ABSTRACT
Skeletal myogenesis is regulated by signal transduction, but the factors and mechanisms involved are not well understood. The group I Paks Pak1 and Pak2 are related protein kinases and direct effectors of Cdc42 and Rac1. Group I Paks are ubiquitously expressed and specifically required for myoblast fusion in Drosophila. We report that both Pak1 and Pak2 are activated during mammalian myoblast differentiation. One pathway of activation is initiated by N-cadherin ligation and involves the cadherin coreceptor Cdo with its downstream effector, Cdc42. Individual genetic deletion of Pak1 and Pak2 in mice has no overt effect on skeletal muscle development or regeneration. However, combined muscle-specific deletion of Pak1 and Pak2 results in reduced muscle mass and a higher proportion of myofibers with a smaller cross-sectional area. This phenotype is exacerbated after repair to acute injury. Furthermore, primary myoblasts lacking Pak1 and Pak2 display delayed expression of myogenic differentiation markers and myotube formation. These results identify Pak1 and Pak2 as redundant regulators of myoblast differentiation in vitro and in vivo and as components of the promyogenic Ncad/Cdo/Cdc42 signaling pathway.
KEYWORDS: Pak, cell adhesion, cell differentiation, myogenesis, regeneration, signal transduction
INTRODUCTION
Cell differentiation is a complex process whereby precursor cells take on tissue-specific structure and function. Lineage-restricted transcription factors lie at the heart of cell differentiation, but the process is often initiated and fortified by ubiquitous signaling pathways that function in many biological contexts. Skeletal myogenesis serves as a paradigm for cell differentiation. Differentiation of skeletal myoblasts is a coordinated process involving adoption of a cell-type-specific transcriptional program and morphological changes, including fusion into multinucleated myofibers (1, 2). MyoD family proteins (MyoD, Myf5, myogenin, and MRF4) are muscle-specific transcription factors that act in concert with other, more broadly expressed transcription factors to establish the muscle phenotype (1, 3). The activities of these factors are regulated posttranslationally by non-muscle-specific signal transduction pathways. One such pathway is the p38α/β mitogen-activated protein kinase (MAPK; here simply p38) pathway (4). p38 is activated during myogenic differentiation in vitro, and its inhibition results in impaired differentiation (5–7). Furthermore, mice lacking p38α exhibit delayed myofiber growth and maturation (8).
The signals that initiate p38 activity during myoblast differentiation are poorly understood. One mechanism is via activation of a signaling complex nucleated at sites of cadherin-based cell-cell adhesion (9, 10). The transmembrane IgSF coreceptor Cdo (also called Cdon) is bound in cis to N-cadherin (Ncad) in myoblasts. During myoblast differentiation, or acutely upon Ncad ligation, the Cdo intracellular region associates directly with (i) Bnip-2, a scaffold protein for Cdc42, and (ii) JLP, a scaffold protein for the p38 pathway. Formation of these complexes stimulates Cdc42 activity, which in turn signals to activate p38 within these complexes to promote myoblast differentiation. Cdo−/− mice display delayed skeletal muscle development, and Cdo−/− myoblasts express reduced levels of muscle-specific gene products and form multinucleated myotubes inefficiently (11, 12).
The small GTPase Cdc42 functions as a signaling hub, transducing a variety of upstream cues via multiple direct effector proteins to regulate myriad cellular functions (13). The concentration of active Cdc42 rises during myogenic differentiation in vitro, and reduction of Cdc42 activity delays expression of muscle-specific genes and diminishes myotube formation (14). Rac1 is related to Cdc42, and they utilize overlapping sets of effector proteins (13, 15). Conditional genetic removal of Rac1 and Cdc42 from myogenic precursor cells in the mouse embryo reveals that they are required, nonredundantly, for myoblast fusion in vivo and in vitro (16).
The effector proteins that transduce Cdc42-dependent (or Rac1-dependent) signals during mammalian myoblast differentiation and fusion are largely unknown. Among the direct effectors of Cdc42 and Rac1 are the group I Paks (Pak1, Pak2, and Pak3), members of the Ste20 family of Ser/Thr kinases (17–19). Paks are conserved regulators of many cellular functions, including cell polarity, cytoskeletal dynamics, and transcription (17, 18). In the yeast Saccharomyces cerevisiae, Ste20 transduces signals from Cdc42 activated in response to mating factor and increases in external osmolarity (20). In each response, Ste20 triggers a MAPK cascade, serving as a MAPK kinase kinase kinase (MAP4K) to phosphorylate and activate Ste11, a MAP3K. Depending on the stimulus, Ste11 activates pathway-specific MAP2Ks and MAPKs to result in polarized cell growth or hyperosmotic stress resistance, respectively. Mammalian group I Paks (Pak1, Pak2, and Pak3) are capable of complementing a Ste20 deletion in yeast; in contrast, the more divergent group II Paks (Pak4, Pak5, and Pak6) are unable to do so (17). Evidence that mammalian group I Paks function as MAP4Ks is lacking, but they can serve as links between Cdc42 (or Rac1) and p38, though the details are less clear than in yeast (21–24).
Group I Paks may play multiple roles in skeletal muscle development. In Drosophila, interaction of cell surface adhesion molecules on adjacent myoblasts initiates a pathway leading to Rac1-dependent activation of dPak3 and dPak1 (orthologous to mammalian Pak2 and Pak1, respectively), a process specifically required for embryonic myoblast fusion (25). In mammalian systems, Pak1 and Pak2 are widely expressed, including in skeletal muscle (26, 27), whereas Pak3 is specific to the nervous system (27). Pak1 acts downstream of the tyrosine kinase MuSK to promote agrin-induced acetylcholine receptor (AChR) clustering in C2C12 myotubes (28). Moreover, denervation of adult mouse muscle results in increased levels of Pak1, which phosphorylates the corepressor CtBP1 to promote transcription of myogenin in extrasynaptic nuclei (29). Therefore, group I Paks may be involved in processes as varied as myoblast fusion and maturation of neuromuscular junctions (NMJs). However, the effects of genetic loss of Pak1 or Pak2 on skeletal myogenesis in mice have not been reported. Mice that are germ line null for Pak1 (Pak1−/−) display defects in mast cells and macrophages but are viable and fertile (27). Pak2 germ line mutation (Pak2−/−) results in multiple developmental defects and embryonic lethality at embryonic day 8.5 (E8.5), prior to skeletal muscle development (27, 30).
We report here studies on the role of Pak1 and Pak2 in mammalian developmental and regenerative myogenesis. Pak1 and Pak2 are expressed in myoblasts and activated specifically during differentiation; they are also activated by Ncad ligation as components of the Cdo-containing signaling complex. Moreover, Pak1 and Pak2 associate with p38 in a JLP-dependent manner during differentiation, and their RNA interference (RNAi)-mediated depletion impairs Ncad-initiated p38 activation. Mice with muscle-specific deletion of both Pak1 and Pak2 have reduced myofiber size in uninjured tissue, as well as after regeneration, but modest defects in neuromuscular synapses. Primary myoblasts from these mice display delayed myotube formation, but this is associated with delayed expression of differentiation markers, revealing that the defect is not specific to myoblast fusion. These results identify group I Paks as regulators of skeletal myoblast differentiation in vivo and in vitro.
RESULTS
Pak1 and Pak2 are activated during myogenic differentiation.
Activation of Pak1 and Pak2 by small GTPases leads to their phosphorylation at Ser144/199/204 and Ser141/192/197, respectively, which can be detected with phospho-Pak (pPak)-specific antibodies (31–34). To assess the roles of Pak1 and Pak2 in myogenic differentiation, we initially analyzed their phosphorylation status in proliferating and differentiating primary mouse myoblasts. Cell lysates were immunoblotted with antibodies against the following: (i) total Pak1, (ii) total Pak2, (iii) pPak1(S144)/pPak2(S141) and pPak1(S199/S204)/pPak2(S192/S197) (note that the two pPak antibodies recognize the respective phosphorylated forms of both Pak1 and Pak2 but not other Paks), and (iv) myosin heavy chain (MHC), a marker of differentiation. Primary myoblasts proliferating in growth medium (primary myoblast GM [see Materials and Methods]) expressed both Pak1 and Pak2 but did not display any pPak1/2 signal (Fig. 1A). In contrast, cells cultured for 3 days in differentiation medium (primary myoblast DM [see Materials and Methods]) displayed abundant pPak1/2. Total levels of Pak1 and Pak2 increased slightly in DM, but this was insufficient to explain the striking difference in pPak1/2 signals. This pattern of phosphorylation was the same as that of p38 (Fig. 1A). A similar pattern of Pak1 and Pak2 expression and phosphorylation was observed in a differentiation time course of C2C12 myoblasts (Fig. 1B).
FIG 1.

Pak1 and Pak2 activation during myoblast differentiation. (A) Western blot analysis of primary myoblasts cultured in GM (G) or in DM (D) for 3 days. (B) Western blot analysis of C2C12 cells proliferating at low density in GM (G), cultured in GM to ∼80% confluence (0) or transferred to DM for the indicated times. β-Tubulin was used as a loading control. (C) Lysates of C2C12 cells cultured in GM (G) or in DM (D) for 36 h were immunoprecipitated (IP) with Pak1 or Pak2 antibodies and then blotted with Pak1, Pak2, or pPak1(S144)/pPak2(S141) antibodies. (D) Lysates of C2C12 cells cultured in GM (G) or in DM (D) for 36 h were immunoprecipitated with Pak1 or Pak2 antibodies, and IPs were incubated with [32P]ATP and myelin basic protein (MBP) as a substrate. Reaction products were fractionated by SDS-PAGE and visualized by autoradiography (top panels) and Coomassie blue staining (bottom panels).
Two pPak1/2 bands were sometimes resolved on SDS-PAGE, but the relative strengths of the bands were somewhat variable (Fig. 1A and B; see below). To more definitively address whether both Pak1 and Pak2 were phosphorylated during myoblast differentiation, C2C12 cells proliferating in GM or cultured in DM for 36 h were harvested, and lysates were immunoprecipitated with Pak1- or Pak2-specific antibodies and blotted for Pak1, Pak2, and pPak1(S144)/pPak2(S141) (Fig. 1C). Both Pak1 and Pak2 were reactive with the phosphospecific antibody when immunoprecipitated from cultures in DM but not GM, consistent with the straight Western blots in Fig. 1A and B. Pak1 and Pak2 did not coimmunoprecipitate with each other, indicating not only the specificity of the antibodies but also that Pak1 and Pak2 did not appreciably heterodimerize in myoblasts (Fig. 1C). Therefore, Pak1 and Pak2 homodimers are presumably activated independently during differentiation. Finally, we analyzed the kinase activities of Pak1 and Pak2 in C2C12 cells proliferating in GM or cultured in DM for 3 days (Fig. 1D). Pak1 and Pak2 immunoprecipitates (IPs) were incubated with myelin basic protein (MBP) as a substrate and [32P]ATP. Consistent with their phosphorylation status, the kinase activities of Pak1 and Pak2 were significantly induced during myoblast differentiation.
Ncad/Cdo signaling stimulates Pak1/2 activation.
Cdc42 and Rac1 are activated by many signaling stimuli, and the cues that activate them during mammalian myogenesis are not well documented. One identified pathway is initiated upon Ncad ligation, which triggers interaction of a Bnip-2/Cdc42 complex with the intracellular region of the Ncad-associated coreceptor Cdo. Cdc42 in such complexes is active and in turn signals to activate p38 bound indirectly to Cdo via its scaffold protein, JLP (10, 12, 14) (see Fig. 10 for a model). These Cdo-dependent multiprotein complexes play a significant, but not exclusive, role in activating Cdc42 and p38 during myoblast differentiation. We hypothesized that Pak1 and/or Pak2 might reside between Cdc42 and p38 in this pathway. To assess whether Paks are activated by Ncad ligation, C2C12 myoblasts were plated at low density on culture dishes coated with recombinant Ncad ectodomain or, as a control, poly-l-lysine (PLL) for 2 h. As previously described (10), cells plated on Ncad but not on PLL produced phosphorylated p38 (pp38) (Fig. 2A). Similarly, pPak1(S144)/pPak2(S141) and pPak1(S199/S204)/pPak2(S192/S197) were produced by cells cultured on the Ncad substrate but not on PLL (Fig. 2A). To address whether Cdo and JLP are required for Ncad-initiated Pak1/2 phosphorylation, C2C12 cells depleted of Cdo or JLP by RNAi were plated on Ncad substrate; cells that expressed a control RNAi sequence were also analyzed. Depletion of either Cdo or JLP had no effect on total Pak1 or Pak2 expression but diminished production of pPak1/2, as well as pp38, upon Ncad ligation (Fig. 2B). The role of Cdc42 in Pak1/2 activation in this pathway was analyzed by stable overexpression of Cdc42GAP, a negative regulator of Cdc42 activity, in C2C12 cells. Cdc42GAP expression reduces steady-state levels of GTP-bound Cdc42 (and, consequently, Ncad ligation-associated p38 activation and myoblast differentiation itself) but is not as disruptive to cell morphology as the expression of dominant negative Cdc42 mutants or small interfering RNA (siRNA) to Cdc42 (14). Like depletion of Cdo and JLP, overexpression of Cdc42GAP did not alter Pak1 or Pak2 levels but reduced Ncad ligation-induced phosphorylation of Pak1/2 (Fig. 2C).
FIG 10.

Model of group I Paks as components of the Ncad/Cdo signaling pathway. Ncad ligation triggers assembly of signaling modules for Cdc42 and p38 MAPK on the intracellular region of the Ncad coreceptor Cdo. Cdo-dependent activation of Cdc42 activates Pak1 and Pak2, initiating a MAPK cascade that culminates in activation of p38, which in turn promotes muscle-specific gene expression and myogenic differentiation. TAK1 and ASK1 are the likely MAP3Ks in the pathway (61), and MKK3 and MKK6 are promyogenic, direct activators of p38 kinases (6), although the work in this paper does not demonstrate that these kinases are those that reside between group I Paks and p38.
FIG 2.
Ncad/Cdo signaling stimulates Pak1/2 activation. (A) Western blot analysis of C2C12 cells plated on Ncad or PLL substrates for 2 h. (B) Western blot analysis of C2C12 cells stably expressing siRNA against Cdo or Jlp or a control siRNA (Con) and plated on Ncad substrate for 2 h. (C) Western blot analysis of C2C12 cells stably transfected with control (Con) or Flag-tagged Cdc42GAP expression vectors and plated on Ncad substrate for 2 h. (D) Photomicrographs of Ncad-coated beads attached to cells that coexpress nonfluorescent Cdo plus GFP, Pak1-DsRed, or Pak2-GFP. Bar, 10 μm. (E) Percentage of Ncad-coated beads that clustered the indicated fluorescent protein. Values are means ± standard deviations (SD); n > 100 beads. *, P < 0.01, with differences referring to both the respective −Cdo and +Cdo/+GFP controls. (F) Western blot analysis of Cdo+/+ (+/+) and Cdo−/− (−/−) myoblasts proliferating at low density in GM (G), cultured in GM to ∼80% confluence (0), or transferred to DM for the indicated times.
Ncad, Cdo, JLP, Bnip-2, and activated Cdc42 all cluster at sites of Ncad ligation in an assay in which Ncad ectodomain-coated microspheres bind to the surface of fibroblasts expressing fluorescently tagged versions of these proteins (10). The assay is specific, in that (i) clustering of proteins downstream of Cdo is dependent on exogenous expression of Cdo (fibroblasts express low levels of Cdo) and (ii) the percentage of Ncad-coated beads that cluster a given protein diminishes in a manner predicted by the model (i.e., wherein Ncad binds Cdo, Cdo binds JLP and Bnip-2, Bnip-2 binds Cdc42, etc.). To monitor clustering of activated Cdc42 in this assay, we previously used N-Wasp-GFP (where GFP is green fluorescent protein), and this protein was found at ∼45% of Ncad-coated beads (10). Like N-Wasp, Paks bind directly to activated Cdc42. Results similar to those with N-Wasp were obtained with fluorescently tagged forms of Pak1 and Pak2: ∼45% of Ncad-coated beads clustered Pak1-DsRed or Pak2-GFP in a Cdo-dependent manner, significantly above the ∼20% of beads that clustered GFP, which was used as a control (Fig. 2D and E).
Cdo−/− myoblasts display reduced levels of GTP-bound Cdc42 and pp38, express low levels of muscle-specific proteins, and form myotubes inefficiently (12, 14). Cdo+/+ and Cdo−/− myoblasts were analyzed for Pak1 and Pak2 expression and phosphorylation over a 3-day time course of differentiation (Fig. 2F). Pak1 levels were similar for Cdo+/+ and Cdo−/− myoblasts. Pak2 levels were reduced in the mutant cells compared to wild-type cells, but Pak2 was still easily detectable. Similar to results with C2C12 cells, pPak1/2 was not observed in cells in GM, whereas both species of pPak1/2 were detectable at day 0, further induced after 1 day in DM, and maintained through 3 days in DM (Fig. 2F). In contrast, production of pPak1/2 in Cdo−/− myoblasts was barely detectable through 2 days in DM, correlating with the defective differentiation program in these cells. Notably, at day 3 in DM, Cdo+/+ and Cdo−/− myoblasts had similar levels of both pPak1/2 species (Fig. 2F), indicating that Cdo-independent mechanisms of Pak activation exist and occur in differentiating myoblasts.
Pak1 and Pak2 form JLP-dependent complexes with p38 during myoblast differentiation.
We next asked whether endogenous Pak1, Pak2, and p38 associate during myogenesis. Lysates from C2C12 cells in GM or in DM for 36 h were immunoprecipitated with Pak1 or Pak2 antibodies and blotted for Pak1, Pak2, and p38 (Fig. 3A). p38 coimmunoprecipitated with Pak1 and Pak2 much more efficiently from cells in DM than in GM, even though the total levels of the three proteins were not substantially different under the two conditions. Similar to results shown in Fig. 1C, Pak1 and Pak2 did not coimmunoprecipitate with each other, indicating that the Pak1/p38 and Pak2/p38 complexes are distinct. To assess whether JLP, the p38 MAPK pathway scaffold protein that links Cdo and p38, is required for association of p38 with Paks, the experiment was repeated with cells from which Jlp had been depleted by RNAi. RNAi-mediated knockdown of JLP led to a substantial decrease in the amount of p38 that coimmunoprecipitated with Pak1 or Pak2, even though total levels of p38 were unaffected (Fig. 3B).
FIG 3.
Pak1 and Pak2 form complexes with p38, and Pak2 is important for Ncad-initiated p38 activity. (A) Lysates of C2C12 cells cultured in GM (G) or in DM (D) for 36 h were immunoprecipitated with Pak1 or Pak2 antibodies and blotted with Pak1, Pak2, or p38 antibodies. (B) Lysates of C2C12 cells stably expressing siRNA against Jlp or a control siRNA (Con) cultured in DM for 36 h were immunoprecipitated with Pak1 or Pak2 antibodies and blotted with Pak1, Pak2, JLP, or p38 antibodies. (C) Western blot analysis of C2C12 cells stably expressing siRNA against Pak1 or Pak2 or a control siRNA (Con) and plated on Ncad substrate for 2 h. (D) Western blot analysis of C2C12 cells stably expressing siRNA against Pak2 or a control siRNA (Con) and plated on Ncad substrate for 2 h. Con cells were also plated on PLL. (E) Western blot analysis of C2C12 cells cultured with 2.5 μM SB203580 (SB) or DMSO (Con) and plated on Ncad for 2 h. Con cells were also plated on PLL.
To address whether Paks are required for p38 activation by Ncad ligation, Pak1 and Pak2 were individually knocked down by RNAi in C2C12 cells, and the cells were plated on Ncad substrate. Cells depleted of Pak2, but not cells depleted of Pak1, had much lower levels of pp38 than control cells (Fig. 3C). In contrast, Ncad-dependent phosphorylation of another MAPK, extracellular signal-regulated kinase (ERK), was not affected by depletion of Pak2 (Fig. 3D), indicating a specific requirement in p38 signaling. To assess whether Pak activation occurs upstream of p38, C2C12 cells pretreated with the p38α/β inhibitor SB203580 or dimethyl sulfoxide (DMSO; vehicle control) were plated on Ncad or PLL substrates. Cells treated with SB203580 or DMSO induced pPak1/2 on the Ncad substrate equivalently (Fig. 3E), indicating that Pak2 is necessary for full p38 activation but p38 activity is not necessary for Pak phosphorylation, consistent with the notion that Paks lie upstream of p38 in the Ncad pathway. (Note that the SB203580-treated cells maintained in culture with the drug failed to differentiate, demonstrating the effectiveness of the treatment.)
Pak1−/− and Pak2cKO mice have no overt muscle defects.
By Western blotting muscle extracts from mice of different ages, we observed robust levels of Pak1 and Pak2 through 3 weeks of age (Fig. 4A). Expression was diminished by 2 months, although both Pak1 and Pak2 could be detected in mice of this age when a greater amount of extract was used (Fig. 4B). To assess the role of group I Paks in muscle development and regeneration in vivo, we studied mice with targeted individual deletions of Pak1 and Pak2. Muscles in 2-month-old Pak1−/− mice showed no obvious defects (Fig. 4B and C). Furthermore, these animals were proficient in regeneration of the tibialis anterior (TA) muscle following a freeze injury (Fig. 4C). As Pak2−/− mice have early embryonic lethality, we generated a muscle-specific Pak2 mutation by crossing mice with a Pak2 conditional allele (Pak2f) with those carrying a MyoDiCre allele (Pak2f/f;MyoDiCre; here referred to as Pak2cKO). Pak2cKO mice were viable and fertile, were comparable in size to their control littermates, and had skeletal muscles with normal appearance (Fig. 4C). Western blotting confirmed efficient loss of Pak2 expression in PakcKO muscles (Fig. 4B). Regenerative myogenesis in 2-month-old Pak2cKO animals subjected to TA freeze injury also revealed no overt differences between mutants and controls (Fig. 4C). These results indicate that, individually, Pak1 and Pak2 are not essential for developmental or regenerative myogenesis in vivo.
FIG 4.
Pak1−/− and Pak2cKO mice have no overt muscle defects. (A) Western blot analyses of muscle extracts from wild-type (WT) mice at postnatal day 7 (P7), P14, and P21 and at 2 months (2M) of age. Whole-brain extract was used as a positive control for Pak1 and Pak2 antibodies and Gapdh as a loading control. (B) Western blot analyses of muscle extracts from mice of the indicated genotypes. Whole-brain extract was used as a positive control and Gapdh as a loading control. (C) H&E-stained sections of uninjured and D14 postinjury TA muscle from 2-month-old animals.
Mice lacking group I Paks display reduced muscle mass.
We investigated potential redundancy of Pak1 and Pak2 function by generating mice lacking both kinases in skeletal muscle. Pak1−/− mice were crossed to Pak2cKO animals to generate Pak1−/−;Pak2cKO double knockout (dKO) mutant mice. dKO animals were viable, fertile, and born at Mendelian ratios. Skeletal muscle from these mice lacked Pak1 and Pak2 protein (Fig. 4B). Age-matched dKO animals and controls (Pak−/+; Pak2f/f) were of similar body weight at postnatal day 11, but by 2 months of age, both male and female dKO mice weighed significantly less than control animals (Fig. 5A and B).
FIG 5.
Mice lacking group I Paks display reduced muscle mass. (A) Body weights of control (n = 7) and dKO (n = 8) mice at P11. (B) Body weights of 2-month-old male and female control and dKO mice (n = 4 for each sex and genotype). (C) Weights of individual muscle groups from animals as described for panel B. (D) H&E-stained sections of TA muscles from 2-month-old mice. (E) Cross-sectional areas of individual fibers from muscles shown in panel D. Frequency is the number of fibers with a given cross-sectional area as a percentage of the total number of fibers. n > 500 fibers measured per animal. (F) TA sections from 2-month-old mice stained for Pax7 (magenta, arrows), laminin (green), and DAPI (blue). The graph shows the number of Pax7/DAPI-positive cells per section (n = 4 per sex and genotype). Values are means ± SD; *, P < 0.05.
We measured the wet weights of TA, quadriceps, extensor digitorum longus, and soleus muscles from age- and sex-matched 2-month-old animals. The TA, quadriceps, and soleus in male animals were smaller in dKO mice than controls (Fig. 5C). A similar pattern was observed in female mutant animals, with the TA and quadriceps having significantly reduced muscle mass (Fig. 5C). Other organs did not show significant differences in weight (data not shown), indicating that the lower body weight in dKO animals was largely a result of reduced muscle mass.
Hematoxylin and eosin (H&E) staining of TA sections showed that despite lower muscle mass, dKO mice formed intact musculature with well-organized myofibers (Fig. 5D). The average cross-sectional area of TA myofibers of control mice was not different from that of dKO mice (data not shown). However, the range of fiber sizes from dKO TA muscles was significantly shifted toward those with smaller cross-sectional areas (Fig. 5E). Two-month-old dKO and control TA muscles had similar numbers of Pax7+ satellite cells (Fig. 5F), suggesting that decreased numbers of muscle stem cells were likely not a cause of the reduced muscle mass.
Pak1 and Pak2 are not essential for formation of neuromuscular synapses in vivo.
Muscle fiber size is controlled, in part, by muscle use. Because defects in synaptic transmission can lead to a decrease in muscle activity and myofiber size (35, 36), we studied the arrangement and organization of neuromuscular synapses in dKO mice. We evaluated neuromuscular synapses by examining the distribution of motor axons and synapses in diaphragm muscles from embryonic day 18.5 (E18.5) mice. Motor axons and nerve terminals were visualized by staining for neurofilaments and synapsin, and postsynaptic differentiation was assessed by staining for acetylcholine receptors (AChRs). We found no gross defects in the arrangement of motor axons and the distribution of synapses in dKO mice compared to control mice (Fig. 6A). Furthermore, control and dKO animals had a comparable number of synapses (Fig. 6B). The average synaptic size was also similar for control and dKO mice, but the density of synaptic AChRs at individual synapses was reduced by 30% in the mutants (Fig. 6C to E).
FIG 6.
Pak1 and Pak2 are not essential for formation of neuromuscular synapses in vivo. (A) Representative immunofluorescence staining of neurofilament and synapsin (for nerve branching and synapses, respectively, in green) and α-bungarotoxin (for AChRs, in magenta) in diaphragm of E18.5 control and dKO mice. (B) Numbers of synapses (synapsin/α-bungarotoxin costaining) in the left diaphragm hemisphere were determined for control (n = 5) and dKO (n = 6) animals. (C) Images of synapse morphology, showing AChR cluster (red), nerve terminal (green), and synapse (merge). Quantification of synapse area (D) and AChR density (E) for control (n = 4) and dKO (n = 5) mice. Values are means ± SD; *, P < 0.05.
Analysis of the Akt signaling pathway in dKO muscle.
The Akt pathway is a key regulator of skeletal muscle mass during postnatal growth (37–39). Deficiencies in Akt pathway activity result in reduced myofiber size and muscle mass. We assessed the activity of this pathway in control and dKO muscle at postnatal day 2 (P2) and at 2 months of age (Fig. 7). Extracts were blotted with antibodies for (i) the differentiation marker, MHC (both the embryonic form, eMHC, and total MHC); (ii) Akt and its activated form, pAkt(S473); (iii) S6 kinase and pS6 ribosomal protein(S235/236); and (iv) FoxO1, FoxO3a, pFoxO3a(S253)/pFoxO1(S251), and pFoxO3a(S318/321)/pFoxO1(S322/325).
FIG 7.
Analysis of the Akt signaling pathway in dKO muscle. Western blot analyses of muscle extracts from postnatal day 2 (P2) and 2-month-old (2 mos.) control and dKO mice. Gapdh was used as a loading control. Results for two mice of each genotype at each age are shown.
We observed similar expression of eMHC and MHC in P2 control and dKO muscle with, as expected, eMHC strongly downregulated in 2-month-old tissue. Both control and dKO P2 animals showed robust expression of total Akt, the levels of which were reduced in 2-month-old animals. dKO mice had slightly higher levels of Akt than controls at 2 months of age. We noted comparable levels of pAkt(S473) in control and dKO P2 animals. However, whereas pAkt(S473) expression was strongly diminished in 2-month-old control animals, dKO mice maintained detectable levels of activated Akt. Despite this difference, similar levels of the Akt effector S6 kinase and the phosphorylated form of the S6 kinase substrate, pS6 ribosomal protein(S235/236), were seen in P2 and 2-month-old mice of both genotypes.
FoxO1 and FoxO3a were expressed at similar levels in P2 control and dKO animals. FoxO1 levels were slightly reduced and FoxO3 levels strongly reduced in 2-month-old mice, again irrespective of genotype. However, we observed no major differences in the levels of pFoxO3a(S318/S321)/pFoxO1(S322/325) or pFoxO3a(S253)/pFoxO1(S251) for either genotype at both time points. Given the strong reduction in total FoxO3a, but not FoxO1, levels from P2 to 2 months of age, it seems likely that the signals detected with the phosphoantibodies represent mainly FoxO1, the levels of which were only modestly changed between these time points. Therefore, apart from the incomplete downregulation of activated Akt in 2-month-old dKO muscle, no obvious alterations in pathway activity were seen in these animals. We also note that the elevated levels of pAkt(S473) in 2-month-old dKO mice are the reverse of what would be predicted if perturbations in Akt signaling were the cause of their decreased muscle mass.
Skeletal muscle is largely resistant to apoptosis, due to expression of prosurvival proteins (40, 41). However, aberrant apoptosis could lead to a lower muscle mass. We evaluated apoptosis by Western blotting of the proapoptotic protein caspase 3 and its active form, cleaved caspase 3 (Fig. 7). Expression of caspase 3 was not elevated in P2 dKO muscles compared to controls. At this age, muscle from mice of both genotypes also expressed cleaved caspase 3, with dKO mice displaying somewhat less than controls. Caspase 3 and cleaved caspase 3 levels were strongly diminished in 2-month-old control muscle. Residual caspase 3 was detected in 2-month-old dKO mice, although cleaved caspase 3 was not detected. Caspase 3 plays a role in myoblast differentiation (42), and its expression and downregulation may reflect this role rather than an apoptotic one. Taken together, major defects in Akt and cell survival signaling were not associated with the lower muscle mass found in dKO mice. However, some changes associated with muscle maturation (e.g., diminished Akt, pAkt, and caspase 3) were incomplete in dKO muscle, suggesting that the maturation process in these mice may also be incomplete.
Myoblasts from dKO mice display delayed myogenic differentiation in vitro.
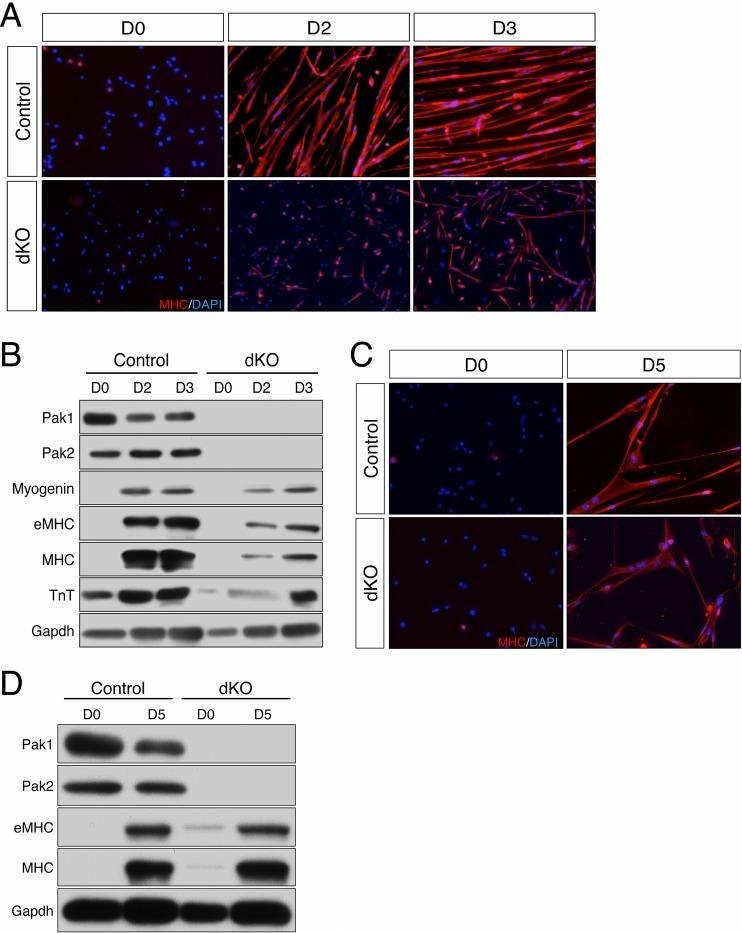
To assess the roles of Pak1/2 in myoblast differentiation, primary myoblasts from dKO and control mice were cultured in GM (day 0) and then switched to DM over a 3-day time course. We assayed cell proliferation in GM and after 24 h in DM by 5-ethynyl-2′-deoxyuridine (EdU) incorporation. At day zero (D0), ∼12% of both control and dKO myoblasts were EdU+. After 24 h in DM, <1% of both cell types were EdU+. Therefore, loss of Pak1/2 did not alter proliferative ability or cell cycle withdrawal. After 2 days in DM, control myoblasts formed elongated, myosin heavy chain-positive (MHC+), multinucleated myotubes (Fig. 8A). Conversely, dKO myoblasts formed myotubes very inefficiently at day 2 (Fig. 8A). By day 3, dKO cultures had formed some multinucleated myotubes, though they were not as elongated and still contained fewer nuclei than control myoblast cultures (Fig. 8A).
FIG 8.
Myoblasts from dKO mice display delayed differentiation in vitro. (A) Primary myoblasts from control and dKO mice cultured in GM (D0) or in DM for 2 or 3 days (D2, D3) were subjected to immunofluorescence staining for MHC (red) and DAPI (blue). (B) Western blot analysis of control and dKO primary myoblasts at D0, D2, and D3. Gapdh was used as a loading control. (C) Control and dKO primary myoblasts cultured in GM (D0) or in DM for 5 days (D5) were analyzed as described for panel A. (D) Western blot analysis of control and dKO primary myoblasts at D0 and D5. Gapdh was used as a loading control.
Cell lysates from primary myoblast cultures at days 0, 2, and 3 were immunoblotted with antibodies against the early differentiation marker, myogenin, and the later differentiation markers, MHC (both eMHC and total MHC) and troponin T. dKO cultures expressed reduced levels of myogenin, eMHC, and total MHC at both day 2 and day 3, compared to control myoblasts (Fig. 8B). Troponin T expression was delayed but was similar for control and dKO cultures at day 3. Primary dKO myoblasts therefore differentiated inefficiently by both biochemical and morphological criteria. Differentiation of primary mouse myoblasts is largely complete by D3 in DM. To address whether dKO myoblasts were defective or delayed in differentiation, control and dKO cultures were further evaluated at D5 in DM. Apart from some further elongation, we observed no major difference between control myotubes at D3 and D5 (Fig. 8C). However, dKO cultures maintained in DM for 5 days had formed myotubes that appeared similar to those of controls at this time point (Fig. 8C). Furthermore, expression of eMHC and total MHC in dKO cultures was equivalent to that of control cultures at D5 (Fig. 8B and D). Therefore, the alteration in differentiation by dKO myoblasts is best characterized as a delay.
These results are similar to those seen with Cdo−/− myoblasts, which display a partial deficiency in activation of p38 MAPK (12, 14). Because Pak1 and Pak2 functioned as components of the Ncad/Cdo/p38 pathway (Fig. 2 and 3), we assessed production of total p38 and pp38 by primary dKO myoblasts during differentiation in vitro. Over the course of several experiments with multiple independent isolates of cells, we found that although the total levels of p38 were similar, the levels of pp38 were highly variable, including cultures with levels that were lower than, similar to, and higher than those seen in parallel cultures of control myoblasts (data not shown). We hypothesize that loss of group I Paks resulted in feedback regulation of p38 activity by unknown mechanisms that were not well controlled under standard culture conditions.
Muscle regeneration is impaired in dKO mice.
The freeze injury model was used to evaluate regeneration in 2-month-old dKO mice. Muscles were harvested at days 5 and 14 postinjury (D5PI and D14PI, respectively) to assess the progression of repair. H&E staining of TA sections at D5PI revealed nascent fibers in both control and dKO muscle (Fig. 9A). Control mice had largely completed the repair process by D14PI, presenting with centrally nucleated myofibers (Fig. 9A). dKO mice also successfully repaired the injury at this time point, although there remained pockets of mononuclear cells between fibers, which were not seen in controls (Fig. 9A). Next, we measured the cross-sectional areas of uninjured and injured TAs at D14PI. Uninjured TAs from control animals had a significantly larger area than those from dKO mice, and this difference was magnified in the injured TAs when measured at D14PI (Fig. 9B). Analysis of regenerated myofibers from D14PI muscle again showed that dKO fibers had significantly smaller cross-sectional areas than controls (Fig. 9C). Additionally, significantly fewer dKO myofibers showed multiple central nuclei in cross sections (Fig. 9A and D).
FIG 9.
Muscle regeneration is impaired in dKO mice. (A) H&E-stained sections of TA muscles from 2-month-old control and dKO animals at D5 and D14 after freeze injury. (B) Cross-sectional areas of TA muscles from sections shown in panel A, measured prior to injury (control and dKO, n = 8) and at D14 postinjury (control, n = 3; dKO, n = 5). (C) Cross-sectional areas of individual regenerated fibers from muscles at D14 postinjury. Frequency equals the number of fibers with a given cross-sectional area as a percentage of the total number of fibers. n > 500 fibers per animal. (D) Numbers of central nuclei per individual regenerated myofiber in sections whose results are shown in panel C were determined. Frequency equals the number of fibers with a given number of central nuclei as a percentage of the total number of fibers. n > 500 fibers per animal. (E) Western blot analysis of muscle extracts from uninjured and postinjury tissue from 2-month-old mice. Gapdh was used as a loading control. Values are means ± SD; *, P < 0.05.
We also assessed the injury repair process by Western blotting of extracts from uninjured and injured quadriceps of dKO and control animals, with Pak2cKO and Pak1−/− mice included for comparison. Lysates were immunoblotted with antibodies for group I Paks (Pak1, Pak2, and Pak3), pPak1(S144)/pPak2(S141), and eMHC. As seen in Fig. 4, Pak1 and Pak2 were expressed at very low levels in uninjured muscle of control and single mutant animals (Fig. 9E). At D5PI, Pak1 and/or Pak2 expression was induced to readily detectable levels in regenerating muscles of animals of the appropriate genotypes. We note that the induction of Pak2 (and presumably Pak1) at D5PI must occur in cells of the skeletal muscle lineage, as Pak2 expression is not observed in Pak2cKO or dKO mice and the MyoDiCre driver is highly specific for this lineage (43). Pak1 and Pak2 expression was downregulated at D14PI, with residual expression of Pak1 seen only in Pak2cKO muscle (Fig. 9E). pPak1(S144)/pPak2(S141) was also detected with a pattern that paralleled Pak1 expression during regeneration. Although this pattern of pPak1(S144)/pPak2(S141) expression could be interpreted to mean that pPak1(S144), but not pPak2(S141), is formed during regeneration, based on the redundancy of Pak1 and Pak2 in muscle development and regeneration, we suspect that pPak2(S141) is present but difficult to detect due to the antibody being directed against the pPak1(S144) epitope and the variability we have also seen with the pPak1/2 antibodies in Western blot experiments with cultured cells (Fig. 1 to 3). Finally, there was no compensatory upregulation of Pak3, the remaining group I Pak, in uninjured or regenerating dKO muscle (Fig. 9E), indicating that dKO mice lacked all group I Pak activity in this tissue.
The developmental marker eMHC is expressed in newly forming myofibers (44, 45). Consistent with this, we observed that it was strongly expressed in regenerating control and Pak1−/− muscle at D5PI, with a slight reduction in Pak2cKO mice (Fig. 9E). eMHC expression was notably diminished in dKO muscle at D5PI, compared to all other genotypes (Fig. 9E). By D14, regenerated muscle from mice of all genotypes no longer expressed detectable eMHC (Fig. 9E).
DISCUSSION
Myogenic differentiation is regulated by signal transduction pathways, but the identities and mechanisms of the factors involved in this process are incompletely understood. The ubiquitous small GTPase Cdc42 is required for fusion of muscle precursor cells into myofibers in vivo and for efficient and complete differentiation (including fusion) of myoblasts in vitro (14, 16), but the effector proteins that transduce myogenic signals from Cdc42 are not known. Group I Paks are direct effectors of Cdc42 (17, 18). They are versatile proteins, with diverse functions in transcriptional and cytoskeletal regulation, which suggested that they may act during mammalian myogenesis. Furthermore, the Drosophila group I Paks are necessary for myoblast fusion during embryonic myogenesis (25). Pak1 has been reported to be involved in AChR clustering in cultured mammalian myotubes (28) as well as in transcriptional upregulation of myogenin in response to muscle denervation in mice (29). Here we investigated the roles of Pak1 and Pak2 in myogenesis via conditional mutagenesis in the mouse. Our results demonstrated that the combined loss of Pak1 and Pak2 resulted in reduced myofiber and muscle size during both developmental and regenerative myogenesis. These defects appear to be best explained by a need for Pak1 and Pak2 for efficient myoblast differentiation.
Pak1 and Pak2 were individually phosphorylated and activated in differentiating, but not proliferating, myoblasts in vitro. We have previously reported that a promyogenic signaling pathway is initiated by cell-cell contact and consequent Ncad-dependent adhesion (10, 14). Ncad ligation leads to assembly of signaling complexes on the intracellular region of the Ncad coreceptor, Cdo, which in turn promote Cdc42 activity and Cdc42-dependent activation of p38 MAPK (Fig. 10). We found that Pak1/2 phosphorylation was stimulated by acute Ncad ligation in a manner dependent on Cdo, JLP (a p38 scaffold protein with a central role in the complex), and Cdc42 activity. Furthermore, Pak1 and Pak2 accumulated at sites of acute Ncad ligation. Pak1 and especially Pak2 also function in this pathway as effectors of Cdc42 in the activation of p38. Pak1 and Pak2 associated with p38 during myoblast differentiation in a JLP-dependent manner, and knockdown of Pak2 diminished p38 activation by Ncad ligation. Despite the clear role for group I Paks in this pathway as observed upon direct, acute Ncad ligation, analysis of Pak1/2 activation and p38 activation during DM-induced differentiation of Cdo−/− and dKO myoblasts, respectively, revealed that there are additional mechanisms for activation of these kinases in these cells. Cdo−/− myoblasts displayed a defect in Pak1/2 phosphorylation early in a differentiation time course, but at later points, Cdo mutant cells had levels of pPak1/2 equivalent to those seen in control cells. Additionally, Pak1/2 dKO myoblasts showed highly variable levels of pp38 when cultured in DM, suggesting activation of feedback mechanisms that were not well controlled when the cells were cultured in DM. This places some limitations on what can be concluded about the relationship between Pak1/2 and p38 activation during myoblast differentiation. Nevertheless, Cdo−/− and dKO myoblasts displayed similar delays in differentiation (12, 14; this study). We speculate that the mechanisms leading to Pak1/2 activation in Cdo−/− myoblasts and the mechanisms leading to p38 activation in dKO myoblasts are unable to provide optimal differentiation signals. This may be due to alterations in the timing, location, or duration of these signals.
dKO myoblasts displayed delays in both expression of muscle-specific gene products and myotube formation. Similarly, we found that during regeneration dKO mice produced low levels of eMHC. These results are consistent with the notion that Pak1 and Pak2 signal to promote myoblast differentiation in vitro and in vivo and that their combined absence resulted in a substantial but incomplete defect in this process. We found that the density of synaptic AChRs was reduced by 30% in E18.5 dKO mice, consistent with previous studies reporting ∼50% decrease in Agrin-induced AChR clusters in C2C12 cell myotubes that express a dominant negative form of Pak1 (which should inhibit both Pak1 and Pak2) (28). However, the number and size of neuromuscular synapses were unaffected in dKO mice. AChRs are present in excess at neuromuscular synapses. Because the safety factor for synaptic transmission is ∼2 at developing rodent neuromuscular synapses and 3 to 4 at adult neuromuscular synapses (46), a 30% decrease in the density of synaptic AChRs is unlikely to lead to a decrease in the efficiency of synaptic transmission and to be responsible for the decrease in myofiber size in dKO mice (47). Instead, it seems likely that inefficient myoblast differentiation is the major contributor to the defect in developmental and regenerative myogenesis seen in the dKO mice. Nevertheless, it will be interesting to assess neuromuscular synapses and AChR density in dKO animals as they age.
Previous reports suggested that Pak1 and Pak2 might have distinct roles in myogenesis. In Drosophila, loss of dPak3 (similar to Pak2) was associated with defective myoblast fusion; loss of dPak1 (similar to Pak1) produced no phenotype, but dPak1;dPak3 double mutants had a more severe fusion defect than dPak3 mutants (25). Additionally, Pak1 but not Pak2 expression was induced upon muscle denervation in mice (29). On the other hand, Pak1 and Pak2 display nearly identical substrate specificity in vitro, and both complement an Ste20 deletion in S. cerevisiae (17, 48). Furthermore, in some studies with mammalian cells, knockdown of both Pak1 and Pak2 is required to lose Pak signaling, revealing overlapping function (49). We found that Pak1 and Pak2 were both expressed in developing postnatal muscle, with each of their levels diminished by 2 months of age. Moreover, unlike what is observed with denervation, both Pak1 and Pak2 were induced during regeneration in response to freeze injury. Consistent with the similar biochemical properties of Pak1 and Pak2 and their similar expression patterns during myogenesis, we found that Pak1−/− and Pak2cKO single mutant mice developed and regenerated muscle comparably to control mice. In contrast, dKO mice had myofibers with small cross-sectional areas and reduced muscle mass relative to controls, and this phenotype was exacerbated after injury repair. Therefore, Pak1 and Pak2 display genetic redundancy in postnatal and regenerative myogenesis.
The fact that dKO myoblasts displayed delays in both muscle-specific gene expression and myoblast fusion suggests that much of the fusion defect is secondary to a generalized defect in differentiation. This is in contrast to the rather specific and complete block to embryonic myoblast fusion seen in Drosophila that lacks group I Paks (25). We believe that dKO mice have lost all group I Pak activity because the mutations appear to be nulls and there was no compensatory expression in muscle of the third mammalian group I Pak, Pak3. The essential role of group I Paks in Drosophila myoblast fusion is therefore not evolutionarily conserved in mammalian myogenesis in vivo. It is possible that one or more of the group II Paks (Pak4, Pak5, and Pak6) compensate for Pak1 and Pak2 in mammalian myogenesis, and there is some evidence of shared substrates between group I and II enzymes (48, 50, 51). However, the mechanisms of activation of group I versus group II Paks are distinct, and the two groups have divergent preferred phosphorylation consensus sites (17, 48, 51). Therefore, the fact that group I Paks are essential for myoblast fusion in Drosophila but not in mice may reflect an evolutionary divergence in some of the mechanisms or components that underlie this process. It should be noted that genetic removal of Cdc42 from muscle progenitors in the mouse embryo results in a very strong block to myoblast fusion (16). Therefore, group I Paks cannot be the sole effectors of Cdc42 in this process. One likely Cdc42 effector in myoblast fusion is N-Wasp, as mouse embryonic myoblasts lacking this factor fail to fuse (52). Finally, Pak1 has been implicated in agrin-stimulated clustering of AChRs in vitro (28), but we did not see obvious defects in neuromuscular synapse formation in dKO mice, suggesting either that additional kinases can perform this function in vivo or that in vitro systems are more sensitive to loss of a role for Paks than is observed in vivo.
In summary, we have shown here that group I Paks play a rate-limiting but nonessential role in myoblast differentiation in vivo and in vitro and that the Ncad/Cdo/Cdc42 pathway signals to activate Pak1 and Pak2 during this process. Identification and mechanistic understanding of fundamental signaling factors that promote muscle precursor cell differentiation, like Paks, may provide potential targets for regulation of this process in therapy of muscle diseases and atrophy (53).
MATERIALS AND METHODS
Cell culture.
C2C12 and NIH 3T3 cells were cultured as previously described (54, 55). For C2C12 cells, cells were maintained as proliferating cultures in growth medium (GM; Dulbecco modified Eagle medium [DMEM] plus 15% fetal bovine serum) and were transferred to differentiation medium (DM; DMEM plus 2% horse serum) at approximately 80% confluence. Primary myoblasts were derived from hind limbs of P14 mice by the method of Rando and Blau (56) and cultured as described previously (11, 12). Primary myoblasts were maintained in primary myoblast GM (Ham F-10 plus 20% fetal bovine serum plus 5% horse serum plus 2.5 ng/ml basic fibroblast growth factor; R&D Systems) and plated 24 h prior to being switched to primary myoblast DM (DMEM plus 5% horse serum) for a 5-day differentiation time course. DM was refreshed at day 3. Primary myoblast proliferation was assessed in GM and after 24 h in DM using the Click-iT EdU Alexa Fluor 488 Imaging kit (Invitrogen). Briefly, primary myoblast cultures were plated for 24 h either in GM or in DM, after which the medium was removed and replaced with fresh medium containing 50 μM EdU, and cells were incubated for 2 h. The manufacturer's protocol was followed to reveal EdU+ cells, and 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes) was used for nuclear staining. The population of EdU+ cells was determined as a percentage of the total number of DAPI+ cells.
For RNAi-mediated depletion of Cdo and JLP, pSUPER.puro vectors containing previously validated siRNA sequences to Cdo and JLP (12, 57) and a control pSUPER.puro vector harboring an irrelevant sequence were used. C2C12 cells were transfected with Lipofectamine 2000 (Invitrogen), and cultures were selected with 4 μg/ml puromycin. Drug-resistant cells were pooled and analyzed. For RNAi-mediated depletion of Pak1 and Pak2, the following sequences were inserted into pSUPER.puro vectors (Oligoengine): Pak1 number 1, 5′-GATCCCCAAGGTTGACATCTGGTCCCTGTTCAAGAGACAGGGACCAGATGTCAACCTTTTTTTA-3′; Pak1 number 3, 5′-GATCCCCCATCAAATATCACTAAGTCTTTTCAAGAGAAAGACTTAGTGATATTTGATGTTTTTA-3′; Pak2 number 1, 5′-GATCCCCAGAAGGAACTGATCATTAATTCAAGAGATTAATGATCAGTTCCTTCTTTTTTA-3′; Pak2 number 4, 5′-GATCCCCAGTTCTACGACTCCAACACTTCAAGAGAGTGTTGGAGTCGTAGAACTTTTTTA-3′.
Cells were transfected and selected as described above. Similar results were obtained with both sequences for Pak1 and Pak2, and the results shown in Fig. 3 were obtained with Pak1 number 1 and Pak2 number 1.
Preparation of adhesion substrates and microspheres.
Production of Ncad-Fc and preparation and use of adhesion substrates and microspheres were performed as previously described (10). Briefly, culture plates (Nunc) were first coated with protein A and then with Ncad-Fc. As a control, plates were coated with poly-l-lysine (PLL; molecular weight [MW], 70,000 to 150,000; Sigma). After coating with ligands, plates were washed with phosphate-buffered saline (PBS), blocked with 1.5% bovine serum albumin (BSA) in PBS for 2 h, and used immediately. To preserve cell surface cadherins, C2C12 cells were treated under trypsin-free conditions with cell-dissociation buffer (Invitrogen) for 5 to 10 min to obtain a single-cell suspension. Cells were then plated at 20 to 30% confluence for 2 h. To assess the effects of p38 inhibition on Ncad-initiated Pak1/2 phosphorylation in this assay, C2C12 cells were treated with 5 μM SB203580 for 1 h prior to dissociating cells and plating onto Ncad substrate in medium also containing 5 μM SB203580. Protein A-coated latex microspheres (5.5 μm; Bangs Laboratories) were further coated with Ncad-Fc as described previously (10) and added to the cell culture medium of adherent NIH 3T3 cells that had been electroporated 36 h earlier with expression vectors encoding GFP, Pak2-GFP (58), or Pak1-DsRed with an Amaxa Nucleofector II. The cells were fixed and imaged 1 h after addition of the microspheres.
Western blot analysis.
Western blot analyses were performed as described previously (54). For Western blot analyses of cells cultured on Ncad-coated plates, protein A and Ncad-Fc were removed by addition of magnetic goat anti-mouse IgG beads (Pierce) to the lysates, incubation for 1 h with rotation, and magnetic removal of the beads before sample buffer was added (10). The antibodies used were as follows: anti-Cdo (Zymed); anti-p38α, anti-glutathione-S-transferase, anti-Flag, anti-TNNT1, and antipancadherin (Sigma-Aldrich); anti-pp38α/β, anti-ERK2, anti-pERK, anti-Pak1, anti-Pak2, anti-Pak3, anti-phospho-Pak1(S144)/Pak2(S141), anti-phospho-Pak1(S199/S204)/Pak2(S192/S197), anti-Akt, anti-phospho-Akt(S473), anti-S6 kinase, anti-phospho-S6 ribosomal protein(S235/236), and anti-caspase 3 (Cell Signaling Technology); anti-JLP (Abcam); antimyogenin, anti-β-tubulin (Santa Cruz); anti-glyceraldehyde-3-phosphate dehydrogenase (anti-Gapdh; Ambion); anti-MHC (MF-20) and anti-eMHC (Developmental Studies Hybridoma Bank). Anti-FoxO1, anti-FoxO3a, anti-phospho-FoxO3a(S253), and anti-phospho-FoxO3a(S318/321) were from the Cell Signaling Technology Forkhead Signaling Antibody sampler kit. Although the phospho-FoxO3a antibodies are listed under that name, the manufacturer's information indicates that they cross-react with the analogous phospho-FoxO1 proteins, and we describe them as such in Results.
Immunoprecipitations (IPs) were performed as described previously (14). Briefly, cells were lysed in extraction buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 1.5 mM MgCl2, 10 mM NaF, 2 mM dithiothreitol [DTT], 1 mM Na3VO4, and 0.5% Triton X-100 supplemented with one tablet/40 ml of Complete protease inhibitor cocktail [Roche]). Whole-cell extract from each sample was immunoprecipitated with antibodies against Pak1 or Pak2 overnight at 4°C. Immunocomplexes were washed three times with, and suspended in, extraction buffer, and samples were analyzed by Western blotting.
Pak IP-kinase assay.
Total protein lysate from C2C12 cells cultured in either GM or DM for 3 days was extracted in TNE buffer (10 mM Tris [pH 7.4], 200 mM NaCl, 1 mM EDTA, 0.1% Triton X-100) supplemented with protease inhibitory cocktail (Sigma-Aldrich). Two hundred fifty to 500 μg total protein lysate was incubated on a rotator overnight at 4°C with 10 μl of Pak1 or Pak2 antibody (Cell Signaling). The protein-antibody complexes were incubated on a rotator at 4°C with 30 μl protein A/G beads (Pierce). The beads were washed 5 times with cold PBS. Washed beads were incubated at 30°C for 30 min with 500 ng myelin basic protein and 2.5 μCi [γ-32P]ATP in 30 μl kinase buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 10 mM MgCl2, 0.5 mM DTT); 10 μl 6× sample loading buffer was added to the kinase assay mixture, and the sample was boiled for 10 min. Forty microliters of the sample was centrifuged, electrophoresed, and transferred to nitrocellulose membrane. The membrane was exposed to X-ray film at −80°C. The rest of 10 μl of the sample was centrifuged and electrophoresed, and the gel was stained with Coomassie blue for 30 min. The gel was destained for 3 h with destaining solution (5% methanol, 10% acetic acid).
Mice.
All animal procedures were conducted in accordance with institutional guidelines for the care and use of laboratory animals as approved by the Institutional Animal Care and Use Committee (IACUC) of the Icahn School of Medicine at Mount Sinai. Pak1−/− and Pak2f/f mice were derived and maintained as described previously (30, 59). Pak2f/f mice were crossed to FVB Cg-Myod1tm2.1(icre)Glh/J animals (43) obtained from the Jackson Laboratory to produce Pak2f/f; MyoDiCre/+ conditional knockout (cKO) mice. Pak1−/−; Pak2cKO double knockout (dKO) animals were generated by crossing Pak2f/f; MyoDiCre/+ and Pak1−/− mice. Because Pak1 and MyodiCre are both located on chromosome 7, Pak1+/−; Pak2f/f; Myod1iCre/+ and Pak2f/f mice were crossed and offspring screened for those that carried a recombinant chromosome 7 carrying both Pak1− and Myod1iCre alleles. These mice were in turn interbred, ultimately to generate dKO animals. Control mice were of a Pak1+/−; Pak2f/f genotype, and all mice were maintained on a mixed C57BL/6/FVB background.
Preparation of tissue extracts for Western blotting.
Quadriceps and brain samples were snap-frozen in liquid nitrogen and stored at −70°C prior to processing. For processing, frozen tissue was finely chopped in 100 μl ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer (Tris lysis buffer [50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1% Triton X-100], SDS, deoxycholate, 0.5 M EDTA, protease and phosphotase inhibitor cocktail). Another 100 μl of RIPA lysis buffer was added, and tissues were ground using a loose pestle on ice until able to freely pass through a 200-μl pipette. RIPA lysis buffer was then added to a total volume of 400 μl, and extracts were placed on a rotator at 4°C for 30 min. Samples were then microcentrifuged for 30 min at 4°C, after which the supernatant was transferred to a fresh tube and diluted 1:2 in RIPA lysis buffer. Protein extracts were stored at −70°C until used for analysis.
Histology and microscopy.
Isolated tibialis anterior (TA) muscles were mounted in 10% Tragacanth (Sigma-Aldrich) and flash-frozen in 2-methylbutane (Fisher Scientific) chilled in liquid nitrogen. Cryosectioning was performed with a Leica CM3050 S at 10-μm thickness. For H&E staining, tissue was fixed in 4% paraformaldehyde on ice for 10 min and rinsed briefly with water five times. Sections were submerged in Harris modified hematoxylin (Fisher) for 1 min, rinsed five times in water, and then flushed with water for 5 min. Following this, they were quickly rinsed in acid wash solution (70% ethanol–1% hydrochloric acid in water) three times and again rinsed five times in water. Eosin Y stain (Ricca) was applied for 1 min, and sections were rinsed in water five times. Tissue was then dehydrated in increasing concentrations of ethanol, i.e., 50%, 70%, 95% for 1 min each, 100% for 3 min, and 100% for 5 min, and then cleared in xylene (Fisher Scientific) for 3 min. Sections were mounted in Permount (Fisher Scientific). Images for myofiber analysis were captured using a Nikon 26 Eclipse TS100 microscope, coupled with a ProgRes CF cool camera (Jenoptik, Germany), and ProgRes Mac CapturePro software (Jenoptik, Germany). Images of whole TA muscle were taken using a Nikon SMZ 1500 Stereomicroscope, with a ProgRes C3 camera (Jenoptik, Germany) and ProgRes Mac CapturePro software (Jenoptik, Germany). Cross-sectional areas of TA muscles and individual myofibers were measured using ImageJ software.
Immunofluorescence and microscopy.
Primary myoblast cultures were washed with PBS and fixed with 4% paraformaldehyde for 10 min on ice. They were then washed with PBS and permeabilized with 0.1% Triton X-100 in PBS for 5 min on ice. The preparations were blocked with 5% fetal bovine serum (Biowest) in PBS for 1 h at room temperature and then incubated with MF20 supernatant at 4°C overnight. Primary antibody was revealed with Alexa Fluor 568-conjugated anti-mouse secondary antibody (1:1,000; Invitrogen) incubated for 1 h at room temperature. Nuclei were counterstained with DAPI (Molecular Probes). Fluorescent images were acquired with a Nikon 26 Eclipse TS100 Microscope with a Plan Fluor ELWD 20×/0.45 differential interference contrast (DIC) L lens, using ProgRes Mac CapturePro software (Jenoptik, Germany) coupled with a camera ProgRes CF cool (Jenoptik, Germany). Images were exported to ImageJ, and if necessary, brightness and contrast were adjusted for the entire image, and similarly in all related panels. Channels were also merged using ImageJ software.
Dissected diaphragm muscles from E18.5-P0 mice were fixed in 1% paraformaldehyde in PBS for 90 min at room temperature. Muscles were washed three times for 15 min each in PBS and were permeabilized in blocking solution containing 3% bovine serum albumin and 0.5% Triton X-100 in PBS for 1 h. Axons and nerve terminals were labeled with rabbit polyclonal antibodies against Neurofilament-L (1:2,000; Synaptic Systems) and Synapsin-1/2 (1:2,000; Synaptic Systems) in blocking solution overnight at 4°C. After four 1 h washes in PBT (PBS containing 0.5% Triton X-100), muscles were incubated at 4°C overnight with Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:2,000; Life Technologies) and Alexa Fluor 594-conjugated α-bungarotoxin (1:1,000; Life Technologies) in PBT. After four 1-h washes in PBT and one 15-min wash in PBS, diaphragm muscles were mounted in Vectashield (Vector Laboratories). Confocal images of the left hemidiaphragm were captured on a Zeiss 700 confocal laser scanning microscope, where detector gain was set as constant between control and dKO preparations. Confocal image stacks were compiled into a reconstructed image of the left hemidiaphragm muscle, and the synapses were quantified using ImageJ software. AchR cluster size and density were quantified using Volocity 3D imaging software (PerkinElmer) as described previously (60).
Statistical analysis.
The unpaired Student's t test was used to determine statistical significance and was conducted using GraphPad Prism 5.0 software.
ACKNOWLEDGMENTS
We thank Marysia Rieder for technical assistance, Rolf Jakobi for providing the Pak2-GFP expression vector, and Neeta Bala for critical reading of the manuscript.
This work was supported by grants from the NIH to R.S.K. (R01AR46207), J.C. (R01CA148805; R01CA142928), and S.J.B (R37NS36193; R01NS075124).
REFERENCES
- 1.Tapscott SJ. 2005. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- 2.Bentzinger CF, Wang YX, Rudnicki MA. 2012. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 4(2):a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bismuth K, Relaix F. 2010. Genetic regulation of skeletal muscle development. Exp Cell Res 316:3081–3086. doi: 10.1016/j.yexcr.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Lluis F, Perdiguero E, Nebreda AR, Munoz-Canoves P. 2006. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol 16:36–44. doi: 10.1016/j.tcb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Cuenda A, Cohen P. 1999. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem 274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, Karin M, Wang JY, Puri PL. 2000. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol 20:3951–3964. doi: 10.1128/MCB.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zetser A, Gredinger E, Bengal E. 1999. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem 274:5193–5200. [DOI] [PubMed] [Google Scholar]
- 8.Perdiguero E, Ruiz-Bonilla V, Gresh L, Hui L, Ballestar E, Sousa-Victor P, Baeza-Raja B, Jardi M, Bosch-Comas A, Esteller M, Caelles C, Serrano AL, Wagner EF, Munoz-Canoves P. 2007. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J 26:1245–1256. doi: 10.1038/sj.emboj.7601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krauss RS. 2010. Regulation of promyogenic signal transduction by cell-cell contact and adhesion. Exp Cell Res 316:3042–3049. doi: 10.1016/j.yexcr.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu M, Krauss RS. 2010. N-cadherin ligation, but not Sonic hedgehog binding, initiates Cdo-dependent p38alpha/beta MAPK signaling in skeletal myoblasts. Proc Natl Acad Sci U S A 107:4212–4217. doi: 10.1073/pnas.0908883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole F, Zhang W, Geyra A, Kang JS, Krauss RS. 2004. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev Cell 7:843–854. doi: 10.1016/j.devcel.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Takaesu G, Kang JS, Bae GU, Yi MJ, Lee CM, Reddy EP, Krauss RS. 2006. Activation of p38alpha/beta MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J Cell Biol 175:383–388. doi: 10.1083/jcb.200608031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffe AB, Hall A. 2005. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 14.Kang JS, Bae GU, Yi MJ, Yang YJ, Oh JE, Takaesu G, Zhou YT, Low BC, Krauss RS. 2008. A Cdo-Bnip-2-Cdc42 signaling pathway regulates p38alpha/beta MAPK activity and myogenic differentiation. J Cell Biol 182:497–507. doi: 10.1083/jcb.200801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vega FM, Ridley AJ. 2007. SnapShot: Rho family GTPases. Cell 129:1430. [DOI] [PubMed] [Google Scholar]
- 16.Vasyutina E, Martarelli B, Brakebusch C, Wende H, Birchmeier C. 2009. The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc Natl Acad Sci U S A 106:8935–8940. doi: 10.1073/pnas.0902501106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arias-Romero LE, Chernoff J. 2008. A tale of two Paks. Biol Cell 100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 18.Zhao ZS, Manser E. 2005. PAK and other Rho-associated kinases–effectors with surprisingly diverse mechanisms of regulation. Biochem J 386:201–214. doi: 10.1042/BJ20041638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radu M, Semenova G, Kosoff R, Chernoff J. 2014. PAK signalling during the development and progression of cancer. Nat Rev Cancer 14:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen RE, Thorner J. 2007. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1773:1311–1340. doi: 10.1016/j.bbamcr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan PM, Lim L, Manser E. 2008. PAK is regulated by PI3K, PIX, CDC42, and PP2Calpha and mediates focal adhesion turnover in the hyperosmotic stress-induced p38 pathway. J Biol Chem 283:24949–24961. doi: 10.1074/jbc.M801728200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mainiero F, Soriani A, Strippoli R, Jacobelli J, Gismondi A, Piccoli M, Frati L, Santoni A. 2000. RAC1/P38 MAPK signaling pathway controls beta1 integrin-induced interleukin-8 production in human natural killer cells. Immunity 12:7–16. doi: 10.1016/S1074-7613(00)80154-5. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau S, Dolado I, Beardmore V, Shpiro N, Marquez R, Nebreda AR, Arthur JS, Case LM, Tessier-Lavigne M, Gaestel M, Cuenda A, Cohen P. 2006. CXCL12 and C5a trigger cell migration via a PAK1/2-p38alpha MAPK-MAPKAP-K2-HSP27 pathway. Cell Signal 18:1897–1905. doi: 10.1016/j.cellsig.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Wang JR, Wang CJ, Xu CY, Wu XK, Hong D, Shi W, Gong Y, Chen HX, Long F, Wu XM. 2016. Signaling cascades governing Cdc42-mediated chondrogenic differentiation and mensenchymal condensation. Genetics 202:1055–1069. doi: 10.1534/genetics.115.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan R, Jin P, Luo F, Zhang G, Anderson N, Chen EH. 2012. Group I PAKs function downstream of Rac to promote podosome invasion during myoblast fusion in vivo. J Cell Biol 199:169–185. doi: 10.1083/jcb.201204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teo M, Manser E, Lim L. 1995. Identification and molecular cloning of a p21cdc42/rac1-activated serine/threonine kinase that is rapidly activated by thrombin in platelets. J Biol Chem 270:26690–26697. doi: 10.1074/jbc.270.44.26690. [DOI] [PubMed] [Google Scholar]
- 27.Kelly ML, Chernoff J. 2012. Mouse models of PAK function. Cell Logist 2:84–88. doi: 10.4161/cl.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, Mei L. 2002. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron 35:489–505. doi: 10.1016/S0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- 29.Thomas JL, Moncollin V, Ravel-Chapuis A, Valente C, Corda D, Mejat A, Schaeffer L. 2015. PAK1 and CtBP1 regulate the coupling of neuronal activity to muscle chromatin and gene expression. Mol Cell Biol 35:4110–4120. doi: 10.1128/MCB.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radu M, Lyle K, Hoeflich KP, Villamar-Cruz O, Koeppen H, Chernoff J. 2015. p21-Activated kinase 2 regulates endothelial development and function through the Bmk1/Erk5 pathway. Mol Cell Biol 35:3990–4005. doi: 10.1128/MCB.00630-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong C, Tan L, Lim L, Manser E. 2001. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem 276:17347–17353. [DOI] [PubMed] [Google Scholar]
- 32.Gatti A, Huang Z, Tuazon PT, Traugh JA. 1999. Multisite autophosphorylation of p21-activated protein kinase gamma-PAK as a function of activation. J Biol Chem 274:8022–8028. doi: 10.1074/jbc.274.12.8022. [DOI] [PubMed] [Google Scholar]
- 33.Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L. 1997. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol 17:1129–1143. doi: 10.1128/MCB.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sells MA, Pfaff A, Chernoff J. 2000. Temporal and spatial distribution of activated Pak1 in fibroblasts. J Cell Biol 151:1449–1458. doi: 10.1083/jcb.151.7.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tintignac LA, Brenner HR, Ruegg MA. 2015. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev 95:809–852. doi: 10.1152/physrev.00033.2014. [DOI] [PubMed] [Google Scholar]
- 36.Washabaugh CH, Ontell MP, Shand SH, Bradbury N, Kant JA, Ontell M. 2007. Neuronal control of myogenic regulatory factor accumulation in fetal muscle. Dev Dyn 236:732–745. doi: 10.1002/dvdy.21078. [DOI] [PubMed] [Google Scholar]
- 37.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 38.Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. 2004. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24:9295–9304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. 2004. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 40.Fujio Y, Guo K, Mano T, Mitsuuchi Y, Testa JR, Walsh K. 1999. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol Cell Biol 19:5073–5082. doi: 10.1128/MCB.19.7.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dominov JA, Houlihan-Kawamoto CA, Swap CJ, Miller JB. 2001. Pro- and anti-apoptotic members of the Bcl-2 family in skeletal muscle: a distinct role for Bcl-2 in later stages of myogenesis. Dev Dyn 220:18–26. doi:. [DOI] [PubMed] [Google Scholar]
- 42.Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. 2002. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A 99:11025–11030. doi: 10.1073/pnas.162172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanisicak O, Mendez JJ, Yamamoto S, Yamamoto M, Goldhamer DJ. 2009. Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev Biol 332:131–141. doi: 10.1016/j.ydbio.2009.05.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyons GE, Ontell M, Cox R, Sassoon D, Buckingham M. 1990. The expression of myosin genes in developing skeletal muscle in the mouse embryo. J Cell Biol 111:1465–1476. doi: 10.1083/jcb.111.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiaffino S, Rossi AC, Smerdu V, Leinwand LA, Reggiani C. 2015. Developmental myosins: expression patterns and functional significance. Skelet Muscle 5:22. doi: 10.1186/s13395-015-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood SJ, Slater CR. 2001. Safety factor at the neuromuscular junction. Prog Neurobiol 64:393–429. doi: 10.1016/S0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- 47.Friese MB, Blagden CS, Burden SJ. 2007. Synaptic differentiation is defective in mice lacking acetylcholine receptor beta-subunit tyrosine phosphorylation. Development 134:4167–4176. doi: 10.1242/dev.010702. [DOI] [PubMed] [Google Scholar]
- 48.Rennefahrt UE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, Knapp S, Turk BE, Peterson JR. 2007. Specificity profiling of Pak kinases allows identification of novel phosphorylation sites. J Biol Chem 282:15667–15678. doi: 10.1074/jbc.M700253200. [DOI] [PubMed] [Google Scholar]
- 49.Beeser A, Jaffer ZM, Hofmann C, Chernoff J. 2005. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem 280:36609–36615. doi: 10.1074/jbc.M502306200. [DOI] [PubMed] [Google Scholar]
- 50.Ha BH, Morse EM, Turk BE, Boggon TJ. 2015. Signaling, regulation, and specificity of the type II p21-activated kinases. J Biol Chem 290:12975–12983. doi: 10.1074/jbc.R115.650416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baskaran Y, Ng YW, Selamat W, Ling FT, Manser E. 2012. Group I and II mammalian PAKs have different modes of activation by Cdc42. EMBO Rep 13:653–659. doi: 10.1038/embor.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gruenbaum-Cohen Y, Harel I, Umansky KB, Tzahor E, Snapper SB, Shilo BZ, Schejter ED. 2012. The actin regulator N-WASp is required for muscle-cell fusion in mice. Proc Natl Acad Sci U S A 109:11211–11216. doi: 10.1073/pnas.1116065109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. 2010. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest 120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang JS, Mulieri PJ, Miller C, Sassoon DA, Krauss RS. 1998. CDO, a robo-related cell surface protein that mediates myogenic differentiation. J Cell Biol 143:403–413. doi: 10.1083/jcb.143.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang JS, Yi MJ, Zhang W, Feinleib JL, Cole F, Krauss RS. 2004. Netrins and neogenin promote myotube formation. J Cell Biol 167:493–504. doi: 10.1083/jcb.200405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rando TA, Blau HM. 1994. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. 2006. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell 10:657–665. doi: 10.1016/j.devcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Jakobi R, Moertl E, Koeppel MA. 2001. p21-activated protein kinase gamma-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J Biol Chem 276:16624–16634. doi: 10.1074/jbc.M007753200. [DOI] [PubMed] [Google Scholar]
- 59.Allen JD, Jaffer ZM, Park SJ, Burgin S, Hofmann C, Sells MA, Chen S, Derr-Yellin E, Michels EG, McDaniel A, Bessler WK, Ingram DA, Atkinson SJ, Travers JB, Chernoff J, Clapp DW. 2009. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood 113:2695–2705. doi: 10.1182/blood-2008-06-160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaworski A, Burden SJ. 2006. Neuromuscular synapse formation in mice lacking motor neuron- and skeletal muscle-derived Neuregulin-1. J Neurosci 26:655–661. doi: 10.1523/JNEUROSCI.4506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tran P, Ho SM, Kim BG, Vuong TA, Leem YE, Bae GU, Kang JS. 2012. TGF-beta-activated kinase 1 (TAK1) and apoptosis signal-regulating kinase 1 (ASK1) interact with the promyogenic receptor Cdo to promote myogenic differentiation via activation of p38MAPK pathway. J Biol Chem 287:11602–11615. doi: 10.1074/jbc.M112.351601. [DOI] [PMC free article] [PubMed] [Google Scholar]