Abstract
Deficits in attention are a common and devastating consequence of traumatic brain injury (TBI), leading to functional impairments, rehabilitation barriers, and long-term disability. While such deficits are well documented, little is known about their underlying pathophysiology hindering development of effective and targeted interventions. Here we evaluate the integrity of brain systems specific to attentional functions using quantitative assessments of electroencephalography recorded during performance of the Attention Network Test (ANT), a behavioral paradigm that separates alerting, orienting, and executive components of attention. We studied 13 patients, at least 6 months post-TBI with cognitive impairments, and 24 control subjects. Based on performance on the ANT, TBI subjects showed selective impairment in executive attention. In TBI subjects, principal component analysis combined with spectral analysis of the EEG after target appearance extracted a pattern of increased frontal midline theta power (2.5–7.5 Hz) and suppression of frontal beta power (12.5–22.5 Hz). Individual expression of this pattern correlated (r = − 0.67, p < 0.001) with executive attention impairment. The grading of this pattern of spatiotemporal dynamics with executive attention deficits reflects impaired recruitment of anterior forebrain resources following TBI; specifically, deafferentation and variable disfacilitation of medial frontal neuronal populations is proposed as the basis of our findings.
Keywords: EEG, Traumatic brain injury, Attention network test, Executive attention, Frontal lobe, Medial frontal theta
Highlights
-
•
Electrophysiological correlate of impaired executive attention after Traumatic Brain Injury is derived.
-
•
Theta increases in medial frontal and beta suppression in frontal regions is linked to behavioral performance.
-
•
Individual-specific pathophysiology allows for tracking of recovery/interventions and studies of function-structure.
1. Introduction
Impaired attention is the most common and debilitating cognitive deficit following traumatic brain injury (TBI), leading to rehabilitation barriers and long-term disability (Stierwalt and Murray, 2002, Ashman et al., 2006, Brenner, 2011). Chronic deficiencies selective to executive attention, such as the capacity to monitor and resolve conflict (Fan et al., 2002), have been demonstrated in both mild and severe TBI (Halterman et al., 2006, Rodríguez-Bailón et al., 2012). Lesion and neuroimaging studies have linked these impairments, measured using tests of conflict processing, to focal cortical and white matter damage in the medial frontal (Niogi et al., 2008), fronto-parietal (Hu et al., 2013), fronto-striatal (Hartikainen et al., 2010) and thalamic regions (Kubat-Silman et al., 2002, Van der Werf et al., 2002, Little et al., 2010, Baker et al., 2016). However, these correlations with structural injuries alone do not provide insight into the underlying pathophysiological mechanisms associated with executive attention deficits after TBI.
In severe TBI and related structural brain injuries, the selective functional vulnerability of anterior forebrain systems has been accounted for by a mesocircuit hypothesis that links the graded deafferentation of central thalamic neurons (Maxwell et al., 2006) to behavioral outcomes as a result of their specializations for activation of frontal and striatal neuronal populations (Schiff, 2010, Fridman et al., 2014, Liu et al., 2015). Neurons within the central thalamus have extensive afferent connections to the anterior cingulate (ACC) (Barbas et al., 1991) and provide important complementary support to frontal cortical contributions during sustained attention (Kinomura et al., 1996, Paus et al., 1997). This is supported by evidence of joint increases in theta power and decreases in beta power in the local field potentials of central thalamic neurons that project to medial frontal populations during the short-term allocation of sustained attention in non-human primate studies (Schiff et al., 2013). Human subject studies of short-term attention also demonstrate increased blood flow in the central thalamus during attentional shifts in visual attention reaction time tasks (Kinomura et al., 1996), comparable BOLD activity increases in both ACC and central thalamus during forewarned reaction time tasks (Nagai et al., 2004), and indexing of performance by co-variation of decreasing blood flow within the ACC and central thalamus during auditory attention tasks performed over long vigils (Paus et al., 1997). The continuity of this account of the role of the anterior forebrain mesocircuit network in cognitive impairment after TBI is more specifically supported by recent studies that correlate focal neuronal damage within medial frontal cortices, anterior cingulate, and the thalamus with executive function measures using 11C Flumazenil-PET and 18FDG-PET measurements (Kato et al., 2007, Kawai et al., 2010).
The goal of the present study is to obtain a direct and quantifiable measure of attention specific neuronal activity that reflects the pathophysiology at the individual level in TBI subjects. We hypothesize that executive attention performance reflects the integrity of anterior forebrain resources known to be vulnerable to TBI and that EEG dynamics can provide a direct measure of their functional engagement. Here, we combine the well-vetted Attention Network Test (ANT) paradigm (Fan et al., 2002, Fan et al., 2005), which measures three attention networks - alerting, orienting, and executive, with simultaneously measured EEG. Executive attention, also known as conflict attention, is supported by specific brain regions including the anterior cingulate, medial frontal, lateral prefrontal cortices and striatum (Petersen and Posner, 2012). The EEG dynamics during the ANT have been reported before in healthy subjects (Fan et al., 2007) and in a comparison of young and elderly subjects (Deiber et al., 2013), but not to our knowledge in subjects with brain injury.
Here we develop a novel methodology of EEG analysis to reveal the pathophysiology at the individual subject level; because the TBI subjects in our study have heterogeneous structural damage, we do not source model the scalp signal to predefined regions of interest (Fan et al., 2007) or average across the subjects (Deiber et al., 1996). Instead, we employ principal component analysis (PCA) to reduce data dimensionality and as a way to filter and identify unique dynamical elements that grade with measured behavior. Taking this approach, we find evidence for a physiological measure that indexes performance at the individual subject level and has a direct mechanistic interpretation in the context of traumatic brain injuries.
2. Materials & methods
2.1. Subjects
Thirteen participants who had sustained a TBI were recruited at the JFK-Johnson Rehabilitation Institute in Edison, NJ. All TBI subjects had sustained a TBI (mild, moderate or severe) as a result of a blow to the head followed by a loss of consciousness or period of being dazed and confused, a period of post-traumatic amnesia, or clinical signs of altered neurological function. Based on prior neuropsychological evaluation or self-report with medical documentation of TBI, at the time of the experiment, all 13 participants had persistent cognitive difficulties of varying severity. We only included subjects who were at least 6 months post-TBI. We excluded subjects who had a history of drug or alcohol abuse, and visual, auditory, and/or motor impairments that would interfere with cognitive testing. Clinical profiles of the TBI subjects are shown in Table 1. Injury severity was calculated using the American Congress of Rehabilitation Medicines guideline (Ruff et al., 2009), which uses the duration of alteration of consciousness. All participants were oriented to time, place, and person, spoke English, and were able both to provide informed consent and to complete questionnaires and cognitive testing. Twenty-four control subjects with no history of neurological disease (mean age 43 years, range 23–65 years; 20 males) also participated in the study. The control subjects were age-matched to the TBI subjects as follows. For each of 11 TBI subjects, 2 age-matched control subjects (± 5 years) were included (n = 22). For 2 TBI subjects, 1 age-matched control subject each (± 5 years) was included (n = 2). Both the Weill Cornell Medical College Institutional Review Board and the JFK-Johnson Rehabilitation Institutional Review Board approved the studies described herein. Written consent was obtained from all study participants.
Table 1.
Patient demographics.
| ID | Gender | Education (yrs) | Age at time of injury | Injuries | Loss of consciousness | Altered consciousness | Post-traumatic amnesia | Injury severity score | Post-injury (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 12 | 20 | 1 | 20 min | 24 h | 2 months | 1 | 24 |
| 2 | F | 13 | 47 | 1 | 3–4 days | Minimal | 1.5 months | 3 | 9 |
| 3 | M | 14 | 21 | 1 | 22 days | 2 days | 2 weeks | 3 | 6 |
| 4 | M | 16 | 50 | 2 | 30 min | > 7 days | 60 min | 2 | 8 |
| 5 | M | 18 | 62 | 3 | 2 min | 1 h | Seconds | 1 | 16 |
| 6 | F | 15 | 61 | 1 | < 30 min | 3 days | 0 | 1 | 89 |
| 7 | M | 14 | 61 | 3 | < 30 min | 30–60 min | 0 | 1 | 8 |
| 8 | M | 16 | 48 | 25 | < 20 min | 6 months | 30 min | 1 | 31 |
| 9 | M | 12 | 49 | 3 | 2 months | Unknown | Unknown | 3 | 8 |
| 10 | F | 17 | 51 | 3 | Few min | > 30 min | > 30 min | 2 | 46 |
| 11 | M | 19 | 29 | 3 | 1 month | Few months | Unknown | 3 | 25 |
| 12 | M | 12 | 19 | 3 | > 30 min | > 30 min | > 30 min | 2 | 25 |
| 13 | F | 12 | 43 | 1 | 10 days | 2 months | 2 months | 3 | 28 |
2.2. Attention network test
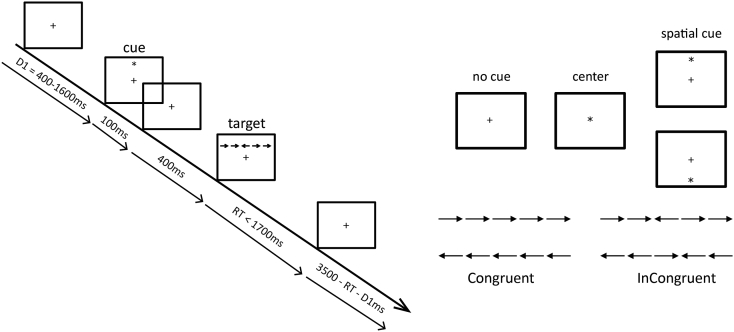
Subjects performed the ANT paradigm (Fan et al., 2002), shown in Fig. 1, which is designed to examine three attention networks: 1) alerting, 2) orienting, and 3) executive. The ANT was presented using Eprime (Psychology Software Tools) on a standard flat screen monitor. The total duration of the experimental session was approximately 25 min. The ANT began with a training block of 24 trials with feedback on performance, followed by three blocks of 96 trials, each, with no feedback. Each trial began with a variable fixation period during which the subject fixated on a cross on the center of the screen. There were three cue conditions that preceded the target – no cue, center cue and spatial cue (96 trials each). Both center and spatial cues preceded the target by a fixed time (400 ms). Center cue gave subjects information about timing of the upcoming target, while spatial cues additionally alerted subjects to location of the subsequent target (i.e. above or below fixation). Following this, a set of 5 arrows was presented above or below center fixation. There were two target conditions – congruent (all arrows point in the same direction) and incongruent (center arrow in opposite direction to flanker arrows) (144 trials each). Subjects were asked to push the left or right mouse button indicating the direction of the target (center) arrow. The trials were randomized to present all possible combinations of the three cue and two target conditions. All trials were self-paced, and subjects took brief breaks between the blocks if needed.
Fig. 1.
Attention Network Test. Network scores are calculated as differences in median reaction times (RT) from all accurate trials as follows: Alerting = RT no cue − RT central cue; Orienting = RT central cue − RT spatial cue; Executive = Incongruent − Congruent.
Reaction time (RT) was calculated as the time interval between target onset and response button press. Accuracy was defined as the percentage of correct responses. Following the standard approach (Fan et al., 2005), we calculated network measures by subtracting median reaction times between contrasting trials, as follows: 1) alerting = RTno cue − RTcentral cue; 2) orienting = RTcenter cue − RTspatial cue; and 3) executive = RTincongruent flanker − RTcongruent flanker. While larger alerting and orienting measures indicate faster cue-related performance, larger executive measures indicate worse performance (longer time to resolve conflict).
2.3. EEG acquisition and overview of analysis
Electroencephalographic data were recorded from 129 scalp sites using the Geodesic EEG Net Station (EGI, Eugene, OR) with the 129-channel Geodesic Sensor Net (Tucker, 1993). The impedance of all electrodes was < 75 kΩ at the beginning of the recording. The signals were sampled at 250 samples per second and filtered from DC to 100 Hz. ANT-related visual stimuli and button presses were marked in the continuous EEG file.
Spectral analysis of the EEG was performed with in-house software written in Matlab (The Mathworks, Natick, MA). EEG was first segmented into epochs based on markers time-locked to cue and/or target onset. Epochs with significant artifact from line noise, eye blink, or muscle activity (as determined by visual inspection) were removed. EEG signals were next converted to the Hjorth Laplacian montage (HLM) (Hjorth, 1975), a well validated method for computing the surface Laplacian (Cimenser et al., 2011, Goldfine et al., 2011, Mattia et al., 2012). To make this conversion, we calculated the difference between the voltages at each individual electrode placement and the weighted average (at the same time point) of the voltages at the surrounding electrodes (four nearest neighbors for electrodes on the interior of the array; three for electrodes on the edge). The power spectral density for each HLM channel was then calculated separately for each epoch using Thomson's multitaper method (Thomson, 1982, Percival and Walden, 1993, Mitra and Pesaran, 1999), as implemented by mtspectrumc in the Chronux Matlab toolbox (Mitra and Bokil, 2007). The multitaper method is designed for non-stationary data (see Babadi and Brown, 2014 for review) and is the preferred method for obtaining precise frequency resolution (van Vugt et al., 2007). EEG data is multiplied by Slepian windows (tapers), which are designed to suppress sidelobe power associated with neighboring frequencies. Use of the multitaper method allows for optimal tradeoff between bias and variance in spectral estimation (Babadi and Brown, 2014). Only the 76 channels based on the 10–10 numbering system were used for further analysis as the electrodes on the face/neck are generally more affected by EMG artifact.
2.4. EEG analysis of task activity (ANT conditions)
We analyzed each individual condition type (i.e., no cue, center cue, spatial cue, congruent, and incongruent) by contrasting the activity after the condition marker to the immediately preceding baseline (within each trial) as was done before (Deiber et al., 2013). First, the 288 trials were separated by each of the five conditions: cue (no cue, center cue, or spatial cue: 96 trials each) and target type (congruent or incongruent: 144 trials each). Then EEG data were epoched (Supplementary Fig. 1) as follows: a) congruent and incongruent conditions - 400 ms before and after target, b) no, spatial and center conditions - 800 ms before target and then cut into two 400 ms segments (i.e. 400 ms before and 400 ms after cue).
The 400 ms epoch, for the congruent and incongruent conditions, was chosen because the minimum reaction time of the control and TBI groups were 478 and 640 ms respectively. The average reaction times for each group were 609 ms and 815 ms. Because our goal is to restrict the measured physiological activity to only response planning as opposed to motor activity (reaction time/button press) and to allow comparison between controls and TBI subjects, we restrict the analysis to 400 ms. Restricting to 400 ms allows for an adequate comparison of post-target to baseline (see Fig. 1, baseline is 400 ms between cue marker and target marker). Therefore, the same number of data samples was available (400 ms before and after) to guarantee the use of the same number of data tapers and same frequency resolution of the analysis using multi-taper power spectral analyses.
For each of the conditions, power spectra were calculated separately for each 400 ms segment (see Supplementary Fig. 8 for spectral estimates from all 13 TBI subjects) and then the log power spectra measured from the baseline (first 400 ms) was subtracted from the latter segment (400 ms after cue or target). We choose as baseline the immediately preceding 400 ms, rather than a predefined resting state, as it controls for overall changes in state (e.g. drowsiness or artifact). For the multi-taper spectral analysis one taper was used, resulting in a frequency resolution of 5 Hz and estimates equally spaced 2.5 Hz apart (Mitra and Bokil, 2007). Further analysis was restricted to the frequencies 2.5 to 20 Hz to avoid overlap with frequencies typically contaminated with artifact (eye blink below 2.5 Hz and muscle above 20 Hz). Results of analyses including gamma (up to 50 Hz) are shown in Supplementary Fig. 3.
The above described analysis pipeline is in contrast to a previous study by Fan et al., where they first modeled the EEG scalp data to pre-defined sources (Fan et al., 2007) before contrasting between the conditions (please see Supplementary data Section 4, Supplementary 4, for results of similarly contrasting between executive conditions). Because the TBI subjects in our study had heterogeneous structural damage, we did not source model the scalp signal to predefined regions of interest or average across the subjects.
2.5. Principal component analysis (EEG spectra – ANT conditions)
Principal component analysis (PCA) is a standard statistical tool that derives orthogonal components that can be used for feature extraction and data reduction (Jolliffe, 2002). Here, we use PCA to reduce dimensionality of the EEG dataset and explore the relationship between each subject's EEG and the measured ANT behavior. Each subject's EEG dataset is represented as 76 electrode channels with 8 frequencies each. By employing PCA, we reduce dimensionality and quantify the spatial-frequency patterns of covariance within each group. By conducting PCA on the TBI group (13 TBI subjects × 76 channels × 8 frequencies), we extract principal components that explain the variance across the group (in descending order). We then represent each TBI subject's (spatial-frequency power in individual EEG dataset) in each of the extracted principal component's space by multiplying with the extracted principal components. This representation of individual subjects, in each principal component space, can then be evaluated for a relationship with measured behavior (ANT). However, because we evaluate every extracted principal component for a relationship with behavior we further evaluate the significance of our findings through a correction for multiple comparisons (see Results Section 3.3.2.3). Without the application of PCA, each of the varying factors (e.g. 1 freq band per EEG channel) would need to be examined for a relationship with behavior either individually (13 × 8 × 76 in TBI subjects alone) or only averaged across subjects, channels or frequency bands. Our approach is in contrast to previously conducted studies that report on the average EEG of a group and do not report on EEG dynamics specific to individual behavioral performance (Fan et al., 2007, Deiber et al., 2013).
This analysis was conducted, first, separately for each condition type (i.e., congruent, incongruent, no-cue, spatial-cue and center-cue). The log spectral power values of the post-cue/target period (with pre-cue/target baseline subtracted as described above) for the frequencies at 2.5 Hz to 20 Hz were used for all subjects. For each group and each condition, we created a data matrix with the columns consisting of the spectral power at each frequency band, for each of the 10–10 scalp locations (i.e., 8 frequencies × 76 channels), and the rows of the matrix corresponding to the subjects (24 control subjects or 13 TBI subjects).
For TBI subjects, the matrices for congruent and incongruent flanker conditions (used to measure the executive network) were first analyzed separately - 8 × 76 columns and 13 rows for TBI subjects. Due to comparable results (see Results 3.3.2.1), the conditions were combined. The combined matrix for TBI group had 8 × 76 columns and 13 × 2 (congruent and incongruent) rows.
We also conducted a similar analysis to explore the contrast between conditions (congruent-incongruent) as reported by other investigators using source modeled data (not raw electrode data) (Fan et al., 2007). We report these results in the Supplementary data (Supplementary Section 4, Supplementary Fig. 4). Our results demonstrate the lack of sensitivity for analyses of raw scalp (electrode) EEG power to detect meaningful changes between congruent and incongruent conditions (also reported in Fan et al., 2007).
2.6. Statistical analysis
Unless otherwise noted, comparisons of ANT network behavior are based on unpaired t-tests, two-tailed, and p-values < 0.05 are described as significant. Correlation between PCs and ANT behavior was also implemented in Matlab, using the code corrcoef, with the p-value calculated by converting to a t-statistic. All analyses were implemented in Matlab.
3. Results
3.1. Accuracy and reaction time
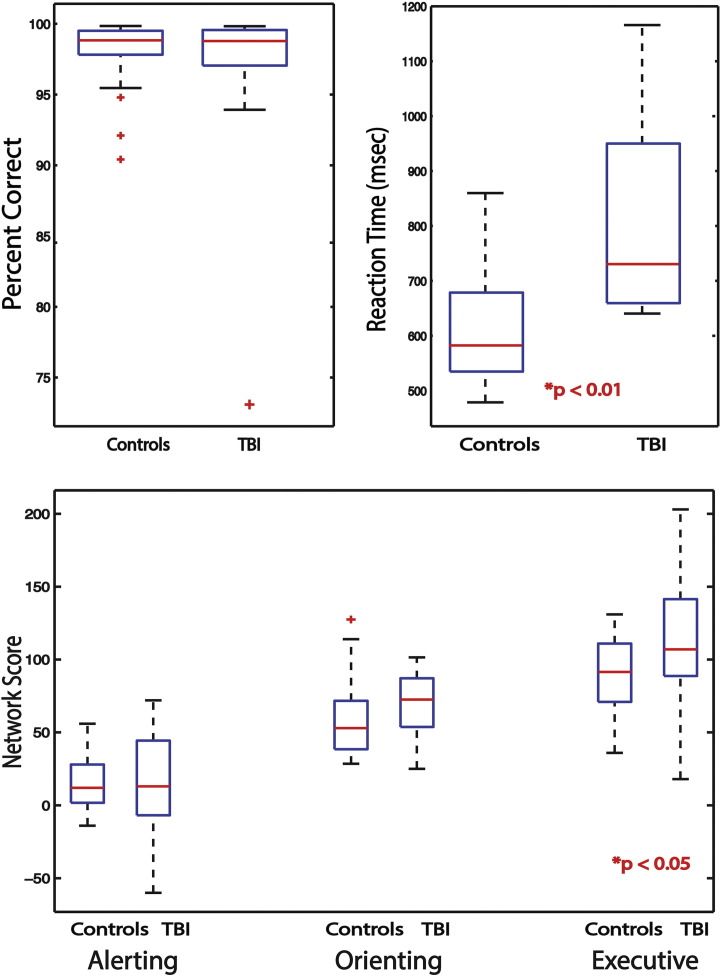
For all subjects, Fig. 2 summarizes the behavioral results. Fig. 2 (top left) shows the performance on the ANT for 24 control subjects and 13 TBI subjects. Across both groups, accuracy rates during the ANT task were very high (mean 98, SD 2.5, for control subjects and mean 96.23, SD 7.22, for TBI subjects). No significant difference (p = 0.28) was found between the two groups. In the TBI subject population, reaction time (mean 815.94, SD 182.54 ms) across all conditions was significantly (p = 0.00008) slower compared with control subjects (mean 609.95, SD 99.98 ms; Fig. 2, top right).
Fig. 2.
Group differences in behavior. Analysis of behavior reveals differences between TBI subjects and control subjects in reaction time and executive network scores.
3.2. ANT network behavior
With regard to the ANT parcellation into alerting, orienting and executive components (Fig. 2, bottom), calculated from differences of median reaction times between conditions (see Methods), TBI subjects differed from control subjects (p = 0.0473) only in the executive network (TBI subjects - mean 115.27, SD 48.30; control subjects - mean 90.60, SD 25.08). Alerting network (mean 15.25, sd 25.57, for control subjects, and mean 17.73, sd 18.43, for TBI subjects) and orienting network (mean 58.5, sd 24.07, for control subjects, and mean 67.84, sd 26.07, for TBI subjects) were comparable between the groups (p = 0.78 and p = 0.29 respectively).
3.3. Electrophysiological results
3.3.1. Executive network/condition activity: individual subjects
In Supplementary Fig. 7 we show the individual results of the EEG power spectral analyses as a topographical map for all thirteen TBI subjects and for all twenty four control subjects, with the incongruent and congruent flanker trials separated. Examinations of EEG spectral power changes showed variable patterns across individuals with no consistent patterns across condition and group.
3.3.2. Executive conditions: principal components (PC) and behavior
3.3.2.1. Condition specific analyses
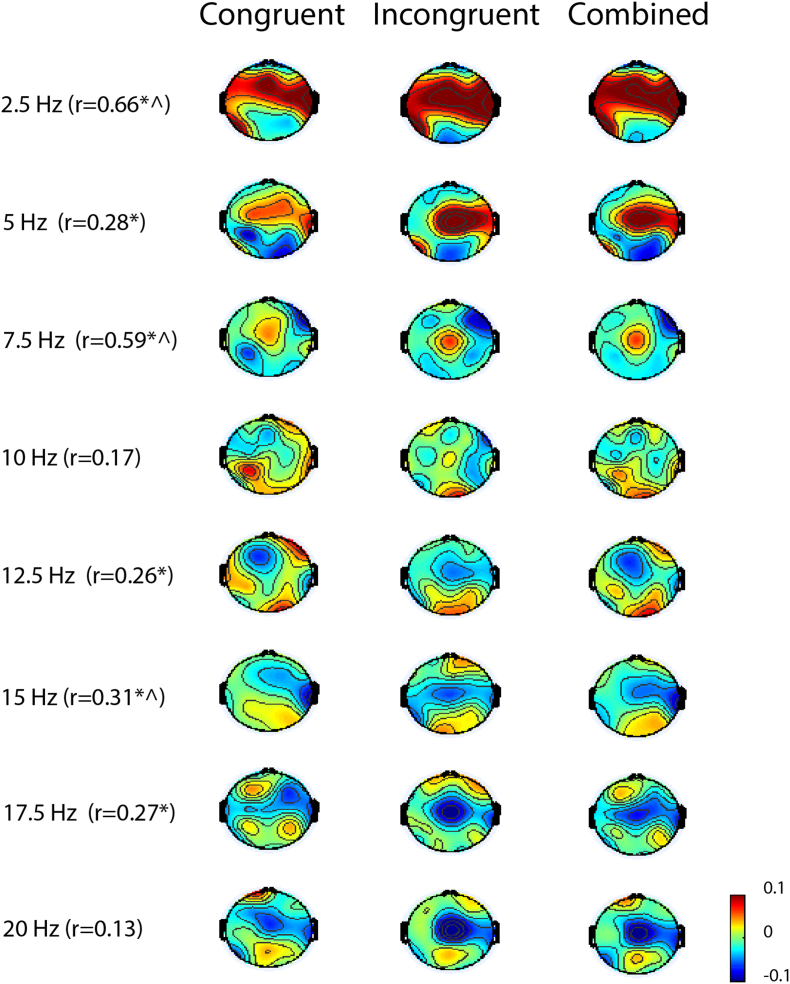
We first conducted PCA on each of the conditions (congruent and incongruent) separately. For TBI subjects, this resulted in 12 (13 subjects minus 1) principal components (PC) for each of the conditions. We then represented the individual subject (power at each frequency) in each of the extracted PCs. Then, we sought the relationship of this representation (for each of the extracted PC's) with executive attention (as this network showed evidence of difference in comparison of control versus TBI subjects). For both conditions, the second PC, only, had a significant correlation with executive attention: r = 0.62, p = 0.0221 for congruent and r = − 0.66, p = 0.0137 for incongruent (see Supplementary Fig. 2 for correlations with every PC). In the congruent condition, the second PC (shown in Fig. 3, the sign of the PC is reversed for display purposes) shows increased delta activity (2.5 Hz), increased midline frontal theta (5 Hz) and suppression of frontal (left and midline) beta. In the incongruent condition (Fig. 3), similar increases in delta are seen but the midline frontal theta increases and midline frontal beta decreases are more focused. Note that the increases and decreases shown here are in comparisons with the period following the target to the period before it (i.e. baseline). The second PC from both the congruent and incongruent conditions had a similar correlation with behavior (r = 0.62 and r = − 0.66) and was similar to each other in frequency and spatial patterns. This visual similarity is supported by high levels of correlation of the scalp topographies in several frequency bands (see Fig. 3).
Fig. 3.
Second principal components for executive network (TBI subjects): Frequency bands shown have a ± 2.5 Hz frequency resolution width. Correlation between the second principal component of congruent and incongruent conditions are shown (r) with * (p < 0.05) and ^ (Benjamini-Hochberg false discovery rate correction). Correlation between TBI subjects represented in the second principal components and the executive network: congruent (r = 0.62, p < 0.05, sign reversed for display), incongruent (r = − 0.66, p < 0.05), combined (r = − 0.67, p < 0.01).
3.3.2.2. Condition combined analyses
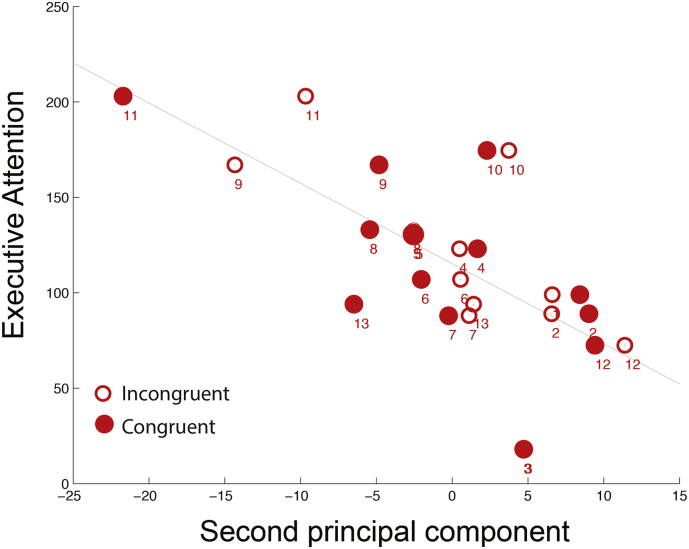
Because of the similarities between each of the target conditions (see Fig. 3) we combined them and repeated the analysis to extract twenty five (26 rows: 13 × 2 minus 1) principal components. We find that representation of the TBI subjects in the second PC space, only (see Supplementary Fig. 2), had a strong and significant relationship with behavior: r = − 0.67, p = 0.0064 (13 degrees of freedom), s.e. 0.03 (jackknife). The second PC (PC2) of this combined analysis, showed the same focused strong increase in theta power over the frontal midline regions with a concurrent decrease in beta power over the same localized regions (Fig. 3). In Fig. 4, we display the relationship between the representation of subjects in PC2 and their executive network (shown separately for each flanker condition).
Fig. 4.
Second principal component from combined analysis vs Executive network (TBI subjects): Significant correlation between executive network and PC2 (score) for TBI subjects: r = − 0.67, p < 0.0064 (13 degrees of freedom), jackknife s.e. 0.03.
Similar analyses were conducted for control subjects' only. Correlating the PC's generated from controls only with their executive attention did not result in any significant relationship. This could be due to homogeneity in their performance (control: variance to mean ratio = 6.94, variance = 629.11; TBI: variance to mean ratio = 20.24, variance = 2333.1, p = 0.0068 (two-sample F-test)). However, projecting the TBI subjects' EEG data into the control subjects' principal component space (see Supplementary data 5) and correlating with their (TBI subject) executive attention score resulted in a significant relationship with PC2 (r = − 0.4794, p = 0.0132). Importantly, the PC2 extracted from the control subjects' only shows an increased midline frontal theta (also extending into posterior regions) and suppression of frontal high beta, similar to the PC2 (extracted from TBI subjects) shown in Fig. 3.
3.3.2.3. Multiple corrections for principal components
The finding of a PC that is so strongly correlated (r = − 0.67) with executive attention is unlikely to be due to chance, and finding one that explains as large a fraction of the variance (2nd PC) is even more unlikely. To show this, we created 5000 surrogate datasets in which the executive attention measure was randomly permuted across subjects, while maintaining the same pairings of measures across the two conditions for each subject. In each surrogate dataset, we determined whether any PC had a correlation as high as what we observed, thus taking multiple comparisons (all PC's) into account. Of all surrogates, only 3% had any PC with a correlation at this level. When a PC did have a correlation at this level, it was rarely as large as the component found in the data: The first PC was never responsible, and in only 0.8% of the 5000 cases, was the second PC responsible.
3.4. Results conclusion
Because the executive attention network was the only one to show a difference between the groups (TBI and healthy controls) we focus here only on these results. However, similar analyses exploring a relationship between the EEG during the cued conditions (EEG data before and after cue) and their respective ANT alerting and orienting networks were conducted. None of the principal components in the TBI group only analyses had a significant relationship with alerting and orienting networks.
The results of both the TBI and control subjects executive network analyses (condition specific and combined) demonstrate the consistency of PC2; midline frontal theta increase and frontal beta suppression appear consistently and has a significant relationship with TBI subjects' executive attention score. The significant relationship between the TBI subjects' executive attention behavior and the control subjects' only PC2 is also promising for future studies of single subjects.
4. Discussion
In this study, we sought to derive an individually specific physiological measure of the executive attention deficit in chronic TBI subjects. Here we find evidence that dynamic activity in cortical regions reflected in a specific spatiotemporal EEG pattern of joint variance - increase in midline theta (5 ± 2.5 Hz) EEG power, and decreases in frontal midline beta power (17.5 ± 5 Hz), specifically indexes deficits in executive attention after TBI. Importantly, this specific pattern, as expressed in the extracted feature (PC2) of the ANT executive network, can be interpreted in the context of known functional networks supporting executive attention and the specific anatomic pathologies underlying TBI. As discussed below, PC2 likely reveals the functional recruitment of the anterior forebrain network across medial frontal regions and because of their strong anatomical connections and joint activity patterns, central thalamic neuronal populations known to be selectively vulnerable to dysfunction across the spectrum of injury severity following TBI (Azouvi, 2000, Cazalis et al., 2006, Maxwell et al., 2006, Kawai et al., 2010, Little et al., 2010, Schiff, 2008, Schiff, 2010)
4.1. PC2 identifies frontal midline systems supporting executive attention and vulnerable to functional deafferentation after multi-focal injuries
The increase in theta, coupled with the decrease in beta within the frontal midline, extracted by PCA, supports the hypothesis that the extracted component reflects the integrity of anterior forebrain resources during the period in the task when decision-making occurs i.e. for holding set and conflict monitoring. It is important to note that the activity in PC2 reflects the activity post-target after accounting for any pre-target (post-cue) changes (baseline subtracted). However, the similarities between the electrophysiological patterns extracted for both congruent (no conflict) and incongruent (conflict) trials demonstrate that the contributions of these regions are not specific to executive decision making but have a more general role in attentional effort, as lesion and imaging studies indicate (Floden et al., 2011). Specifically, this activity pattern is likely to reflect the adaptive control, response inhibition and task monitoring (as discussed below) required for successful completion of the task and not the processing delays (significantly slower reaction time), which may additionally reflect motor impairments and motor planning deficits in TBI subjects. Combining both of these conditions results in the strong statistical correlation with PC2 (Fig. 4), which likely originates in the engagement of the midline frontal resources independent of the condition (congruent vs incongruent).
The most prominent and spatially focused finding in PC2 is the joint increase of frontal midline theta power and decrease of beta power. In isolation, frontal midline theta power is known to grade and modulate with cognitive demands during attentive behaviors (Hsieh and Ranganath, 2014). These include specific executive attention functions such as task monitoring and error detection (Luu et al., 2004, Cohen and Donner, 2013), sensorimotor integration (Caplan et al., 2003) and time-pressure reaction time responses (Slobounov et al., 2000). While our methods preclude inferences about the source, the involvement of the midline frontal regions, particularly anterior cingulate cortex (ACC) is suggested by the following studies. Frontal theta is known to originate from frontal midline generators (ACC) as studied in both humans (Raghavachari et al., 2001, Wang et al., 2005) and non-human primates (Tsujimoto et al., 2010). More generally, the contribution of the ACC towards executive or conflict attention and simple reaction time tasks have also been characterized using blood flow (Naito et al., 2000), event-related fMRI in healthy controls (Fan et al., 2005), schizophrenic patients (Carter and van Veen, 2007), and non-human primates (Paus, 2001). Frontal beta suppression also arises prominently in the second PC and is a reliable marker of the engagement of attentional and executive resources for an expected stimulus and response (Cohen et al., 2008, Cohen and Donner, 2013) and with motor preparation in visuomotor attention tasks (Engel and Fries, 2010, Gola et al., 2012, Brinkman et al., 2014).
The identification of a single component with a distinct frontal midline origin is also consistent with the known pathology of TBI. Severe TBI is more likely to damage medial frontal cortical and subcortical regions, including fronto-striatal and thalamo-frontal projections (Trexler and Zappala, 1988, Zappalà et al., 2012), whereas orbitofrontal and temporal polar zones are usually implicated in mild and moderate TBI (Engel and Fries, 2010, Gola et al., 2012). Moreover, damage to the medial frontal cortices and the thalamus after TBI has been directly linked to executive attention deficits in other studies (Niogi et al., 2008, Little et al., 2010, Hu et al., 2013) and can be understood in the context of the vulnerabilities of the connections between the medial frontal cortices and thalamus as a function of the severity of injury. In severe TBI a graded relationship between deafferentation of central thalamic neurons and outcomes ranging from moderate disability to vegetative state has been established in autopsy studies (Maxwell et al., 2006). The central lateral nucleus neurons show the first evidence of cell loss, reflecting their broad innervation across the frontal cortices and striatum; these neurons have been demonstrated to strongly activate the entire fronto-striatal system (Liu et al., 2015). The same central thalamic neurons have extensive afferent connections to the ACC (Barbas et al., 1991) and likely account for the functional imaging studies demonstrating co-activation of thalamic and ACC populations during allocation of short-term attention (Nagai et al., 2004). Similar joint increases in theta power and decreases in beta power arise with recruitment of central thalamic neurons during the short-time allocation of sustained attention (Schiff et al., 2013). Finally, the selective loss of neuronal populations within medial frontal cortices, anterior cingulate, and the thalamus in a comparable group of TBI in subjects with measured executive function deficits using 11C Flumazenil-PET and 18FDG-PET supports the pathophysiological origin of impaired function across these regions (Kato et al., 2007, Kawai et al., 2010). Thus, the ability to dynamically recruit frontal midline resources observed here may reflect reduced capacity to dynamically allocate resources across the frontal and pre-frontal cortical regions that underlies an impaired response to complex task demands (Luu et al., 2004, Hanif et al., 2012) as a result of functional deafferentation of the medial frontal cortices and central thalamus.
4.2. Comparison with other studies
In previous studies, deficiencies in both orienting and executive attention have been identified in subjects immediately following mild TBI (van Donkelaar et al., 2005) while long-term deficiencies in executive attention, similar to our findings, have been shown for both mild and severe TBI (Halterman et al., 2006, Rodríguez-Bailón et al., 2012). Decreased processing speed has also been associated with attentional impairment in TBI (Willmott et al., 2009). The EEG correlates of ANT-derived attentional networks have been characterized in both healthy adults and the elderly (Fan et al., 2007, Deiber et al., 2013). Fan et al. (2007) analyzed group-averaged dipole-modeled spectrograms, which showed task-related theta, alpha, and beta power decreases and associated increases in gamma power, in predefined cortical and subcortical regions of interest. Similarly, a study of averaged surface EEG found that young subjects showed more widespread alpha oscillations while the elderly subjects demonstrated more beta suppression (Deiber et al., 2013). In comparing our findings with these prior studies, we note that using PCA allowed us to extract spatial patterns of joint variance across theta, and beta rhythms, which could be compared across individuals, an advantage over the existing methods.
We also examined ERPs, which showed no evidence of statistically significant group effects (using t-test and corrected for multiple comparisons, Supplementary Fig. 6). Prior ERP studies in TBI have revealed markers of cognitive deficits (for review, see Dockree and Robertson, 2011), but in this study the high accuracy (96% for TBI subjects and not significantly different from control subjects) and the small group size (n = 13) may have contributed to the negative ERP finding. Additionally, the high dimensionality of the data and averaging might obscure individual subject differences. Thus, when using this paradigm, the ability to resolve both group and individual differences in TBI subjects versus control subjects using PCA and the multi-channel within-task EEG is an advantage over a more traditional method.
Finally, while we did not find a significant correlation between the EEG activity of the orienting and alerting networks and their behavioral measures in this chronic (average 24 months post-injury) population, the results might be different with a larger sample size and in a more acute population with more pronounced deficiencies (van Donkelaar et al., 2005).
4.3. Limitations
The primary limitations of this current study are related to the small group size limiting generalizability of this study. However, despite the small patient group size and the known heterogeneity of TBI (Goldstein et al., 2010, León-Carrión et al., 2012), we found a strong and significant correlation of executive attention with its associated electrophysiological patterns extracted using PCA. Most importantly, the robustness of our results is further validated by a similar PC extracted from an out-of-sample group of control subjects only (Supplementary Fig. 5).
4.4. Theoretical implications
This study, which is the first to link impaired executive attention to physiological measures in the EEG at the individual subject level, has several theoretical implications. Most importantly, it provides evidence that the underlying neuronal deficit producing executive attentional impairment after traumatic brain injury takes its primary origins in the dynamic recruitment of frontal lobe resources. Specifically, it identifies the impaired recruitment of midline frontal regions and invites further characterization of the structural and functional alterations of networks within these regions. Further, the individualized analyses developed here can be applied not only to tracking of spontaneous recovery, but also to examine the impact of instrumental manipulations (such as targeted pharmacotherapy or electrical stimulation) of the medial frontal systems inferred, here, as generators of the reporting signal in PC2. Such approaches will allow for linkage of the dynamical element identified in PC2 to the local neuronal populations and specific network activity supporting their contribution to executive attention.
5. Conclusions
Our findings that dynamic recruitment of frontal lobe resources during effortful attention indexes attentional deficits have implications for rehabilitation and therapeutic interventions in TBI. As reviewed above, recruitment of frontal midline theta power and suppression of beta power, as shown here, are both consistently linked to engagement of frontal-thalamocortical systems responsible for executive attention and more broadly with arousal regulation. Thus the failure to adequately recruit these resources after TBI is a proxy for the functional integrity of subsystems supporting on-demand resource allocation within the anterior forebrain. Our results also indicate that greater recruitment of these resources is evident in patients with better behavioral performance, which may represent compensatory mechanisms. As such our findings further direct efforts that modulate and target brain regions within the anterior forebrain arousal regulation mesocircuit, as they may prove to be efficacious in the recovery after traumatic brain injury (Laureys and Schiff, 2012, Liu et al., 2015). Our findings also suggest that the effectiveness of cognitive rehabilitation may depend on facilitating the allocation of adequate executive-attention resources in response to adaptive, task-dependent cognitive challenge, rather than the exercise of isolated cognitive functions (Cicerone and Maestas, 2014).
Funding
The Jerold B Katz Foundation supported this work.
Acknowledgements
The authors are grateful to Drs. Jonathan Victor and Amy Kuceyeski for their invaluable comments on the analysis and manuscript. The authors also thank Tanya Nauvel, Jennifer Hersh-Goldman and Laura Simon-Pearson for their assistance with data collection. This investigation received support from the Clinical and Translational Science Center (UL1TR000457) at Weill Cornell Medical College.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.01.010.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4
References
- Ashman T.A., Gordon W.A., Cantor J.B., Hibbard M.R. Neurobehavioral consequences of traumatic brain injury. Mt Sinai J Med N Y. 2006;73:999–1005. [PubMed] [Google Scholar]
- Azouvi P. Neuroimaging correlates of cognitive and functional outcome after traumatic brain injury. Curr. Opin. Neurol. 2000;13:665–669. doi: 10.1097/00019052-200012000-00009. [DOI] [PubMed] [Google Scholar]
- Babadi B., Brown E.N. A review of multitaper spectral analysis. IEEE Trans. Biomed. Eng. 2014;61:1555–1564. doi: 10.1109/TBME.2014.2311996. [DOI] [PubMed] [Google Scholar]
- Baker J.L., Ryou J.-W., Wei X.F., Butson C.R., Schiff N.D., Purpura K.P. Robust modulation of arousal regulation, performance and frontostriatal activity through central thalamic deep brain stimulation in healthy non-human primates. J Neurophysiol. 2016 doi: 10.1152/jn.01129.2015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H., Henion T.H., Dermon C.R. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1991;313:65–94. doi: 10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- Brenner L.A. Neuropsychological and neuroimaging findings in traumatic brain injury and post-traumatic stress disorder. Dialogues Clin. Neurosci. 2011;13:311–323. doi: 10.31887/DCNS.2011.13.3/lbrenner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman L., Stolk A., Dijkerman H.C., de Lange F.P., Toni I. Distinct roles for alpha- and beta-band oscillations during mental simulation of goal-directed actions. J. Neurosci. 2014;34:14783–14792. doi: 10.1523/JNEUROSCI.2039-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan J.B., Madsen J.R., Schulze-Bonhage A., Aschenbrenner-Scheibe R., Newman E.L., Kahana M.J. Human theta oscillations related to sensorimotor integration and spatial learning. J. Neurosci. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C.S., van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Cazalis F., Feydy A., Valabrègue R., Pélégrini-Issac M., Pierot L., Azouvi P. fMRI study of problem-solving after severe traumatic brain injury. Brain Inj. 2006;20:1019–1028. doi: 10.1080/02699050600664384. [DOI] [PubMed] [Google Scholar]
- Cicerone K.D., Maestas K.L. Handbook on the Neuropsychology of Traumatic Brain Injury. Springer-Verlag New York, LLC; 2014. Rehabilitation of attention and executive function impairments; pp. 191–212. [Google Scholar]
- Cimenser A., Purdon P.L., Pierce E.T., Walsh J.L., Salazar-Gomez A.F., Harrell P.G., Tavares-Stoeckel C., Habeeb K., Brown E.N. Tracking brain states under general anesthesia by using global coherence analysis. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8832–8837. doi: 10.1073/pnas.1017041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.X., Donner T.H. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol. 2013;110:2752–2763. doi: 10.1152/jn.00479.2013. [DOI] [PubMed] [Google Scholar]
- Cohen M.X., Ridderinkhof K.R., Haupt S., Elger C.E., Fell J. Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res. 2008;1238:127–142. doi: 10.1016/j.brainres.2008.07.114. [DOI] [PubMed] [Google Scholar]
- Deiber M.-P., Ibañez V., Missonnier P., Rodriguez C., Giannakopoulos P. Age-associated modulations of cerebral oscillatory patterns related to attention control. NeuroImage. 2013;82:531–546. doi: 10.1016/j.neuroimage.2013.06.037. [DOI] [PubMed] [Google Scholar]
- Deiber M.P., Ibanez V., Sadato N., Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J. Neurophysiol. 1996;75:233–247. doi: 10.1152/jn.1996.75.1.233. [DOI] [PubMed] [Google Scholar]
- Dockree P.M., Robertson I.H. Electrophysiological markers of cognitive deficits in traumatic brain injury: a review. Int J Psychophysiol Off J Int Organ Psychophysiol. 2011;82:53–60. doi: 10.1016/j.ijpsycho.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Fan J., Byrne J., Worden M.S., Guise K.G., McCandliss B.D., Fossella J., Posner M.I. The relation of brain oscillations to attentional networks. J. Neurosci. 2007;27:6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Fossella J., Flombaum J.I., Posner M.I. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Sommer T., Raz A., Posner M.I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Floden D., Vallesi A., Stuss D.T. Task context and frontal lobe activation in the Stroop task. J. Cogn. Neurosci. 2011;23:867–879. doi: 10.1162/jocn.2010.21492. [DOI] [PubMed] [Google Scholar]
- Fridman E.A., Beattie B.J., Broft A., Laureys S., Schiff N.D. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc. Natl. Acad. Sci. 2014;111:6473–6478. doi: 10.1073/pnas.1320969111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M., Kamiński J., Brzezicka A., Wróbel A. β band oscillations as a correlate of alertness–changes in aging. Int J Psychophysiol Off J Int Organ Psychophysiol. 2012;85:62–67. doi: 10.1016/j.ijpsycho.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Goldfine A.M., Victor J.D., Conte M.M., Bardin J.C., Schiff N.D. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2011;122:2157–2168. doi: 10.1016/j.clinph.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Allen D.N., Caponigro J.M. A retrospective study of heterogeneity in neurocognitive profiles associated with traumatic brain injury. Brain Inj. 2010;24:625–635. doi: 10.3109/02699051003670882. [DOI] [PubMed] [Google Scholar]
- Halterman C.I., Langan J., Drew A., Rodriguez E., Osternig L.R., Chou L.-S., van Donkelaar P. Tracking the recovery of visuospatial attention deficits in mild traumatic brain injury. Brain J. Neurol. 2006;129:747–753. doi: 10.1093/brain/awh705. [DOI] [PubMed] [Google Scholar]
- Hanif A., Ferrey A.E., Frischen A., Pozzobon K., Eastwood J.D., Smilek D., Fenske M.J. Manipulations of attention enhance self-regulation. Acta Psychol. 2012;139:104–110. doi: 10.1016/j.actpsy.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Hartikainen K.M., Wäljas M., Isoviita T., Dastidar P., Liimatainen S., Solbakk A.-K., Ogawa K.H., Soimakallio S., Ylinen A., Öhman J. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. J. Clin. Exp. Neuropsychol. 2010;32:767–774. doi: 10.1080/13803390903521000. [DOI] [PubMed] [Google Scholar]
- Hjorth B. An on-line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr. Clin. Neurophysiol. 1975;39:526–530. doi: 10.1016/0013-4694(75)90056-5. [DOI] [PubMed] [Google Scholar]
- Hsieh L.-T., Ranganath C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. NeuroImage. 2014;85(Pt 2):721–729. doi: 10.1016/j.neuroimage.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Fan J., Xu P., Zhou S., Zhang L., Tian Y., Wang K. Attention network impairments in patients with focal frontal or parietal lesions. Neurosci. Lett. 2013;534:177–181. doi: 10.1016/j.neulet.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Jolliffe I.T. Springer; New York: 2002. Principal Component Analysis.http://site.ebrary.com/id/10047693 Available at: (Accessed August 27, 2015) [Google Scholar]
- Kato T., Nakayama N., Yasokawa Y., Okumura A., Shinoda J., Iwama T. Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J. Neurotrauma. 2007;24:919–926. doi: 10.1089/neu.2006.0203. [DOI] [PubMed] [Google Scholar]
- Kawai N., Maeda Y., Kudomi N., Yamamoto Y., Nishiyama Y., Tamiya T. Focal neuronal damage in patients with neuropsychological impairment after diffuse traumatic brain injury: evaluation using 11C-flumazenil positron emission tomography with statistical image analysis. J. Neurotrauma. 2010;27:2131–2138. doi: 10.1089/neu.2010.1464. [DOI] [PubMed] [Google Scholar]
- Kinomura S., Larsson J., Gulyás B., Roland P.E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Kubat-Silman A.K., Dagenbach D., Absher J.R. Patterns of impaired verbal, spatial, and object working memory after thalamic lesions. Brain Cogn. 2002;50:178–193. doi: 10.1016/s0278-2626(02)00502-x. [DOI] [PubMed] [Google Scholar]
- Laureys S., Schiff N.D. Coma and consciousness: paradigms (re)framed by neuroimaging. NeuroImage. 2012;61:478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- León-Carrión J., Domínguez-Morales M., Martín J., Leon-Dominguez U. Recovery of cognitive function during comprehensive rehabilitation after severe traumatic brain injury. J. Rehabil. Med. 2012;44:505–511. doi: 10.2340/16501977-0982. [DOI] [PubMed] [Google Scholar]
- Little D.M., Kraus M.F., Joseph J., Geary E.K., Susmaras T., Zhou X.J., Pliskin N., Gorelick P.B. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lee H.J., Weitz A.J., Fang Z., Lin P., Choy M., Fisher R., Pinskiy V., Tolpygo A., Mitra P., Schiff N., Lee J.H. Frequency-selective control of cortical and subcortical networks by central thalamus. eLife. 2015;4 doi: 10.7554/eLife.09215. http://elifesciences.org/lookup/doi/10.7554/eLife.09215 Available at: (Accessed July 5, 2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P., Tucker D.M., Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Mattia M., Spadacenta S., Pavone L., Quarato P., Esposito V., Sparano A., Sebastiano F., Di Gennaro G., Morace R., Cantore G., Mirabella G. Stop-event-related potentials from intracranial electrodes reveal a key role of premotor and motor cortices in stopping ongoing movements. Front. Neuroenerg. 2012;5:12. doi: 10.3389/fneng.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell W.L., MacKinnon M.A., Smith D.H., McIntosh T.K., Graham D.I. Thalamic nuclei after human blunt head injury. J. Neuropathol. Exp. Neurol. 2006;65:478–488. doi: 10.1097/01.jnen.0000229241.28619.75. [DOI] [PubMed] [Google Scholar]
- Mitra P., Bokil H. first ed. Oxford University Press; USA: 2007. Observed Brain Dynamics. [Google Scholar]
- Mitra P.P., Pesaran B. Analysis of dynamic brain imaging data. Biophys. J. 1999;76:691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Critchley H.D., Featherstone E., Fenwick P.B.C., Trimble M.R., Dolan R.J. Brain activity relating to the contingent negative variation: an fMRI investigation. NeuroImage. 2004;21:1232–1241. doi: 10.1016/j.neuroimage.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Naito E., Kinomura S., Geyer S., Kawashima R., Roland P.E., Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J. Neurophysiol. 2000;83:1701–1709. doi: 10.1152/jn.2000.83.3.1701. [DOI] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P., Ghajar J., Johnson C., Kolster R.A., Sarkar R., Lee H., Meeker M., Zimmerman R.D., Manley G.T., McCandliss B.D. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am. J. Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Paus T., Zatorre R.J., Hofle N., Caramanos Z., Gotman J., Petrides M., Evans A.C. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J. Cogn. Neurosci. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- Percival D.B., Walden A.T. first ed. Cambridge University Press; Cambridge; New York, NY, USA: 1993. Spectral Analysis for Physical Applications. [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S., Kahana M.J., Rizzuto D.S., Caplan J.B., Kirschen M.P., Bourgeois B., Madsen J.R., Lisman J.E. Gating of human theta oscillations by a working memory task. J. Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Bailón M., Triviño M., Lupiáñez J. Executive attention and personality variables in patients with frontal lobe damage. Span J Psychol. 2012;15:967–977. doi: 10.5209/rev_sjop.2012.v15.n3.39388. [DOI] [PubMed] [Google Scholar]
- Ruff R.M., Iverson G.L., Barth J.T., Bush S.S., Broshek D.K., NAN Policy and Planning Committee Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2009;24:3–10. doi: 10.1093/arclin/acp006. [DOI] [PubMed] [Google Scholar]
- Schiff N.D. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann. N. Y. Acad. Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Schiff N.D. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff N.D., Shah S.A., Hudson A.E., Nauvel T., Kalik S.F., Purpura K.P. Gating of attentional effort through the central thalamus. J. Neurophysiol. 2013;109:1152–1163. doi: 10.1152/jn.00317.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S.M., Fukada K., Simon R., Rearick M., Ray W. Neurophysiological and behavioral indices of time pressure effects on visuomotor task performance. Brain Res. Cogn. Brain Res. 2000;9:287–298. doi: 10.1016/s0926-6410(00)00009-4. [DOI] [PubMed] [Google Scholar]
- Stierwalt J.A.G., Murray L.L. Attention impairment following traumatic brain injury. Semin. Speech Lang. 2002;23:129–138. doi: 10.1055/s-2002-24989. [DOI] [PubMed] [Google Scholar]
- Thomson D.J. Spectrum estimation and harmonic analysis. Proc. IEEE. 1982;70:1055–1096. [Google Scholar]
- Trexler L.E., Zappala G. Neuropathological determinants of acquired attention disorders in traumatic brain injury. Brain Cogn. 1988;8:291–302. doi: 10.1016/0278-2626(88)90056-5. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T., Shimazu H., Isomura Y., Sasaki K. Theta oscillations in primate prefrontal and anterior cingulate cortices in forewarned reaction time tasks. J. Neurophysiol. 2010;103:827–843. doi: 10.1152/jn.00358.2009. [DOI] [PubMed] [Google Scholar]
- Tucker D.M. Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalogr. Clin. Neurophysiol. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Van der Werf Y.D., Witter M.P., Groenewegen H.J. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P., Langan J., Rodriguez E., Drew A., Halterman C., Osternig L.R., Chou L.-S. Attentional deficits in concussion. Brain Inj BI. 2005;19:1031–1039. doi: 10.1080/02699050500110363. [DOI] [PubMed] [Google Scholar]
- van Vugt M.K., Sederberg P.B., Kahana M.J. Comparison of spectral analysis methods for characterizing brain oscillations. J. Neurosci. Methods. 2007;162:49–63. doi: 10.1016/j.jneumeth.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Ulbert I., Schomer D.L., Marinkovic K., Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J. Neurosci. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmott C., Ponsford J., Hocking C., Schönberger M. Factors contributing to attentional impairments after traumatic brain injury. Neuropsychology. 2009;23:424–432. doi: 10.1037/a0015058. [DOI] [PubMed] [Google Scholar]
- Zappalà G., Thiebaut de Schotten M., Eslinger P.J. Traumatic brain injury and the frontal lobes: what can we gain with diffusion tensor imaging? Cortex J Devoted Study Nerv Syst Behav. 2012;48:156–165. doi: 10.1016/j.cortex.2011.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3
Supplementary material 4