Abstract
Current treatment for chronic hepatitis C (CHC) is highly efficacious, well-tolerated, and of short duration for the majority of patients. Despite the dramatic advances in therapy, there remain several barriers to disease eradication. These include deficiencies in screening, diagnosis, and access to care, and high cost of the direct-acting antiviral medications. In addition, incident cases and reinfection associated with injection drug use contribute to the persistent worldwide disease burden. This article will review the current CHC treatments, and outline the remaining gaps in therapy and barriers to disease eradication.
INTRODUCTION
Available treatment options for chronic hepatitis C (CHC) infection have rapidly evolved over the last 5 years. There have been dramatic advances in terms of treatment efficacy and side effect profile of the new direct-acting antiviral agents (DAAs) with sustained virologic response (SVR) rates >95% and minimal associated side effects.1 Although these treatment advances have been revolutionary for the care of patients with CHC, there are multiple barriers that need to be overcome to move toward disease eradication. These include increasing screening and confirmatory diagnosis of at-risk populations, and improving access to care for treatment initiation and clinical monitoring of disease complications. Continuing to refine and simplify treatment algorithms to broaden the pool of providers who feel comfortable initiating hepatitis C virus (HCV) treatment would greatly increase the ability of these treatment advances to translate into real-world improvement in outcomes.
EFFICACY OF CURRENTLY AVAILABLE THERAPIES
Interferon (IFN)-free regimens with DAAs are the mainstay of therapy for patients with all HCV genotypes. Approved DAAs currently in use in western countries include (i) NS5B polymerase inhibitors (including nucleos(t)ide and non-nucleoside inhibitors): sofosbuvir (SOF) and dasabuvir; (ii) NS5A inhibitors: ledipasvir, daclatasvir, elbasvir (EBR), ombitasvir, and velpatasvir; and (iii) NS3/4A protease inhibitors: simeprevir, paritaprevir, and grazoprevir (GZR). Combination regimens comprising two or three DAAs with or without ribavirin have consistently produced SVR rates of ~95% in most patients including patients with compensated cirrhosis or HIV coinfection, and nonresponders to prior IFN-based therapies.2, 3, 4, 5, 6 Registries of patients treated in clinical practice showed that these high SVR rates are reproduced in the real world.7, 8
Given the high efficacy and tolerability of IFN-free DAA regimens, the American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (IDSA) HCV guidance recommends all patients with CHC should be considered for treatment irrespective of the stage of disease except those with estimated life expectancy <1 year. Although monitoring for response and adverse events have been greatly simplified, many factors have to be considered to determine the most appropriate DAA regimen for each patient. These factors include: (i) HCV genotype (including associated subtypes, however, this is becoming less relevant with available pangenotypic regimens), (ii) prior treatment history (naive vs. experienced and any prior use of DAA), (iii) stage of liver disease (cirrhosis or no cirrhosis), (iv) HCV RNA level (for certain regimens), (v) presence of decompensation for patients with cirrhosis, (vi) renal function, and (vii) concomitant medications that may interact with DAAs.
Assessing the stage of liver disease is crucial not only in determining the duration of treatment, but in many instances also to determine whether DAAs will be approved by the patient's insurance. Traditionally, staging of liver fibrosis had relied on liver biopsy. There are several validated, noninvasive methods to assess liver fibrosis that have largely supplanted biopsy in current clinical care. Laboratory methods include indices based on routinely available tests such as the aspartate aminotransferase to platelet ratio index and fibrosis 4 marker panel. From an imaging standpoint, elastography, a technique that assesses tissue deformation or elastic properties of soft tissue after applying a force, has been commonly used. Vibration-controlled transient elastography (Fibroscan, Echosens, Paris, France) is the most common method used in clinical practice, though acoustic radiation force impulse imaging and magnetic resonance elastography have also been shown to correlate with histologic staging of fibrosis.9 These noninvasive tests can be limited by other contributing processes that can affect the components of the tests, e.g., high aspartate aminotransferase and alanine aminotransferase (ALT) from alcohol use or increased hepatic inflammation can lead to falsely high aspartate aminotransferase to platelet ratio index or fibrosis 4 marker panel scores.10 Similarly, high aspartate aminotransferase or ALT, moderate/severe hepatic inflammation, and hepatic congestion can lead to increased liver stiffness and falsely high readings on Fibroscan.
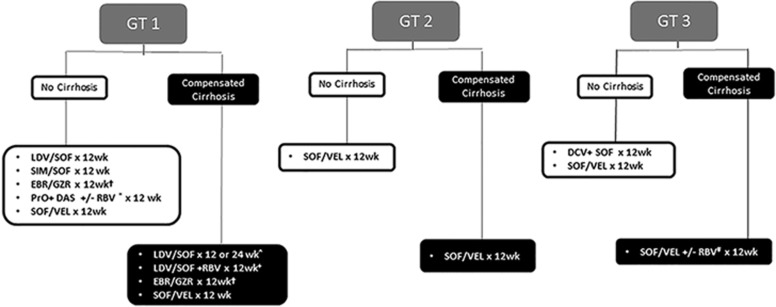
A concise overview of the primary recommended (Class I) treatment regimens by the AASLD and IDSA HCV guidance for the most common HCV genotypes in the United States are outlined in Figure 1.1
Figure 1.
Class I treatment recommendations for chronic hepatitis C. Treatment experienced defined as PEG-IFN/RBV only. †In GT1a, need to test for NS5A RAV for EBR; need 16 weeks of therapy with RBV for high-fold RAV. *RBV needed for GT1a but not GT1b in treatment-naive patients; RBV needed for all GT1 treatment-experienced patients. ^A total of 24 weeks of therapy required for treatment-experienced patients. +This regimen only applies to treatment-experienced patients. #RBV only needed in treatment-experienced patients. EBR, ebasvir; DAS, dasabuvir; DCV, daclatasvir; GT, genotype; GZR, grazoprevir; IFN, interferon; LDV, ledipasvir; PrO, paritaprevir+ritonavir+ombitasvir; RAV, resistance-associated variant; RBV, ribavirin; SIM, simeprevir; SOF, sofosbuvir; VEL, velpatasvir.
PATIENT SUBGROUPS OF INTEREST
Although current therapies are highly efficacious, simple, and well-tolerated for most patients, there are gaps in existing therapies. These include suboptimal SVR rates in patients with HCV genotype 3 infection and patients with child C cirrhosis of any genotype. Fortunately, the recent approval of SOF/velpatasvir has in large part addressed gaps in efficacy for patients with genotype 3 infection.11, 12 Patients with end-stage renal disease had until recently remained a subgroup with very limited treatment options, but the approval of daclatasvir and EBR/GZR has been a significant breakthrough for patients with genotype 1 and 4 infections. Impaired renal function remains a significant limitation in therapeutic treatment options for patients with genotype 2, 3, 5, or 6. Treating patients with decompensated cirrhosis remains complex, given their baseline tenuous hepatic function; therefore, it is recommended that they are managed in a liver transplant center.1 Therapeutic options are more restricted for patients with decompensated cirrhosis because several DAAs (simeprevir, dasabuvir, GZR, and EBR) are not approved for use in patients with child B or C cirrhosis, and there is a Food and Drug Administration warning against the use of paritaprevir/ritonavir/ombitasvir. These additional complexities have led to a debate whether treatment should be deferred until after liver transplant as several DAA combination regimens with minimal drug interactions with calcineurin inhibitors are available. On the other hand, SVR has been associated with improvement in liver function and decrease in model for end-stage liver disease score in patients with decompensated cirrhosis, raising the possibility that treatment in the pretransplant setting may obviate the need for transplant.
Patients coinfected with HIV have more rapid fibrosis progression than patients with HCV monoinfection, and were considered to be a “difficult-to-treat” population in the era of IFN-based therapies.13 However, SVR rates with currently available DAA regimens are comparable and the AASLD/IDSA HCV guidance recommends the same treatment approach for HIV–HCV coinfected patients as for patients with HCV monoinfection.1 Hepatitis B virus (HBV)–HCV coinfection prevalence is lower than HIV–HCV coinfection, but the prevalence is up to 10% globally.14 Patients with HBV–HCV coinfection tend to have more rapid progression of liver disease including higher rates of decompensation and hepatocellular carcinoma (HCC). Although the recommended HCV treatment regimens are the same for this patient population, it is important to recognize that HBV replication may increase after successful eradication of chronic HCV infection with resultant hepatitis flares.15 Thus, it is important to monitor HBV DNA levels during and following completion of HCV therapy in patients with HBV–HCV coinfection.
Another patient subgroup of interest is patients with a prior history of HCC. One study raised concern about the increased risk of HCC recurrence after treatment with DAAs.16 This observation was not confirmed in another study that included a larger number of patients.17 It has also been suggested that patients treated with DAAs have increased incidence of HCC.16 Further studies on this topic are needed; in the meantime, patients with prior HCC should be monitored for HCC recurrence and those with cirrhosis should continue HCC surveillance even if they achieve SVR.
There are also gaps in safety data in some patient populations, specifically children and pregnant women. The seroprevalence of hepatitis C antibody (anti-HCV) among children is estimated to be 0.2% in those aged 6–11 years and 0.4% in those who are 11–19 years old.18, 19 The most recent AASLD/ISDA HCV guidance does not provide specific recommendations for the treatment of pediatric patients as clinical trials of DAAs in children are ongoing. There is a similar lack of guidance for pregnant patients. Treatment during pregnancy may be beneficial in that it could decrease the estimated 5% risk of perinatal transmission; however, safety data of DAAs in pregnancy are limited and most young women have early-stage liver disease and can defer treatment until after delivery. Among the DAAs, ledipasvir and SOF are pregnancy category B, and simeprevir, dasabuvir, and paritaprevir+ritonvir+ombitasvir are category C. There is no data regarding safety of daclatasvir, EBR, velpatasvir, or GZR. Ribavirin is pregnancy category X.
Finally, there are limited data regarding retreatment of patients who have failed one of the current DAA combination regimens and the utility of testing for resistance-associated variants (RAVs) to guide the choice of rescue therapy. NS5A RAVs have been shown to persist for a few years after treatment discontinuation, whereas NS3 RAVs generally become undetectable within 12 months after treatment discontinuation. Preliminary data of one study involving two second-generation DAAs: ABT-493 (NS3/4A protease inhibitor) and ABT-530 (NS5A inhibitor) showed that high (95%) SVR rates can be achieved even in patients who failed prior regimens of first-generation DAAs.20 NS3 and NS5A RAVs can also be present in treatment-naive patients. Baseline NS5A-resistance testing is recommended in patients with GT1a infection in whom treatment with EBR/GZR is being considered to determine treatment duration.
BRIDGING GAPS IN EXISTING THERAPIES AND UPCOMING THERAPIES
Several DAA regimens are currently in phase 3 or late phase 2 trials. These regimens include DAAs with pangenotypic activity, and/or have higher barrier to resistance and activity against RAVs that are selected by first-generation DAAs. One example is a combination of ABT-493 and ABT-530, that has pangenotypic activity with high SVR rates including patients with genotype 3 infection and cirrhosis.20, 21
As HCV treatment evolves, applicability of DAA regimens in resource-limited countries, where reliable tests for HCV genotype, quantitative HCV RNA, and assessment of liver fibrosis are lacking must be considered. Treatment regimens that have pangenotypic activity, and are equally effective and safe irrespective of disease stage (cirrhosis vs. no cirrhosis and compensated vs. decompensated cirrhosis) and kidney function would enable all patients to be treated with the same regimen with minimal pretreatment evaluation and on-treatment monitoring, thus increasing the total number of patients who can be treated.
BARRIERS TO HCV ERADICATION
Deficiencies in Screening and Diagnosis
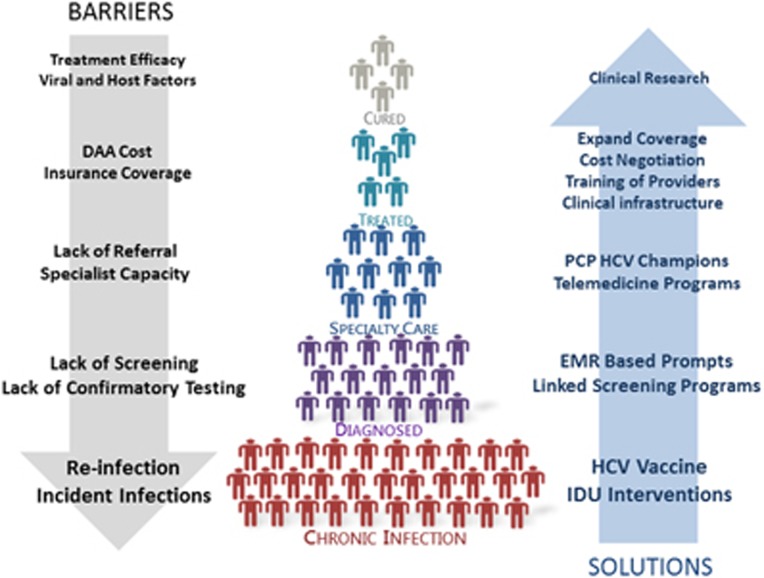
The revolutionary advances in HCV therapy can only benefit patients who have been diagnosed and are linked to care. However, multiple deficiencies in the “HCV care cascade” pose barriers to the goal of elimination of HCV infection (Figure 2).22, 23 It is estimated that <50% of the ~3.2 million persons with CHC in the United States are aware of the diagnosis.22, 24 The Centers for Disease Control and Prevention and United States Preventive Services Task Force recommended that HCV screening should be expanded to all baby boomers, persons born between 1945 and 1965, regardless of the presence of any of the traditional risk factors for HCV, given the fivefold higher prevalence of HCV in this cohort and the failure of risk-based screening.25, 26 However, uptake of this screening recommendation remains low with screening rates ranging from 6 to 20% across several studies on screening of baby boomers.27, 28 Integrating screening into preventive care and other health-screening-related settings like screening colonoscopy or HIV testing has been shown to be an effective way to increase uptake.29, 30 Application of health information technology-based tools has also been shown to increase screening rates. Implementation of a birth cohort “best practice advisory” in our electronic medical record increased screening by fivefold.31
Figure 2.
HCV care cascade and path to disease eradication: barriers and potential solutions. DAA, direct-acting antiviral; EMR, electronic medical record; HCV, hepatitis C virus; IDU, injection drug use; PCP, primary care physician.
Compounding the difficulties in screening is the need for confirmatory testing with HCV RNA in persons found to be anti-HCV-positive on screening. In most clinical settings, the two tests are disconnected and testing for HCV RNA requires calling back of patients who tested positive for anti-HCV. It is estimated that 30–50% of anti-HCV-positive persons never receive confirmatory HCV RNA testing.23, 32, 33, 34 Reflex testing of all anti-HCV-positive samples for HCV RNA can bridge this gap and has been shown to be cost-effective.35, 36 However, many laboratories have been hesitant to implement reflex testing owing to concerns about sample contamination and billing for a test not in the original order.37 At the global level, barriers to screening and diagnosis are even more pronounced as many regions have limited health-care resources that make it difficult to dedicate funds to HCV screening programs. Access to HCV RNA testing is limited in many low-income countries making confirmatory testing and monitoring for treatment response challenging.38, 39
Gaps in Linkage to Care
Even among persons who have been diagnosed, it is estimated that only a minority (32–38%) of those diagnosed in the United States had been connected to specialty care (Figure 2).40, 41 This is in part attributed to the overall lower likelihood of patients with CHC to have health insurance and/or an established primary care physician (PCP).42 A recent study demonstrated that even with implementation of a structured intervention for high risk patients, only 52% of patients newly diagnosed with CHC attended HCV specialist appointments.43 In this study, ongoing alcohol use and lack of insurance were identified as cofactors in decreased linkage to specialist care (odds ratio 0.4 for each).43 Low efficacy of IFN-based regimens, frequent adverse events, medical/psychiatric contraindications, and need for intense on-treatment monitoring had been cited as reasons for non-referral in the past. Recent advances in HCV therapeutics might have increased referral to specialty care, but the magnitude of the change has not been quantified. The time frame from referral to assessment by specialist has also been cited as a potential barrier. Previous studies estimated that >80% of patients with CHC were managed by 20% of gastroenterologists and hepatologists, most of whom were associated with academic medical centers. This funneling of patients to a minority of specialists creates a bottleneck that results in long appointment wait times and logistical strain on these practices, given the labor-intensive nature of obtaining approval for DAAs.44, 45, 46, 47 A proposed intervention to improve access to care had been the administration of HCV treatment by PCPs, particularly for patients without advanced fibrosis or cirrhosis.48 However, most PCPs are hesitant to assume this responsibility. A recent survey of PCPs associated with an academic medical center demonstrated that most PCPs are unable to keep up to date with the rapid advances in HCV treatment. The majority grossly underestimated efficacy, tolerability, and ease of administration, and overestimated treatment duration. Moreover, only 9% of PCPs reported they felt comfortable administering treatment for HCV, even among patients without cirrhosis.49
Access to care for patients globally is an even more pronounced problem. In resource-limited countries, there is an overall shortage of health-care workers and facilities. HCV treatment that does not require individualized regimens based on viral parameters and stage of liver disease from clinical diagnostics that are unavailable in these countries would greatly increase the capacity to deliver effective care. Treatment simplifications must be coupled with training of more providers and expansion of the necessary infrastructure.39
High Cost of DAAs
For those who have been diagnosed and linked to care, a major barrier to HCV treatment remains the cost of the DAAs (Table 1). However, it should be realized that although the wholesale acquisition cost of DAAs is publicly available, the actual price insurers pay is unknown as the negotiated discount is considered confidential information.50 In general, it is estimated that the negotiated cost in the United States is around 46% of the wholesale acquisition cost, but this is highly variable and continuously changing.51, 52 In response to the high cost of the DAAs, many payers in the United States have restricted DAA treatment only to patients with advanced fibrosis or cirrhosis, and some have imposed requirements for drug and alcohol screening prior to the approval of treatment.53 Restricting treatment to patients with advanced disease creates multiple issues from both a patient and provider standpoint. Patients with cirrhosis often need a longer duration of treatment or the addition of ribavirin, which in turn requires additional monitoring. In addition, patients with cirrhosis remain at risk of HCC after achieving SVR, and patients with early-stage disease who are denied treatment will require continual monitoring to assess for disease progression until a time they become eligible for therapy. Modeling studies have demonstrated that there is a likely survival benefit if treatment is initiated in patients with early-stage (<F3) disease and is cost-effective if the cost is <$22,200.54, 55, 56 A Veterans Affairs Hospital System study evaluated 187,860 patients with chronic HCV infection and predicted a 36% decrease in mortality if HCV treatment was initiated prior to fibrosis 4 marker panel level reaching above 1.00 (cutoff for advanced fibrosis >3.25).54
Table 1. Wholesale acquisition cost for DAAs in the United States. *Regimens that require RBV would add additional $500 for 12 weeks and $1,000 for 24 weeks.
| Medication | WAC for 1 day of treatment | WAC for 12 weeks | WAC for 24 weeks |
|---|---|---|---|
| Ledipasvir/sofosbuvir | $1,125 | $94,500 | $189,000 |
| Simeprevir+sofosbuvir | $1,790 | $150,000 | $300,000 |
| Daclatasvir+sofosbuvir | $1,750 | $147,000 | $294,000 |
| Sofosbuvir | $1,000 | $84,000 | $168,000 |
| Paritaprevir/ritonavir/ombitasvir | $912 | $77,000 | |
| Paritaprevir/ritonavir/ombitasvir+dasabuvir | $992 | $84,000 | $168,000 |
| Ebasvir/grazoprevir | $650 | $54,600 | $72,980a |
| Sofosbuvir/velpatasvir | $890 | $74,760 |
RBV, ribavirin; WAC, wholesale acquisition cost.
16 weeks, not 24.
With successive introduction of additional drugs, the price of DAAs has come down. Integrated health systems have also negotiated bulk discounts. The Veterans Affairs Hospital System and the Medicaid consortium represent two prime examples. Individual insurance companies have also negotiated for discounts. Thus, scenarios where DAA treatment were shown not to be cost-effective using wholesale acquisition cost might actually be cost-effective using discounted prices. Some resource-limited countries have been able to make arrangements to access DAAs at greatly reduced costs. A prime example is Egypt where the government negotiated a price for a 12-week course of ledipasvir/SOF at ~$900 (less than the wholesale acquisition cost for 1 day of ledipasvir/SOF in the United States).57 In addition, individual pharmaceutical companies have agreed to work with companies in India and select countries to make generic DAAs that will be distributed to low-income countries.35
New Infections, Reinfection, and Lack of a Vaccine
Worldwide, the incidence of new HCV infections has been decreasing owing to screening of blood products, universal precautions in health-care settings, and counseling, and needle-exchange programs for people with injection drug use (IDU). However, blood donor screening is not implemented in all countries, nosocomial infections still occur even in developed countries like the United States, and many countries including the United States have seen a resurgence of new HCV infections among young injection drug users.58, 59 Multidisciplinary programs aimed at reducing IDU and/or reducing transmission of HCV associated with IDU are needed to curb the new epidemic of HCV. Studies have shown that interventions that result in SVR in a modest number of persons with IDU can lead to a decrease in both incidence and prevalence of CHC.60 One modeling study estimated that treatment with DAAs among persons with IDU could decrease prevalence of HCV infection in half over the subsequent 15 years.60 Patients with recent or prior IDU have historically been less likely to be referred to specialty care for treatment and remains a patient population to prioritize in terms of optimizing access to care if CHC eradication is to be achieved.33 Prior studies have identified multiple factors contributing to this patient populations' hesitation to start HCV treatment including poor relations with health providers, lack of knowledge, low priority, fear of side effects, and lack of financial resources.61, 62
Persons who achieve SVR after treatment lack protective immunity and remain at risk of new HCV infection if reexposed. Therefore, counseling and enrollment in formal substance abuse programs remain critical pieces of the overall care for patients with chronic HCV infection. A recent meta-analysis demonstrated that SVR appears durable in the vast majority of patients with IDU.63 Another hurdle to eradication of hepatitis C is the lack of a vaccine. Despite the rapid advances in HCV treatment, there is still a role for a preventive vaccine against HCV infection, and research in this area should not be abandoned.
CONCLUSIONS
The rapid advances in treatment for CHC in the past few years have been remarkable, and have provided curative options for patients who otherwise may have experienced significant morbidity and mortality associated with HCV infection. Multiple real-world hurdles prevent disease eradication presently. The Institute of Medicine and the World Health Organization have prioritized optimizing outcomes in CHC. These entities have outlined that HCV disease eradication is feasible, but will require worldwide efforts to provide universal access to health care, implement and promote preventative health programs, markedly reduce the cost of diagnostics and treatment agents for CHC, and provision of high-quality chronic care.64, 65
Guarantor of the article: Monica A. Konerman, MD, MSc.
Specific author contributions: MAK and ASFL drafted and edited the manuscript. Both authors have approved the final submitted draft of the manuscript.
Financial support: MAK is supported by the AASLD Advanced/Transplant Hepatology Fellowship.
Potential competing interests: None.
References
- AASLD-IDSA. Recommendations for Testing, Managing, and Treating Hepatitis C; http://www.hcvguidelines.org.
- Afdhal N, Zeuzem S, Kwo P et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370: 1889–1898. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Ghalib R, Reddy KR et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 2015; 163: 1–13. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Dusheiko GM, Salupere R et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014; 370: 1993–2001. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Gardiner DF, Rodriguez-Torres M et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370: 211–221. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Eron JJ, Wyles D et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA 2015; 313: 1223–1231. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Vargas HE, Di Bisceglie AM et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology 2016; 150: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus LI, Belperio PS, Shahoumian TA et al. Real world effectiveness of ledipasvir/sofosbuvir in 4365 treatment-naive genotype 1 hepatitis C infected patients. Hepatology 2016; 64: 405–414. [DOI] [PubMed] [Google Scholar]
- Branchi F, Conti CB, Baccarin A et al. Non-invasive assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol 2014; 20: 14568–14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GL, Wong VW, Choi PC et al. Increased liver stiffness measurement by transient elastography in severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol 2009; 24: 1002–1007. [DOI] [PubMed] [Google Scholar]
- Feld JJ, Zeuzem S. Sofosbuvir and velpatasvir for patients with HCV infection. N Engl J Med 2016; 374: 1688–1689. [DOI] [PubMed] [Google Scholar]
- Pianko S, Flamm SL, Shiffman ML et al. Sofosbuvir plus velpatasvir combination therapy for treatment-experienced patients with genotype 1 or 3 hepatitis C virus infection: a randomized trial. Ann Intern Med 2015; 163: 809–817. [DOI] [PubMed] [Google Scholar]
- Konerman MA, Mehta SH, Sutcliffe CG et al. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology 2014; 59: 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Hou J. Hepatitis B virus (HBV) and hepatitis C virus (HCV) dual infection. Int J Med Sci 2006; 3: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff A, Wedemeyer H, Boecher WO et al. The HEP-NET B/C co-infection trial: a prospective multicenter study to investigate the efficacy of pegylated interferon-alpha2b and ribavirin in patients with HBV/HCV co-infection. J Hepatol 2008; 49: 688–694. [DOI] [PubMed] [Google Scholar]
- Conti F, Buonfiglioli F, Scuteri A et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct acting antivirals. J Hepatol 2016; e-pub ahead of print 24 June 2016. [DOI] [PubMed]
- Pol S. Lack of evidence of an effect of direct acting antivirals on the recurrence of hepatocellular carcinoma: the ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CIRVIR and CO23 CUPILT cohorts). J Hepatol 2016; e-pub ahead of print 7 June 2016. [DOI] [PubMed]
- Denniston MM, Jiles RB, Drobeniuc J et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014; 160: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri R. Diagnosis and management of hepatitis C virus-infected children. Pediatr Infect Dis J 2011; 30: 983–985. [DOI] [PubMed] [Google Scholar]
- Poordad F, Gordon SC, Asatryan A et al High-efficacy of ABT-493 and ABT-530 in HCV genotype-1-infected patients who have failed direct-acting antiviral-containing regimens: the Magellan-1 study. The International Liver Congress, 13–17 April 2016, Barcelona, Abstract GS11.
- Kwo P. 100% SVR4 with ABT-493 and ABT-530 with or without Ribavirin in Treatment-Naïve HCV Genotype 3-Infected Patients with Cirrhosis. EASL International Liver Congress. 2016.
- Holmberg SD, Spradling PR, Moorman AC et al. Hepatitis C in the United States. N Engl J Med 2013; 368: 1859–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia BR, Schranz AJ, Umscheid CA et al. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9: e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong GL, Wasley A, Simard EP et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144: 705–714. [DOI] [PubMed] [Google Scholar]
- Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 159: 349–357. [DOI] [PubMed] [Google Scholar]
- Smith BD, Morgan RL, Beckett GA et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep 2012; 61: 1–32. [PubMed] [Google Scholar]
- Litwin AH, Smith BD, Drainoni ML et al. Primary care-based interventions are associated with increases in hepatitis C virus testing for patients at risk. Dig Liver Dis 2012; 44: 497–503. [DOI] [PubMed] [Google Scholar]
- Adebajo CO, Aronsohn A, Te HS et al. Digestive Diseases Week 2015. Gastroenterology 2015; 148: S1101–1102. [Google Scholar]
- Sears DM, Cohen DC, Ackerman K et al. Birth cohort screening for chronic hepatitis during colonoscopy appointments. Am J Gastroenterol 2013; 108: 981–989. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care and treatment of viral hepatitis; http://www.hhs.gov/ash/initiatives/hepatitis.
- Konerman MA, Thomson M, Lok AS. Digestive Diseases Week 2016. Gastroenterology 2016; 150: S1159–1160. [Google Scholar]
- Reau N. HCV testing and linkage to care: expanding access. Clin Liver Dis 2014; 4: 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggmann P. Accessing hepatitis C patients who are difficult to reach: it is time to overcome barriers. J Viral Hepat 2012; 19: 829–835. [DOI] [PubMed] [Google Scholar]
- McGibbon E, Bornschlegel K, Balter S. Half a diagnosis: gap in confirming infection among hepatitis C antibody-positive patients. Am J Med 2013; 126: 718–722. [DOI] [PubMed] [Google Scholar]
- Spradling PR, Tong X, Rupp LB et al. Trends in HCV RNA testing among HCV antibody-positive persons in care, 2003-2010. Clin Infect Dis 2014; 59: 976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affairs DoV. Reflex Confirmatory Testing for Chronic Hepatitis C Virus Infection. VHA Directive;2009-063.
- Prevention CfDCa. Guidelines for laboratory testing and result reporting of antibody to hepatitis c virus. MMWR Recomm Rep 52:1–16. [PubMed] [Google Scholar]
- Organization WH. Access to hepatitis c medicines. Bull World Health Organ 2015;93:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine M, Thursz M. Hepatitis C, a global issue: access to care and new therapeutic and preventive approaches in resource-constrained areas. Semin Liver Dis 2014; 34: 89–97. [DOI] [PubMed] [Google Scholar]
- Moorman AC, Gordon SC, Rupp LB et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis 2013; 56: 40–50. [DOI] [PubMed] [Google Scholar]
- McWilliam R, Dundas P, Fraser A. Follow-up of HCV patients referred to specialist services- where do they all go? Gut A46–A47.
- Stepanova M, Kanwal F, El-Serag HB et al. Insurance status and treatment candidacy of hepatitis C patients: analysis of population-based data from the United States. Hepatology 2011; 53: 737–745. [DOI] [PubMed] [Google Scholar]
- Falade-Nwulia O, Mehta SH, Lasola J et al. Public health clinic-based hepatitis C testing and linkage to care in Baltimore. J Viral Hepat 2016; 23: 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera JL. Patients with hepatitis C are best managed by a specialist in liver diseases. PRO: management of hepatitis C by liver disease specialists. Am J Gastroenterol 2007; 102: 1837–1839. [DOI] [PubMed] [Google Scholar]
- Shiffman ML. A balancing view: we cannot do it alone. Am J Gastroenterol 2007; 102: 1841–1843. [DOI] [PubMed] [Google Scholar]
- Volk ML. Antiviral therapy for hepatitis C: why are so few patients being treated? J Antimicrob Chemother 2010; 65: 1327–1329. [DOI] [PubMed] [Google Scholar]
- McGovern BH. Editorial commentary: hepatitis C virus and the infectious disease physician: a perfect match. Clin Infect Dis 2012; 55: 414–417. [DOI] [PubMed] [Google Scholar]
- Asrani SK, Davis GL. Impact of birth cohort screening for hepatitis C. Curr Gastroenterol Rep 2014; 16: 381. [DOI] [PubMed] [Google Scholar]
- Thomson M, Konerman MA, Choxi H et al. Primary care physician perspectives on hepatitis C management in the era of direct-acting antiviral therapy. Dig Dis Sci 2016. [DOI] [PMC free article] [PubMed]
- Hepatitis C Online. Cost and Access to Direct-acting Antiviral Agents. 2016. http://www.hepatitisc.uw.edu/pdf/evaluation-treatment/cost-access-medications/core-concept/all.
- Saag MS. Editorial commentary: getting smart in how we pay for HCV drugs: KAOS vs CONTROL. Clin Infect Dis 2015; 61: 169–170. [DOI] [PubMed] [Google Scholar]
- Pollack A. Sales of Sovaldi, New Gilead Hepatitis C Drug, Soar to $10.3 Billion. New York Times, 3 February 2015.
- Barua S, Greenwald R, Grebely J et al. Restrictions for medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163: 215–223. [DOI] [PubMed] [Google Scholar]
- McCombs JS, Tonnu-MiHara I, Matsuda T et al. O003: Can hepatitis c treatment be safely delayed? evidence from the veterans administration healthcare system. J Hepatol 2015; 62: S191. [Google Scholar]
- Zahnd C, Salazar-Vizcaya L, Dufour JF et al Impact of Deferring HCV Treatment on Liver-related Events in HIV+ Patients. Conference on Retroviruses and Opportunistic Infections (CROI). 2015.
- Leidner AJ, Chesson HW, Xu F et al. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology 2015; 61: 1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Sciences. Chronic Hepatitis C Treatment Expansion: Generic Manufacturing for Developing Countries. 2015. http://www.gilead.com/~/media/files/pdfs/other/hcv%20generic%20agreement%20fast%20facts%2072815.pdf.
- Akyar E, Seneca KH, Akyar S et al. Linkage to care for suburban heroin users with hepatitis C virus infection, New Jersey, USA. Emerg Infect Dis 2016; 22: 907–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibbell JE, Iqbal K, Patel RC et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ⩽30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep 2015; 64: 453–458. [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Vickerman P, Grebely J et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013; 58: 1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickmund SL, Campbell SA, Tirado CF et al. Perceived barriers to hepatitis C therapy for patients receiving opioid agonist treatment. J Addict Med 2012; 6: 233–239. [DOI] [PubMed] [Google Scholar]
- Walley AY, White MC, Kushel MB et al. Knowledge of and interest in hepatitis C treatment at a methadone clinic. J Subst Abuse Treat 2005; 28: 181–187. [DOI] [PubMed] [Google Scholar]
- Simmons B, Saleem J, Hill A et al. Risk of late relapse or reinfection with hepatitis c virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis 2016. [DOI] [PMC free article] [PubMed]
- Organization WH. Draft Global Health Sector Strategies: Viral Hepatitis, 2016-2021. 2016.
- Buckley GJ, Strom BL; Committee on a National Strategy for the Elimination of Hepatitis B and C. Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase One Report. National Academies Press: Washington, DC, 2016. [PubMed]