Abstract
OBJECTIVES:
Therapeutic drug monitoring of infliximab improves treatment outcomes, but available assays to monitor infliximab lack speed to implement treatment algorithms immediately. Our aim is to validate a rapid, lateral flow-based assay (LFA) for quantitative determination of infliximab and to assess thresholds associated with mucosal healing in patients with ulcerative colitis.
METHODS:
Samples (n=190) from 29 anti-tumor necrosis factor naive patients with ulcerative colitis starting infliximab induction therapy between June 2010 and February 2012 were prospectively collected. All patients had a Mayo endoscopic sub-score ≥2 at baseline. Mucosal healing (MH), defined as a Mayo endoscopic sub-score ≤1, was evaluated at week 10–14. Infliximab trough concentrations (TC) were determined with a novel LFA, which was benchmarked with the RIDASCREEN infliximab Monitoring (ELISA).
RESULTS:
The LFA showed an excellent agreement with enzyme-linked immunosorbent assay (ELISA) for quantification of infliximab, as observed from Pearson and intraclass correlation coefficients of 0.95 and 0.95 during induction and 0.93 and 0.87 during maintenance therapy, respectively. In total, 45% of patients achieved MH. Using the LFA, week 14 TC ≥2.1 μg/ml (AUROC: 0.819, P=0.008) were associated with MH. After 2 years follow-up, 77% of patients with MH were still receiving infliximab therapy vs. 25% of patients without MH.
CONCLUSIONS:
We validated a LFA for quantification of infliximab and identified TC associated with MH. With a time-to-result of 20 min, individual sample analysis and user-friendliness, the LFA outplays ELISA as a rapid, accurate tool to monitor infliximab concentrations.
Introduction
Therapeutic drug monitoring of infliximab has become increasingly important in optimizing therapy of patients with inflammatory bowel diseases. The trough concentration (TC) of infliximab, which is the drug concentration in serum just before the next infusion, has been linked to biological, clinical, and endoscopic outcomes.1, 2, 3 Recently, several prospective trials have identified concentration-based dosing as a cost-effective alternative to clinically based dosing.4, 5 However, dosing could only be adapted at the next infusion of infliximab, typically 8 weeks later, because of the lack of a rapid infliximab assay.
The conventional assays used for therapeutic drug monitoring of infliximab are enzyme-linked immunosorbent assays (ELISA), radio-immunoassays, or homogeneous mobility shift assays.6, 7, 8, 9 Several comparison studies have been performed and although all assays were shown to correlate very well, small differences in measured infliximab concentration between assays were observed.10, 11, 12, 13, 14 However, these differences are supposed to have limited effect on clinical outcome, as demonstrated by Steenholdt et al.15 Infliximab assays are usually performed in a hospital laboratory, are time-consuming and require analysis of multiple samples at a time to reduce costs. Consequently, these assays do not allow immediate dose optimization and hamper the implementation of therapeutic drug monitoring in a hospital or infusion setting.
In this study, we present a novel lateral flow-based assay (LFA) for rapid infliximab quantification. The LFA was benchmarked to a reference infliximab ELISA using 190 serum samples, prospectively collected from a cohort of 29 patients with ulcerative colitis starting infliximab. In addition, we determined infliximab concentration thresholds associated with mucosal healing (MH) following induction treatment.
Methods
Study design, definitions, and patient population
In this prospective observational study, we included anti-tumor necrosis factor naive patients diagnosed with moderate–severe ulcerative colitis that started infliximab treatment.16 Only patients with active disease, as confirmed by an endoscopic evaluation at baseline, and with an endoscopic evaluation after scheduled infliximab induction therapy (w10–14) were included. Active disease was defined as a Mayo endoscopic sub-score of 2 or 3. MH was defined as a Mayo endoscopic sub-score of 0 or 1 after infliximab induction therapy. Serum samples were prospectively collected during infliximab induction (w0, 2, and 6) and maintenance therapy (w14 onward for up to 2 years), just before an infliximab infusion, centrifuged and stored at −20 °C. Long-term infliximab survival was defined as continuation of infliximab therapy for over 2 years. Written informed consent was provided by all patients in the framework of the Institutional Review Board-approved Flemish Inheritance study for Crohn's and colitis (VLECC; B322201213950/S53684).
Infliximab concentrations
Infliximab concentrations in serum samples (including but not limited to w0, w2, w6, w14, w30, and w54 samples) were determined using a novel LFA (R-Biopharm AG, Darmstadt, Germany) and the RIDASCREEN infliximab Monitoring (ELISA) (R-Biopharm AG)—both using the specific monoclonal anti-infliximab antibody mAb-IFX6B7.8
In the LFA, serum samples were measured according to the manufacturer's instructions, consisting of a 5 min pre-incubation of diluted sample with “Solution A” and “Solution B” and 15 min developing time on the lateral flow strip. Subsequently, the lateral flow strips were read using a portable LFA reader. The LFA revealed a dose-response curve in the 0.5–40 ng/ml range (r2=0.9995), which was fitted using four parameter logistic regression 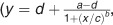 Supplementary Figure S1). The dose-response curve was validated in the 1–20 ng/ml range. For the determination of serum samples withdrawn during the induction phase of infliximab (w2 and 6), a 1:2000 dilution was applied. For anti-tumor necrosis factor naive samples and samples withdrawn during the maintenance phase (including but not limited to w0, w14, w30, and w54), a 1:500 dilution was applied.
Supplementary Figure S1). The dose-response curve was validated in the 1–20 ng/ml range. For the determination of serum samples withdrawn during the induction phase of infliximab (w2 and 6), a 1:2000 dilution was applied. For anti-tumor necrosis factor naive samples and samples withdrawn during the maintenance phase (including but not limited to w0, w14, w30, and w54), a 1:500 dilution was applied.
The RIDASCREEN infliximab Monitoring (ELISA) was carried out according to the manufacturer's instructions and has a lower limit of quantification of 0.5 μg/ml infliximab. For the determination of serum samples, a 1:400 dilution was applied. All other serum samples were determined as described before using an in-house developed and clinically validated infliximab ELISA with a lower limit of quantification of 0.5 μg/ml.8
Anti-infliximab antibodies
Anti-infliximab antibodies (ATI) were measured using a drug-sensitive and novel drug-tolerant ELISA as described in the Supplementary Data section.17
Statistical analysis
All statistics were carried out using the statistical programs Graphpad Prism 5.0 (Graphpad software, San Diego, CA, USA) and IBM SPSS Statistics 23 (IBM SPSS, Costa Mesa, CA, USA). Data are expressed as median and interquartile range for continuous variables and frequency and percentage for categorical variables unless stated otherwise. Normality was assessed using the D′Agostino–Pearson test. For the univariate analysis of unpaired continuous variables, either an unpaired t-test or independent 2-group Mann–Whitney U-test was used. Univariate analysis of discrete variables was performed using the Fisher's exact test. Correlations and agreements between LFA and ELISA were assessed using the Pearson r correlation coefficient and the intraclass correlation coefficient (ICC; two-way mixed, absolute agreement—single measures). The ICC allows to reveal systematic differences between two methods that would go unnoticed using a Pearson r. The proportion of positive and negative agreement between LFA and ELISA was determined by the average number of patient samples identified as positive and negative by either method, respectively, assuming none of the two methods is the golden standard. Infliximab concentrations exceeding the lower and upper assay quantification limits were not taken into account for statistical analysis. Receiver operating characteristic (ROC) analysis was performed to assess infliximab concentration thresholds associated with MH. Optimal thresholds were selected using the Youden's index to maximize the sum of specificity and sensitivity. A log-rank test was performed to assess differences in long-term infliximab survival.
Results
Study population
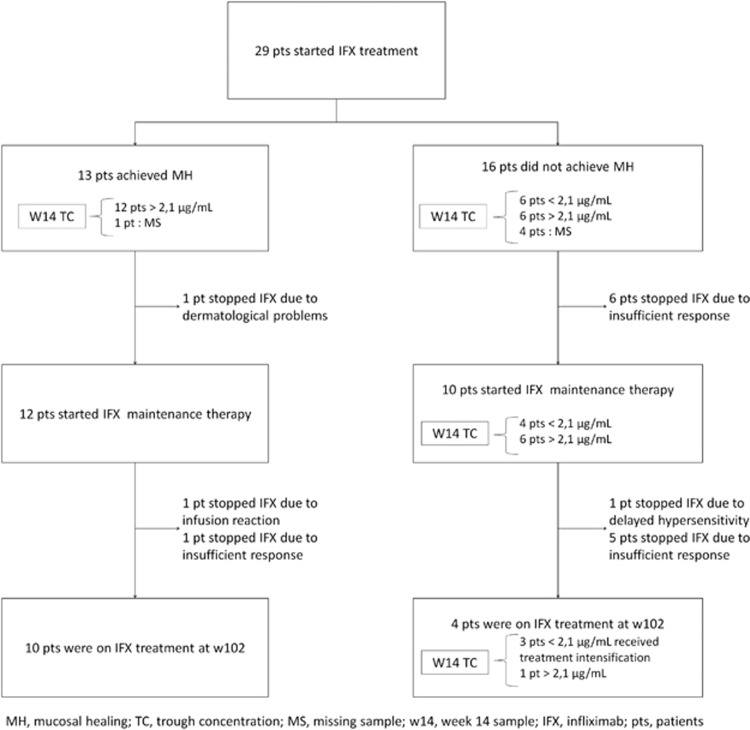
Twenty-nine patients met the study inclusion criteria and were included in this post hoc analysis. Demographic characteristics of these patients are provided in Table 1. All but two patients received the standard 5 mg/kg induction treatment on week 2, 6, and 14. The two other patients received an intensified dosing regimen. In total, 13/29 (45%) patients achieved MH. Seven out of 29 (24%) patients discontinued infliximab treatment after induction, of which six patients because of lack of response (no MH) and one because of dermatological side reactions (Figure 1). A significant difference in long-term infliximab survival was observed between patients with and without MH (P=0.004, log-rank test).
Table 1. Baseline characteristics of patients with ulcerative colitis.
| All patients | MH | No MH | P-value | |
|---|---|---|---|---|
| n (%) | 29 (100) | 13 (45) | 16 (55) | |
| Gender (male/female) | 19/10 | 7/6 | 12/4 | 0.3 |
| BMI, kg/m2, mean±s.d. | 25.6±4.1 | 26.4±4.6 | 25.0±3.6 | 0.5 |
| UC extension: E3 (pancolitis), n (%)a | 11 (44) | 4 (31) | 7 (44) | 0.4 |
| Mayo endoscopic sub-score before IFX, Mayo 3 (%) | 12 (41) | 4 (31) | 8 (50) | 0.5 |
| Acute severe colitis, n | 2 | 1 | 1 | 1.0 |
| Age at first IFX, y, median (IQR) | 43 (28–51) | 29 (24–53) | 45 (31–51) | 0.4 |
| Duration of disease at first IFX, y, median (IQR) | 3.0 (1.4–8.4) | 3.4 (1.9–8.0) | 2.3 (1.3–9.4) | 0.5 |
| CRP concentration, mg/l, median (IQR) | 3.7 (2.1–13.4) | 3.7 (1.5–14.2) | 3.5 (2.2–12.0) | 0.8 |
| Albumin, g/l, median (IQR) | 42.5 (39.7–45.4) | 42.1 (40.7–44.9) | 43.2 (38.4–45.9) | 0.9 |
| Current smokers, n | 4 | 3 | 1 | 0.3 |
| Immunomodulator use, n (%) | 12 (41) | 8 (62) | 4 (25) | 0.07 |
| Corticosteroid use, n (%) | 9 (31) | 3 (23) | 6 (38) | 0.5 |
BMI, body mass index; CRP, C-reactive protein; IFX, infliximab; IQR, interquartile range; MH, mucosal healing; UC, ulcerative colitis.
According to Montreal classification.
Figure 1.
Short- and long- term response of 29 patients with ulcerative colitis treated with infliximab.
Infliximab concentrations
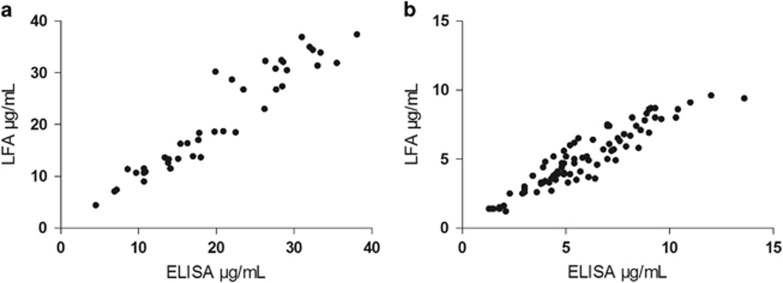
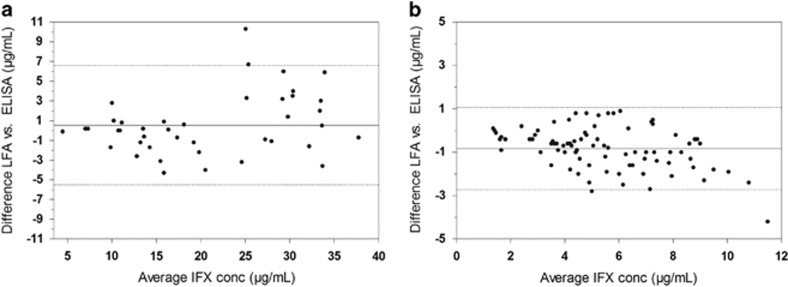
In total, 190 unique patient samples were analyzed in parallel by the LFA and ELISA (Table 2). A very good correlation and ICC was observed for samples withdrawn during induction (Figure 2a, Pearson r of 0.95 and ICC of 0.95, n=41) and maintenance of infliximab treatment (Figure 2b, Pearson r of 0.93 and ICC of 0.87, n=84). In addition, a good agreement was observed in the Bland–Altman plots, showing an average bias of 0.5 (95% confidence interval: −5.5 to 6.6) μg/ml infliximab for induction treatment samples (Figure 3a) and −0.8 (95% confidence interval: −2.7 to 1.1) μg/ml for maintenance treatment samples (Figure 3b) between the LFA and ELISA.
Table 2. Intention-to-diagnose analysis of patient samples.
| Sampling date | w0 | w2 | w6 | w14 | w30 | w54 | Other | Total |
|---|---|---|---|---|---|---|---|---|
| Intention-to-diagnose | 29 | 29 | 29 | 24 | 19 | 18 | 44 | 192 |
| Measured samples | 28 | 29 | 28 | 24 | 19 | 18 | 44 | 190 |
| Missing samples | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
Figure 2.
Correlation analysis of week 2 and 6 serum samples (a, n=41) and maintenance treatment serum samples (b, n=84).
Figure 3.
Bland–Altman plots of week 2 and 6 serum samples (a, n=41) and maintenance treatment serum samples (b, n=84), including the average bias (solid line) and 95% limits of agreement (dotted line) between lateral flow-based assay (LFA) and enzyme-linked immunosorbent assay (ELISA).
Out of 28 available anti-tumor necrosis factor naive samples (w0), only one sample revealed a slightly elevated infliximab concentration of 0.51 μg/ml, which is just above the quantification limit of the assay (0.50 μg/ml). The positive and negative percent agreement between LFA and the reference ELISA are 99.7% and 98.6%, respectively. In addition, 10 samples were re-tested using LFA and ELISA demonstrating excellent reproducibility with an average coefficient of variation of 8±6% and 5±2%, respectively.
Association of infliximab trough concentrations and mucosal healing
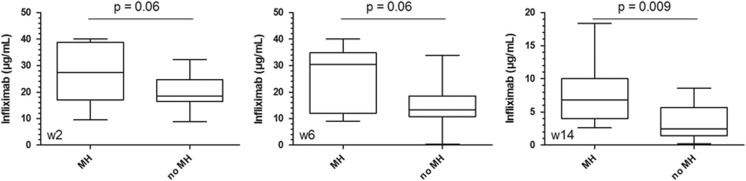
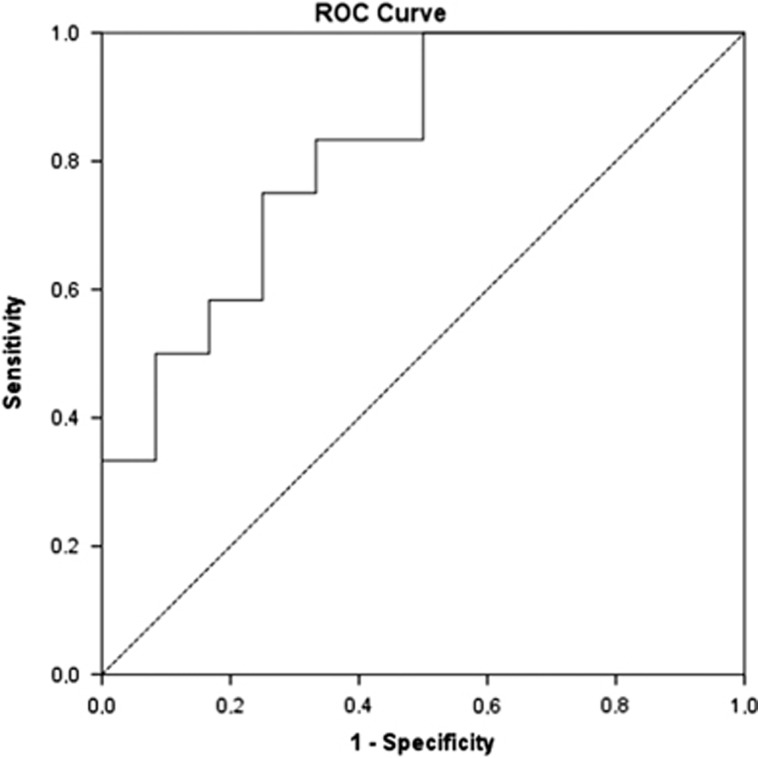
Infliximab concentrations were higher in patients with MH than in patients without MH (Figure 4), but the difference was only significant at week 14 (P=0.007). Receiver operating characteristic analysis identified an infliximab concentration threshold ≥2.1 μg/ml at week 14 using the LFA (Figure 5, area under the ROC curve (AUROC) of 0.819, P=0.008, sensitivity 100%, specificity 50%) and ≥2.7 μg/ml using ELISA (AUROC of 0.802, P=0.012, sensitivity 100%, specificity 50%) to be associated with MH. This cut-off implies that 50% of patients without MH have infliximab concentrations lower than 2.1 μg/ml. Our results, however, indicate that these patients benefit from an intensified dosing regimen as three out of four patients with a TC<2.1 μg/ml at w14 who started infliximab maintenance therapy and received intensified therapy, successfully completed 2 years of infliximab treatment. In addition, the positive and negative percent agreement of the LFA with ELISA at the ELISA cut-off of 2.7 μg/ml was determined to be 94% and 100%, respectively.
Figure 4.
Infliximab concentrations at w2, w6, and w14 in patients with and without mucosal healing.
Figure 5.
Receiver operating characteristic analysis of week 14 infliximab trough concentrations, measured with the lateral flow-based assay, showing an AUROC of 0.819 (P=0.008, 95% confidence interval: 0.652–0.987). An infliximab concentration threshold of 2.1 μg/ml was found to be associated with mucosal healing.
Anti-infliximab antibodies
In total, 221 serum samples were tested using the drug-sensitive and novel drug-tolerant assay. During the 1 year follow-up, the drug-sensitive anti-infliximab assay detected ATI in 4/29 patients (14%), whereas the drug-tolerant anti-infliximab assay identified 10/29 patients (34%) with ATI on at least one time point (Supplementary Figure S2). In total, 5/221 (2%) samples and 35/221 (16%) samples were ATI positive using the drug-sensitive and drug-tolerant assay, respectively. All four patients with positive ATI in the drug-sensitive bridging ELISA had at that time no detectable TC and were also identified as ATI positive using the drug-tolerant assay.
Two out of eight patients who failed infliximab maintenance treatment suffered from an infusion reaction and delayed hypersensitivity associated with high ATI, which were detected 48 and 33 weeks before the adverse event using the drug-tolerant assay and 40 and 17 weeks using the drug-sensitive assay, respectively (Supplementary Figure S3). The six other patients stopped because of insufficient response to therapy despite quantifiable TC—only one of these patients had detectable ATI using the drug-tolerant approach at the time of infliximab discontinuation.
Discussion
The advent of biological therapies, such as infliximab, revolutionized the treatment of patients with refractory inflammatory bowel diseases, but raised as well-significant pharmaco-economic concerns. Although hospitalizations and surgical interventions decreased, cost of drug strongly increased. The main challenging goal for the future is to use biologicals as effective as possible. Therapeutic drug monitoring has, therefore, been introduced to guide the treatment of patients with inflammatory bowel disease and improve clinical outcomes.
Sufficient evidence in favor of therapeutic drug monitoring during maintenance infliximab therapy has been demonstrated in a number of clinical trials linking TC with clinical outcome.1, 2, 18 In a post hoc analysis of the SONIC trial, TC>3.0 μg/ml at week 30 were significantly associated with MH at week 26.19 In a meta-analysis of Moore et al.,20 TC>2.0 μg/ml were associated with a greater probability of clinical remission and MH. A study of Arias et al.21 identified a w14 TC>2.5 μg/ml to be associated with relapse-free infliximab survival and colectomy-free survival. In addition, prospective trials such as the TAXIT trial demonstrated that a TC target window of 3–7 μg/ml increases the number of patients in clinical remission at a lower cost.4
Recently, the scope of therapeutic drug monitoring has been widened to the induction phase of infliximab therapy.22, 23, 24, 25 A Dutch group reported an infliximab serum concentration of 6.6 μg/ml at week 6 as a cut-off value for endoscopic response in patients with moderate–severe UC.24 Another study identified infliximab concentration thresholds of 28.3, 15.0, and 2.1 μg/ml at week 2, 6, and 14, respectively, to be associated with short-term MH in UC patients.25 It is hypothesized that in some patients, infliximab is cleared more rapidly due to a high baseline disease burden, early anti-infliximab antibody formation, or other pharmaco-kinetic and -dynamic mechanisms leading to an underexposure of patients to infliximab and subsequently insufficient clinical response. These patients may benefit from an intensified infliximab dosing regimen. However, the current available assays to perform drug monitoring only allow a dose optimization at the next patient's hospital visit, limiting their use.6, 7, 8, 9 The feasibility of a rapid assay for infliximab monitoring has previously been demonstrated in two studies using either a LFA or a fiber optic-surface plasmon resonance technology.26, 27 However, both assays still require multiple manipulations, extensive validation and are not available for routine use.
Here, we present a novel, rapid LFA for infliximab quantification that is accurate, accessible, and affordable. The LFA is accurate. A blindly performed, parallel analysis of 190 serum samples of patients with ulcerative colitis using the LFA and a reference ELISA revealed an excellent correlation for induction and maintenance treatment samples. Re-analysis of 10 patient samples on an independent time point revealed a high reproducibility of the LFA. The analysis of 28 anti-tumor necrosis factor naive samples on LFA revealed one false-positive quantification of infliximab (0.51 μg/ml), which was only marginally higher than the assay cut-off (0.50 μg/ml). This value is considered not to alter clinical decisions. The LFA is accessible. The bench top-sized reader (Supplementary Figure S4) can be placed in any hospital/infusion center and the comprehensive assay format does not require extensive laboratory skills. With a time-to-result of roughly 20 min clinicians have immediate access to the results and may adapt the infliximab dosing regimen within a patient's hospital visit. One drawback of the current assay format is that it still requires a blood collection and serum preparation, but this allows adequate comparison of the LFA with the current available standard methods and application of the established therapeutic drug monitoring-based treatment algorithms. In addition, the LFA is CE-marked and is currently only available in Europe. There is no need to collect a series of samples before an analysis can be performed as a single sample can be measured. Moreover, it avoids the need of costly sample transportation to a centralized, fully equipped, laboratory. The final pricing will depend on distributor, country, laboratory, and end user.
In our study, using the LFA, a TC≥2.1 μg/ml infliximab at week 14 was identified as a cut-off associated with MH, which is equal to the cut-off determined in a larger cohort of 101 patients (of which 20/101 patients overlap in this study cohort).25 In contrast to the study of Papamichael et al.,25 we could not identify cut-offs at week 2 and week 6 associated with MH, probably related to the small sample size used. The cut-off of 2.1 μg/ml infliximab has a sensitivity of 100%, but a specificity of only 50%, indicating that 50% of patients without MH have infliximab concentrations <2.1 μg/ml at week 14. Our results, however, indicate that these patients may benefit from an intensified dosing regimen as three out of four patients with a TC<2.1 μg/ml at w14 who started infliximab maintenance therapy and received intensified therapy, successfully completed 2 years of infliximab treatment. These results are to be confirmed in a larger prospective trial.
In addition, we determined the evolution of ATI over time using a drug-sensitive and novel drug-tolerant ELISA; the latter assay was expected to increase the number of ATI-positive samples. We could strengthen this as four patients (14%) or 5/221 samples (2%) were ATI positive using the drug-sensitive assay vs. 10 patients (34%) or 35/221 samples (16%) with the drug-tolerant assay. Two patients had to stop infliximab maintenance treatment because of an adverse event associated with high ATI titers. These ATI were already detected 48 and 33 weeks before the adverse event using the drug-tolerant assay. It is hypothesized that these patients could have had benefit from an early treatment optimization.
This study has some limitations. As the amount of lateral flow strips available was restricted, we opted to apply these strips in a well-defined cohort of only 29 patients to validate the LFA performance. Hence, the modest sample size used may limit our associations of TCs with MH. In addition, because a post hoc analysis was performed, not all factors that could explain the variability in response, such as fecal calprotectin levels, were included in this study.
In conclusion, we validated a novel LFA which is sensitive, selective, and highly specific for infliximab. Using the LFA we identified an infliximab threshold ≥2.1 μg/ml at week 14 to be associated with MH in patients with ulcerative colitis. With a time-to-result of 20 min, individual sample analysis and user-friendliness, the LFA outplays ELISA as a rapid, accurate tool to monitor infliximab concentrations facilitating clinical decision-making. The novel drug-tolerant ATI assay improves the detection of ATI and allows an early identification of patients at risk for developing excessive ATI and associated treatment failure.
Study Highlights

Acknowledgments
We would like to thank R-Biopharm AG for providing us with the lateral flow cassettes, the reagentia, and the portable reader. We acknowledge the valuable expertise of Chris Barthel, Yessica Kölmel, Nasim Zali, and Steffen Rameil.
Guarantor of the article: Ann Gils, PharmD, PhD.
Specific author contributions: TVS helped with the design of the study, performed research, interpreted data, implemented statistical analyses, and drafted the manuscript. LB and KP helped with the acquisition of data and critically revised the manuscript. NVC critically revised the manuscript. GVA and MF provided patient samples and critically revised the manuscript. SV provided patient samples, helped with the acquisition and interpretation of data, and critically revised the manuscript. AG designed the study, coordinated the experiments, interpreted data, and critically revised the manuscript for intellectual content. All the authors read and approved the final version of the manuscript.
Financial support: This study was financially supported in part by C2 grant C22/15/025 from the KU Leuven. TVS is a PhD fellow of the Agency for the Promotion and Innovation through Science and Technology in Flanders (IWT, Flanders). NVC is a Postdoctoral Fellow of the Research Foundation—Flanders (FWO), Belgium; grant number 1260714N and received speakers fees from AbbVie and consultancy fees from MSD, Janssen Biologics, Pfizer, UCB, and Takeda. KP received a Fellowship Grant from the Hellenic Society of Gastroenterology. GVA received financial support for research from Abbott and Ferring Pharmaceuticals, lecture fees from Janssen, MSD and Abbott, and consultancy fees from PDL BioPharma, UCB Pharma, Sanofi-Aventis, Abbott, Abbvie, Ferring, Novartis, Biogen Idec, Janssen Biologics, NovoNordisk, Zealand Pharma A/S, Millenium/Takeda, Shire, Novartis, and Bristol Mayer Squibb. MF received financial support for research from Takeda, lecture fees from MSD, Janssen, Abbvie, Boehringer-Ingelheim, Ferring, Chiesi, Tillotts, Zeria, and Mitsubishi Tanabe, and consultancy fees from MSD, Janssen, Abbvie, Boehringer-Ingelheim, and Ferring. SV received grant support from MSD, Abbvie and Takeda, lecture fees from Abbvie, MSD, Ferring Pharmaceuticals, Takeda, Hospira and consultancy fees from Abbvie, Takeda, Pfizer, Ferring Pharmaceuticals, Shire Pharmaceuticals Group, MSD, Hospira, Mundipharma, Celgene, Galapagos, Genentech/Roche. AG has served as a speaker for MSD, Janssen Biologicals, Pfizer, Takeda and Abbvie, as consultant for UCB and has received Investigator Initiated Research Grants from Pfizer. KU Leuven licensed the infliximab ELISA to apDia and R-Biopharm and the use of monoclonal antibody—mAb-IFX6B7—in the LFA to R-Biopharm.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Maser EA, Villela R, Silverberg MS et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol 2006; 4: 1248–1254. [DOI] [PubMed] [Google Scholar]
- Seow CH, Newman A, Irwin SP et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010; 59: 49–54. [DOI] [PubMed] [Google Scholar]
- Paul S, Del Tedesco E, Marotte H et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis 2013; 19: 2568–2576. [DOI] [PubMed] [Google Scholar]
- Vande Casteele N, Ferrante M, Van Assche G et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015; 148: 1320–1329. [DOI] [PubMed] [Google Scholar]
- Steenholdt C, Brynskov J, Thomsen OO et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014; 63: 919–927. [DOI] [PubMed] [Google Scholar]
- Bendtzen K, Geborek P, Svenson M et al. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum 2006; 54: 3782–3789. [DOI] [PubMed] [Google Scholar]
- Wang SL, Ohrmund L, Hauenstein S et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods 2012; 382: 177–188. [DOI] [PubMed] [Google Scholar]
- Van Stappen T, Brouwers E, Tops S et al. Generation of a highly specific monoclonal anti-infliximab antibody for harmonization of TNF-coated infliximab assays. Ther Drug Monit 2015; 37: 479–485. [DOI] [PubMed] [Google Scholar]
- Ternant D, Mulleman D, Degenne D et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther Drug Monit 2006; 28: 169–174. [DOI] [PubMed] [Google Scholar]
- Vande Casteele N, Buurman DJ, Sturkenboom MG et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther 2012; 36: 765–771. [DOI] [PubMed] [Google Scholar]
- Schmitz EM, van de Kerkhof D, Hamann D et al. Therapeutic drug monitoring of infliximab: performance evaluation of three commercial ELISA kits. Clin Chem Lab Med 2016; 54: 1211–1219. [DOI] [PubMed] [Google Scholar]
- Ruiz-Arguello B, del Agua AR, Torres N et al. Comparison study of two commercially available methods for the determination of infliximab, adalimumab, etanercept and anti-drug antibody levels. Clin Chem Lab Med 2013; 51: e287–e289. [DOI] [PubMed] [Google Scholar]
- Malickova K, Duricova D, Bortlik M et al. Serum trough infliximab levels: a comparison of three different immunoassays for the monitoring of CT-P13 (infliximab) treatment in patients with inflammatory bowel disease. Biologicals 2016; 44: 33–36. [DOI] [PubMed] [Google Scholar]
- Steenholdt C, Ainsworth MA, Tovey M et al. Comparison of techniques for monitoring infliximab and antibodies against infliximab in Crohn's disease. Ther Drug Monit 2013; 35: 530–538. [DOI] [PubMed] [Google Scholar]
- Steenholdt C, Bendtzen K, Brynskov J et al. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn's disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014; 109: 1055–1064. [DOI] [PubMed] [Google Scholar]
- Bollen L, Vande Casteele N, Peeters M et al. Short-term effect of infliximab is reflected in the clot lysis profile of patients with inflammatory bowel disease: a prospective study. Inflamm Bowel Dis 2015; 21: 570–578. [DOI] [PubMed] [Google Scholar]
- Van Stappen T, Billiet T, Vande Casteele N et al. An optimized anti-infliximab bridging enzyme-linked immunosorbent assay for harmonization of anti-infliximab antibody titers in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2015; 21: 2172–2177. [DOI] [PubMed] [Google Scholar]
- Baert F, Noman M, Vermeire S et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003; 348: 601–608. [DOI] [PubMed] [Google Scholar]
- Reinisch W, Colombel JF, Sandborn WJ et al. Factors associated with short- and long-term outcomes of therapy for Crohn's disease. Clin Gastroenterol Hepatol 2015; 13: 539–547. [DOI] [PubMed] [Google Scholar]
- Moore C, Corbett G, Moss AC. Systematic review and meta-analysis: serum infliximab levels during maintenance therapy and outcomes in inflammatory bowel disease. J Crohns Colitis 2016; 10: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias MT, Vande Casteele N, Vermeire S et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2015; 13: 531–538. [DOI] [PubMed] [Google Scholar]
- Adedokun OJ, Sandborn WJ, Feagan BG et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014; 147: 1296–1307. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Suzuki Y, Motoya S et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J Gastroenterol 2016; 51: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandse JF, Mathot RA, van der Kleij D et al. Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2016; 14: 251–258. [DOI] [PubMed] [Google Scholar]
- Papamichael K, Van Stappen T, Vande Casteele N et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016; 14: 543–549. [DOI] [PubMed] [Google Scholar]
- Corstjens PL, Fidder HH, Wiesmeijer KC et al. A rapid assay for on-site monitoring of infliximab trough levels: a feasibility study. Anal Bioanal Chem 2013; 405: 7367–7375. [DOI] [PubMed] [Google Scholar]
- Lu J, Van Stappen T, Spasic D et al. Fiber optic-SPR platform for fast and sensitive infliximab detection in serum of inflammatory bowel disease patients. Biosens Bioelectron 2016; 79: 173–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.