Abstract
Objectives:
Diabetes has become an epidemic in developed and developing countries alike, with an increased demand for new efficacious treatments. A large body of pre-clinical evidence suggests that the gut–brain axis may be exploited as a potential therapeutic target for defective glucose homeostasis. This clinical study aimed to investigate a comprehensive panel of glucoregulatory peptides, released by both the gut and brain, in individuals after acute pancreatitis.
Methods:
Fasting levels of glucagon-like peptide-1 (GLP-1), glicentin, oxyntomodulin, peptide YY, ghrelin, cholecystokinin, vasoactive intestinal peptide (VIP), and secretin were studied. Modified Poisson and multivariable linear regression analyses were conducted. Pre-determined concentration ranges were used to categorize each peptide into quartiles.
Results:
A total of 83 individuals were included, of who 30 (36%) developed abnormal glucose metabolism (AGM) after acute pancreatitis. In individuals with AGM, the highest quartile of oxyntomodulin differed most significantly from the lowest quartile with a prevalence ratio (PR; 95% confidence interval) of 0.50 (0.21, 1.20; P=0.005); of glicentin with a PR of 0.26 (0.13, 0.54; P<0.001); and of VIP with a PR of 0.34 (0.13, 0.89; P=0.043). Peptide YY, GLP-1, cholecystokinin, ghrelin, and secretin were not significantly associated with AGM.
Conclusions:
Fasting circulating oxyntomodulin, glicentin, and VIP levels are significantly decreased in patients with defective glucose homeostasis after acute pancreatitis. Oxyntomodulin appears to be a promising therapeutic target for future clinical studies on diabetes associated with diseases of the exocrine pancreas.
Introduction
Diabetes is a pervasive disease with a tremendous health and economic burden; an estimated 285 million people worldwide were affected by the disease in 2010, and it is projected to increase to 439 million by 2030.1 This is a 69% increase in number of adults with diabetes in developing countries, and 20% increase in developed countries over the span of 20 years.1 Therapeutic armamentarium for treating diabetes has broadened considerably in the last decade (glucagon-like peptide-1 (GLP-1) agonists, inhibitors of dipeptidyl peptidase-4), driven partly by improved understanding of the role of the gut in diabetes,2, 3, 4 while metformin remains first choice oral glucose-lowering drug in type 2 diabetes and has profound effects on the gut.5
The gastrointestinal tract had first been implicated in the pathogenesis of diabetes in 1930s and the discovery of glucose-dependent insulinotropic peptide and sequence of the human proglucagon in 1970–1980s ushered in the era of incretins.6 Although original concepts suggested that glucoregulatory peptides affecting pancreatic islet cells are secreted by enteroendocrine cells only, more recent studies have established that the same peptides (e.g., GLP-1, oxyntomodulin, ghrelin, and cholecystokinin) are also produced by the brain. Moreover, there is a bidirectional communication system involving endocrine, neural, and immunological signaling pathways by which the digestive system and central nervous system interact and play an important role in energy regulation and metabolism. This is termed the gut–brain axis.7, 8, 9
Peptides of the gut–brain axis exert their actions through structurally related receptors belonging to 7-transmembrane domain, heterotrimeric G protein-coupled receptors superfamily.10 They are promising new targets for treatment of diabetes11, 12 but specific drug development must be informed by sound knowledge of the pathogenesis of diabetes. However, diabetes is not a single homogeneous disease but rather composed of several diseases with hyperglycemia as a common feature; hence, the need for precise patient characterization in clinical studies is being increasingly recognized.13, 14, 15 Diabetes associated with diseases of the exocrine pancreas is a form of secondary diabetes that accounts for up to 10% of diabetes in the Western population.16, 17, 18 Further, this estimate is based on the studies focused on new onset diabetes after chronic pancreatitis and pancreatic cancer, but not acute pancreatitis (AP). Given the recently emerged evidence from clinical and population-based studies of a high incidence of diabetes after AP (regardless of the magnitude of macroscopic mechanical destruction of the pancreas)19, 20, 21, 22 and taking into account that AP is the most frequent disease of the pancreas,23 it is very likely that the relative contribution of diabetes associated with diseases of the exocrine pancreas has actually been underestimated in the literature.
We hypothesized that defective glucose homeostasis, secondary to disease of the exocrine pancreas, can be associated with dysfunction of the gut–brain axis. The aim of this study was to investigate the relationships between peptides known to be produced in both the gut and brain and glucose metabolism in patients after AP as an exemplar of a relatively homogeneous population with high risk of developing incident diabetes.
Methods
Study design
This was a cross-sectional follow-up study of patients with AP admitted to Auckland City Hospital, a tertiary center in Auckland, New Zealand, from January 2010 to December 2014. The Health and Disability Ethics Committee (13/STH/182) and the Auckland District Health Board (ADHB) Institution (A+ 6139) approved the study protocol.
Study population
All eligible individuals were telephoned, after their contact details were extracted from the ADHB clinical database (Concerto software, Orion Health Group Ltd, Auckland, New Zealand), and invited to participate in the study. Eligible individuals were recruited if they had a primary diagnosis of AP based on the international guidelines;24 resided in Auckland at the time of the study; were at least 18 years of age; and provided informed consent. Home visits, by a certified phlebotomist, were arranged for those individuals unable to visit the hospital. Individuals were not eligible if they had diabetes or prediabetes (as defined below) before the first hospital admission due to AP; chronic pancreatitis; intra-operative diagnosis of pancreatitis; post-ERCP pancreatitis; malignancy; or were pregnant at the time of AP or afterwards.
Sample acquisition and storage
All participants were required to fast for at least 8 h and attend the clinic at 08:00 hours. Patients were then accompanied to the International Accreditation New Zealand (IANZ) accredited tertiary referral medical laboratory, LabPlus, at Auckland City Hospital. A certified phlebotomist collected venous blood into two ethylene-diamine-tetra-acetic acid tubes, one plasma separation tube, one fluoride tube, and one lithium heparin tube. The tubes were centrifuged at 4,000 g for 7.5 min at 4 °C, followed by plasma separation into aliquots of 400 μl, and storage of the eppendorf tubes at −80 °C until use.
Definitions
Normal glucose metabolism was defined as fasting blood glucose (FBG) ≤5.6 mmol/l and/or glycated hemoglobin A1c (HbA1c) ≤38 mmol/mol. 25
Abnormal glucose metabolism (AGM) was defined as FBG >5.6 mmol/l and/or HbA1c ≥39 mmol/mol. 25
HOMA-%β: insulin (pmol/l) and glucose (mmol/l) values were entered into the validated HOMA2 calculator (HOMA2 v2.2.3 Diabetes Trials Unit, University of Oxford) 26 to calculate the β-cell mass percentage for each participant.
HOMA-IR: insulin (pmol/l) and glucose (mmol/l) values were entered into the validated HOMA2 calculator (HOMA2 v2.2.3 Diabetes Trials Unit, University of Oxford) 26 to calculate insulin resistance for all participants.
Current smoking status was recorded as a binary response, yes or no, based on a questionnaire asking participants if they smoked cigarettes or tobacco related products on a daily basis.
Physical activity was recorded as a binary response, active or inactive, based on a questionnaire asking participants if they engaged in physical activity for at least half an hour per day, five days a week.
Body mass index (BMI) (kg/m2) was determined using a digital medical scale with stadiometer. For weight measurement (kg), patients were asked to remove shoes, jacket, belt, watch, and to empty their pockets of all items. For height measurement (cm), study participants were asked to remove their shoes and any head attire.
Etiology was categorized as biliary, alcohol-induced, or other (including but not limited to pancreatic divisum, idiopathic pancreatitis, and triglyceride-induced pancreatitis). Cause of AP was ascetained from patients' discharge notes.
Duration from first attack of AP was defined as months elapsed from first hospital admission due to AP to the time of the study. For each patient, the date of the first AP admission was recorded from the ADHB clinical database (Concerto software, Orion Health Group Ltd).
Recurrence of AP: individuals admitted with one or more episodes of confirmed AP since their first admission with AP to the time of their participation in the study were considered to have recurrent AP.
Severity of AP was defined according to the Determinant-Based Classification. 27
Laboratory assays
Blood tests for insulin, FBG, and HbA1c were conducted at LabPlus, an IANZ accredited medical laboratory at Auckland City Hospital. An enzymatic colourimetric assay (2015F. Hoffmann-La Roche Ltd., Basel, Switzerland) was used to measure FBG while insulin was measured using chemiluminescence sandwich immunoassay (2015 Roche Products NZ Ltd and Roche Diagnostics NZ Ltd). Glycated hemoglobin A1c was measured using the boronate affinity chromatography assay (2015 Trinity Biotech, Bray, Co Wicklow, Ireland).
Glucagon-like peptide-1, glicentin, oxyntomodulin, peptide YY (PYY), ghrelin, cholecystokinin, vasoactive intestinal peptide (VIP), and secretin were measured using the Merck-Millipore (MA, USA) ELISA kits as per the user's manual. The Rayto Microplate Reader (V-2100C, Santa Fe, Granada, Spain) with an absorbance of 405–630 nm was used to read the results. The intra-assay and inter-assay variation was <10% and 15%, respectively. Glucagon-like peptide-1, oxyntomodulin, PYY, ghrelin, cholecystokinin, VIP, and secretin results were reported in ng/ml, while results for glicentin were reported in pmol/l.
Statistical analyses
The χ2 test was used to evaluate the differences in baseline characteristics between participants with normal glucose metabolism and AGM. All data were presented either as frequency or as mean and standard deviation (s.d.).
Modified Poisson regression analysis, using the Generalised Linear Model, was used to study the association between AGM and each glucoregulatory peptide. The peptides were categorized into quartiles based on pre-determined concentration ranges. These were calculated using the frequencies function. The p-trend was then calculated by assigning each patient the median value in their quartile and evaluating this as a continuous variable. Each glucoregulatory peptide was investigated as an independent variable in both unadjusted and three adjusted models. Model 1 was adjusted for age, sex, and ethnicity, whereas model 2 was adjusted for age, sex, ethnicity, BMI, smoking status, physical activity, recurrence of AP, etiology, duration from first attack of AP, and severity of AP. Covariates found to be statistically significant in model 2 for each glucoregulatory peptide were then adjusted for in corresponding model 3. Offset value was set as one and a main effects model was fit for all four models to obtain the most conservative estimates. Potential over-dispersion was accounted for by fitting Pearson's χ2 as the scale parameter. Robust estimator for covariance matrix was selected to obtain the most robust estimates. All data were presented as prevalence ratio (PR) with corresponding 95% confidence intervals (CI).
Linear regression analysis, using the linear regression function, was used to study first the associations between oxyntomodulin, glicentin, and VIP and the other glucoregulatory peptides. Second, it was used to study the associations between oxyntomodulin and cholecystokinin, VIP, and secretin. For the first linear regression analysis, model 1 was adjusted for age, sex, and ethnicity, whereas model 2 was adjusted for age, sex, ethnicity, BMI, smoking status, physical activity, recurrence of AP, etiology, duration from first attack of AP, and severity of AP. Covariates found to be statistically significant in model 2 for each glucoregulatory peptide were then adjusted for in corresponding model 3. For the second linear regression analysis, model 1 was adjusted for cholecystokinin and VIP; model 2 was adjusted for cholecystokinin and secretin; model 3 was adjusted for secretin and VIP; and model 4 was adjusted for cholecystokinin, secretin, and VIP. All data were presented as β coefficients with 95% CI.
All analyses were conducted using SPSS for Windows Version 23 (SPSS, Chicago, IL). P value <0.05 was deemed to be statistically significant in all analyses.
Results
Study population
Eighty-three individuals were recruited into the study. Of them, 30 (36%) developed AGM after AP and 53 did not. Table 1 shows the baseline characteristics of the two study groups. The median (interquartile range) concentrations of the glucoregulatory peptides in the overall cohort were as follows: cholecystokinin, 0.98 (0.45–1.19) ng/ml; ghrelin, 7.64 (4.24–14.36) ng/ml; glicentin, 6.15 (3.00–16.69) pmol/l; GLP-1, 2.31 (0.39–2.88) ng/ml; oxyntomodulin, 15.48 (0.78–23.80) ng/ml; PYY, 34.76 (9.20–166.05) ng/ml; VIP, 0.66 (0.30–0.69) ng/ml; and secretin 0.43 (0.35–0.56) ng/ml.
Table 1. Characteristics of study participants.
| Characteristic | NGM (n=53) | AGM (n=30) | Pvalue |
|---|---|---|---|
| Age (years)a | 47±15 | 57±13 | 0.005 |
| Sex | 0.485 | ||
| Male | 30 | 20 | |
| Female | 23 | 10 | |
| Ethnicity | 0.005 | ||
| NZ European | 29 | 18 | |
| Maori | 1 | 5 | |
| Pacific Islanders | 2 | 1 | |
| Asian | 4 | 5 | |
| Other | 17 | 1 | |
| Severity | <0.001 | ||
| Mild | 50 | 18 | |
| Moderate | 2 | 9 | |
| Severe/Critical | 1 | 3 | |
| Etiology | 0.906 | ||
| Biliary | 24 | 13 | |
| Alcohol | 12 | 6 | |
| Other | 17 | 11 | |
| Smoking | 0.179 | ||
| No | 44 | 21 | |
| Yes | 9 | 9 | |
| Physical activity | 1.000 | ||
| No | 15 | 8 | |
| Yes | 38 | 22 | |
| Recurrence | 0.448 | ||
| No | 40 | 20 | |
| Yes | 13 | 10 | |
| Duration from 1st attack of AP (months)a | 33±30 | 23±19 | 0.112 |
| BMI (kg/m2)a | 26.86±4.82 | 29.93±6.07 | 0.016 |
| HbA1c (mmol/mol)a | 33.57±2.62 | 39.13±3.00 | <0.001 |
| FBG (mmol/l)a | 4.98±0.29 | 5.87±0.77 | <0.001 |
| HOMA-IRa | 1.09±0.63 | 1.71±1.16 | 0.002 |
| HOMA-%βa | 99.81±33.39 | 98.32±45.11 | 0.865 |
AGM, abnormal glucose metabolism; AP, acute pancreatitis; BMI, body mass index; FBG, fasting blood glucose; HbA1c, glycated hemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-%β, homeostasis model assessment of percentage beta-cell mass; NGM, normal glucose metabolism.
Data are presented as mean±s.d. Significant (P<0.05) associations are shown in bold.
Association between the glucoregulatory peptides and AGM after AP
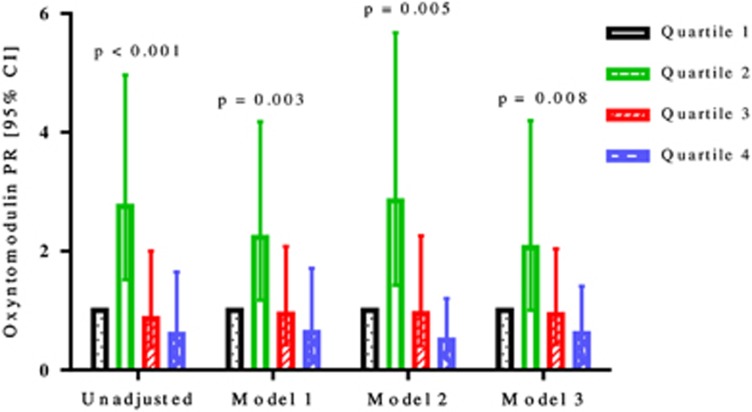
Of the eight glucoregulatory peptides studied, oxyntomodulin, glicentin, and VIP were significantly associated with AGM (Table 2). Oxyntomodulin was significantly associated with AGM in both the unadjusted and the three adjusted models (Figure 1). The highest quartile of oxyntomodulin differed most significantly from the lowest quartile in model 2, with a prevalence ratio (PR; 95% CI) of 0.50 (0.21, 1.20; P=0.005), followed by the unadjusted model with a PR of 0.60 (0.22, 1.65; P<0.001), model 3, with a PR of 0.61 (0.26, 1.41; P=0.008), and model 1, with a PR of 0.63 (0.23, 1.71; P=0.003).
Table 2. Associations between glucoregulatory peptides and AGM.
| Peptide model | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend |
|---|---|---|---|---|---|
| GLP-1 | |||||
| Crude | 1.00 (reference) | 1.27 (0.52, 3.09) | 0.75 (0.30, 1.88) | 1.22 (0.52, 2.88) | 0.579 |
| Model 1 | 1.00 (reference) | 1.12 (0.49, 2.58) | 0.77 (0.33, 1.80) | 0.95 (0.42, 2.16) | 0.735 |
| Model 2 | 1.00 (reference) | 0.88 (0.39, 1.97) | 0.60 (0.26, 1.36) | 0.55 (0.20, 1.52) | 0.727 |
| Model 3 | 1.00 (reference) | 1.00 (0.44, 2.30) | 0.64 (0.27, 1.49) | 0.64 (0.24, 1.70) | 0.720 |
| Glicentin | |||||
| Crude | 1.00 (reference) | 1.15 (0.55, 2.43) | 0.92 (0.43, 1.97) | 0.58 (0.24, 1.37) | 0.550 |
| Model 1 | 1.00 (reference) | 1.37 (0.60, 3.14) | 1.08 (0.53, 2.19) | 0.47 (0.21, 1.04) | 0.274 |
| Model 2 | 1.00 (reference) | 1.27 (0.44, 3.67) | 0.87 (0.33, 2.27) | 0.26 (0.13, 0.54) | <0.001 |
| Model 3 | 1.00 (reference) | 1.60 (0.68, 3.74) | 0.77 (0.33, 1.76) | 0.32 (0.16, 0.62) | 0.001 |
| Oxyntomodulin | |||||
| Crude | 1.00 (reference) | 2.75 (1.52, 4.96) | 0.86 (0.37, 2.00) | 0.60 (0.22, 1.65) | <0.001 |
| Model 1 | 1.00 (reference) | 2.23 (1.18, 4.18) | 0.94 (0.42, 2.08) | 0.63 (0.23, 1.71) | 0.003 |
| Model 2 | 1.00 (reference) | 2.85 (1.43, 5.68) | 0.95 (0.40, 2.26) | 0.50 (0.21, 1.20) | 0.005 |
| Model 3 | 1.00 (reference) | 2.06 (1.01, 4.20) | 0.93 (0.43, 2.04) | 0.61 (0.26, 1.41) | 0.008 |
| PYY | |||||
| Crude | 1.00 (reference) | 1.27 (0.33, 4.84) | 1.71 (0.45, 6.54) | 2.48 (0.67, 9.17) | 0.240 |
| Model 1 | 1.00 (reference) | 1.26 (0.38, 4.17) | 1.56 (0.50, 4.89) | 1.74 (0.47, 6.42) | 0.608 |
| Model 2 | 1.00 (reference) | 1.04 (0.26, 4.16) | 1.08 (0.30, 3.90) | 1.16 (0.20, 6.65) | 0.916 |
| Model 3 | 1.00 (reference) | 1.19 (0.29, 5.93) | 1.64 (0.40, 6.66) | 1.94 (0.48, 7.87) | 0.495 |
| Cholecystokinin | |||||
| Crude | 1.00 (reference) | 2.00 (0.96, 4.16) | 1.14 (0.54, 2.41) | 0.75 (0.30, 1.87) | 0.150 |
| Model 1 | 1.00 (reference) | 1.72 (0.76, 3.88) | 1.14 (0.58, 2.26) | 0.84 (0.37, 1.92) | 0.284 |
| Model 2 | 1.00 (reference) | 1.73 (0.73, 4.07) | 1.01 (0.49, 2.12) | 0.82 (0.35, 1.91) | 0.267 |
| Model 3 | 1.00 (reference) | 1.68 (0.76, 3.72) | 1.06 (0.55, 2.06) | 0.76 (0.34, 1.68) | 0.340 |
| Ghrelin | |||||
| Crude | 1.00 (reference) | 0.83 (0.35, 1.97) | 1.05 (0.45, 2.45) | 1.24 (0.59, 2.58) | 0.837 |
| Model 1 | 1.00 (reference) | 0.88 (0.39, 1.99) | 0.95 (0.42, 2.15) | 0.98 (0.51, 1.89) | 0.961 |
| Model 2 | 1.00 (reference) | 1.40 (0.56, 3.45) | 0.98 (0.41, 2.36) | 0.71 (0.29, 1.71) | 0.975 |
| Model 3 | 1.00 (reference) | 1.01 (0.49, 2.05) | 0.87 (0.42, 1.82) | 0.72 (0.35, 1.49) | 0.999 |
| VIP | |||||
| Crude | 1.00 (reference) | 1.24 (0.55, 2.78) | 1.21 (0.60, 2.43) | 0.56 (0.18, 1.74) | 0.497 |
| Model 1 | 1.00 (reference) | 1.46 (0.63, 3.41) | 1.18 (0.63, 2.22) | 0.55 (0.19, 1.56) | 0.248 |
| Model 2 | 1.00 (reference) | 2.03 (0.88, 4.69) | 0.95 (0.42, 2.12) | 0.34 (0.13, 0.89) | 0.043 |
| Model 3 | 1.00 (reference) | 1.84 (0.78, 4.33) | 0.94 (0.47, 1.90) | 0.46 (0.19, 1.11) | 0.111 |
| Secretin | |||||
| Crude | 1.00 (reference) | 1.69 (0.77, 3.68) | 1.17 (0.44, 3.12) | 1.05 (0.41, 2.72) | 0.472 |
| Model 1 | 1.00 (reference) | 1.02 (0.46, 2.27) | 0.85 (0.35, 2.06) | 0.74 (0.32, 1.73) | 0.930 |
| Model 2 | 1.00 (reference) | 0.82 (0.33, 2.04) | 0.77 (0.30, 1.97) | 0.48 (0.18, 1.27) | 0.613 |
| Model 3 | 1.00 (reference) | 1.10 (0.52, 2.30) | 0.88 (0.37, 2.08) | 0.63 (0.27, 1.46) | 0.733 |
GLP-1, glucagon-like peptide-1; PYY, peptide YY; VIP, vasoactive intestinal peptide. All data are presented as PR (95% CI); Model 1 for all glucoregulatory peptides was adjusted for age, sex, and ethnicity; Model 2 for all glucoregulatory peptides was adjusted for age, BMI, duration from first attack of AP, sex, ethnicity, etiology, recurrence, severity, smoking, and physical activity; Model 3 was adjusted for only those risk factors found to be significant in Model 2. Model 3 for GLP-1 was adjusted for severity, ethnicity, and age. Model 3 for oxyntomodulin was adjusted for severity, ethnicity, and age. Model 3 for glicentin was adjusted for etiology, severity, ethnicity, age, and BMI. Model 3 for PYY was adjusted for severity. Model 3 for cholecystokinin was adjusted for severity, ethnicity, and BMI. Model 3 for ghrelin was adjusted for severity, ethnicity, age, and BMI. Model 3 for VIP was adjusted for severity, ethnicity, age, and smoking. Model 3 for secretin was adjusted for ethnicity, age, and severity. Significant (P<0.05) associations are shown in bold.
Figure 1.
Prevalence ratios with 95% confidence intervals of oxyntomodulin by unadjusted and three adjusted models for patients with abnormal glucose metabolism after acute pancreatitis.
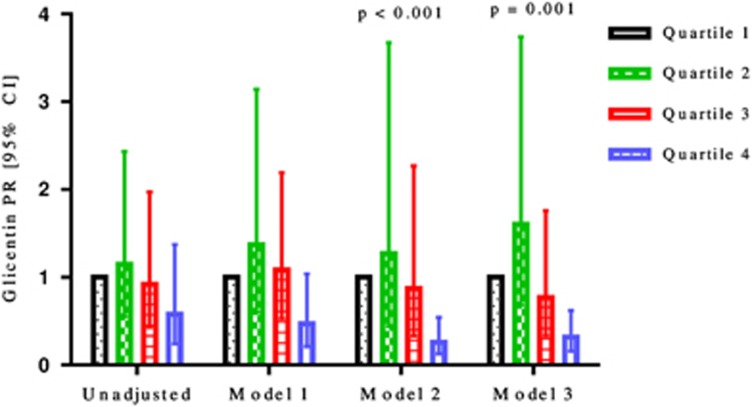
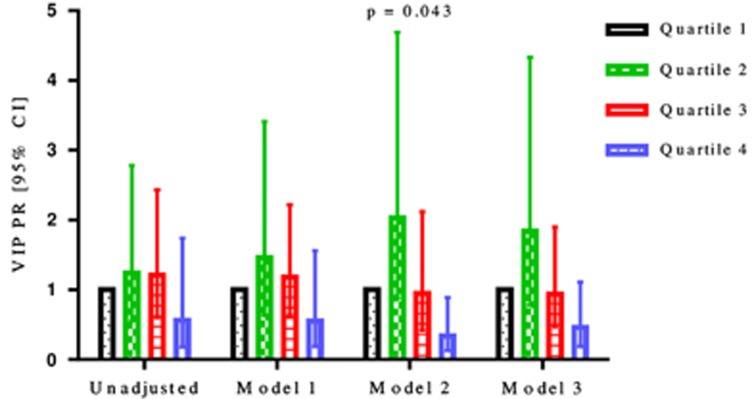
Glicentin and VIP were significantly associated with AGM in adjusted models only (Figures 2 and 3, respectively). The highest quartile of glicentin differed significantly from the lowest quartile with a PR of 0.26 (0.13, 0.54; P<0.001) in model 2, and with a PR of 0.32 (0.16, 0.62; P=0.001) in model 3. The highest quartile of VIP differed significantly from the lowest quartile with a PR of 0.34 (0.13, 0.89; P=0.043) in model 2. Glucagon-like peptide-1, PYY, cholecystokinin, ghrelin, and secretin were not significantly associated with AGM after AP in both the unadjusted and the three adjusted models (Table 2).
Figure 2.
Prevalence ratios with 95% confidence intervals of glicentin by unadjusted and three adjusted models for patients with abnormal glucose metabolism after acute pancreatitis.
Figure 3.
Prevalence ratios with 95% confidence intervals of vasoactive intestinal peptide by unadjusted and three adjusted models for patients with abnormal glucose metabolism after acute pancreatitis.
Associations between oxyntomodulin, glicentin, vasoactive intestinal peptide and other glucoregulatory peptides
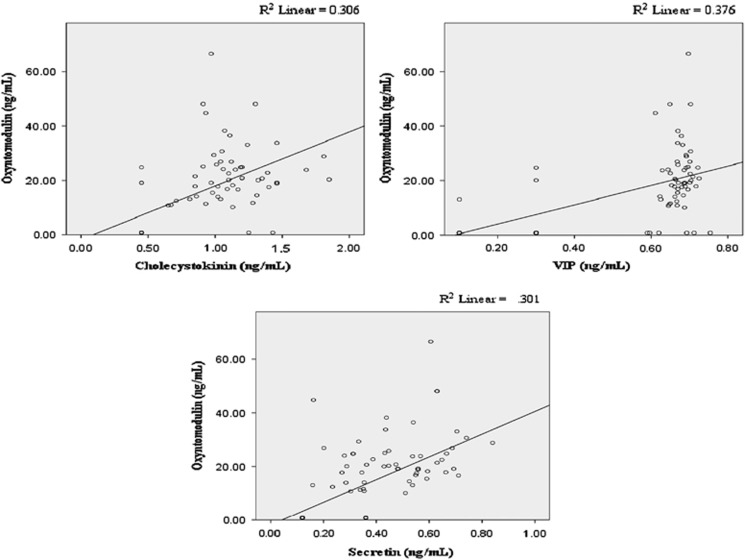
The associations between oxyntomodulin, glicentin, VIP, and other glucoregulatory peptides are shown in Table 3. Oxyntomodulin was significantly associated with cholecystokinin (Figure 4, panel A), VIP (Figure 4, panel B), and secretin (Figure 4, panel C) in both the unadjusted and three adjusted models. For every 1 ng/ml increase in cholecystokinin, oxyntomodulin increased by 20.09 ng/ml (P<0.001) in model 2, by 19.76 ng/ml (P<0.001) in the unadjusted model and model 3, and by 19.56 ng/ml (P<0.001) in model 1. For every one ng/ml increase in VIP, oxyntomodulin increased by 38.34 ng/ml (P<0.001) in model 2, by 36.14 ng/ml (P<0.001) in model 1, and by 35.61 ng/ml (P<0.001) in both the unadjusted model and model 3. For every 1 ng/ml increase in secretin, oxyntomodulin increased by 53.89 ng/ml (P<0.001) in model 2, by 45.25 ng/ml (P<0.001) in model 1, and by 42.32 ng/ml (P<0.001) in both the unadjusted model and model 3.
Table 3. Associations between oxyntomodulin, glicentin, and vasoactive intestinal peptide and all glucoregulatory peptides.
| Peptide model |
Oxyntomodulin |
Glicentin |
VIP |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P value | R2 | β (95% CI) | P value | R2 | β (95% CI) | P value | R2 | |
| Oxyntomodulin | |||||||||
| Unadjusted | – | – | – | 0.24 (−0.05, 0.52) | 0.103 | 0.032 | 0.01 (0.01, 0.02) | <0.001 | 0.376 |
| Model 1 | – | – | – | 0.26 (−0.03, 0.54) | 0.075 | 0.072 | 0.01 (0.01, 0.02) | <0.001 | 0.385 |
| Model 2 | – | – | – | 0.24 (−0.03, 0.52) | 0.083 | 0.132 | 0.01 (0.01, 0.03) | <0.001 | 0.450 |
| Model 3 | – | – | – | 0.24 (−0.05, 0.52) | 0.103 | 0.032 | 0.01 (0.01, 0.03) | <0.001 | 0.428 |
| Glicentin | |||||||||
| Unadjusted | 0.14 (−0.03, 0.30) | 0.111 | 0.032 | – | – | – | 0.00 (−0.01, 0.01) | 0.405 | 0.009 |
| Model 1 | 0.15 (−0.02, 0.33) | 0.087 | 0.044 | – | – | – | 0.00 (−0.01, 0.01) | 0.425 | 0.025 |
| Model 2 | 0.15 (−0.02, 0.32) | 0.083 | 0.103 | – | – | – | 0.00 (−0.01, 0.01) | 0.460 | 0.128 |
| Model 3 | 0.14 (−0.03, 0.30) | 0.101 | 0.060 | – | – | – | 0.00 (−0.01, 0.01) | 0.420 | 0.096 |
| Cholecystokinin | |||||||||
| Unadjusted | 19.76 (13.02, 26.51) | <0.001 | 0.306 | 8.96 (−2.31, 20.23) | 0.119 | 0.033 | 0.31 (0.19, 0.43) | <0.001 | 0.262 |
| Model 1 | 19.56 (12.90, 26.21) | <0.001 | 0.308 | 8.81 (−2.34, 19.96) | 0.121 | 0.076 | 0.31 (0.19, 0.43) | <0.001 | 0.273 |
| Model 2 | 20.09 (13.48, 26.69) | <0.001 | 0.338 | 7.97 (−2.88, 18.83) | 0.150 | 0.133 | 0.32 (0.21, 0.42) | <0.001 | 0.417 |
| Model 3 | 19.76 (13.02, 26.51) | <0.001 | 0.306 | 8.96 (−2.31, 20.23) | 0.119 | 0.033 | 0.30 (0.19, 0.41) | <0.001 | 0.387 |
| Ghrelin | |||||||||
| Unadjusted | 0.13 (−0.16, 0.41) | 0.381 | 0.009 | 0.31 (−0.06, 0.68) | 0.105 | 0.032 | 0.00 (−0.02, 0.08) | 0.199 | 0.020 |
| Model 1 | 0.13 (−0.16, 0.41) | 0.379 | 0.017 | 0.34 (−0.03, 0.71) | 0.069 | 0.072 | 0.03 (−0.02, 0.08) | 0.189 | 0.027 |
| Model 2 | 0.14 (−0.16, 0.43) | 0.359 | 0.077 | 0.24 (−0.13, 0.62) | 0.199 | 0.121 | 0.04 (−0.01, 0.08) | 0.130 | 0.155 |
| Model 3 | 0.13 (−0.16, 0.41) | 0.381 | 0.009 | 0.31 (−0.06, 0.68) | 0.105 | 0.032 | 0.01 (−0.01, 0.07) | 0.261 | 0.104 |
| VIP | |||||||||
| Unadjusted | 35.61 (25.61, 45.61) | <0.001 | 0.376 | 7.45 (−10.08, 24.98) | 0.405 | 0.009 | – | – | – |
| Model 1 | 36.14 (26.29, 45.99) | <0.001 | 0.387 | 7.12 (−10.38, 24.62) | 0.425 | 0.045 | – | – | – |
| Model 2 | 38.34 (27.96, 48.71) | <0.001 | 0.413 | 6.71 (−11.09, 24.52) | 0.460 | 0.115 | – | – | – |
| Model 3 | 35.61 (25.61, 45.61) | <0.001 | 0.376 | 7.45 (−10.08, 24.98) | 0.405 | 0.009 | – | – | – |
| GLP-1 | |||||||||
| Unadjusted | 0.01 (−0.16, 0.18) | 0.915 | 0.000 | 0.10 (−0.13, 0.32) | 0.402 | 0.009 | 0.00 (0.00, 0.01) | 0.081 | 0.036 |
| Model 1 | 0.02 (−0.15, 0.19) | 0.826 | 0.010 | 0.09 (−0.14, 0.31) | 0.457 | 0.041 | 0.00 (0.00, 0.01) | 0.099 | 0.040 |
| Model 2 | 0.05 (−0.15, 0.25) | 0.610 | 0.071 | 0.03 (−0.23, 0.29) | 0.826 | 0.104 | 0.03 (−0.01, 0.06) | 0.051 | 0.185 |
| Model 3 | 0.01 (−0.16, 0.18) | 0.915 | 0.000 | 0.10 (−0.13, 0.32) | 0.402 | 0.009 | 0.02 (0.00, 0.05) | 0.087 | 0.122 |
| PYY | |||||||||
| Unadjusted | 0.01 (−0.02, 0.03) | 0.538 | 0.005 | 0.02 (−0.02, 0.05) | 0.342 | 0.011 | 0.00 (−0.01, 0.01) | 0.331 | 0.012 |
| Model 1 | 0.01 (−0.01, 0.04) | 0.307 | 0.019 | 0.01 [−0.02, 0.05) | 0.494 | 0.042 | 0.00 (−0.01, 0.01) | 0.226 | 0.021 |
| Model 2 | 0.02 (−0.01, 0.04) | 0.213 | 0.093 | 0.01 (−0.03, 0.04) | 0.622 | 0.108 | 0.00 (0.00, 0.01) | 0.240 | 0.143 |
| Model 3 | 0.01 (−0.02, 0.03) | 0.538 | 0.005 | 0.02 (−0.02, 0.05) | 0.342 | 0.011 | 0.00 (−0.01, 0.01) | 0.366 | 0.100 |
| Secretin | |||||||||
| Unadjusted | 42.32 (28.44, 56.20) | <0.001 | 0.301 | 32.94 (11.95, 53.93) | 0.002 | 0.106 | 0.76 (0.51, 1.00) | <0.001 | 0.313 |
| Model 1 | 45.25 (31.72, 58.76) | <0.001 | 0.323 | 32.27 (11.30, 53.24) | 0.003 | 0.130 | 0.78 (0.53, 1.01) | <0.001 | 0.331 |
| Model 2 | 53.89 (39.76, 68.02) | <0.001 | 0.376 | 29.76 (7.26, 52.27) | 0.010 | 0.183 | 0.82 (0.58, 1.06) | <0.001 | 0.447 |
| Model 3 | 42.32 (28.44, 56.20) | <0.001 | 0.301 | 32.94 (11.95, 53.93) | 0.002 | 0.106 | 0.82 (0.58, 1.07) | <0.001 | 0.353 |
GLP-1, glucagon-like peptide-1; PYY, peptide YY; VIP, vasoactive intestinal peptide. All data are presented as β coefficient (95% CI); Model 1 for all glucoregulatory peptides was adjusted for age, sex, and ethnicity; Model 2 for all glucoregulatory peptides was adjusted for age, BMI, duration from first attack of AP, sex, ethnicity, etiology, recurrence, severity, smoking, and physical activity; Model 3 was adjusted for only those risk factors found to be significant in Model 2. Model 3 for glicentin and oxyntomodulin was adjusted for duration. Model 3 for oxyntomodulin and VIP was adjusted for etiology, and duration. Model 3 for oxyntomodulin and secretin was adjusted for etiology, ethnicity, recurrence, and duration. Model 3 for glicentin and VIP was adjusted for duration. Model 3 for glicentin and secretin was adjusted for etiology, recurrence, and duration. Model 3 for VIP and cholecystokinin was adjusted for etiology and duration. Model 3 for VIP and ghrelin, GLP-1, and PYY were adjusted for duration. Model 3 for VIP and secretin was adjusted for etiology. Significant (P<0.05) associations are shown in bold.
Figure 4.
Unadjusted associations between oxyntomodulin and cholecystokinin (a), vasoactive intestinal peptide (b), and secretin (c).
Glicentin was found to be significantly associated with secretin. For every 1 ng/ml increase in secretin, glicentin increased by 32.94 pmol/l (P=0.002) in both the unadjusted model and model 3, by 32.27 pmol/l (P=0.003) in model 1, and by 29.76 pmol/l (P=0.010) in model 2.
Vasoactive intestinal peptide was significantly associated with oxyntomodulin, cholecystokinin, and secretin. For every 1 ng/ml increase in cholecystokinin, VIP increased by 0.32 ng/ml (P<0.001) in model 2, by 0.31 ng/ml (P<0.001) in the unadjusted model and model 1, and by 0.30 ng/ml (P<0.001) in model 3. For every 1 ng/ml increase in secretin, VIP increased by 0.82 ng/ml (P<0.001) in models 2 and 3, by 0.78 ng/ml (P<0.001) in model 1, and by 0.76 ng/ml (P<0.001) in the unadjusted model.
Associations between oxyntomodulin and cholecystokinin, vasoactive intestinal peptide, and secretin
The associations between oxyntomodulin and cholecystokinin, VIP, and secretin are shown in Figure 4. Cholecystokinin was significantly associated with oxyntomodulin in models 1, 2, and 4 (P=0.003, P=0.001, and P=0.015, respectively). Vasoactive intestinal peptide was significantly associated with oxyntomodulin in models 1, 3, and 4 (P<0.001, P<0.001, and P=0.001, respectively). Secretin was significantly associated with oxyntomodulin in models 2 and 3 only (P=0.003, and P=0.010, respectively; Table 4).
Table 4. Associations between oxyntomodulin and cholecystokinin, vasoactive intestinal peptide, and secretin.
| Glucoregulatory peptide model |
Oxyntomodulin |
||
|---|---|---|---|
| β (95% CI) | P value | R2 | |
| Model 1 | 0.442 | ||
| Cholecystokinin | 11.34 (3.97, 18.71) | 0.003 | |
| VIP | 26.11 (14.01, 38.20) | <0.001 | |
| Model 2 | 0.384 | ||
| Cholecystokinin | 13.66 (5.95, 21.38) | 0.001 | |
| Secretin | 25.40 (8.72, 42.07) | 0.003 | |
| Model 3 | 0.427 | ||
| Secretin | 21.56 (5.39, 37.73) | 0.010 | |
| VIP | 26.68 (14.72, 38.64) | <0.001 | |
| Model 4 | 0.459 | ||
| Cholecystokinin | 9.61 (1.94, 17.28) | 0.015 | |
| Secretin | 12.99 (−4.50, 30.49) | 0.143 | |
| VIP | 21.99 (8.78, 35.20) | 0.001 | |
VIP, vasoactive intestinal peptide. All data are presented as β coefficient (95% CI). For oxyntomodulin, Model 1 was adjusted for cholecystokinin and VIP; Model 2 was adjusted for cholecystokinin and secretin; Model 3 was adjusted for VIP and secretin; and Model 4 was adjusted for cholecystokinin, VIP, and secretin. Significant (P<0.05) associations are shown in bold.
Discussion
This is the first human study that has investigated the association between defective glucose homeostasis after diseases of the exocrine pancreas, in particular AP, and a comprehensive panel of peptides, released by both the gut and brain, that were previously shown to have a glucoregulatory effect in physiological and animal studies. The study had a respectable sample size, with the diagnoses of both AGM and AP established prospectively based on the most up-to-date international guidelines (as opposed to International Classification of Diseases codes, prone to misclassification bias). To yield the most robust results, a number of patient-related and pancreatitis-related characteristics as well as risk factors for diabetes were adjusted for by means of multi-level statistical modeling (as opposed to usual correlative analysis). The study has shown a significant decrease in circulating levels of oxyntomodulin, glicentin, and VIP in individuals with AGM. Further, cholecystokinin, secretin, and VIP contributed to nearly half of circulating oxyntomodulin variance in our study population. These findings have translational implications on the pathogenesis of diabetes associated with diseases of the exocrine pancreas and the use of specific drugs to treat it.
The gastrointestinal tract contains a variety of enteroendocrine cells, including the more proximal cells within the stomach (A cells producing ghrelin), duodenum and jejunum (I cells producing cholecystokinin and S cells producing secretin) and more distal cells in the ileum and large intestine (L cells producing GLP-1, oxyntomodulin, glicentin, PYY). There are multiple putative signaling mechanisms by which peptides derived from enteroendocrine cells can influence interactions between the gastrointestinal tract, the nervous system, including the brain, and modulate glucose metabolism. Under physiological conditions, food ingestion is thought to be the primary trigger for activation of the gut–brain axis via the release of regulatory peptides from enteroendocrine cells, via afferent neural pathways (including the vagus nerve), and via cytokine release from immune cells. The feedback information from the brain to the gut (and peripheral organs involved in maintaining blood glucose homeostasis such as the liver, pancreas, adipose tissue, and muscles) is thought to be predominantly carried via autonomic neurons and neuroendocrine factors.28 It is also traditionally believed that plasma levels of most regulatory peptides investigated in this study (with the exception of ghrelin and, perhaps, VIP) rise transiently during and after eating and then fall rapidly below the minimum detectable concentrations as a result of enzymatic inactivation and clearance by kidneys. However, there have been more review articles and news stories on this topic than original studies reporting the initial clinical observations8 and, hence, caution is advised when extrapolating findings from studies in animals and healthy volunteers to human pathophysiology.
There is limited information about changes in the actions of glucoregulatory peptides in the setting of gastrointestinal disorders.29 This study in patients after AP has found that fasting blood levels of each of the eight studied peptides were above the minimum detectable concentrations in more than 90% of patients and none of the patients had all the peptides below their minimum detectable concentrations. Given that the direct effect of food intake on enteroendocrine cells can be ruled out, this finding suggests that, in addition to food, there must be other stimulators for the production of these peptides. It is possible they are being made in the brain and regulating glucose homeostasis via the autonomic nervous system and neurotransmitters. Taking into account the recent study suggesting that the α-melanocyte stimulating hormone can stimulate the release of PYY and GLP-1 from L cells,30 we hypothesize that the gut hormones produced in the brain could be regulating the neurotransmitters which then influence gut hormones that are produced by enteroendocrine cells and released into the circulation. This is further supported by findings of this study that VIP (which is found in the enteric nerves and may function as a neurotransmitter) was significantly associated with both AGM and several glucoregulatory peptides. What induces this alternative path is a matter of speculation but we believe that alterations in the intestinal microbiota might be a potential trigger. The fact that intestinal microbiota undergoes substantial changes in patients with AP (irrespective of its severity) has been recently proven with the use of both polymerase chain reaction-denaturing gradient gel electrophoresis and real-time quantitative polymerase chain reaction in a prospective clinical study.31 It is also known that normal epithelial lining of the gut possesses specialized microvilli that project into the lumen as well as provides information to the brain via ascending neural circuits, strongly suggesting a functional communication between the gut microorganisms and the brain.32 Moreover, a recent systematic review of 44 prospective clinical studies in patients with AP showed that gut barrier dysfunction occurs in almost 60% of patients with AP (irrespective of its severity), thus amplifying the effect of intestinal microbiota on gut–brain communications.33 Carefully designed translational studies are now warranted to investigate molecular mechanisms involved in these complex interactions.
The other notable finding of this study is a 50% decrease in oxyntomodulin (P=0.005) and 74% decrease in glicentin (P<0.001) in individuals with AGM after AP. Oxyntomodulin and glicentin are proglucagon-derived peptides, generated by prohormone convertase 1 in enteroendocrine L cells (predominantly located in the ileum and colon) and the central nervous system (particularly in the brainstem).34, 35, 36 Both peptides are structurally related to glucagon produced in the pancreas. Whereas oxyntomodulin exerts its effect through both the glucagon (GCGR) and GLP-1 (GLP-1 R) receptors, no separate glicentin receptor has been identified yet. The physiological actions of oxyntomodulin and glicentin described to date include stimulation of insulin secretion, stimulation of gut motility, and inhibition of gastric acid secretion.37 However, their individual role in human physiology is difficult (if not impossible) to ascertain as the same actions are also ascribed to other proglucagon-derived peptides such as glucagon and GLP-1.
Although GLP-1 (along with glucose-dependent insulinotropic peptide) has been studied extensively in original pre-clinical and clinical studies, the knowledge base has been extrapolated to other peptides secreted by enteroendocrine cells, in particular oxyntomodulin and glicentin, in the absence of robust clinical studies. It is commonly believed that nutrient ingestion is the primary stimulus for release of oxyntomodulin and glicentin. Our study adds to the literature by delineating, for the first time, the contribution of peptides produced by both the gut and brain. In particular, cholecystokinin contributed independently to 34% of oxyntomodulin variance (after adjustment for age, sex, ethnicity, BMI, smoking, physical activity, duration from first attack of AP, etiology, recurrence, and severity of initial episode of AP). Similarly, secretin, and VIP contributed to 38 and 41% of circulating oxyntomodulin variance (after adjustment for the above confounders), respectively. Other studied peptides (GLP-1, PYY, ghrelin) did not affect the level of oxyntomodulin. Interestingly, glicentin was influenced by fewer peptides and to a much lesser degree, with only secretin contributing independently to 18% of glicentin variance (after adjustment for the above confounders). Cholecystokinin,38, 39 secretin,40, 41, 42 and VIP43, 44 have been repeatedly demonstrated to participate in regulation of glucose homeostasis but the exact signaling pathways remain unknown. Findings from multiple linear regression analyses in this study raise the possibility that they all exert their glucoregulatory effects via signaling pathways converging at oxyntomodulin.
This clinical study in a relatively homogeneous population at risk of developing diabetes also suggests that manipulating signaling of some of the studied peptides may have a therapeutic potential in patients with diabetes associated with diseases of the exocrine pancreas. First, subcutaneously administered oxyntomodulin may prove an efficacious therapeutic option. Several randomized trials have demonstrated that direct administration of oxyntomodulin results in a significant weight loss and reduced energy intake.45, 46 Second, increase in oxyntomodulin secretion could also be achieved indirectly by preventing bile acid absorption and thus increasing the level of cholecystokinin. In this regard, the use of bile acid sequestrants appear to be promising as a recent clinical study showed an improved glycemic control in individuals with impaired glucose tolerance after 8-week treatment with colesevelam.47 Third, given that the intestinal microbiota might be a potential trigger of the derangements of gut–brain axis, transfer of intestinal microbiota from individuals with normal glucose homeostasis may be considered in future studies.48 Findings from a recent proof-of-concept study showed improvements in insulin sensitivity in patients with metabolic syndrome six weeks after infusion of intestinal microbiota from lean individuals.49
Our study has several limitations. First, all blood parameters were investigated in the fasted state. This was done deliberately to decipher and better understand the stand-alone and basal effects of the studied peptides before the effect of nutrient ingestion. Studies that use meal-induced tests and frequently sampled oral glucose tolerance tests are now required to discern further changes in glucose homeostasis in this study population. Second, patients with AGM in this study had a significantly higher BMI, and BMI is known to affect glucose metabolism. However, BMI was adjusted for in all analyses, and BMI alone did not have a significant effect on any of the studied peptides. Further, results from linear regression analysis showed that BMI had no effect in the entire study cohort. Third, although all measures were taken to exclude all patients with known pre-existing abnormalities of glucose metabolism, it is possible that some genetically susceptible patients were not identified, in particular homozygous carriers of the G allele of the glucagon gene50 that are prone to decreased fasting levels of proglucagon-derived peptides. Fourth, some of the studied peptides are not secreted exclusively in the gut and brain. In particular, ghrelin is also secreted in the pancreas.51, 52 Fifth, we assumed that the different circulating level of the peptides investigated in this study reflect their level of secretion while, in theory, it might also be attributed to a differential inactivation of the peptides by corresponding exopeptidases.53 However, with the exception of dipeptidyl peptidase-4 for GLP-1 (which was not significantly changed in this study), there is no commercially available assay to measure them. Last, the cross-sectional study design prevents drawing of inferences as to whether the observed changes in the gut–brain axis are a consequence of defective glucose metabolism or factors causing it.54, 55 A prospective longitudinal study would be the best study design. Yet, the current study provides the most robust evidence on the role of gut–brain axis in derangement of glucose homeostasis after disease of the exocrine pancreas.
In conclusion, the present study shows that defective glucose metabolism after diseases of the exocrine pancreas is associated with decreased fasting level of oxyntomodulin, which is in turn influenced by cholecystokinin, secretin, and VIP. Research that focuses on these peptides should provide further important insights into the physiological mechanisms and pathophysiological role of the cross-talk between the gut and the brain. The molecular machinery involved in this interwoven network could potentially be exploited to develop efficacious new treatments for diabetes.
Study Highlights
Acknowledgments
This study was part of the Clinical and epidemiOlogical inveStigations in Metabolism, nutritiOn, and pancreatic diseaseS (COSMOS) program (Principal Investigator - Dr Petrov).
Footnotes
Guarantor of the article: Maxim S. Petrov.
Specific author contributions: Data collection: Sayali A. Pendharkar and Varsha M. Asrani; analysis of the data and drafting the manuscript: Sayali A. Pendharkar; critical reviewing of the manuscript for intellectual content: Varsha M. Asrani, Rinki Murphy, Richard Cutfield, John A. Windsor, and Maxim S. Petrov; study supervision: Maxim S. Petrov.
Financial support: The Health Research Council of New Zealand (grant 15/035 to Dr Petrov).
Potential competing interests: None.
References
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014; 383: 1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C, Kramer CK, Zinman B et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 2014; 384: 2228–2234. [DOI] [PubMed] [Google Scholar]
- Smits MM, van Raalte DH, Tonneijck L et al. GLP-1 based therapies: clinical implications for gastroenterologists. Gut 2016; 65: 702–711. [DOI] [PubMed] [Google Scholar]
- McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia 2016; 59: 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest 2015; 125: 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014; 146: 1500–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendharkar SA, Singh RG, Petrov MS. Cross-talk between innate cytokines and the pancreatic polypeptide family in acute pancreatitis. Cytokine 2017; 90: 161–168. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Deciphering metabolic messages from the gut drives therapeutic innovation: the 2014 Banting Lecture. Diabetes 2015; 64: 317–326. [DOI] [PubMed] [Google Scholar]
- Obrenovich M, Flückiger R, Sykes L et al. The co-metabolism within the gut-brain metabolic interaction: potential targets for drug treatment and design. CNS Neurol Disord Drug Targets 2016; 15: 127–134. [DOI] [PubMed] [Google Scholar]
- Fredman G, Ozcan L, Tabas I. Common therapeutic targets in cardiometabolic disease. Sci Transl Med 2014; 6: 239ps5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomi T, Santoro N, Caprio S et al. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014; 383: 1084–1094. [DOI] [PubMed] [Google Scholar]
- Leslie RD, Palmer J, Schloot NC et al. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia 2016; 59: 13–20. [DOI] [PubMed] [Google Scholar]
- Li L, Cheng W-Y, Glicksberg BS et al. Identification of type 2 diabetes subgroups through topological analysis of patient similarity. Sci Transl Med 2015; 7: 311ra174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt PD, Brendel MD, Kloer HU et al. Is pancreatic diabetes (type 3c diabetes) underdiagnosed and misdiagnosed? Diabetes Care 2008; 31: S165–S169. [DOI] [PubMed] [Google Scholar]
- Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology 2011; 11: 279–294. [DOI] [PubMed] [Google Scholar]
- Ewald N, Kaufmann C, Raspe A et al. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c). Diabetes Metab Res Rev 2012; 28: 338–342. [DOI] [PubMed] [Google Scholar]
- Das SL, Singh PP, Phillips AR et al. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut 2014; 63: 818–831. [DOI] [PubMed] [Google Scholar]
- Ho T-W, Wu J-M, Kuo T-C et al. Change of both endocrine and exocrine insufficiencies after acute pancreatitis in non-diabetic patients: a nationwide population-based study. Medicine (Baltimore) 2015; 94: e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SL, Kennedy JIC, Murphy R et al. Relationship between the exocrine and endocrine pancreas after acute pancreatitis. World J Gastroenterol 2014; 20: 17196–17205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-K, Huang M-Y, Hsu C-Y et al. Bidirectional relationship between diabetes and acute pancreatitis: a population-based cohort study in Taiwan. Medicine (Baltimore) 2016; 95: e2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao AY, Tan ML, Wu LM et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016; 1: 45–55. [DOI] [PubMed] [Google Scholar]
- Maraví-Poma E, Zubia Olascoaga F, Petrov MS et al. Recommendations for intensive care management of acute pancreatitis. Med Intensiva 2013; 37: 163–179. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. (2) Classification and Diagnosis of Diabetes. Diabetes Care 2015; 38: S8–S16. [DOI] [PubMed] [Google Scholar]
- Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998; 21: 2191–2192. [DOI] [PubMed] [Google Scholar]
- Petrov MS, Windsor JA. Conceptual framework for classifying the severity of acute pancreatitis. Clin Res Hepatol Gastroenterol 2012; 36: 341–344. [DOI] [PubMed] [Google Scholar]
- Latorre R, Sternini C, De Giorgio R et al. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil 2016; 28: 620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman HS, Mallinson CN, Modigliani R et al. Gut hormones in inflammatory bowel disease. Scand J Gastroenterol 1983; 18: 845–852. [DOI] [PubMed] [Google Scholar]
- Panaro BL, Tough IR, Engelstoft MS et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab 2014; 20: 1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Ling Z, Huang Y et al. Dysbiosis of intestinal microbiota associated with inflammation involved in the progression of acute pancreatitis. Pancreas 2015; 44: 868–875. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012; 10: 735–742. [DOI] [PubMed] [Google Scholar]
- Wu LM, Sankaran SJ, Plank LD et al. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg 2014; 101: 1644–1656. [DOI] [PubMed] [Google Scholar]
- Blache P, Kervran A, Bataille D. Oxyntomodulin and glicentin: brain-gut peptides in the rat. Endocrinology 1988; 123: 2782–2787. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ et al. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997; 77: 257–270. [DOI] [PubMed] [Google Scholar]
- Dong CX, Brubaker PL. Ghrelin, the proglucagon-derived peptides and peptide YY in nutrient homeostasis. Nat Rev Gastroenterol Hepatol 2012; 9: 705–715. [DOI] [PubMed] [Google Scholar]
- Naito H, Ohneda A, Kojima R et al. Plasma glicentin in diabetic and gastrectomized patients. Regul Pept 1999; 79: 55–61. [DOI] [PubMed] [Google Scholar]
- Cheung GWC, Kokorovic A, Lam CKL et al. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab 2009; 10: 99–109. [DOI] [PubMed] [Google Scholar]
- Rasmussen BA, Breen DM, Luo P et al. Duodenal activation of cAMP-dependent protein kinase induces vagal afferent firing and lowers glucose production in rats. Gastroenterology 2012; 142: 834–843.e3. [DOI] [PubMed] [Google Scholar]
- Chu JYS, Yung WH, Chow BKC. Secretin: a pleiotrophic hormone. Ann N Y Acad Sci 2006; 1070: 27–50. [DOI] [PubMed] [Google Scholar]
- Hisatomi A, Unger RH. Secretin inhibits glucagon in the isolated perfused dog pancreas. Diabetes 1983; 32: 970–973. [DOI] [PubMed] [Google Scholar]
- Kofod H, Hansen B, Lernmark A et al. Secretin and its C-terminal hexapeptide potentiates insulin release in mouse islets. Am J Physiol 1986; 250: E107–E113. [DOI] [PubMed] [Google Scholar]
- Sanlioglu AD, Karacay B, Balci MK et al. Therapeutic potential of VIP vs PACAP in diabetes. J Mol Endocrinol 2012; 49: R157–R167. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. Tissue specific expression of different human receptor types for pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide: implications for their role in human physiology. J Neuroendocrinol 1996; 8: 811–817. [DOI] [PubMed] [Google Scholar]
- Wynne K, Park AJ, Small CJ et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 2005; 54: 2390–2395. [DOI] [PubMed] [Google Scholar]
- Wynne K, Park AJ, Small CJ et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes 2006; 30: 1729–1736. [DOI] [PubMed] [Google Scholar]
- Marina AL, Utzschneider KM, Wright LA et al. Colesevelam improves oral but not intravenous glucose tolerance by a mechanism independent of insulin sensitivity and β-cell function. Diabetes Care 2012; 35: 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut 2014; 63: 1513–1521. [DOI] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913–916.e7. [DOI] [PubMed] [Google Scholar]
- Torekov SS, Ma L, Grarup N et al. Homozygous carriers of the G allele of rs4664447 of the glucagon gene (GCG) are characterised by decreased fasting and stimulated levels of insulin, glucagon and glucagon-like peptide (GLP)-1. Diabetologia 2011; 54: 2820–2831. [DOI] [PubMed] [Google Scholar]
- Dezaki K, Sone H, Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther 2008; 118: 239–249. [DOI] [PubMed] [Google Scholar]
- Wu LM, Premkumar R, Phillips AR et al. Ghrelin and gastroparesis as early predictors of clinical outcomes in acute pancreatitis. Pancreatology 2016; 16: 181–188. [DOI] [PubMed] [Google Scholar]
- Petrov MS, Windsor JA. Nutritional management of acute pancreatitis: the concept of ‘gut rousing'. Curr Opin Clin Nutr Metab Care 2013; 16: 557–563. [DOI] [PubMed] [Google Scholar]
- Pendharkar SA, Asrani VM, Xiao AY et al. Relationship between pancreatic hormones and glucose metabolism: a cross-sectional study in patients after acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2016; 311: G50–G58. [DOI] [PubMed] [Google Scholar]
- Gillies N, Pendharkar SA, Asrani VM et al. Interleukin-6 is associated with chronic hyperglycemia and insulin resistance in patients after acute pancreatitis. Pancreatology 2016; 16: 748–755. [DOI] [PubMed] [Google Scholar]