This is the first study to show increases in vestibular-evoked responses of the lower body muscles under conditions of increased threat of postural perturbation. While robust findings were observed in hip and leg muscles, less consistent results were found in muscles of the trunk. The present findings provide further support in the ongoing debate for arguments that vestibular-evoked balance responses are influenced by fear and anxiety and explain previous threat-related changes in balance.
Keywords: postural threat, fear, vestibular-evoked response, electrical vestibular stimulation
Abstract
Standing balance is significantly influenced by postural threat. While this effect has been well established, the underlying mechanisms of the effect are less understood. The involvement of the vestibular system is under current debate, and recent studies that investigated the effects of height-induced postural threat on vestibular-evoked responses provide conflicting results based on kinetic (Horslen BC, Dakin CJ, Inglis JT, Blouin JS, Carpenter MG. J Physiol 592: 3671–3685, 2014) and kinematic (Osler CJ, Tersteeg MC, Reynolds RF, Loram ID. Eur J Neurosci 38: 3239–3247, 2013) data. We examined the effect of threat of perturbation, a different form of postural threat, on coupling (cross-correlation, coherence, and gain) of the vestibulo-muscular relationship in 25 participants who maintained standing balance. In the “No-Threat” conditions, participants stood quietly on a stable surface. In the “Threat” condition, participants' balance was threatened with unpredictable mediolateral support surface tilts. Quiet standing immediately before the surface tilts was compared to an equivalent time from the No-Threat conditions. Surface EMG was recorded from bilateral trunk, hip, and leg muscles. Hip and leg muscles exhibited significant increases in peak cross-correlation amplitudes, coherence, and gain (1.23–2.66×) in the Threat condition compared with No-Threat conditions, and significant correlations were observed between threat-related changes in physiological arousal and medium-latency peak cross-correlation amplitude in medial gastrocnemius (r = 0.408) muscles. These findings show a clear threat effect on vestibular-evoked responses in muscles in the lower body, with less robust effects of threat on trunk muscles. Combined with previous work, the present results can provide insight into observed changes during balance control in threatening situations.
NEW & NOTEWORTHY This is the first study to show increases in vestibular-evoked responses of the lower body muscles under conditions of increased threat of postural perturbation. While robust findings were observed in hip and leg muscles, less consistent results were found in muscles of the trunk. The present findings provide further support in the ongoing debate for arguments that vestibular-evoked balance responses are influenced by fear and anxiety and explain previous threat-related changes in balance.
while recent evidence suggests that changes in threat-related factors can influence balance control and increase the sensitivity of the sensory systems involved in balance, the underlying neural mechanisms mediating these changes are not well understood (de Souza et al. 2015). Neuroanatomical evidence shows interconnections between vestibular nuclei and threat-related areas, such as the amygdala, the parabrachial nucleus network, infralimbic cortex, bed nucleus of stria terminalis, and hypothalamus (Balaban 2002; Balaban and Thayer 2001; Öhman 2005). There are also direct and indirect connections between the vestibular nuclei and both the locus coeruleus (Schuerger and Balaban 1999) and dorsal raphe nuclei (Jacobs and Azmitia 1992). Additionally, human neuroimaging suggests that areas of the vestibular cortex are directly and indirectly linked to limbic and prefrontal regions of the brain (Carmona et al. 2009; Indovina et al. 2014) and may influence the vestibular output (Dieterich and Brandt 2008). These numerous anatomical connections, therefore, provide clear pathways for threat-related psychological and autonomic state changes to modulate the vestibular system's output (Staab et al. 2013). This is supported by evidence of increased vestibuloocular reflex gains in humans under increased states of arousal (Yardley et al. 1995) and alertness (Kasper et al. 1992) or when standing with increased threat (Naranjo et al. 2016). However, the extent to which these anatomical connections contribute to changes in vestibular-evoked balance responses in humans has been under recent debate (Horslen et al. 2015; Reynolds et al. 2015).
The debate stems from contrasting studies that used electrical vestibular stimulation (EVS) to examine the effects of height-induced threat on vestibular-evoked balance responses. EVS evokes early short-latency and medium-latency peak responses of opposite polarity, both of which are thought to represent responses to a vestibular disturbance (Fitzpatrick and Day 2004), as well as later responses involving multisensory interactions. Horslen et al. (2014) reported increases in coupling between EVS and early ground reaction forces in subjects standing at the edge of a high compared with a low platform. In contrast, Osler et al. (2013) found no changes in early vestibular-evoked trunk or head kinematic responses at high compared with low heights. Methodological differences may explain some of the contradictory results [i.e., stance task, orientation to platform edge(s), and EVS characteristics]. However, one limitation of both studies was the reliance on net vestibular-evoked kinetic (Horslen et al. 2014) and kinematic (Osler et al. 2013) responses, which may be insensitive to short-duration or small-amplitude effects due to mechanical filtering (Loram et al. 2005) and do not distinguish changes in individual muscles. Furthermore, prior work is limited by the sole use of height-induced postural threat. This directionally specific threat is thought to engage balance strategies to avoid the edge(s) (Huffman et al. 2009), which may limit the interpretation of any observed changes in vestibular-evoked balance responses beyond the unique context of standing on elevated surfaces.
To overcome some of these limitations, we used EVS to examine changes in vestibular-evoked muscular responses in trunk, hip, and leg muscles under threat of unexpected support surface perturbations to upright stance. Participants stood on a servo-controlled rotating platform in conditions in which they were explicitly informed that they would experience a series of balance perturbations (Threat) or would stand quietly without balance perturbations (No-Threat). Threat of perturbation avoids the potential limitation of an edge effect while inducing similar psychological and autonomic responses and eliciting sensory and postural changes similar to those observed with height-induced postural threat (Horslen et al. 2013; Phanthanourak et al. 2016; Shaw et al. 2012). We hypothesized that 1) a threat of perturbation would increase vestibular-evoked responses in all recorded muscles and 2) threat-related changes in vestibular-evoked balance responses would be significantly correlated with threat-related changes in psychological and autonomic arousal responses.
METHODS
Twenty-eight healthy young adults volunteered for this study. Participants had no known neurological, vestibular, postural, skeletal, or orthopedic deficits that affect balance and were not prone to motion sickness. The study was approved by the University of British Columbia Clinical Research Ethics Board and performed in accordance with the Declaration of Helsinski. All participants provided written informed consent before their participation. One participant withdrew during testing because of EVS discomfort; two others were withdrawn by the experimenter because of technical issues. Therefore, data from 25 participants (age: 24 ± 3 yr, height: 172.5 ± 10.6 cm; 11 women, 14 men) were analyzed.
Procedure.
Participants stood on a custom-built servo-controlled rotating platform with heels 15 cm apart, feet lightly strapped in position, and ankles centered to the axes of rotation. For safety purposes, handrails were placed on both sides of the platform and a spotter was present at all times to prevent a fall (no falls occurred). Participants were blindfolded, stood with arms at their sides, and leaned slightly forward to increase triceps surae muscle activation. Participants faced forward with Reid's plane tilted 18° above horizontal to optimize the vestibular-evoked response (Fitzpatrick and Day 2004). Head position was maintained by verbal feedback from the experimenter who monitored online position through motion capture markers on the participants' head (Optotrak Certus, Northern Digital). Participants stood quietly in this position for 1 min to establish baseline anteroposterior and mediolateral moments from a forceplate mounted on the platform.
Participants then performed the first block of the No-Threat condition (No-Threat1), in which they were instructed to stand quietly for 3 min and were assured in advance that no balance perturbations would be experienced (none occurred). To assess the effect of increased postural threat, two 10-min blocks of the Threat condition were experienced in which they were instructed to maintain balance after random platform perturbations. Finally, to account for any order effect, a second 3-min No-Threat condition (No-Threat2) was experienced. To avoid fatigue, mandatory seated rests (minimum 5 min) were provided between every block.
Data collection.
EVS was delivered during both the No-Threat and Threat conditions through 9-cm2 carbon rubber electrodes with conductive gel (Spectra 360 electrode gel, Parker Laboratories) placed on the mastoid processes and secured by tensor bands. EVS stimulus was constructed (LabVIEW, National Instruments) from white noise filtered to 0–25 Hz, peak amplitudes of ±4.5 mA, and average root-mean-square amplitude of 1.05 mA (Dakin et al. 2007). EVS was delivered as an analog signal (NI PCI-MIO-16E-1 with NI BNC-2110, National Instruments) to an isolated constant-current stimulator (model 2200 Analog Stimulus Isolator, AM Systems and Stimsola, BioPac Systems). A copy of the EVS signal was sampled at 2,000 Hz (Power 1401 with Spike2 software, Cambridge Electronic Design).
Surface electromyography (EMG) data were collected from bilateral paraspinals (PARA) at L1-L2 level and obliquus externus (OE), gluteus medius (GM), vastus lateralis (VL), medial gastrocnemius (mGAS), and soleus (SOL) muscles. These muscles all have roles in quiet stance and/or regaining balance from lateral rotational support surface perturbations (Carpenter et al. 2004). Surface electrode pairs were placed 2 cm apart over muscle bellies. Data were collected at 3,000 Hz, amplified 500×, and analog band-pass filtered between 10 and 500 Hz (Telemyo 2400R, Noraxon). The data were then A/D converted and sampled at 2,000 Hz (Power 1401 with Spike2). Off-line, the bias was removed from the entire EMG signal by subtracting an unrectified 500-ms mean from before the first perturbation (MATLAB R2016a, MathWorks). EMG data were high-pass filtered (50 Hz, 4th-order dual-pass Butterworth) to remove heart rate and movement artifacts and full-wave rectified.
Physiological arousal was assessed using electrodermal activity, which is sensitive to sympathetic changes in skin (Venables and Mitchell 1996). It was collected with electrodes placed on the thenar and hypothenar eminences of the nondominant hand (model 2502, CED) and sampled at 2,000 Hz (Power 1401 with Spike2). Electrodermal activity was not analyzed for 3 of 25 participants because of technical difficulties. Questionnaires were used to assess psychological responses to threat (Hauck et al. 2008). Before each block, participants reported their confidence in their ability to avoid falling and maintain balance (0%: not confident, 100%: completely confident). After each block, participants filled out a series of questionnaires related to fear of falling (0%: no fear, 100%: completely fearful) and anxiety (15 items, sum 135 points, larger score = higher anxiety).
Perturbations only occurred in the Threat conditions. Support surface rotations (peak amplitude 7.7°, average angular velocity of 55°/s) were in the roll direction, with 85% occurring in the rightward direction. A majority of rightward perturbations were included to investigate secondary questions unrelated to the present study. However, previous work has demonstrated that threat of repeated unidirectional perturbations significantly increases arousal, fear, and anxiety and decreases confidence (Phanthanourak et al. 2016). After the 7.7° displacement was reached, the platform remained in place for 5 s before slowly (1.1°/s) returning back to horizontal (0°). Participants were encouraged and verbally guided to quickly return to baseline position. Perturbations were triggered once participants' ground reaction moments were within ±1 Nm of their baseline stance for a minimum of 5 s.
Analysis.
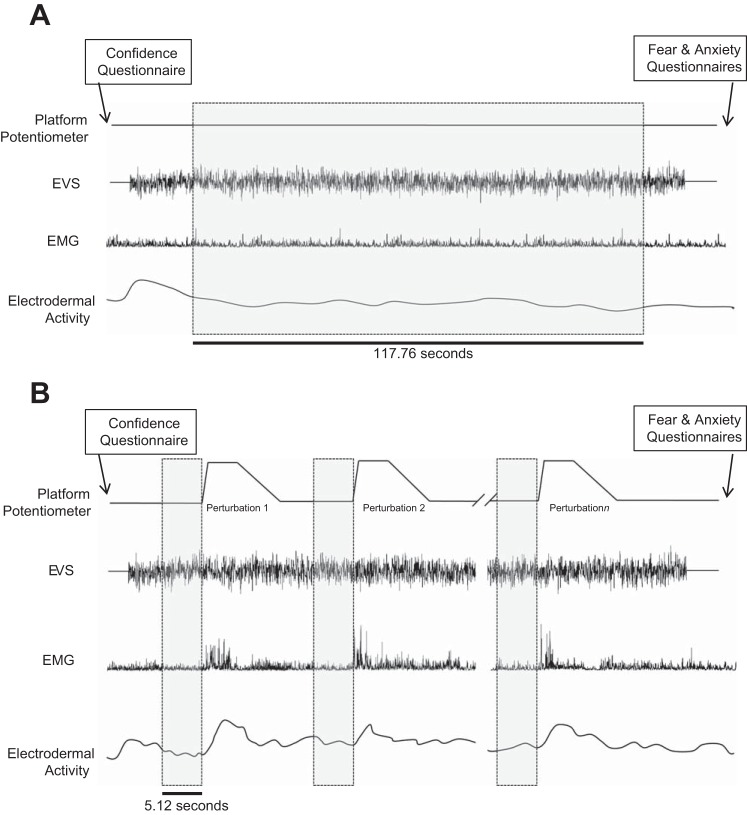
The No-Threat data were analyzed between 10 and 127.76 s after EVS onset (115 segments of 1.024 s; Fig. 1). Threat condition data were extracted from 5.12-s time windows immediately preceding each perturbation (5 segments of 1.024 s), when participants stood quietly and received limited verbal cues. Preperturbation data were combined (using 5 complete 1.024-s segments to avoid discontinuities in the windowed-Fourier analysis; see below) into a single array of data for each participant and condition. The number of perturbations experienced by each participant varied depending on their ability to regain baseline stance. Therefore, the participant who experienced the fewest perturbations dictated the amount of data clipped for all participants (23 perturbations × 5 segments of 1.024 s/perturbation = 115 segments of 1.024 s/participant).
Fig. 1.
Schematic of the experimental protocol and data collected for the 2 No-Threat conditions (A) and the Threat condition (B). Shaded boxes indicate the regions extracted for data analysis. EVS, electrical vestibular stimulation.
Vestibulo-muscular coupling between EVS and EMG signals was examined through cross-correlation (time domain), coherence (frequency domain), and gain (magnitude of cross-spectrum between the input EVS and output EMG divided by the input EVS auto-spectrum) (Neurospec 2.0 using NFFT = 2,048 and sampling frequency = 2,000 Hz, i.e., 1.024-s segments; Halliday et al. 1995). Calculations and statistical tests were replicated from Horslen et al. (2014); only details pertinent to the present study are discussed below.
Cross-correlations were analyzed on a participant-by-participant basis for each muscle and used to calculate short-latency and medium-latency peaks of the biphasic response, which are comparable to responses elicited by square-wave EVS (Dakin et al. 2007). Responses were amplitude normalized (Dakin et al. 2010), and significant peaks were identified when values exceeded 95% confidence intervals. If no significant peak was observed for a given muscle within a condition, then zero was used; if no peaks were observed in either condition, then that participant's muscle response was not included in the statistical analysis.
Peak short- and medium-latency amplitudes and average background EMG were analyzed with a 3 × 2 within-subject ANOVA for Condition (No-Threat1, Threat, No-Threat2) and Side (left, right). Significant Condition and interaction effects were explored post hoc with paired t-tests between each No-Threat condition and the Threat condition, with Bonferroni corrections.
Coherence and gain estimates were calculated after combining data segments for all subjects within a single array (2,875 segments of 1.024 s, 0.977-Hz resolution). Significant within-condition coherence was classified as values exceeding the 95% confidence interval (i.e., 0.00107). Significant between-condition changes in coherence were identified with difference of coherence tests for each muscle (Amjad et al. 1997). Significant changes in signal gain between conditions were identified for frequencies where No-Threat and Threat conditions had significant within-condition coherence and pointwise 95% confidence intervals did not overlap (Horslen et al. 2014).
Threat effects on electrodermal activity and psychological measures were compared with a one-way repeated-measures ANOVA (No-Threat1, Threat, NoThreat2). Significant main effects were explored post hoc with paired t-tests between each No-Threat condition and the Threat condition, with Bonferroni corrections. Threat-related changes in short- and medium-latency peak amplitudes were pooled across sides and No-Threat conditions and correlated with changes in electrodermal activity, confidence, fear, and anxiety, with either Pearson's r or Spearman's ρ correlations, where applicable. In all cases, level of significance was set at P ≤ 0.05.
RESULTS
Significant main effects of Condition were found for arousal [F(2,36) = 4.17, P = 0.023], anxiety [F(2,48) = 33.46, P < 0.001], fear [F(2,48) = 44.03, P ≤ 0.001], and confidence [F(2,48) = 38.93, P < 0.001]. Post hoc tests revealed that arousal, anxiety, and fear significantly increased in the Threat condition compared with both No-Threat conditions and balance confidence significantly decreased with Threat (all P < 0.03).
Significant main effects of Condition were observed for background EMG activity in OE, GM, and VL muscles, with greater activity in the Threat condition compared with both No-Threat conditions. Significantly larger background activity was calculated for right GM and SOL compared with the left side. No Condition × Side interactions were observed (Table 1).
Table 1.
Summary of results
| Cross-Correlation |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coherence |
Gain |
Short latency |
Medium latency |
Background Activity |
|||||||||
| Muscle | NT1, Hz | T, Hz | NT2, Hz | NT1-T, Hz | Ratio, T/NT1 | NT2-T, Hz | Ratio, T/NT2 | F value | P value | F value | P value | F value | P value |
| LPARA | 0–20.5 | 0–20.5 | 0–20.5 | 4.8–5.9 | 1.30 | 2.9–6.8 | 1.40 | 0.408 (n = 21) | 0.668 | 3.765 (n = 21) | 0.032* | 2.118 | 0.131 |
| RPARA | 0–20.5 | 0–23.4 | 0–19.5 | 4.8–5.9 | 1.26 | 2.9–5.9 | 1.35 | ||||||
| LOE | 0–8.8 | 2.0–9.8,12.7–13.7 | 0–13.7 | n.s. | 2.66 | n.s. | 1.23 | 1.476 (n = 14) | 0.247 | 0.628 (n = 14) | 0.542 | 3.853 | 0.028* |
| ROE | 0–10.7 | 0–12.7 | 0–8.8,12.7–14.7 | n.s. | 1.70 | n.s. | 1.35 | ||||||
| LGM | 0–24.4 | 0–24.4 | 0–23.4 | 0–15.6 | 2.09 | 0–13.7 | 2.07 | 45.349 (n = 24) | <0.001* | 19.714 (n = 24) | <0.001* | 5.532 | 0.007* |
| RGM | 0–25.4 | 0–24.4 | 0–23.4 | 0–15.6 | 2.16 | 0–18.6 | 1.87 | ||||||
| LVL | 2.9–12.7,15.6–16.6 | 0–21.5 | 2.0–15.6 | 6.8–7.8,10.7–11.7 | 2.12 | 6.8–7.8,10.7–11.7 | 2.57 | 38.064 (n = 13) | <0.001* | 14.853 (n = 13) | <0.001* | 4.471 | 0.027* |
| RVL | 5.9–12.7 | 0–23.4 | 2.0–6.8,9.8–12.7 | 2.0–2.9,5.9–6.8,13.7–15.6 | 2.23 | 5.9–6.8 | 2.39 | ||||||
| LmGAS | 0–21.5 | 0–21.5 | 0–22.5 | 0–5.9 | 1.26 | 0–6.8,9.8–10.7,13.7–15.6 | 1.63 | 42.836 (n = 25) | <0.001* | 10.43 (n = 25) | 0.001* | 2.454 | 0.116 |
| RmGAS | 0–24.4 | 0–25.4 | 0–22.5 | 0–7.8 | 1.39 | 0–17.6 | 2.00 | ||||||
| LSOL | 0–21.5 | 0–23.4 | 0–21.5 | 0–6.8 | 1.60 | 0–11.7,14.7–15.6 | 1.70 | 23.056 (n = 19) | <0.001* | 15.553 (n = 19) | <0.001* | 0.54 | 0.586 |
| RSOL | 0–20.5 | 0–25.4 | 0–19.5 | 0–14.7 | 2.01 | 0–12.7 | 2.02 | ||||||
Frequencies of significant coherence within each condition and frequencies of significant gain differences between the Threat and No-Threat conditions for all muscles are reported. Significant coherence was determined by values that are above a 95% confidence level. Significant gain was only assessed for frequencies that exhibited significant coherence for both the No-Threat and Threat conditions. Gain ratio values were calculated as an average of Threat gain/No-Threat gain for the frequencies that exhibited significant within-condition coherence. Cross-correlation main effects of Condition results are reported for short-latency and medium latency responses, with n representing the number of participants analyzed. Finally, the background activity main effects of Condition are reported.
n.s., no significant values; NT1, No-Threat1 condition; T, Threat condition; NT2, No-Threat2 condition; L, left-side muscle; R, right-side muscle.
Significant main effects of Condition for 2×3 ANOVA.
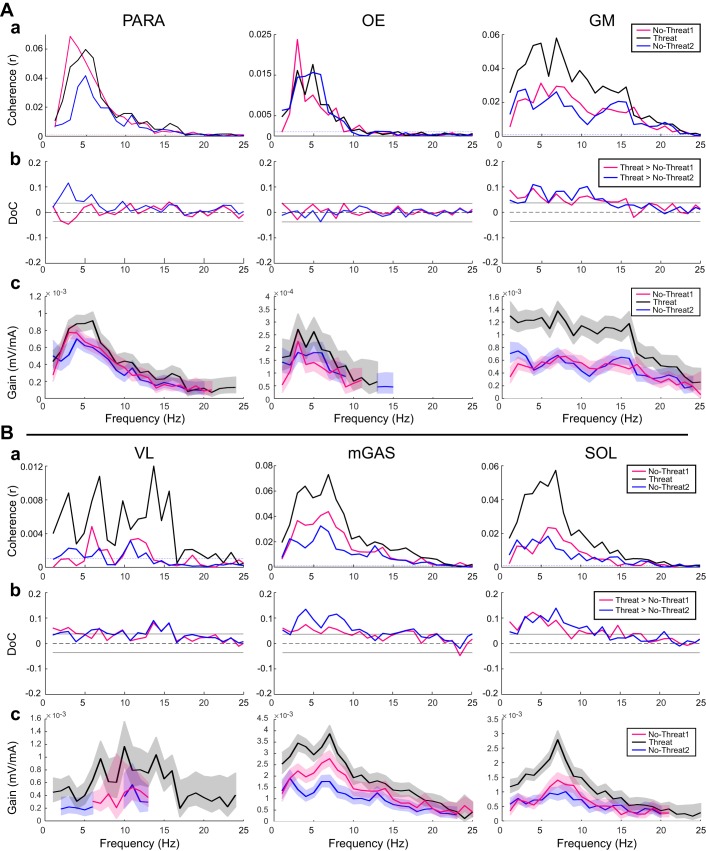
Significant EVS-EMG coherence was observed in all recorded muscle groups for both No-Threat and Threat conditions (Fig. 2, Aa and Ba; Table 1). Significant differences of coherence were observed for bilateral GM, VL, mGAS, and SOL, with larger coherence in the Threat condition compared with both No-Threat conditions. Right PARA had a significant increase in coherence in Threat compared with No-Threat2 but not No-Threat1. No significant differences in coherence were observed between Threat and No-Threat conditions for left PARA or bilateral OE muscles (Fig. 2, Ab and Bb).
Fig. 2.
Vestibulo-muscular coupling for muscles on the right side only. A: PARA, OE, and GM muscles. a: Pooled coherence plots. Dotted horizontal lines indicate the 95% confidence limits. b: Difference of coherence (DoC) plots. Traces above the 95% confidence limit (horizontal lines) indicate significantly larger coherence in the Threat condition compared with the No-Threat1 condition and compared with the No-Threat2 condition. Traces below the confidence limit indicate a significantly lower coherence in the Threat condition with No-Threat1 and No-Threat2 conditions. c: Gain of the vestibulo-muscular signal and their pointwise 95% confidence interval (shaded) for the No-Threat1, Threat, and No-Threat2 conditions. Nonoverlapping regions of the confidence limits represent significantly different gain values at the respective frequencies. B: same as A for VL, mGAS, and SOL muscles.
Significantly larger gains were found in the Threat condition compared with both No-Threat conditions for bilateral PARA, GM, VL, mGAS, and SOL muscles. There were no significant changes in gain for bilateral OEs (Fig. 2Ac; Table 1).
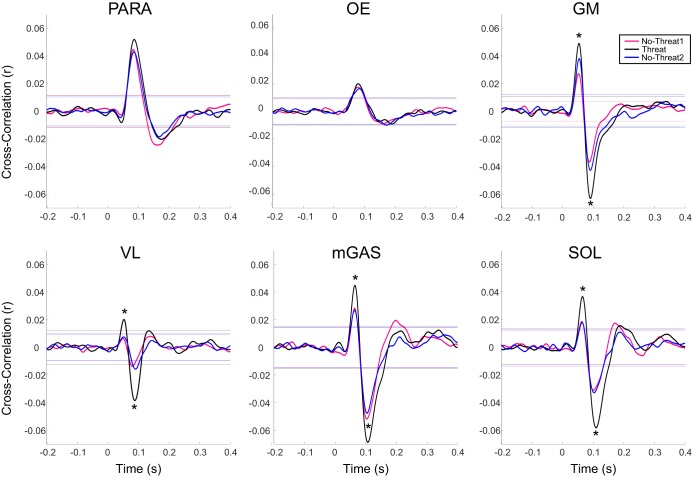
Significant main effects of Condition were observed in both short-latency and medium-latency peaks for GM, VL, mGAS, and SOL and in medium-latency peaks for PARAs (Table 1); post hoc tests revealed significant increases in the Threat condition compared with both No-Threat conditions for short- and medium-latency peaks in all lower body muscles but no significant change in PARAs (Fig. 3). No significant main effects of Condition were observed for short-latency peaks in PARAs or short- or medium-latency peaks in OEs. No significant main effects of Side or Condition × Side interactions were observed.
Fig. 3.
Average cross-correlation traces for the right-side muscles. Horizontal lines indicate the 95% confidence interval. Cross-correlations were positive for anodal currents applied to the right ear (cathode on the left ear) inducing a muscle facilitation for right-side muscles. *Significant differences in post hoc analysis (P < 0.025).
Threat-related increases in arousal were significantly correlated with increases in the medium-latency peaks of mGAS [r(21) = 0.408, P = 0.05]. No other significant correlations were observed.
DISCUSSION
This is the first study to investigate changes in vestibular-evoked trunk, hip, and leg muscle responses under threat of unexpected support surface perturbations. Threat of perturbation significantly increased EVS-EMG coupling in leg (VL, mGAS, SOL) and hip (GM) muscles. While threat-related increases in background EMG levels were observed in some muscles and were greater on the right side for some muscles, this was not evident or consistent across all muscles. Furthermore, although gain changes may be affected by background EMG, the cross-correlations and coherence are normalized by background EMG levels and are less likely to be affected by these changes. Therefore, the observations of threat-related changes in cross-correlation, coherence, and gain across lower body muscles provide strong converging evidence of a postural threat effect on vestibular-evoked responses. These results are congruent with previous reports of increased EVS-ground reaction force coupling (Horslen et al. 2014), increased vestibular-evoked myogenic potentials in the leg and neck muscles (Naranjo et al. 2015), and increased vestibuloocular reflex gains (Naranjo et al. 2016) when standing at height. Thus the results suggest a general vestibular response to postural threat and not a specific strategy to avoid the edge of high surfaces used in prior studies (Horslen et al. 2014; Naranjo et al. 2015; Osler et al. 2013).
Threat effects on vestibulo-muscular coupling in trunk muscles were less robust. While PARAs had significantly increased gain with threat, and significant differences in coherence between Threat and No-Threat2 for right PARA, post hoc analysis showed no significant increases in cross-correlations between the conditions for short-latency or medium-latency responses. Likewise, there was no evidence of threat-related changes in coherence, cross-correlation, or gain in OE muscles. The lack of robust threat effects observed in muscles of the trunk compared with the lower body may explain the absence of early vestibular-evoked trunk displacements changes reported by Osler et al. (2013). These results are also consistent with proposed differences in vestibular contributions to leg and trunk muscles during balance based on the early timing of stretch reflexes in the trunk compared with head movements (Allum et al. 2008) and differences in trunk and leg responses to support surface perturbation in individuals with bilateral vestibular loss compared with healthy control subjects (Carpenter et al. 2001).
The threat-related increases in vestibular coupling in leg and hip muscles may explain a number of balance changes previously observed with threat. For instance, increased vestibular coupling could contribute to the threat-induced stiffening response observed during quiet stance [increased postural sway frequency, tonic muscle activity, and decreased sway amplitude (Carpenter et al. 1999)] as well as increased amplitude of balance-correcting responses observed during dynamic balance perturbations (Carpenter et al. 2004), particularly during reflexive periods that may be modulated by changes in vestibular input (Carpenter et al. 2001; Cenciarini and Peterka 2006).
The present results, coupled with prior evidence of threat-related increases in vestibular coupling and gain in early kinetic (Horslen et al. 2014) and muscular (Naranjo et al. 2015, 2016) responses, are most likely explained by a common mechanism for modulation involving the vestibular nuclei (Staab et al. 2013). Alternatively, modulation could arise from other nuclei known to be sensitive to threat, including the reticular formation, tectum, or cortex (Naranjo et al. 2016; Staab et al. 2013). Although the present results only showed significant correlations between mGAS and arousal, this is consistent with prior reports of weak correlations between self-reported emotional states and reflexive responses (Naranjo et al. 2015, 2016). Additional work is needed to further understand the mechanisms that contribute to vestibular modulation with threat.
Limitations.
Although there was a directional bias for the perturbations to the right, there was no evidence of an interaction between threat and side for any muscle. Furthermore, the slight forward lean used to ensure tonic activation of triceps surae muscles during baseline stance may have altered OE activation levels and its role in balance control compared with a normal upright stance position. While this change in balance control role may explain the relatively small amplitude of coupling observed in OEs, significant coherence and cross-correlation were still calculated in both conditions and thus should not limit the potential for threat to influence vestibulo-muscular coupling in this muscle, especially since forward leaning was held constant across conditions.
Conclusions.
Standing in a situation of increased postural threat elicits an increase in the vestibular-evoked muscle responses of the lower body with little change observed in muscles of the trunk. The present results help reconcile previously contrasting results (Horslen et al. 2014; Osler et al. 2013) by demonstrating the different threat-related modulations of vestibular-evoked muscle responses in the hip/leg and trunk. These results are important to understand how the vestibular system may contribute to normal balance responses during threat and help underscore how the vestibular-emotional links may contribute to clinical balance and dizziness symptoms in patients with increased fear and anxiety.
GRANTS
The authors thank the University of British Columbia Faculty of Education and the Natural Sciences and Engineering Research Council (NSERC) for financial support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.B.L., T.W.C., and B.C.H. performed experiments; S.B.L. analyzed data; S.B.L., T.W.C., B.C.H., J.-S.B., J.T.I., and M.G.C. interpreted results of experiments; S.B.L. prepared figures; S.B.L. and M.G.C. drafted manuscript; S.B.L., T.W.C., B.C.H., J.-S.B., J.T.I., and M.G.C. edited and revised manuscript; S.B.L., T.W.C., B.C.H., J.-S.B., J.T.I., and M.G.C. approved final version of manuscript.
ACKNOWLEDGMENTS
These data have been published in part as a thesis by S. B. Lim.
Present address of S. B. Lim: Rehabilitation Research Program, G. F. Strong Rehabilitation Centre and Graduate Studies in Rehabilitation Sciences, University of British Columbia, Vancouver, British Columbia, Canada.
REFERENCES
- Allum JH, Oude Nijhuis LB, Carpenter MG. Differences in coding provided by proprioceptive and vestibular sensory signals may contribute to lateral instability in vestibular loss subjects. Exp Brain Res 184: 391–410, 2008. [DOI] [PubMed] [Google Scholar]
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods 73: 69–79, 1997. [DOI] [PubMed] [Google Scholar]
- Balaban CD. Neural substrates linking balance control and anxiety. Physiol Behav 77: 469–475, 2002. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Thayer JF. Neurological bases for balance-anxiety links. J Anxiety Disord 15: 53–79, 2001. [DOI] [PubMed] [Google Scholar]
- Carmona JE, Holland AK, Harrison DW. Extending the functional cerebral systems theory of emotion to the vestibular modality: a systematic and integrative approach. Psychol Bull 135: 286–302, 2009. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Allum JH, Honegger F. Vestibular influences on human postural control in combinations of pitch and roll planes reveal differences in spatiotemporal processing. Exp Brain Res 140: 95–111, 2001. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Adkin AL, Paton A, Allum JH. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol 92: 3255–3265, 2004. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP. Surface height effects on postural control: a hypothesis for a stiffness strategy for stance. J Vestib Res 9: 277–286, 1999. [PubMed] [Google Scholar]
- Cenciarini M, Peterka RJ. Stimulus-dependent changes in the vestibular contribution to human postural control. J Neurophysiol 95: 2733–2750, 2006. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Luu BL, van den Doel K, Inglis JT, Blouin JS. Frequency-specific modulation of vestibular-evoked sway responses in humans. J Neurophysiol 103: 1048–1056, 2010. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Son GM, Inglis JT, Blouin JS. Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol 583: 1117–1127, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza NS, Martins AC, Alexandra DJ, Orsini M, Bastos VH, Laite MA, Teixeira S, Velasques B, Ribeiro P, Bittencourt J, Matta AP, Filho PM. The influence of fear of falling on orthostatic postural control: a systematic review. Neurol Int 7: 62–65, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain 131: 2538–2552, 2008. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol 96: 2301–2316, 2004. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278, 1995. [DOI] [PubMed] [Google Scholar]
- Hauck LJ, Carpenter MG, Frank JS. Task-specific measures of balance efficacy, anxiety, and stability and their relationship to clinical balance performance. Gait Posture 27: 676–682, 2008. [DOI] [PubMed] [Google Scholar]
- Horslen BC, Dakin CJ, Inglis JT, Blouin JS, Carpenter MG. Modulation of human vestibular reflexes with increased postural threat. J Physiol 592: 3671–3685, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horslen BC, Dakin CJ, Inglis JT, Blouin JS, Carpenter MG. CrossTalk proposal: Fear of falling does influence vestibular-evoked balance responses. J Physiol 593: 2979–2981, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horslen BC, Murnaghan CD, Inglis JT, Chua R, Carpenter MG. Effects of postural threat on spinal stretch reflexes: evidence for increased muscle spindle sensitivity? J Neurophysiol 110: 899–906, 2013. [DOI] [PubMed] [Google Scholar]
- Huffman JL, Horslen BC, Carpenter MG, Adkin AL. Does increased postural threat lead to more conscious control of posture? Gait Posture 30: 528–532, 2009. [DOI] [PubMed] [Google Scholar]
- Indovina I, Riccelli R, Staab JP, Lacquaniti F, Passamonti L. Personality traits modulate subcortical and cortical vestibular and anxiety responses to sound-evoked otolithic receptor stimulation. J Psychosom Res 77: 391–400, 2014. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 72: 165–229, 1992. [DOI] [PubMed] [Google Scholar]
- Kasper J, Diefenhardt A, Mackert A, Thoden U. The vestibulo-ocular response during transient arousal shifts in man. Acta Otolaryngol 112: 1–6, 1992. [DOI] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. Human postural sway results from frequent, ballistic bias impulses by soleus and gastrocnemius. J Physiol 564: 295–311, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo EN, Allum JH, Inglis JT, Carpenter MG. Increased gain of vestibulospinal potentials evoked in neck and leg muscles when standing under height-induced postural threat. Neuroscience 293: 45–54, 2015. [DOI] [PubMed] [Google Scholar]
- Naranjo EN, Cleworth TW, Allum JH, Inglis JT, Lea J, Westerberg BD, Carpenter MG. Vestibulo-spinal and vestibulo-ocular reflexes are modulated when standing with increased postural threat. J Neurophysiol 115: 833–842, 2016. [DOI] [PubMed] [Google Scholar]
- Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology 30: 953–958, 2005. [DOI] [PubMed] [Google Scholar]
- Osler CJ, Tersteeg MC, Reynolds RF, Loram ID. Postural threat differentially affects the feedforward and feedback components of the vestibular-evoked balance response. Eur J Neurosci 38: 3239–3247, 2013. [DOI] [PubMed] [Google Scholar]
- Phanthanourak AL, Cleworth TW, Adkin AL, Carpenter MG, Tokuno CD. The threat of a support surface translation affects anticipatory postural control. Gait Posture 50: 145–150, 2016. [DOI] [PubMed] [Google Scholar]
- Reynolds RF, Osler CJ, Tersteeg MC, Loram ID. CrossTalk opposing view: Fear of falling does not influence vestibular-evoked balance responses. J Physiol 593: 2983–2984, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerger RJ, Balaban CD. Organization of the coeruleo-vestibular pathway in rats, rabbits, and monkeys. Brain Res Rev 30: 189–217, 1999. [DOI] [PubMed] [Google Scholar]
- Shaw JA, Stefanyk LE, Frank JS, Jog MS, Adkin AL. Effects of age and pathology on stance modifications in response to increased postural threat. Gait Posture 35: 658–661, 2012. [DOI] [PubMed] [Google Scholar]
- Staab JP, Balaban CD, Furman JM. Threat assessment and locomotion: clinical applications of an integrated model of anxiety and postural control. Semin Neurol 33: 297–306, 2013. [DOI] [PubMed] [Google Scholar]
- Venables PH, Mitchell DA. The effects of age, sex and time of testing on skin conductance activity. Biol Psychol 43: 87–101, 1996. [DOI] [PubMed] [Google Scholar]
- Yardley L, Watson S, Britton J, Lear S, Bird J. Effects of anxiety arousal and mental stress on the vestibulo-ocular reflex. Acta Otolaryngol 115: 597–602, 1995. [DOI] [PubMed] [Google Scholar]