Abstract
Objectives:
The mechanisms responsible for the development of nonalcoholic fatty liver disease (NAFLD) and progression to nonalcoholic steatohepatitis (NASH) are incompletely understood. Growing evidence suggests that growth hormone (GH) and insulin-like growth factor-1 (IGF-1) may have roles in the development and progression of NAFLD. We hypothesized that lower serum IGF-1 levels would be associated with increased liver fat accumulation, inflammation, and fibrosis in a group of meticulously phenotyped obese subjects with liver biopsies.
Methods:
A retrospective, cross-sectional study was performed at Massachusetts General Hospital, Boston, MA, USA and St. Mary's Hospital, Richmond, VA, USA. Liver biopsies were performed in 142 subjects during NAFLD work-up or bariatric surgery and were graded by a single, blinded pathologist. Main outcome measures included liver histology and serum IGF-1.
Results:
Mean age was 52±10 years and body mass index (BMI) was 43±9 kg/m2. Mean serum IGF-1 was lower in subjects with lobular inflammation (112±47 vs. 136±57 ng/ml, P=0.01), hepatocyte ballooning (115±48 vs. 135±57 ng/ml, P=0.05), higher fibrosis stage (stage 2–4 vs. 0–1; 96±40 vs. 125±51 ng/ml, P=0.005), and NASH (109±45 vs. 136±57 ng/ml, P=0.002). All results remained significant after controlling for age, BMI, and a diagnosis of diabetes, and all but hepatocyte ballooning (trend, P=0.06) remained significant after excluding individuals with cirrhosis. Steatosis was not significantly associated with mean serum IGF-1 levels.
Conclusions:
Low serum IGF-1 levels are associated with increased histologic severity of NAFLD when rigorously controlled for age, BMI, the presence of diabetes, and after the exclusion of subjects with cirrhosis. Further investigation is warranted to determine the differential effects of GH and IGF-1 on the development and progression of NAFLD, which could further elucidate pathophysiology and identify therapeutic targets.
Introduction
Nonalcoholic fatty liver disease (NAFLD), fatty infiltration of the liver in the absence of alcohol use, is a serious complication of obesity. Nonalcoholic steatohepatitis (NASH), the progressive form of NAFLD, is characterized by inflammation and hepatocellular injury, and can progress to cirrhosis in a subset of patients.1, 2 NASH-related cirrhosis is expected to be the leading cause of liver transplant by the year 2020.3, 4 There are currently no Food and Drug Administration-approved treatments for NAFLD and NASH, and weight loss remains the only effective management strategy.5, 6, 7 However, the mechanisms responsible for the development of NAFLD and progression to NASH and cirrhosis are incompletely understood, and further insight could inform the development of future therapies.8
Growth hormone (GH) is an anterior pituitary hormone that is a key regulator of lipolysis in adipose tissue and a cytokine with anti-inflammatory properties both systemically and at the macrophage level. Furthermore, obesity is a well-established state of relative GH deficiency.9, 10, 11, 12, 13 Insulin-like growth factor-1 (IGF-1), which is released by the liver in response to GH stimulation, is also reduced in obesity, but not to the same degree as GH.10, 14, 15 IGF-1 induces insulin sensitivity and has been shown to have antifibrotic properties in rodent models of liver disease, including models of NAFLD and NASH.16, 17, 18, 19 Thus, the state of relative GH deficiency of obesity has potential mechanistic implications in the development of NAFLD and NASH, both with respect to the deposition of liver fat and the progression of inflammation and fibrosis, through reductions in both GH and IGF-1.
Consistent with this hypothesis, mice with liver-specific mutations in the GH receptor or downstream signaling pathways (JAK/STAT) develop hepatic steatosis.20, 21, 22, 23, 24 Although there are few data with regard to the effects of GH in humans with NAFLD, adults with pituitary GH deficiency have a higher incidence of NAFLD and, in one small study, GH administration has been shown to reduce liver enzymes, markers of fibrosis and liver fat in this population.25, 26 GH administration has also been shown to reduce markers of inflammation such as high-sensitivity C-reactive protein in hypopituitary individuals with frank GH deficiency and obese individuals with relative GH deficiency.27, 28, 29 Moreover, IGF-1 has also been implicated in the pathogenesis of NASH in animal models.16, 30
These data raise the question of whether downregulation of the GH/IGF-1 axis in obesity contributes to the development and progression of NAFLD. However, data regarding the GH/IGF-1 axis and NAFLD in obese subjects remains limited, in particular given the challenges of studying the GH/IGF-1 axis. For example, GH is pulsatile and a single, fasting GH measurement is not reflective of an individual's overall GH status. Formal stimulation testing is required for full assessment of a peak-stimulated GH level, in order to provide meaningful data, which limits the study of GH in large study groups due to practical constraints.31 In contrast, IGF-1, which is produced in response to GH, is easily measurable in serum and is not pulsatile. However, it is well established that IGF-1 declines with age, body mass index (BMI), and cirrhosis—factors that are generally associated with increasing severity of NAFLD and NASH.12, 32 Thus, careful consideration must be given to existing literature regarding the GH/IGF-1 axis and the severity of NAFLD. In particular, prior studies of the relationship between the histologic severity of NAFLD and IGF-1 levels have been affected by these methodological issues, with no one study controlling for age, BMI, and the presence of cirrhosis.33, 34, 35, 36 Moreover, the GH/IGF-1 axis interacts in a complex manner with glucose and insulin homeostasis.37 Thus, we sought to determine the effects of IGF-1 on the histologic severity of NAFLD independent of age, BMI, diagnosis of diabetes, and the presence of cirrhosis.
We hypothesized that lower serum IGF-1 levels would be associated with increased liver fat accumulation, inflammation, and fibrosis in a group of 142 meticulously phenotyped obese subjects with liver biopsies even after controlling for age, BMI, and the presence of diabetes, as well as after exclusion of subjects with cirrhosis.
Methods
Subjects
One hundred and forty-two subjects (46% males and 54% postmenopausal females) with liver biopsies obtained during work-up for NAFLD or during bariatric surgery were recruited for an Institutional Review Board-approved (approval date: 11/10/2009) longitudinal repository study between 2010 and 2015. Exclusionary criteria for alcohol intake included more than two drinks per day for men and more than one drink per day for women. Subjects with possible drug-induced NAFLD due to steroid, methotrexate, or tamoxifen were excluded. Overall, 34% (n=48) of subjects in the whole cohort were on diabetes medications (Table 1), with some subjects taking more than one such medication. Eighty-six percent (n=38) were on metformin, 44% (n=21) on insulin, 38% (n=18) on sulfonylureas, 8% (n=4) on glucagon-like peptide-1 agonists, and 2% (n=1) was on both a dipeptidyl peptidase-4 inhibitor and thiazolidinedione. Six subjects with a diagnosis of diabetes were not taking a diabetes-related medication. Baseline characteristics for different subsets of this cohort have been reported elsewhere.38, 39, 40, 41, 42 IGF-1 results have not been reported elsewhere for any of the cohort. Presence of diabetes, diabetes medication use, hypertension, and sleep apnea were self-reported by subjects and confirmed by physician chart review.
Table 1. Subject demographics and laboratory values.
| Controls n=21 (15%) | Steatosis n=41 (29%) | NASH n=80 (56%) | Overall modela P-value | Controls vs. steatosis P-value | Controls vs. NASH P-value | Steatosis vs. NASH P-value | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, years | 50±10 | 55±8 | 50±11 | NS | |||
| BMI, kg/m2 | 42.7±8.3 | 43.9±6.8 | 41.4±9.9 | NS | |||
| Female sex, n (%) | 15 (71%) | 25 (61%) | 36 (45%) | 0.05 | NS | 0.03 | NS |
| Race | |||||||
| White, n (%) | 8 (38%) | 32 (78%) | 65 (81%) | ||||
| African American, n (%) | 13 (62%) | 8 (20%) | 9 (11%) | ||||
| Hispanic, n (%) | 0 (0%) | 0 (0%) | 7 (9%) | ||||
| Other, n (%) | 0 (0%) | 1 (2%) | 6 (8%) | ||||
| Obesity comorbidities | |||||||
| Diabetes mellitus, n (%) | 3 (14%) | 12 (29%) | 39 (49%) | 0.004 | NS | 0.006 | 0.05 |
| Diabetes medications, n (%) | 2 (10%) | 11 (27%) | 35 (44%) | 0.003 | NS | 0.004 | NS |
| Hypertension, n (%) | 13 (62%) | 26 (63%) | 51 (64%) | NS | NS | NS | NS |
| Hyperlipidemia, n (%) | 4 (19%) | 25 (61%) | 50 (63%) | 0.01 | 0.003 | 0.0005 | NS |
| Obstructive sleep apnea, n (%) | 5 (24%) | 15 (37%) | 45 (56%) | 0.01 | NS | 0.01 | 0.04 |
| Laboratory values | |||||||
| ALT (U/l) | 33±18 | 45±29 | 71±57 | <0.0001 | NS | <0.0001 | 0.0007 |
| AST (U/l) | 18±9 | 27±22 | 46±38 | <0.0001 | NS | <0.0001 | <0.0001 |
| Alkaline phosphatase (U/l) | 80±23 | 88±31 | 86±38 | NS | |||
| hsCRP (mg/l) | 0.7±0.6 | 1.1±0.9 | 1.0±0.8 | NS | |||
| HbA1cb (%) | NA | 5.7±0.3 | 6.7±1.7 | NS | |||
| Lipids | |||||||
| Total cholesterol (mg/dl) | 190±58 | 173±39 | 171±40 | NS | |||
| LDL (mg/dl) | 114±50 | 100±36 | 99±36 | NS | |||
| VLDL (mg/dl) | 19±10 | 30±13 | 30±15 | 0.0023 | 0.0016 | 0.001 | NS |
| HDL (mg/dl) | 55±18 | 42±9 | 41±10 | <0.0001 | 0.0006 | <0.0001 | NS |
| Non-HDL Cholesterol (mg/dl) | 122±39 | 130±41 | 128±36 | NS | |||
| Triglycerides (mg/dl) | 104±52 | 150±83 | 164±109 | 0.0295 | 0.0403 | 0.0081 | NS |
ANOVA, analysis of variance; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; NA, not available; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NS, not significant; VLDL, very low density lipoprotein.
Values are reported as n (%) or mean±s.d.
Overall model represents ANOVA for linear variables and Pearson's χ2-test for categorical variables.
HbA1c available for a subset of n=31 subjects, 5 Steatosis, and 26 NASH.
Liver biopsy analysis
Biopsies were reviewed by a single, blinded hepatopathologist (JM) and scored for steatosis grade (0=<5%, 1=5–33%, 2=33–66%, and 3>66%), lobular inflammation per 200 × (0=no foci, 1 corresponding to <2 foci, 2 corresponding to 2–4 foci, and 3 corresponding to >4 foci) and hepatocyte ballooning (0=no ballooning, 1=few, and 2=many) as per Kleiner et al.43 NAFLD activity score was then assigned from 0 to 8 as a sum of the steatosis grade, lobular inflammation, and hepatocyte ballooning values.43 Fibrosis stage was assessed according to the modified Brunt stage (0–4), with four representing cirrhosis.43
Subject classification
Subjects were classified into subgroups for further analysis based on histologic assessment (Table 1). Controls (n=21/142, 15%) were defined as steatosis grade of 0 with no lobular inflammation, steatosis or fibrosis. Steatosis (n=41/142, 29%) was defined by grade 1 or higher steatosis not meeting the criteria for NASH. NASH (n=80/142, 56%) was defined as grade ≥1 in steatosis, ≥1 in lobular inflammation, and ≥1 in hepatocyte ballooning as per the American Association for the Study of Liver Disease.44 Cirrhosis (n=8/142, 6%) was defined as stage 4/4 fibrosis.
Insulin-like growth factor-1
Serum IGF-1 was measured in batch for all subjects using an Immulite 2000 Immunoassay System (Siemens Medical Systems, Erlangen, Germany), a solid-phase enzyme-labeled chemiluminescent immunometric assay with a coefficient of variation below 5%.
Statistical analysis
JMP Pro Statistical Database Software (version 11.0.0; SAS Institute, Cary, NC) was used for statistical analyses. Results are expressed as mean±s.d. unless otherwise noted. Variables were log-transformed and means compared with Fisher's least significance testing for three-way comparisons,45 t-test for two-way comparisons for continuous variables, and the χ2-test for categorical variables. Multivariable analysis was performed using linear or nominal logistic regression as appropriate. All multivariable analyses were controlled for both age and BMI. Sensitivity analyses were performed with regard to gender and the presence of cirrhosis where noted.
Results
Clinical characteristics
Clinical characteristics are reported in Table 1. Overall mean subject age was 52±10 years and mean BMI was 43±9 kg/m2 with a cohort that was 53% female (67/142). There was no difference in age, BMI, or sex between the control, NAFLD, or NASH groups. The NASH group had a significantly higher rate of diabetes mellitus and obstructive sleep apnea than the control and NAFLD groups. The NASH and NAFLD groups had similar rates of hyperlipidemia that were both higher than the rate in controls. Hypertension was equally prevalent across all three groups. Liver histology characteristics of each group are reported in Table 2.
Table 2. Liver histology characteristics.
| Controls (n=21) | Steatosis (n=41) | NASH (n=80) | |
|---|---|---|---|
| Steatosis grade (0–3) | |||
| Grade 0, n (%) | 21 (100%) | ||
| Grade 1, n (%) | 28 (68%) | 24 (30%) | |
| Grade 2, n (%) | 11 (27%) | 31 (39%) | |
| Grade 3, n (%) | 2 (5%) | 25 (31%) | |
| Lobular inflammation score (0–2) | |||
| Grade 0, n (%) | 21 (100%) | 31 (76%) | |
| Grade 1, n (%) | 8 (20%) | 60 (75%) | |
| Grade 2, n (%) | 2 (5%) | 20 (25%) | |
| Ballooning grade (0–2) | |||
| Grade 0, n (%) | 21 (100%) | 16 (39%) | |
| Grade 1, n (%) | 20 (49%) | 38 (48%) | |
| Grade 2, n (%) | 5 (12%) | 42 (53%) | |
| Fibrosis stage (0–4) | |||
| Stage 0, n (%) | 21 (100%) | 23 (56%) | 18 (23%) |
| Stage 1, n (%) | 18 (44%) | 38 (48%) | |
| Stage 2, n (%) | 12 (15%) | ||
| Stage 3, n (%) | 4 (5%) | ||
| Stage 4, n (%) | 8 (10%) | ||
| NAS | |||
| 0–2 n (%) | 21 (100%) | 27 (66%) | |
| 2–4 n (%) | 13 (32%) | 30 (38%) | |
| 5–8 n (%) | 1 (2%) | 50 (63%) | |
NAS, nonalcoholic fatty liver disease activity score; NASH, nonalcoholic steatohepatitis.
Liver histology characteristics by group reported as n (%).
IGF-1 and histologic assessment
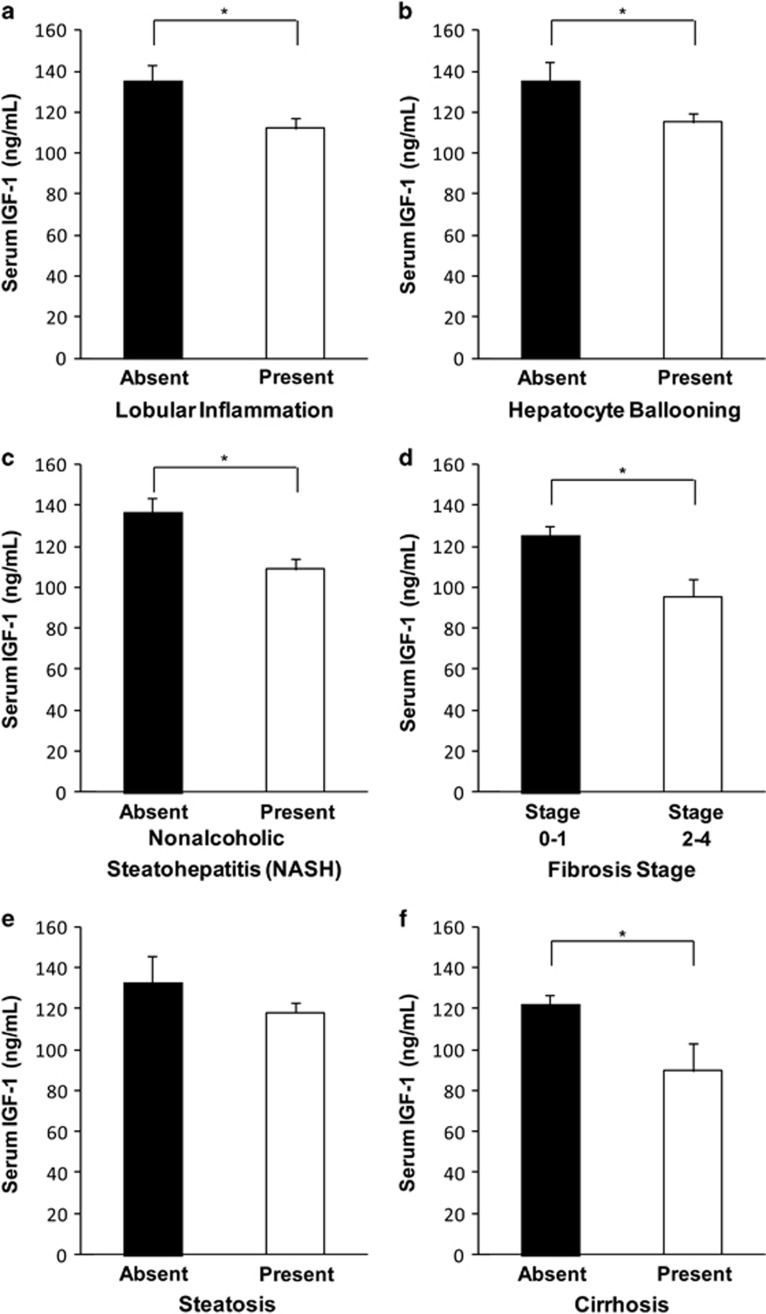
The presence of lobular inflammation was associated with lower mean serum IGF-1 (112±47 vs. 136±57 ng/ml, P=0.01; Figure 1a). Hepatocyte ballooning was associated with lower mean serum IGF-1 levels (115±48 vs. 135±57 ng/ml, P=0.05; Figure 1b). Subjects with NASH had lower mean serum IGF-1 levels than those without NASH (controls or steatosis alone; 109±45 vs. 136±57 ng/ml, P=0.002; Figure 1c). In addition, mean serum IGF-1 was lower in subjects with higher fibrosis stage (stage 2–4 vs. 0–1; 96±40 vs. 125±51 ng/ml, P=0.005; Figure 1d). Steatosis by any measure was not significantly associated with mean serum IGF-1 levels (steatosis absent 133±56 vs. steatosis present 118±54 ng/ml, P=NS; Figure 1e).
Figure 1.
Reported as mean serum insulin-like growth factor-I (IGF-1) in ng/ml±s.e.m. *P<0.05 as specified below. (a) Mean serum IGF-1 was significantly lower in subjects with the presence (“present”) vs. the absence (“absent”) of lobular inflammation (P=0.01). (b) Subjects with hepatocyte ballooning had significantly lower mean serum IGF-1 levels than those without (P<0.05). (c) Subjects with nonalcoholic steatohepatitis (NASH) (“present”) had significantly lower mean serum IGF-1 when compared with those without NASH (“absent”)(P=0.002). (d) Subjects with more severe fibrosis (Stages 2–4) had significantly lower mean serum IGF-1 levels than those with less severe fibrosis (Stages 0–1) (P<0.005). All results remained significant when excluding individuals with cirrhosis with the exception of hepatocyte ballooning, which remained a trend at P=0.06. (e) Mean serum IGF-1 level was not significantly different in subjects with steatosis (“present”) vs. controls without steatosis (“absent”) (P=NS), which was unchanged after excluding those with cirrhosis. (f) Mean serum IGF-1 was lower in subjects with cirrhosis (“present”) vs. those without cirrhosis (“absent”) (P<0.05).
As expected, based on known physiology, the presence of cirrhosis was associated with lower mean serum IGF-1 levels (90±38 vs. 122±51 ng/ml, P<0.05; Figure 1f). Thus, a sensitivity analysis was performed for each of the remaining variables excluding individuals with cirrhosis. Lobular inflammation, the presence of NASH, and higher fibrosis stage continued to be significantly associated and hepatocyte ballooning a trend (P=0.06) with lower mean serum IGF-1 when subjects with cirrhosis were excluded. To assess whether lower levels of fibrosis could be contributing to a decrease in IGF-1 levels, we performed a subset analysis of all individuals with minimal fibrosis (F0–1, n=117). Despite a significant loss in power, individuals with NASH still had a significantly lower mean serum IGF-1 than those without NASH (115±8 vs. 137±8 ng/ml, P=0.02).
Models controlling for diabetes and glucose homeostasis
When controlled for the presence or absence of diabetes in addition to age and BMI, lower mean serum IGF-1 remained significantly associated with fibrosis (P=0.003), lobular inflammation (P=0.002), hepatocyte ballooning (P=0.02), and NASH (P=0.0003) but not steatosis. Hemoglobin A1c (HbA1c) measurements were available in a subset of individuals without cirrhosis from the cohort (n=27, 19%), including 13 women and 14 men. Five individuals in this subgroup had NAFLD and 22 had NASH. Mean age was 49±11 years, mean BMI was 34±7 kg/m2, and mean HbA1c was 6.5±1.6% (range 4.8–11.5%). When controlled for age and BMI in this subgroup, lower mean serum IGF-1 was associated with NASH (P=0.01) and hepatocyte ballooning (P=0.03), but not lobular inflammation, fibrosis, or steatosis. When controlled for age, BMI, and HbA1c, lower mean serum IGF-1 remained significantly associated with the presence of NASH (P=0.002) but the relationship between mean serum IGF-1 and hepatocyte ballooning was lost (P=0.9). Of note, in this small subset, higher HbA1c was significantly associated with the presence of NASH (P=0.001) and by trend with steatosis (P=0.06), lobular inflammation (P=0.06), and fibrosis (P=0.07). Finally, we repeated the primary analysis controlling for the use of any medication used to treat diabetes mellitus rather than diagnosis of diabetes itself. All findings in Figure 1 remained unchanged and the use of medications for the treatment of diabetes mellitus did not have an impact on mean serum IGF-1 levels in any model. This was true even after excluding the n=5 subjects on a glucagon-like peptide-1 agonist or thiazolidinedione.
Models controlling for race
The effect of Caucasian and African American groups was examined with respect to this analysis. There was no difference in mean serum IGF-1 levels between the Caucasian and African American groups (128±53 vs. 125±59 ng/ml), and in addition there was no effect of race on mean serum IGF-1 levels (model was also controlled for BMI and age). The central analysis investigating the relationship between mean serum IGF-1 levels and histology (Figure 1) was unchanged when controlling for race, with significantly lower mean serum IGF-1 levels in individuals with lobular inflammation, hepatocyte ballooning, NASH, increasing fibrosis severity, and cirrhosis. In addition, race was not significant in these models (P>0.1 in all models). The only difference noted was that controlling for race strengthened the difference between mean serum IGF-1 levels in the steatosis group and controls (133±5 vs. 110±11 ng/ml, respectively, P=0.09), which was previously not significant.
Discussion
In this study, we demonstrate that markers of inflammation, hepatocyte ballooning, and fibrosis, but not steatosis, are associated with low serum IGF-1 levels in meticulously phenotyped patients with biopsy-proven NAFLD, even when controlling for age and BMI, and after excluding individuals with cirrhosis. In addition, our data show that serum IGF-1 remained a significant predictor of NASH even after additionally controlling for a diagnosis of diabetes or HbA1c, demonstrating that the GH/IGF-1 axis may have a role in the development and progression of NAFLD and NASH, independent of its association with insulin resistance and glucose homeostasis. These data thus implicate the GH/IGF-1 axis in the development and progression of NAFLD.
This work builds on the existing literature that suggests a link between GH/IGF-1 axis deficiency and NAFLD, while employing a rigorous approach to histological assessment, hormonal evaluation, and adjustment for potential confounders that has been absent from many prior studies. As obesity is a well-defined state of relative GH deficiency, further study of the GH/IGF-1 axis dysfunction in the development and progression of NAFLD and NASH is of critical importance.
Multiple mouse models have implicated the GH/IGF-1 axis in the development of NAFLD.20, 21, 23, 24, 46 Liver-specific GH receptor knockout mice develop significant steatosis compared with controls.22, 24 In addition, liver-specific knockouts of GH receptor signaling pathway components, including JAK2 and STAT, cause significant hepatic steatosis in mice.20, 21 Moreover, low levels of GH in mice with fatty liver have been associated with epidermal growth factor receptor downregulation, a pathway that is critical to liver regeneration. GH administration in these mice normalized hepatocyte proliferation and epidermal growth factor receptor activation after partial hepatectomy, suggesting that GH-dependent activation of epidermal growth factor receptor is critical to hepatic regeneration.46
It is difficult to tease out the direct vs. indirect effects of GH on NAFLD, as GH has a complex relationship with insulin resistance, which itself is a factor that has been implicated in the development and progression of NAFLD. Both GH deficiency and GH excess are both associated with insulin resistance and worsening glucose homeostasis. For example, Qin et al.47 demonstrated that GH administration worsened insulin resistance in normally fed control rats but improved insulin resistance in rats with NAFLD and associated metabolic comorbidities from a high-fat diet. Interestingly, List et al.48 revealed even more complexity in this relationship, demonstrating improvement in glucose tolerance but no change in insulin levels in response to GH administration in a high-fat fed mouse model. Thus, the reduction in liver fat with GH administration in this cohort was not mediated by a change in absolute insulin levels but possibly a reduction in insulin resistance.48 Finally, Cordoba-Chacon et al.24 implicated an increase in de novo lipogenesis in the deposition of liver fat in adult GH receptor knockout mice. Interestingly, the typical pathway by which insulin regulates de novo lipogenesis (via SREBP-1) was not upregulated in this model, suggesting the effect of GH on de novo lipogenesis is not mediated via insulin.24 Thus, the relationship between GH and insulin resistance, as well as the exact mechanisms of the impact of GH on NAFLD require further study.
IGF-1, which is produced in the liver in response to GH, is an integral part of the GH/IGF-1 axis and of growing interest with respect to NAFLD and NASH.30 Although the IGF-1 receptor is not widely expressed in normal hepatocytes,49, 50 it is expressed in other types of hepatic cells. For example, hepatic stellate cells, which are involved in liver regeneration, have been shown to express the IGF-1 receptor, and overexpression of this receptor has been shown to enhance hepatic regeneration in mouse models.17 There may also be direct effects of IGF-1 on hepatocytes in the presence of liver disease, as studies have demonstrated increased IGF-1 receptor expression in human hepatoma cells in the regenerative state.49 Human hepatic tissue from patients with cirrhosis and chronic hepatitis C confirmed this finding if IGF-1 receptor upregulation.50 Moreover, IGF-1 administration has been shown to improve fibrosis in a mouse model of cirrhosis.18, 19, 51 In addition, IGF-1 has direct insulin-sensitizing effects,52 which could have a role in the amelioration of the NAFLD/NASH phenotype in these models.
In fact, both GH and IGF-1 have been implicated concurrently in the pathogenesis of NAFLD and progression to NASH in animal models. As an example, spontaneous dwarf rats have been studied as a murine model of NASH, as they produce no GH and low levels of IGF-1 compared with normal rats, and develop hepatic steatosis, elevated liver enzymes, and fibrosis by 20 weeks of age.16 In separate groups, both GH replacement and IGF-1 replacement normalized IGF-1 levels, reduced hepatic triglyceride content, decreased measures of oxidative stress, and ameliorated hepatocyte mitochondrial abnormalities. Interestingly, IGF-1 replacement alone also led to a significant reduction in liver fibrosis.16 In short, there is growing evidence that both GH and IGF-1 have a role in the development of NAFLD and progression to NASH.
Human data also support the role of the GH/IGF-1 axis in NAFLD and NASH. Studies of hypopituitary patients with frank GH deficiency have a higher rate of NAFLD compared with age- and BMI-matched controls (77 vs. 12%, respectively, P<0.001). In addition, administration of physiologic GH replacement decreased alanine transaminase, aspartate transaminase, high-sensitivity C-reactive protein, and markers of fibrosis in a group of hypopituitary individuals with GH deficiency, more than half of whom had NAFLD. Histologic steatosis and fibrosis scores improved in a small subset of patients (n=5) with pre- and post-GH treatment biopsies.25 Although this is a complex model of NAFLD and NASH, as hypopituitary patients often have other hormonal deficiencies that require adrenal, gonadal, and thyroid hormone replacement, these data suggest a possible effect of the GH/IGF-1 axis on NAFLD.
Obesity is a well-established state of relative GH deficiency in humans, with multiple studies showing a decrease in measures of GH with increasing BMI.9, 10, 11, 12, 13 However, the GH/IGF-1 axis has not been well characterized in biopsy-proven NAFLD. Existing studies of the GH/IGF-1 axis in patients with NAFLD have led to exciting data that potentially implicate the GH/IGF-1 axis in NAFLD, but they have also been confounded by methodological difficulties.33, 34, 35, 36, 53, 54, 55, 56, 57 GH is most often assessed by one fasting measurement rather than a gold-standard GH stimulation test or frequent sampling.34, 54, 56 Moreover, most studies rely on ultrasound for the diagnosis of NAFLD,53, 54, 55, 56, 57, 58, 59 including the most rigorous one showing lower mean peak stimulated GH levels in 65 subjects with NALFD compared with 55 controls of similar mean age and BMI.53 These data are very suggestive for an effect of relative GH deficiency on development of steatosis. However, ultrasound cannot quantify intrahepatic lipid content or assess for inflammation and fibrosis, and, in addition, has poor sensitivity for the detection of NAFLD, in particular with increasing BMI and at more mild levels of steatosis.60, 61
A few studies before ours have also explored the relationship between histologic severity of NAFLD and IGF-1 levels, which provide an integrated measure of GH but additionally reflect the independent hormonal actions of IGF-1 throughout the body. However, IGF-1 decreases significantly with both increasing BMI and age,14, 62 and no studies are controlled for both of these variables.33, 34, 35, 36 In addition, end-stage liver disease has been shown to blunt the production of IGF-1 in response to GH,32 yet patients with cirrhosis are often included in analyses of IGF-1 levels.33 Thus, it is imperative to control for these factors that are concurrently associated with decreased activation of the GH/IGF-1 axis and increased severity of NAFLD when investigating this relationship.
In short, although prior histologic studies of NAFLD overall suggest a role of the GH/IGF-1 axis in this disease process, rigorously controlled studies are still lacking. Our study confirmed the association between low IGF-1 levels and lobular inflammation, hepatocyte ballooning, fibrosis, and NASH, while controlling for age and BMI. We additionally controlled for race and performed a sensitivity analysis excluding those with cirrhosis with similar results. Interestingly, our data suggest that the GH/IGF-1 axis could potentially be involved in the progression of inflammation, hepatocyte ballooning, fibrosis, and NASH. These findings could reflect a combination of the anti-inflammatory properties of GH and anti-fibrotic properties of IGF-1. Given that it is poorly understood why some individuals with simple steatosis progress to NASH while others do not, our data implicate the GH/IGF-1 axis in this process and suggest that low-dose GH may be a potential treatment for patients with NASH.
In addition, the GH/IGF-1 axis is closely interlaced with insulin resistance and glucose homeostasis. This relationship is complex, given that both GH deficiency and GH excess can lead to insulin resistance, whereas IGF-1 generally acts as an insulin sensitizer.37 Given this complex relationship and that insulin resistance has been implicated in the development and progression of NAFLD and NASH, we sought to identify independent effects of the GH/IGF-1 axis on these histologic endpoints. Interestingly, our findings were independent of a diagnosis of diabetes mellitus, and in the small subset of patients for which HbA1c data were available, we found that IGF-1 was independently associated with NASH, even after controlling for HbA1c. Furthermore, a significant proportion of our cohort had diabetes mellitus and were receiving at least one diabetes mellitus-related medication. Although metformin, sulfonylureas, and insulin have not been found to prevent or reverse NAFLD,63 there are some limited data that glucagon-like peptide-1 agonists64 and thiazolidinediones63 may improve NAFLD. However, all results were unchanged when excluding subjects (n=5) who were on a glucagon-like peptide-1 agonist or thiazolidinedione, suggesting that taking medications for diabetes mellitus did not have an impact on the relationship between IGF-1 and NAFLD histology in this cohort.
This study is limited by a cross-sectional design. In addition, we did not have fasting glucose and insulin measurements on this cohort and only had HbA1c on a subgroup, which limited our overall evaluation of the interaction between the GH/IGF-1 axis, glucose homeostasis, and NAFLD. In addition, we were not able to perform GH stimulation testing and measurement of random levels of this pulsatile hormone is not useful. Although low IGF-1 is a reflection of low GH levels, IGF-1 is relatively preserved in obesity compared with GH. Thus, it is possible that GH levels would show an even stronger association with features of NASH, including inflammation, hepatocyte ballooning, and fibrosis.
This work suggests that dysregulation of the GH/IGF-1 axis in obesity may contribute to the progression from NAFLD to NASH. Further investigation is warranted to determine the differential effects of the GH/IGF-1 axis on the development and progression of NAFLD. Future research in this area could clarify the impact of the relative GH deficiency of obesity on the development of NAFLD and progression to NASH, with the potential for the identification of new therapeutic targets.
Study Highlights

Footnotes
Guarantor of the article: Karen K. Miller, MD.
Specific author contributions: L.E.D., M.A.B., K.E.C., and K.K.M. contributed to study design. K.E.C., S.A.O., and L.E.D. contributed to data collection. J.M. performed all pathology review. L.E.D., K.E.C., M.A.B., B.J.Y., and J.C.S. performed statistical analysis and data interpretation. L.E.D., K.E.C., M.S., and K.K.M. contributed to writing the manuscript. All authors reviewed and approved the manuscript before submission.
Financial support: This work was supported by National Institutes of Health grants R01 HL 077674, K24 HL092902, K23 RR023090, K23 DK099422, and T32 DK007028, as well as the Harvard Clinical and Translational Science Center (CTSC) grant UL1 RR025758.
Potential competing interests: None.
References
- Browning JD, Kumar KS, Saboorian MH et al. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol 2004; 99: 292–298. [DOI] [PubMed] [Google Scholar]
- Wong VW, Wong GL, Choi PC et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 2010; 59: 969–974. [DOI] [PubMed] [Google Scholar]
- Charlton MR, Burns JM, Pedersen RA et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011; 141: 1249–1253. [DOI] [PubMed] [Google Scholar]
- Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014; 59: 2188–2195. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol 2012; 107: 811–826. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Chalasani N, Kowdley KV et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promrat K, Kleiner DE, Niemeier HM et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy T, Oakley F, Anstee QM et al. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol 2016; 11: 451–496. [DOI] [PubMed] [Google Scholar]
- Pijl H, Langendonk JG, Burggraaf J et al. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 2001; 86: 5509–5515. [DOI] [PubMed] [Google Scholar]
- Utz AL, Yamamoto A, Sluss P et al. Androgens may mediate a relative preservation of IGF-I levels in overweight and obese women despite reduced growth hormone secretion. J Clin Endocrinol Metab 2008; 93: 4033–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makimura H, Stanley T, Mun D et al. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab 2008; 93: 4254–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 1991; 73: 1081–1088. [DOI] [PubMed] [Google Scholar]
- Beck P, Koumans JH, Winterling CA et al. Studies of insulin and growth hormone secretion in human obesity. J Lab Clin Med 1964; 64: 654–667. [PubMed] [Google Scholar]
- Maccario M, Ramunni J, Oleandri SE et al. Relationships between IGF-I and age, gender, body mass, fat distribution, metabolic and hormonal variables in obese patients. Int J Obes Relat Metab Disord 1999; 23: 612–618. [DOI] [PubMed] [Google Scholar]
- Rasmussen MH, Juul A, Hilsted J. Effect of weight loss on free insulin-like growth factor-I in obese women with hyposomatotropism. Obesity (Silver Spring) 2007; 15: 879–886. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, Takahashi M, Fukuoka H et al. GH-independent IGF-I action is essential to prevent the development of nonalcoholic steatohepatitis in a GH-deficient rat model. Biochem Biophys Res Commun 2012; 423: 295–300. [DOI] [PubMed] [Google Scholar]
- Sanz S, Pucilowska JB, Liu S et al. Expression of insulin-like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut 2005; 54: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguerza B, Castilla-Cortazar I, Garcia M et al. Antifibrogenic effect in vivo of low doses of insulin-like growth factor-I in cirrhotic rats. Biochim Biophys Acta 2001; 1536: 185–195. [DOI] [PubMed] [Google Scholar]
- Castilla-Cortazar I, Garcia M, Muguerza B et al. Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology 1997; 113: 1682–1691. [DOI] [PubMed] [Google Scholar]
- Barclay JL, Nelson CN, Ishikawa M et al. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology 2011; 152: 181–192. [DOI] [PubMed] [Google Scholar]
- Nordstrom SM, Tran JL, Sos BC et al. Disruption of JAK2 in adipocytes impairs lipolysis and improves fatty liver in mice with elevated GH. Mol Endocrinol 2013; 27: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Menon RK, Cohen P et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem 2009; 284: 19937–19944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Fang X, Tajima A et al. Evolution of hepatic steatosis to fibrosis and adenoma formation in liver-specific growth hormone receptor knockout mice. Front Endocrinol (Lausanne) 2014; 5: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Chacon J, Majumdar N, List EO et al. Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes 2015; 64: 3093–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa H, Iguchi G, Murawaki A et al. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur J Endocrinol 2012; 167: 67–74. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Fukuoka H, Iguchi G et al. Long-term effects of growth hormone replacement therapy on liver function in adult patients with growth hormone deficiency. Growth Hormone IGF Res 2014; 24: 174–179. [DOI] [PubMed] [Google Scholar]
- Sesmilo G, Biller BM, Llevadot J et al. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Int Med 2000; 133: 111–122. [DOI] [PubMed] [Google Scholar]
- Bredella MA, Lin E, Brick DJ et al. Effects of GH in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. Eur J Endocrinol 2012; 166: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Gerweck AV, Lin E et al. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab 2013; 98: 3864–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y. Essential roles of growth hormone (GH) and insulin-like growth factor-I (IGF-I) in the liver. Endocr J 2012; 59: 955–962. [DOI] [PubMed] [Google Scholar]
- Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol 2007; 157: 695–700. [DOI] [PubMed] [Google Scholar]
- Assy N, Pruzansky Y, Gaitini D et al. Growth hormone-stimulated IGF-1 generation in cirrhosis reflects hepatocellular dysfunction. J Hepatol 2008; 49: 34–42. [DOI] [PubMed] [Google Scholar]
- Sumida Y, Yonei Y, Tanaka S et al. Lower levels of insulin-like growth factor-1 standard deviation score are associated with histological severity of non-alcoholic fatty liver disease. Hepatol Res 2015; 45: 771–781. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Nakao K, Hamasaki K et al. Role of growth hormone, insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 in development of non-alcoholic fatty liver disease. Hepatol Int 2007; 1: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Galiano D, Sanchez-Garrido MA, Espejo I et al. IL-6 and IGF-1 are independent prognostic factors of liver steatosis and non-alcoholic steatohepatitis in morbidly obese patients. Obes Surg 2007; 17: 493–503. [DOI] [PubMed] [Google Scholar]
- Colak Y, Senates E, Ozturk O et al. Serum concentrations of human insulin-like growth factor-1 and levels of insulin-like growth factor-binding protein-5 in patients with nonalcoholic fatty liver disease: association with liver histology. Eur J Gastroenterol Hepatol 2012; 24: 255–261. [DOI] [PubMed] [Google Scholar]
- O'Connell T, Clemmons DR. IGF-I/IGF-binding protein-3 combination improves insulin resistance by GH-dependent and independent mechanisms. J Clin Endocrinol Metab 2002; 87: 4356–4360. [DOI] [PubMed] [Google Scholar]
- Mueller JL, Feeney ER, Zheng H et al. Circulating soluble CD163 is associated with steatohepatitis and advanced fibrosis in nonalcoholic fatty liver disease. Clin Trans Gastroenterol 2015; 6: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey KE, Misdraji J, Zheng H et al. The absence of obstructive sleep apnea may protect against non-alcoholic fatty liver in patients undergoing bariatric surgery. PLoS ONE 2013; 8: e62504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey KE, Misdraji J, Gelrud L et al. Nonalcoholic steatohepatitis is associated with an atherogenic lipoprotein subfraction profile. Lipids Health Dis 2014; 13: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey KE, Misdraji J, Gelrud L et al. Obstructive sleep apnea is associated with nonalcoholic steatohepatitis and advanced liver histology. Dig Dis Sci 2015; 60: 2523–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey KE, Lai M, Gelrud LG et al. Non-high-density lipoprotein cholesterol as a biomarker for nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2012; 10: 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 1998; 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Brunt EM, Kleiner DE et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011; 54: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U. A note on the power of Fisher's least significant difference procedure. Pharm Stat 2006; 5: 253–263. [DOI] [PubMed] [Google Scholar]
- Collin de l'Hortet A, Zerrad-Saadi A, Prip-Buus C et al. GH administration rescues fatty liver regeneration impairment by restoring GH/EGFR pathway deficiency. Endocrinology 2014; 155: 2545–2554. [DOI] [PubMed] [Google Scholar]
- Qin Y, Tian YP. Preventive effects of chronic exogenous growth hormone levels on diet-induced hepatic steatosis in rats. Lipids Health Dis 2010; 9: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List EO, Palmer AJ, Berryman DE et al. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia 2009; 52: 1647–1655. [DOI] [PubMed] [Google Scholar]
- Caro JF, Poulos J, Ittoop O et al. Insulin-like growth factor I binding in hepatocytes from human liver, human hepatoma, and normal, regenerating, and fetal rat liver. J Clin Invest 1998; 81: 976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano JT, Correa-Giannella ML, Ribeiro CM et al. Increased hepatic expression of insulin-like growth factor-I receptor in chronic hepatitis C. World J Gastroenterol 2006; 12: 3821–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez R, Garcia-Fernandez M, Diaz-Sanchez M et al. Mitochondrial protection by low doses of insulin-like growth factor- I in experimental cirrhosis. World J Gastroenterol 2008; 14: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR. Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol 2006; 6: 620–625. [DOI] [PubMed] [Google Scholar]
- Fusco A, Miele L, D'Uonnolo A et al. Nonalcoholic fatty liver disease is associated with increased GHBP and reduced GH/IGF-I levels. Clin Endocrinol 2012; 77: 531–536. [DOI] [PubMed] [Google Scholar]
- Xu L, Xu C, Yu C et al. Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study. PLoS ONE 2012; 7: e44136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arturi F, Succurro E, Procopio C et al. Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. J Clin Endocrinol Metab 2011; 96: E1640–E1644. [DOI] [PubMed] [Google Scholar]
- Lonardo A, Loria P, Leonardi F et al. Growth hormone plasma levels in nonalcoholic fatty liver disease. Am J Gastroenterol 2002; 97: 1071–1072. [DOI] [PubMed] [Google Scholar]
- Runchey SS, Boyko EJ, Ioannou GN et al. Relationship between serum circulating insulin-like growth factor-1 and liver fat in the United States. J Gastroenterol Hepatol 2014; 29: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volzke H, Nauck M, Rettig R et al. Association between hepatic steatosis and serum IGF1 and IGFBP-3 levels in a population-based sample. Eur J Endocrinol 2009; 161: 705–713. [DOI] [PubMed] [Google Scholar]
- Mallea-Gil MS, Ballarino MC, Spiraquis A et al. IGF-1 levels in different stages of liver steatosis and its association with metabolic syndrome. Acta Gastroenterol Latinoam 2012; 42: 20–26. [PubMed] [Google Scholar]
- Mottin CC, Moretto M, Padoin AV et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg 2004; 14: 635–637. [DOI] [PubMed] [Google Scholar]
- de Moura Almeida A, Cotrim HP, Barbosa DB et al. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol 2008; 14: 1415–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifke E, Gorenoi V, Wichers C et al. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 2000; 53: 689–695. [DOI] [PubMed] [Google Scholar]
- Rakoski MO, Singal AG, Rogers MA et al. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2010; 32: 1211–1221. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Gaunt P, Aithal GP et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016; 387: 679–690. [DOI] [PubMed] [Google Scholar]