Abstract
The gastrointestinal tract consists of an enormous surface area that is optimized to efficiently absorb nutrients, water, and electrolytes from food. At the same time, it needs to provide a tight barrier against the ingress of harmful substances, and protect against a reaction to omnipresent harmless compounds. A dysfunctional intestinal barrier is associated with various diseases and disorders. In this review, the role of intestinal permeability in common disorders such as infections with intestinal pathogens, inflammatory bowel disease, irritable bowel syndrome, obesity, celiac disease, non-celiac gluten sensitivity, and food allergies will be discussed. In addition, the effect of the frequently prescribed drugs proton pump inhibitors and non-steroidal anti-inflammatory drugs on intestinal permeability, as well as commonly used methods to assess barrier function will be reviewed.
INTRODUCTION
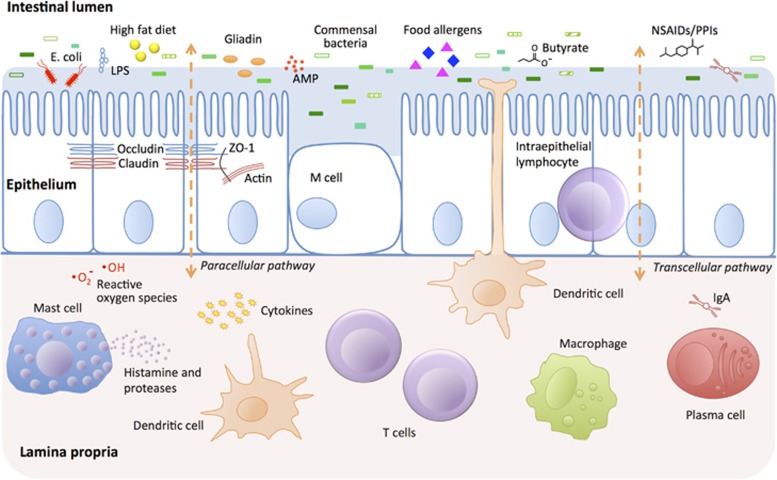
The intestine is the main organ involved in the uptake of nutrients and water. At the same time, it constitutes an essential barrier against harmful substances and pathogens from the external environment. The intestinal barrier is mainly composed of the mucus layer, the epithelial layer, and the underlying lamina propria. Tight junction (TJ) proteins connect the intestinal epithelial cells and regulate the paracellular permeability. In addition, components such as immune cells, the intestinal microbiota, and anti-microbial peptides have crucial roles in supporting appropriate gut barrier function (see Figure 1).
Figure 1.
Schematic figure of the intestinal barrier and affecting factors. The intestinal barrier is composed of several layers providing protection against microbial invasion. The intestinal lumen contains anti-microbial peptides (AMPs), secreted immunoglobulin A (IgA), and commensal bacteria, which inhibit the colonization of pathogens by competitive inhibition and by production of, e.g., butyrate, which has barrier-protective properties. A mucus layer covers the intestinal surface providing a physical barrier. The epithelial layer consists of a single layer of epithelial cells that are sealed by tight junction proteins such as occludin, claudin, and zonulin-1 preventing paracellular passage. This layer also harbors intraepithelial lymphocytes, M cells (overlying Peyer's patches and lymphoid follicles), mucus-producing Goblet cells and bacteriocin-producing Paneth cells (not shown). The lamina propria contains a large amount of immune cells, both of the innate immune system (e.g., macrophages, dendritic cells, mast cells) and the adaptive immune system (e.g., T cells, IgA producing plasma cells). In addition, cells of the central and enteric nervous system innervate in the lamina propria (not shown). Factors affecting the intestinal barrier function include pathogenic bacteria such as enteropathogenic E. coli, high-fat diet, lipopolysaccharides (LPS), drugs such as non-steroidal anti-inflammatory drugs (NSAIDs), and proton pump inhibitors (PPIs), as well as various food allergens and the gluten component gliadin.
Disruption of this barrier results in increased intestinal permeability, which in turn facilitates translocation of harmful substances and pathogens to the bloodstream. The pathophysiology of a number of diseases is associated with a dysfunctional intestinal barrier, and some of these diseases and their underlying mechanisms will be discussed in this review. To date, the key work has been done in animal models and in vitro, and little is known about the equivalent processes in humans.
THE INTESTINAL EPITHELIAL BARRIER IN INFECTION
Infectious intestinal pathogens, including various bacteria and viruses, have different mechanisms of gaining access to the host. Some directly adhere to and invade the intestinal epithelial barrier, whereas others disrupt this barrier via the secretion of toxins. In either case, various common pathogens have developed mechanisms that target the host's TJ proteins. By disrupting the TJ complex, epithelial permeability increases and the pathogens' invasion process is facilitated.
TJ proteins
TJs are protein complexes that connect adjacent epithelial cells near their apical pole.1 The core TJ complex is composed of transmembrane proteins, including occludin, junctional adhesion molecules, and members of the claudin family, depending partly on the location of the epithelium and its permeability. Occludin and claudins interact with the zonula occludens (ZO) proteins that link to the actin cytoskeleton, thereby regulating cell cycle control and linking it with cell polarity and permeability function. TJs have a crucial role in paracellular permeability by conferring selectivity to the flow of ions, small molecules, and solutes between cells. Additionally, they regulate cellular polarity by preventing the diffusion of receptors from the apical membrane above the TJs to the basolateral membrane. This can have a crucial role in the responsiveness of cells to directional stimuli and transport functions, as well as proliferation.
In all epithelium, TJ assembly and disassembly is a dynamic process involving endocytosis, migration, and recycling. This is influenced by the activity of multiple cytokines and kinases including subsets of the protein kinase C family, which can phosphorylate occludin and affect stability in the dynamic TJ complex.2, 3 Several cytokines can modulate TJ dynamics,4 e.g., tumor necrosis factor-α (TNF-α) induces TJ permeability through the extracellular signal-regulated kinase 1/2 pathway.5, 6 Intracellular cAMP, energy levels,7 oxidative stress, and calcium imbalance also impact TJ dynamics and assembly through their varied effects on cellular kinases.8, 9, 10
TJ proteins as targets in infections
TJs have an important role in the infection mechanisms of a range of viral and bacterial pathogens, by acting as receptors or targets of bacterial virulence factors (Table 1). The end result is typically disruption of the TJs, leading to increased epithelial permeability, and facilitation of the translocation and colonization of pathogens into the body.
Table 1. Modulation of TJ structures by human intestinal pathogens.
| Pathogen | Effector molecules | Effects on TJs and epithelial barrier |
|---|---|---|
| EPEC | T3SS, EspF, EspG, Map20, 176, 177 | Altered localization of claudin, ZO-1 and occludin; loss of TER and increased flux of small molecules |
| EHEC | Altered TJ protein expression.178 TNF-α produced by infection increases expression of claudin-2 20 | |
| Salmonella typhimurium | T3SS, SPI1 effectors; SopB, SopE SopE2 and SipA have been implicated179, 180 | Decreased ZO-1 expression, and decreased phosphorylation of occludin180 |
| Helicobacter pylori | T4SS, CagA | Mislocalization of ZO-1 in the cytoplasm181, 182 |
| Shigella flexneri | Disruption of TJ structures, decreased expression of claudin-1 and TER after 90 min | |
| Clostridium perfringens | Enterotoxin binding to claudin proteins | The C-terminal region of C. perfringens enterotoxin can bind to specific claudin proteins, resulting in the disintegration of TJs and an increase in paracellular permeability183 |
| Vibrio cholerae | ZOT26 | Altered flux and ZO-1 density in the TJs |
| Reovirus | Protein σ1 | Binding of σ1 to TJ protein N-terminal part of JAM-A promotes internalization184 |
| Rotaviruses | VP8, NSP4 | VP8 is released from the protein core by trypsin leading to disruption of barrier integrity; toxin NSP4 blocks TJ formation185, 186 |
Abbreviations: EHEC, enterohemorrhagic E. coli; EPEC, enteropathogenic E. coli; JAM, junctional adhesion molecule; NSP4, nonstructural protein 4; TER, transepithelial electrical resistance; TJ, tight junction; TNF-α, tumor necrosis factor-α ZO-1, zonulin-1; ZOT, zonula occludens toxin.
TJ rearrangements are implicated in the pathology of gastrointestinal infections with different pathogenic Escherichia coli (Table 1). Enteropathogenic E. coli (EPEC) are a common cause of diarrheal disease, particularly in infants and characteristically cause a loss of enterocyte microvilli (also known as effacement) and formation of a raised pedestal structure for firm bacterial attachment.11 These cellular effects are mediated by the formation of a Type III secretion system (encoded in the locus of enterocyte effacement) and by injection of multiple effector proteins into the cell cytoplasm (reviewed in Frankel and Phillips12). One of these effectors (Tir) gets phosphorylated by the host and thereby inserts into the apical membrane to serve as a receptor for bacterial intimin, leading to firm attachment of EPEC. The other effectors elicit numerous cellular responses through the activation of various protein kinases, including myosin light-chain kinase, which leads to TJ disruption and increased paracellular permeability.13, 14, 15 The myriad events leading to TJ disruption contribute to the pathogenesis of diarrhea caused by EPEC and are still being investigated at the molecular level.
Similar to EPEC, enterohemorrhagic E. coli also possess an attaching and effacement locus, but exert less profound effects on the barrier.16 One reported difference is the increased expression of claudin-2, which forms cation-selective channels in the paracellular space, resulting in water transportation across the TJs.17, 18 Increased expression of claudin-2 is also observed in the intestinal epithelium of inflammatory bowel disease (IBD) patients with active disease and is associated with barrier dysfunction and ‘leak-flux' diarrhea.19 TNF-α has been shown to upregulate the expression of claudin-2 via phosphatidylinositol-3-kinase signaling.20
Enteroaggregative E. coli and enterotoxigenic E. coli colonize the epithelium via specific interactions with pilli and produce enterotoxins that cause diarrhea through effects on chloride secretion in the intestinal epithelium.21 The enterotoxins responsible for diarrhea are the heat-labile toxins I, II and heat-stable toxins STa, STb, and EAST1 (enteroaggregative heat-resistant toxin 1), all of which increase chloride ion secretion from the intestinal epithelial cells.22, 23 Recently, STb was shown to cause a redistribution of claudin-1, ZO-1, and occludin in T84 intestinal cell monolayers, which is likely to be involved in the observed increase in permeability, although the mechanisms by which these changes are brought about remain to be elucidated.24
During pathogenesis, Helicobacter pylori causes disruption of cell–cell adhesions and loss of cell polarity. CagA toxin, which is secreted into the host cells by a type 4 secretion system, induces multiple signaling events leading to cytoskeleton disruption, disruption of TJs, and the loss of cell polarization, with severe physiological consequences.25 These events are considered to increase the diffusion of iron and nutrients to support bacterial growth during colonization. Ultimately, barrier disruption would also allow H. pylori to invade the paracellular space and gain access to the lamina propria.
Production of zonula occludens toxin (ZOT) in culture supernatants of Vibrio cholerae was shown to correlate with their capacity to cause diarrhea by decreasing strand complexity of ZO and increasing intestinal permeability.26 Subsequently, the activity of ZOT was mapped to the hexapeptide immediately downstream of the ZOT cleavage site27 and was shown to cause TJ disassembly via increased phosphorylation of ZO-1 and mycosin 1C, leading to decreased protein interactions with ZO-1 and rearrangement of actin filaments via proteinase-activated receptor 2 activation.28
In summary, several gastrointestinal pathogens mediate changes in TJs to disrupt paracellular permeability to facilitate release of nutrients and to gain access to the lamina propria. Pharmacological or nutritional approaches to maintain the integrity of TJ and the epithelium may interfere with the pathogenesis of disease caused by these gastrointestinal pathogens. This could be due to the intake of specific probiotics that strengthen the function of TJs29 or are able to replace existing pathogens or inhibit their adherence.30 Accordingly, several probiotic strains have been shown to successfully prevent traveler's diarrhea.31
THE ROLE OF INTESTINAL BARRIER FUNCTION IN IBD
The causes of IBD are still not understood, but there is no doubt that the intestinal tissue injury is caused by an excessive immune/inflammatory process in the gut wall. Consequently, immune suppression is the mainstay of therapy. In terms of the relationship between gut barrier function and IBD, the critical question is whether impaired barrier function is secondary to gut inflammation and damage, or if it is important as an independent event, which may either protect or confer risk of IBD.
Gut barrier defect and inflammation
In 1995, Gordon and co-workers32 developed a chimeric mouse model in which some of the small bowel epithelium expressed N-cadherin instead of E-cadherin, thereby disrupting the E-cadherin homotypic interactions that help maintain barrier integrity. At the regions of the intestine expressing N-cadherin, the epithelium was leaky and the mice developed focal inflammation in these areas. Several subsequent studies using gut epithelial gene-specific knockout mice confirmed that a dysfunctional epithelial barrier results in spontaneous intestinal inflammation. Markedly, genes associated with uncontrolled cell death seem to be involved.33, 34, 35 Also, defects in mucus assembly and production can lead to spontaneous development of colitis in mice models.36, 37, 38, 39
The clear lesson from this work is that if the gut epithelium is disrupted, ingress of bacterial components into the lamina propria is sufficient to trigger IBD. However, many animal models have shown that even in the presence of an apparently normal gut epithelial barrier, changes in immune regulation can result in exaggerated mucosal immune responses and IBD phenotypes. For example, in dirty animal houses, all interleukin-10 (IL-10)-null mice develop small and large bowel inflammation early in life, whereas in clean animal houses, only a colitis develops and the onset of IBD is delayed.40 The importance of IL-10 is further demonstrated by the fact that children with IL-10R loss-of-function mutations develop a severe enteritis early in life.41 Regulation of inflammatory responses is also crucial to intestinal homeostasis, as shown by the identification of IL-23R variants as risk factors for both Crohn's disease and ulcerative colitis.42, 43 Thus, in the absence of immune regulation, the low levels of bacterial antigens that cross into the lamina propria are sufficient to trigger inflammation. In healthy individuals, translocating bacteria and bacterial antigens are mopped up by macrophages in the lamina propria. However, e.g., in children with defects in their ability to deal with low-grade bacterial infections, such as chronic granulomatous disease or glycogen storage disease type 1b, ~40% of patients develop a lesion similar to Crohn's disease.44
Epithelial barrier dysfunction in IBD
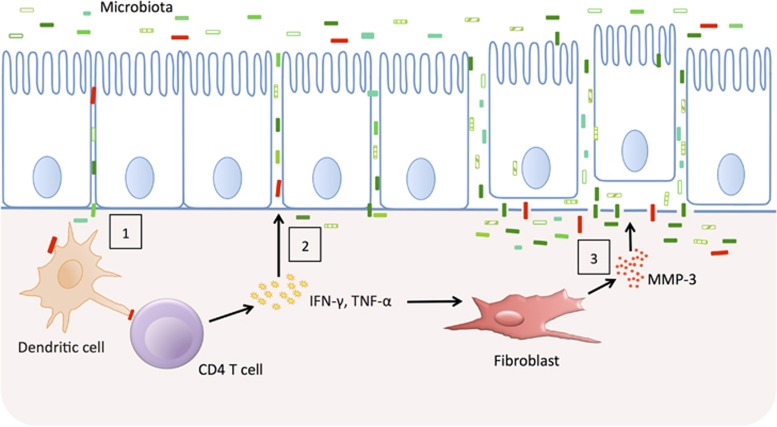
There is a widely held perception that a determinant of susceptibility to IBD, especially to Crohn's disease, is an inherent/genetic defect in the intestinal barrier, which allows greater ingress of luminal antigens into the tissues (see Figure 2). Patients with active IBD have clear epithelial barrier defects, exemplified most typically by overt ulceration. When patients enter remission, barrier function improves; however, it rarely returns to normal. This is most probably due to the fact that inflammation continues at a relatively low level.45 One way to determine if there is indeed a tendency for those who develop IBD to have a leaky gut is to study unaffected relatives who are known to have a 30-fold increase in the risk of developing IBD. Permeability tests in these individuals are normal; however, in response to an insult, such as a non-steroidal anti-inflammatory drug (NSAID), a subset of relatives (35%) did show markedly increased permeability (+2 s.d. of the controls).46 Overall, these first-degree relatives to Crohn's disease patients had a 110% increase in permeability compared with a 57% increase in healthy controls after the NSAID challenge. Therefore, while the epithelial barrier per se may not be intrinsically leaky in IBD, the response to injury may be impaired, perhaps because of impaired healing or delayed epithelial restitution.
Figure 2.
Epithelial barrier dysfunction and inflammation in inflammatory bowel disease (IBD). Genetically encoded variation in the epithelial barrier function may allow microbes to cross the barrier and trigger a T-cell response (1). The cytokines produced by activated T cells and macrophages loosen tight junctions allowing more antigens to cross (2). Finally, degradation of the basement membrane causes the epithelial cells to be shed and massive penetration of microbes into the gut wall occurs (3).
A particularly attractive way to determine if the epithelial barrier function is important in IBD is to attempt to treat active IBD with agents that help to restore the barrier, such as trefoil peptides or growth factors. In a very small clinical study of patients with distal colitis, epidermal growth factor enemas were superior to placebo enemas in inducing remission.47 Ten out of 12 patients in the treatment group were in remission after 2 weeks compared with 1 out of 12 in the control group. However, this work has not been replicated in a larger properly powered study.
A number of proinflammatory cytokines produced in excess in IBD also change the epithelial barrier function. It is very well known that interferon-γ, TNF-α, and IL-13 increase the epithelial permeability.48
Many studies have shown that in active IBD there is a dysbiosis of the microbiota, which could be a cause for a disturbed epithelial barrier function. Reduced complexity of the phylum Firmicutes is a common signature of fecal microbiota of Crohn's disease patients and in particular decreased abundance of Faecalibacterium prausnitzi.49 Several species within the phylum Firmicutes ferment complex carbohydrates in the colon and produce butyrate, which has been reported to increase production of secreted mucus and has other potential barrier-protecting functions (see section on Irritable Bowel Syndrome).50 Additionally, E. coli pathobionts exhibiting pathogen-like behaviors that disrupt the epithelial barrier are more frequently cultured from IBD patients.51
IBD susceptibility loci associated with epithelial barrier function
About 10 years ago, candidate gene studies suggested that two loci, OCTN1 (organic cation transporter 1) and DLG5 (Disks large homolog 5), were susceptibility loci for Crohn's disease. Subsequent studies could, however, only show weak associations.52, 53, 54 More recent genome-wide association studies have identified risk variants of five epithelial-associated loci in ulcerative colitis. Briefly, these are the CDH1 locus that encodes the E-cadherin gene, LAMB1, which encodes laminin, HNF4α (hepatocyte nuclear factor 4 alpha), an epithelial-specific transcriptional regulator, GNA12, a guanine nucleotide-binding protein and ECM-1, which encodes an extracellular matrix protein.55 Interestingly, HNF4α-null mice develop a spontaneous colitis.56 An additional prominent variant consistently associated with an increased risk of IBD is NOD2 (nucleotide-binding oligomerization domain-containing protein 2), which encodes a receptor recognizing bacterial cell wall components and activating a respective immune response.57 It is suggested that defects in NOD2 result in lower α-defensin secretion from Paneth cells and thus in an increased amount of mucosal bacteria.58, 59 Patients with mutations in the gene that encodes cytoplasmic phospholipase A2 suffer from devastating intestinal inflammation as prostaglandins are needed to maintain gut barrier function.60
In conclusion, it needs to be noted that genetically encoded variants are not causal and have only a very small effect compared with environmental factors. In the small bowel at least, environmental agents such as spices and NSAIDs (see also section on Proton Pump Inhibitors and NSAIDs) can alter barrier function, and it may be the case that the effects of small genetically encoded variants synergize with environmental insults. For Crohn's disease, which is not seen in the developing world, it may well be that infectious agents that disrupt barrier function early in life may be protective against the immune hyper-reactivity, which drives the disease, perhaps by raising the threshold at which T-cell immunity is triggered, or by activating healing and regulatory circuits. In addition, the intestinal microbiota composition seems to be an important environmental factor in IBD, either as an excessive immune response to a normal microbiota or an appropiate immune response to an altered microbiota (or components thereof).
It is still unclear if it is possible to uncouple the inflammation of IBD in remission from secondary effects of the inflammation on barrier function, and whether maintaining the barrier would allow patients to remain in remission longer. The challenge is to identify nutritional or pharmaceutical compounds that show a positive effect on barrier function and could thus be used to prolong remission.
IRRITABLE BOWEL SYNDROME AND INTESTINAL BARRIER FUNCTION
Irritable bowel syndrome (IBS) is a common disorder affecting 3–22% of the adult population in Western countries.61 It is characterized by recurrent abdominal pain or discomfort that occurs in association with altered bowel habits. The pathogenesis of IBS is unclear, but is considered to involve aberrations along the gut–brain axis. Interestingly, 4–32% of patients suffering from acute gastroenteritis develop IBS during the 3–12 months follow-up, predominantly of diarrhea-predominant character.62 This subtype of IBS is defined as postinfectious IBS (PI-IBS). The prevalence of mood or anxiety disorders in IBS patients exceeds 35%, which is considerably higher compared with control populations, and these disorders seem also to be a risk factor for the development of PI-IBS.63, 64, 65
Visceral hypersensitvity and intestinal barrier function in IBS
In PI-IBS, it is assumed that the initial infectious agent triggers a sustained immune and inflammatory response with direct consequences for intestinal barrier function. This low-grade inflammation is especially characterized by mast cell activation,66 which is directly linked to impaired barrier function by release of, e.g., proteases and histamine.67
Given the high prevalence of sensory abnormalities in IBS, and the correlation of visceral sensitivity with symptoms, an increased colorectal perception is considered to be a biological hallmark of IBS. Increased visceral perception (hypersensitivity) and motor responses (compliance, contraction, accommodation) may be caused by peripheral (e.g., inflammation, infection)66 or central (e.g., attention, anticipation, and mood) sensitization mechanisms.68 Soluble mediators in the gut lumen may partly be responsible for this sensitization and contribute to alterations of intestinal barrier function with systemic implications.69 Accordingly, the supernatant of cultured colonic biopsies from IBS patients (n=39) significantly reduced barrier function in Caco-2 cells compared with healthy controls (n=14) and control solutions (n=12), measured as transepithelial resistance (P<0.0001) as well as paracellular permeability (P=0.001).70 In addition, the supernatant of colonic biopsies from PI-IBS patients increased the degranulation rate of rat peritoneal mast cells, concomitant with an increased expression of protease-activated receptor 2.71 These effects could be attenuated by treating the mast cells with phenyl N-tert-butylnitrone, a potent scavenger of reactive oxygen species (ROS). ROS are important mediators of adaptive and innate immune regulatory function and a delicate balance between induction of immune responses and prevention of inadequate oxidative stress and damage should be maintained. Increased levels of ROS may lead to increased recruitment of regulatory T cells and downregulation of the forkhead box O3a transcription factors, with a subsequent decrease of intestinal antioxidants such as catalase and superoxide dismutase,71 potentially leading to damages of the intestinal barrier function.
The role of intestinal microbiota and intestinal barrier function in IBS
The question arises about what role the intestinal microbiota have in IBS in general and more specifically, with respect to sensitization and intestinal barrier function. Evidence increases that IBS is associated with distinct changes in microbiota, involving several groups of Firmicutes and Proteobacteria.72, 73, 74, 75, 76 This is consistent with findings of altered toll-like receptor expression in colonic biopsies,77 as well as toll-like receptor-related cytokine responses in the peripheral blood of IBS patients.69
The altered host–microbe interplay in IBS fits with a pathophysiologic concept integrating the intestinal ecosystem, immune activation, intestinal barrier, afferent sensory signaling, and the brain. The intestinal barrier probably has a rather central role in this interplay and may serve as a “surrogate” marker of aberrations in its regulation. Disturbed intestinal barrier function coincides with changes in TJ function and hence altered expression of genes coding for TJ proteins. On a functional level, this can result in increased mucosal permeability. Ex vivo experiments using the Ussing chamber showed an almost twofold increased permeability in colonic biopsies of IBS patients (n=12) compared with healthy controls (n=5, P=0.01), which was correlated with lower expression of ZO-1 mRNA in biopsies from IBS patients (n=21) compared with controls (n=12, P=0.04).70 As described above, further in vitro analysis showed that soluble factors in the colonic epithelium may mediate this effect.70 In addition, Zhou et al.78 found that a subset of diarrhea-predominant IBS patients (21/54, 39%) had signs of increased intestinal permeability using the lactulose/mannitol test, which correlated with an increased Functional Bowel Disorder Severity Index. In this study, patients with increased visceral hypersensitivity and increased permeability had a twofold higher Functional Bowel Disorder Severity Index score compared with patients with normal sensitivity and permeability, and a 16-fold higher score compared with controls.78 A subsequent study found an increased barrier function in 8 out of 19 patients with IBS (42%).79 Mujagic et al.80 assessed the intestinal permeability at different sites of the gastrointestinal tract of IBS patients. After adjusting for confounders, they found an increased small intestinal permeability (lactulose/rhamnose ratio) only in diarrhea-predominant IBS patients, while the colonic permeability was unchanged.
Similar to IBD, alterations of the gut microbiota in IBS also affect butyrate-producing bacteria. Butyrate, a short-chain fatty acid, is an important end-product of intestinal microbial fermentation of mainly dietary fiber. It has been shown to reduce visceral perception in humans.81 This could be linked to beneficial effects on intestinal barrier function by the anti-inflammatory properties of butyrate, via inhibition of nuclear factor-kappa B activation, as well as through the activation of specific G-protein-coupled receptors, GPR41 and GPR43. These receptors are expressed on polymorphonuclear leukocytes highly present in the colonic mucosa and are supposed to have a role in the immune surveillance of the colonic mucosa towards the microbial activity.82
Stress and intestinal barrier function in IBS
As it is well known that acute stress may affect intestinal barrier function negatively,83, 84 the question arises whether this may have a role in the pathogenesis and pathophysiology of visceral hypersensitivity in IBS. Corticotropin-releasing factor and poststress intestinal mast cell activation have the central role in this interplay.85 Although most studies have been using animal models, functional brain imaging by magnetic resonance imaging has opened up new possibilities for pathophysiologic studies in humans. Recently, the role of hyperactivation of corticotropin-releasing factor/corticotropin-releasing factor 1 signaling in IBS patients has been elucidated, as well as the possibility of pharmacologic intervention with a corticotropin-releasing factor 1 receptor antagonist, both by applying functional magnetic resonance imaging.86
The intestinal barrier function has an important part in the pathophysiology of IBS, and its role needs to be further elucidated. There is an unmet need for non-pharmaceutical treatments for patients suffering from this life-long disorder, and nutritional compounds that can strengthen the barrier function could be a promising treatment option. Easy to perform clinical tests to assess barrier function (see section later on) support the identification of subgroups of patients with barrier dysfunctions.
IMPAIRED INTESTINAL BARRIER FUNCTION IN OBESITY
Obesity is classically associated with metabolic alterations related to glucose homeostasis such as glucose intolerance, type 2 diabetes, and insulin resistance, as well as cardiovascular risk factors, including hypertension and dyslipidemia.87 Systemic low-grade inflammation is a hallmark of metabolic syndrome and its related disorders,88 and is directly related to intestinal barrier dysfunction. Investigation is ongoing to determine the factors triggering this low-grade inflammation and to understand how they affect disease progression.
Obesity, impaired barrier function, and metabolic endotoxemia
Gut microbiota-derived lipopolysaccharide (LPS) has been identified as a factor involved in the onset and progression of inflammation and of metabolic diseases associated with obesity (insulin resistance, type 2 diabetes).89 LPS are cell wall components of Gram-negative bacteria. They are potent inducers of inflammation and can initiate severe systemic effects. Under healthy physiological conditions, the intestinal epithelium acts as a barrier that prevents translocation of LPS. Cani et al.89 demonstrated that in mice a high-fat diet resulted in chronically increased plasma LPS levels. A continuous infusion of LPS over 4 weeks in these mice led to a metabolic state comparable to high-fat feeding, including increased macrophage infiltration into the adipose tissue, hepatic steatosis, and an increase in inflammation markers, as well as onset of liver insulin resistance. High LPS plasma levels are defined as metabolic endotoxemia.89
Among the factors explaining metabolic endotoxemia after a high-fat diet, changes in gut barrier function seem to have an especially important role. A high-fat diet led to reduced expression of epithelial TJ proteins in mice.90 In addition, it was shown that high-fat diet feeding reduced mucus layer thickness and impaired anti-microbial peptide production, which could be counteracted by prebiotic treatment as well as treatment with Akkermansia muciniphila.91, 92 The administration of prebiotics improved intestinal barrier function via a proglucagon-derived peptide (GLP-2)-dependent mechanism in genetically obese mice.90 GLP-2 is produced by L-cells and is involved in gut barrier function via TJ protein regulation.90 Muccioli et al.93 showed that the gut microbiota can also regulate gut permeability via the endocannabinoid system, the tone of which is increased in adiposity.
Recent evidence suggests that emulsifiers commonly used in processed food have a negative effect on gut lining by altering the intestinal microbiota composition, which results in increased microbiota encroachment of the intestinal mucus layer94 (for a review see also Cani and Van Hul95).
Lipid-induced metabolic endotoxemia in humans
The relationship between a high-fat diet, obesity, type 2 diabetes, type 1 diabetes, and metabolic endotoxemia is also becoming established in humans. Erridge et al.96 demonstrated in a study with healthy human subjects (n=12) that a high-fat meal induced metabolic endotoxemia, which fluctuated rapidly to concentrations sufficient to induce a significant degree of inflammation. In this study, plasma endotoxin levels increased from 8 to 12 pg/ml after the meal. Amar et al.97 found a positive relationship between energy intake and metabolic endotoxemia in a cohort of 201 healthy men. This relationship between fatty acid ingestion and metabolic endotoxemia has been confirmed in multiple independent studies.98, 99, 100 Furthermore, it has been shown that metabolic endotoxemia increases adipose tissue markers of inflammation such as TNF-α and IL-6, as well as insulin resistance in healthy volunteers.101
Creely et al.102 showed that circulating LPS levels were 76% higher in type 2 diabetic subjects (n=25) compared with controls (n=25, P<0.0001), reinforcing the hypothesis that LPS might act as a gut microbiota-related factor involved in the development of type 2 diabetes and obesity in humans. This was further confirmed by Pussinen et al.103 who investigated the FINRISK97 cohort comprising 7,169 subjects aged 25–74 years, which were followed up for 10 years. Also in this study, a strong relationship between metabolic endotoxemia and type 2 diabetes was found. Both the subjects with prevalent diabetes (n=537) and those with incident diabetes (n=462) had higher metabolic endotoxemia compared with the non-diabetic subjects (P<0.001). In addition, metabolic endotoxemia was significantly associated with an increased risk for incident diabetes (52% increased risk in the highest quartile compared with the lowest one).103 Metabolic endotoxemia positively correlated with several markers of cardiovascular risk factors and type 2 diabetes such as triglycerides, cholesterol, glucose, and insulin in diabetic patients.104
Endotoxemia and gut-derived toxins are suggested to also have causative roles in the onset and progression of liver inflammation and damage in chronic liver diseases in humans.105, 106 This hepatic component of metabolic syndrome involves a complex spectrum of pathological changes, including steatosis, nonalcoholic steatohepatitis, fibrosis, and cirrhosis.107, 108 Changes in TJ protein expression and distribution are suggested as critical factors in the impairment of gut barrier function and subsequent alterations in gut permeability observed in nonalcoholic fatty liver disease patients.109
Although the mechanisms linking metabolic endotoxemia with the gut microbiota remain to be demonstrated in humans, one study has shown a positive correlation between waist circumference and large intestinal permeability markers (6–12 h ratio sucralose/mannitol). Moreover, by combining computed tomography and dual-energy X-ray absorptiometry to measure abdominal fat (visceral and subcutaneous), liver fat, and total body fat, it was shown that gut permeability was significantly and positively correlated with visceral fat and liver fat, but not with subcutaneous or whole-body fat. Thus, this study strongly supports that intestinal permeability may link gut microbiota dysbalance, inflammation, steatosis, and visceral fat accumulation,110 whereas the small intestinal permeability seems undisturbed in obese subjects.110, 111
These findings and future studies will hopefully provide the basis for new therapeutic possibilities for obesity based on diets that can improve intestinal barrier function.
INTESTINAL PERMEABILITY IN CELIAC DISEASE, NON-CELIAC GLUTEN SENSITIVITY, AND FOOD ALLERGY
Intestinal permeability in celiac disease
Celiac disease is an immune-mediated disorder of the small intestine that occurs in genetically susceptible individuals (HLA-DQ2/DQ8 haplotype). It is triggered by an abnormal reaction towards gliadin, a component of gluten proteins found in wheat and related proteins of other grains. Celiac disease is characterized by various degrees of villous atrophy of the small bowel mucosa, malabsorption, and impaired integrity of the small bowel epithelium with increased lymphocytic infiltration.
Celiac disease patients are known to have an abnormal TJ structure112, 113, 114 and increased intestinal permeability.115, 116, 117, 118 An early study by Van Elburg et al.118 showed that even in relatives of patients with celiac disease, an increased intestinal permeability is present, with a mean lactulose/mannitol ratio of 0.243 in celiac disease patients and 0.158 in relatives compared with 0.043 in healthy controls. It is hypothesized that in celiac disease, gliadin passes through the intestinal epithelium into the lamina propria, where it then triggers an immune reaction, whereas a healthy intestinal epithelium is impermeable to gliadin.114 In addition, it has been shown in vitro and in animal studies that gliadin itself increases intestinal permeability by enhancing the release of zonulin,119, 120, 121 presumably via binding to the chemokine receptor CXCR3.122 Zonulin, a protein that reduces intestinal barrier function by modulating TJ proteins (such as ZO-1), is about sixfold increased in the intestinal submucosa of patients with active celiac disease compared with healthy controls.123 Accordingly, ZO-1 is reduced in duodenal biopsies in those patients, an effect which is reversed following a gluten-free diet.113, 114
Intestinal permeability in non-celiac gluten sensitvity
In the past years, it has become more and more recognized that a clinical reaction to food containing gluten can also occur without the involvement of allergic or autoimmune mechanisms. This condition is classified as non-celiac gluten sensitivity.124 It is not completely clear if gluten or other components in wheat are responsible for the symptoms,125 and not much is known about its pathophysiology yet. Gluten-sensitive individuals are negative for anti-tissue transglutaminase antibodies, and epithelial lesions in the small intestine or villous atrophy are absent, but some studies have shown signs of a mild mucosal immune activation.126, 127 Only two small studies have investigated the role of the intestinal permeability in this condition. Sapone et al.126 did not find an increased intestinal permeability in the small bowel of non-celiac gluten-sensitive individuals (n=13) using the lactulose/mannitol test compared with dyspeptic controls (n=14), whereas celiac disease patients (n=11) had a significantly increased permeability (P=0.01). Instead, the permeability was significantly decreased in gluten sensitives compared with the dyspeptic controls (P=0.03), which was paralleled by an increased expression of claudin 4 in jejunal biopsies. Another study measured the increase in permeability of duodenal biopsies ex vivo after exposure to gliadin using a microsnapwell system, and the increase was higher in biopsies from patients with celiac disease (n=6, P<0.05) but not in those from non-celiac gluten-sensitive subjects (n=6) compared with healthy controls (n=5).128 The permeability of biopsies from gluten-sensitive subjects was, however, increased compared with biopsies from patients with celiac disease in remission (n=6, P<0.05). Further studies with larger numbers of participants are necessary before conclusions regarding an increased permeability in non-celiac gluten sensitvity can be drawn.
Intestinal permeablity in food allergy
In children and adults suffering from food allergy or intolerance, an increased intestinal permeability that can persist even in an allergen-free diet has been reported.129, 130, 131, 132 Ventura et al.130 measured the intestinal permeability in 21 patients with food allergy and in 20 patients with food hypersensitivity who had been on an allergen-free diet for 6 months, and found an ~3-fold increase compared with healthy controls (n=40) using the lactulose/mannose test. The intestinal permeability correlated positively with symptom severity. Järvinen et al.132 used the same test to investigate the intestinal permeability in 131 asymptotic children with food allergy, and found that 38% had an increased permeability even though they were on strict elimination diets. It is hypothesized that in those patients, food particles/allergens can cross the epithelial barrier and cause an allergic reaction characterized by mast cell recruitment and allergen-specific IgE production. In turn, inflammatory mediators (cytokines, proteases) lead to further disintegration of barrier function and increased passage of allergens.131 These studies suggest that an increased permeability can be a risk factor for developing food allergy in a subset of patients; however, the increased intestinal permeability could also be a consequence of the allergic reaction, and more clinical studies are needed to investigate this further.
THE EFFECT OF PPIS AND NSAIDS ON INTESTINAL BARRIER FUNCTION
Various drugs have been associated with the occurrence of a disturbed intestinal epithelial barrier function. Of those, proton pump inhibitors (PPIs) and non-steroidal anti-inflammatory drugs (NSAIDs) belong to the most prescribed medications worldwide, and are often administered in combination.
Proton pump inhibitors
PPIs specifically target the proton pumps (H+/K+ ATPases), which are responsible for the gastric acid secretion in the stomach, and also occur in the colon, where they contribute to whole-body potassium homeostasis.133 Inhibition of these colonic proton pumps may therefore affect the local electrolyte balance, thereby compromising fluid acidification, and, consequently, immune reactions in colonic mucosa.134
Effect of PPIs on intestinal barrier function
The PPIs omeprazole and lansoprazole were shown to induce smooth muscle relaxation and to inhibit contractile activity,135 indicating that they do not only affect the proton pumps. In this manner, they may affect the regulation of the TJ complex and hence the intestinal epithelial barrier function, because the TJs are directly linked to the intracellular actin–myosin cytoskeleton. Omeprazole, and to a lesser extent also lansoprazole or esomeprazole, have been shown to cause an increase in paracellular permeability in the mucosa of gastric corpus mucosa of rats in an ex vivo setting.136, 137 This confirmed the earlier observations of Hopkins et al.,138 who observed an increase in the paracellular permeability of rat gastric mucosa upon addition of omeprazole. In GERD (gastroesophageal reflux disease) patients, esomeprazole treatment resulted in a significant increase in upper gastrointestinal permeability as evaluated by the sucrose permeability test in 21 out of 26 patients (84%, P=0.001). In healthy controls, the effects of esomeprazole were shown to be positively correlated with the duration of therapy.139 The use of PPIs thus seems to induce a paracellular transepithelial leak in the gastric corpus, which allows compounds to cross the mucosa. This may be considered as a harmful event; however, it might also have a beneficial application by facilitating the absorption of small-sized drugs or other molecules, which normally could not cross the gastrointestinal barrier or would have been digested in the gastrointestinal lumen before absorption. Gabello et al.136, 140 showed that addition of omeprazole to rat gastric tissue increased the absorption of the peptide bradykinin and of the drug digoxin, whereas passage of the peptide oxytocin and of the drug phenytoin was not affected by the presence of the PPI. Future studies should establish the inclusionary and exclusionary structural criteria of peptide passage through the PPI-induced leak. The possibly increased bioavailability of small-sized structures should be considered when prescribing PPIs to patients who are on multidrug therapy.
Mechanisms behind the effect of PPIs on barrier function
The underlying mechanism of the disturbing effects of PPIs on the intestinal epithelial barrier function is poorly understood. Given the fact that PPI intake is not associated with serious adverse effects, it is unlikely that PPIs induce cell death. It has been shown in an in vitro study that the PPIs omeprazole, lansoprazole, and SCH 28080 (2-methyl-8-(phenylmethoxy)-imidazo[1,2-a]pyridine-3-acetonitrile) decreased the contractile response of rat vas deferens to electrical stimulation. This effect was at least, in part, due to an inhibitory effect of the PPIs on Ca2+ entry into the cells.141 Similarly, omeprazole and lansoprazole inhibited spontaneous contractions and caused dose-dependent relaxation of smooth muscle in guinea-pigs. As the contraction induced by the addition of Ca2+ was completely relaxed by omeprazole and lansoprazole, calcium channel blockade by the PPIs seemed to be involved in this effect. Calcium influx in cells is known to modulate contractions of the intracellular cytoskeleton. Hence, the effect of PPIs on calcium homeostasis probably has a role in the effects of PPIs on the regulation of intestinal epithelial barrier function. The effects on local potassium homeostasis, which may affect mucosal immune activation as mentioned above, may also contribute to the modulation of this barrier function. Mullin et al.139 postulated that we should consider the fact that PPIs directly act on a phosphatase. It could be that PPIs do not only inhibit the H,K-ATP-ase but also other phosphatases. As phosphatase-mediated dephosphorylation of specific TJ proteins is known to alter TJ permeability, this may contribute to the observed disturbances in PPI-induced barrier dysfunction.
Non-steroidal anti-inflammatory drugs
NSAID therapy is associated with the occurrence of serious adverse effects, such as epigastric pain, abdominal pain, constipation, abdominal distension, mucosal inflammation, erythema, erosions, and ulcers.142, 143 In severe cases, it may induce bleeding, ileus, and perforation. The prevalence of NSAID-induced enteropathy (determined by means of fecal occult blood test, assessment of intestinal inflammation, and intestinal permeability) is observed in 19–72% of patients.144 Acetylsalicylic acid causes less damage to intestinal mucosa because, in contrast to the other NSAIDs, it does not undergo enterohepatic circulation.145
Effect of NSAIDs on intestinal barrier function and possible mechanisms behind
The pathogenesis of NSAIDs is well investigated, but still not fully understood. It has been suggested that the toxic topical effect of NSAIDs has separate phases. First, NSAIDs are incorporated into biological membranes because of their lipophilic properties. They interact with brush border phospholipids, thereby causing direct damage to intestinal epithelium.146 NSAIDs also uncouple oxidative phosphorylation, which leads to mitochondrial dysfunction and, consequently, to a reduction in intracellular ATP.147, 148 This ATP depletion results in a decreased intestinal epithelial barrier function, as the regulation of the intracellular actin–myosin complex is an ATP-dependent process. The modulation of membrane phospholipids and intracellular ATP levels are followed by leakage of intracellular calcium and increased production of free oxygen radicals. Taken together, these processes will directly modulate intestinal permeability by affecting the contraction of the intracellular cytoskeleton, and the integrity of the TJ complex. This increased permeability subsequently induces the last phase of NSAID-induced enteropathy, which is infiltration of luminal compounds (such as bile acids, bacterial breakdown products, acid, pepsin) into the intestinal mucosa, leading to immune activation and, in some cases, inflammation.149, 150, 151 The inflammation may then progress to erosions and ulcers, which, in turn, can lead to bleeding and perforations.
In addition to the phases mentioned above, which have a topical effect leading to mucosal damage, NSAIDs can also induce mucosal damage by its prostaglandin-inhibiting properties. After absorption, NSAIDs inhibit cyclooxygenase-1 and -2 (COX-1 and -2). COX-1 inhibition leads to a decrease in mucosal blood flow, whereas inhibition of COX-2 probably has an effect on immune modulation.152
The intestinal barrier function is disturbed in 60–80% of patients using NSAID therapy.144, 153, 154, 155 Some investigators believe that especially bacteria and their breakdown products are responsible for the initial inflammatory response, because of their neutrophil chemoattractant properties.151, 156 Increased intestinal epithelial permeability, which occurs within hours after ingestion of virtually all conventional NSAIDs, is associated with inflammatory enteropathy,156 and with significant complications.157, 158 At present, there is no consensus on the best strategy to prevent this damage. Several studies showed promising results, but follow-up confirmation studies are lacking. Prostaglandin administration may inhibit NSAID-induced enteropathy, especially during a short-term therapy.159, 160, 161 The topical administration of ATP by nasointestinal tube significantly reduced the intestinal permeability increase after indomethacin intake to control levels in healthy volunteers, measured using the lactulose/rhamnose test (P<0.01).162 Enteric-coated capsules containing ATP however showed no effect.163 Also, the intake of recombinant human lactoferrin reduced the NSAID-mediated increase in small intestinal permeability from 0.036 to 0.028 (lactulose/rhamnose ratio, P<0.05).164
CLINICAL ASSESSMENT OF THE INTESTINAL BARRIER FUNCTION
A variety of methods are currently used to assess the intestinal barrier function, all of which come with their own advantages and drawbacks. It is important to note that each method is specific for a certain section of the gastrointestinal tract and measures different functional aspects of epithelial integrity of the intestine.
For an in vivo assessment of the barrier function, intestinal permeability assays using orally administered, non-degradable sugars or other molecules such as [51Cr]EDTA or polyethylene glycol can be used. Common methods use probes of two different sizes. The larger size molecule (e.g., lactulose) can only cross the intestinal barrier by paracellular passage if it is compromised, and is not taken up actively. The smaller molecule (e.g., rhamnose) crosses the epithelial barrier transcellularly and acts as a control for gastric emptying and dilution, transit time, and epithelial absorptive area, as well as systemic distribution and renal function. The urinary excretion ratio is then used as a standardized assessment of intestinal permeability of the intestinal segment where the permeability probes are absorbed. Nowadays, small bowel and colon permeability are mostly analyzed using multisugar tests.165 The noninvasiveness of this method is certainly an advantage; however, the analytical analysis by high pressure liquid chromatography or liquid chromatography and mass spectrometry is rather laborious and necessitates advanced laboratory skills.
In addition, there are several biomarkers that can be measured in the blood that can act as indicators of deteriorated barrier function. These include, for example, LPS and intestinal fatty acid-binding protein (for more detail refer to van Wijck et al.165 and Bischoff et al.1). Different to measurements in portal vein blood of animals, LPS measurements in peripheral blood of humans is still a technical challenge and results need be interpreted with caution.1
The most established system to assess intestinal permeability ex vivo is the Ussing chamber, which is commonly used to measure the transport of ions, nutrients, and drugs across various epithelial tissues such as intestinal biopsies or tissue specimen.166 It allows for a more complex experimental setup than in vivo methods, but is invasive due to the need for fresh intestinal tissue specimens. Furthermore, the method is highly dependent on experimental and laboratory skills and very labor intensive.
CONCLUSION
Maintenance of the intestinal barrier function is important for our health, and a dysfunction is a risk factor for a variety of disorders and diseases. Various viral and bacterial pathogens exert their harmful effects through modulating TJ proteins, thus disrupting intestinal permeability and facilitating their access to the host. The inflammatory processes occurring in the intestinal epithelium in IBD are associated with a clearly damaged intestinal barrier. It is still unknown if the disrupted barrier is a consequence of the ongoing inflammation, or if it is an independent process involved in the pathophysiology of IBD. On the one hand, animal studies have shown that a primary gut barrier defect can lead to inflammation, and on the other hand, IBD can also develop in the presence of an intact barrier, with T-cell regulation being a critical factor. Genetic variants of genes involved in barrier function are associated with the risk of developing IBD; however, environmental factors such as the gut microbiota composition seem to have an even more important role. In IBS, the involvement of the intestinal barrier is not as clear as in IBD, but at least a subset of patients present with increased intestinal permeability. There is evidence that the intestinal barrier has a central role in the pathophysiological concept of a dysregulated microbe–gut–brain axis in IBS. In addition, an altered gut microbiota, which is often found in IBS, as well as the presence of specific bacterial metabolites or other soluble factors can influence intestinal permeability.
In animal models, gut microbiota-derived LPS, which passes through a dysfunctional intestinal barrier, has been shown to be one of the triggering factors in the development of obesity and associated disorders by contributing to a low-grade inflammatory state with systemic effects. Even though there is increasing evidence in humans that points to an altered gut barrier in obesity, the link between intestinal permeability, increased metabolic endotoxemia, inflammation, and the development of obesity still remains to be demonstrated. In celiac disease and food allergy, an increased intestinal permeability is clearly established and is known to have a major role in their pathophysiology.
Also, other disorders that were not discussed in this review have been associated with a disturbed intestinal barrier function. An increased intestinal permeability in children with autism spectrum disorder as well as their first-degree relatives could be shown in one study.167 Also in depression, a disturbed intestinal barrier seems to a have a role. Significantly increased serum levels of IgM and IgA towards LPS derived from Gram-negative bacteria belonging to the normal commensal gut microbiota have been detected in patients with chronic depression, suggesting that there is an increased permeability in the gut of depressed patients facilitating bacterial translocation.168 A ‘leaky gut' is also hypothesized to be involved in the pathophysiology of other psychological disorders such as schizophrenia, even though concrete evidence is still missing.169, 170, 171 Additional factors known to affect intestinal permeability include alcohol abuse,172 strenuous exercise,173, 174 and enteral feeding.175
Hopefully, future studies will increase our knowledge on how intestinal barrier dysfunction is caused and how it can be prevented or restored, providing new therapeutic strategies for a variety of diseases.
Acknowledgments
All authors contributed to discussions and had input into writing the article. JK had responsibility for producing the final version of the article, and is the corresponding author (Email: publications@ilsieurope.be). ILSI Europe facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work. For further information about ILSI Europe, please email info@ilsieurope.be or call +32 2 771 00 14.
Financial support: This work was conducted by an expert group of the European branch of the International Life Sciences Institute, ILSI Europe. The expert group received funding from the ILSI Europe Probiotics Task Force. Industry members of this task force are listed on the ILSI Europe website at www.ilsi.eu/task-forces/nutrition/probiotics/. Experts are not paid for the time spent on this work; however, the non-industry members within the expert group were offered support for travel and accommodation costs to attend meetings to discuss the manuscript, and a small compensatory sum (honoraria) with the option to decline. The expert group carried out the work, i.e. collecting/analysing data/information and writing the scientific paper separate to other activities of the task forces. The research reported is the result of a scientific evaluation in line with ILSI Europe's framework to provide a precompetitive setting for public-private partnership (PPP). The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies.
References
- Bischoff SC, Barbara G, Buurman W et al. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol 2014; 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörfel M, Huber O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. Biomed Res Int 2012; 2012: 807356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörfel MJ, Huber O. A phosphorylation hotspot within the occludin C-terminal domain. Ann NY Acad Sci 2012; 1257: 38–44. [DOI] [PubMed] [Google Scholar]
- Petecchia L, Sabatini F, Usai C et al. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab Invest 2012; 92: 1140–1148. [DOI] [PubMed] [Google Scholar]
- Al-Sadi R, Guo S, Ye D et al. TNF-a modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol 2013; 183: 1871–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R, Guo S, Ye D et al. Mechanism of IL-1b modulation of intestinal epithelial barrier involves p38 kinase and activating transcription factor-2 activation. J Immunol 2013; 190: 6596–6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci USA 2007; 104: 819–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Tsukamoto T, Sun A et al. A role for intracellular calcium in tight junction reassembly after ATP depletion-repletion. Am J Physiol Physiol 1999; 277: F524–F532. [DOI] [PubMed] [Google Scholar]
- Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci 2008; 13: 7210–7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Rill BK, Carlson SL et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol Physiol 1997; 273: C1378–C1385. [DOI] [PubMed] [Google Scholar]
- Lapointe TK, O'Connor PM, Buret AG. The role of epithelial malfunction in the pathogenesis of enteropathogenic E. coli-induced diarrhea. Lab Invest 2009; 89: 964–970. [DOI] [PubMed] [Google Scholar]
- Frankel G, Phillips AD. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell Microbiol 2008; 10: 549–556. [DOI] [PubMed] [Google Scholar]
- Muza-Moons MM, Schneeberger EE, Hecht GA. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol 2004; 6: 783–793. [DOI] [PubMed] [Google Scholar]
- Simonovic I, Arpin M, Koutsouris A et al. Enteropathogenic Escherichia coli activates ezrin, which participates in disruption of tight junction barrier function. Infect Immun 2001; 69: 5679–5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjarrez-Hernandez HA, Amess B, Sellers L et al. Purification of a 20 kDa phosphoprotein from epithelial cells and identification as a myosin light chain phosphorylation induced by enteropathogenic Escherichia coli and phorbol ester. FEBS Lett 1991; 292: 121–127. [DOI] [PubMed] [Google Scholar]
- Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol 2004; 2: 123–140. [DOI] [PubMed] [Google Scholar]
- Amasheh S, Meiri N, Gitter AH et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 2002; 115: 4969–4976. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Milatz S, Krug SM et al. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 2010; 123: 1913–1921. [DOI] [PubMed] [Google Scholar]
- Zeissig S, Bürgel N, Günzel D et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 2007; 56: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankertz J, Amasheh M, Krug SM et al. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res 2009; 336: 67–77. [DOI] [PubMed] [Google Scholar]
- Dubreuil JD. The whole Shebang: the gastrointestinal tract, Escherichia coli enterotoxins and secretion. Curr Issues Mol Biol 2012; 14: 71–82. [PubMed] [Google Scholar]
- Okhuysen PC, DuPont HL. Enteroaggregative Escherichia coli (EAEC): a cause of acute and persistent diarrhea of worldwide importance. J Infect Dis 2010; 202: 503–505. [DOI] [PubMed] [Google Scholar]
- Dubreuil JD. Escherichia coli STb toxin and colibacillosis: knowing is half the battle. FEMS Microbiol Lett 2008; 278: 137–145. [DOI] [PubMed] [Google Scholar]
- Ngendahayo MC, Dubreuil JD. Escherichia coli heat-stable toxin b impairs intestinal epithelial barrier function by altering tight junction proteins. Infect Immun 2013; 81: 2819–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnoli F, Buti L, Tompkins L et al. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci USA 2005; 102: 16339–16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Baudry B, Pumplin DW et al. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA 1991; 88: 5242–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Pandey N, Tamiz AP et al. Mechanism of action of ZOT-derived peptide AT-1002, a tight junction regulator and absorption enhancer. Int J Pharm 2009; 365: 121–130. [DOI] [PubMed] [Google Scholar]
- Goldblum SE, Rai U, Tripathi A et al. The active Zot domain (aa 288–293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J 2011;25:144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski J, Troost FJ, Konings I et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarumin vivo and protective effects on the epithelial barrier. Am J Physiol Liver Physiol 2010; 298: G851–G859. [DOI] [PubMed] [Google Scholar]
- Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 2004; 28: 405–440. [DOI] [PubMed] [Google Scholar]
- McFarland LV. Meta-analysis of probiotics for the prevention of traveler's diarrhea. Travel Med Infect Dis 2007; 5: 97–105. [DOI] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 1995; 270: 1203–1207. [DOI] [PubMed] [Google Scholar]
- Nenci A, Becker C, Wullaert A et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007; 446: 557–561. [DOI] [PubMed] [Google Scholar]
- Guenther C, Martini E, Wittkopf N et al. Caspase-8 regulates TNF-[agr]-induced epithelial necroptosis and terminal ileitis. Nature 2011; 477: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Vereecke L, Bertrand MJM et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 2014; 513: 95–99. [DOI] [PubMed] [Google Scholar]
- Heazlewood CK, Cook MC, Eri R et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 2008; 5: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Lee AH, Franke A et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008; 134: 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis M, De Koning BAE, de Bruijn ACJM et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006; 131: 117–129. [DOI] [PubMed] [Google Scholar]
- Sovran B, Loonen LMP, Lu P et al. IL-22-STAT3 pathway plays a key role in the maintenance of ileal homeostasis in mice lacking secreted mucus barrier. Inflamm Bowel Dis 2015; 21: 531–542. [DOI] [PubMed] [Google Scholar]
- Kuehn R, Loehler J, Rennick D et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993; 75: 263–274. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 2009; 361: 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006; 314: 1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg MS, Cho JH, Rioux JD et al. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet 2009; 41: 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeppi MG, Smith V V, Goldblatt D et al. Colitis in chronic granulomatous disease. Arch Dis Child 2001; 84: 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan GT, Melton SD, Spechler SJ et al. Clinical implications of histologic abnormalities in ileocolonic biopsies of patients with Crohn's disease in remission. J Clin Gastroenterol 2016. http://journals.lww.com/jcge/Abstract/publishahead/Clinical_Implications_of_Histologic_Abnormalities.98321.aspx. [DOI] [PubMed]
- Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn's disease. Gastroenterology 1996; 110: 1395–1403. [DOI] [PubMed] [Google Scholar]
- Sinha A, Nightingale JMD, West KP et al. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med 2003; 349: 350–357. [DOI] [PubMed] [Google Scholar]
- Salim SY, Soederholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis 2011; 17: 362–381. [DOI] [PubMed] [Google Scholar]
- Miquel S, Martin R, Rossi O et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 2013; 16: 255–261. [DOI] [PubMed] [Google Scholar]
- Finnie IA, Dwarakanath AD, Taylor BA et al. Colonic mucin synthesis is increased by sodium butyrate. Gut 1995; 36: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011; 140: 1720–1728. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Yang H et al. Contribution of the IBD5 locus to inflammatory bowel disease: a meta-analysis. Hum Genet 2011; 129: 597–609. [DOI] [PubMed] [Google Scholar]
- Noble CL, Nimmo ER, Drummond H et al. The contribution of OCTN1/2 variants within the IBD5 locus to disease susceptibility and severity in Crohn's disease. Gastroenterology 2005; 129: 1854–1864. [DOI] [PubMed] [Google Scholar]
- Tremelling M, Waller S, Bredin F et al. Genetic variants in TNF-alpha but not DLG5 are associated with inflammatory bowel disease in a large United Kingdom cohort. Inflamm Bowel Dis 2006; 12: 178–184. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Boucher G, Lees CW et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 2011; 43: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsigny M, Babeu JP, Dupuis AA et al. Loss of hepatocyte-nuclear-factor-4alpha affects colonic ion transport and causes chronic inflammation resembling inflammatory bowel disease in mice. PLoS One 2009; 4: e7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 2001; 411: 599–603. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Ladhoff A, Pernthaler A et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002; 122: 44–54. [DOI] [PubMed] [Google Scholar]
- Strober W, Asano N, Fuss I et al. Cellular and molecular mechanisms underlying NOD2 risk-associated polymorphisms in Crohn's disease. Immunol Rev 2014; 260: 249–260. [DOI] [PubMed] [Google Scholar]
- Brooke MA, Longhurst HJ, Plagnol V et al. Cryptogenic multifocal ulcerating stenosing enteritis associated with homozygous deletion mutations in cytosolic phospholipase A2-α. Gut 2012; 63: 96–104. [DOI] [PubMed] [Google Scholar]
- Delvaux M. Functional bowel disorders and irritable bowel syndrome in Europe. Aliment Pharmacol Ther 2003; 18: 75–79. [DOI] [PubMed] [Google Scholar]
- Ghoshal UC, Ranjan P. Post-infectious irritable bowel syndrome: the past, the present and the future. J Gastroenterol Hepatol 2011; 26: 94–101. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Hamdani N et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014; 264: 651–660. [DOI] [PubMed] [Google Scholar]
- Levy RL, Olden KW, Naliboff BD et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology 2006; 130: 1447–1458. [DOI] [PubMed] [Google Scholar]
- Spiller R, Lam C. An update on post-infectious irritable bowel syndrome: role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil 2012; 18: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol 2011; 46: 421–431. [DOI] [PubMed] [Google Scholar]
- Steck N, Mueller K, Schemann M et al. Republished: bacterial proteases in IBD and IBS. Postgrad Med J 2013; 89: 25–33. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry 2000; 62: 28–36. [PubMed] [Google Scholar]
- McKernan DP, Gaszner G, Quigley EM et al. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther 2011; 33: 1045–1052. [DOI] [PubMed] [Google Scholar]
- Piche T, Barbara G, Aubert P et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 2009; 58: 196–201. [DOI] [PubMed] [Google Scholar]
- Han W, Lu X, Jia X et al. Soluble mediators released from PI-IBS patients' colon induced alteration of mast cell: involvement of reactive oxygen species. Dig Dis Sci 2012; 57: 311–319. [DOI] [PubMed] [Google Scholar]
- Chassard C, Dapoigny M, Scott KP et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther 2012; 35: 828–838. [DOI] [PubMed] [Google Scholar]
- Koenig J, Brummer RJ. Alteration of the intestinal microbiota as a cause of and a potential therapeutic option in irritable bowel syndrome. Benef Microbes 2014; 5: 247–261. [DOI] [PubMed] [Google Scholar]
- Rajilic-Stojanovic M, Biagi E, Heilig HGHJ et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011; 141: 1792–1801. [DOI] [PubMed] [Google Scholar]
- Saulnier DM, Riehle K, Mistretta T et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011; 141: 1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin J, Rangel I, Fuentes S et al. Altered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment Pharmacol Ther 2015; 41: 342–351. [DOI] [PubMed] [Google Scholar]
- Brint EK, MacSharry J, Fanning A et al. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol 2011; 106: 329–336. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhang B, Nicholas Verne G. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009; 146: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Souba WW, Croce CM et al. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010; 59: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujagic Z, Ludidi S, Keszthelyi D et al. Small intestinal permeability is increased in diarrhea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther 2014; 40: 288–297. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Jonkers DMAE, Bast A et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr 2009; 28: 88–93. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K et al. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 2008; 27: 104–119. [DOI] [PubMed] [Google Scholar]
- Wallon C, Yang PC, Keita AV et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 2008; 57: 50–58. [DOI] [PubMed] [Google Scholar]
- Vanuytsel T, van Wanrooy S, Vanheel H et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014; 63: 1293–1299. [DOI] [PubMed] [Google Scholar]
- Larauche M. Novel insights in the role of peripheral corticotropin-releasing factor and mast cells in stress-induced visceral hypersensitivity. Neurogastroenterol Motil 2012; 24: 201–205. [DOI] [PubMed] [Google Scholar]
- Hubbard CS, Labus JS, Bueller J et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional–arousal circuit during expectation of abdominal pain. J Neurosci 2011; 31: 12491–12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH. Obesity. Circulation 2005; 111: 257–259. [DOI] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72: 219–246. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56: 1761–1772. [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57: 1470–1481. [DOI] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 2013; 110: 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A, Geurts L, Caesar R et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun 2014; 5: 5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli GG, Naslain D, Backhed F et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 2010; 6: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Koren O, Goodrich JK et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015; 519: 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Van Hul M. Novel opportunities for next-generation probiotics targeting metabolic syndrome. Curr Opin Biotechnol 2015; 32: 21–27. [DOI] [PubMed] [Google Scholar]
- Erridge C, Attina T, Spickett CM et al. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007; 86: 1286–1292. [DOI] [PubMed] [Google Scholar]
- Amar J, Burcelin R, Ruidavets JB et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008; 87: 1219–1223. [DOI] [PubMed] [Google Scholar]
- Laugerette F, Vors C, Geloen A et al. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem 2011; 22: 53–59. [DOI] [PubMed] [Google Scholar]
- Deopurkar R, Ghanim H, Friedman J et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 2010; 33: 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanim H, Abuaysheh S, Sia CL et al. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 2009; 32: 2281–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PD, Mehta NN, Wolfe ML et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab 2007; 92: 2272–2279. [DOI] [PubMed] [Google Scholar]
- Creely SJ, McTernan PG, Kusminski CM et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007; 292: E740–E747. [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Havulinna AS, Lehto M et al. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 2011; 34: 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attas OS, Al-Daghri NM, Al-Rubeaan K et al. Changes in endotoxin levels in T2DM subjects on anti-diabetic therapies. Cardiovasc Diabetol 2009; 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JP. The role of endotoxin in liver injury. Gastroenterology 1975; 69: 1346–1356. [PubMed] [Google Scholar]
- Nolan JP, Leibowitz AI. Endotoxins in liver disease. Gastroenterology 1978; 75: 765–766. [PubMed] [Google Scholar]
- Bedogni G, Miglioli L, Masutti F et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 2005; 42: 44–52. [DOI] [PubMed] [Google Scholar]
- Farhadi A, Gundlapalli S, Shaikh M et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int 2008; 28: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele L, Valenza V, La TG et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009; 49: 1877–1887. [DOI] [PubMed] [Google Scholar]
- Gummesson A, Carlsson LM, Storlien LH et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity 2011; 19: 2280–2282. [DOI] [PubMed] [Google Scholar]
- Brignardello J, Morales P, Diaz E et al. Pilot study: alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther 2010; 32: 1307–1314. [DOI] [PubMed] [Google Scholar]
- Schulzke JD, Bentzel CJ, Schulzke I et al. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res 1998; 43: 435–441. [DOI] [PubMed] [Google Scholar]
- Montalto M, Cuoco L, Ricci R et al. Immunohistochemical analysis of ZO-1 in the duodenal mucosa of patients with untreated and treated celiac disease. Digestion 2002; 65: 227–233. [DOI] [PubMed] [Google Scholar]
- Pizzuti D, Bortolami M, Mazzon E et al. Transcriptional downregulation of tight junction protein ZO-1 in active celiac disease is reversed after a gluten-free diet. Dig Liver Dis 2004; 36: 337–341. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Peters T, Veall N. A persistent defect in intestinal permeability in celiac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet 1983; 321: 323–325. [DOI] [PubMed] [Google Scholar]
- Duerksen DR, Wilhelm-Boyles C, Parry DM. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig Dis Sci 2005; 50: 785–790. [DOI] [PubMed] [Google Scholar]
- Heyman M, Abed J, Lebreton C et al. Intestinal permeability in celiac disease: insight into mechanisms and relevance to pathogenesis. Gut 2012; 61: 1355–1364. [DOI] [PubMed] [Google Scholar]
- Van Elburg RM, Uil JJ, Mulder CJ et al. Intestinal permeability in patients with celiac disease and relatives of patients with celiac disease. Gut 1993; 34: 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago S, El Asmar R, Di Pierro M et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 2006; 41: 408–419. [DOI] [PubMed] [Google Scholar]
- Sander GR, Cummins AG, Powell BC. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett 2005; 579: 4851–4855. [DOI] [PubMed] [Google Scholar]
- Clemente MG, De Virgiliis S, Kang JS et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 2003; 52: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers KM, Lu R, Brownley J et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008; 135: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Not T, Wang W et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in celiac disease. Lancet 2000; 355: 1518–1519. [DOI] [PubMed] [Google Scholar]
- Sapone A, Bai JC, Ciacci C et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med 2012; 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebener S, Tanaka CK, Uhde M et al. Specific nongluten proteins of wheat are novel target antigens in celiac disease humoral response. J Proteome Res 2015; 14: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapone A, Lammers KM, Casolaro V et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med 2011; 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brottveit M, Beitnes A-CR, Tollefsen S et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol 2013; 108: 842–850. [DOI] [PubMed] [Google Scholar]
- Hollon J, Puppa EL, Greenwald B et al. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015; 7: 1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre C, Andre F, Colin L et al. Measurement of intestinal permeability to mannitol and lactulose as a means of diagnosing food allergy and evaluating therapeutic effectiveness of disodium cromoglycate. Ann Allergy 1987; 59: 127–130. [PubMed] [Google Scholar]
- Ventura MT, Polimeno L, Amoruso AC et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis 2006; 38: 732–736. [DOI] [PubMed] [Google Scholar]
- Perrier C, Corthesy B. Gut permeability and food allergies. Clin Exp Allergy 2011; 41: 20–28. [DOI] [PubMed] [Google Scholar]
- Jaervinen KM, Konstantinou GN, Pilapil M et al. Intestinal permeability in children with food allergy on specific elimination diets. Pediatr Allergy Immunol 2013; 24: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin JM, Gabello M, Murray LJ et al. Proton pump inhibitors: actions and reactions. Drug Discov Today 2009; 14: 647–660. [DOI] [PubMed] [Google Scholar]
- Keszthelyi D, Jansen S V, Schouten GA et al. Proton pump inhibitor use is associated with an increased risk for microscopic colitis: a case–control study. Aliment Pharmacol Ther 2010; 32: 1124–1128. [DOI] [PubMed] [Google Scholar]
- Aydin C, Sarac B, Koyuncu A et al. Relaxant effect of omeprazole and lansoprazole in guinea pig gallbladder muscle strips in vitro. J Gastroenterol 2003; 38: 765–771. [DOI] [PubMed] [Google Scholar]
- Gabello M, Valenzano MC, Zurbach EP et al. Omeprazole induces gastric transmucosal permeability to the peptide bradykinin. World J Gastroenterol 2010; 16: 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LJ, Gabello M, Rudolph DS et al. Transmucosal gastric leak induced by proton pump inhibitors. Dig Dis Sci 2009; 54: 1408–1417. [DOI] [PubMed] [Google Scholar]
- Hopkins AM, McDonnell C, Breslin NP et al. Omeprazole increases permeability across isolated rat gastric mucosa pre-treated with an acid secretagogue. J Pharm Pharmacol 2002; 54: 341–347. [DOI] [PubMed] [Google Scholar]
- Mullin JM, Valenzano MC, Whitby M et al. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther 2008; 28: 1317–1325. [DOI] [PubMed] [Google Scholar]
- Gabello M, Valenzano MC, Barr M et al. Omeprazole induces gastric permeability to digoxin. Dig Dis Sci 2010; 55: 1255–1263. [DOI] [PubMed] [Google Scholar]
- Yenioehirli A, Onur R. Specific H+/K+-ATPase inhibitors decreased contractile responses of isolated rat vas deferens. Pharmacol Res 2006; 54: 397–405. [DOI] [PubMed] [Google Scholar]
- Tachechi I, Kopacova M, Rejchrt S et al. Non-steroidal anti-inflammatory drug induced injury to the small intestine. Acta Med 2010; 53: 3–11. [PubMed] [Google Scholar]
- Higuchi K, Umegaki E, Watanabe T et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol 2009; 44: 879–888. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, So A, Levi AJ et al. Intestinal permeability and inflammation in rheumatoid arthritis: effects of non-steroidal anti-inflammatory drugs. Lancet 1984; 324: 1171–1174. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Price AB, Zanelli G et al. Clinicopathological features of nonsteroidal antiinflammatory drug-induced small intestinal strictures. Gastroenterology 1988; 94: 1070–1074. [DOI] [PubMed] [Google Scholar]
- Darling RL, Romero JJ, Dial EJ et al. The effects of aspirin on gastric mucosal integrity, surface hydrophobicity, and prostaglandin metabolism in cyclooxygenase knockout mice. Gastroenterology 2004; 127: 94–104. [DOI] [PubMed] [Google Scholar]
- Somasundaram S, Sigthorsson G, Simpson RJ et al. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment Pharmacol Ther 2000; 14: 639. [DOI] [PubMed] [Google Scholar]
- Somasundaram S, Rafi S, Hayllar J et al. Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut 1997; 41: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortun PJ, Hawkey CJ. Nonsteroidal antiinflammatory drugs and the small intestine. Curr Opin Gastroenterol 2005; 21: 169–175. [DOI] [PubMed] [Google Scholar]
- Reuter BK, Davies NM, Wallace JL. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology 1997; 112: 109–117. [DOI] [PubMed] [Google Scholar]
- Scarpignato C. NSAID-induced intestinal damage: are luminal bacteria the therapeutic target? Gut 2008; 57: 145–148. [DOI] [PubMed] [Google Scholar]
- Whittle BJR. Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur J Pharmacol 2004; 500: 427–439. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Williams P, Smethurst P et al. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut 1986; 27: 1292–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigthorsson G, Simpson RJ, Walley M et al. COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology 2002; 122: 1913–1923. [DOI] [PubMed] [Google Scholar]
- Smecuol E, Bai JC, Sugai E et al. Acute gastrointestinal permeability responses to different non-steroidal anti-inflammatory drugs. Gut 2001; 49: 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J. Gastroenterol. 2009; 44: 23–29. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Prouse P, Smith T et al. Blood and protein loss via small-intestinal inflammation induced by non-steroidal anti-inflammatory drugs. Lancet 1987; 330: 711–714. [DOI] [PubMed] [Google Scholar]
- Langman MJ, Morgan L, Worrall A. Use of anti-inflammatory drugs by patients admitted with small or large bowel perforations and haemorrhage. BMJ 1985; 290: 347–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I, Smethurst P, Fenn CG et al. Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci 1989; 34: 407–411. [DOI] [PubMed] [Google Scholar]
- Davies GR, Wilkie ME, Rampton DS. Effects of metronidazole and misoprostol on indomethacin-induced changes in intestinal permeability. Dig Dis Sci 1993; 38: 417–425. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Murray L, Sturrock RD et al. Short report: the effect of misoprostol on the anaemia of NSAID enteropathy. Aliment Pharmacol Ther 1994; 8: 343–346. [DOI] [PubMed] [Google Scholar]
- Bours MJ, Troost FJ, Brummer R-JM et al. Local effect of adenosine 5′-triphosphate on indomethacin-induced permeability changes in the human small intestine. Eur J Gastroenterol Hepatol 2007; 19: 245–250. [DOI] [PubMed] [Google Scholar]
- Bours MJL, Bos HJ, Meddings JB et al. Effects of oral adenosine 5′-triphosphate and adenosine in enteric-coated capsules on indomethacin-induced permeability changes in the human small intestine: a randomized cross-over study. BMC Gastroenterol 2007; 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troost FJ, Saris WHM, Brummer RJM. Recombinant human lactoferrin ingestion attenuates indomethacin-induced enteropathy in vivo in healthy volunteers. Eur J Clin Nutr 2003; 57: 1579–1585. [DOI] [PubMed] [Google Scholar]
- van Wijck K, Verlinden TJM, van Eijk HMH et al. Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: a randomized controlled crossover trial. Clin Nutr 2013; 32: 245–251. [DOI] [PubMed] [Google Scholar]
- Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 2009; 296: G1151–G1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magistris L, Familiari V, Pascotto A et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 2010; 51: 418–424. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis J-C et al. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord 2012; 141: 55–62. [DOI] [PubMed] [Google Scholar]
- Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res 2016; 176: 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res 2013; 148: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Williams LJ, Jacka FN et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013; 11: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1984; 1: 179–182. [DOI] [PubMed] [Google Scholar]
- Pals KL, Chang RT, Ryan AJ et al. Effect of running intensity on intestinal permeability. J Appl Physiol 1997; 82: 571–576. [DOI] [PubMed] [Google Scholar]
- van Nieuwenhoven MA, Brouns F, Brummer RJ. Gastrointestinal profile of symptomatic athletes at rest and during physical exercise. Eur J Appl Physiol 2004; 91: 429–434. [DOI] [PubMed] [Google Scholar]
- Maxton DG, Menzies IS, Slavin B et al. Small-intestinal function during enteral feeding and starvation in man. Clin Sci 1989; 77: 401–406. [DOI] [PubMed] [Google Scholar]
- Dean P, Kenny B. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol Microbiol 2004; 54: 665–675. [DOI] [PubMed] [Google Scholar]
- Viswanathan VK, Koutsouris A, Lukic S et al. Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infect Immun 2004; 72: 3218–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxas JL, Koutsouris A, Bellmeyer A et al. Enterohemorrhagic E. coli alters murine intestinal epithelial tight junction protein expression and barrier function in a Shiga toxin independent manner. Lab Investig 2010; 90: 1152–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen LS, Paesold G, Marcus SL et al. Modulation of chloride secretory responses and barrier function of intestinal epithelial cells by the Salmonella effector protein SigD. Am J Physiol Physiol 2004; 287: C939–C948. [DOI] [PubMed] [Google Scholar]
- Boyle EC, Brown NF, Finlay BB. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell Microbiol 2006; 8: 1946–1957. [DOI] [PubMed] [Google Scholar]
- Amieva MR, Vogelmann R, Covacci A et al. Disruption of the epithelial apical–junctional complex by Helicobacter pylori CagA. Science 2003; 300: 1430–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger S, Hundertmark T, Kuester D et al. Helicobacter pylori alters the distribution of ZO-1 and p120ctn in primary human gastric epithelial cells. Pathol Pract 2007; 203: 433–444. [DOI] [PubMed] [Google Scholar]
- Romanov V, Whyard TC, Waltzer WC et al. A claudin 3 and claudin 4-targeted Clostridium perfringens protoxin is selectively cytotoxic to PSA-producing prostate cancer cells. Cancer Lett 2014; 351: 260–264. [DOI] [PubMed] [Google Scholar]
- Forrest JC, Campbell JA, Schelling P et al. Structure–function analysis of reovirus binding to junctional adhesion molecule 1 implications for the mechanism of reovirus attachment. J Biol Chem 2003; 278: 48434–48444. [DOI] [PubMed] [Google Scholar]
- Lopez S, Arias CF. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol 2004; 12: 271–278. [DOI] [PubMed] [Google Scholar]
- Nava P, Lopez S, Arias CF et al. The rotavirus surface protein VP8 modulates the gate and fence function of tight junctions in epithelial cells. J Cell Sci 2004; 117: 5509–5519. [DOI] [PubMed] [Google Scholar]