Abstract
OBJECTIVES:
We aimed to investigate prognostic effects of plasma levels of ghrelin before and after gastrectomy in gastric cancer (GC).
METHODS:
We followed 81 GC patients up to 3 years in this study. They were candidates for curative gastrectomy with or without neoadjuvant chemotherapy. Plasma levels of total and active ghrelins before and after the operation were assessed. Association of plasma levels of ghrelin with survival were assessed and adjusted for other potential prognostic factors using Cox regression analyses.
RESULTS:
Both total and active ghrelins dropped after gastrectomy (P<0.001 for both). Multiple Cox models revealed worse survival for patients with postoperative total ghrelins below median (hazards ratio (HR)=2.33, 95% confidence interval (CI): 1.01–5.41) or 25th percentile (HR=4.29, 95% CI: 1.48–12.44) compared with patients with higher ghrelin levels. In case of preoperative total ghrelin, patients with either second or third quartiles of plasma ghrelin showed worse survival compared with patients with the lowest quartile (HR=2.67, 95% CI: 1.11–6.38 for second quartile, and HR=2.32, 95% CI: 1.01–5.35 for third quartile vs. the lowest quartile). However, there was no difference between patients with the highest and lowest quartiles (HR=0.78, 95% CI: 0.22–2.73). Similar pattern was observed for preoperative active ghrelin (HR=4.92, 95% CI: 1.80–13.54 for second quartile, and HR=2.87, 95% CI: 1.11–7.38 for third quartile vs. the lowest quartile). Advanced TNM stage (HR=4.88, 95% CI: 1.10–21.77), cachexia (HR=2.99, 95% CI: 1.35–6.63), and receiving no neoadjuvant chemotherapy (HR=2.02, 95% CI: 1.04–3.92) were other poor prognostic factors.
CONCLUSIONS:
Preoperative and postoperative plasma levels of ghrelin could predict survival of GC patients with different patterns. This prognostic effect was independent of stage and cachexia. Measurement of plasma ghrelin in GC patients could complement conventional staging for more precise risk-stratification of the patients. Extrinsic admirations of ghrelin after total gastrectomy has potentials to improve survival of GC patients.
INTRODUCTION
Gastric cancer (GC) is the third leading cause of cancer-related death world-wide.1 Yet, it is associated with a higher health burden in some countries such as Iran, where stomach cancer is the most common cancer among male.2 Several studies have reported a low survival rates of GC in Iran and other low and middle income countries.2, 3, 4 Clinical parameters, including stage at the diagnosis and appropriate treatment (e.g., extent of lymphadenectomy) are the main prognostic factors in GC;3, 4, 5 however, patients with similar clinical stages may experience different survival rates.6 Therefore, research for exploring new diagnostic and prognostic factors is still warranted.
Ghrelin is a 28-amino acid peptide, which is mainly produced by the stomach. Lower amount of this peptide is also produced in other organs such as bowel, immune system, and kidneys.7, 8, 9 Main physiological functions of ghrelin proposed include stimulation of growth hormone, prolactin, and adrenocorticotropic hormone secretion, negative effect on pituitary-gonadal axis, stimulation of appetite and a positive energy balance, control of gastric motility and acid secretion, and regulation of glucose levels.9
Ghrelin can modulate pathogenesis, progression and clinical course of cancers through anti-inflammation,10 regulation of cell proliferation and apoptosis,11, 12 and stimulation of appetite.13 Although ghrelin could increase proliferation and invasion capabilities of tumor cells, it prevents carcinogenesis and orexigenic effects of ghrelin could provide survival advantage for cancer patients.14, 15 In addition, response to ghrelin seems to be varied by cancer type, which make the inter-relation between ghrelin and cancer more complicated.15 Therefore, the role of ghrelin in each cancer in regards to different aspects of tumor behavior should be investigated specifically. Since stomach is the main source of ghrelin in body, contribution of ghrelin to different aspects of tumor biology is expected to be more pronounced in this cancer. Likewise, many studies have investigated different roles of ghrelin in GC.16, 17, 18 Follow-up studies showed that low basal levels of ghrelin increased the risk of stomach cancer development.16 Similarly, administration of extrinsic ghrelin could ameliorate cachexia and increase energy intake in patients with GC;19 however Tian et.al reported that ghrelin may stimulate proliferation and invasion of GC cell lines.18
Despite identification of various local and systemic effects in GC, prognostic effects of ghrelin in this cancer remains to be well investigated. A recent study investigated associations of blood ghrelin with survival in 16 individuals with gastric cancer and 14 with pancreatic or gallbladder cancers. They reported association of low ghrelin levels just prior to adjuvant chemo radiotherapy with poor survival;20 however, low sample size and lack of adjustment for main confounding factors such as stage of tumor limit the inference of this finding. In addition, the prognostic effect of preoperative and postoperative plasma ghrelin levels could be different. By removal of stomach, gastric cancerous cells are no longer exposed to local high amounts of ghrelin and its stimulatory effects on proliferation and invasiveness;18 at this time systemic effects of ghrelin would dominate its contribution to GC prognosis, which could be contrary to effects of preoperative ghrelin. We aimed to assess the independent prognostic effects of preoperative and postoperative plasma ghrelin levels in GC patients. Prognostic role of postoperative ghrelin could also shed further light on safety of extrinsic ghrelin administration after the gastrectomy.
METHODS
In this follow-up study, we enrolled GC patients who were referred to the Cancer Institute of Iran, Tehran, Iran between December 2011 and October 2012. We followed up the patients up to 3 years (until October 2015) after recruitment; median follow-up time was 568 days. We obtained written informed consent from all patients before they were included in the study. Medical Ethics Committee of Tehran University of Medical Sciences approved the study protocol in Octobor 2011.
Patients were screened for eligibility criteria by the time they were referred to oncological surgery wards for hospitalization. Inclusion criteria were gastric adenocarcinoma patients with tumor stages of I–III, who were candidates for curative gastrectomy (total or subtotal) with or without neoadjuvant chemotherapy based on the clinic rules. Our exclusion criteria included: stage IV GC patients, palliative gastrectomy, anorexia or bulimia nervosa; chronic intestinal disorders (e.g., inflammatory bowel disease, congenital bowel disorders); chronic infection except a history of Helicobacter pylori infection; major depressive disorders; chronic rheumatologic disorders; long-time use of corticosteroids, insulin, oral anti-diabetic drugs, psychotherapeutic drugs, or drugs used for treatment of obesity; self-reported cannabinoid abuse; history of surgery for treatment of obesity; history of major surgery during the last six months; prior history of gastric or other cancers; and inoperable GC. By considering 5% as level of significance, to have 90% power to detect a hazards ratio (HR) of 0.5 among different quartiles of plasma level of ghrelin with assumed s.d. of 0.7, we would need to observe at least 45 deaths.21 Considering an estimate of 30% for 3-year overall survival rate of gastric cancer patients3, 4 and a 10% loss to follow-up, we would need to follow at least 75 gastric cancer patients to expect 45 deaths.
Trained interviewers interviewed the patients and collected personal and clinical data such as the date of first symptom(s), type of symptom(s), percentage of weight loss and cachexia, which is defined as the history of weight loss ≥5% during the last 6 months,22 status of feeding and past medical and drug history. Percentage of weight loss was calculated by dividing the weight loss by body weight before weight loss in kilogram. The history given by the patient was confirmed against any available clinical records that contained past medical and drug history and anthropometric data. We collected further clinical and para clinical data from patient records, laboratory tests (i.e., serum levels of hemoglobin and albumin), consultation reports, imaging, and pathological reports. Patients were considered to have a cardiovascular or pulmonary problems based on the consultation reports. Acute postoperative complications such as infection, bleeding, profuse leak from anastomosis of surgical site, and cardiovascular complications (e.g., acute coronary syndrome) were also registered. After the discharge, we collected the follow-up information from hospital records. If the data were not available in hospital, we followed the patients by phone calls every 6-month. Status of the patient (i.e., alive or dead) and precise date of death in case of mortality were registered. We used tumor-node-metastasis (TNM) staging guidelines of the American Joint Committee on Cancer and Union for international Cancer Control.23
Tumor size, location, and histopathological features were considered for selecting the type of gastrectomy. A minimum of five cm proximal margin was kept. Accordingly, patients with tumors located in cardia underwent total gastrectomy. In addition, in case of tumors with diffuse type adenocarcinoma histopathology, total gastrectomy was selected. On the other hand, small tumors in prepyloric area with a histopathology report other than diffuse type adenocarcinoma, were subject to subtotal gastrectomy. After tumor resection, extended D1 (also called D1 plus) lymphadenectomy was performed to dissect lymph nodes at the level of I–VI lymph node stations as well as all significant lymph nodes in D2 zone. After the operation, nasogastric tube (NGT) was fixed for all patients. Enteric feeding was resumed when bowel sounds could be heard and NGT secretions was lower than 500 cc/day, if there was no leakage of bile or gastrointestinal secretions from surgical site, and if there was no sign or symptoms of peritonitis. It usually occurred 3–5 days after the operation. Initially, patients were given pure liquid diet followed by semi-solid and solid diets. There was no difference regarding timing, method, and formula of enteric feeding between patients who underwent total or subtotal gastrectomy.
Plasma levels of active and total ghrelin were assessed before the surgery and afterwards, when the patients resumed enteric feeding. Post-operative assessment was done consistently 4 days after initiating enteral feeding. In patients who had received neoadjuvant chemotherapy, the last session of chemotherapy was 4–5 weeks before current hospitalization for surgery. Blood sampling was done at early morning (around 0600 to 0630 hours), almost two hours before breakfast. Samples were collected in the tubes containing Na2 EDTA 1.25 mg/ml and Aprotinin 500 U/ml. Within seven minutes from sampling, the tubes were quickly centrifuged at 3,000 r.p.m. for 15 min in a cold temperature. We added HCL with a final concentration of 0.05 N to the plasma samples considered for active ghrelin assay. All the samples were stored at −70 degrees of centigrade until the assay.
We used enzyme-linked immunosorbent assay (ELISA) kits (EMD Millipore Corporation, St Charles, MO) to assess the plasma concentration of active and total ghrelin. Measurements were performed by a well-trained and well-experienced technician in doing ELISA. A comprehensive quality control (QC) check was performed before main assessments. It included calibration of the equipments (e.g., pipette, reader), in-house validation of the ELISA kit by comparing standard and in-house pool plasma serial dilution curves, monitoring the environmental setting, particularly the temperature, confirming the quality of specimens, and checking QC samples of the kit. All the samples were read at duplicates. The technician was blind to study end-points. According to the manufacturer, maximum intra-assay and inter-assay coefficients of variation (CV) for total ghrelin kit were 1.91% and 7.81%, respectively (catalog number EZGRT-89K); for active ghrelin kit, these coefficients were 7.53% and 12.9%, respectively (catalog number EZGRA-88K). The sensitivity of the assay was 50 pg/ml for total ghrelin and 15 pg/ml for active ghrelin when using a 20-μl sample. Our intra-assay and inter-assay CVs for total ghrelin were calculated 2.09% and 8.85%, respectively. Intra-assay and inter-assay CVs for active ghrelin were 7.92% and 13.17%, respectively.
Statistical analyses
We mainly aimed to investigate association of plasma levels of ghrelin with patients' survival. For this, plasma levels of ghrelin were categorized into two or four subgroups based on median or quartile values, respectively. Then, we used life-table method to estimate overall survival rates and Log-rank test to compare the overall survival rates between subgroups of patients with different plasma levels of ghrelin. In addition, Kaplan–Meier survival curves demonstrated an estimate of overall survival patterns in whole sample or different subgroups of patients. Parametric paired t-test or non-parametric Wilcoxon test compared the mean values of preoperative and postoperative assessments. In case of patients who were lost to follow up, we did not exclude them; rather we included their last survival status in our total person-time survival data.
We also aimed to adjust the association between plasma levels of ghrelin and survival (overall survival rate) for other potential confounding or suppressing factors. We used univariate and multiple Cox regression analyses to detect these potential parameters and adjust their effects. Hosmer and Lemeshow approach for model building24 was used to fit multiple Cox models. In brief, we first used univariate analyses and considered P-value <0.2 as the cut-point to screen the variables and detect primary candidates for multivariable model. Subsequently, we fitted the primary multivariable model by the variables detected in addition to plasma ghrelin. A backward elimination method was employed to reach to the final model. Plausible interaction terms between final determinants were also checked. Final models were screened for collinearity. Preoperative total ghrelin, preoperative active ghrelin, postoperative total ghrelin and postoperative active ghrelin were separately entered in the multiple models for sensitivity analysis. Cases with missing data for ghrelin were excluded from analysis. Cases with missing data for other variables of interest were included in model building as a new subgroup of corresponding variable designated "undetermined". We used Stata statistical software version SE 11.2 (StataCorp LP, College Station, TX) for statistical analyses.
RESULTS
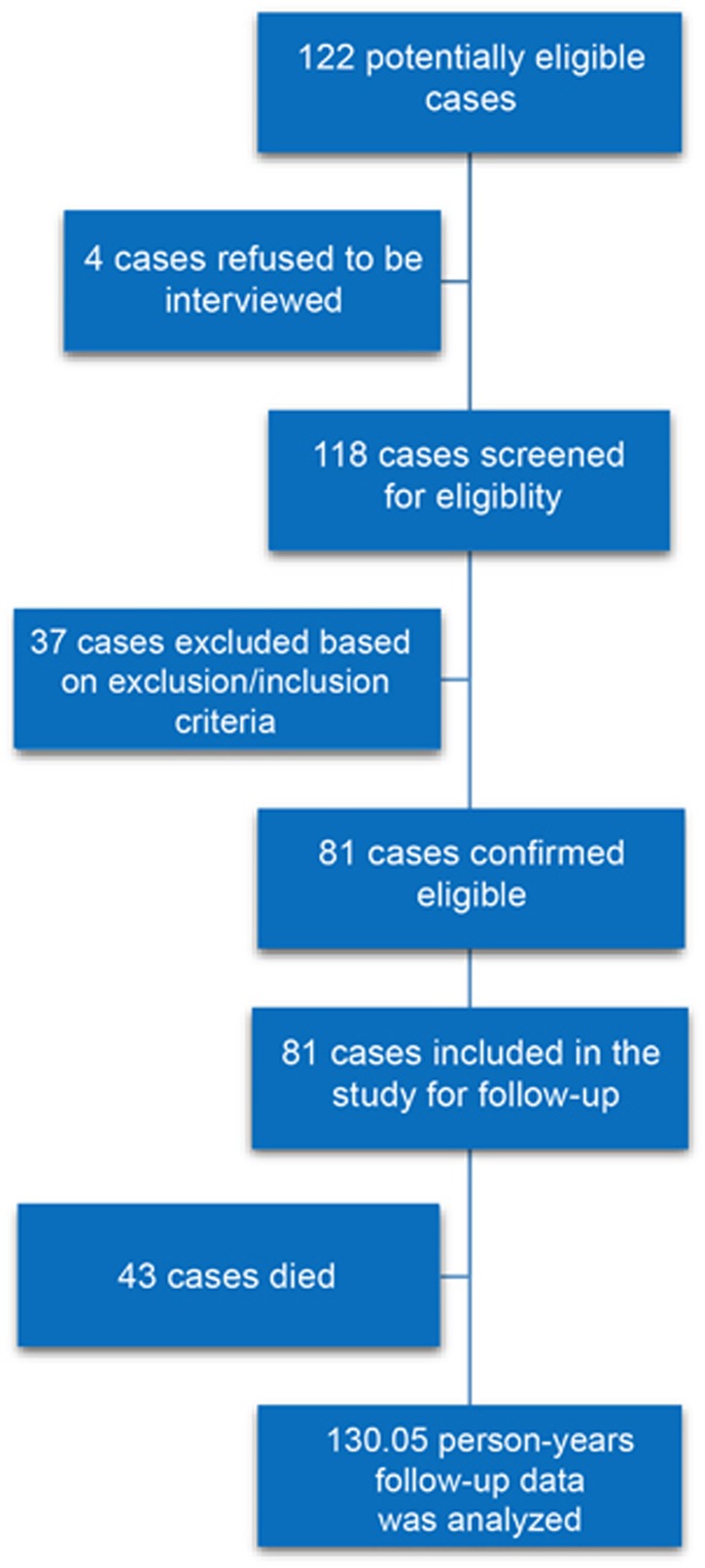
From 118 patients screened for eligibility 81 were included in the study based on inclusion and exclusion criteria (Figure 1). We followed 81 GC patients for 3 years in this study. Finally, data collected for a total of 130.05 person-years follow-up was analyzed. The mean (±s.d.) age of the patients was 60.11 (±14.22) and 76.54% of the patients were male. Most of the tumors (38.27%) originated from distal stomach (body, antrum, and pylorus). Less than half (43.21%) of the patients were diagnosed at stage III of the disease. Before the gastrectomy, 51 (62.96%) patients received neoadjuvant chemotherapy. Cachexia was observed in 42 (51.85%) patients at the time of diagnosis. Maximum body mass index (BMI) was 28.3 kg/m2 (Table 1).
Figure 1.
Flow diagram of study participants. The Figure demonstrates the number of participants in different stages of the study.
Table 1. Personal, clinical, and para clinical characteristics of gastric cancer patients hospitalized in cancer institute of Iran within 2011–2012.
| Age, mean (s.d.), years | 60.11 (14.22) |
| Gender | |
| Male | 62 (76.54) |
| Female | 19 (23.46) |
| Tumor site | |
| Cardia | 25 (30.86) |
| Fundus | 3 (3.70) |
| Distal | 27 (33.33) |
| Overlap | 26 (32.10) |
| T stage | |
| T 0–1 | 7 (8.64) |
| T 2 | 20 (24.69) |
| T 3 | 40 (49.36) |
| T 4 | 14 (17.28) |
| N stage | |
| N 0–1 | 44 (54.32) |
| N 2 | 21 (25.93) |
| N 3 | 16 (19.75) |
| N 4 | 0 |
| Metastasis | |
| Yes | 0 |
| No | 81 (100.00) |
| TNM stage | |
| I | 6 (7.41) |
| II | 40 (49.38) |
| III | 35 (43.21) |
| IV | 0 |
| Grade | |
| Well-differentiated | 10 (12.35) |
| Moderately differentiated | 43 (53.09) |
| Poorly differentiated | 28 (34.57) |
| Tumor size, mean (s.d.), cm | 4.55 (1.89) |
| Neoadjuvant chemotherapy | 54 (66.67) |
| Type of gastrectomy | |
| Total | 66 (81.48) |
| Subtotal | 15 (18.52) |
| BMI, mean (s.d.), kg/m2 | 22.80 (3.91) |
| First symptom—diagnosis delay, mean (s.d.), month | 7.10 (6.34) |
| Loss of appetite | 34 (41.97) |
| Dysphagia | 27 (33.33) |
| Cachexia | |
| Yes | 42 (51.85) |
| No | 29 (35.80) |
| Undetermined | 10 (12.35) |
| Weight loss, mean (s.d.), % | |
| Cachectic patients | 18.47 (9.26) |
| Non-cachectic patients | 2.49 (1.05) |
| Cardiovascular problem | |
| Yes | 15 (18.52) |
| No | 62 (76.54) |
| Undetermined | 4 (4.94) |
| Pulmonary problem | 5 (6.17) |
| Acute postoperative complication(s) | |
| Yes | 6 (7.41) |
| No | 73 (90.12) |
| Undetermined | 2 (2.47) |
| Hemoglobin, mean (s.d.), mg/dl | 11.63 (1.95) |
| Anemia | |
| Yes | 59 (72.84) |
| No | 20 (24.69) |
| Undetermined | 2 (2.15) |
| Albumin, mean (s.d.), mg/dl | 3.81 (0.47) |
| Hypoalbuminemia | |
| Yes | 24 (29.63) |
| No | 52 (64.20) |
| Undetermined | 5 (6.17) |
| Plasma Ghrelin, mean (s.d.), median (IQR), pg/ml | |
| Total (N=81) | |
| Preoperative | 309.14 (276.88), 229.38 (123.81, 348.10) |
| Postoperative | 148.32 (91.41), 123.22 (76.78, 170.53) |
| Active (N=81) | |
| Preoperative | 74.54 (64.29), 54.70 (30.94, 95.16) |
| Postoperative | 50.43 (30.26), 41.72 (25.75, 63.78) |
| Survival, mean (s.d.), median (IQR), year | 1.58 (0.99), 1.55 (0.76, 2.22) |
BMI, body mass index; IQR, interquartile (25th–75th percentile) range.
Values are number (percentages) unless stated otherwise.
We observed statistically significant decrease in the average of both total (P-value <0.001) and active plasma ghrelin levels (P-value <0.001). Although preoperative ghrelin levels were similar in two subgroups of patients who underwent subtotal or total gastrectomy (307.3±316.8 vs. 309.8±263.5 pg/ml, P-value=0.9 for total ghrelin; and 79.2±79.0 vs. 72.9±59.0 pg/ml, P-value=0.7 for active ghrelin), postoperative plasma levels of total and active ghrelin were higher in subtotal gastrectomy subgroup (179.3±99.3 vs. 137.4±78.3 pg/ml, P-value=0.003 for total ghrelin; and 60.2±39.2 vs. 46.6±25.4 pg/ml, P-value=0.01 for active ghrelin).
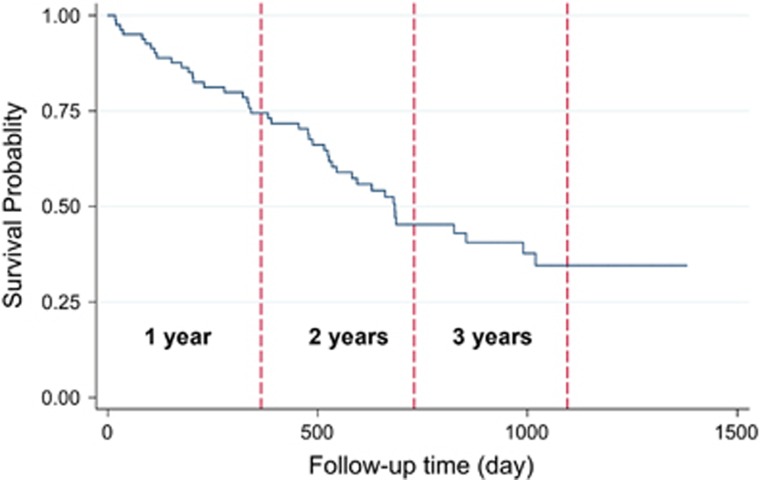
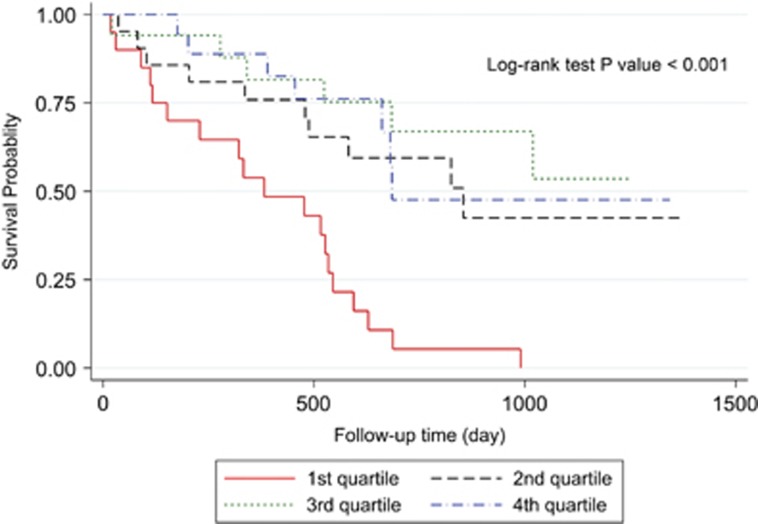
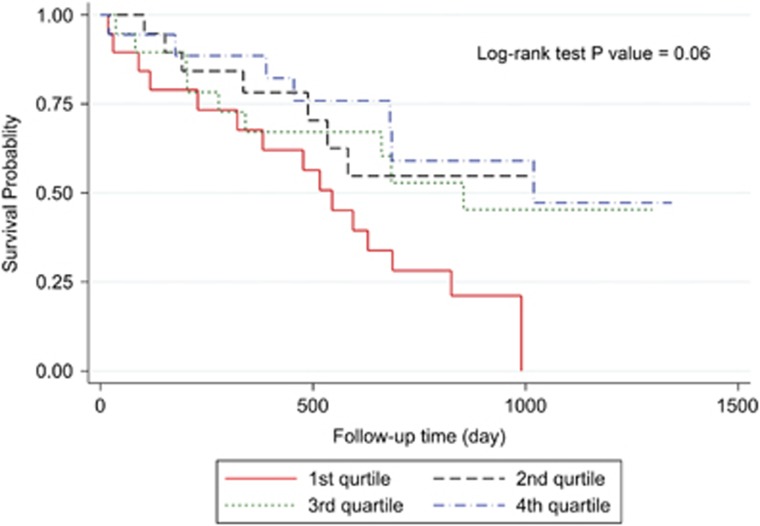
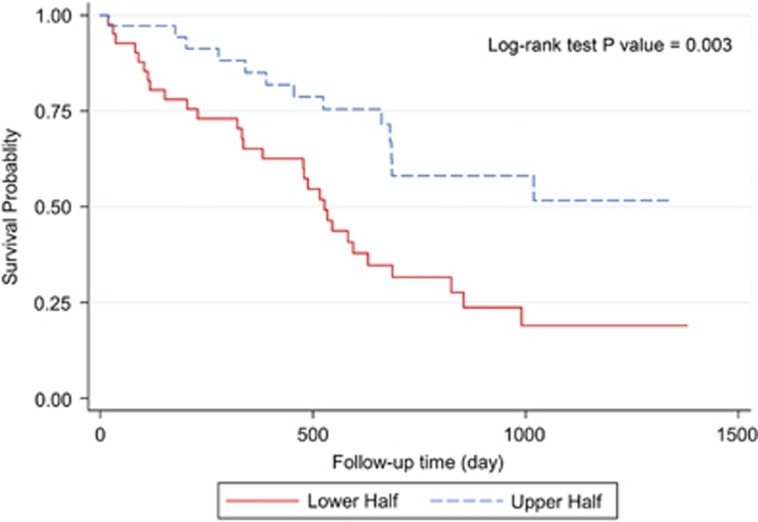
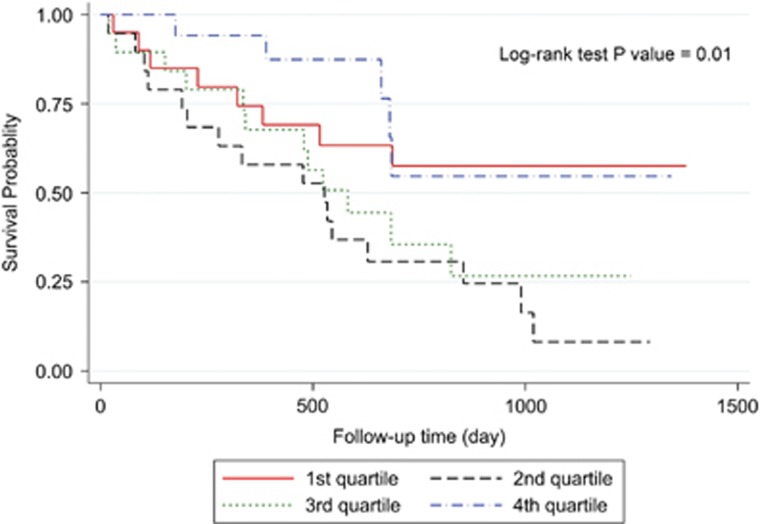
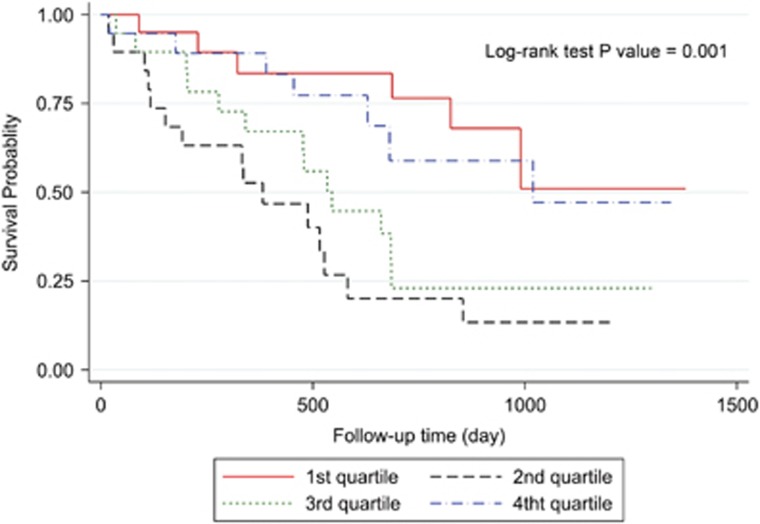
Three-year overall survival rate of the patients was 34.56% (Figure 2). Kaplan–Meier survival curves demonstrated that patients with higher values of postoperative ghrelin had better survivals (Figures 3 and 4). Patients with postoperative total ghrelin above 123 pg/ml as the median (Figure 5) had better overall survival compared with those patients with postoperative total ghrelin below the median (P-value=0.003). The difference was also detected when the 25th percentile value of postoperative total ghrelin (77 pg/ml) was considered as cut-off point. Patients with postoperative total ghrelin of more than 77 pg/ml had significantly higher survival compared with those with ghrelin level of equal or lower than 77 pg/ml (P-value <0.001). In addition, patients with either the lowest or the highest quartiles of preoperative ghrelin showed significantly better survival (P-value=0.01 for total ghrelin and P-value=0.001 for active ghrelin) compared with patients with second or third quartiles of ghrelin levels (Figures 6 and 7). There was no difference between patients with the highest and lowest quartiles, or between patients with the second and third quartiles of preoperative ghrelin. Sum of patients with the lowest or highest quartiles of preoperative ghrelins as one subgroup, had statistically significant better prognosis compared with the subgroup of patients with ghrelin levels in range of second and third quartiles (P-value=0.001 for preoperative total ghrelin and P-value <0.001 for preoperative active ghrelin). Furthermore, Log-rank tests between subgroups of patients categorized using median levels of postoperative active ghrelin or preoperative total and active ghrelins did not reveal significant differences (P-value=0.07, 0.38, and 0.84, respectively).
Figure 2.
Overall survival of total gastric cancer patients. The Figure demonstrates Kaplan–Meier survival curve of 1-year, 2-year, and 3-year overall survivals of total gastric cancer patients.
Figure 3.
Overall survival in subgroups of patients with different quartiles of postoperative total ghrelin. The Figure demonstrates Kaplan–Meier survival curves of overall survival in subgroups of patients with different quartiles of postoperative total ghrelin.
Figure 4.
Overall survival in subgroups of patients with different quartiles of postoperative active ghrelin. The Figure demonstrates Kaplan–Meier survival curves of overall survival in subgroups of patients with different quartiles of postoperative active ghrelin.
Figure 5.
Overall survival in two subgroups of patients based on median postoperative total ghrelin. The Figure demonstrates Kaplan–Meier survival curves of overall survival in subgroups of patients with lower and upper halves of postoperative total ghrelin.
Figure 6.
Overall survival in subgroups of patients with different quartiles of preoperative total ghrelin. The Figure demonstrates Kaplan–Meier survival curves of overall survival in subgroups of patients with different quartiles of preoperative total ghrelin.
Figure 7.
Overall survival in subgroups of patients with different quartiles of preoperative active ghrelin. The Figure demonstrates Kaplan–Meier survival curves of overall survival in subgroups of patients with different quartiles of preoperative active ghrelin.
Univariate Cox analysis demonstrated that TNM stage; postoperative plasma levels of total ghrelin; preoperative plasma levels of total and active ghrelin; and cachexia were significantly associated with survival. In addition, grade of the tumor and receiving no neoadjuvant chemotherapy showed a trend of association with survival of the patients (Table 2). Other parameters did not show notable associations with survival. We also found significant inverse correlation between BMI and both active (r=−0.47, P-value=0.001) and total ghrelin (r=−0.28, P-value=0.04).
Table 2. Hazards ratio (HR) and corresponding 95% confidence interval (95% CI) attained for different clinical factors and plasma levels of ghrelin on prognosis of gastric cancer among patients hospitalized in the cancer institute of Iran in 2011–2012.
| Determinant parameter | Subgroup(s) |
Univariate Cox analyses |
Multiple Cox model | ||
|---|---|---|---|---|---|
| Person-years (Total=130.05) | Deaths (N=43) | HR (95% CI) | HR (95% CI) | ||
| TNM stage | I | 9.97 | 1 | Reference | Reference |
| II | 70.59 | 20 | 2.84 (0.78–11.19) P=0.33 | 2.62 (0.47–14.46) P=0.35 | |
| III | 49.49 | 22 | 4.41 (1.21–14.78) P=0.032 | 4.88 (1.10–21.77) P=0.041 | |
| Cachexia | No | 55.90 | 11 | Reference | Reference |
| Yes | 59.39 | 27 | 2.49 (1.22–5.07) P=0.012 | 2.99 (1.35–6.63) P=0.007 | |
| Undetermined | 14.76 | 5 | 1.72 (0.60–5.00) P=0.32 | 1.85 (0.52–6.53) P=0.35 | |
| Neoadjuvant Chemotherapy | Yes | 85.11 | 25 | Reference | Reference |
| No | 44.94 | 18 | 1.41 (0.92–2.19) P=0.17 | 2.02 (1.04–3.92) P=0.04 | |
| Preoperative total Ghrelin | ≤25th percentile | 37.83 | 8 | Reference | Reference |
| 25th–50th percentile | 26.92 | 16 | 2.75 (1.47–6.46) P=0.02 | 2.67 (1.11–6.38) P=0.03 | |
| 50th–75th percentile | 29.07 | 12 | 1.95 (1.11–4.98) P=0.15 | 2.32 (1.01–5.35) P=0.048 | |
| ≥75th percentile | 30.02 | 5 | 0.75 (0.24–2.30) P=0.61 | 0.78 (0.22–2.73) P=0.70 | |
| Grade | Well-differentiated | 21.81 | 4 | Reference | Reference |
| Moderately differentiated | 68.59 | 22 | 1.77 (0.61–5.14) P=0.30 | 2.65 (0.81–8.70) P=0.11 | |
| Poorly differentiated | 39.65 | 17 | 2.29 (0.76–6.87) P=0.14 | 1.53 (0.52–4.52) P=0.41 | |
| Adjusted HRs (95% CI) attained for other ghrelins when substituted for preoperative total ghrelin in the modela | |||||
| Preoperative active Ghrelin | ≤25th percentile | 38.31 | 6 | Reference | Reference |
| 25th–50th percentile | 21.62 | 15 | 4.78 (1.83–12.47) P=0.001 | 4.92 (1.80–13.54) P=0.002 | |
| 50th–75th percentile | 25.75 | 13 | 3.40 (1.28–9.01) P=0.01 | 2.87 (1.11–7.38) P=0.03 | |
| ≥75th percentile | 35.94 | 7 | 1.30 (0.44–3.89) P=0.64 | 1.97 (0.59–6.54) P=0.27 | |
| Postoperative total Ghrelin | ≥75th percentile | 35.97 | 7 | Reference | Reference |
| 50th–75th percentile | 30.50 | 6 | 0.95 (0.32–2.82) P=0.9 | 1.02 (0.28–3.67) P=0.9 | |
| 25th–50th percentile | 34.77 | 9 | 1.32 (0.49–3.54) P=0.58 | 1.23 (0.34–4.40) P=0.7 | |
| ≤25th percentile | 22.72 | 20 | 4.86 (2.01–11.73) P<0.001 | 4.29 (1.48–12.44) P=0.007 | |
| Postoperative total Ghrelin | ≥50th percentile | 66.47 | 13 | Reference | Reference |
| <50th percentile | 57.50 | 29 | 2.64 (1.37–5.11) P=0.004 | 2.33 (1.01–5.41) P=0.049 | |
| Postoperative active Ghrelin | ≥75th percentile | 33.86 | 7 | Reference | Reference |
| 50th–75th percentile | 34.63 | 9 | 1.25 (0.46–3.36) P=0.57 | 0.75 (0.26–2.16) P=0.6 | |
| 25th–50th percentile | 26.44 | 7 | 1.31 (0.45–3.78) P=0.54 | 0.87 (0.28–2.75) P=0.8 | |
| ≤25th percentile | 25.80 | 15 | 2.80 (1.13–6.96) P=0.02 | 1.68 (0.68–4.14) P=0.26 | |
TNM, tumor-node-metastasis staging.
HRs attained by other ghrelins in sensitivity analysis when they were separately substituted for preoperative total ghrelin in the base model developed.
Because prognostic effect of postoperative ghrelin might be confounded by extent of gastrectomy, we repeated univariate Cox analysis of postoperative ghrelin in subgroups of patients with total and subtotal gastrectomy, exclusively. Association of postoperative total ghrelin with survival remained significant in both groups of patients (HR=4.12, P-value=0.005 in total gastrectomy, and HR=5.26, P-value=0.04 in subtotal gastrectomy).
Association of ghrelin with survival still remained significant after adjusting for other factors. Based on the multiple Cox regression model, patients with advanced TNM stage had almost five folds lower survival compared with patients with earlier stages of GC (HR=4.88, 95% confidence interval (CI): 1.01–5.35; Table 2). In addition, patients with cachexia at the time of diagnosis had threefolds higher risk of mortality compared with patients without cachexia (HR=2.99, 95% CI: 1.35–6.63). We also found that patients who did not receive neoadjuvant chemotherapy had two-fold increase in risk of mortality compared with those who received neoadjuvant chemotherapy before surgery (HR=2.02, 95% CI: 1.04–3.92). Compared with patients with first quartile of preoperative plasma levels of total ghrelin, those with second and third quartiles of plasma ghrelin had 2.7- and 2.3-folds, respectively, less survival (HR=2.67, 95% CI: 1.11–6.38 for second quartile, and HR=2.32, 95% CI: 1.01–5.35 for third quartile vs. first quartile). In sensitivity analysis, substituting other ghrelins for total preoperative ghrelin in the model showed that preoperative active and postoperative total ghrelins retained significant associations with prognosis; in addition, association of TNM stage, cachexia, and neoadjuvant chemotherapy with survival did not substantially change and they retained their prognostic effects. Similar to preoperative total ghrelin, patients with second and third quartiles of preoperative active ghrelin had, respectively 4.9 and 2.7 folds higher risk for mortality compared with patients with first quartile of plasma levels of active ghrelin (HR=4.92, 95% CI: 1.79–13.54 for second quartile, and HR=2.87, 95% CI: 1.11–7.38 for third quartile vs. first quartile). In contrast, patients with first quartile of postoperative total ghrelin had 4.3 folds less survival compared with higher quartiles of plasma ghrelin levels (HR=4.29, 95% CI: 1.48–12.44). Notably, postoperative total ghrelin remained a significant predictor of survival when using the median value as the cut-off. Patients with postoperative total ghrelin levels below median had 2.3 folds higher risk for mortality compared with patients with ghrelin levels above the median (HR=2.33, 95% CI: 1.01–5.41). Postoperative active ghrelin did not retain significant association with prognosis (HR=1.68, 95% CI: 0.68–4.14 for first quartile vs. fourth quartile). After construction of the final model for postoperative total ghrelin, adding extent of gastrectomy (i.e., total vs. subtotal) to the model revealed no significant association between this variable and GC survival. In addition, it did not modify prognostic effect of ghrelin in the model.
DISCUSSION
In this survival study, we found considerable drop in the total and active ghrelin levels after gastrectomy in patients with GC. Because the stomach is the main source of ghrelin secretion in humans,8 this finding was expected. We also showed that both preoperative and postoperative plasma levels of ghrelin had significant prognostic roles in survival of GC patients; however, the prognostic patterns of preoperative and postoperative ghrelin were different. These prognostic effects of ghrelin in GC patients remained significant even after adjustment for other factors such as stage, grade and cachexia.
A recent study on 16 stage I–III gastric cancer patients reported associations between plasma levels of ghrelin and survival. The authors measured plasma levels of ghrelin just prior to adjuvant chemoradiotherpay (after gastrectomy), and reported that patients with plasma levels of ghrelin below 35 pg/ml had significantly shorter survival compared with patients with higher levels of ghrelin.20 Their findings demonstrated association between low postoperative plasma ghrelin and poor survival in gastric cancer, which are consistent with our results in current study; however, they did not assess prognostic role of preoperative plasma levels of ghrelin. In addition, the sample size was low and the authors did not adjust for confounding factors such as stage or weight loss. To the best of our knowledge, our findings in this study is the first evidence for significant prognostic roles of both preoperative and postoperative ghrelin in GC patients, adjusted for other prognostic factors. We conducted a comprehensive approach to adjust for many parameters with potential confounding effects. We found a HR range of 2.3–4.9 for effects of preoperative and postoperative ghrelin on GC prognosis. Our findings suggest that ghrelin could be considered as an important prognostic biomarker for GC. It is more pronounced in case of postoperative ghrelin, regarding its consistent prognostic behavior. Along with other molecular prognostic biomarkers proposed for GC,6, 25 assessment of plasma ghrelin could complement conventional clinical staging to better stratify patients with poor survival.
Our findings add evidence in favor of prognostic effects of ghrelin in cancer. Reports from survival studies for prognostic effect of ghrelin in different types of cancer is controversial. A study reported that breast cancer patients with positive immunostaining of tumor specimen for ghrelin showed threefold increase in survival compared with patients with negative immunostaining.26 Another study reported that patients with renal cell carcinoma who had high expression of ghrelin experienced almost two folds decrease in survival compared with patients with low expression of ghrelin.27 In contrast, a third survival study reported no significant prognostic role for ghrelin in patients with non-small cell and small cell lung cancer.28 Similar inconsistency is observed in in vitro studies.14 Treatment with ghrelin inhibited proliferation and invasion of some types of human breast cancerous cell lines,12 promoted migration capabilities of renal cell carcinoma cell lines,27 and inhibited proliferation of human lung cancer cell line11 or had no effect on this cell line.29 Different distribution of ghrelin-specific receptors on different cancerous cell lines could account in part to this controversy. Altogether, as supported by our findings, we believe that various local and systemic effects of ghrelin in cancer could more likely affect clinical course of this disease.
We found different patterns in contribution of preoperative and postoperative levels of plasma ghrelin to course of GC. The stomach is the main source of ghrelin secretion;8 thereafter gastric cancer cells are exposed to paracrine effects of locally high amounts of ghrelin. This would further highlight cellular and local effects of ghrelin in case of gastric cancer. After gastrectomy, the microenvironment for exposure of cancerous cells to this local high amount of ghrelin is removed. It implies that contribution to clinical course of gastric cancer observed for level of ghrelin before and after gastrectomy could be different. This specific circumstance may not apply to other types of cancer. Expectedly, we found that while higher quartiles of plasma ghrelin after the operation were associated with better survival, both the lowest and the highest quartiles of preoperative ghrelin predicted a better survival. We postulated that the summary effect of preoperative level of plasma ghrelin on GC prognosis is a trade-off between probably contrary effects of ghrelin on behavior and clinical course of this type of cancer. Ghrelin was shown to stimulate proliferation, migration and invasion of GC cell lines.18 This could be the reason for better survival of patients with the lowest quartile of preoperative ghrelin. From the other side, ghrelin has anti-inflammatory effects,30 which can limit the progression of the tumor. The latter might have overwhelmed the former stimulatory effect in higher doses and could justify better survival in patients with the highest quartile of preoperative ghrelin. In addition, although the role of cachexia was adjusted, residual confounding effect of appetite stimulation by elevated ghrelin could have contributed to better survival of patients with the highest quartile of preoperative ghrelin. After the gastrectomy, the main microenvironment for paracrine stimulatory effect of ghrelin on tumor cells no longer exists. In this case, orexigenic and anti-inflammatory effects of ghrelin seem to be the dominant mechanisms for contribution of ghrelin to survival. That could be the reason patients with the lowest quartile of postoperative ghrelin experienced worse survival.
Different prognostic patterns observed for preoperative and postoperative ghrelin in GC could help understand more details about contribution of ghrelin to tumor biology. In a simplified scheme, the summary effect of ghrelin on cancer prognosis could be considered as result of its systemic and local effects. These effects could be in the same direction or could be contrary. Although positive contribution of orexigenic and anti-inflammatory systemic effects of ghrelin seems to be common among different cancers, direction (stimulatory vs. suppressive) and magnitude of its local effects in case of each specific type of cancer are variable and might be more important determining factors for resultant summary effect. Extent of ghrelin local access to cancerous cell lines and distribution of ghrelin-specific receptors on different cancerous cell lines could be important factors that regulate local effects of ghrelin on a specific cancer.
Better prognosis of patients with higher quartiles of postoperative ghrelin in current study could help address the concerns raised about administration of extrinsic ghrelin after gastrectomy in patients with GC.18, 31 After total gastrectomy, the level of plasma ghrelin remains almost steady.32, 33 Therefore, our assay after the operation could also estimate the level of plasma ghrelin during the survival period of the patient. Our findings support the hypothesis that increasing the plasma level of ghrelin by administration of extrinsic ghrelin after total gastrectomy could augment efficacy of treatment and improve GC patients' survival. This would not be as appropriate in case of subtotal gastrectomy, where in remaining gastric tissue would still be in danger of exposure to stimulatory effects of ghrelin administrated. Thereafter, ghrelin in addition to many other new anti-cancer agents introduced34, 35 could complement current conventional therapeutic regimens to augment efficacy, reduce dosage needed, and restrict exposure to toxic effects.36
Receiving neoadjuvant chemotherapy was associated with an almost two-fold increase in overall survival of GC patients in our study. Similarly, most of interventional studies report survival advantage of GC patients when receiving neoadjuvant chemotherapy compared with surgery alone;37, 38 however, some trials failed to show a survival benefit for neoadjuvant chemotherapy in GC patients.39 Finally, recent meta-analyses of clinical trials demonstrated that it slightly improves survival of GC patients.40, 41 Altogether, the advantage of neoadjuvant chemotherapy in GC remains to be completely established. Our findings in this study add another evidence in support of significant survival benefit for GC patients who received neoadjuvant chemotherapy.
There is mutual associations between obesity and ghrelin. Although higher levels of plasma ghrelin lead to increase in adipose tissue and body weight,42, 43 ghrelin levels are reduced in obese cases.44, 45 In addition, BMI was reported to be reversely associated with plasma ghrelin.46, 47 None of our cases in this study were obese (BMI>30). Similar to previous reports,46, 47 we noted a negative association between BMI and plasma levels of ghrelin in our gastric cancer patients, more pronounced in case of active ghrelin. However, BMI did not show notable associations with survival and would not confound or modify prognostic role of ghrelin.
Majority of the current literature indicates that there is more advanced disease in GC patients negative for H. pylori infection.48, 49, 50 On the other hand, H. pylori can alter plasma levels of ghrelin.51 We did not assess current status of H. pylori infection in our cases. Therefore, the concern remains that apparent relationship between plasma ghrelin and survival in GC patients in our study might be confounded by status of gastric H. pylori infection and we may not be able to definitely conclude plasma ghrelin as an independent biomarker of survival in patients with GC without assessing status of this infection. To address this concern, a future study would exclusively investigate prognostic role of ghrelin in GC patients positive and negative for H. pylori, and adjust prognostic role of plasma ghrelin in GC for status of infection. It can provide more confident conclusions on independent prognostic roles of ghrelin in GC taking into account the status of H. pylori infection.
We recruited our cases from a referral center for cancer. Patients from all parts of the country could be referred to this center. Thereafter, our study sample was not limited to a specific ethnical or socioeconomic group of patients. Our data for cachexia and some other clinical parameters was based on history taken from the patients. This is prone to recall bias and may lead to misclassification; however, it is not expected to be a differential misclassification. Access to previous clinical records of the patients that contain their past medical and drug history and anthropometric data could be a better source to prevent this information bias. Our findings were from an observational prospective study. Future long-term interventional trials assessing long-term outcomes of extrinsic ghrelin administration in gastric cancer patients would yield more robust and more practical evidences in the topic.
CONCLUSION
We found strong evidence in favor of clinically significant prognostic roles for preoperative and postoperative levels of plasma ghrelin. Different prognostic patterns observed for preoperative and postoperative levels of ghrelin shed further light on various physiological and pathological functions of ghrelin in cancer. Ghrelin, particularly postoperative ghrelin, could be considered a prognostic biomarker to stratify patients at higher risk in GC. In addition, our findings is an evidence in support of potential benefit for extrinsic ghrelin administration after total gastrectomy, where in whole stomach tissue is resected. This could not be recommend for subtotal gastrectomy, where in remaining gastric tissue would still be exposed to stimulatory effects of ghrelin. Further observational as well as interventional studies with longer follow-ups and taking into account the status of H. pylori infection will shed further light on the prognostic and therapeutic roles of plasma ghrelin in GC.
Study Highlights
Acknowledgments
We thank Fatemeh Hoseinpoor for her assistance in follow-up of the patients.
Guarantor of the article: Kazem Zendehdel, MD, PhD.
Specific author contributions: Conception and design, data collection, analysis and interpretation of data, writing the manuscript, critical revision of the manuscript, statistical expertize, obtaining funding, supervision, approval of final draft submitted: S.S.-J.; conception and design, critical revision of the manuscript, administrative support, approval of final draft submitted: A.A.; conception and design, data collection, writing the manuscript, critical revision of the manuscript, approval of final draft submitted: A.A.F.; data collection, critical revision of the manuscript, approval of final draft submitted: S.G.; data collection, critical revision of the manuscript, approval of final draft submitted: F.S.; data collection, critical revision of the manuscript, approval of final draft submitted: R.H.; critical revision of the manuscript, approval of final draft submitted, administrative support: H.M.; conception and design, analysis and interpretation of data, writing the manuscript, critical revision of the manuscript, statistical expertize, obtaining funding, administrative support, supervision, approval of final draft submitted: K.Z.
Financial support: The study was supported by a grant (grant number: 90-02-51-14147) from Tehran University of Medical Sciences. The funder had no role in the study design; In the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
Potential competing interests: The authors declare no conflict of interest.
References
- Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- Zendehdel K, Marzban M, Nahvijou A et al. Six-fold difference in the stomach cancer mortality rate between northern and southern Iran. Arch Iran Med 2012; 15: 741–746. [PubMed] [Google Scholar]
- Khedmat H, Panahian M, Mashahdian M et al. Prognostic factors and survival in stomach cancer—analysis of 15 years of data from a referral hospital in iran and evaluation of international variation. Onkologie 2011; 34: 178–182. [DOI] [PubMed] [Google Scholar]
- Zeraati H, Mahmoudi M, Kazemnejad A et al. Postoperative survival in gastric cancer patients and its associated factors: A time dependent covariates model. Iranian J Publ Health 2006; 35: 40–46. [Google Scholar]
- Posteraro B, Persiani R, Dall'Armi V et al. Prognostic factors and outcomes in Italian patients undergoing curative gastric cancer surgery. Eur J Surg Oncol 2014; 40: 345–351. [DOI] [PubMed] [Google Scholar]
- Sawada T, Yashiro M, Sentani K et al. New molecular staging with G-factor supplements TNM classification in gastric cancer: a multicenter collaborative research by the Japan Society for Gastroenterological Carcinogenesis G-Project committee. Gastric Cancer 2015; 18: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999; 402: 656–660. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000; 141: 4255–4261. [DOI] [PubMed] [Google Scholar]
- Van Der Lely AJ, Tschöp M, Heiman ML et al. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004; 25: 426–457. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Kanemaru A, Fukushima T et al. Ghrelin administration suppresses inflammation-associated colorectal carcinogenesis in mice. Cancer Sci 2015; 106: 1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoni P, Allia E, Marrocco T et al. Ghrelin and cortistatin in lung cancer: expression of peptides and related receptors in human primary tumors and in vitro effect on the H345 small cell carcinoma cell line. J Endocrinol Invest 2006; 29: 781–790. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Papotti M, Ghe C et al. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab 2001; 86: 1738–1745. [DOI] [PubMed] [Google Scholar]
- Akamizu T, Kangawa K. Ghrelin for cachexia. J Cachexia Sarcopenia Muscle 2010; 1: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin LK, Seim I, Walpole CM et al. The ghrelin axis—does it have an appetite for cancer progression? Endocr Rev 2012; 33: 849–891. [DOI] [PubMed] [Google Scholar]
- Jeffery PL, Herington AC, Chopin LK. The potential autocrine/paracrine roles of ghrelin and its receptor in hormone-dependent cancer. Cytokine Growth Factor Rev 2003; 14: 113–122. [DOI] [PubMed] [Google Scholar]
- Murphy G, Kamangar F, Dawsey SM et al. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst 2011; 103: 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadjadi A, Yazdanbod A, Lee YY et al. Serum ghrelin; a new surrogate marker of gastric mucosal alterations in upper gastrointestinal carcinogenesis. PLoS One 2013; 8: e74440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Zhang L, Hu D et al. Ghrelin induces gastric cancer cell proliferation, migration, and invasion through GHS-R/NF-κB signaling pathway. Mol Cell Biochem 2013; 382: 163–172. [DOI] [PubMed] [Google Scholar]
- Adachi S, Takiguchi S, Okada K et al. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology 2010; 138: 1312–1320. [DOI] [PubMed] [Google Scholar]
- Karaca F, Afsar CU, Gunaldi M et al. Alterations of ghrelin with weights and correlation among ghrelin, cytokine and survival in patients receiving chemoradiotherapy for gastrointestinal cancers. In J Clin Exp Med 2015; 8: 876–882. [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics 1983; 39: 499–503. [PubMed] [Google Scholar]
- Fearon K, Strasser F, Anker SD et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12: 489–495. [DOI] [PubMed] [Google Scholar]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- Hosmer DWJr, Lemeshow S, Sturdivant RX. Model-building strategies and methods for logistic regression. Applied Logistic Regression. 3rd edn. John Wiley & Sons, Inc: Hoboken, NJ, 2000, pp 89–151. [Google Scholar]
- Soleyman-Jahi S, Nedjat S, Abdirad A et al. Prognostic significance of matrix metalloproteinase-7 in gastric cancer survival: a meta-analysis. PLoS One 2014; 10: e0122316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronberg M, Fjallskog ML, Jirstrom K et al. Expression of ghrelin is correlated to a favorable outcome in invasive breast cancer. Acta Oncol 2012; 51: 386–393. [DOI] [PubMed] [Google Scholar]
- Lin TC, Liu YP, Chan YC et al. Ghrelin promotes renal cell carcinoma metastasis via Snail activation and is associated with poor prognosis. J Pathol 2015; 237: 50–61. [DOI] [PubMed] [Google Scholar]
- Kerenidi T, Lada M, Tsaroucha A et al. Clinical significance of serum adipokines levels in lung cancer. Med Oncol 2013; 30: 507. [DOI] [PubMed] [Google Scholar]
- Ghe C, Cassoni P, Catapano F et al. The antiproliferative effect of synthetic peptidyl GH secretagogues in human CALU-1 lung carcinoma cells. Endocrinology 2002; 143: 484–491. [DOI] [PubMed] [Google Scholar]
- Baatar D, Patel K, Taub DD. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol 2011; 340: 44–58. [DOI] [PubMed] [Google Scholar]
- Xu JL, Xia R, Min X et al. Ghrelin and its emerging role in tumor pathogenesis and progression. Obes Res Clin Pract 2015; 9: 184–185. [DOI] [PubMed] [Google Scholar]
- Kizaki J, Aoyagi K, Sato T et al. Production of ghrelin by the stomach of patients with gastric cancer. Kurume Med J 2014; 60: 99–104. [DOI] [PubMed] [Google Scholar]
- Takiguchi S, Takata A, Murakami K et al. Clinical application of ghrelin administration for gastric cancer patients undergoing gastrectomy. Gastric Cancer 2014; 17: 200–205. [DOI] [PubMed] [Google Scholar]
- Soleyman-Jahi S, Zendehdel K, Akbarzadeh K et al. In vitro assessment of antineoplastic effects of deuterium depleted water. Asian Pac J Cancer Prev 2014; 15: 2179–2183. [DOI] [PubMed] [Google Scholar]
- Chaudhary AK, Pandya S, Ghosh K et al. Matrix metalloproteinase and its drug targets therapy in solid and hematological malignancies: an overview. Mutat Res 2013; 753: 7–23. [DOI] [PubMed] [Google Scholar]
- Mahmoodi M, Soleyman-Jahi S, Zendehdel K et al. Chromosomal aberrations, sister chromatid exchanges, and micronuclei in lymphocytes of oncology department personnel handling anti-neoplastic drugs. Drug Chem Toxicol 2016: 1–6; doi:10.1080/01480545.2016.1209678. [DOI] [PubMed]
- Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- Ychou M, Boige V, Pignon JP et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011; 29: 1715–1721. [DOI] [PubMed] [Google Scholar]
- Schuhmacher C, Gretschel S, Lordick F et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010; 28: 5210–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronellenfitsch U, Schwarzbach M, Hofheinz R et al. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev 2013; 5: CD008107. [DOI] [PubMed] [Google Scholar]
- Xiong BH, Cheng Y, Ma L et al. An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Invest 2014; 32: 272–284. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Gomez-Ambrosi J, Catalán V et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 2009; 33: 541–552. [DOI] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000; 407: 908–913. [DOI] [PubMed] [Google Scholar]
- Gomez G, Han S, Englander EW et al. Influence of a long-term high-fat diet on ghrelin secretion and ghrelin-induced food intake in rats. Regul Pept 2012; 173: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handjieva-Darlenska T, Boyadjieva N. The effect of high-fat diet on plasma ghrelin and leptin levels in rats. J Physiol Biochem 2009; 65: 157–164. [DOI] [PubMed] [Google Scholar]
- Tschöp M, Weyer C, Tataranni PA et al. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001; 50: 707–709. [DOI] [PubMed] [Google Scholar]
- Williams DL, Grill HJ, Cummings DE et al. Overfeeding-induced weight gain suppresses plasma ghrelin levels in rats. J Endocrinol Invest 2006; 29: 863–868. [DOI] [PubMed] [Google Scholar]
- Huang JQ, Sridhar S, Chen Y et al. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998; 114: 1169–1179. [DOI] [PubMed] [Google Scholar]
- Hwang JJ, Lee DH, Lee AR et al. Characteristics of gastric cancer in peptic ulcer patients with Helicobacter pylori infection. World J Gastroenterol 2015; 21: 4954–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HW, Choi IJ, Cho SJ et al. Characteristics of gastric cancer according to Helicobacter pylori infection status. J Gastroenterol Hepatol 2014; 29: 1671–1677. [DOI] [PubMed] [Google Scholar]
- Paoluzi OA, Blanco del VG, Caruso R et al. Impairment of ghrelin synthesis in Helicobacter pylori-colonized stomach: new clues for the pathogenesis of H. pylori-related gastric inflammation. World J Gastroenterol 2014; 20: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]