Abstract
OBJECTIVES:
The accuracy of available noninvasive biomarkers for diagnosis, stratification, and prediction of inflammatory bowel disease (IBD) courses is limited. We analyzed volatile organic compounds (VOCs) in the breath of IBD patients and controls for diagnosis and differentiation of IBD as well as their link with disease location, activity, and phenotype.
METHODS:
A prospective study of diagnostic testing was conducted, recruiting Crohn's disease (CD), ulcerative colitis (UC), other inflammatory gastrointestinal diseases (OGDs), and healthy controls (HCs), as well as subjects with ileal pouch anal anastomosis (IPAA). The breath VOC profile was analyzed using selective ion flow tube-mass spectrometry.
RESULTS:
One hundred and twenty-four subjects (n=24 CD, n=11 UC, n=6 OGD, n=53 HC, n=30 IPAA) were included. The breath metabolome was significantly different in patients with IBD, CD, or UC compared with OGD and HC (7 out of 22 VOCs), but not between CD and UC. No link between the level of VOCs with complications, disease location, and clinical or radiologic disease activity, as well as lab parameters or type of medication was found. Breath VOCs were markedly different in patients with IPAA compared with any other group (17 out of 22 VOCs) and the presence of pouch inflammation did not alter the VOC levels.
CONCLUSIONS:
A specific breath metabolome is associated with IBD and markedly changes in patients with IPAA. Analysis of a broader spectrum of VOCs can potentially aid in the development of breath prints to diagnose or differentiate inflammatory bowel disorders.
INTRODUCTION
The diagnosis of inflammatory bowel diseases (IBDs) and differentiation between Crohn's disease (CD) and ulcerative colitis (UC) requires a multimodal approach involving clinical, endoscopic, histologic, serologic, and radiologic modalities.1, 2 IBD patients commonly suffer from a lag in time from first occurrence of symptoms to diagnosis, with a median diagnostic delay of 9 months.3 The length of diagnostic delay positively correlates with the later occurrence of bowel stenosis and need for intestinal surgery. Hence, the diagnostic delay may hinder our ability to alter the progression of disease.3 In addition, once the diagnosis of IBD is made, its subcategorization into CD or UC is critical to determine the optimal treatment strategy. It remains unclear which patients with an initially inflammatory disease classification will develop into a more severe vs. benign disease phenotype. Thus, it would be beneficial to identify at-risk populations that could benefit from a tailored therapeutic approach. The use of biologic and/or immunomodulator agents for therapy might be justified early in the disease course for patients at risk for rapid disease progression.4, 5
Metabolomics are defined as the investigation of a group of intermediate or end-point metabolites of a physiologic or pathophysiologic process,6, 7 which have the potential to provide a signature pattern for specific disease conditions. Metabolomic studies have entered the field of IBD, derived from serum, urine, or tissue samples in humans and IBD animal models.7 Recent technical advances now allow the measurement of some metabolites in the form of volatile organic compounds (VOCs) in the breath.7 This is important because certain VOC patterns are linked to disease inside and outside of the intestine, such as asthma, chronic obstructive pulmonary disease, chronic kidney disease, heart failure, alcoholic hepatitis, colon cancer, and others.8, 9, 10, 11, 12, 13, 14, 15 In the past, volatility and very low concentrations of breath components as well as difficulties with standardization and normalization have limited our ability to analyze them. However, these challenges have been largely overcome with advanced analysis techniques such as selective ion flow tube mass spectrometry (SIFT-MS). This technique has already shown a high discriminatory capability analyzing breath of pediatric IBD patients and controls.16
Limited data are available using this technique in adult IBD patients for diagnosis and differentiation, and most studies focus on single or only few VOCs.17, 18, 19, 20, 21 Information is missing about a more comprehensive evaluation of multiple VOCs at the same time and a link between the breath metabolome and disease phenotypes. In addition, the origin of the VOC changes remains to be defined. This study was designed to fill these knowledge gaps.
METHODS
Study population
We performed a single-center prospective study of diagnostic testing at a tertiary care academic referral hospital. Subjects in the age group 18–85 years were recruited from May 2013 to April 2015 from the general medical wards, gastroenterology in-patient and outpatient facilities, and the radiology department at the Cleveland Clinic. Inclusion criteria were the following conditions: IBD (CD and UC), other gastrointestinal (GI) inflammatory disease controls (OGDs) (diverticulitis, infectious enteritis, microscopic colitis, celiac disease, ischemic colitis, non-steroidal anti-inflammatory drug-induced colitis, radiation enteritis), and non-inflammatory controls (HCs) (subjects with no intestinal symptoms or no known GI disorders, irritable bowel syndrome, chronic diarrhea without intestinal inflammation). Subjects were categorized into each group after review of in-patient and outpatient medical records and a structured medical interview at the time of sample procurement. Subjects were identified to have IBD based on a combination of clinical, endoscopic, histologic, or serologic tests, following the consensus guidelines of the European Crohn's and Colitis Foundation.1, 2 Exclusion criteria were: subjects refusing to sign informed consent, younger than 18 years and older than 85 years of age, on oral or intravenous antibiotics within a 2-week period from breath testing, current diverting ileostomy and/or total abdominal colectomy, not having command over the English language, and subjects who could not be nil orally for 8 h owing to any medical reasons. Given a possible effect on the VOC profile, patients who underwent bowel preparation were excluded as well.
As a comparator, we also performed an analysis on a group of patients with ileal pouch anal anastomosis (IPAA). The inclusion criteria for IPAA patients were: patients who had IPAA for refractory UC, UC-associated dysplasia or cancer, familial adenomatosis polyposis, and pouchoscopy to document endoscopic findings at the time of breath sample procurement. Exclusion criteria were age younger than 18 years or older than 85 years, closure of diverting ileostomy <3 months from the time of sample collection, subjects refusing to sign informed consent, subjects not having command over the English language, and who could not be nil orally for 8 h owing to any medical reasons. Also in this group patients who underwent full bowel preparation were excluded. Subjects were recruited into each of the three different groups under the following categories: normal pouch (which included patients with irritable pouch syndrome), refractory pouchitis (RP), and CD of pouch. Subjects were classified into each category after review of in-patient and outpatient medical records, a structured medical interview at the time of sample procurement, and review of endoscopy and biopsy reports performed immediately after recruitment and sample procurement. Irritable pouch syndrome was defined as the presence of abdominal pain, pelvic discomfort and diarrhea with no inflammation of the afferent limb, and pouch or the rectal cuff on endoscopy.22, 23 Pouchitis was defined as a clinical syndrome characterized by the onset of increased stool frequency often with bloody diarrhea, pelvic discomfort, urgency, malaise, and fever.22 RP was defined as the requirement for continuous antibiotic treatment for symptom relief or symptoms refractory to antibiotic treatment for >4 weeks as well as patients needing any additional therapy besides antibiotics.22, 23 CD of the pouch was defined as involvement of the small bowel mucosa proximal to the ileal pouch or the development of perianal complications or pouch fistula more than 3 months after ileostomy closure.23, 24 Mechanical complications of surgery were excluded. Stool studies to rule out infection as a cause for the pouchitis were available. This study was performed with approval from the institutional review board at Cleveland Clinic, Cleveland, Ohio.
Data collection
Informed consent was obtained before breath sample collection. A chart review and structured medical interview was conducted to gather the following information: demographics (age, gender, race), body metrics (height, weight, body mass index), type of diagnosis, date of onset of symptoms, date of diagnosis, anatomic location of disease, current medications for IBD, smoking history, extraintestinal manifestations, presence of perianal disease or complications (stricture, fistula), surgery, and clinical disease activity score at the time of sample procurement (Harvey Bradshaw index for CD25 and Lichtiger score for UC26). To obtain objective information of disease activity, medical records were also reviewed to obtain the white blood cell count, and in a subgroup of patients, the breath sample was obtained immediately before CT enterography or MR enterography examination. CT enterography and MR enterography were performed as part of routine clinical practice and standard institutional protocols were used. To assess the quality and quantity of bowel inflammation, we modified a previously published radiologic score27 that includes assessment of mural inflammation and length of involved bowel segment. The scoring system is shown in Supplementary Table 1 online. In IPAA subjects, in addition to the data obtained for the rest of the groups, the following information was procured: reason for pouch, type of pouch, preoperative diagnosis, extent of colitis before surgery, use of preoperative medications, current pouch status, and current pouch disease activity index (clinical or endoscopic).22
Sample procurement and processing
Subjects were ensured to be nil per orally for 8 h before breath collection and they rinsed their mouths and gargled with tap water immediately before obtaining the breath sample to eliminate contamination from oral VOCs. Subjects were encouraged to exhale to release residual air from the lungs followed by inhalation to total lung capacity through a disposable mouth filter. The filter helps eliminate exogenous VOCs and potential contaminating agents. The inhaled ambient air was filtered through an attached N7500-2 acid gas cartridge (North Safety Products, Smithfield, RI). The subjects then exhaled through the mouth filter against 10 cm of water pressure into a Mylar bag (Convertidora Industrial, Jalisco, Mexico) at a steady flow rate. This allowed for the exhaled breath to be trapped in the Mylar bags once the bags were capped. Breath samples were analyzed within 2 h of collection after incubation to 37 °C for 10 min using the SIFT-MS (Syft Technologies, Christchurch, New Zealand) available at the Respiratory Institute at Cleveland Clinic (Cleveland, OH). Mylar bags were reused after flushing them with nitrogen.
Selective ion flow tube-mass spectrometry
SIFT-MS works on the principle of creation of reagent ions such as H3O+, NO+, and O2+ by a quadrupole. The reagent ions are selected one at a time by a quadrupole mass analyzer. These reagent ions then ionize individual gases of a complex gaseous mixture, such as the breath. These ionized compounds are then introduced into another quadrupole, which helps separate the individual ionized reaction products. Using the SIFT-MS, we measured the concentration of 22 identifiable VOCs in exhaled breath as described previously.16 The identified compounds include: 2-propanol, acetaldehyde, acetone, acrylonitrile, benzene, carbon disulfide, dimethyl sulfide, ethanol, isoprene, pentane, 1-decene, 1-heptene, 1-nonene, 1-octene, 3-methylhexane, (E)-2-nonene, ammonia, ethane, hydrogen sulfide, triethylamine, and trimethylamine.
Statistical analysis
Demographic and baseline comparisons were calculated in mean±standard deviations, or medians (P25, P75). P values were derived from appropriate statistical analytic tests like analysis of variance, Kruskal–Wallis test, Pearson's χ2 test or Fisher's exact test. VOC values were represented in means and P values were derived using Kruskal–Wallis test. VOC concentration values were adjusted to age and sex as mean (95% confidence interval (CI)) and were obtained using analysis of covariance analysis. When comparing VOCs of UC vs. CD, RP, and CD of pouch subjects, the logarithm of each VOC was modeled as the outcome variable, with age at diagnosis and disease duration at the time of breath test as the independent variables. Receiver operating characteristics of each VOC were used to obtain area under the curve (AUC) for each subject group as a measure of accuracy. Rho values were determined by using Spearman's correlations between each VOC and certain characteristics such as clinical score of disease activity, white blood cell counts, radiologic scores, ileal involvement, type of medications used, occurrence of complications such as strictures, fistulae, surgery in IBD subgroup analyses, and clinical and endoscopic pouchitis disease activity index in pouch disorders.
RESULTS
Cohort characteristics
The cohort characteristics is found in Table 1. The age at sample procurement, gender, and body mass index were comparable between the cohorts. Disease location in CD and UC is reflective of a tertiary referral center population. When comparing the groups, differences were detected in race (more African Americans in the HC group) and age at diagnosis (older age at diagnosis for the OGD group). As expected, patients with UC had a higher frequency of 5-aminosalicylic acid use.
Table 1. Demographic and clinical characteristics.
| Factor | CD (N=24) | UC (N=11) | IPAA (N=30) | OGD (N=6) | HC (n=53) | P value |
|---|---|---|---|---|---|---|
| Age at breath test (years) | 45.9±12.9 | 43.9±15.9 | 46.8±12.5 | 59.8±16.4 | 42.6±14.6 | 0.069a |
| Male | 11(45.8) | 4(36.4) | 15(50.0) | 1(16.7) | 17(32.1) | 0.36c |
| BMI | 26.3±8.6 | 26.7±7.3 | — | 30.6±13.1 | 26.4±8.9 | 0.75a |
| Race | 0.016d | |||||
| Caucasian | 21(87.5) | 10(90.9) | 30(100.0) | 6(100.0) | 14(73.7) | |
| African American | 1(4.2) | 0(0.0) | 0(0.0) | 0(0.0) | 5(26.3) | |
| Asian | 1(4.2) | 1(9.1) | 0(0.0) | 0(0.0) | 0(0.0) | |
| Hispanic | 1(4.2) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | |
| Age at diagnosis | 30.7±11.7 | 31.0±10.5 | 24.8±11.5 | 56.7±16.9 | — | <0.001a |
| Upper GI | 0(0.0) | — | — | — | — | — |
| Jejunum/proximal ileum | 2(9.1) | — | — | — | — | — |
| Ileocecal | 10(45.5) | — | — | — | — | — |
| Colon w/o cecum | 2(9.1) | — | — | — | — | — |
| Ileum/colon | 12(54.5) | — | — | — | — | — |
| Rectum | 2(9.1) | — | — | — | — | — |
| Ileal involvement | 19(79.2) | — | — | — | — | — |
| Proctosigmoiditis | — | 7(63.6) | — | — | — | — |
| Left-sided colitis | — | 5(45.5) | — | — | — | — |
| Pancolitis/extensive colitis | — | 3(27.3) | — | — | — | — |
| IBD duration at pouch (years) | — | — | 9.3±7.1 | — | — | — |
| Reason for pouch | — | |||||
| Dysplasia/cancer | — | — | 10(33.3) | — | — | |
| Refractory disease | — | — | 20(66.7) | — | — | |
| Pouch type | — | |||||
| J | — | — | 29(96.7) | — | — | |
| S | — | — | 1(3.3) | — | — | |
| Preop diagnosis | — | |||||
| UC | — | — | 27(90.0) | — | — | |
| CD | — | — | 1(3.3) | — | — | |
| IC | — | — | 2(6.7) | — | — | |
| Medications | ||||||
| 5-ASA | 6(25.0) | 11(100.0) | 5(16.7) | — | — | <0.001c |
| Immunosuppressants | 8(40.0) | 5(45.5) | 8(26.7) | — | — | 0.43d |
| Anti-TNF | 6(30.0) | 4(36.4) | 5(16.7) | — | — | 0.33d |
Abbreviations: ANOVA, analysis of variance; ASA, 5-aminosalicylic acid; BMI, body mass index; CD, Crohn's disease; GI, gastrointestinal; HC, non-inflammatory controls; IBD, inflammatory bowel disease; IC, inflammatory control; IPAA, ileal pouch anal anastomosis; OGD, inflammatory controls; TNF, tumor necrosis factor; UC, ulcerative colitis.
Values are presented as mean plus/minus s.d., median (P25, P75) or N (column %).
P values: a=ANOVA, b=Kruskal–Wallis test, c=Pearson's χ2 test, d=Fisher's exact test.
Bold and italic values are significant.
Diagnosis of IBD
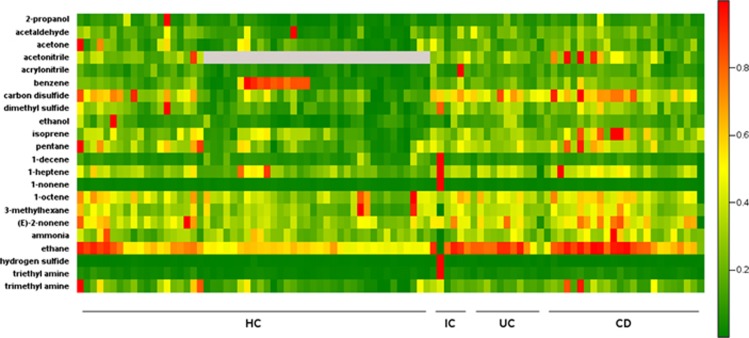
We next assessed the utility of breath VOCs to differentiate IBD from inflammatory and non-inflammatory controls. Age- and gender-adjusted analysis of the VOC concentration showed significant differences for IBD vs. HC in 7/22 (2-propanol, acrylonitrile, carbon disulfide, dimethylsulfide, ethanol, isoprene, triethylamine), for CD vs. HC in 7/22, (2-propanol, acrylonitrile, carbon disulfide, dimethylsulfide, ethanol, isoprene, triethylamine), for UC vs. HC in 2/22 (carbon disulfide, acrylonitrile), and OGD vs. HC in 2/22 (hydrogen sulfide, triethylamide) (Table 2 and Figure 1). The AUCs for differentiation of IBD compared with HC can be found in Table 3, with ethanol having the highest discriminatory capacity of 0.809. Six out of 22 VOCs showed an AUC ≥0.7 (2-propanol, acrylonitrile, carbon disulfide, dimethyl sulfide, ethanol, triethylamine), indicating strong discrimination.
Table 2. Breath VOCs in IBD: adjusted for age and gender.
| Factor | CD (N=24) | UC (N=11) | IPAA (N=30) | OGD (N=6) | HC (N=53) | P value |
|---|---|---|---|---|---|---|
| 2-Propanol | 83.3 (61.0, 113.8)a,b | 86.3 (54.3, 136.9)b | 322.1 (243.7, 425.9)a,c,d,e | 41.2 (21.6, 78.6)b | 44.5 (35.8, 55.3)b,e | <0.001 |
| Acetaldehyde | 38.8 (30.9, 48.6)b | 40.8 (29.2, 57.0)b | 170.7 (139.5, 208.9)a,c,d,e | 35.4 (22.2, 56.5)b | 26.8 (22.9, 31.4)b | <0.001 |
| Acetone | 220.9 (156.6, 311.6)b | 197.2 (118.4, 328.3)b | 853.3 (627.1, 1161.0)a,c,d,e | 247.6 (121.4, 504.6)b | 157.0 (123.4, 199.6)b | <0.001 |
| Acetonitrilef | 15.0 (11.8, 19.0) | 12.4 (8.7, 17.6) | 21.3 (17.3, 26.3) | 14.5 (8.9, 23.7) | 14.1 (10.7, 18.5) | 0.038 |
| Acrylonitrile | 1.08 (0.91, 1.3)a | 1.2 (0.89, 1.5)a | 1.08 (0.92, 1.3)a | 1.3 (0.89, 1.9) | 0.76 (0.67, 0.86)b,d,e | <0.001 |
| Benzene | 4.2 (3.1, 5.6)b | 3.6 (2.3, 5.6)b | 13.3 (10.3, 17.4)a,c,d,e | 3.9 (2.1, 7.1)b | 3.3 (2.7, 4.1)b | <0.001 |
| Carbon disulfide | 4.3 (3.4, 5.4)a,b | 4.3 (3.1, 5.9)a,b | 11.6 (9.5, 14.2)a,c,d,e | 4.2 (2.6, 6.6)b | 2.4 (2.1, 2.8)b,d,e | <0.001 |
| Dimethyl sulfide | 2.9 (2.2, 3.7)a,b | 3.0 (2.1, 4.3)b | 27.6 (22.1, 34.6)a,c,d,e | 3.4 (2.0, 5.7)b | 1.8 (1.5, 2.2)b,e | <0.001 |
| Ethanol | 111.5 (83.0, 149.6)a,b | 113.4 (73.3, 175.5)b | 256.5 (197.1, 333.9)a,c,d,e | 63.0 (34.2, 115.9)b | 57.3 (46.6, 70.4)b,e | <0.001 |
| Isoprene | 30.3 (23.3, 39.4)a,b | 22.6 (15.3, 33.3)b | 150.3 (118.8, 190.2)a,c,d,e | 24.5 (14.2, 42.2)b | 16.8 (14.0, 20.2)b,e | <0.001 |
| Pentane | 20.2 (16.2, 25.0)b | 18.5 (13.4, 25.5)b | 87.0 (71.7, 105.6)a,c,d,e | 18.9 (12.1, 29.6)b | 14.1 (12.1, 16.4)b | <0.001 |
| 1-Decene | 5.4 (3.7, 7.8)b | 5.3 (3.0, 9.1)b | 0.86 (0.62, 1.2)a,c,d,e | 9.8 (4.5, 21.1)b | 6.7 (5.1, 8.6)b | <0.001 |
| 1-Heptene | 12.2 (7.9, 19.0)b | 9.4 (4.9, 18.0)b | 2.2 (1.5, 3.3)a,c,d,e | 16.7 (6.7, 41.4)b | 11.0 (8.1, 14.9)b | <0.001 |
| 1-Nonene | 4.3 (3.0, 6.0)b | 4.2 (2.5, 7.0)b | 0.97 (0.72, 1.3)a,c,d,e | 11.4 (5.6, 23.1)b | 4.9 (3.9, 6.2)b | <0.001 |
| 1-Octene | 20.5 (14.3, 29.3)b | 20.8 (12.3, 35.3)b | 3.1 (2.2, 4.2)a,c,d,e | 21.9 (10.5, 45.8)b | 19.3 (15.1, 24.8)b | <0.001 |
| 3-Methylhexane | 32.6 (26.0, 41.0)b | 31.0 (22.1, 43.4)b | 67.3 (55.0, 82.5)a,c,d,e | 19.6 (12.3, 31.4)b | 25.3 (21.6, 29.7)b | <0.001 |
| (E)-2-nonene | 2.7 (1.9, 3.9)b | 2.3 (1.3, 3.9)b | 0.84 (0.61, 1.2)a,c,d,e | 3.2 (1.5, 6.7)b | 2.3 (1.8, 3.0)b | <0.001 |
| Ammonia | 76.5 (60.0, 97.6)c | 70.1 (48.9, 100.5) | 60.2 (48.4, 74.8) | 33.9 (20.5, 56.0)e | 62.0 (52.3, 73.4) | 0.065 |
| Ethane | 94.1 (82.3, 107.6)b | 92.0 (75.4, 112.2)b | 167.0 (148.1, 188.3)a,c,d,e | 69.4 (52.5, 91.6)b | 76.9 (70.0, 84.4)b | <0.001 |
| Hydrogen sulfide | 0.46 (0.35, 0.60) | 0.51 (0.34, 0.75) | 0.32 (0.25, 0.40)c | 1.01 (0.58, 1.8)a,b | 0.41 (0.34, 0.50)c | 0.003 |
| Triethyl amine | 1.3 (1.05, 1.6)a,b | 1.2 (0.86, 1.6) | 0.75 (0.62, 0.91)c,e | 2.2 (1.4, 3.4)a,b | 0.89 (0.77, 1.03)c,e | <0.001 |
| Trimethyl amine | 11.0 (8.2, 14.7)b | 8.8 (5.7, 13.5)b | 25.4 (19.6, 32.9)a,c,d,e | 9.8 (5.4, 17.7)b | 7.5 (6.1, 9.2)b | <0.001 |
Abbreviations: ANCOVA, analysis of covariance; CD, Crohn's disease; CI, confidence interval; HC, non-inflammatory controls; IBD, inflammatory bowel disease; IC, inflammatory control; IPAA, ileal pouch anal anastomosis; OGD, inflammatory controls; UC, ulcerative colitis; VOC, volatile organic compound.
Values presented as mean (95% CI) and were obtained using ANCOVA analysis. The logarithm of each VOC was modeled as the outcome variable with group, age, and gender as the independent variables. VOC values are presented as parts per billion.
Bonferroni correction was used for all post hoc comparisons.
Significantly different from healthy controls.
Significantly different from pouch.
Significantly different from ICs.
Significantly different from UC.
Significantly different from CD.
Acetonitrile measured in only 19 healthy controls. Bold and italic values are significant.
Figure 1.
Heat map depicting mass scans for the relative concentrations of examined volatile organic compounds (VOCs) in the breath of healthy controls (HCs), inflammatory controls (ICs), ulcerative colitis (UC) and Crohn's disease (CD) subjects. Red color depicts high and green color depicts low concentrations. OGD, other inflammatory GI diseases.
Table 3. Breath VOCs in IBD: ROC analysis.
| VOC | IBD vs. HC | CD vs. UC | IBD vs. OGD |
|---|---|---|---|
| 2-Propanol | 0.741 (0.639, 0.843) | 0.500 (0.302, 0.698) | 0.714 (0.506, 0.923) |
| Acetaldehyde | 0.700 (0.591, 0.808) | 0.523 (0.301, 0.744) | 0.524 (0.233, 0.815) |
| Acetone | 0.644 (0.528, 0.761) | 0.629 (0.428, 0.829) | 0.557 (0.277, 0.837) |
| Acetonitrile | 0.498 (0.341, 0.655) | 0.648 (0.464, 0.831) | 0.433 (0.214, 0.653) |
| Acrylonitrile | 0.769 (0.669, 0.868) | 0.538 (0.343, 0.733) | 0.510 (0.143, 0.876) |
| Benzene | 0.370 (0.252, 0.488) | 0.659 (0.456, 0.862) | 0.481 (0.180, 0.781) |
| Carbon disulfide | 0.798 (0.703, 0.892) | 0.542 (0.341, 0.742) | 0.562 (0.374, 0.750) |
| Dimethyl sulfide | 0.733 (0.628, 0.837) | 0.473 (0.254, 0.693) | 0.633 (0.338, 0.928) |
| Ethanol | 0.809 (0.717, 0.901) | 0.470 (0.235, 0.705) | 0.700 (0.413, 0.987) |
| Isoprene | 0.681 (0.570, 0.792) | 0.625 (0.436, 0.814) | 0.462 (0.231, 0.693) |
| Pentane | 0.710 (0.602, 0.818) | 0.572 (0.379, 0.765) | 0.590 (0.321, 0.860) |
| 1-Decene | 0.506 (0.382, 0.629) | 0.598 (0.385, 0.812) | 0.633 (0.383, 0.884) |
| 1-Heptene | 0.654 (0.534, 0.775) | 0.610 (0.414, 0.806) | 0.524 (0.293, 0.755) |
| 1-Nonene | 0.520 (0.397, 0.643) | 0.621 (0.405, 0.838) | 0.738 (0.506, 0.970) |
| 1-Octene | 0.608 (0.489, 0.726) | 0.598 (0.395, 0.802) | 0.567 (0.294, 0.840) |
| 3-Methylhexane | 0.647 (0.533, 0.762) | 0.583 (0.390, 0.776) | 0.476 (0.212, 0.740) |
| (E)-2-nonene | 0.673 (0.556, 0.790) | 0.636 (0.432, 0.841) | 0.576 (0.372, 0.780) |
| Ammonia | 0.627 (0.508, 0.746) | 0.568 (0.371, 0.765) | 0.748 (0.468, 1.000) |
| Ethane | 0.793 (0.689, 0.896) | 0.568 (0.369, 0.768) | 0.510 (0.238, 0.781) |
| Hydrogen sulfide | 0.616 (0.497, 0.735) | 0.549 (0.328, 0.770) | 0.619 (0.291, 0.948) |
| Triethyl amine | 0.735 (0.628, 0.842) | 0.614 (0.421, 0.807) | 0.686 (0.446, 0.926) |
| Trimethyl amine | 0.655 (0.541, 0.769) | 0.633 (0.438, 0.827) | 0.462 (0.175, 0.749) |
Abbreviations: AUC, are under ROC curve; CD, Crohn's disease; CI, confidence interval; HC, non-inflammatory controls; IBD, inflammatory bowel disease; OGD, inflammatory controls; ROC, receiver operating characteristics; UC, ulcerative colitis; VOC, volatile organic compound.
Values presented as AUC (95% CI).
Differentiation of IBDs
After detecting marked differences between IBD and HC, we next assessed whether VOCs can be used to differentiate CD from UC and OGD. There was no difference in any VOC levels between CD and UC and, in addition, no VOCs reached an AUC ≥0.7, suggesting a common breath metabolome in IBD that is shared between CD and UC (Table 3 and Supplementary Table 2). Only three compounds were significantly different between IBD and OGD (2-propanol, ethanol, and ammonia), with an AUC ≥0.7 (Table 3 and Supplementary Table 3).
Discriminant analysis diagnosis and differentiation
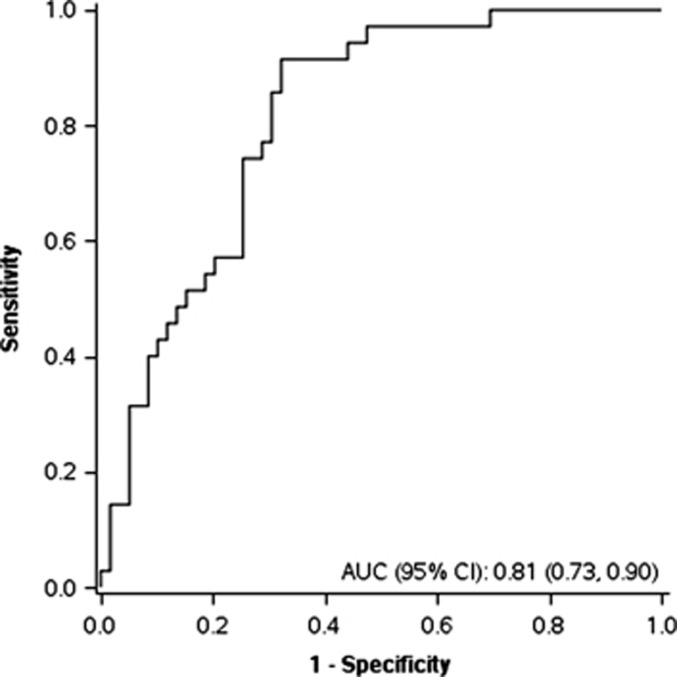
To assess the accuracy of the combined set of VOCs to differentiate IBD from non-IBD, we completed a discriminant analysis. Stepwise variable selection was performed using the VOC data. Acetone, acrylonitrile, carbon disulfide, and triethylamine were used to classify patients into the groups IBD or non-IBD. Considering the VOCs chosen by a discriminant analysis, the receiver operating characteristics for discrimination of IBD vs. non-IBD combined is 0.81 (95% CI: 0.73, 0.90) (Figure 2).
Figure 2.
Receiver operating characteristic (ROC) curve demonstrating discrimination of inflammatory bowel disease (IBD) compared with non-IBD. Acetone, acrylonitrile, carbon disulfide, and triethylamine were used to classify patients into the two groups. AUC, area under the curve; CI, confidence interval.
Disease activity
Given the pronounced difference in VOCs between IBD and all other groups, but a lack of difference between CD and UC, we speculated whether intestinal disease activity could influence the breath metabolome profile. Colonic preparation for endoscopy could potentially influence the breath VOC profile and hence assessment of endoscopic disease activity has its limitations in this scenario. We therefore decided to evaluate clinical disease activity using established clinical scoring systems,25, 26 the white blood cell count, and a modification of a previously published radiologic disease activity score27 at the time of breath measurements. Given the multitude of comparisons we set the significance level at P<0.01. None of the VOCs was associated to clinical or radiologic disease activity or white blood cell count (data not shown).
Disease phenotype
We next evaluated a link between the breath VOCs and CD location, medication, and the presence of complicated CD or the need for surgery. Significance level was again set at P<0.01. None of the VOCs was linked to ileal disease location, the intake of steroids, immunosuppressants or biologics, a history of complications, or the need for surgery (data not shown).
VOCs in patients with ileal pouch anal anastomosis
Given the distinct VOC profile in IBD compared with all other groups and no association with the disease activity parameters, we speculated about a possible influence of the colonic microflora and aimed at investigating a possible colonic origin of the breath VOCs found in IBD. We therefore recruited patients with IPAA after diagnosis of IBD or familial adenomatosis polyposis, hence being diagnosed with IBD or not, but lacking a colon. There was no difference in the demographics of IPAA subjects compared with the subjects with IBD, OGD, or HC with respect to age, gender, and body mass index (Table 1).
Seventeen out of 22 VOCs were different compared with all other groups, suggesting an entirely distinct breath metabolome compared with patients with a colon, regardless of normal or diseased (Table 2 and Supplementary Figure 1). We next compared HC vs. IPAA subjects with a normal pouch and found marked differences in VOCs in 18 out of 22 VOCs (Supplementary Table 4). The same was true for 16 out of 22 VOCs when comparing UC patients with a colon in situ with IPAA subjects with a normal pouch (Supplementary Table 5).
We again tested for a potential influence of inflammatory activity on the VOC profile. For this purpose, we compared normal, non-inflamed pouches with the most severe inflammatory pouch disorders, namely RP and CD of the pouch. Demographics of the groups can be found in Supplementary Table 6. Patients with CD of the pouch had a shorter time from diagnosis to pouch creation and the RP patients had a higher rate of preoperative immunomodulators. Patients with RP and CD of the pouch had a higher frequency of antibiotics at the time of sample procurement compared with normal pouches and more patients with RP were on 5-aminosalicylic acid compared with the two other groups (data not shown). Only one out of 22 VOCs (acrylonitrile) was higher in CD of the pouch compared with a normal pouch with an AUC of 0.846. All other VOCs remains unchanged (Table 4). We furthermore examined the endoscopic and clinical pouch disease activity index and none of the VOCs correlated with either of the two scores (data not shown). This supports the notion that active inflammation of the pouch does not alter the examined VOCs. We additionally assessed the pouch subjects for an association between antibiotic intake and VOC profile. Pouch patients with any antibiotic intake within the past 3 months had significantly higher acetaldehyde and benzene levels (P<0.05). None of those VOCs was used in the DCA.
Table 4. Breath VOCs in pouch disorders: adjusted for use of antibiotics.
| Factor | Normal pouch (N=7) | Refractory pouchitis (N=10) | CD (N=13) | P value |
|---|---|---|---|---|
| 2-Propanol | 245.1 (133.7, 449.3) | 254.8 (159.1, 408.3) | 484.9 (303.2, 775.2) | 0.14 |
| Acetaldehyde | 141.2 (88.1, 226.3) | 158.9 (110.1, 229.3) | 206.2 (143.1, 297.1) | 0.46 |
| Acetone | 1017.3 (416.7, 2483.2) | 632.8 (316.1, 1266.9) | 983.0 (492.5, 1962.0) | 0.56 |
| Acetonitrile | 19.5 (10.0, 38.1) | 28.5 (16.9, 47.9) | 17.2 (10.3, 28.9) | 0.36 |
| Acrylonitrile | 0.73 (0.52, 1.03)a | 1.00 (0.77, 1.3) | 1.5 (1.1, 1.9)b | 0.014 |
| Benzene | 13.0 (7.0, 24.3) | 16.6 (10.2, 26.9) | 11.2 (6.9, 18.1) | 0.5 |
| Carbon disulfide | 10.5 (5.6, 19.7) | 14.4 (8.8, 23.5) | 10.3 (6.3, 16.8) | 0.55 |
| Dimethyl sulfide | 21.8 (12.1, 39.3) | 20.8 (13.1, 32.9) | 41.8 (26.5, 66.1) | 0.1 |
| Ethanol | 193.3 (90.2, 414.2) | 215.8 (119.3, 390.4) | 373.1 (206.8, 673.3) | 0.35 |
| Isoprene | 123.4 (76.1, 199.8) | 120.3 (82.7, 175.1) | 207.1 (142.5, 300.9) | 0.12 |
| Pentane | 95.5 (53.7, 169.9) | 117.0 (74.7, 183.1) | 64.2 (41.1, 100.4) | 0.19 |
| 1-Decene | 0.67 (0.23, 1.9) | 0.43 (0.19, 0.97) | 1.9 (0.84, 4.4) | 0.052 |
| 1-Heptene | 2.9 (0.73, 11.6) | 0.83 (0.28, 2.4) | 4.4 (1.5, 12.9) | 0.082 |
| 1-Nonene | 1.2 (0.46, 3.3) | 0.49 (0.23, 1.05) | 1.6 (0.73, 3.3) | 0.087 |
| 1-Octene | 3.0 (0.82, 11.2) | 1.4 (0.52, 4.0) | 6.1 (2.2, 16.7) | 0.15 |
| 3-Methylhexane | 61.5 (42.2, 89.6) | 75.5 (56.3, 101.2) | 64.9 (48.5, 86.9) | 0.6 |
| (E)-2-nonene | 0.90 (0.26, 3.1) | 0.37 (0.14, 0.95) | 1.7 (0.65, 4.3) | 0.088 |
| Ammonia | 36.6 (17.4, 76.8) | 74.4 (41.8, 132.5) | 69.5 (39.2, 123.4) | 0.28 |
| Ethane | 151.6 (114.8, 200.4) | 144.2 (116.1, 179.1) | 203.6 (164.1, 252.7) | 0.092 |
| Hydrogen sulfide | 0.24 (0.14, 0.41) | 0.34 (0.23, 0.51) | 0.37 (0.25, 0.56) | 0.44 |
| Triethyl amine | 0.81 (0.50, 1.3) | 0.55 (0.38, 0.80) | 0.93 (0.64, 1.3) | 0.13 |
| Trimethyl amine | 37.2 (19.5, 70.8) | 29.0 (17.5, 47.8) | 18.0 (10.9, 29.6) | 0.23 |
Abbreviations: ANCOVA, analysis of covariance; CD, Crohn's disease; CI, confidence interval; VOC, volatile organic compound.
Values are presented as mean (95% CI) and were obtained using ANCOVA analysis. The logarithm of each VOC was modeled as the outcome variable with pouch type and use of antibiotics as the independent variables. VOC values are presented as parts per billion.
Bonferroni correction was used for all post hoc comparisons.
Significantly different from CD.
Significantly different from healthy controls. Bold and italic values are significant.
DISCUSSION
The main findings of our study are (1) the human breath metabolome can distinguish IBD from non-IBD with high accuracy; (2) the breath VOCs are not different between CD and UC; (3) the changes observed in IBD are not linked to clinical or radiologic disease activity; (4) VOCs do not differ among CD phenotypes; and (5) the breath metabolome is markedly different in the absence of a colon, but is not altered by inflammation of the pouch.
Recent technical advances allow for the measurement of metabolites in the form of VOCs in the breath. SIFT-MS technology is a new method allowing the detection of breath gases in complex mixtures regardless of water vapor content in real time. Compounds in concentrations as low as parts per billion can be distinguished from each other on the basis of their unique reaction with precursor ions. Pathologic GI conditions, such as alcoholic hepatitis,12 non-alcoholic fatty liver disease,15 or colorectal cancer9 can lead to a distinct breath pattern of VOCs that can aid in their diagnosis. In IBD most studies used single or a limited number of VOCs.
For diagnosis and differentiation of IBD from HC, elevated levels of pentane have been demonstrated with an AUC reported to be 0.927.17, 21 Additional VOCs found to be linked to IBD were NO, ethane, and propane.20, 21, 28 In our own cross-sectional study examining 21 VOCs in the breath of 62 pediatric IBD patients via SIFT-MS, six VOCs differentiated between IBD and HC: 1-octene, 3-methylhexane, and 1-decene were increased and 1-nonene, 2-nonene, and hydrogen sulfide were decreased. The AUC for a discriminant analysis IBD vs. HC was 0.96.16 In one very recent study, a panel of 26 VOCs was analyzed in 56 patients (38 IBD and 18 healthy controls).19 Concentrations of dimethyl sulfide, hydrogen sulfide, butanal, and nonanal were significantly different between CD and HC, ammonia was different in UC compared with HC, and hydrogen cyanide, hydrogen sulfide, and butanal differed in CD vs. UC. The AUC for distinguishing CD from healthy controls was 0.86 for UC vs. controls 0.74 and for CD vs. UC 0.82. In this small study, clinically active disease was not associated with changes in VOC patterns.
In the present investigation, 7/22 VOCs discriminated between IBD and HC, namely 2-propanol, acrylonitrile, carbon disulfide, dimethylsulfide, ethanol, isoprene, and trimethylamine. The major metabolic themes arising from the VOC differences between IBD and controls are bacterial fermentation, fatty acid and carbohydrate metabolism, and changes induced by an increase in reactive oxygen species.7 Data suggest that the intestinal microbiota may generate isoprene, dimethylsufilde, and ethanol.29, 30 Isoprenes are also products of cholesterol metabolism.7, 29 The presence of pentane in exhaled breath is considered a result of lipid peroxidation of polyunsaturated fatty acids in cellular membranes, a process mediated by free radicals and oxidative stress.7, 31 Dimethylsulfide has been established as a source of extra oral halitosis, which is thought to be derived from unexplained metabolic processes and is directly derived from the blood stream.32 Endogenous and exogenous sources of sulfur, mucin, or taurocholic acid are usually metabolized by bowel bacteria to produce toxigenic sulfur compounds such as hydrogen sulfide, methanethiol, and dimethylsulfide.33 These compounds have been implicated in the pathogenesis of UC.34 Dietary phosphatidylcholine is degraded by the intestinal microflora to form the volatile compound trimethylamine.7, 35 The influence of IBD on these areas of metabolism has been previously described and fits with the previously published VOC patterns. Considering the fact that we used an identical technical procedure to measure the breath VOCs in adults and pediatric patients in the same center, the pattern of VOCs that differentiate IBD in adult and pediatric populations were found to be different.16 Even though single gases, in which differences were detected, might be different to some prior reported studies, the pathways they belong to are shared among the published studies.7 This finding is in concordance with our observation that there was no difference in the breath pattern between CD and UC, given that metabolic pathways that we found to be altered are presumably shared between both entities of IBD. In addition, the patients in the pediatric study were not nil per os and hence diet could have influenced the expression of the VOCs.
Much less information is available in the literature on VOCs and their link with disease activity in CD. Pentane correlated with disease activity as measured by white blood cell scintigraphy in IBD18 and ethane, propane, and isoprene were linked to clinical and/or endoscopic disease activity in UC.20 No data are available on associating the breath metabolome with disease phenotypes, location, or medications and no studies have been performed in subjects without a colon. None of the VOCs in our study was associated with any of the above-mentioned parameters. This was also true for the quality and quantity of inflammation on cross-sectional imaging. This is novel information and suggests that IBD-associated factors other than inflammation could lead to a distinct expression of metabolic pathways measureable in the breath.
One such factor could be the intestinal microbiota, known to be distinct in IBD compared with controls.36 We therefore assessed whether the absence of the colon, the site of the largest amount of microbes, influences the breath metabolome. Subjects lacking a colon had a marked alteration of their breath metabolome. The difference between IPAA subjects and all other groups was significantly stronger compared with the difference between IBD patients and all other groups (data not shown). This was true when comparing HC with IPAA patients (normal pouch), and UC with IPAA patients (normal pouch). We again assessed intestinal inflammatory activity and VOCs. Given the fact that no full colon preparation is necessary for pouchoscopy, we were able to compare endoscopic and histologic disease activity with breath VOCs. For this purpose we chose extreme phenotypes, RP and CD of the pouch. The absence of an association of intestinal inflammation with changes in the VOCs was confirmed in this setting.
How could the breath VOC differences in IBD and their marked changes after colectomy be explained? The combined findings indicate that colon-derived factors in IBD lead to a distinct and inflammation-independent VOC profile. Given these data, we can speculate that this is either due to colonic microbial factors, changes in diet or an altered metabolism of luminal components (including diet), or all of them combined. The gut microbiome is critical in maintaining mucosal homeostasis and it is altered in IBD compared with healthy controls, showing reduced diversity.36, 37 The VOCs of fecal matter are distinct in IBD compared with healthy controls38 supporting this link. Walton et al.39 demonstrated that several VOCs in the headspace of feces differ markedly between patients with CD and other gastrointestinal conditions including UC and irritable bowel syndrome. The authors, using gas chromatography-mass spectrometry, showed that patients with CD had significant elevations in the concentrations of ester and alcohol derivatives of short-chain fatty acids and indole compared with patients in the other groups. After therapy, the levels of many of the VOCs significantly decreased and were similar to healthy controls. The authors concluded that intestinal dysbiosis in IBD may contribute to different fecal metabolite profiles. They also concluded that the normalization of the fecal VOC profile following therapy suggests re-establishment of relatively normal microbiota. Interestingly, the intake of probiotics in the preceding 3 months did not have an influence on the VOC profile in our cohort (data not shown). Based on our study protocol, the prior intake of antibiotics was an exclusion criterion with the exception of the pouch patients. In this group, the intake of antibiotics in the preceding 3 months had a minimal effect on the VOC expression profile. Intriguing is the finding of largely increased VOC levels in our pouch subjects, despite the removal of the colon. Our study results cannot explain this finding and this may invite further investigations on the contribution of the intestinal microbiota to breath VOCs.
Our study has several limitations. Our population is a single referral center study possibly introducing a referral bias. The VOCs were determined at a single time point and no longitudinal samples are available. Even though utmost care was taken to avoid an immediate influence of dietary factors, we cannot control for other environmental exposures that might influence the exhaled breath collection. This represents a pilot study and patient numbers are limited, which may influence the power to detect differences in phenotypes. The lack of association with disease activity and phenotype, however, is likely robust, given the absence of any statistical trends in the analysis. While we used filtered air in a controlled setting some of the VOCs may be of exogenous origin. Concomitant diagnoses of chronic obstructive pulmonary disease, asthma, or interstitial lung disease may confound the VOC profile, but the number of patients with concomitant lung diseases in our cohort were negligible (<3 per lung disease). The residence time of the chyme in the whole colon varies between 15 and 50 h. An 8 h fasting period may hence not allow normalization of VOC production. Administration of a standardized diet 4–5 days before the test would be optimal.
In conclusion, our study shows that exhaled VOC are a promising noninvasive method to discriminate IBD from non-IBD. The breath metabolome could not distinguish CD from UC and was not linked to clinical, radiologic, or endoscopic disease activity or disease phenotypes. The absence of a colon leads to a marked change in the exhaled VOCs, suggesting a critical role of the colon in their generation. This is a pilot study and the results need to be confirmed before they can be applied in clinical practice.
Study Highlights

Guarantor of the article: Florian Rieder, MD.
Specific author contributions: Study concept and design: FR, SK, CF, RD, RL, NP, NA; acquisition of data: SK, DG, CF, RL, BS, AB, MB; analysis and interpretation of data: FR, SK, DG, FC, RL, MB, CF, RD; drafting of the manuscript and critical revision of the manuscript for important intellectual content: all authors; statistical analysis: RL, DG; obtained funding: FR, CF, RD; administrative, technical, or material support: BS, PN, NA; study supervision: FR, RD.
Financial support: N.A. is funded by the American College of Gastroenterology Junior Faculty Development Award. C.F. is funded by NIH DK050984. R.D. is supported by BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development (ODOD) and by NIH 1U01AA021890. F.R. is funded by T32DK083251, 1K08DK110415, and P30DK097948.
Potential competing interests: FR is on the advisory board of AbbVie and UCB and on the speakers bureau for AbbVie.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Dignass A, Eliakim R, Magro F et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis 2012; 6: 965–990. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Dignass A, Panes J et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis 2010; 4: 7–27. [DOI] [PubMed] [Google Scholar]
- Schoepfer AM, Dehlavi MA, Fournier N et al. Diagnostic delay in Crohn's disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol 2013; 108: 1744–1753; quiz 1754. [DOI] [PubMed] [Google Scholar]
- D'Haens G, Baert F, van Assche G et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 2008; 371: 660–667. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L, Oussalah A, Williet N et al. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn's disease. Gut 2011; 60: 930–936. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Hatano N, Nishiumi S et al. Diagnosis of gastroenterological diseases by metabolome analysis using gas chromatography-mass spectrometry. J Gastroenterol 2012; 47: 9–20. [DOI] [PubMed] [Google Scholar]
- Kurada S, Alkhouri N, Fiocchi C et al. Review article: breath analysis in inflammatory bowel diseases. Aliment Pharmacol Ther 2015; 41: 329–341. [DOI] [PubMed] [Google Scholar]
- Grob NM, Aytekin M, Dweik RA. Biomarkers in exhaled breath condensate: a review of collection, processing and analysis. J Breath Res 2008; 2: 037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare DF, Di Lena M, Porcelli F et al. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg 2013; 100: 144–150. [DOI] [PubMed] [Google Scholar]
- Pagonas N, Vautz W, Seifert L et al. Volatile organic compounds in uremia. PLoS One 2012; 7: e46258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samara MA, Tang WH, Cikach FJr et al. Single exhaled breath metabolomic analysis identifies unique breathprint in patients with acute decompensated heart failure. J Am Coll Cardiol 2013; 61: 1463–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanouneh IA, Zein NN, Cikach F et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikach FSJr, Tonelli AR, Barnes J et al. Breath analysis in pulmonary arterial hypertension. Chest 2014; 145: 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhouri N, Eng K, Cikach F et al. Breathprints of childhood obesity: changes in volatile organic compounds in obese children compared with lean controls. Pediatr Obes 2014; 10: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhouri N, Cikach F, Eng K et al. Analysis of breath volatile organic compounds as a noninvasive tool to diagnose nonalcoholic fatty liver disease in children. Eur J Gastroenterol Hepatol 2014; 26: 82–87. [DOI] [PubMed] [Google Scholar]
- Patel N, Alkhouri N, Eng K et al. Metabolomic analysis of breath volatile organic compounds reveals unique breathprints in children with inflammatory bowel disease: a pilot study. Aliment Pharmacol Ther 2014; 40: 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryahina K, Spanel P, Pospisilova V et al. Quantification of pentane in exhaled breath, a potential biomarker of bowel disease, using selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom 2013; 27: 1983–1992. [DOI] [PubMed] [Google Scholar]
- Kokoszka J, Nelson RL, Swedler WI et al. Determination of inflammatory bowel disease activity by breath pentane analysis. Dis Colon Rectum 1993; 36: 597–601. [DOI] [PubMed] [Google Scholar]
- Hicks LC, Huang J, Kumar S et al. Analysis of exhaled breath volatile organic compounds in inflammatory bowel disease: a pilot study. J Crohns Colitis 2015; 9: 731–737. [DOI] [PubMed] [Google Scholar]
- Sedghi S, Keshavarzian A, Klamut M et al. Elevated breath ethane levels in active ulcerative colitis: evidence for excessive lipid peroxidation. Am J Gastroenterol 1994; 89: 2217–2221. [PubMed] [Google Scholar]
- Pelli MA, Trovarelli G, Capodicasa E et al. Breath alkanes determination in ulcerative colitis and Crohn's disease. Dis Colon Rectum 1999; 42: 71–76. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Tremaine WJ, Batts KP et al. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc 1994; 69: 409–415. [DOI] [PubMed] [Google Scholar]
- Shen B. Acute and chronic pouchitis—pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol 2012; 9: 323–333. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ. Pouchitis following ileal pouch-anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology 1994; 107: 1856–1860. [DOI] [PubMed] [Google Scholar]
- Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980; 1: 514. [DOI] [PubMed] [Google Scholar]
- Lichtiger S, Present DH, Kornbluth A et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994; 330: 1841–1845. [DOI] [PubMed] [Google Scholar]
- Faubion WAJr., Fletcher JG, O'Byrne S et al. EMerging BiomARKers in Inflammatory Bowel Disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn's disease activity: role of cross-sectional imaging. Am J Gastroenterol 2013; 108: 1891–1900. [DOI] [PubMed] [Google Scholar]
- Koek GH, Verleden GM, Evenepoel P et al. Activity related increase of exhaled nitric oxide in Crohn's disease and ulcerative colitis: a manifestation of systemic involvement? Respir Med 2002; 96: 530–535. [DOI] [PubMed] [Google Scholar]
- Ajibola OA, Smith D, Spanel P et al. Effects of dietary nutrients on volatile breath metabolites. J Nutr Sci 2013; 2: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curson AR, Sullivan MJ, Todd JD et al. Identification of genes for dimethyl sulfide production in bacteria in the gut of Atlantic Herring (Clupea harengus. ISME J 2010; 4: 144–146. [DOI] [PubMed] [Google Scholar]
- Van Gossum A, Decuyper J. Breath alkanes as an index of lipid peroxidation. Eur Respir J 1989; 2: 787–791. [PubMed] [Google Scholar]
- Tangerman A, Winkel EG. Intra- and extra-oral halitosis: finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J Clin Periodontol 2007; 34: 748–755. [DOI] [PubMed] [Google Scholar]
- Suarez F, Furne J, Springfield J et al. Production and elimination of sulfur-containing gases in the rat colon. Am J Physiol 1998; 274: G727–G733. [DOI] [PubMed] [Google Scholar]
- Roediger WE, Duncan A, Kapaniris O et al. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology 1993; 104: 802–809. [DOI] [PubMed] [Google Scholar]
- Zeisel SH, daCosta KA, Youssef M et al. Conversion of dietary choline to trimethylamine and dimethylamine in rats: dose–response relationship. J Nutr 1989; 119: 800–804. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008; 134: 577–594. [DOI] [PubMed] [Google Scholar]
- Ott SJ, Schreiber S. Reduced microbial diversity in inflammatory bowel diseases. Gut 2006; 55: 1207. [PMC free article] [PubMed] [Google Scholar]
- Probert CS, Reade S, Ahmed I. Fecal volatile organic compounds: a novel, cheaper method of diagnosing inflammatory bowel disease? Expert Rev Clin Immunol 2014; 10: 1129–1131. [DOI] [PubMed] [Google Scholar]
- Walton C, Fowler DP, Turner C et al. Analysis of volatile organic compounds of bacterial origin in chronic gastrointestinal diseases. Inflamm Bowel Dis 2013; 19: 2069–2078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.