Abstract
Objectives:
The ability to differentiate histological characteristics between serrated polyps (SPs) and make a pathological diagnosis of a sessile serrated polyp (SSP) is highly variable. Recent studies have shown that immunohistochemical (IHC) expression of Annexin A10 (ANXA10) is a marker of a SSP. However, the clinical utility of ANXA10 expression in patients with SPs is unknown. The objective of this study was to evaluate the utility of ANXA10 expression in SPs in predicting the development of subsequent polyps at follow-up colonoscopy.
Methods:
Specimens from patients with SPs assessed in the Department of Pathology between 2006 and 2010 were identified. Patients whose colon harbored only SPs including either an SSP and/or hyperplastic polyp (HP) and who had complete polyp resection, no remaining polyps, and a follow-up colonoscopy were analyzed. ANXA10 IHC expression was performed in all baseline SPs. The rate of metachronous polyps on follow-up colonoscopy based on baseline maximal ANXA10 expression (low vs. high) was determined.
Results:
One hundred and seventy-nine patients were included. Sixty-seven patients had SPs with low ANXA10 expression (30 SSP and 37 HP) and 112 had polyps with high ANXA10 expression (105 SSP and 7 with HP). Individuals with SPs with high ANXA10 expression had a threefold higher risk of SSP on follow-up colonoscopy (hazard ratio (HR)=2.7; P=0.048) particularly, in the proximal colon (HR=4.0; P=0.02). ANXA10 expression did not predict patients at an increased risk of subsequent adenomas (18.8% vs. 19.4%, P=0.52).
Conclusions:
Individuals who harbor SPs with high ANXA10 expression are at an increased risk of metachronous serrated neoplasms. ANXA10 may be a reproducible tool to stratify patients with SPs into higher- and lower-risk groups of metachronous serrated neoplasia, allowing a more aggressive colonoscopic surveillance in patients at high risk.
Introduction
Hyperplastic polyps (HPs), which are non-neoplastic, compose the greatest proportion of lesions within the family of serrated polyps (SPs). The most recent World Health Organization classification of serrated colorectal lesions includes HP, sessile serrated polyp (SSP) with and without cytological dysplasia, and traditional serrated adenoma (TSA).1 A normal continuous and symmetric proliferation of the epithelium at the base of the crypts defines an HP. SSPs demonstrate abnormal cellular proliferation characterized by a proximally displaced proliferative zone, epithelial serrations, and characteristic distorted basilar crypt architecture with crypt dilation, branching, and lateral growth along the muscularis mucosa, which is the hallmark of SSP. Nuclear atypia, cytoplasmic eosinophilia, ectopic crypt foci, and serrated crypts are the reported characteristics of the rare TSA.2
Prior to the recognition that SSPs are the precursor of a substantial percentage of colorectal cancers (CRCs), most SSPs were diagnosed as HPs. However, molecular and genetic differences exist between these two types of polyps.2 SSPs frequently have a BRAF mutation, CpG island methylation, and occasional loss of MLH1 due to MLH1 promoter methylation, hallmarks of microsatellite unstable CRC2 and supporting the clinical observation linking SSPs to CRC.2, 3, 4
Recognition of the pathological characteristics differentiating SPs with premalignant potential from those without is essential in assessing CRC risk and guiding postpolypectomy surveillance recommendations.5, 6 However, the distinction between SPs can be challenging, even to the experienced gastrointestinal pathologist. As a major criterion for the diagnosis of SSP and distinction from HP is the basilar crypt morphology, complete polyp resection and well-oriented biopsies are needed but often not achieved.7, 8 Furthermore, concordance in the diagnosis of optimally oriented sections of SPs (HP, SSP, TSA) by pathologists with a special interest in gastrointestinal pathology is only moderate (k=0.55).7 Given these challenges, identifying molecular markers that more accurately predict the biological behavior of SPs and individuals at greatest risk of recurrent neoplasms would be of great clinical importance.
RNA-sequencing studies identified many highly and differentially expressed genes in SSPs compared with adenomas and HPs. Annexin A10 (ANXA10) was among one of the highly expressed genes.9
ANXA10, member of the Annexin family, is a calcium-and-phospholipid-binding protein. It is implicated in multiple physiological processes, including growth regulation, cell division, apoptosis, and differentiation.10 Increased expression was reported in Barrett's esophagus, oral cancers, and pancreatic cancer.11 Its role in CRC remains to be determined. A recent study12 demonstrated that ANXA10 gene and protein expression by immunohistochemistry (IHC) differentiated SSPs from HPs. High ANXA10 IHC expression had a sensitivity of 73% and a specificity of 95% in the diagnosis of an SSP. Additionally, ANXA10 showed to be highly expressed in serrated colon carcinoma13 compared with conventional colon cancer, perhaps proving valuable in the progression from SSP to colon cancer.
Therefore, we conducted a study aiming at determining the utility of ANXA10 expression in baseline SPs in predicting patients at increased risk of metachronous SPs and/or adenomas.
Methods
The study was approved by the Cleveland Clinic Institutional Review Board. Patients with serrated colorectal lesions reviewed in the Department of Pathology between 2006 and 2010 were identified through a natural language search. The electronic records of these patients were reviewed. Only patients with an SSP with or without dysplasia or an HP who had a complete baseline colonoscopy, no remaining polyps, and a follow-up colonoscopy by 2014 were included and analyzed. A gastrointestinal pathologist (R.K.P.) re-reviewed the SPs at baseline for histological confirmation. The diagnosis of SSP was based on the consensus criteria provided by an expert panel.5 The presence of at least one unequivocal architecturally distorted, dilated, and/or horizontally branched crypt was sufficient for a diagnosis of SSP. Individuals with TSA or a combination of serrated and adenomatous polyps at baseline colonoscopy were not included in the study group. We also excluded individuals with a history of colectomy, personal history of CRC, hereditary CRC syndrome (familial adenomatous polyposis, hereditary nonpolyposis CRC, and serrated polyposis syndrome), incomplete colonoscopy, or poor/inadequate bowel preparation.
Demographic data collection
Demographic factors, including age, gender, height, weight, smoking status (never, former, current), and family history of CRC, were obtained from the electronic medical record. The number, size, and location of the polyps were obtained from the colonoscopic reports. The location of the polyps was described as either proximal (defined as from cecum inclusive of splenic flexure) or distal (from the rectum inclusive of the descending colon). An advanced adenoma was defined as an adenoma ≥10 mm, adenoma with villous component or high-grade dysplasia. An advanced SSP was defined as an SSP ≥10 mm or SSP with dysplasia.
The primary objective was to determine the rate of development of SPs and adenomas on follow-up colonoscopy in relationship to baseline ANXA10 IHC expression. The secondary end points were the rates of advanced serrated neoplasia (defined as an SSP ≥10 mm, SSP with cytological dysplasia, TSA, or adenocarcinoma) and advanced adenoma (presence of villous component, size ≥10 mm, presence of high-grade dysplasia) in patients with SPs with high and low ANXA10 expression.
IHC for Annexin A10
IHC of all HPs and SSPs was performed on 4-μm sections of formalin-fixed, paraffin-embedded tissues using a Discovery XT automated stainer (Ventana Medical Systems, Tucson, AZ). Rabbit polyclonal, affinity purified anti-ANXA10 (NBP1–90156, Novus, Littleton, CO; 1:250) was applied for 1 h at 37 °C. A secondary antibody (OmniMap anti-rabbit HRP; Ventana Medical Systems) was applied for 32 min at 37 °C. The chromogenic substrate (ChromoMapDAB; Ventana Medical Systems) was applied for 8 min at 37 °C. Slides were counterstained and visualized by a light microscope. The percentage of serrated crypts positively staining for ANXA10 were scored according to the following scoring system:
0—No crypts stained positive for ANXA10;
1—<5% of crypts stained positive for ANXA10;
2—5–25% of crypts stained positive for ANXA10;
3—26–50% of crypts stained positive for ANXA10;
4—51–75% of crypts stained positive for ANXA10; and
5—76–100% of crypts stained positive for ANXA10.
Follow-up colonoscopy
The electronic records of all included patients were reviewed to document completeness of exam, adequacy of bowel preparation and polyp resection and the number, size, location, and histology of all metachronous lesions.
Statistical analysis
Patients were divided into two cohorts based on maximal baseline SP ANXA10 expression: low (ANXA score 0–3, stain positive in ≤50% of serrated crypts) and high (ANXA score ≥4, stain positive in >50% of crypts). Data were presented as mean±s.d., median (25th, 75th percentiles) or N (%)). As the consensus criteria for the diagnosis of SSPs was not established during the baseline colonoscopic period, all baseline SPs were re-reviewed by R.K.P. and the revised pathological diagnosis was used in the statistical analysis. A univariable analysis was carried out to compare demographic characteristics and baseline colonoscopic findings between the two groups. Analysis of variance or non-parametric Kruskal–Wallis tests were used to assess differences in continuous variables and Pearson's chi-square tests or Fisher's Exact tests were used for categorical factors. In addition, a time-to-event analysis was performed to assess the effect of baseline ANXA10 expression on metachronous polyps. Patients were followed until the most advanced end point or the last colonoscopy occurred, whichever was earlier. Kaplan–Meier plots were constructed and log-rank tests were used to compare the groups. In addition, univariable and multivariable Cox regression analysis was also performed. ANXA10 hazard ratios (HRs) for SSP were estimated adjusting for one confounder at a time. A P<0.05 was considered statistically significant. SAS (version 9.4, The SAS Institute, Cary, NC) was used for all analyses.
Results
Patient demographics, baseline endoscopic findings, and ANXA10 expression
One hundred and eighty-seven patients with SPs detected and resected on a complete baseline colonoscopy, with a follow-up colonoscopy by 2014, were identified during the study period. Eight patients were excluded owing to lack of available polyp tissue to perform ANXA10 expression studies. One hundred and seventy-nine patients were included in the analysis. Sixty-seven patients had polyps with low ANXA10 expression and 112 patients had polyps with high ANXA10 expression. High ANXA10 expression was more common in SPs from females (66% vs. 52%, P=0.066) and in younger patients (55.6 vs. 58.6 years, P=0.027) (Table 1). Body mass index, smoking exposure, and family history of CRC was unrelated to ANXA10 expression.
Table 1. Demographic and clinical characteristics.
| Factor | Low ANXA10 (N=67) | High ANXA10 (N=112) | P value |
|---|---|---|---|
| Female | 35 (52.2) | 74 (66.1) | 0.066a |
| Age (years)b | 58.6±8.3 | 55.6±8.8 | 0.027c |
| BMI (kg/m2)b | 29.6±6.0 | 29.2±6.5 | 0.67c |
| Smokingb | |||
| Never | 30 (45.5) | 47 (43.1) | |
| Current | 9 (13.6) | 16 (14.7) | 0.95a |
| Ex-smoker | 27 (40.9) | 46 (42.2) | |
| Family history of CRCb | 13 (20.3) | 30 (27.5) | 0.29a |
| Indication for colonoscopyb | |||
| Screening | 43 (69.4) | 56 (51.9) | |
| Family history | 9 (14.5) | 24 (22.2) | 0.083a |
| Diagnostic | 10 (16.1) | 28 (25.9) |
ANXA10, Annexin 10; BMI, body mass index; CRC, colorectal cancer.
Values are presented as mean±s.d. or N (%).
Analysis of variance.
Data are not available for all subjects. Missing values: age=2, BMI=13, smoking=4, family history of CRC=6, indication for colonoscopy=9.
Pearson's chi-square test.
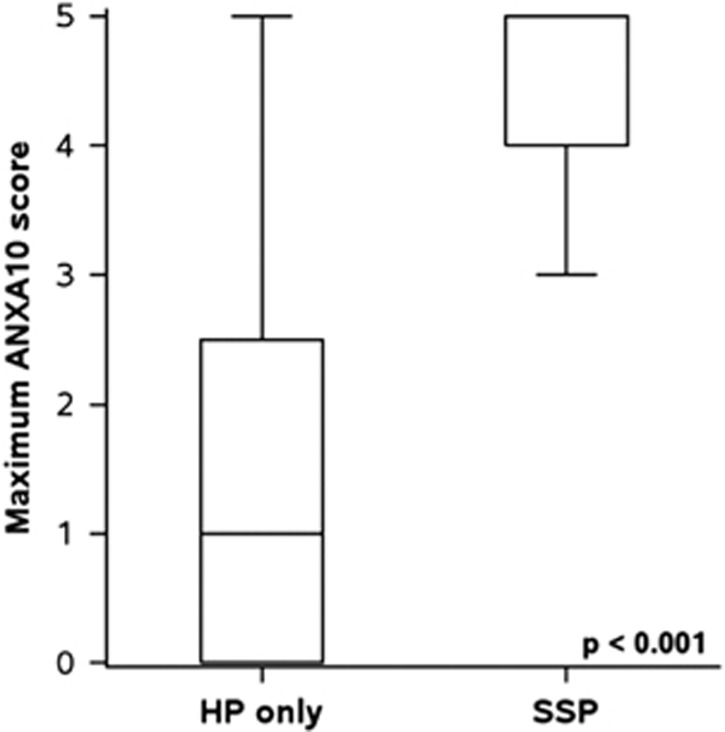
On baseline colonoscopy, 54% of patients had only 1 SP while 24% had ≥3 SPs (Table 2). High ANXA10 expression was related to the number of baseline SPs (P=0.032). SSPs comprised 43% of the SPs with low ANXA10 expression and 92% of SPs with high ANXA10 expression (<0.001). Patients with high ANXA10 expression near universally had only SSPs (94%, P<0.001; Figure 1) and more numerous SSPs (P<0.001) The SPs with high ANXA10 expression were most often proximal (84.7% vs. 48.5%, P<0.001) and larger in size (7 vs. 5 mm, P=0.009). Patients with low ANXA10 expression were more likely to have exclusively HPs and more numerous HPs (P=0.003).
Table 2. Baseline colonoscopic findings and relationship with ANXA10.
| Factor | Low ANXA10 (N=67) | High ANXA10 (N=112) | P value |
|---|---|---|---|
| Number of baseline HPs | |||
| 0 | 22 (32.8) | 68 (60.7) | |
| 1 | 30 (44.8) | 23 (20.5) | 0.003a |
| >1 | 15 (22.4) | 21 (18.7) | |
| Number of baseline SSP | |||
| 0 | 37 (55.2) | 7 (6.3) | |
| 1 | 25 (37.3) | 82 (73.2) | <0.001a |
| >1 | 5 (7.4) | 23 (20.5) | |
| Baseline polyp types | |||
| HP only | 37 (55.2) | 7 (6.3) | |
| SSP only | 22 (32.8) | 68 (60.7) | <0.001b |
| HP+SSP | 8 (11.9) | 37 (33.0) | |
| Size of index polypc,d | 5.0 (5.0,10.0) | 7.0 (5.0,10.0) | 0.009a |
| Pathology of index polyp c | |||
| HP | 38 (56.7) | 9 (8.0) | |
| SSP | 28 (41.8) | 101 (90.2) | <0.001e |
| SSP-LGD | 1 (1.5) | 1 (0.89) | |
| SSP-HGD | 0 (0.0) | 1 (0.89) | |
| Location of index polyp c | |||
| Proximal | 32 (48.5) | 94 (84.7) | <0.001b |
| Distal | 34 (51.5) | 17 (15.3) |
ANXA10, Annexin 10; HGD, high-grade dysplasia; HP, hyperplastic polyp; LGD, low-grade dysplasia; SSP, sessile serrated polyp.
Values are presented as median (P25, P75) or N (%).
Kruskal–Wallis test.
Pearson's chi-square test.
cIndex polyp: polyp with the highest ANXA10 expression.
dData are not available for all subjects. Missing values: size of index polyp ANXA=3, location of index polyp=2.
eFisher's Exact test.
Figure 1.
Distribution of Annexin A (ANXA) score according to baseline pathology. HP, hyperplastic polyp; SSP, sessile serrated polyp.
Finding on follow-up colonoscopy
The median follow-up times was 44 months (interquartile range (36.8–60) months). Patients with SPs with low ANXA10 expression had a longer follow-up time compared with patients with polyps with high ANXA10 expression (51 vs. 42 months, P=0.017).
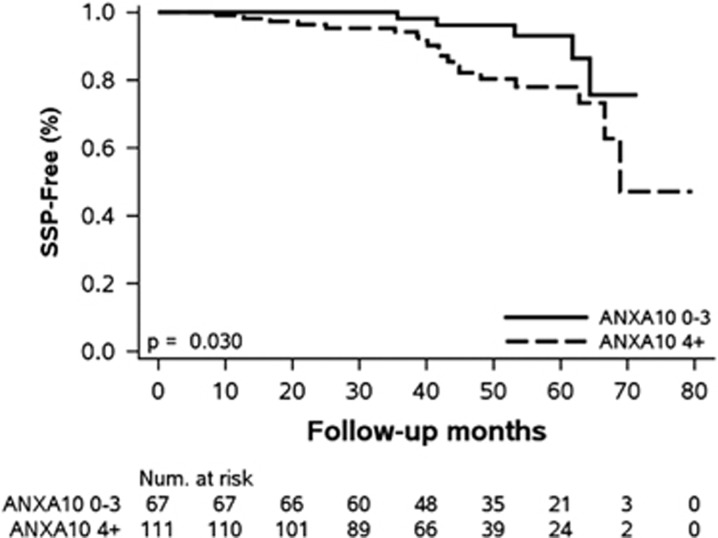
Ninety-eight patients (55%) had polyps detected on follow-up colonoscopy, including 32% with ≥2 polyps (Table 3). SSPs were identified in 17% of patients with baseline polyps with high ANXA10 expression and 7.5% of patients with polyps with low ANXA10 expression (Figure 2, P=0.03). Almost all (95%) of the metachronous SSPs in patients with polyps with high ANXA10 expression were proximal in location while only 60% of SSPs with low ANXA10 expression were noted above the descending colon. The third of recurrent SPs in both groups (8/24) were in the same segment of the colon than the baseline SP. Four patients with polyps with high ANXA10 expression developed an SSP ≥10 mm or an SSP with dysplasia compared with 1 patient with polyps with low ANXA10 expression. There was no difference in the detection rate of adenomas on follow-up colonoscopy in patients with polyps with high or low ANXA10 expression, 18.8% vs. 19.4%, respectively (P=0.52). Five patients with polyps with high ANXA10 expression and two patients with polyps with low ANXA10 expression had an advanced adenoma on follow-up. In univariate analysis, patients with polyps with high ANXA10 expression were three times at higher risk of SSP recurrence (HR 2.7; P=0.048). Particularly, the recurrence risk of proximal SSPs was four times higher in this group of patient (HR=4; P=0.02). High ANXA10 expression remained significantly associated with higher risk of SSP recurrence after adjusting for gender, age, smoking, or family history of CRC.
Table 3. Follow-up colonoscopic findings.
| Outcome | Low ANXA10 at baseline colonoscopy | High ANXA10 at baseline colonoscopy | HR (95% CI) | P value |
|---|---|---|---|---|
| (N=67) | (N=112) | |||
| HPs | 26 (38.8) | 37 (33.0) | 1.04 (0.62, 1.7) | 0.89 |
| Proximal HPs | 8 (11.9) | 7 (6.3) | 0.66 (0.24, 1.8) | 0.43 |
| HP above rectosigmoid | 9 (13.4) | 10 (8.9) | 0.83 (0.34, 2.0) | 0.68 |
| SSPs | 5 (7.5) | 19 (17.0) | 2.7 (1.01, 7.0) | 0.048 |
| Proximal SSPs | 3 (4.5) | 18 (16.1) | 4.0 (1.3, 13.0) | 0.02 |
| SSP with dysplasia or ≥10 mm | 1 (1.5) | 4 (3.6) | 2.5 (0.32, 18.6) | 0.38 |
| SSP or HP above rectosigmoid | 13 (19.4) | 27 (24.1) | 1.5 (0.79, 3.0) | 0.21 |
| Any adenomas | 13 (19.4) | 21 (18.8) | 1.2 (0.62, 2.5) | 0.55 |
| Any advanced adenomas | 2 (3.0) | 5 (4.5) | 1.8 (0.36, 9.0) | 0.47 |
ANXA10, Annexin 10; CI, confidence interval; HP, hyperplastic polyp; HR, hazard ratio; SSP, sessile serrated polyp.
Values are presented as N (%). HR and P values correspond to univariable Cox regression models for each outcome.
Figure 2.
Kaplan–Meier plot for metachronous sessile serrated polyps (SSPs) based on baseline Annexin A10 (ANXA10) staining.
In the regression analysis, the association between high ANXA10 expression and risk of recurrent SSPs weakened (P=0.098) (Table 4). When the analysis was repeated using the baseline polyp diagnosis from the original pathologist, the association of high ANXA10 and SSP recurrence was stronger (P=0.054).
Table 4. Adjusted Cox regression analysis: ANXA10 expression and the presence of SSP on follow-up.
| Adjusted fora |
High vs. low ANXA10 expression |
|
|---|---|---|
| HR (95% CI) | Pvaluea | |
| Female | 2.97 (1.10, 8.04) | 0.032 |
| Age | 3.51 (1.19, 10.37) | 0.023 |
| Smoking | 3.13 (1.16, 8.44) | 0.024 |
| Family history of cancer | 2.90 (1.08, 7.81) | 0.035 |
| Original SSP diagnosis on baseline scope | 2.7 (0.98, 7.3) | 0.054 |
| Revised SSP diagnosis on baseline scope | 2.45 (0.85, 7.10) | 0.098 |
ANXA10, Annexin 10; CI, confidence interval; HR, hazard ratio; SSP, sessile serrated polyp.
Six separate Cox regression models were built; occurrence of follow-up SSP was modeled as the outcome with high vs. low ANXA10 expression and each confounder (one at a time) as the independent variable.
Discussion
In the present study, we found that patients with baseline SPs with high ANXA10 expression had a higher risk of SSP but not adenomas on follow-up colonoscopy compared with patients with SP with low ANXA10 expression. This association remained valid after adjustment for age, gender, smoking, or family history of CRC. The present large study is the first to demonstrate an association between high ANXA10 expression and metachronous SSPs, including proximal SSPs. We also found a non-significant 2.5-fold increase in large SSPs in the high vs. low ANXA10 cohort.
Several recent studies14, 15, 16 indicate that ANXA10 is not only a marker of SSPs but more importantly a marker of the serrated pathway of CRC. Microsatellite unstable CRC expressing ANXA10 were significantly associated with CpG island methylator predictor status, MLH1 promoter gene methylation, and female predominance and were most often in the proximal colon, known features of the serrated carcinoma pathway. Our results indicate that ANXA10 expression in SPs is associated with a moderate risk of SSP recurrence in the proximal colon and a possible increased risk of metachronous advanced serrated lesions. These findings confirm the results of prior research and strongly suggest that ANXA10 expression might be maintained during the multistep transformation from SSP to carcinoma.
Multiple studies7, 17 have shown variable SSP diagnosis rates among pathologists, up to 13-fold in one study suggesting perhaps that the use of an adjunct tool such as IHC for ANXA10 may improve the ability of pathologists to make the diagnosis of SSPs. In this large study, we observed that high ANXA10 expression was only associated with the development of SSPs but not adenomas on follow-up colonoscopy. In the multivariate analysis, the relationship between high ANXA expression and metachronous SSPs weakened (P=0.098), suggesting that ANXA10 may not be a better predictor of SSPs on follow-up compared with baseline histology interpreted by an expert pathologist. However, when we adjusted for the diagnosis from the original pathologist, the utility of ANXA10 was more compelling (P=0.054). It is likely that the association of ANXA10 expression and recurrent SSPs would only be strengthened using non-expert pathologists where greater variability in diagnosis exists. Despite having a fairly large number of patients with SPs at baseline, the number of patients in each subgroup is small and does not allow a comparison between SP recurrence in patients with ANXA10 low SSPs with ANXA high SSPs. Nonetheless, an important question remains unanswered. If not better than histology, is IHC for ANXA10 an adjunct to histopathology for the risk stratification of patients with SPs? Additional studies to elucidate the role of ANXA10 stain in clinical practice would be valuable in guiding surveillance colonoscopy in patients with SPs.
ANXA10 was not associated with metachronous adenomas (P=0.52). Although this negative finding may be due to the small size of the cohort, previous research indicates that SPs and adenomatous polyps arise through different molecular and biological processes. Some authors suggested that SPs may flourish in a milieu where hypermethylation leads to increased serrated neoplasia.16 Therefore, based on observations acknowledging ANXA10 as a supportive marker of the serrated pathway, one might not anticipate to find an association with metachronous adenomas.
To the best of our knowledge, the present study is the first study to evaluate the utility of ANXA10 expression in predicting the development of subsequent polyps. In our study, all SPs at baseline were re-reviewed by a single gastrointestinal pathologist and the majority of the SPs (75%) included in the analysis were SSP and proximal in location (71%), which are considered as high-risk lesions. Nevertheless, our study has several limitations. First, the primary end point of the study, recurrence of SSPs, is only a surrogate marker of CRC development and its true significance is still to be determined. Second, it is well known that the awareness and detection of SPs has improved over time. If so, it is possible that some serrated lesions detected on follow-up were missed rather than newly developed, which may decrease the predictive value of ANXA10 for metachronous lesions. Third, it is possible that some of the follow-up polyps were incompletely resected at baseline. Fourth, the pathology of serrated lesions were not re-reviewed on follow-up. We believe that this is less of a concern as the consensus criteria for the diagnosis of SSPs was utilized at our institution since 2011. Finally, the small number of patients included in the study and the small number of metachronous polyps may have diminished the power of the study to show statistical significance of the results.
Albeit these limitations, our findings suggest that ANXA10 is a potential marker of “high-risk” patients with SSPs who are at considerable risk of recurrent SSPs, in particular proximal SSPs. Additional studies to confirm the role of ANXA10 expression in predicting metachronous neoplasia, particularly large and dysplastic serrated lesions, are needed. If additional studies confirm our findings, ANXA10 may be an adjunct molecular tool to stratify patients with SPs into high- and low-risk groups allowing personalized CRC surveillance. Given the minimal cost associated with performing an IHC stain, cost savings may occur if ANXA10 better stratifies patients and results in more efficient usage of colonoscopies.
Study Highlights
Footnotes
Guarantor of the manuscript: Carol A. Burke, MD.
Specific authors contribution: Carole Macaron: collecting and interpreting the data and drafting the manuscript. Rocio Lopez: statistical analysis and interpreting the data. Rish K. Pai: planning, interpreting the data, and reviewing the manuscript. Carol A. Burke: planning, interpreting the data, and drafting the manuscript. All authors have reviewed and approved the final draft submitted.
Financial support: This work was supported by American College of Gastroenterology Clinical Research Grant 2014.
Potential competing interests: None.
References
- Snover D, Ahnen DJ, Burt RW et alSerrated polyps of the colon and rectum and serrated (“hyperplastic”) polyposis. In: Bozman FT, CarneiroF, Hruban RH et al (eds). WHO Classification of Tumours Pathology and Genetics: Tumours of the Digestive System, 4th edn.Springer-Verlag: Berlin, Germany, 2010. [Google Scholar]
- Torlakovic E, Skovlund E, Snover DC et al. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27: 65–81. [DOI] [PubMed] [Google Scholar]
- O'Brien MJ, Shi Y, Mack E et al. Comparision of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30: 1491–1501. [DOI] [PubMed] [Google Scholar]
- Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst 2001; 93: 1307–1313. [DOI] [PubMed] [Google Scholar]
- Rex DK, Ahnen DJ, Baron JA et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107: 1315–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DA, Rex DK, Winawer SJ et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143: 844–857. [DOI] [PubMed] [Google Scholar]
- Farris AB, Misdraji J, Srivastava A et al. Sessile serrated adenoma: challenging discrimination from other serrated colonic polyps. Am J Surg Pathol 2008; 32: 30–35. [DOI] [PubMed] [Google Scholar]
- Morales SJ, Bodian CA, Kornacki S et al. A simple tissue-handling technique performed in the endoscopy suite improves histologic section quality and diagnostic accuracy for serrated polyps. Endoscopy 2013; 45: 897–905. [DOI] [PubMed] [Google Scholar]
- Delker DA, McGettigan BM, Kanth P et al. RNA sequencing of sessile serrated colon polyps identifies differentially expressed genes and immunohistochemical markers. PLoS One 2014; 9: e88367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussunoor S, Murray GI. The role of annexins in tumour development and progression. J Pathol 2008; 216: 131–140. [DOI] [PubMed] [Google Scholar]
- Lu SH, Yuan RH, Chen YL et al. Annexin A10 is an immunohistochemical marker for adenocarcinoma of the upper gastrointestinal tract and pancreatobiliary system. Histopathology 2013; 63: 640–648. [DOI] [PubMed] [Google Scholar]
- Gonzalo DH, Lai KK, Shadrach B et al. Gene expression profiling of serrated polyps identifies annexin A10 as a marker of a sessile serrated adenoma/polyp. J Pathol 2013; 230: 420–429. [DOI] [PubMed] [Google Scholar]
- Laiho P, Kokko A, Vanharanta S et al. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene 2007; 26: 312–320. [DOI] [PubMed] [Google Scholar]
- Sajanti SA, Vayrynen JP, Sirnio P et al. Annexin A10 is a marker for the serrated pathway of colorectal carcinoma. Virhows Arch 2015; 466: 5–12. [DOI] [PubMed] [Google Scholar]
- Kim JH, Rhee Y, Kim K et al. Annexin A10 expression correlates with serrated pathway features in colorectal carcinoma with microsatellite instability. APMIS 2014; 122: 1187–1195. [DOI] [PubMed] [Google Scholar]
- Pai RK, Shadrach BL, Carver P et al. Immunohistochemistry for Annexin A10 can distinguish sporadic from Lynch syndrome- associated microsatellite unstable colorectal carcinoma. Am J Surg Pathol 2014; 38: 518–525. [DOI] [PubMed] [Google Scholar]
- Hetzel JT, Huang CS, Coukos JA et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105: 2656–2664. [DOI] [PubMed] [Google Scholar]