Abstract
Cyclic vomiting syndrome (CVS) is an uncommon, idiopathic disorder defined by recurrent, sudden-onset attacks of repetitive retching and vomiting that are separated by symptom-free intervals. CVS was long regarded as a disorder primarily experienced by children but is now known to present de novo in adulthood. Adult CVS has garnered more research attention over the past 20 years, and these efforts have identified some acute and prophylactic treatments for this disorder. However, CVS still lacks a unifying disease model, and this has hindered the development of new therapies. Here adult CVS is reframed as a neurogenic disorder, driven by various endophenotypic factors that shape patterns of activity within the neural circuits required for disease expression. The concept of the “CVS threshold” is put forth in parallel with exploring the remarkable similarity of adult CVS with features of chronic migraine, epilepsy, and panic disorder. Because of such shared neural mechanisms and overlapping endophenotypes, many therapies that have been developed for these other disorders could also be useful in managing CVS. This review seeks to achieve three primary aims: (1) to develop a comprehensive, explanatory framework for adult CVS pathogenesis, (2) to use this framework for identifying potentially novel therapies for CVS, and (3) to describe future research directions that are needed to move the field forward.
INTRODUCTION
Cyclic vomiting syndrome (CVS) is an uncommon, idiopathic disorder that, in its most classical form, is characterized by recurrent, sudden-onset attacks of repetitive retching and vomiting that are separated by symptom-free intervals. CVS was long regarded as a disorder primarily experienced by children but is now known to present de novo in adulthood.1, 2 Because of the negative impact on patients' quality of life and the increased clinical recognition of the syndrome, CVS in adults has garnered more research attention over the past 20 years. These research efforts have led to substantial progress in identifying various core clinical features of adult CVS, a range of associated medical conditions, and potential biomarkers of the disorder. Importantly, several therapies have been suggested to be effective in the acute treatment and prophylaxis of CVS attacks, and these therapies currently form the cornerstone of clinical management. However, adult CVS is an uncommon disorder that may present in varied clinical contexts, and individual regional and academic medical centers may only treat relatively small, heterogeneous populations of adult CVS patients. Clinicians with expertise in recognizing and treating CVS in adults are not widely distributed throughout the healthcare system. Thus, patients often suffer for years without receiving a “correct” diagnosis,2 and are offered an array of idiosyncratic and ineffective treatments. The heterogeneity and scarcity of adult patients with CVS poses a significant barrier to performing prospective randomized therapeutic trials in this population. Indeed, such a trial has yet to be performed. Thus, the evidence basis supporting current diagnostic criteria and treatment options for adult CVS has been primarily derived from cross-sectional and mostly retrospective studies in tertiary care populations.
But progress in the field of CVS research has been hampered not only by structural healthcare delivery or organizational problems in conducting clinical trials. A major issue is that CVS remains an idiopathic, syndromic disorder without a pathophysiological explanatory framework. To be clear, several potentially important pathophysiological mechanisms have been identified to date. These putative contributing mechanisms include genetic predispositions,3, 4, 5 physiological abnormalities involving mitochondrial function,6 autonomic regulation,7, 8, 9 or neuroendocrine function,10 as well as co-morbid affective disorders,2, 11, 12 substance abuse,13 or neurological conditions.14, 15 Yet, as a complex syndrome, CVS is unlikely to be driven by any single mechanism that has been identified in such associational studies. Rather, CVS could be characterized as a complex medical disorder with a final common “phenotype” (i.e., recurrent episodes of vomiting), which is driven by the cumulative impact of multiple, discrete endophenotype factors. Endophenotypes are increasingly recognized as typically subclinical traits with potential pathophysiological relevance for a wide range of complex medical disorders.16 Endophenotypes have a strong genetic basis (i.e., either purely heritable or driven by gene-environmental interactions) and lead to some functional biological variation either associated with a specific symptom and/or objective physiological biomarker. A unified disease model for adult CVS proposes that multiple endophenotypes (both those already identified and that remain to be discovered) each contribute risk to drive the cumulative, moment-to-moment probability of developing a CVS attack. The component endophenotypes for complex medical disorders are not necessarily individually pathogenic, nor are they necessarily specific for the disorder. Therefore, CVS could share multiple endophenotypes with other complex medical disorders, yet have a different final phenotype. Viewed in this way, it is not surprising that there are tremendous overlaps between the pathophysiological associations documented in CVS with those described in other recurrent, triggered syndromes such as migraine, epilepsy, or panic disorder.
A comprehensive disease model for CVS, which is based on identifying relevant endophenotypes and comorbidities could potentially shift the mode of therapeutic discovery in adult CVS from one that has been opportunistic and anecdotal, to one that is mechanistic and predictive. Thus, the identification of the presence or absence of core endophenotypes and comorbidities in a specific CVS patient should provide the means to develop targeted, personalized treatments with a high likelihood of success. This review is not intended to be an exhaustive accounting of adult cyclic vomiting syndrome. The reader is referred to other comprehensive reviews of CVS both in children17 and adults1, 18 for more details about disease features, associations, diagnosis, and treatment in these populations. Rather, this review seeks to achieve three primary aims: (1) to develop a comprehensive, explanatory framework for CVS pathogenesis in adults, (2) to use this framework for identifying potential novel therapies for adult CVS, and (3) to describe future research directions needed to move the field forward.
Establishing a paradigm: shared endophenotypes in CVS, migraines, epilepsy, and panic disorder
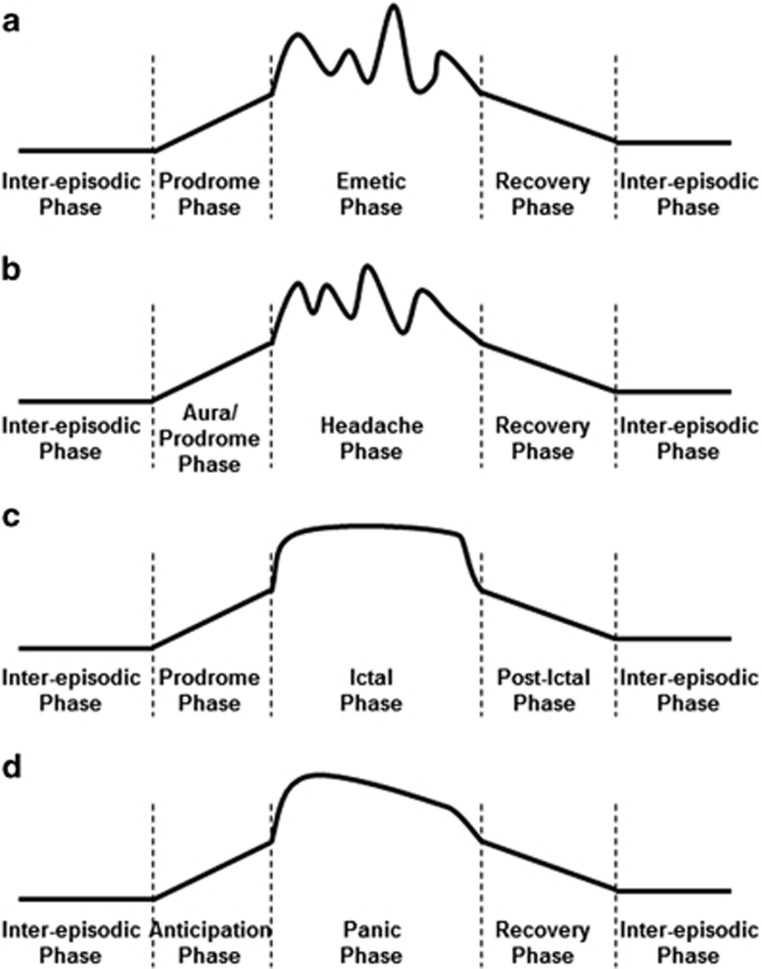
It is now well accepted that CVS is characterized by four primary temporal phases of the disorder: (1) the “well” phase (classically asymptomatic, but not necessarily so), (2) the prodromic phase, (3) the emetic phase, and (4) the recovery phase (Figure 1a). Prototypically, CVS patients experience nausea during the prodromic phase before the abrupt, sudden evolution to the emetic phase.19 However, multiple symptoms other than nausea are often experienced by patients during the prodromic phase through the acute emetic phase and even during the recovery phase.2 These symptoms may be driven by autonomic (salivation, sweating, pallor, flushing, rapid/irregular heartbeat, and diarrhea), affective and cognitive (anxiety/panic, food aversion, irritability, insomnia/restlessness, and depersonalization), constitutional (fatigue, listlessness, light-headedness, intense feeling hot or chilled, and intense thirst), skeletal motor (shivering or shaking, retching, and tachypnea), and sensory (abdominal pains, cramps/bloating, limb paresthesias, hyperesthesia, photophobia, phonophobia, headache, and dyspnea) phenomena.1, 2 There is also evidence that subsets of CVS patients demonstrate dysregulation within one or more of these domains even during the inter-episodic phase.1, 7, 8 Collectively, these observations suggest that CVS is more than a brain-gut disorder characterized by episodes of nausea and repetitive vomiting.1, 20 Rather, CVS could be regarded as a multi-system disorder involving aberrant autonomic, neuroendocrine, affective, cognitive, and sensorimotor function. All of these functions are under some influence by the central nervous system (i.e., the brain). The varied clinical features of CVS predict that aberrant function in the specific neural circuits capable of influencing each of these varied functions is the neurobiological basis of the disorder. Thus, CVS could be reframed as a neurogenic disorder, driven by the cumulative impact of endophenotypic “building blocks” that shape the structure and activity of the neural circuits required for disease expression.
Figure 1.
Overlap in temporal patterns of illness between (a) cyclic vomiting syndrome (CVS), (b) chronic migraine, (c) epilepsy, and (d) panic disorder. Each illness has a quiescent, inter-episodic phase, punctuated by the sudden onset of a prodrome, followed immediately by the acute attack, and then a recovery phase with lingering symptoms before returning to baseline.
Viewed from this neurological perspective, remarkable parallels between CVS, migraine, epilepsy, and panic disorder begin to emerge quite clearly. Each of these distinct illnesses blend into a web of interrelated disorders with shared clinical features due to their shared endophenotypes and mechanisms dependent on neuronal activity within specific neural circuits. The evidence to support this claim will be detailed below. However, it is important to note that much of evidence is derived from smaller, retrospective studies performed in tertiary care centers using cross-sectional, patient reported data, often without control groups. Thus, the literature reporting on patterns of presentation (triggers and prodromic symptoms) and disease associations in CVS, migraine, epilepsy, and panic disorder could be subject to observer, recall, or tertiary care biases. Nonetheless, the similarities in the features reported to be associated with CVS, migraine, seizures, and panic attacks are quite striking.
First, CVS attacks, migraines, seizures, and panic attacks each demonstrate a characteristic episodic pattern of disease with inter-episodic “well” phases, punctuated by prodromic, attack, and recovery phases (Figures 1a–d). Most adult CVS patients report experiencing some form of prodrome,2 as do a substantial number of patients report experiencing individually stereotypical (yet complex) prodromic symptoms before migraines,21 seizures,22, 23 or panic attacks.24 The prodomic symptoms reported by migraineurs and epileptics may vary across autonomic/neuroendocrine, affective, cognitive, and sensorimotor domains as they do for CVS and panic disorder.2, 21, 22, 23, 24, 25 Second, CVS, migraine, epilepsy, and panic disorder all are “triggered” by similar internal and external conditions. For example, an increased sensitivity to acute physiological or psychological stressors,1, 2, 7, 24, 26, 27, 28, 29, 30, 31, 32, 33 impaired sleep,1, 7, 27, 34, 35 hypoglycemia,1, 7, 36, 37 and even hormonal variations during the menstrual cycle,1, 27, 38, 39, 40 among others, may increase the likelihood of developing a CVS, migraine, seizure, or panic attack. Some of these associations may be driven, in part, by mitochondrial dysfunction.5, 41, 42 Third, many patients with CVS, migraines, seizures, or panic attacks experience symptom onset dependent upon a circadian pattern.2, 7, 25, 43, 44, 45 In particular, CVS attacks, migraines, and frontal seizures may cluster in the early AM hours.2, 7, 45, 46 Fourth, prior exposure to adverse or traumatic life events is linked with increased likelihood of developing CVS,1, 2 migraine,47 epilepsy,48 or panic disorder.49 Finally, alterations in both basal autonomic activity and provoked autonomic responses have been observed in many patients during the inter-episodic phase in CVS,7, 8, 9 migraine,26 epilepsy,30, 50 and panic disorder,51 suggesting that impaired allostatic regulation is a common feature of each of these disorders.
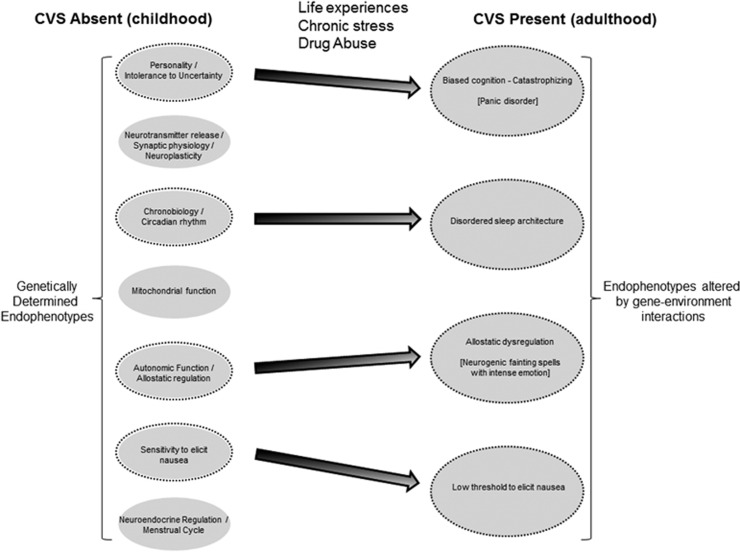
Because of the common endophenotypes shared between CVS, migraine, epilepsy, and panic disorder, individual patients may experience more than one of these disorders during their lifetime. For example, CVS patients often suffer from co-morbid migraines,1 and it is intriguing that patients who initially developed CVS during childhood may undergo a phenotypic switch to become adult migraineurs without CVS.2 Co-morbid anxiety disorders are especially common in those with CVS,2 migraines,26 and epilepsy.30 There is a notably high prevalence of epilepsy in those with chronic migraines, as well as a high prevalence of migraines in those with epilepsy.52 This particular overlap is so clinically prominent that some neurologists have termed the phenomenon “migralepsy,” in part to reinforce the notion that these disorders exist in a spectrum with often overlapping mechanisms.52 One case report even suggested the co-existence of CVS, migraines, and seizures in a young adult patient,15 and another report described EEG-demonstrable epileptic seizures in two adults that presented primarily with acute onset nausea and vomiting.53 Collectively, these observations reinforce the notion that common endophenotypic mechanisms contribute to the expression of CVS, chronic migraines, epilepsy, and anxiety disorders. The endophenotype concept for CVS in adults is further detailed in Figure 2.
Figure 2.
Conceptual model of endophenotypes that mediate the development of CVS in adults. CVS is envisioned as a complex disorder derived from the summative contribution of individual endophenotypes that lead to the final common phenotype. The left side of the figure lists a few potential endophenotypes, which are genetically determined and present in childhood. With life experiences, chronic stress, and/or exposure to drug abuse, and based upon genetic susceptibility to those exposures, some endophenotypic factors may undergo changes over time (represented by black dashed outlines and changes in the size of the ovals). Thus, these changed endophenotypes and symptoms become risk factors that predict the development of adult CVS (right side of the figure). In this particular example, the adult CVS patient has developed disordered cognition and a tendency to catastrophize, as well as comorbid panic disorder. They experience wildly dysregulated autonomic patterns during intense emotional stress, occasionally to the point of causing neurocardiogenic syncope. The patient also has developed subclinical endophenotypes with a disordered sleep architecture and a generally decreased threshold to experience nausea. Other innate endophenotypes on the left side of the figure that are not circled are relatively insensitive to change in this patient. They are present in adulthood (not shown), but do not contribute significantly to CVS in this individual.
Neuronal excitability: a therapeutic target for CVS
There have been tremendous advances in the neurobiological understanding of migraine and epilepsy that could inform our understanding of cyclic vomiting syndrome and suggest new treatments. For example, cortical spreading depression is now recognized as a major pathophysiological process that contributes to the evolution of a migraine.54 This process is driven by increases in basal neuronal hyperexcitability that exceed a critical threshold to initiate waves of neuronal depolarization that ripple through the cerebral cortex, followed by a wake of hyperpolarization/inhibition associated with lingering, multi-systemic symptoms.54 A broad range of triggers are hypothesized to initiate cortical spreading depression, including emotional or physiological stress, inflammation, and sleep deprivation.54 Triptan medications act primarily as 5HT1B/1D agonists,55 which lead to increased presynaptic inhibition via 5HT1B/1D receptors that are broadly expressed in the CNS.56 Thus, triptans may directly impact neuronal excitability and interfere with the development of cortical spreading depression. Finally, calcitonin gene-related peptide (CGRP) release during cortical spreading depression has recently been identified as an important factor required for sustaining a migraine, and pharmacological inhibition of the CGRP-receptor system shows therapeutic promise in both preventing and aborting migraines.54, 55, 57, 58
Similar advances in understanding the pathophysiology of epilepsy have focused on identifying factors that lead to increased neuronal excitability within the cerebral cortex.59, 60 Multiple genetic variants that alter ion channel conformation or their expression (“channelopathies”) directly influence neuronal excitability and are associated with epilepsy.60, 61, 62, 63 Genetic variants that influence the conformation or expression of neurotransmitter receptors may also contribute to epileptogenesis, particularly within the gamma-aminobutyric acid64 and endocannabanoid systems.65 Furthermore, energy depletion and varying degrees of mitochondrial dysfunction have long been recognized as significant etiological factors in epilepsy.37 Lastly, increased systemic inflammatory cytokines may directly impact neuronal function, leading to increased excitability that is associated with an increased risk of seizures. Thus, nonsteroidal anti-inflammatory drugs may have a novel role in epilepsy management.59 It is particularly interesting that exposure to early life stress is independently associated with increased inflammatory cytokine levels that may secondarily drive an increased incidence of epilepsy in this population.66
Increased neuronal excitability that decreases the threshold for attacks may be a common link between cyclical vomiting syndrome, chronic migraine, epilepsy, and panic disorder. This would account for the shared associations of each of these disorders with gamma-aminobutyric acid and endocannabanoid receptor physiology,7, 67, 68, 69, 70 and the fact that benzodiazepines and exogenous cannabinoid use may be effective in aborting acute CVS attacks,1, 13 migraines,70 seizures,65, 67 and panic attacks.69 It would account for the utility of triptan medications in aborting migraines and CVS attacks.1, 18, 55 It may also explain some reports of beneficial effects of nonsteroidal anti-inflammatory drugs in treating CVS.71, 72 Indeed, given these general observations, CVS patients could benefit from trials of therapies that are currently available for the treatment of migraines, seizures, or panic disorder (Table 1).
Table 1. Newer therapeutic targets in the treatment of migraine, epilepsy, or panic disorders with potential utility in CVS.
| Treatments | Pharmacological target | Potential medications | Reference # |
|---|---|---|---|
| Calcitonin gene-related peptide system | Small molecule CGRP receptor anatagonist | Tacagepant | 74 |
| Ubrogepant | 75 | ||
| Monoclonal Ab to CGRP receptor | LY2951742, ALD403, and TEV-48125 (Phase II) | 76 | |
| Monoclonal Ab to CGRP ligand | AMG 334 | 76 | |
| Gap junctions | Connexin-36 | Quinine, Quinidine, and Mefloquine | 77 |
| Glial-neuronal gap junction | Tonabersat | 77 | |
| Angiotensin system | Angiotensin II receptors | Candesartan | 78 |
| Atypical neuroleptics | Dopamine/5-HT2 receptors | Olanzapine | 79 |
| Glutamate receptor system | AMPA/Kainate receptors | Perampane | 80 |
| NMDA receptors | Ketamine | 81, 82 | |
| Calcium channels | Cavα2δ subunits | Gabapentin, Pregabalin | 83 |
| R-type channels | Lamotrigine, Zonisamide, | 84 | |
| T-type channels | Valproate | 85 | |
| Z944 (Phase II) | 86 | ||
| Flunarizine Ethosuxamide | 87 | ||
| Serotonin system | Serotonin receptors | SSRIs and SNRIs | 88 |
| Atypical/Unclear | Mirtazapine | 88 | |
| Tianeptine | 89, 90 | ||
| Biobehavioral treatments | |||
| Cognitive behavioral therapy; mindful meditation; exposure therapy | Unknown | N/A | 91, 92 |
| Yoga/Pilates/Tai Chi | Unknown | N/A | 93 |
The therapeutic armamentarium capable of influencing neural excitability and neural circuit function is not restricted to pharmacological interventions, but extends to bio-behavioral interventions that can potently and durably induce neuroplasticity to ameliorate the propensity for attacks.7 For example, viewed from this neural circuit perspective, the neurobiological underpinnings of arousal and fear may be as relevant for CVS as they are for panic disorder. “Mind-body” interventions include psychological therapies such as cognitive behavioral therapy or exposure therapy, stress management techniques, biofeedback therapy, mindfulness meditation, and movement-based therapies such as yoga, Tai Chi, or Pilates (Table 1). Many of these mind-body interventions, coupled with general wellness interventions such as regular sleep habits, exercise, and a balanced diet, could also have positive influence on CVS disease, via pleiotropic impacts on neuronal circuit function.7
Lastly, a focus on neuronal excitability and endophenotypes in CVS may resolve some potential paradoxes in its disease associations and management. For example, it has long been recognized that a substantial number of adult patients develop CVS in the context of chronic heavy marijuana use and that eliminating its use can improve symptoms.1, 18 Yet, not everyone that regularly uses marijuana develops CVS, and in many patients, intermittent use of marijuana reduces anxiety and can even abort a CVS attack.13 Similarly, chronic exposure to opiates may worsen the CVS disease course to drive a more coalescent form of the disorder, yet acutely administered opiates can be effective in alleviating acute pain during a CVS attack.7 However, if viewed through an endophenotype lens and considering different neural circuits, these paradoxes can be resolved. For example, in susceptible individuals, chronic marijuana and opiate may increase neuronal excitability within the neural circuits that regulate autonomic function to drive allostatic dysregulation and increased CVS attack frequency,7 whereas at the same time leaving preserved the sensitivity of neural circuits that alleviate pain or nausea and vomiting to acute opiate and cannabinoid exposure, respectively.
The “CVS threshold”: an integrative model of a complex disorder
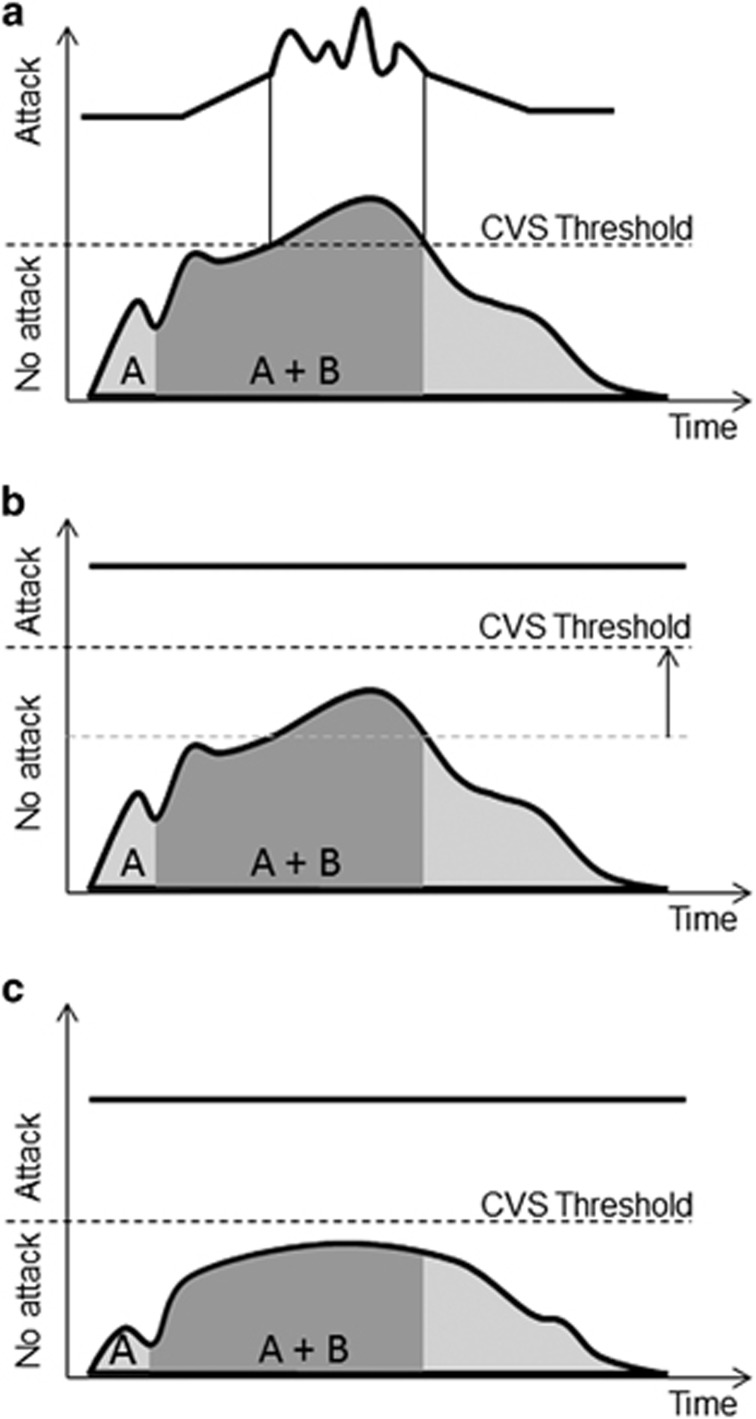
Given the importance of neuronal excitability in their pathogenesis, the migraine and epilepsy fields have long used the terms “migraine threshold” and “seizure threshold” to link the biological and clinical features of the disorders. Indeed, a threshold concept is also discussed for panic disorder.73 Because of shared mechanisms with migraine, epilepsy, and panic disorder, it would appear that the CVS field should adopt the term “CVS threshold.” The term “CVS threshold” links CVS to its multiple-contributing endophenotypes and anchors the concept of CVS disease expression to neuronal excitability and plasticity in neuronal circuits (Figure 3a). Thus, the “CVS threshold” not only has semantic, descriptive appeal, but also offers an explanatory framework to account for the efficacy of current therapies for CVS patients. For example, prophylactic medications with efficacy in CVS such as tricylic antidepressants or anti-epileptic drugs may each raise the CVS threshold by decreasing neuronal excitability (Figure 3b).1, 18 This effect would predict a decreased probability for developing a CVS attack, even in the face of an exposure to established CVS triggers. Alternatively, individual endophenotypes could be targeted to decrease the sensitivity to a relevant trigger, such that the CVS threshold is not breached during the exposure (Figure 3c). For example, an individual with CVS driven by mitochrondrial dysfunction and allostatic dysregulation during stress may benefit significantly from avoiding prolonged fasting and developing improved coping mechanisms to deal with perceived stresses. Finally, acute therapies that abort a CVS attack may do so by temporarily decreasing neuronal activity below the CVS threshold, accounting for the utility of gamma-aminobutyric acid agonists and triptans in this context. Indeed, some of the newer therapeutic approaches emerging for migraine, epilepsy, and panic disorder prophylaxis and abortive treatment could become potentially useful for preventing and aborting CVS attacks (Table 1).
Figure 3.
Graphical representation of the CVS threshold concept. In panel (a), two successive CVS triggers (a and b) summate to increase neuronal excitability to a point that crosses the CVS threshold. This then precipitates a CVS attack (represented at the top of the panel to mirror Figure 1a). In panel (b), the same two successive CVS triggers (a and b) now do not reach the CVS threshold, which has been raised by prophylactic medications that decrease neuronal excitability within key neural circuits. The lack of a CVS attack is represented by a solid black line at the top of the panel. In panel (c), the CVS threshold remains the same, but the impact of CVS triggers is minimized by interventions such that the summative impact of exposure to the triggers is now not sufficient to precipitate a CVS attack. Again, the lack of a CVS attack is represented by a solid black line at the top of the panel.
The CVS threshold concept mirrors management strategies widely recognized to be useful in CVS management: avoiding triggers, using prophylactic therapies, and providing access to abortive therapies in the case of an attack. However, an additional advantage of the CVS threshold concept is that it provides not only a neurophysiological framework that can predict which therapies could be useful in managing CVS, but also provides a framework for developing precision medicine interventions. Such interventions could be tailored to patients with specific therapeutic choices driven by the identification of dominant endophenotypic traits that drive their own unique version of CVS (Figures 2 and 3).
Future directions: a call to action
Adult CVS has a devastating impact on patients and their families,2 and the associated burden of the disorder has broad direct and indirect impacts on the economy and the healthcare system. But research in the adult CVS field has been hampered by the uncommon and episodic nature of the disorder, which makes it more difficult to identify the true prevalence of the illness, its relevant endophenotype components, and the true efficacy of therapeutic interventions. Thus, short-term, prospective, and randomized placebo-controlled trials have been essentially impossible to conduct, and no single academic center would ever likely be able to do so. Rather, progress with CVS research would be substantially accelerated by developing a nationwide (or international) disease registry with parallel recruitment of CVS patients from multiple academic medical centers. Such a registry would allow for increased precision in determining the nature and prevalence of underlying endophenotypes and comorbid conditions in adult patients with CVS. Patients enrolled in these registries could markedly facilitate the recruitment into multi-center therapeutic trials. However, a prospective CVS disease registry should be crafted to enable linkages with clinical data and health system information to explore patterns and costs of healthcare utilization in the CVS population. Establishing the financial burden of adult CVS could shape regulatory and funding agency priorities, which then may be leveraged into investments in an expanded national research program. A prospective CVS registry should also be linked to the prospective procurement of genomic information and biomarker data. Such information would provide critical insight into the genetic basis for CVS and treatment responses. By segregating CVS patients based on their dominant endophenotypes, genome-wide association studies should have markedly enhanced power to detect relevant polymorphisms associated with distinct subgroups of CVS patients. For example, patients with distinct circadian patterns to their CVS attacks may harbor variation in genes known to have a role in chronobiological regulation. Those with markedly altered stress reactivity may have variations in genes associated with allostatic regulation of the autonomic nervous system. Such genetic data could also provide the basis for prospective, pharmacogenetic approaches essential for predicting individual responses to specific therapies. Given that the cost of such genetic studies have rapidly decreased in recent years, at the same time that bioinformatics approaches have revolutionized the analysis of complex data sets, the time seems ripe for exploiting these methods to further the understanding of a complex disorder such as CVS. In doing so, these efforts would quite effectively expand the evidence base for personalized medicine.
However, these efforts should not be restricted to the research domain. Right now, endophenotypes of CVS patients should be actively searched for in the clinic, and then incorporated into tailored treatment plans. The CVS threshold concept described in this manuscript is grounded in the recognition that several distinct factors likely drive CVS in any particular patient. Clearly, patients will vary in the combination of potential contributing mechanisms, but such individual variations provide important clues to optimal management. A detailed history identifying specific CVS triggers and comorbidities should direct both pharmacological and non-pharmacological interventions. This precision medicine approach should target the relevant mechanism in a particular patient with the right therapy, using a combination of interventions that both raise the CVS threshold and diminish the impact of relevant triggers. Neural excitability is a key factor in CVS pathogenesis that impacts both the CVS threshold and the impact of an exposure to triggers. Thus, therapies that decrease neural excitability should be a useful foundation in the therapeutic plan for most patients with CVS. Perhaps close attention to progress in the fields of migraine, epilepsy, and panic disorder could quickly expand the therapeutic armamentarium available for use in adult patients with CVS.
Study Highlights
Guarantor of the article: David J. Levinthal, MD, PhD.
Specific author contributions: The sole author (DJL) was responsible for the conceptualization and drafting of the manuscript. He has approved the submitted final draft.
Financial support: This work was conducted independently from efforts supported by NIH K08 DK101756 (DJL).
Potential competing interests: None.
References
- Abell TL, Adams KA, Boles RG et al. Cyclic vomiting syndrome in adults. Neurogastroenterol Motil 2008; 20: 269–284. [DOI] [PubMed] [Google Scholar]
- Fleisher DR, Gornowicz B, Adams K et al. Cyclic vomiting syndrome in 41 adults: the illness, the patients, and problems of management. BMC Med 2005; 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles RG, Chun N, Senadheera D et al. Cyclic vomiting syndrome and mitochondrial DNA mutations. Lancet 1997; 350: 1299–1300. [DOI] [PubMed] [Google Scholar]
- Boles RG, Zaki EA, Lavenbarg T et al. Are pediatric and adult-onset cyclic vomiting syndrome (CVS) biologically different conditions? Relationship of adult-onset CVS with the migraine and pediatric CVS-associated common mtDNA polymorphisms 16519 T and 3010 A. Neurogastroenterol Motil 2009; 21: 936–972. [DOI] [PubMed] [Google Scholar]
- Lee J, Wong SA, Li BU et al. NextGen nuclear DNA sequencing in cyclic vomiting syndrome reveals a significant association with the stress-induced calcium channel (RYR2). Neurogastroenterol Motil 2015; 27: 990–996. [DOI] [PubMed] [Google Scholar]
- Boles RG, Williams JC. Mitochondrial disease and cyclic vomiting syndrome. Dig Dis Sci 1999; 44: 103 S–107 S. [PubMed] [Google Scholar]
- Levinthal DJ, Bielefeldt K. Adult cyclical vomiting syndrome: a disorder of allostatic regulation? Exp Brain Res 2014; 232: 2541–2547. [DOI] [PubMed] [Google Scholar]
- Venkatesan T, Prieto T, Barboi A et al. Autonomic nerve function in adults with cyclic vomiting syndrome: a prospective study. Neurogastroenterol Motil 2010; 22: e1339. [DOI] [PubMed] [Google Scholar]
- Hejazi RA, Lavenbarg TH, Pasnoor M et al. Autonomic nerve function in adult patients with cyclic vomiting syndrome. Neurogastroenterol Motil 2011; 23: 439–443. [DOI] [PubMed] [Google Scholar]
- Tache Y. Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Dig Dis Sci 1999; 44: 79S–86S. [PubMed] [Google Scholar]
- Tarbell S, Li BU. Psychiatric symptoms in children and adolescents with cyclic vomiting syndrome and their parents. Headache 2008; 48: 259–266. [DOI] [PubMed] [Google Scholar]
- Tarbell SE, Li BU. Anxiety measures predict health-related quality of life in children and adolescents with cyclic vomiting syndrome. J Pedriatr 2015; 167: e631. [DOI] [PubMed] [Google Scholar]
- Venkatesan T, Sengupta J, Lodhi A et al. An Internet survey of marijuana and hot shower use in adults with cyclic vomiting syndrome (CVS). Exp Brain Res 2014; 232: 2563–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles RG, Baldwin EE, Prezant TR. Combined cyclic vomiting syndrome and Kearns-Sayre syndromes. Pediatr Neurol 2007; 26: 135–136. [DOI] [PubMed] [Google Scholar]
- Cupini LM, Santorelli FM, Iani C et al. Cyclic vomiting syndrome, migraine, and epilepsy: a common underlying disorder? Headache 2003; 43: 407–409. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK et al. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004; 29: 1765–1781. [DOI] [PubMed] [Google Scholar]
- Li BU, Lefevre F, Chelimsky GG et al. North American Society for pediatric gastroenterology, hepatology, and nutrition consensus statement on the diagnosis and management of cyclic vomiting syndrome. J Pediatr Gastroenterol Nutr 2008; 47: 379–393. [DOI] [PubMed] [Google Scholar]
- Hejazi RA, McCallum RW. Review article: cyclic vomiting syndrome in adults–-rediscovering and redefining an old entity. Aliment Pharmacol Ther 2011; 34: 263–273. [DOI] [PubMed] [Google Scholar]
- Sunku B. Cyclic vomiting syndrome: a disorder of all ages. Gastroenterol Hepatol (NY) 2009; 5: 507–515. [PMC free article] [PubMed] [Google Scholar]
- Li BU, Misiewicz L. Cyclic vomiting syndrome: a brain-gut disorder. Gastroenterol Clin North Am 2003; 32: 997–1019. [DOI] [PubMed] [Google Scholar]
- Cutrer FM, Charles A. The neurogenic basis of migraine. Headache 2008; 48: 1411–1414. [DOI] [PubMed] [Google Scholar]
- Alving J, Beniczky S. Epileptic prodromes: are they nonconvulsive status epilepticus? Seizure 2013; 22: 522–527. [DOI] [PubMed] [Google Scholar]
- Scaramelli A, Braga P, Avellanal A et al. Prodromal symptoms in epileptic patients: clinical characterization of the pre-ictal phase. Seizure 2009; 18: 246–250. [DOI] [PubMed] [Google Scholar]
- Taylor CB. Panic disorder. BMJ 2006; 332: 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Craske MG, Epstein AM et al. Subtypes of panic attacks: a critical review of the empirical literature. Depress Anxiety 2009; 26: 878–887. [DOI] [PubMed] [Google Scholar]
- Borsook D, Maleki N, Becerra L et al. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron 2012 26; 73: 219–234. [DOI] [PubMed] [Google Scholar]
- Andress-Rothrock D, King W, Rothrock J. An analysis of migraine triggers in a clinic-based population. Headache 2010; 50: 1366–1370. [DOI] [PubMed] [Google Scholar]
- Kanner AM. The treatment of depressive disorders in epilepsy: what all neurologists should know. Epilepsia 2013; 54 (Suppl 1): 3–12. [DOI] [PubMed] [Google Scholar]
- Mula M. Treatment of anxiety disorders in epilepsy: an evidence-based approach. Epilepsia 2013; 54 (Suppl 1): 13–18. [DOI] [PubMed] [Google Scholar]
- Maguire J, Salpekar JA. Stress, seizures, and hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Epilepsy Behav 2013; 26: 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Hamilton SP. Panic attack as a marker of core psychopathological processes. Psychopathology 2001; 34: 278–288. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Richter J, Pané-Farré C et al. Panic disorder with agoraphobia from a behavioral neuroscience perspective: applying the research principles formulated by the Research Domain Criteria (RDoC) initiative. Psychophysiology 2016; 53: 312–322. [DOI] [PubMed] [Google Scholar]
- van Campen JS, Hompe EL, Jansen FE et al. Cortisol fluctuations relate to interictal epileptiform discharges in stress sensitive epilepsy. Brain 2016; 139 (Pt 6): 1673–1679. [DOI] [PubMed] [Google Scholar]
- Carreño M, Fernández S. Sleep-related epilepsy. Curr Treat Options Neurol 2016; 18: 23. [DOI] [PubMed] [Google Scholar]
- Babson KA, Feldner MT, Trainor CD et al. An experimental investigation of the effects of acute sleep deprivation on panic-relevant biological challenge responding. Behav Ther 2009; 40: 239–250. [DOI] [PubMed] [Google Scholar]
- Borkum JM. Migraine triggers and oxidative stress: a narrative review and synthesis. Headache 2016; 56: 12–35. [DOI] [PubMed] [Google Scholar]
- Rahman S. Pathophysiology of mitochondrial disease causing epilepsy and status epilepticus. Epilepsy Behav 2015; 49: 71–75. [DOI] [PubMed] [Google Scholar]
- Nierenburg Hdel C, Ailani J, Malloy M et al. Systematic review of preventive and acute treatment of menstrual migraine. Headache 2015; 55: 1052–1071. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Sperling MR et al. Progesterone Trial Study Group. Distribution of seizures across the menstrual cycle in women with epilepsy. Epilepsia 2015; 56: e58–e62. [DOI] [PubMed] [Google Scholar]
- Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clin Psychol Rev 2011; 31: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbiroli B, Montagna P, Cortelli P et al. Complicated migraine studied by phosphorus magnetic resonance spectroscopy. Cephalalgia 1990; 10: 263–272. [DOI] [PubMed] [Google Scholar]
- Barbiroli B, Montagna P, Cortelli P et al. Abnormal brain and muscle energy metabolism shown by 31 P magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology 1992; 42: 1209–1214. [DOI] [PubMed] [Google Scholar]
- Nesbitt AD, Leschziner GD, Peatfield RC. Headache, drugs and sleep. Cephalalgia 2014; 34: 756–766. [DOI] [PubMed] [Google Scholar]
- Hofstra WA, Spetgens WP, Leijten FS et al. Diurnal rhythms in seizures detected by intracranial electrocorticographic monitoring: an observational study. Epilepsy Behav 2009; 14: 617–621. [DOI] [PubMed] [Google Scholar]
- Pavlova MK, Lee JW, Yilmaz F et al. Diurnal pattern of seizures outside the hospital: is there a time of circadian vulnerability? Neurology 2012; 78: 1488–1492. [DOI] [PubMed] [Google Scholar]
- Fox AW, Davis RL. Migraine chronobiology. Headache 1998; 38: 436–441. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Khubchandani J, Herial NA et al. Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache 2012; 52: 920–929. [DOI] [PubMed] [Google Scholar]
- van Campen JS, Jansen FE, de Graan PN et al. Early life stress in epilepsy: a seizure precipitant and risk factor for epileptogenesis. Epilepsy Behav 2014; 38: 160–171. [DOI] [PubMed] [Google Scholar]
- Serretti A, Souery D, Antypa N et al. The impact of adverse life events on clinical features and interaction with gene variants in mood disorder patients. Psychopathology 2013; 46: 384–389. [DOI] [PubMed] [Google Scholar]
- Lotufo PA, Valiengo L, Benseñor IM et al. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 2012; 53: 272–282. [DOI] [PubMed] [Google Scholar]
- Berle D, Starcevic V, Milicevic D et al. The structure and intensity of self-reported autonomic arousal symptoms across anxiety disorders and obsessive-compulsive disorder. J Affect Disord 2016; 199: 81–86. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Striano P, Belcastro V et al. Migralepsy and related conditions: advances in pathophysiology and classification. Seizure 2011; 20: 271–275. [DOI] [PubMed] [Google Scholar]
- Sekimoto M, Kato M, Kaneko T et al. Ictal nausea with vomiting as the major symptom of simple partial seizures: Electroencephalographic and magnetoencepalographic analysis. Epilepsy Behav 2007; 11: 582–587. [DOI] [PubMed] [Google Scholar]
- Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci 2015; 35: 6619–6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Charles A, Goadsby PJ et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol 2015; 14: 1010–1022. [DOI] [PubMed] [Google Scholar]
- Guo JD, Rainnie DG. Presynaptic 5-HT(1B) receptor-mediated serotonergic inhibition of glutamate transmission in the bed nucleus of the stria terminalis. Neuroscience 2010; 165: 1390–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TW, Ferrari MD, Dodick DW et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 2008; 372: 2115–2123. [DOI] [PubMed] [Google Scholar]
- Ho AP, Dahlöf CG, Silberstein SD et al. Randomized, controlled trial of telcagepant over four migraine attacks. Cephalalgia 2010; 30: 1443–1457. [DOI] [PubMed] [Google Scholar]
- Iori V, Frigerio F, Vezzani A. Modulation of neuronal excitability by immune mediators in epilepsy. Curr Opin Pharmacol 2016; 26: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Carney KE, Falgoust L et al. Emerging roles of Na(+)/H(+) exchangers in epilepsy and developmental brain disorders. Prog Neurobiol 2016; 138-140: 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striano P, Vari MS, Mazzocchetti C et al. Management of genetic epilepsies: from empirical treatment to precision medicine. Pharmacol Res 2016; 107: 426–429. [DOI] [PubMed] [Google Scholar]
- Bagal SK, Marron BE, Owen RM et al. Voltage gated sodium channels as drug discovery targets. Channels (Austin) 2015; 9: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov 2016; 15: 19–34. [DOI] [PubMed] [Google Scholar]
- Yuan H, Low CM, Moody OA et al. Ionotropic GABA and glutamate receptor mutations and human neurologic diseases. Mol Pharmacol 2015; 88: 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Alger BE, Kano M et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci 2015; 16: 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S et al. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 2016; 21: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy RA, Freestone DR, Lai A et al. Epilepsy: ever-changing states of cortical excitability. Neuroscience 2012; 222: 89–99. [DOI] [PubMed] [Google Scholar]
- Quintas M, Neto JL, Pereira-Monteiro J et al. Interaction between γ-aminobutyric acid A receptor genes: new evidence in migraine susceptibility. PLoS One 2013; 8: e74087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges LM, Fyer AJ, Weissman MM et al. Evidence for linkage and association of GABRB3 and GABRA5 to panic disorder. Neuropsychopharmacology 2014; 39: 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyne DN, Anderson SL, Gedde M et al. Effects of medical marijuana on migraine headache frequency in an adult population. Pharmacotherapy 2016; 36: 505–510. [DOI] [PubMed] [Google Scholar]
- Pasricha PJ, Schuster MM, Saudek CD et al. Cyclic vomiting: association with multiple homeostatic abnormalities and response to ketorolac. Am J Gastroenterol 1996; 91: 2228–2232. [PubMed] [Google Scholar]
- Vidula MK, Wadhawani A, Roberts K et al. Use of a once-daily NSAID in treatment of cyclic vomiting syndrome. J Gen Intern Med 2013; 29: 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pané-Farré CA, Fenske K, Stender JP et al. Sub-threshold panic attacks and agoraphobic avoidance increase comorbidity of mental disorders: results from an adult general population sample. J Anxiety Disord 2013; 27: 485–493. [DOI] [PubMed] [Google Scholar]
- Ho TW, Conner KM, Zhang Y et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 2014; 83: 958–966. [DOI] [PubMed] [Google Scholar]
- Voss T, Lipton RB, Dodick DW et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia 2016; 36: 887–898. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Rapoport AM. New players in the preventive treatment of migraine. BMC Med 2015; 13: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrouilhe D, Dejean C, Mesnil M. Involvement of gap junction channels in the pathophysiology of migraine with aura. Front Physiol 2014; 5: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovner LJ, Linde M, Gravdahl GB et al. A comparative study of candesartan versus propranolol for migraine prophylaxis: a randomised, triple-blind, placebo-controlled, double cross-over study. Cephalalgia 2014; 34: 523–532. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Peres MF, Hopkins MM et al. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache 2002; 42: 515–518. [DOI] [PubMed] [Google Scholar]
- French JA, Krauss GL, Wechsler RT et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy: a randomized trial. Neurology 2015; 85: 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati A, L'Erario M, Ilvento L et al. Efficacy and safety of ketamine in refractory status epilepticus in children. Neurology 2012; 79: 2355–2358. [DOI] [PubMed] [Google Scholar]
- Afridi SK, Giffin NJ, Kaube H et al. A randomized controlled trial of intranasal ketamine in migraine with prolonged aura. Neurology 2013; 80: 642–647. [DOI] [PubMed] [Google Scholar]
- Johannessen-Landmark C, Beiske G, Baftiu A et al. Experience from therapeutic drug monitoring and gender aspects of gabapentin and pregabalin in clinical practice. Seizure 2015; 28: 88–91. [DOI] [PubMed] [Google Scholar]
- Glauser TA, Cnaan A, Shinnar S et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: initial monotherapy outcomes at 12 months. Epilepsia 2013; 54: 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matar N, Jin W, Wrubel H et al. Zonisamide block of cloned human T-type voltage-gated calcium channels. Epilepsy Res 2009; 83: 224–234. [DOI] [PubMed] [Google Scholar]
- Tringham E, Powell KL, Cain SM et al. T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci Transl Med 2012; 4: 121ra–19. [DOI] [PubMed] [Google Scholar]
- Vajda FJ, Eadie MJ. The clinical pharmacology of traditional antiepileptic drugs. Epileptic Disord 2014; 16: 395–408. [DOI] [PubMed] [Google Scholar]
- Freire RC, Machado S, Arias-Carrión O et al. Current pharmacological interventions in panic disorder. CNS Neurol Disord Drug Targets 2014; 13: 1057–1065. [DOI] [PubMed] [Google Scholar]
- Carli V, Sarchiapone M, Camardese G et al. Mirtazapine in the treatment of panic disorder. Arch Gen Psychiatry 2002; 59: 661–662. [DOI] [PubMed] [Google Scholar]
- Moon J, Jung KH, Shin JW et al. Safety of tianeptine use in patients with epilepsy. Epilepsy Behav 2014; 34: 116–119. [DOI] [PubMed] [Google Scholar]
- Sullivan A, Cousins S, Ridsdale L. Psychological interventions for migraine: a systematic review. J Neurol 2016; doi:10.1007/s00415-016-8126-z. [DOI] [PMC free article] [PubMed]
- Pompoli A, Furukawa TA, Imai H et al. Psychological therapies for panic disorder with or without agoraphobia in adults: a network meta-analysis. Cochrane Database Syst Rev 2016; 4: CD011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John PJ, Sharma N, Sharma CM et al. Effectiveness of yoga therapy in the treatment of migraine without aura: a randomized controlled trial. Headache 2007; 47: 654–661. [DOI] [PubMed] [Google Scholar]