthis C, Schieppati M. Coordinated modulation of locomotor muscle synergies constructs straight-ahead and curvilinear walking in humans. Exp Brain Res 170: 320–335, 2005. [DOI] [PubMed] [Google Scholar]
- d'Avella A, Bizzi E. Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci USA 102: 3076–3081, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The mammalian startle response. In: Neural Mechanisms of Startle Behavior. Boston, MA: Springer, 1984, p. 287–351. [Google Scholar]
- de Leva P. Adjustments to Zatsiorsky-Seluyanov's segment inertia parameters. J Biomech 29: 1223–1230, 1996. [DOI] [PubMed] [Google Scholar]
- De Marchis C, Severini G, Castronovo AM, Schmid M, Conforto S. Intermuscular coherence contributions in synergistic muscles during pedaling. Exp Brain Res 233: 1907–1919, 2015. [DOI] [PubMed] [Google Scholar]
- Delbono O. Neural control of aging skeletal muscle. Aging Cell 2: 21–29, 2003. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Stumbling reactions in man—release of a ballistic movement pattern. Brain Res 362: 355–357, 1986. [DOI] [PubMed] [Google Scholar]
- Dietz V, Zijlstra W, Duysens J. Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101: 513–520, 1994. [DOI] [PubMed] [Google Scholar]
- Ding L, Yang F. Muscle weakness is related to slip-initiated falls among community-dwelling older adults. J Biomech 49: 238–243, 2016. [DOI] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, d'Avella A, Mondi V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, Poppele RE, Lacquaniti F. Locomotor primitives in newborn babies and their development. Science 334: 997–999, 2011. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Winter DA, Patla AE. Strategies for recovery from a trip in early and late swing during human walking. Exp Brain Res 102: 339–349, 1994. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. J Physiol 463: 83–105, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber R, Osternig LR, Woollacott MH, Wasielewski NJ, Lee JH. Reactive balance adjustments to unexpected perturbations during human walking. Gait Posture 16: 238–248, 2002. [DOI] [PubMed] [Google Scholar]
- Fingerhut LA, Warner M. Health, United States, 1996-97 and Injury Chartbook. Hyattsville, MD: National Center for Health Statistics, 1997. [Google Scholar]
- Fox EJ, Tester NJ, Kautz SA, Howland DR, Clark DJ, Garvan C, Behrman AL. Modular control of varied locomotor tasks in children with incomplete spinal cord injuries. J Neurophysiol 110: 1415–1425, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. J Neurophysiol 80: 1868–1885, 1998. [DOI] [PubMed] [Google Scholar]
- Grönqvist R, Abeysekera J, Gard G, Hsiang SM, Leamon TB, Newman DJ, Gielo-Perczak K, Lockhart TE, Pai CY. Human-centred approaches in slipperiness measurement. Ergonomics 44: 1167–1199, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Yang F. Strength or power, which is more important to prevent slip-related falls? Hum Mov Sci 44: 192–200, 2015. [DOI] [PubMed] [Google Scholar]
- Hart CB. Modular premotor drives and unit bursts as primitives for frog motor behaviors. J Neurosci 24: 5269–5282, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Chvatal SA, French MA, Ting LH, Trumbower RD. Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury. Clin Neurophysiol 125: 2024–2035, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen MJ, Rietdyk S. Falls in young adults: perceived causes and environmental factors assessed with a daily online survey. Hum Mov Sci 46: 86–95, 2016. [DOI] [PubMed] [Google Scholar]
- Houry D, Florence C, Baldwin G, Stevens J, McClure R. The CDC Injury Center's response to the growing public health problem of falls among older adults. Am J Lifestyle Med 10: 74–77, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Pai Y. Predicted region of stability for balance recovery: motion at the knee joint can improve termination of forward movement. J Biomech 33: 1619–1627, 2000. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP. Motor control programs and walking. Neuroscientist 12: 339–348, 2006. [DOI] [PubMed] [Google Scholar]
- Krouchev N, Kalaska JF, Drew T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol 96: 1991–2010, 2006. [DOI] [PubMed] [Google Scholar]
- Kutch JJ, Kuo AD, Bloch AM, Rymer WZ. Endpoint force fluctuations reveal flexible rather than synergistic patterns of muscle cooperation. J Neurophysiol 100: 2455–2471, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacquaniti F, Ivanenko YP, Zago M. Patterned control of human locomotion. J Physiol 590: 2189–2199, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Wolstenholme C, van der Linden M, Pang MY, Yang JF. Stumbling corrective responses during treadmill-elicited stepping in human infants. J Physiol 553: 319–331, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis C, Hunt W. The Startle Pattern. New York: Farrar and Rinehart, 1939. [Google Scholar]
- Latash ML. The bliss (not the problem) of motor abundance (not redundancy). Exp Brain Res 217: 1–5, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature 401: 788–791, 1999. [DOI] [PubMed] [Google Scholar]
- Lee WA. Neuromotor synergies as a basis for coordinated intentional action. J Mot Behav 16: 135–170, 1984. [DOI] [PubMed] [Google Scholar]
- Lockhart TE, Kim S. Relationship between hamstring activation rate and heel contact velocity: factors influencing age-related slip-induced falls. Gait Posture 24: 23–34, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart TE, Woldstad JC, Smith JL. Effects of age-related gait changes on the biomechanics of slips and falls. Ergonomics 46: 1136–1160, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart TE, Woldstad JC, Smith JL, Ramsey JD. Effects of age related sensory degradation on perception of floor slipperiness and associated slip parameters. Safety Sci 40: 689–703, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigold DS, Bethune AJ, Patla AE. Role of the unperturbed limb and arms in the reactive recovery response to an unexpected slip during locomotion. J Neurophysiol 89: 1727–1737, 2003. [DOI] [PubMed] [Google Scholar]
- Martino G, Ivanenko YP, d'Avella A, Serrao M, Ranavolo A, Draicchio F, Cappellini G, Casali C, Lacquaniti F. Neuromuscular adjustments of gait associated with unstable conditions. J Neurophysiol 114: 2867–2882, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer BE, Redfern MS, Cham R. Biomechanics of trailing leg response to slipping—evidence of interlimb and intralimb coordination. Gait Posture 29: 565–570, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM. Balance adjustments of humans perturbed while walking. J Neurophysiol 44: 650–664, 1980. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: a simulation study. J Biomech 42: 1282–1287, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijzen PH, Schillings AM, Van Galen GP, Duysens J. Modulation of the startle response during human gait. J Neurophysiol 84: 65–74, 2000. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Carpenter MG, Inglis JT, Duysens J, Weerdesteyn V. What startles tell us about control of posture and gait. Neurosci Biobehav Rev 53: 131–138, 2015. [DOI] [PubMed] [Google Scholar]
- Oliveira AS, Gizzi L, Farina D, Kersting UG. Motor modules of human locomotion: influence of EMG averaging, concatenation, and number of step cycles. Front Hum Neurosci 8: 335, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AS, Gizzi L, Kersting UG, Farina D. Modular organization of balance control following perturbations during walking. J Neurophysiol 108: 1895–1906, 2012. [DOI] [PubMed] [Google Scholar]
- Olree KS, Vaughan CL. Fundamental patterns of bilateral muscle activity in human locomotion. Biol Cybern 73: 409–414, 1995. [DOI] [PubMed] [Google Scholar]
- Oude Nijhuis LB, Allum JH, Valls-Solé J, Overeem S, Bloem BR. First trial postural reactions to unexpected balance disturbances: a comparison with the acoustic startle reaction. J Neurophysiol 104: 2704–2712, 2010. [DOI] [PubMed] [Google Scholar]
- Overduin SA, d'Avella A, Carmena JM, Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron 76: 1071–1077, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell C, Chambers A, Mahboobin A, Cham R. Effects of slip severity on muscle activation of the trailing leg during an unexpected slip. J Electromyogr Kinesiol 28: 61–66, 2016. [DOI] [PubMed] [Google Scholar]
- Pai YC, Patton J. Center of mass velocity-position predictions for balance control. J Biomech 30: 347–354, 1997. [DOI] [PubMed] [Google Scholar]
- Pai YC, Yang F, Bhatt T, Wang E. Learning from laboratory-induced falling: long-term motor retention among older adults. Age (Dordr) 36: 9640–9650, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai YC, Yang F, Wening JD, Pavol MJ. Mechanisms of limb collapse following a slip among young and older adults. J Biomech 39: 2194–2204, 2006. [DOI] [PubMed] [Google Scholar]
- Parijat P, Lockhart TE, Liu J. EMG and kinematic responses to unexpected slips after slip training in virtual reality. IEEE Trans Biomed Eng 62: 593–599, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavol MJ, Owings TM, Foley KT, Grabiner MD. Gait characteristics as risk factors for falling from trips induced in older adults. J Gerontol A Biol Sci Med Sci 54: M583–M590, 1999. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Owings TM, Foley KT, Grabiner MD. Mechanisms leading to a fall from an induced trip in healthy older adults. J Gerontol A Biol Sci Med Sci 56: M428–M437, 2001. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Pai YC. Feedforward adaptations are used to compensate for a potential loss of balance. Exp Brain Res 145: 528–538, 2002. [DOI] [PubMed] [Google Scholar]
- Perkins PJ, Wilson MP. Slip resistance testing of shoes—new developments. Ergonomics 26: 73–82, 1983. [Google Scholar]
- Portney LG, Watkins MP. Foundations of Clinical Research. Upper Saddle River, NJ: Pearson, 2013. [Google Scholar]
- Rodriguez KL, Roemmich RT, Cam B, Fregly BJ, Hass CJ. Persons with Parkinson's disease exhibit decreased neuromuscular complexity during gait. Clin Neurophysiol 124: 1390–1397, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich RT, Fregly BJ, Hass CJ. Neuromuscular complexity during gait is not responsive to medication in persons with Parkinson's disease. Ann Biomed Eng 42: 1901–1912, 2014. [DOI] [PubMed] [Google Scholar]
- Roh J, Cheung VC, Bizzi E. Modules in the brain stem and spinal cord underlying motor behaviors. J Neurophysiol 106: 1363–1378, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders OP, Savin DN, Creath RA, Rogers MW. Protective balance and startle responses to sudden freefall in standing humans. Neurosci Lett 586: 8–12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers A, Allen JL, Ting LH. Long-term training modifies the modular structure and organization of walking balance control. J Neurophysiol 114: 3359–3373, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillings AM, Van Wezel BM, Mulder TH. Muscular responses and movement strategies during stumbling over obstacles. J Neurophysiol 83: 2093–2102, 2000. [DOI] [PubMed] [Google Scholar]
- Shigematsu R, Okura T, Sakai T, Rantanen T. Square-stepping exercise versus strength and balance training for fall risk factors. Aging Clin Exp Res 20: 19–24, 2008. [DOI] [PubMed] [Google Scholar]
- Shuman B, Goudriaan M, Bar-On L, Schwartz MH, Desloovere K, Steele KM. Repeatability of muscle synergies within and between days for typically developing children and children with cerebral palsy. Gait Posture 45: 127–132, 2016. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Blouin JS, Inglis JT. Does startle explain the exaggerated first response to a transient perturbation? Exerc Sport Sci Rev 36: 76–82, 2008. [DOI] [PubMed] [Google Scholar]
- Steele KM, Rozumalski A, Schwartz MH. Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev Med Child Neurol 57: 1176–1182, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele KM, Tresch MC, Perreault EJ. The number and choice of muscles impact the results of muscle synergy analyses. Front Comput Neurosci 7: 105, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev 12: 290–295, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg L. On accident analysis and slip-resistance measurement. Ergonomics 26: 11–32, 1983. [DOI] [PubMed] [Google Scholar]
- Tang PF, Woollacott MH. Phase-dependent modulation of proximal and distal postural responses to slips in young and older adults. J Gerontol A Biol Sci Med Sci 54: M89–M102, 1999. [DOI] [PubMed] [Google Scholar]
- Tang PF, Woollacott MH, Chong RK. Control of reactive balance adjustments in perturbed human walking: roles of proximal and distal postural muscle activity. Exp Brain Res 119: 141–152, 1998. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. J Gerontol A Biol Sci Med Sci 53: M112–M119, 1998. [DOI] [PubMed] [Google Scholar]
- Ting LH, Chiel HJ, Trumbower RD, Allen JL, McKay JL, Hackney ME, Kesar TM. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 86: 38–54, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol 98: 2144–2156, 2007. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Cheung VC, d'Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol 95: 2199–2212, 2006. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol 19: 601–607, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nat Neurosci 2: 162–167, 1999. [DOI] [PubMed] [Google Scholar]
- Weerdesteyn V, Laing AC, Robinovitch SN. The body configuration at step contact critically determines the successfulness of balance recovery in response to large backward perturbations. Gait Posture 35: 462–466, 2012. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. New York: Wiley, 2009. [Google Scholar]
- Yang F, Espy D, Bhatt T, Pai YC. Two types of slip-induced falls among community dwelling older adults. J Biomech 45: 1259–1264, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Pai YC. Correction of the inertial effect resulting from a plate moving under low-friction conditions. J Biomech 40: 2723–2730, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Pai YC. Automatic recognition of falls in gait-slip training: harness load cell based criteria. J Biomech 44: 2243–2249, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]





