The present study shows that unilateral contusion injury at C4 results in substantial loss of phrenic motoneurons but increased diaphragm muscle activity across a range of ventilatory and higher force, nonventilatory behaviors. Measures of neural drive indicate increased descending input to phrenic motoneurons that was more pronounced during higher force, nonventilatory behaviors. These findings reveal novel, complex adaptations in neuromotor control following injury, suggestive of increased recruitment of more fatigable, high-threshold motor units.
Keywords: spinal cord injury, respiratory muscles, neuromotor control, chronic EMG recordings
Abstract
Contusion-type injuries to the spinal cord are characterized by tissue loss and disruption of spinal pathways. Midcervical spinal cord injuries impair the function of respiratory muscles and may contribute to significant respiratory complications. This study systematically assessed the impact of a 100-kDy unilateral C4 contusion injury on diaphragm muscle activity across a range of motor behaviors in rats. Chronic diaphragm electromyography (EMG) was recorded before injury and at 1 and 7 days postinjury (DPI). Histological analyses assessed the extent of perineuronal net formation, white-matter sparing, and phrenic motoneuron loss. At 7 DPI, ∼45% of phrenic motoneurons were lost ipsilaterally. Relative diaphragm root mean square (RMS) EMG activity increased bilaterally across a range of motor behaviors by 7 DPI. The increase in diaphragm RMS EMG activity was associated with an increase in neural drive (RMS value at 75 ms after the onset of diaphragm activity) and was more pronounced during higher force, nonventilatory motor behaviors. Animals in the contusion group displayed a transient decrease in respiratory rate and an increase in burst duration at 1 DPI. By 7 days, following midcervical contusion, there was significant perineuronal net formation and white-matter loss that spanned 1 mm around the injury epicenter. Taken together, these findings are consistent with increased recruitment of remaining motor units, including more fatigable, high-threshold motor units, during higher force, nonventilatory behaviors. Changes in diaphragm EMG activity following midcervical contusion injury reflect complex adaptations in neuromotor control that may increase the risk of motor-unit fatigue and compromise the ability to sustain higher force diaphragm efforts.
NEW & NOTEWORTHY The present study shows that unilateral contusion injury at C4 results in substantial loss of phrenic motoneurons but increased diaphragm muscle activity across a range of ventilatory and higher force, nonventilatory behaviors. Measures of neural drive indicate increased descending input to phrenic motoneurons that was more pronounced during higher force, nonventilatory behaviors. These findings reveal novel, complex adaptations in neuromotor control following injury, suggestive of increased recruitment of more fatigable, high-threshold motor units.
cervical spinal cord injuries (SCIs) commonly result in significant respiratory complications, which contribute considerably to the high mortality and morbidity of SCI patients (National Spinal Cord Injury Statistical Center 2016). Disruption of descending bulbospinal pathways to the phrenic nucleus and loss of phrenic motoneurons can directly impair the function of respiratory muscles, including the diaphragm muscle. Phrenic motoneurons innervating the diaphragm muscle are located from level C3 to C5 in rats (Furicchia and Goshgarian 1987; Goshgarian and Rafols 1981; Mantilla et al. 2009; Prakash et al. 2000) and humans (Keswani and Hollinshead 1955; Routal and Pal 1999). Following unilateral midcervical contusion injuries in rats, there is a substantial loss of phrenic motoneurons, e.g., ∼50% on the side injured (Alvarez-Argote et al. 2016; Nicaise et al. 2013), which should impact phrenic motor output. In support, Nicaise and colleagues (2012, 2013) showed that the amplitude of diaphragm compound muscle action potential, evoked by phrenic nerve stimulation, was markedly reduced in rats following C4 or C3 + C4 injuries. However, assessments of phrenic motor output using neurographic or electromyographic (EMG) recordings have not consistently revealed substantial decrements during ventilatory behaviors (Alvarez-Argote et al. 2016; Choi et al. 2005; el-Bohy et al. 1998; Golder et al. 2011; Lane et al. 2012; Nicaise et al. 2012, 2013).
Diaphragm EMG activity reflects the summation of motor-unit action potentials as they are recruited to accomplish different motor behaviors (Seven et al. 2014). Diaphragm motor units comprise a phrenic motoneuron and the muscle fibers it innervates, and each unit displays homogenous muscle fiber-type composition and contractile and fatigue properties (Sieck 1994; Sieck et al. 1989, 1996). Indeed, across various species, ventilation (i.e., eupneic breathing and breathing in hypoxic/hypercapnic conditions) requires relatively low levels of diaphragm force generation (∼10–30%) and can be accomplished by recruitment of <50% of diaphragm motor units, primarily those that are most fatigue resistant. Only when accomplishing higher force, nonventilatory behaviors (e.g., during sighs, sneezing, coughing, or airway occlusion) does recruitment of up to 90% of phrenic motor units become necessary (Mantilla et al. 2010; Mantilla and Sieck 2011; Seven et al. 2014; Sieck and Fournier 1989). With loss of phrenic motoneurons, the recruitment of diaphragm motor units across motor behaviors will be affected. Changes in the recruitment of diaphragm motor units during different motor behaviors should be reflected by the relative root mean square (RMS) of EMG activity (e.g., normalized to maximum) (Gill et al. 2015; Mantilla et al. 2014).
The present study systematically assessed the impact of a unilateral C4 spinal cord contusion injury on diaphragm RMS EMG activity, 1 and 7 days postinjury (DPI), across a range of ventilatory and higher force, nonventilatory behaviors in rats. We comprehensively characterized the extent of histological injury and quantified phrenic motoneuron loss at 7 DPI. We hypothesized that the loss of phrenic motoneurons following a unilateral midcervical contusion injury increases the relative diaphragm RMS EMG activity across all motor behaviors.
MATERIALS AND METHODS
Experimental animals.
A total of 29 adult male Sprague-Dawley rats (280–300 g) were obtained from Harlan Laboratories (Indianapolis, IN). Rats were randomly assigned to two experimental groups: unilateral C4 contusion (n = 17) or laminectomy (n = 12). All procedures were conducted in accordance with the American Physiological Society's Animal Care Guidelines and were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic. Animals were housed individually in cages under a 12-h light/dark cycle with ad libitum access to food and water. Animals were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) via intramuscular injection for all experimental and surgical procedures.
Assessment of diaphragm muscle activity.
Electrodes were placed in the diaphragm muscle using previously described techniques (Alvarez-Argote et al. 2016; Gransee et al. 2015; Mantilla et al. 2011; Martinez-Galvez et al. 2016). Briefly, a ∼3-mm section was bared on an insulated stainless-steel fine wire (AS631; Cooner Wire, Chatsworth, CA). Following induction of anesthesia, a midline laparotomy was performed, such that the inferior surface of the diaphragm was exposed, and a pair of electrodes was placed into the midcostal region of each hemidiaphragm muscle with an interelectrode distance of ∼3 mm. The midcostal region of the diaphragm muscle is expected to reflect innervation by cervical segments involved in the contusion injury, based on existing topographical information for diaphragm innervation (Laskowski and Sanes 1987). The electrode pair was secured in place using a surgical knot at the muscle surface, looped intra-abdominally, tunneled subcutaneously, and externalized and secured in the dorsum of the animal. A location between the shoulder blades bilaterally was selected to minimize animal tampering with the externalized electrodes. In all animals, diaphragm EMG electrodes were placed 4 days before surgery (laminectomy or contusion).
Baseline diaphragm EMG activity was recorded 1 day before injury, and further recordings were performed at 1 and 7 days postoperatively (laminectomy or contusion). Before each recording, the exposed electrode was dipped in 10% acetic acid and cleaned to remove any biofilm residue. Animals were anesthetized and placed on a heating pad (Model 1060; K&H Manufacturing, Colorado Springs, CO) for the duration of each experiment, except while EMG recordings were being collected. For every session, the EMG signal for each pair of diaphragm muscle electrodes was differentially amplified (gain: 2,000×) and band-pass filtered (20–1,000 Hz) using an analog amplifier (Model 2124; DATA, Ft. Collins, CO). The signal was digitized using a data acquisition board (National Instruments, Austin, TX) with sampling frequency of 2,000 Hz and recorded using a custom-made program (LabVIEW 8.2; National Instruments). The RMS amplitude of the EMG signal was calculated over a 100-ms window. Peak RMS (RMSpeak) was determined from the EMG signal, and the RMS value at 75 ms after the onset of diaphragm activity (RMS75) was used as an estimate of neural drive (Seven et al. 2014). Timing parameters, such as burst duration (in milliseconds), respiratory rate (in minutes−1), and duty cycle (in percent) were determined using the RMS EMG signal from the intact (contralateral) hemidiaphragm.
Experimental behaviors.
Diaphragm EMG activity was recorded for three different behaviors in the following order: 1) eupnea for ∼2 min; 2) hypoxia-hypercapnia (10% O2, 5% CO2) for ∼5 min, and 3) sustained airway occlusion via forced closure of the airway at end-expiration for ∼45 s, as in previous studies (Mantilla et al. 2010; Mantilla and Sieck 2011). Spontaneous deep breaths (“sighs”) were observed during spontaneous ventilatory behaviors and were defined as individual breaths with an RMSpeak EMG amplitude at least two-fold greater than eupneic breaths. In all cases, ∼5–10 min were allowed between behaviors for a stable eupneic breathing pattern to be restored. Animals were periodically checked for depth of anesthesia using a toe pinch, and a change in heart rate, whisker twitch, and hindlimb withdrawal was monitored. An appropriate level of anesthesia was verified before recording each behavior. RMS EMG amplitude was averaged during the entire 120-s exposure for eupnea, the last 60 s for hypoxia-hypercapnia, the last 10 s for occlusion, and for all sighs. In a previously published study, a noninvasive measurement of arterial oxygenation was used to verify that hemoglobin oxygen saturation returned to baseline levels during the recovery period between behaviors (Mantilla et al. 2011). Chronic diaphragm EMG recordings were included in the analysis if all time points were available for analysis on that side (as a result, contralateral diaphragm EMG recordings for 3 animals in the laminectomy group were not included).
Unilateral C4 contusion injury.
Unilateral midcervical contusion injury was performed, as reported previously (Alvarez-Argote et al. 2016). A midline incision was made in a caudal-to-rostral direction, starting from the T2 dorsal process to the base of the skull. Dorsal paravertebral muscles between C2 and T1 were incised and retracted. The posterior portion of cervical vertebrae was exposed, and a laminectomy was performed at the C4 level, while preserving the facet joints and leaving the dura intact. The laminectomy group (n = 12) was subjected to a laminectomy at the C4 level but no contusion injury. In the contusion group (n = 17), the rat was suspended by clamps secured on the C3 and C5 vertebrae. The rats were subjected to a single contusion injury, just lateral to the midline, using a 1.3-mm diameter impactor tip. A desired force of 100 kDy with 0 s dwell time was delivered using the Infinite Horizon Impactor (Precision Systems and Instrumentation, Lexington, KY). Muscle and skin layers were sutured using 3-0 Vicryl (polyglactin 910). Carprofen (50 mg/ml) was administered ad libitum in the drinking water and started 48 h before surgery. A single Buprenorphine SR (0.6 mg/kg) bolus was administered immediately postsurgery. Animals also received Penicillin G (25,000 U/kg) intramuscularly and parenteral dextrose and saline injections subcutaneously. Animals were maintained on a heating pad until alert and awake. Animals were monitored on a daily basis for signs of distress, dehydration, and weight loss, with appropriate veterinary care given as needed. Animals were excluded from the study if the impact achieved a force >110 kDy or if irregular displacement graphs were present, suggesting vertebral contact, impactor slip, or no displacement.
Histological characterization of injury.
A subset of animals was used for analyzing the extent of tissue damage following laminectomy or contusion (n = 4/group). At the terminal time point (7 DPI), animals were deeply anesthetized, euthanized by exsanguination, and transcardially perfused with 4% paraformaldehyde in 0.1 M PBS (pH 7.4) before tissue collection. The spinal cord was resected from C2 to T2, postfixed in 4% paraformaldehyde for 24 h, and transferred to 25% sucrose in 0.1 M PBS (pH 7.4) for 3 days at 4°C. The entire spinal cord was cut in five, 3 mm segments centered on the injury epicenter and assembled systematically, such that the blocks could be sectioned simultaneously in the transverse plane, generating equally spaced sets, representing the entire length of the spinal cord. Each set of ∼15 sections (each 20 μm thick and ∼200 μm apart) was frozen on Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA). One series of sections was used to label the perineuronal net using biotin-conjugated Wisteria floribunda agglutinin (WFA; Vector Laboratories, Burlingame, CA) by incubating serially in unlabeled streptavidin and biotin, biotin-WFA (1:200), and streptavidin Alexa Fluor 568 (1:50; Thermo Fisher Scientific). Another set of sections was processed for eriochrome cyanine staining to measure the extent of white-matter sparing, as previously described (Schonberg et al. 2007). Briefly, acetone-treated sections were sequentially incubated in Eriochrome cyanine solution, 5% iron alum (5 min), and borax-ferricyanide solution (5 min). All tissue sections were dehydrated in graded alcohol concentrations and coverslipped with DPX Mountant (Fluka; Sigma-Aldrich, St. Louis, MO).
Phrenic motoneuron labeling.
In a subset of animals (laminectomy n = 5; contusion n = 6), phrenic motoneurons were retrogradely labeled by injecting Alexa Fluor 488-conjugated cholera toxin subunit B (CTB) solution (Thermo Fisher Scientific) bilaterally into the intrapleural space, as previously described (Alvarez-Argote et al. 2016; Gransee et al. 2013, 2015; Mantilla et al. 2009, 2013a). Two injections of 10 μl 0.2% CTB were administered on each side of the thorax, 5 days before surgery. At the terminal experiment, tissue collection and cryoprotection were performed as above. Spinal cords embedded in cryomolds (VWR, Radnor, PA) were sectioned at 100 μm and mounted on Tissue Tack slides (Polysciences, Warrington, PA), precoated with Cell-Tak adhesive (Becton Dickinson Lab Ware, Bedford, MA). Sections were dehydrated in graded alcohol concentrations, coverslipped with DPX, and stored until imaging, usually within 1 wk of processing.
Microscopy and morphometrics.
Fluorescently labeled sections were imaged using a confocal microscope (Nikon Eclipse A1; Nikon Instruments, Melville, NY) with 488 nm (Argon) and 564 nm (solid-state) lasers. All images (12-bit, 1,024 × 1,024 pixel array) were obtained using a 10× Plan Fluor numerical aperture 0.30 objective (1.24 μm/pixel) with a 5- to 8-μm step size. All images were acquired using the same gain and laser intensity. Maximum intensity projection images from the confocal z-stack were created in tiff format using NIS-Elements Advanced Research Imaging Software (Nikon Instruments) and used for measurements of perineuronal net formation (using WFA) in ImageJ version 1.49 (National Institutes of Health, Bethesda, MD). For each animal, the section with the greatest perineuronal net formation was considered to contain the injury epicenter. Sections rostral and caudal to the injury epicenter (up to 1.2 mm rostral and 1.2 mm caudal) were analyzed for WFA fluorescence (∼200 μm apart). Across animals, measurements of perineuronal net formation were averaged for the respective distance from the injury epicenter.
Eriochrome cyanine-stained sections were imaged using an Olympus 4 × S Plan 4 numerical aperture 0.13 objective on a light microscope (Nikon Instruments). Brightfield images of Eriochrome cyanine-stained sections were stitched in Adobe Photoshop CS6 (Adobe, San Jose, CA). Stitched images were imported into ImageJ. The area encompassed by a normal myelin profile and devoid of cysts and dense granulations was defined as spared white matter. The section with the least spared white matter for each rat was considered the epicenter. The fraction of spared white-matter area (expressed as a percentage of total cross-sectional area) was determined at distances from the injury epicenter, ranging 0.8 mm rostral and caudal. Because ipsilateral-descending bulbospinal pathways to the phrenic motoneuron pool have been mapped in the ventromedial and lateral columns (Lipski et al. 1994), white-matter sparing specifically in the ipsilateral-ventral horn was also assessed.
Fluorescently labeled phrenic motoneurons were counted manually using the Cell Counter plugin in ImageJ. Motoneurons were counted only if the midnuclear portion was contained in the section. Total phrenic motoneuron counts were determined by analyzing consecutive sections on each side of the spinal cord.
Images were converted to 8 bit in NIS-Elements for presentation only. No thresholding or postimaging processing was applied for any images. Each channel was pseudocolored in Adobe Photoshop by changing the color gamut (red-green-blue).
Statistical analysis.
All statistical evaluations were performed using JMP statistical software (version 9.0.1; SAS Institute, Cary, NC) using a mixed linear model with animal as a random effect. For measurements done repeatedly on the same animal (e.g., diaphragm EMG amplitude and other parameters), comparisons were conducted using repeated measures with time, experimental group, and motor behavior (e.g., eupnea vs. hypoxia-hypercapnia) as grouping variables. Single-outcome measures per animal (i.e., motoneuron counts) were analyzed using a one-way ANOVA. Outcome measures done on multiple spinal cord sections (at various distances from the injury epicenter) for each animal (i.e., percent white-matter sparing, WFA fluorescence intensity) were analyzed using distance and experimental group as grouping variables. All experimental data are presented as means ± SE, unless specified otherwise. Statistical significance was established at the 0.05 level, and adjustment for sphericity in the mixed linear model was done using the Greenhouse-Geisser correction. When appropriate, post hoc analyses were conducted using Tukey-Kramer honestly significant difference (HSD).
RESULTS
Unilateral C4 contusion injury.
Twenty-nine animals received bilateral EMG electrodes in the midcostal diaphragm muscle and were randomly assigned to receive a unilateral contusion injury at C4 (n = 17) or laminectomy only (n = 12).
Contusion injury was effective in 12 animals, with a subset of animals excluded from further analyses based on the lack of evidence of tip displacement or hematoma (n = 4) or the impactor tip slipping (n = 1). In all other cases, a hematoma was visually evident under the surgical microscope on the side of contusion only. Average impact force for the contusion group was 102 ± 0 kDy, and average displacement was 1,280 ± 58 μm. In the laminectomy group (n = 12), integrity of the dura mater and lack of accidental bruising were visually verified before surgical closure in all animals. All animals recovered successfully from the surgery without the need for mechanical ventilation. Following surgery, all animals in the contusion group exhibited a motor deficit with clenching of the paw ipsilateral to the injury. No animals in the laminectomy group displayed such deficits. At the terminal experiment, upon dissection of the spinal cord, level and laterality of laminectomy and injury were also confirmed in all animals by locating the cervical vertebral level and dorsal root relationship to the bruise.
Body weight was measured on the day of electrode placement, before contusion or laminectomy, and terminally. There was an overall interaction between time and experimental group (F2,44 = 6.2; P = 0.004) on body weight. There was no effect of experimental group on body weight (F1,22 = 0.4; P = 0.549) but an effect of time (F2,44 = 6.8; P = 0.003). Five days following electrode placement, animals in the laminectomy and contusion groups lost ∼3% of initial body weight (across groups: 310 ± 5 vs. 300 ± 5 g). The laminectomy group subsequently regained most of the weight by 7 DPI (99.2 ± 5.0% of initial), but the contusion group lost more weight by 7 DPI (92.4 ± 6.0%), and this difference was statistically significant.
Characterization of C4 contusion injury.
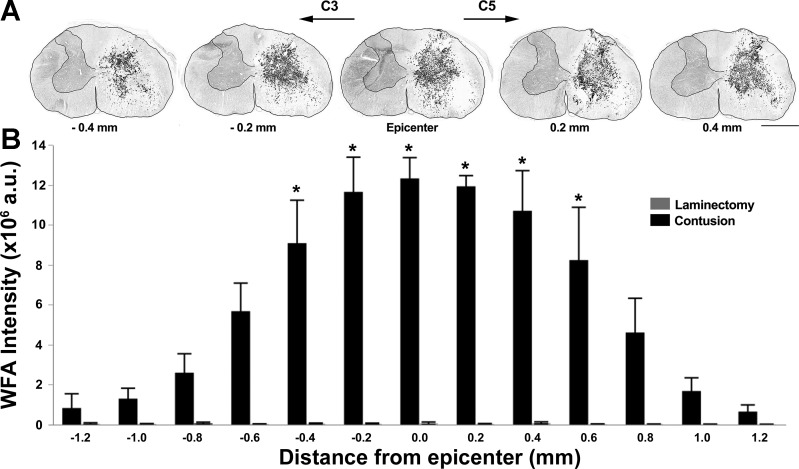
In the first set of analyses, the amount of WFA immunoreactivity in the spinal cord, indicating formation of a perineural net, was used to determine the injury epicenter and extent of injured spinal cord tissue. The level of the injury epicenter was also confirmed by comparing gray-matter horn anatomy in the section with the highest WFA immunoreactivity to a standard rat spinal cord atlas (Sengul et al. 2012). Visually, WFA immunoreactivity was negligible in all animals in the laminectomy group and on the contralateral side of the spinal cord in all unilateral C4 contusion animals (Fig. 1A). There was evidence of increased perineuronal net formation in the C4 contusion animals with WFA immunoreactivity spanning gray- and white-matter regions ipsilaterally. The measurement of integrated fluorescence intensity for WFA showed an interaction between distance from injury epicenter and experimental group (F12,66 = 13.7; P < 0.001), as well as an effect of distance (F12,66 = 13.8; P < 0.001) and experimental group (F1,6 = 38.8; P < 0.001). Overall, increased perineuronal net formation was evident for 1 mm around the injury epicenter in contusion animals (Fig. 1B).
Fig. 1.
Extent of perineuronal net formation around injury epicenter at 7 days following a unilateral C4 contusion injury. A: representative 20 μm-thick transverse sections from an individual unilateral C4 contusion animal displaying Wisteria floribunda agglutinin (WFA) immunoreactivity (grayscale; black reflects greater immunoreactivity) in 5 sections (∼200 μm apart), spanning 0.4 mm rostral to 0.4 mm caudal to the injury epicenter. An outline of the spinal cord section and contralateral gray matter is presented for clarity. B: summary of integrated WFA immunoreactivity (fluorescence intensity), 2.4 mm around the injury epicenter in laminectomy (n = 4) and contusion (n = 4) groups. A significant increase in perineuronal net formation was evident at a distance 0.4 mm rostral to 0.6 mm caudal to the injury site (*P < 0.001). Original scale bar, 1 mm.
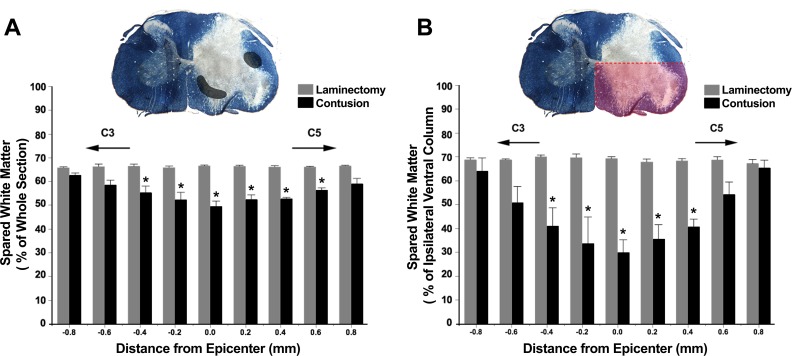
In a second set of analyses, differentiation of white and gray matter in the spinal cord using the Eriochrome cyanine histological stain was used to examine loss of white-matter integrity following a unilateral C4 contusion injury. At the injury epicenter, white-matter pathology was evident in the dorsal, lateral, and ventral white-matter columns (Fig. 2A). The percent of spinal cord area reflecting intact white matter showed an overall interaction between distance from the injury epicenter and experimental group (F8,53 = 4.4; P < 0.001), as well as an effect of distance (F8,53 = 4.0; P < 0.001) and experimental group (F1,18 = 26.5; P < 0.001). Overall, there were significant differences in the percent of spared white matter for 1 mm around the injury epicenter, consistent with the WFA findings. In addition, the percent of white matter spared in the ipsilateral-ventral columns of the spinal cord was quantified, since descending bulbospinal inputs to phrenic motoneurons are located in these columns (Boulenguez et al. 2007; Dobbins and Feldman 1994; Ellenberger and Feldman 1988) (Fig. 2B). There was an overall interaction between distance from epicenter and experimental group (F8,48 = 8.2; P < 0.001), as well as an effect of distance (F8,48 = 7.2; P < 0.001) and experimental group (F1,6 = 22.6; P = 0.003). Overall, significant differences in the fraction of spared white matter were present, 0.8 mm around the injury epicenter.
Fig. 2.
White-matter sparing around the injury epicenter at 7 days following a unilateral C4 contusion injury. A: summary of percent white matter spared, 1.6 mm around the injury epicenter in laminectomy (n = 4) and contusion (n = 4) groups. Evidence of significant white-matter compromise is present, 1 mm around the injury site (*P < 0.001). Spinal cord cross-section is a representative 20-μm-thick eriochrome cyanine stained section at the injury epicenter of a C4 contusion animal. Gray-shaded areas represent expected location of descending bulbospinal inputs to phrenic motoneurons in the ventromedial and lateral columns. B: summary of percent white matter spared in the ipsilateral-ventral columns, 1.6 mm around the injury epicenter in laminectomy (n = 4) and contusion (n = 4) groups (*P < 0.001, mixed linear model; post hoc Tukey-Kramer HSD at P < 0.05). The ipsilateral-ventral area quantified is highlighted in red in the spinal cord cross-section.
Phrenic motoneuron loss.
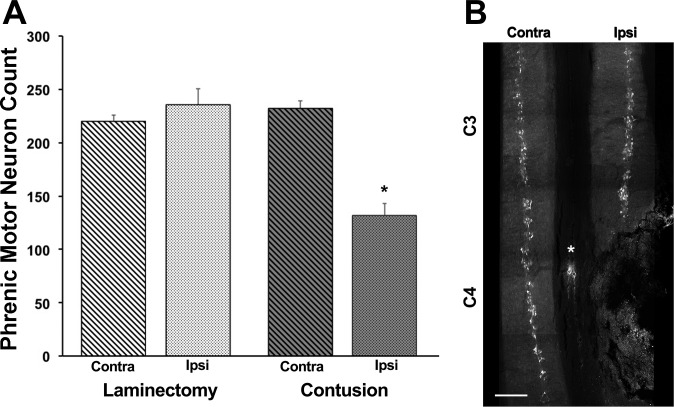
Extensive labeling of phrenic motoneurons in the ventral horn of cervical segments C3–C5 was achieved using Alexa 488-conjugated CTB (Fig. 3). Phrenic motoneuron counts were consistent in the laminectomy animals, with no significant difference between the ipsilateral and contralateral motoneuron counts in laminectomy animals and contralateral to the C4 contusion injury (∼230 motoneurons/side). There was an overall interaction in phrenic motoneuron counts between spinal cord side and experimental group (F1,9 = 54.4; P < 0.001), as well as an effect of side (F1,9 = 29.1; P < 0.001) and group (F1,9 = 14.3; P = 0.004). In the contusion group, the phrenic motoneuron count on the ipsilateral side of the injury was significantly lower (43%) than the contralateral side and laminectomy animals (P < 0.05). Phrenic motoneuron loss appeared to be predominantly at the lower portion of C4 and most of C5.
Fig. 3.
Phrenic motoneuron counts in laminectomy and unilateral C4 contusion animals at 7 days postinjury (DPI). A: summary of phrenic motoneuron counts in the ipsilateral (Ipsi) and contralateral (Contra) spinal cord in laminectomy (n = 5) and contusion (n = 6) groups. Animals in the contusion group had extensive and significant phrenic motoneuron loss at 7 DPI (*P < 0.001, mixed linear model; post hoc Tukey-Kramer HSD at P < 0.05). B: representative image of retrogradely labeled phrenic motoneurons obtained from consecutive maximum intensity projections of 100 μm-thick longitudinal spinal cord sections for an animal with a unilateral C4 contusion injury. *Artifact in fluorescent image. Original scale bar, 0.5 mm.
Diaphragm EMG across motor behaviors.
Diaphragm EMG was successfully recorded at preinjury and at 1 and 7 DPI in both the laminectomy (n = 12 ipsilateral; n = 9 contralateral) and contusion (n = 12 ipsilateral and contralateral) groups. To quantify the amplitude of EMG activity on each side of the diaphragm over time, RMSpeak EMG values for all respiratory behaviors were normalized to the ipsilateral preinjury (−1 DPI) amplitude (RMSpeak value) for sighs in the same animal. We have previously shown that the normalization of RMSpeak EMG values to near-maximal behaviors, such as deep breath (sigh), results in reduced intra-animal variability over time, permitting consistent EMG analyses with chronically implanted diaphragm electrodes (Mantilla et al. 2011).
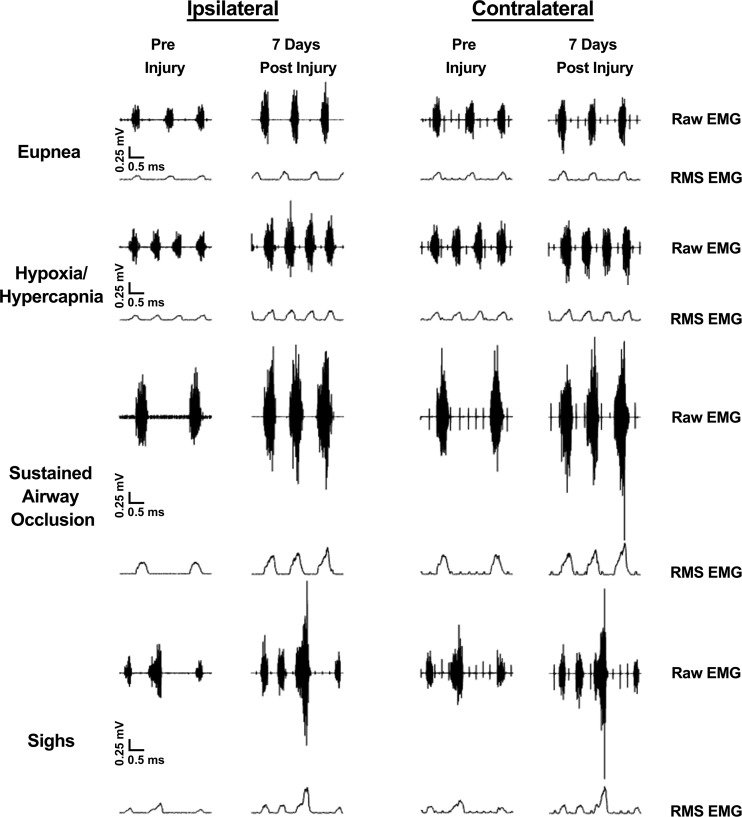
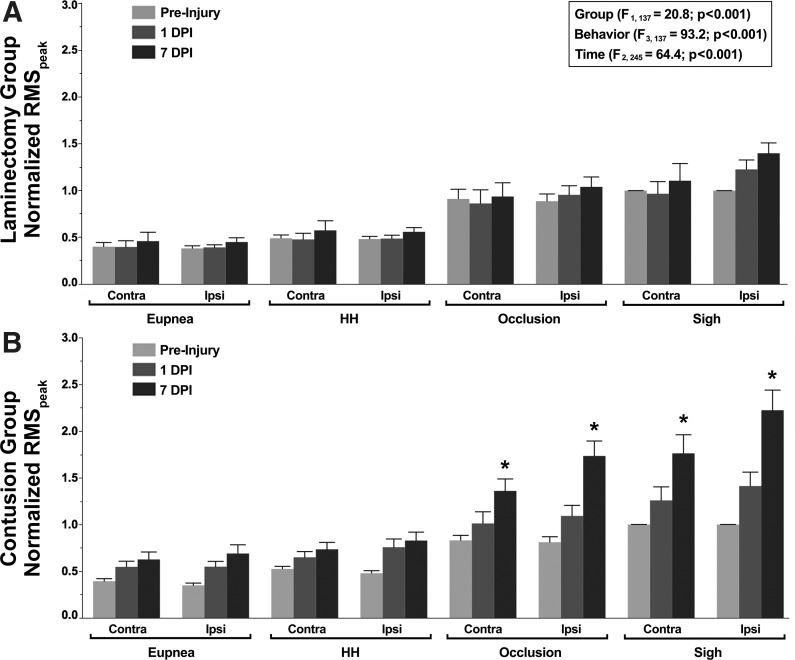
Representative diaphragm EMG recordings and RMSpeak EMG amplitude obtained across ventilatory and higher force, nonventilatory motor behaviors in a contusion animal at −1, 1, and 7 DPI are shown in Fig. 4. Changes in normalized diaphragm RMSpeak EMG amplitude over time across behaviors are summarized in Fig. 5. There was an overall effect on normalized RMSpeak EMG amplitude evident in the repeated-measures analysis (F27,245 = 5.8; P < 0.001) but no interaction among experimental group, behavior, time (DPI), and side (F5,245 = 0.2; P = 0.975). There was an effect of group (F1,137 = 20.8; P < 0.001), behavior (F3,137 = 93.2; P < 0.001), and time (F2,245 = 64.4; P < 0.001). Significant interactions among group·behavior·time (F5,245 = 4.0; P = 0.001), group·time (F2,245 = 33.6; P < 0.001), behavior·time (F5,245 = 6.5; P < 0.001), and time·side (F2,244 = 6.2; P = 0.004) were observed. Overall, normalized RMSpeak EMG amplitude was higher in the contusion group compared with the laminectomy group. Regardless of experimental group or time postinjury, normalized RMSpeak EMG amplitude during sigh and occlusion was significantly greater than during exposure to hypoxia-hypercapnia or eupnea. In the contusion group, a significant increase in normalized RMSpeak EMG amplitude was evident for sighs and occlusion from −1 to 7 DPI on both sides of the diaphragm (1.5- to 2.0-fold increase). In the laminectomy group, there was no significant change in normalized RMSpeak EMG amplitude over time for either side of the diaphragm within each behavior.
Fig. 4.
Representative ipsilateral and contralateral compound diaphragm EMG and root mean square (RMS) EMG recordings across eupnea, hypoxia-hypercapnia (10% O2, 5% CO2), airway occlusion, and spontaneous deep breath (sigh) from a C4 contusion animal at −1 and 7 days postinjury. Note increased diaphragm RMS EMG activity during sighs and airway occlusion compared with the preinjury levels.
Fig. 5.
Bilateral chronic diaphragm EMG activity for animals in the laminectomy (A; n = 9–12) and C4 unilateral contusion (B; n = 12) groups. Summary of diaphragm RMS EMG amplitude (RMSpeak) at preinjury (light gray) and 1 (dark gray) and 7 (black) days postinjury (DPI) normalized to the preinjury RMS EMG amplitude for sigh. In the repeated-measures analysis, there was an effect of group, behavior, and time. Animals in the laminectomy group displayed stable chronic diaphragm EMG values for all respiratory motor behaviors across the 8-day recording period. At 7 DPI, animals in the contusion group displayed significantly higher ipsilateral and contralateral RMS EMG amplitudes compared with the preinjury and 1 DPI values during occlusion and sighs (*post hoc Tukey-Kramer HSD at P < 0.05). HH, hypoxia-hypercapnia.
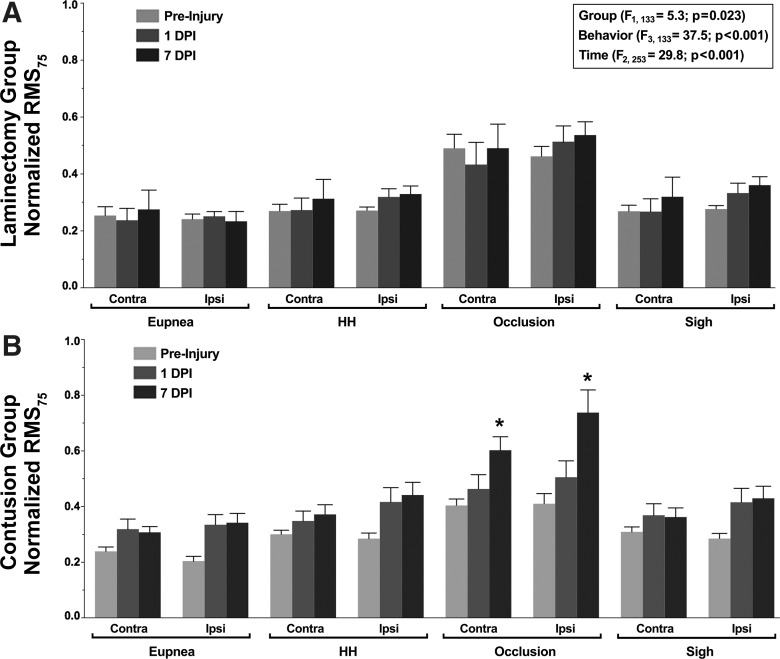
In addition, an estimate of neural drive to phrenic motoneurons was obtained from normalized RMS75 EMG values (Seven et al. 2014). Figure 6 summarizes the changes in RMS75 EMG values over time across ventilatory and higher force, nonventilatory behaviors following a unilateral C4 contusion injury or a laminectomy. There was an overall effect on the amplitude of RMS75 EMG, evident in the repeated-measures analysis (F29,253 = 2.7; P < 0.001), but no overall interaction among experimental group, behavior, time (DPI), and side (F6,253 = 2.7; P = 0.962). There was an effect of group (F1,133 = 5.3; P = 0.023), behavior (F3,133 = 37.5; P < 0.001), and time (F2,253 = 29.8; P < 0.001). Significant interactions among group·behavior·time (F6,253 = 3.2; P = 0.005), group·time (F2,253 = 12.5; P < 0.001), behavior·time (F6,253 = 2.7; P = 0.049), and time·side (F2,253 = 2.2; P = 0.044) were observed. Overall, normalized RMS75 EMG values were higher in the contusion group compared with the laminectomy group. Regardless of experimental group or time postinjury, RMS75 EMG values during occlusion were significantly greater than during sigh or exposure to hypoxia-hypercapnia or eupnea. In the contusion group, there was a significant increase in the RMS75 EMG value during airway occlusion from −1 to 7 DPI for both sides of the diaphragm (1.5- to 1.8-fold increase). In the laminectomy group, there was no significant change in RMS75 EMG amplitude over time for either side of the diaphragm within each behavior.
Fig. 6.
Estimated neural drive to phrenic motoneurons in the laminectomy (A; n = 9–12) and unilateral C4 contusion (B; n = 12) groups. Neural drive was defined as the RMS value at 75 ms after the onset of diaphragm activity (RMS75) and was normalized to the preinjury RMS EMG amplitude during sigh. In the repeated-measures analysis, there was an effect of group, behavior, and time. Animals in the laminectomy group displayed stable chronic diaphragm RMS75 EMG values for all respiratory motor behaviors across the 8-day recording period. Animals in the contusion group displayed RMS75 EMG values during airway occlusion that were significantly higher at 7 DPI compared with preinjury and 1 DPI (*post hoc Tukey-Kramer HSD at P < 0.05).
Parameters of diaphragm EMG recordings across behaviors.
Respiratory rate and duty cycle were measured from diaphragm EMG recordings during eupnea and during hypoxic-hypercapnic conditions at −1, 1, and 7 DPI. Additionally, burst duration for sighs and during sustained airway occlusion was obtained (Table 1). An overall effect on respiratory rate was evident in the repeated-measures analysis (F6,84 = 2.5; P = 0.028) but no interaction among experimental group, behavior, and time (DPI; F2,84 = 0.5; P = 0.591). There was a significant effect of group (F1,42 = 7.4; P = 0.009), behavior (F1,42 = 87.2; P < 0.001), and time (F2,109 = 29.2; P < 0.001), as well as an interaction with group·time (F2,84 = 4.8; P = 0.01). Respiratory rate was greater during hypoxia-hypercapnia compared with eupnea at all time points for both experimental groups. During eupnea and hypoxia-hypercapnia, there were no differences in respiratory rate among groups at any time points in either the laminectomy or contusion group. Sighs were evident across spontaneous ventilatory behaviors in most animals (20 out of 24) at −1, 1, and 7 DPI, independent of group. In the laminectomy group, two animals did not display sighs at 1 DPI and one at 7 DPI (>5 min). In the contusion group, one animal did not display sighs at both 1 and 7 DPI during the recording period. Overall, sighs were observed at a frequency of 1.1 ± 0.4 min−1. Repeated-measures analysis showed no overall effect of experimental group or time (DPI) on sigh frequency (F4,30 = 1.7; P = 0.179).
Table 1.
Ventilatory parameters following unilateral midcervical contusion at C4 or laminectomy were determined from diaphragm EMG recordings over time at preinjury (−1) and 1 and 7 days postinjury (DPI)
| Experimental Group |
|||||||
|---|---|---|---|---|---|---|---|
| Laminectomy (n = 12) |
Contusion (n = 12) |
||||||
| Behavior | Ventilatory Parameters | Preinjury | DPI 1 | DPI 7 | Preinjury | DPI 1 | DPI 7 |
| Eupnea | Respiratory rate, min−1 | 80 ± 14 | 64 ± 6 | 70 ± 8 | 80 ± 15 | 51 ± 12* | 65 ± 11 |
| Burst duration, ms | 248 ± 28 | 279 ± 38 | 291 ± 47 | 282 ± 57 | 380 ± 87*† | 289 ± 63‡ | |
| Duty cycle, % | 32 ± 5 | 29 ± 4 | 33 ± 6 | 37 ± 8 | 31 ± 4 | 31 ± 7 | |
| Hypoxia-hypercapnia | Respiratory rate, min−1 | 101 ± 13 | 95 ± 16 | 100 ± 12 | 100 ± 19 | 74 ± 15*† | 96 ± 19 |
| Burst duration, ms | 267 ± 34 | 279 ± 28 | 288 ± 30 | 269 ± 36 | 364 ± 75* | 305 ± 60 | |
| Duty cycle, % | 44 ± 6 | 44 ± 6 | 47 ± 5 | 44 ± 6 | 43 ± 6 | 48 ± 10 | |
| Airway occlusion | Burst duration, ms | 370 ± 31 | 432 ± 55 | 400 ± 41 | 369 ± 66 | 494 ± 88* | 396 ± 96‡ |
| Sighs | Frequency, min−1 | 1.2 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.3 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.4 |
| Burst duration, ms | 489 ± 70 | 493 ± 50 | 527 ± 45 | 464 ± 42 | 549 ± 77* | 580 ± 57* | |
Comparisons were conducted using a mixed linear model with animal as a random effect (see materials and methods for details). Data presented as means ± SD. Duty cycle was calculated as the percentage of total time corresponding to inspiration.
P < 0.05 different than preinjury values within the same group during the same behavior.
P < 0.05 different than the laminectomy group during the same behavior at the same time point.
P < 0.05 different than DPI 1 within the same group during the same behavior.
An overall effect on burst duration was evident in the repeated-measures analysis (F13,139 = 3.8; P < 0.001) but no interaction among experimental group, behavior, and time (DPI; F6,139 = 1.2; P = 0.322). There was a significant effect of group (F1,75 = 9.9; P = 0.002), behavior (F3,75 = 117.9; P < 0.001), and time (F2,139 = 3.8; P < 0.001), as well as interactions between group·time (F2,139 = 10.7; P < 0.001) and behavior·time (F6,139 = 3.4; P = 0.005). Burst duration was greater during sighs compared with occlusion, which itself was greater than hypoxia-hypercapnia and eupnea, at all time points for both experimental groups. There was no difference in burst duration during hypoxic-hypercapnic and eupneic breaths. During eupnea, burst duration was longer in the contusion group at 1 DPI compared with the laminectomy group, but this difference was no longer present at 7 DPI. Within the contusion group, burst duration during all behaviors was significantly longer at 1 DPI compared with preinjury values. By 7 DPI, this difference was no longer evident during eupnea, hypoxia-hypercapnia, and occlusion but remained elevated at 7 DPI during sighs. Repeated-measures analysis showed no overall effect on duty cycle (percent; F5,70 = 2.0; P = 0.084). Within the groups, there was no significant change in duty cycle at any of the time points across behaviors.
DISCUSSION
The major observation in this study was that 7 days after a unilateral C4 contusion injury, there is a 43% loss of phrenic motoneurons, whereas there is an increase in relative diaphragm RMS EMG activity across a range of motor behaviors, with an associated increase in neural drive (RMS75). However, the increase in diaphragm RMS EMG activity was more pronounced during higher force, nonventilatory motor behaviors. The bilateral increase in diaphragm RMS EMG was evident at 1 DPI but increased markedly by 7 days, especially for higher force, nonventilatory behaviors. There was evidence of increased perineuronal net formation and marked ipsilateral white-matter loss around the injury epicenter that may have affected premotor input (both descending and ascending) to surviving phrenic motoneurons. Taken together, these results highlight compensatory changes in neuromotor control of phrenic motoneurons that maintain force [transdiaphragmatic pressure (Pdi)] generation by the diaphragm muscle and support motor behaviors necessary for airway clearance.
Contusion-type injuries are the most common mechanism of SCI, and midcervical SCIs result in significant respiratory complications in humans, with substantial effects on long-term morbidity, as well as mortality (Winslow and Rozovsky 2003). However, animal models of midcervical contusion injuries are inconsistent in demonstrating alterations in phrenic nerve and diaphragm muscle activity or ventilation (Alvarez-Argote et al. 2016; Choi et al. 2005; el-Bohy et al. 1998; Golder et al. 2011; Lane et al. 2012; Nicaise et al. 2012, 2013). The diaphragm muscle has a considerable reserve capacity for force generation, and it is crucial to conduct systematic, functional assessments that include higher force, nonventilatory motor behaviors. Indeed, complete unilateral denervation and paralysis of the right hemidiaphragm (effectively the same as the total loss of phrenic motoneurons on that side) result in a 37% reduction in maximum Pdi induced by phrenic nerve stimulation but do not impair Pdi during ventilatory behaviors and result in only an ∼20% reduction in Pdi during higher force, nonventilatory behaviors (Gill et al. 2015). Thus it is not unexpected that unilateral contusion injuries, which result in less overall loss of diaphragm motor units, will have minimal impact on Pdi generation across a range of motor behaviors. Indeed, this was confirmed in two animals with C4 contusion injury. However, this Pdi generation must be achieved with fewer diaphragm motor units. Thus contusion injury will affect the recruitment of surviving phrenic motoneurons, as reflected by the increase in diaphragm RMS EMG activity. The impact of cervical contusion injury on respiratory motor output was evident across multiple motor behaviors, including both ventilatory and higher force, nonventilatory behaviors necessary for airway clearance.
Phrenic motoneuron loss.
In the present study, ipsilateral phrenic motoneuron loss was confirmed at 7 days following unilateral C4 contusion injury, based on a validated retrograde-labeling technique that reliably labels the entire phrenic motoneuron pool in rats (Mantilla et al. 2009). The extent of phrenic motoneuron loss following unilateral C4 contusion injury that we observed is consistent with previous studies that reported phrenic motoneuron loss ranging from 39% to 54% (Alvarez-Argote et al. 2016; Nicaise et al. 2012, 2013). The variance in motoneuron loss may relate to the intensity of impact (100–395 kDy) and the level of contusion injury (C3–C4). In agreement with previous studies using a C4 contusion-injury model, nearly all phrenic motoneurons, at and below the injury epicenter, were lost (C4–C5), with the majority of surviving phrenic motoneurons located rostral to the injury epicenter (Nicaise et al. 2012, 2013).
Changes in diaphragm EMG activity following unilateral C4 contusion.
In studies by Nicaise and colleagues (2012, 2013), the loss of phrenic motoneurons, which occurred as early as 24 h after unilateral contusion, was clearly evident by a reduction in the diaphragm compound muscle action potential amplitude. However, the effect of phrenic motoneuron loss on phrenic neurographic (el-Bohy et al. 1998; Golder et al. 2011) or diaphragm EMG activity (Alvarez-Argote et al. 2016; Lane et al. 2012; Nicaise et al. 2012) is more difficult to interpret. In fact, most studies have reported either no change or an increase in phrenic nerve activity (el-Bohy et al. 1998; Golder et al. 2011) and no or minimal change in diaphragm EMG activity (Alvarez-Argote et al. 2016; Lane et al. 2012; Nicaise et al. 2012). Most of these studies examined phrenic nerve or diaphragm muscle EMG activity only at the terminal experiment and did not assess a full range of motor behaviors.
In our previous study (Alvarez-Argote et al. 2016), we did examine the effect of C3 or C5 contusion injury on diaphragm EMG activity using a longitudinal analysis that compared EMG activity at 1–14 DPI with a preinjury baseline (as in the present study). However, we only assessed diaphragm EMG activity during eupnea and found minimal impact. In this previous study, we found that unilateral C3 contusion resulted in ∼39% loss of phrenic motoneurons, whereas C5 contusion resulted in ∼13% loss. The present study extends our previous results by exploring changes in diaphragm EMG across a range of motor behaviors. As in our previous study, we used EMG recordings that allowed longitudinal comparison of diaphragm EMG activities at 1 day before unilateral C4 contusion injury with activities at 1 and 7 DPI.
Diaphragm EMG amplitudes in the control laminectomy group were largely unchanged over the 8-day duration of experimentation. This is consistent with previous studies in which we used chronic diaphragm EMG recordings (Alvarez-Argote et al. 2016; Gransee et al. 2015; Mantilla et al. 2011). In the contusion group, an increase in normalized diaphragm RMS EMG amplitude at 7 DPI was observed on both ipsilateral and contralateral sides and was more pronounced during higher force, nonventilatory behaviors compared with eupnea and hypoxia-hypercapnia. This increase in diaphragm muscle EMG activity was accompanied by an increase in neural drive (as determined by the RMS75). After unilateral midcervical contusion, timing parameters, such as respiratory rate, were mostly unaffected, with either only a transient decrease (at 1 DPI in the present study) or no change in respiratory rate (Alvarez-Argote et al. 2016; el-Bohy et al. 1998; Lane et al. 2012). A transient increase in inspiratory duration during eupneic and hypoxic-hypercapnic breathing was observed at 1 DPI, but there was no change on duty cycle. The minimal changes in respiratory parameters following unilateral midcervical contusion are also consistent with results from studies using a unilateral spinal cord hemisection at C2, where the descending excitatory drive to the phrenic motor pool is removed ipsilaterally, causing hemidiaphragm paralysis (Gransee et al. 2013, 2015, 2016; Mantilla et al. 2013a, b; Martinez-Galvez et al. 2016).
A few studies assessing changes in phrenic nerve activity following cervical contusion normalized integrated activity during eupnea to either activity during hypercapnia (Golder et al. 2011) or asphyxia (el-Bohy et al. 1998). Golder et al. (2011) reported no change in phrenic nerve activity during eupnea (normalized to hypercapnia) after unilateral C4 contusion. In contrast, El-Bohy et al. (1998) reported that phrenic nerve activity during eupnea (normalized to asphyxia) increased following a bilateral midline contusion injury involving C4 and C5. These latter results are consistent with the results of the present study, suggesting that recruitment of surviving phrenic motoneurons increases after contusion injury. We have previously established that the normalization of EMG amplitude to preinjury EMG activity of a higher force behavior, such as a sigh (or airway occlusion), allows for significantly reduced intra-animal variability and permits quantitative assessment of EMG changes over time (Mantilla et al. 2011). The results of the present study highlight the importance of conducting longitudinal assessments across a range of motor behaviors, including higher force, nonventilatory behaviors of the diaphragm muscle.
Significant loss of diaphragm motor units ipsilateral to the C4 contusion did not reduce measures of diaphragm EMG activity, reflecting sampling of spared motor units in diaphragm EMG recordings. Previous studies support extensive overlap in the segmental innervation of the rat diaphragm muscle (Alvarez-Argote et al. 2016; Fournier and Sieck 1988b; Gordon and Richmond 1990; Li et al. 2015). Electrode placement in the midcostal diaphragm was selected based on available information regarding the topographical organization of diaphragm innervation (Laskowski and Sanes 1987). In agreement, a previous study (Alvarez-Argote et al. 2016) found minimal impact of either unilateral C3 or C5 contusion injuries on diaphragm EMG activity. Studies examining diaphragm muscle denervation following unilateral contusion demonstrate segmental innervation with substantial overlap, with C3 contusions primarily (but not exclusively) showing denervation in more ventral regions (Alvarez-Argote et al. 2016) compared with a C4 contusion (Li et al. 2015). Collectively, the findings of the present study suggest that the loss of phrenic motoneurons due to cervical contusion injury activates compensatory mechanisms to maintain the range of motor behaviors.
The increase in diaphragm muscle EMG activity following unilateral C4 contusion was more pronounced during higher force, nonventilatory behaviors compared with ventilatory behaviors. Following midcervical contusion injury and associated phrenic motoneuron loss, increased diaphragm muscle EMG activity could result from the following: 1) recruitment of additional motor units or 2) increased motor-unit firing frequency (Kong and Berger 1986; Seven et al. 2013, 2014), both consistent with the increased neural drive that was observed. Additionally, an increase in RMS EMG amplitude can result from synchronization of motor-unit firing (Yao et al. 2000). Forces generated during nonventilatory behaviors, such as occlusion and sighs, typically require activation of more fatigable motor-unit types across a range of species (Fournier and Sieck 1988a; Mantilla et al. 2010; Mantilla and Sieck 2011; Seven et al. 2014; Sieck and Fournier 1989). Recruitment of these additional, more fatigable motor units may result in motor-unit fatigue, especially if firing frequencies are also increased. As a result, the loss of phrenic motoneurons following contusion would likely predispose animals to fatigue during repetitive contractions and compromise the ability to sustain higher force diaphragm efforts. Although this remains unexplored, as we did not seek to induce fatigue in any of the behaviors, it would be important to ascertain whether higher force behaviors can be sustained with repetitive activation in conditions of traumatic SCI associated with motoneuron loss.
Airway occlusion was performed at end-expiration, and since the occlusion prevents a change in lung volume while increasing transpulmonary pressure, activation of vagal afferents, including pulmonary stretch receptors, is expected to be reduced (Pate and Davenport 2012). In agreement with reduced vagal activity, we observed a prolongation of burst duration during occlusion (Table 1). By 7 DPI, burst duration returns to preinjury levels, consistent with no change in functional residual capacity after cervical SCI (Loveridge et al. 1992) and restoration of vagal-mediated reflexes postinjury (Lee and Kuo 2016).
Contusion injury.
Histological examination of the spinal cord at 7 DPI revealed a consistent lateral contusion at cervical level C4, extending into levels C3 and C5. An established hallmark of SCI is the upregulation of chondroitin sulfate proteoglycans around the injury epicenter (Andrews et al. 2012; Jones et al. 2003). Chondroitin sulfate proteoglycans are integral constituents of perineuronal nets that form a specialized, condensed extracellular matrix around neurons and astrocytes in the central nervous system following SCI and likely contribute to forming a significant barrier to recovery (Silver and Miller 2004). Following a unilateral C4 contusion, there was evidence of increased perineuronal net formation, 1 mm around the injury epicenter. It is important to note that WFA immunoreactivity was evident in laminectomy animals and in rostral and caudal regions of the spinal cord at distances >1 mm from the injury, but WFA levels were negligible compared with the substantial increase around the injury epicenter following contusion. Furthermore, increased perineuronal net formation was evident unilaterally around the injury in both gray- and white-matter regions. The observed extracellular expression appears to enwrap cells in a lattice-like manner in the injured milieu and parallels previous reports of WFA expression pattern (Karetko and Skangiel-Kramska 2009; Massey et al. 2006). The immunolabeling of the perineuronal net reliably assessed the degree of tissue trauma following a spinal cord contusion injury.
White-matter sparing.
In contusion animals, substantial white-matter damage was observed around the lesion epicenter, which would disrupt both descending ipsilateral-bulbospinal innervation (ventromedial and lateral columns) (Lipski et al. 1994) and ascending propriospinal inputs (lateral columns) (Decima and von Euler 1969; Dimarco and Kowalski 2013; Remmers and Tsiaras 1973) to the phrenic nucleus. Of note, other studies, including those using higher contusion-injury forces, report similar patterns of white-matter injury encompassing the dorsal, lateral, and ventral funiculi (Awad et al. 2013; Choi et al. 2005; Lane et al. 2012).
Contusion-injury models may vary in the laterality of the lesion and the subsequent white-matter compromise. In the present study, the unilateral contusion impact was delivered dorsally at the center of the ipsilateral spinal cord. This approach was preferred to maximize trauma to the ventral gray matter containing phrenic motoneurons, as well as the ventromedial and lateral funiculi when using a 100-kDy force. A previous study using a lateralized, 395-kDy unilateral C4 contusion injury reported tissue pathology being more prominent in the lateral funiculus, although lesion to the ventral funiculus was more variable, and the extent of white-matter pathology was not assessed (Nicaise et al. 2012). In the study by Lane et al. (2012), a 150- and 250-kDy C4/C5 midline contusion resulted in near-complete loss of ventromedial white matter, but a distinct portion of the lateral columns remained intact.
Apart from descending innervation from the brain stem, various other anatomical substrates (intercostal-phrenic reflex, interneurons, propriospinal inputs) have been implicated in modulating phrenic motoneuron output (Bellingham and Lipski 1990; Billig et al. 2000; DiMarco and Kowalski 2009; Lane et al. 2008; Lipski et al. 1993). Of interest, intercostal-to-phrenic reflexes can be activated by mechanical stimulation of the ribcage, including chest compression and intercostal muscle stretch, and have been proposed to play a role in phrenic inhibition during airway occlusion (Corda et al. 1965; Homma 1980; Remmers 1970; Remmers and Marttila 1975). These afferent fibers to the phrenic nucleus ascend in the lateral and ventral-lateral columns (Decima and von Euler 1969; Dimarco and Kowalski 2013; Remmers 1970; Remmers and Tsiaras 1973). White-matter damage to these tracts, following a midcervical contusion injury, may contribute to the increase in diaphragm muscle activation by the following: 1) removal of inhibition of outflow from medullary inspiratory neurons (Bolser and Remmers 1989; Shannon 1980) or 2) removal of inhibition of phrenic motoneuron output via disruption of ascending inhibitory projections (Bellingham 1999). Disruption of white-matter tracts following SCI may contribute to significant variability in the extent of motor recovery postinjury. In humans, the segmental level of SCI is the main determinant of long-term motor function (Kramer et al. 2012). Better characterization of injury to funiculi providing motor control (descending, segmental, and ascending) to the motoneuron pools of interest will be necessary to establish mechanisms of neuroplasticity and neuroregeneration in preclinical models of SCI.
GRANTS
Support for this work was provided by the National Heart, Lung, and Blood Institute (Grant R01 HL96750).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.R., G.C.S., and C.B.M. conceived and designed research; S.R. performed experiments; S.R., G.C.S., and C.B.M. analyzed data; S.R., G.C.S., and C.B.M. interpreted results of experiments; S.R., G.C.S., and C.B.M. prepared figures; S.R., G.C.S., and C.B.M. drafted manuscript; S.R., G.C.S., and C.B.M. edited and revised manuscript; S.R., G.C.S., and C.B.M. approved final version of manuscript.
REFERENCES
- Alvarez-Argote S, Gransee HM, Mora JC, Stowe JM, Jorgenson AJ, Sieck GC, Mantilla CB. The impact of midcervical contusion injury on diaphragm muscle function. J Neurotrauma 33: 500–509, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews EM, Richards RJ, Yin FQ, Viapiano MS, Jakeman LB. Alterations in chondroitin sulfate proteoglycan expression occur both at and far from the site of spinal contusion injury. Exp Neurol 235: 174–187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad BI, Warren PM, Steinmetz MP, Alilain WJ. The role of the crossed phrenic pathway after cervical contusion injury and a new model to evaluate therapeutic interventions. Exp Neurol 248C: 398–405, 2013. [DOI] [PubMed] [Google Scholar]
- Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol 82: 1224–1232, 1999. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Lipski J. Respiratory interneurons in the C-5 segment of the spinal cord of the cat. Brain Res 533: 141–146, 1990. [DOI] [PubMed] [Google Scholar]
- Billig I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J Neurosci 20: 7446–7454, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Remmers JE. Synaptic effects of intercostal tendon organs on membrane potentials of medullary respiratory neurons. J Neurophysiol 61: 918–926, 1989. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Gestreau C, Vinit S, Stamegna JC, Kastner A, Gauthier P. Specific and artifactual labeling in the rat spinal cord and medulla after injection of monosynaptic retrograde tracers into the diaphragm. Neurosci Lett 417: 206–211, 2007. [DOI] [PubMed] [Google Scholar]
- Choi H, Liao WL, Newton KM, Onario RC, King AM, Desilets FC, Woodard EJ, Eichler ME, Frontera WR, Sabharwal S, Teng YD. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci 25: 4550–4559, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda M, Eklund G, Von Euler. External intercostal and phrenic alpha-motor responses to changes in respiratory load. Acta Physiol Scand 63: 391–400, 1965. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C. Intercostal and cerebellar influences on efferent phrenic activity in the decerebrate cat. Acta Physiol Scand 76: 148–158, 1969. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol 107: 662–669, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimarco AF, Kowalski KE. Spinal pathways mediating phrenic activation during high frequency spinal cord stimulation. Respir Physiol Neurobiol 186: 1–6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86, 1994. [DOI] [PubMed] [Google Scholar]
- el-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol 150: 143–152, 1998. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol 269: 47–57, 1988. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988a. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Somatotopy in the segmental innervation of the cat diaphragm. J Appl Physiol 64: 291–298, 1988b. [DOI] [PubMed] [Google Scholar]
- Furicchia FV, Goshgarian HG. Dendritic organization of phrenic motoneurons in the adult rat. Exp Neurol 96: 621–634, 1987. [DOI] [PubMed] [Google Scholar]
- Gill LC, Mantilla CB, Sieck GC. Impact of unilateral denervation on transdiaphragmatic pressure. Respir Physiol Neurobiol 210: 14–21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Lovett-Barr MR, Vinit S, Resnick DK, Mitchell GS. Breathing patterns after mid-cervical spinal contusion in rats. Exp Neurol 231: 97–103, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DC, Richmond FJ. Topography in the phrenic motoneuron nucleus demonstrated by retrograde multiple-labelling techniques. J Comp Neurol 292: 424–434, 1990. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of the albino rat: a correlative HRP and Golgi study. J Comp Neurol 201: 441–456, 1981. [DOI] [PubMed] [Google Scholar]
- Gransee HM, Gonzalez-Porras MA, Zhan WZ, Sieck GC, Mantilla CB. Motoneuron glutamatergic receptor expression following recovery from cervical spinal hemisection. J Comp Neurol. First published November 3, 2016; doi: 10.1002/cne.24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J Neurotrauma 32: 185–193, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS One 8: e64755, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma I. Inspiratory inhibitory reflex caused by the chest wall vibration in man. Respir Physiol Neurobiol 39: 345–353, 1980. [DOI] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol 182: 399–411, 2003. [DOI] [PubMed] [Google Scholar]
- Karetko M, Skangiel-Kramska J. Diverse functions of perineuronal nets. Acta Neurobiol Exp (Wars) 69: 564–577, 2009. [DOI] [PubMed] [Google Scholar]
- Keswani NH, Hollinshead WH. The phrenic nucleus. III. Organization of the phrenic nucleus in the spinal cord of the cat and man. Proc Staff Meet Mayo Clin 30: 566–577, 1955. [PubMed] [Google Scholar]
- Kong FJ, Berger AJ. Firing properties and hypercapnic responses of single phrenic motor axons in the rat. J Appl Physiol 61: 1999–2004, 1986. [DOI] [PubMed] [Google Scholar]
- Kramer JL, Lammertse DP, Schubert M, Curt A, Steeves JD. Relationship between motor recovery and independence after sensorimotor-complete cervical spinal cord injury. Neurorehabil Neural Repair 26: 1064–1071, 2012. [DOI] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Salazar K, O'Steen BE, Bloom DC, Fuller DD, Reier PJ. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp Neurol 235: 197–210, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MB, Sanes JR. Topographic mapping of motor pools onto skeletal muscles. J Neurosci 7: 252–260, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Kuo HC. Vagal control of breathing pattern after midcervical contusion in rats. J Neurotrauma. First published October 2016; doi: 10.1089/neu.2016.4645. [DOI] [PubMed] [Google Scholar]
- Li K, Javed E, Hala TJ, Sannie D, Regan KA, Maragakis NJ, Wright MC, Poulsen DJ, Lepore AC. Transplantation of glial progenitors that overexpress glutamate transporter GLT1 preserves diaphragm function following cervical SCI. Mol Ther 23: 533–548, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Duffin J, Kruszewska B, Zhang X. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res 95: 477–487, 1993. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640: 171–184, 1994. [DOI] [PubMed] [Google Scholar]
- Loveridge B, Sanii R, Dubo HI. Breathing pattern adjustments during the first year following cervical spinal cord injury. Paraplegia 30: 479–488, 1992. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol 247C: 101–109, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol 114: 380–386, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol 177: 176–182, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Sieck GC. Convergence of pattern generator outputs on a common mechanism of diaphragm motor unit recruitment. Prog Brain Res 209: 309–329, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol 179: 57–63, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 182: 244–249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Galvez G, Zambrano JM, Diaz Soto JC, Zhan WZ, Gransee HM, Sieck GC, Mantilla CB. TrkB gene therapy by adeno-associated virus enhances recovery after cervical spinal cord injury. Exp Neurol 276: 31–40, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci 26: 4406–4414, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal Cord Injury (SCI): Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham, 2016. [Google Scholar]
- Nicaise C, Frank DM, Hala TJ, Authelet M, Pochet R, Adriaens D, Brion JP, Wright MC, Lepore AC. Early phrenic motor neuron loss and transient respiratory abnormalities following unilateral cervical spinal cord contusion. J Neurotrauma 30: 1092–1099, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise C, Hala TJ, Frank DM, Parker JL, Authelet M, Leroy K, Brion JP, Wright MC, Lepore AC. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp Neurol 235: 539–552, 2012. [DOI] [PubMed] [Google Scholar]
- Pate KM, Davenport PW. Tracheal occlusions evoke respiratory load compensation and neural activation in anesthetized rats. J Appl Physiol 112: 435–442, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol 89: 563–572, 2000. [DOI] [PubMed] [Google Scholar]
- Remmers JE. Inhibition of inspiratory activity by intercostal muscle afferents. Respir Physiol Neurobiol 10: 358–383, 1970. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Marttila I. Action of intercostal muscle afferents on the respiratory rhythm of anesthetized cats. Respir Physiol Neurobiol 24: 31–41, 1975. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Tsiaras WG. Effect of lateral cervical cord lesions on the respiratory rhythm of anaesthetized, decerebrate cats after vagotomy. J Physiol 233: 63–74, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routal RV, Pal GP. Location of the phrenic nucleus in the human spinal cord. J Anat 195: 617–621, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg DL, Popovich PG, McTigue DM. Oligodendrocyte generation is differentially influenced by toll-like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. J Neuropathol Exp Neurol 66: 1124–1135, 2007. [DOI] [PubMed] [Google Scholar]
- Sengul G, Watson C, Tanaka I, Paxinos G. Atlas of the Spinal Cord: Mouse, Rat, Rhesus, Marmoset, and Human. Cambridge, MA: Academic, 2012. [Google Scholar]
- Seven YB, Mantilla CB, Sieck GC. Recruitment of rat diaphragm motor units across motor behaviors with different levels of diaphragm activation. J Appl Physiol 117: 1308–1316, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol 185: 400–409, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R. Intercostal and abdominal muscle afferent influence on medullary dorsal respiratory group neurons. Respir Physiol Neurobiol 39: 73–94, 1980. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med 15: 641–659, 1994. [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol 66: 2539–2545, 1989. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neurosci Lett 97: 29–34, 1989. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Prakash YS, Blanco CE. Myosin phenotype and SDH enzyme variability among motor unit fibers. J Appl Physiol 80: 2179–2189, 1996. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci 5: 146–156, 2004. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 82: 803–814, 2003. [DOI] [PubMed] [Google Scholar]
- Yao W, Fuglevand RJ, Enoka RM. Motor-unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol 83: 441–452, 2000. [DOI] [PubMed] [Google Scholar]