Abstract
The cDC1 subset of classical dendritic cells is specialized for priming CD8 T cell responses through the process of cross-presentation. The molecular mechanisms of cross-presentation remain incompletely understood because of limited biochemical analysis of rare cDC1 cells, difficulty in their genetic manipulation, and reliance on in vitro systems based on monocyte- and bone-marrow-derived dendritic cells. This review will discuss cross-presentation from the perspective of studies with monocyte- or bone-marrow-derived dendritic cells while highlighting the need for future work examining cDC1 cells. We then discuss the role of cDC1s as a cellular platform to combine antigen processing for class I and class II MHC presentation to allow the integration of “help” from CD4 T cells during priming of CD8 T cell responses.
Keywords: Dendritic cell, monocyte-derived dendritic cells, T cell response
Introduction
Dendritic cells (DCs) are a distinct lineage of innate immune cells that was originally defined based on its unique stellate morphology and ability to prime T cell responses 1– 3. DCs broadly segregate into four groups, plasmacytoid DCs (pDCs), classical DCs (cDCs), Langerhans cells, and monocyte-derived DCs (moDCs) based on function and surface markers. pDCs are potent producers of type I interferons in response to viral pathogens 4– 7. cDCs themselves are divided into two lineages, recently renamed 8 as cDC1 (CD8α + DCs) and cDC2 (CD8α ˗ DCs). The cDC2 lineage is heterogeneous and expresses the Irf4 transcription factor 9– 11. Notch 2-dependent cDC2s are required for IL-23 production in response to Citrobacter rodentium infection 12, 13, while a separate Klf4-dependent subset of cDC2s is required for type II responses to house dust mite antigen and Schistosoma mansoni infection 14. By contrast, cDC1 cells require the Irf8 10, 15, 16 and Batf3 transcription factors 10, 16, 17 and produce the IL-12 necessary for protection against Toxoplasma gondii 18, 19. They are also the subset involved in priming CD8 T cell responses to tumors and virally infected cells through cross-presentation 17, 20. All cDCs in vivo arise from a common DC progenitor (CDP) in the bone marrow 21.
Cultures of monocytes in GM-CSF and IL-4 are able to produce DC-like cells, distinct from those that develop from the CDP 22, termed monocyte-derived DCs (moDCs), in large numbers 23. Similar cells that derive from cultures of whole bone marrow with GM-CSF with or without IL-4 in vitro have been referred to as “moDCs”, despite the uncertainty of the origin, or bone-marrow-derived DCs (BMDCs). BMDCs have been the basis for many studies aimed at understanding the properties of cDCs 24, 25. Recent studies have shown that these cultures are actually heterogeneous and that it may not be appropriate to refer to the cells that are generated as moDCs, since many display macrophage characteristics and the precursor to the DC-like cells from whole bone marrow is not known 26. Some investigators object to the use of the term moDC for in-vitro-derived cells from whole bone marrow since it is misleading with regard to their development; however, it has been argued that the DC-like cells that develop from GM-CSF cultures develop from monocytes 27. The term BMDC can also lead to confusion, since DCs can also be derived from bone marrow cultures with fms-related tyrosine kinase 3 ligand (Flt3L) and produce cells that are distinct from those produced in GM-CSF cultures 27. Therefore, in this review, we will refer to cells generated from monocytes as moDCs and cells generated from whole bone marrow GM-CSF cultures as GMDCs. Conceivably, both may be derived from monocytes and distinct from in-vivo-derived cDCs.
In this review, we will first highlight new discoveries regarding cross-presentation and discuss how molecular mechanisms governing cross-presentation by cDC1s may be distinct from the cross-presentation pathways identified in moDCs or GMDCs in vitro. We will then describe how cDC1s initiate and maintain anti-viral responses, including through their interactions with CD4 T cells.
Molecular mechanisms of cross-presentation
Cross-presentation is the process by which exogenous antigens are taken up by antigen-presenting cells and presented on major histocompatibility class I (MHCI) 28. cDC1s are the unique DC subset specialized in cross-presentation in vivo 20. The molecular mechanisms specific to DCs that govern cross-presentation have been the subject of a large body of work over the past decade 29, with much of the early work on cross-presentation carried out in macrophages 30– 32, though the majority of our understanding of cross-presentation is based on experiments carried out using GMDCs. GMDCs are generated from bone marrow cultures with GM-CSF alone or GM-CSF with IL-4 originally developed in the early 1990s 24– 26. While these cells can cross-present in vitro, it is unlikely that these are the cells that operate in vivo, since Batf3 -/- mice that lack cDC1s fail to mount CD8 T cell responses to challenges requiring cross-presentation 17. However, Batf3 -/- mice can generate moDCs that are able to cross-present normally in vitro 33, indicating that any moDCs that may develop in vivo do not compensate for the loss of cDC1s for in vivo cross-presentation.
Surprisingly little work has been done to analyze cross-presentation in DCs derived from bone marrow cultures with Flt3L. DCs that resemble splenic cDC1 and cDC2 by surface markers can be generated in large numbers in bone marrow cultures with Flt3L 34, 35. These cells are able to present antibody-targeted antigens and activate T cells to a similar extent as cDCs of the same lineage derived in vivo 36. Also, Flt3L-derived DCs express Rab43, a molecule necessary for cross-presentation in in vivo cDC1s but not moDCs 37. While more studies may be needed to compare the cross-presentation efficiency of Flt3L-derived DCs to in-vivo-generated cDCs, Flt3L-derived DCs are arguably more appropriate for in vitro studies of DC function than GMDCs. Nonetheless, the examination of macrophages and GMDCs has been useful for identifying the components of two major cross-presentation pathways, the cytosolic and vacuolar pathways.
In the cytosolic pathway, exogenous antigens that are taken up into phagosomes are exported into the cytosol to enter the traditional proteasome- and TAP-dependent MHCI presentation pathway 32, 38, 39. The cytosolic pathway is dependent on the reduced acidification of phagosomes produced by the activity of NADPH oxidase Nox2, leading to delayed antigen degradation 40, 41. Recruitment and localization of NOX2 components was determined to be regulated by the activities of Rac2 and Rab27a 41, 42. Phagosomal alkalization has also been demonstrated to involve Rab3c (a marker of recycling vesicles 43), Rab34 (an LPS-regulated protein that can delay phago-lysosomal fusion 44), and TFEB (a transcription factor that can negatively regulate cross-presentation 45). The delay in antigen degradation caused by phagosomal alkalization acts to allow antigens to move into the cytosol, possibly through channels such as Sec61, promoting antigen processing and presentation through the normal MHCI pathway 46. These pathways have mainly been shown to act in phagosomes containing latex beads, raising the question of whether this process is specific to uptake of beads or if antigens that bind different receptors are processed through similar mechanisms.
NOX2 has been shown to play a role in cross-presentation in vivo 40, 42, suggesting that phagosomal alkalization may also be important for cross-presentation by cDC1s. However, the magnitude of the contribution of this pathway is limited, as loss of NOX2 activity decreased cross-presentation of antibody-targeted antigen only by about 50% 40. The remainder of the molecules in the cytosolic pathway, including Rac2, Rab27a, Rab3c, Sec61, TFEB, and Rab34, have not been examined in in vivo cDCs 41– 45. Genetic studies with mouse models will be necessary to determine the importance of these molecules and the cytosolic pathway in general to cross-presentation in vivo.
The vacuolar pathway involves the loading of MHCI molecules by antigens processed directly within endosomes without transport to the cytosol and is independent of TAP and the proteasome 47, 48. One molecule linked to the vacuolar pathway is the insulin-regulated aminopeptidase (IRAP) 49. IRAP can trim peptides in DC phagosomes to lengths appropriate for loading into MHCI molecules 49, similar to the action of endoplasmic reticulum aminopeptidase associated with antigen processing (ERAAP) in the endoplasmic reticulum 50. The role of IRAP in vivo remains unclear. Although an early study detailing the mechanism of IRAP was conducted using in vitro GMDCs, IRAP-deficient mice were also shown to have reduced cross-presentation 49. However, a subsequent study concluded that IRAP was not required for cross-presentation of soluble OVA or OVA-coated splenocytes by splenic cDC1s in vitro, suggesting that IRAP may not play a role in cDC1-mediated cross-presentation 51. But another study, using OVA-expressing yeast in vitro, showed that IRAP is recruited to endosomes in cDC1 cells and that cross-presentation is reduced in IRAP-deficient cDC1s 52. Conceivably, the use of differing forms of antigen underlies some of these variances. While the ability of cDC1 cells to cross-present is not solely due to their ability to capture antigens 53, 54, it is plausible that distinct antigen internalization and processing pathways are used for different forms of antigen. For example, cell-associated and soluble antigens are not cross-presented equally and cDC2s, which do not cross-present in vivo 20, have the capacity to present soluble antigens in vitro 52, 54. Therefore, work is still needed to compare cross-presentation of different antigens by cDC1s and cDC2s in vitro to find a system that mimics in vivo models where only cDC1s are able to cross-present. Developing standardized assays for the field through careful comparison of DC subsets may help to eliminate confusion between whether or not molecules are necessary for cross-presentation in vivo as in the case of IRAP.
Presentation through the vacuolar pathway requires the loading of MHCI molecules within endosomes. The molecule Sec22b was described in GMDCs to regulate the movement of the peptide-loading complex to endosomes 55. It has also been shown that GMDCs contain pools of MHCI in endosomal recycling compartments marked by Rab11a 56. A model has been proposed where TLR signals induce MHCI movement from these intracellular pools to phagosomes, where they meet antigen and the peptide-loading complex machinery brought by Sec22b 56. A second proposed model involves CD74, the MHCII invariant chain, which was also shown to control the movement of MHCI to endosomes and to regulate cross-presentation in vivo 57. CD74 acts in both splenic cDC1s and GMDCs, meaning CD74 and IRAP are the two molecules shown to be involved in the vacuolar pathway of cross presentation in cDC1s 52, 57. However, as with the cytosolic pathway, many gaps still remain in our understanding of what proteins and signals are involved in regulating the cross-presentation ability of cDC1s.
Role of moDCs in vivo
The discovery that moDCs cannot compensate for the loss of cross-presentation by cDC1s in vivo has called into question their relevance in vivo 33. Bone marrow cultured with GM-CSF produces a heterogeneous population of CD11c + MHCII + cells which contain functionally distinct macrophages and DCs 26. While moDCs have a stellate morphology, express the cDC-specific ZBTB46 transcription factor 58, and can cross-present cell-associated antigens, they do so in a manner distinct from ex vivo cDC1 cells 33, 51.
Further, recent work has called into question if moDCs exist in vivo. Studies of moDCs started with the observation that transferred monocytes are able to generate CD11c + DC-like cells in vivo 22. These moDCs have been observed in numerous models including viral infections 59, alum-OVA immunization 60, arthritis 61, and house dust mite exposure 62. They can be distinguished from cDCs in vivo by expression of CD64 and MAR-1 60, 62 and are dependent on CCR2 and CD115 (MCSF-R) 60, 63. However, it is unclear whether the moDCs identified in these studies in vivo are equivalent to those generated with GM-CSF and IL-4 in vitro. Recent lineage tracing has suggested that the inflammatory cells that develop during house dust mite challenge lack expression of the cDC marker ZBTB46 58 and instead express the macrophage-specific transcription factor MafB 64, 65, suggesting that these cells are not moDCs but rather monocyte-derived macrophages. Furthermore, others have shown little functional difference among moDCs, monocyte-derived macrophages, myeloid-derived suppressor cells, and immature monocytes 8, 66, 67, also suggesting that in vivo moDCs may actually be monocyte-derived macrophages. In addition, no in vivo model has yet to be described where moDCs are required for cross-presentation. Lineage tracing of in vivo moDCs and comparisons to in vitro-derived GMDCs will be necessary to determine whether GM-CSF cultures are an appropriate model to study DC function. Owing to the observed differences between GMDCs and cDC1s, studies of cross-presentation in vitro should rely on either ex vivo cDCs or Flt3L-derived DCs to more appropriately model how cross-presentation occurs in vivo.
Cross-presentation during viral infections
Though cDC1s are the major cell that appears to carry out cross-presentation for expanding CD8 T cells in vivo 20, many cells are able to present antigens on MHCI to CD8 T cells 68. Therefore, it is unclear whether cross-presentation is the only pathway used in priming CD8 T cells to pathogens, or alternately whether direct presentation by infected cells might contribute in some settings. Indeed, the cell type responsible for T cell priming and the pathway of antigen processing may vary with the pathogen and could depend on factors such as viral tropism and the time after infection 69– 71. For example, using DC-tropic vaccinia virus expressing an extended OVA peptide that could not be cross-presented, Xu et al. demonstrated that direct presentation is sufficient for generating a CD8 T cell response 69. However, during infection with mouse cytomegalovirus, another DC-tropic virus, the predominant T cell clones react to epitopes that were presented through cross-presentation 70, 71. It is likely that both direct and cross-presentation can contribute in priming CD8 T cell responses and that the predominant form of presentation may depend on the stage of infection. Early during infection, antigen presentation requires viral replication, suggesting direct presentation is playing a role; however, late during infection most presentation occurs by uninfected DCs through cross-presentation 72 ( Figure 1). Imaging of T cells and cDC1s during vaccinia virus infection showed a similar phenomenon and it was observed that multiple DC subsets could prime CD8 T cell responses early during infection; however, later in infection CD8 T cells interacted with only XCR1-expressing cDC1s 73. cDC1s are also essential for priming CD8 T cell responses during secondary infections and generating T resident memory cells, a process recently shown to depend on cross-presentation 74, 75.
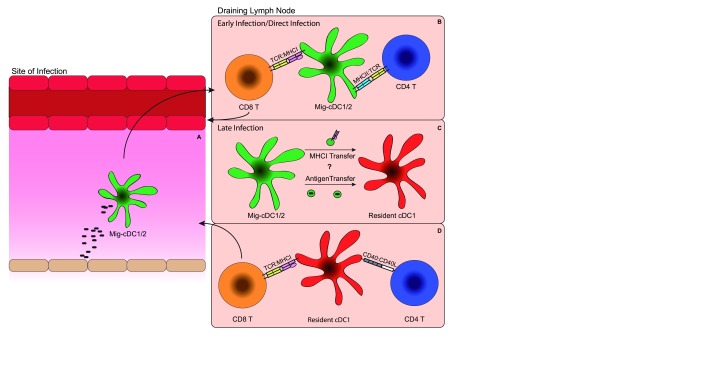
Figure 1. Model for CD8 T cell priming by resident classical CD8α + dendritic cells (cDC1s).
( A) Antigen is captured by migratory cDC1s or CD11b + cDCs (cDC2s) at the site of infection by either direct infection or phagocytosis. ( B) After antigen capture, migratory cDC1s or cDC2s with antigen then migrate to the draining lymph node, where they prime naïve antigen-specific CD4 and possibly CD8 T cells through major histocompatibility (MHC):T cell receptor (TCR) interactions. ( C) Migratory cDCs transfer antigens to resident cDC1s through either “cross-dressing”, the process by which loaded MHCI is transferred between cell membranes, or by transferring the antigen itself, which is then taken up by the resident cDC1s for cross-presentation. ( D) Resident cDC1s receive “help” through CD40:CD40L interactions with CD4 T cells, which allow them to prime antigen-specific naïve CD8 T cells through MHCI:TCR interactions. Mig-DC, migratory dendritic cell.
In lymph nodes, cDC1s can be separated into two categories of migratory and resident DCs that are developmentally related 76, either of which could be involved in the presentation of antigen to CD8 T cells during an infection. Tracking of migratory DCs from the skin during herpes simplex virus (HSV) infection has shown that CD8 T cell priming occurs in lymph nodes and movement of migratory DCs from the skin is required for priming to occur 77. Then in the lymph node, antigens acquired by migratory DCs can be transferred to lymph-node-resident DCs for presentation to CD8 T cells 77 ( Figure 1C). These results imply that there may be two distinct priming events: an initial priming from migratory cDC1s that directly captured antigen and then a secondary priming that occurs after antigen has been transferred to resident cDC1s. Imaging of the anti-viral response to HSV suggested that CD4 T cells are primed before CD8 T cells and that they interact with migratory DCs, while CD8 T cells interact with resident cDC1s in the lymph node 78. However, others have demonstrated that antigen-specific CD8 T cells preferentially interact with migratory cDC1s 79, 80. These results raise the question of whether all CD8 T cell priming occurs through migratory cDC1s, which are directly exposed to antigens, or through resident cDC1s, which can present their antigens through either cross-presentation 73, 77 or cross-dressing, a process by which loaded MHCI is transferred between different cells 81. Conceivably, early CD8 and potentially CD4 T cell priming is mediated by direct presentation from migratory cDC1s, since they encounter antigen first, and then later CD8 T cell priming occurs after antigen transfer to and cross-presentation by lymph-node-resident cDC1s ( Figure 1A and D).
CD4 T cells and cDC1s
For many pathogens, DCs alone are not enough to prime a CD8 T cell response. CD4 T cells and type I interferons have been shown to be involved in the “help” reaction, which stimulates DCs and enables them to prime CD8 T cells 82, 83. Early work on cross-presentation showed that CD4 T cell help to DCs is necessary for the generation of a CD8 T cell response against cell-associated antigens 82. This help is mediated through interactions between CD40 on DCs and CD40L on CD4 T cells 84– 86. These results describe a “bridge” model, where CD4 T cells and CD8 T cells interact with the same dendritic cell, albeit likely at different times, in order to properly prime a cytotoxic T cell response 78, 85. This suggests that CD4 T cells must be activated prior to CD8 T cells, likely by migratory cDCs, in order for them to act on cDC1s through CD40L to help induce CD8 T cell priming ( Figure 1B and D).
Questions remain as to whether the interaction between cDC1s and CD4 T cells is antigen specific. Initial studies that showed that CD4 T cell help for CD8 T cell priming required cognate CD4 T cell interactions 82. However, later it was suggested that CD40 signaling was sufficient to provide help, even when DCs lack MHCII 85. In vitro analysis of presentation by DC subsets using antibody-targeted antigen implied that cDC1s were relatively poor in antigen presentation to CD4 T cells relative to cDC2s, while cDC2s were adept at activating CD4 T cells in vitro 36, 87. This leads to the question of whether cDC1s use MHCII presentation solely to obtain help from previously activated CD4 T cells for CD8 T cell priming or, alternatively, whether cDC1s can also prime naïve CD4 T cells. A recent study has shown that CD8 T cells cluster with cDC1s, while CD4 T cells cluster with cDC2s during OVA immunization 88, suggesting that T cell priming may be DC-subset specific. However, late during viral infection both cDC1 and cDC2 subsets have the capacity to activate CD4 T cells 79. In addition, both CD4 and CD8 T cell priming against insulin in non-obese diabetic mice is decreased in the absence of cDC1s 89. Since CD4 T cells were shown to be primed first by migratory DCs 78, it is possible that migratory cDC1s prime the CD4 T cells that later help lymph-node-resident cDC1s induce CD8 T cell priming ( Figure 1B and D). Further studies will be necessary to determine to what extent each DC subset contributes to T cell priming in different infection contexts.
Conclusion
cDC1s are the predominant cross-presenting cells functioning in CD8 T cell priming in vivo 20. Recent imaging studies suggest that cDC1s also function as a platform for CD4 T cell help during viral infections 74, 78, likely through CD40–CD40L interactions 84, 85. However, it remains unclear whether cDC1s can also prime naive CD4 T cells or whether they receive only help from them 36, 79, 82, 89. More sophisticated in vivo models will need to be generated in order to determine the role of cDC1s in priming CD4 T cell responses in vivo in order to further distinguish the unique roles of DC subsets.
Transcriptional profiling has suggested that moDCs may not be a functional cross-presenting DC subset in vivo 33 and at least in one setting may represent monocyte-derived macrophages 65. Many molecules described previously to be involved in cross-presentation were evaluated in the context of GMDCs and need to be examined in the context of cDC1s 41– 45. Recent advances in DC biology have allowed for the conditional deletion of genes in cDC1s through the use of XCR1-cre 90 and analysis of transcriptional differences between DC subsets 91. Examining molecules described in moDCs also in cDC1s and studying other cDC1-specific genes will aid in our understanding of how cross-presentation against viral and cancer antigens occurs and may provide more insight into whether moDCs are a true DC subset in vivo. Elucidating the mechanisms by which cDC1s activate CD8 T cells and the mechanisms underlying the various interactions between DC subsets and T cells should be of value in designing DC-based cancer vaccines.
Abbreviations
BMDC, bone-marrow-derived dendritic cell; cDC, classical dendritic cell; CDP, common dendritic cell progenitor; DC, dendritic cell; Flt3L, fms-related tyrosine kinase 3 ligand; GMDC, GM-CSF-derived dendritic cell; HSV, herpes simplex virus; IRAP, insulin-regulated aminopeptidase; MHCI, major histocompatibility class I; MHCII, major histocompatibility class II; moDC, monocyte-derived dendritic cell; pDC, plasmacytoid dendritic cell.
Acknowledgements
We thank Prachi Bagadia, Carlos Briseño, and Vivek Durai for their contributions to this review.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Paul Roche, Experimental Immunology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Terri M. Laufer, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA; Philadelphia Veterans Affairs Medical Center, Philadelphia, PA, USA
Caetano Reis e Sousa, Immunobiology Laboratory, The Francis Crick Institute, London, UK
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Steinman RM, Cohn ZA: Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–62. 10.1084/jem.137.5.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steinman RM, Witmer MD: Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978;75(10):5132–6. 10.1073/pnas.75.10.5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nussenzweig MC, Steinman RM, Gutchinov B, et al. : Dendritic cells are accessory cells for the development of anti-trinitrophenyl cytotoxic T lymphocytes. J Exp Med. 1980;152(4):1070–84. 10.1084/jem.152.4.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perussia B, Fanning V, Trinchieri G: A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell Growth Regul. 1985;4(3):120–37. [PubMed] [Google Scholar]
- 5. Cella M, Jarrossay D, Facchetti F, et al. : Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5(8):919–23. 10.1038/11360 [DOI] [PubMed] [Google Scholar]
- 6. Siegal FP, Kadowaki N, Shodell M, et al. : The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–7. 10.1126/science.284.5421.1835 [DOI] [PubMed] [Google Scholar]
- 7. Asselin-Paturel C, Boonstra A, Dalod M, et al. : Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2(12):1144–50. 10.1038/ni736 [DOI] [PubMed] [Google Scholar]
- 8. Guilliams M, Ginhoux F, Jakubzick C, et al. : Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–8. 10.1038/nri3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki S, Honma K, Matsuyama T, et al. : Critical roles of interferon regulatory factor 4 in CD11b highCD8alpha- dendritic cell development. Proc Natl Acad Sci U S A. 2004;101(24):8981–6. 10.1073/pnas.0402139101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamura T, Tailor P, Yamaoka K, et al. : IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174(5):2573–81. 10.4049/jimmunol.174.5.2573 [DOI] [PubMed] [Google Scholar]
- 11. Jaitin DA, Kenigsberg E, Keren-Shaul H, et al. : Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343(6172):776–779. 10.1126/science.1247651 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Lewis KL, Caton ML, Bogunovic M, et al. : Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35(5):780–91. 10.1016/j.immuni.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satpathy AT, Briseño CG, Lee JS, et al. : Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14(9):937–48. 10.1038/ni.2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tussiwand R, Everts B, Grajales-Reyes GE, et al. : Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity. 2015;42(5):916–28. 10.1016/j.immuni.2015.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aliberti J, Schulz O, Pennington DJ, et al. : Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood. 2003;101(1):305–10. 10.1182/blood-2002-04-1088 [DOI] [PubMed] [Google Scholar]
- 16. Schiavoni G, Mattei F, Sestili P, et al. : ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha + dendritic cells. J Exp Med. 2002;196(11):1415–25. 10.1084/jem.20021263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hildner K, Edelson BT, Purtha WE, et al. : Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–100. 10.1126/science.1164206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reis e Sousa C, Hieny S, Scharton-Kersten T, et al. : In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186(11):1819–29. 10.1084/jem.186.11.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mashayekhi M, Sandau MM, Dunay IR, et al. : CD8alpha + dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35(2):249–59. 10.1016/j.immuni.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. den Haan JM, Lehar SM, Bevan MJ: CD8 + but not CD8 - dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192(12):1685–96. 10.1084/jem.192.12.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy KM: Transcriptional control of dendritic cell development. Adv Immunol. 2013;120:239–67. 10.1016/B978-0-12-417028-5.00009-0 [DOI] [PubMed] [Google Scholar]
- 22. Naik SH, Metcalf D, van Nieuwenhuijze A, et al. : Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7(6):663–71. 10.1038/ni1340 [DOI] [PubMed] [Google Scholar]
- 23. Sallusto F, Lanzavecchia A: Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109–18. 10.1084/jem.179.4.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caux C, Dezutter-Dambuyant C, Schmitt D, et al. : GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360(6401):258–61. 10.1038/360258a0 [DOI] [PubMed] [Google Scholar]
- 25. Inaba K, Inaba M, Romani N, et al. : Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–702. 10.1084/jem.176.6.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helft J, Böttcher J, Chakravarty P, et al. : GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c +MHCII + Macrophages and Dendritic Cells. Immunity. 2015;42(6):1197–211. 10.1016/j.immuni.2015.05.018 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Xu Y, Zhan Y, Lew AM, et al. : Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179(11):7577–7584. 10.4049/jimmunol.179.11.7577 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Bevan MJ: Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143(5):1283–8. 10.1084/jem.143.5.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joffre OP, Segura E, Savina A, et al. : Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–69. 10.1038/nri3254 [DOI] [PubMed] [Google Scholar]
- 30. Pfeifer JD, Wick MJ, Roberts RL, et al. : Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361(6410):359–62. 10.1038/361359a0 [DOI] [PubMed] [Google Scholar]
- 31. Kovacsovics-Bankowski M, Clark K, Benacerraf B, et al. : Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci U S A. 1993;90(11):4942–6. 10.1073/pnas.90.11.4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kovacsovics-Bankowski M, Rock KL: A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267(5193):243–6. 10.1126/science.7809629 [DOI] [PubMed] [Google Scholar]
- 33. Briseño CG, Haldar M, Kretzer NM, et al. : Distinct Transcriptional Programs Control Cross-Priming in Classical and Monocyte-Derived Dendritic Cells. Cell Rep. 2016;15(11):2462–74. 10.1016/j.celrep.2016.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naik SH, Proietto AI, Wilson NS, et al. : Cutting edge: generation of splenic CD8 +and CD8 - dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174(11):6592–7. 10.4049/jimmunol.174.11.6592 [DOI] [PubMed] [Google Scholar]
- 35. Mayer CT, Ghorbani P, Nandan A, et al. : Selective and efficient generation of functional Batf3-dependent CD103 + dendritic cells from mouse bone marrow. Blood. 2014;124(20):3081–91. 10.1182/blood-2013-12-545772 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Kamphorst AO, Guermonprez P, Dudziak D, et al. : Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol. 2010;185(6):3426–35. 10.4049/jimmunol.1001205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kretzer NM, Theisen DJ, Tussiwand R, et al. : RAB43 facilitates cross-presentation of cell-associated antigens by CD8α +dendritic cells. J Exp Med. 2016;213(13):2871–83. 10.1084/jem.20160597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brossart P, Bevan MJ: Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90(4):1594–9. [PMC free article] [PubMed] [Google Scholar]
- 39. Morón VG, Rueda P, Sedlik C, et al. : In vivo, dendritic cells can cross-present virus-like particles using an endosome-to-cytosol pathway. J Immunol. 2003;171(5):2242–50. 10.4049/jimmunol.171.5.2242 [DOI] [PubMed] [Google Scholar]
- 40. Savina A, Jancic C, Hugues S, et al. : NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126(1):205–18. 10.1016/j.cell.2006.05.035 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Jancic C, Savina A, Wasmeier C, et al. : Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9(4):367–78. 10.1038/ncb1552 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Savina A, Peres A, Cebrian I, et al. : The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8 + dendritic cells. Immunity. 2009;30(4):544–55. 10.1016/j.immuni.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 43. Zou L, Zhou J, Zhang J, et al. : The GTPase Rab3b/3c-positive recycling vesicles are involved in cross-presentation in dendritic cells. Proc Natl Acad Sci U S A. 2009;106(37):15801–6. 10.1073/pnas.0905684106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alloatti A, Kotsias F, Pauwels AM, et al. : Toll-like Receptor 4 Engagement on Dendritic Cells Restrains Phago-Lysosome Fusion and Promotes Cross-Presentation of Antigens. Immunity. 2015;43(6):1087–100. 10.1016/j.immuni.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 45. Samie M, Cresswell P: The transcription factor TFEB acts as a molecular switch that regulates exogenous antigen-presentation pathways. Nat Immunol. 2015;16(7):729–36. 10.1038/ni.3196 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Zehner M, Marschall AL, Bos E, et al. : The translocon protein Sec61 mediates antigen transport from endosomes in the cytosol for cross-presentation to CD8 + T cells. Immunity. 2015;42(5):850–63. 10.1016/j.immuni.2015.04.008 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Grommé M, Uytdehaag FG, Janssen H, et al. : Recycling MHC class I molecules and endosomal peptide loading. Proc Natl Acad Sci U S A. 1999;96(18):10326–31. 10.1073/pnas.96.18.10326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bertholet S, Goldszmid R, Morrot A, et al. : Leishmania antigens are presented to CD8 + T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol. 2006;177(6):3525–33. 10.4049/jimmunol.177.6.3525 [DOI] [PubMed] [Google Scholar]
- 49. Saveanu L, Carroll O, Weimershaus M, et al. : IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science. 2009;325(5937):213–7. 10.1126/science.1172845 [DOI] [PubMed] [Google Scholar]
- 50. Serwold T, Gonzalez F, Kim J, et al. : ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419(6906):480–3. 10.1038/nature01074 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Segura E, Albiston AL, Wicks IP, et al. : Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci U S A. 2009;106(48):20377–81. 10.1073/pnas.0910295106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weimershaus M, Maschalidi S, Sepulveda F, et al. : Conventional dendritic cells require IRAP-Rab14 endosomes for efficient cross-presentation. J Immunol. 2012;188(4):1840–6. 10.4049/jimmunol.1101504 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Sancho D, Joffre OP, Keller AM, et al. : Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458(7240):899–903. 10.1038/nature07750 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Schnorrer P, Behrens GM, Wilson NS, et al. : The dominant role of CD8 + dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci U S A. 2006;103(28):10729–34. 10.1073/pnas.0601956103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cebrian I, Visentin G, Blanchard N, et al. : Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 2011;147(6):1355–68. 10.1016/j.cell.2011.11.021 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Nair-Gupta P, Baccarini A, Tung N, et al. : TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell. 2014;158(3):506–21. 10.1016/j.cell.2014.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Basha G, Omilusik K, Chavez-Steenbock A, et al. : A CD74-dependent MHC class I endolysosomal cross-presentation pathway. Nat Immunol. 2012;13(3):237–45. 10.1038/ni.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Satpathy AT, KC W, Albring JC, et al. : Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209(6):1135–52. 10.1084/jem.20120030 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Hou W, Gibbs JS, Lu X, et al. : Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119(13):3128–31. 10.1182/blood-2011-09-379479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Langlet C, Tamoutounour S, Henri S, et al. : CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol. 2012;188(4):1751–60. 10.4049/jimmunol.1102744 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Campbell IK, van Nieuwenhuijze A, Segura E, et al. : Differentiation of inflammatory dendritic cells is mediated by NF-κB1-dependent GM-CSF production in CD4 T cells. J Immunol. 2011;186(9):5468–77. 10.4049/jimmunol.1002923 [DOI] [PubMed] [Google Scholar]
- 62. Plantinga M, Guilliams M, Vanheerswynghels M, et al. : Conventional and monocyte-derived CD11b + dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38(2):322–35. 10.1016/j.immuni.2012.10.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Greter M, Helft J, Chow A, et al. : GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36(6):1031–46. 10.1016/j.immuni.2012.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gautier EL, Shay T, Miller J, et al. : Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118–28. 10.1038/ni.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Wu X, Briseño CG, Durai V, et al. : Mafb lineage tracing to distinguish macrophages from other immune lineages reveals dual identity of Langerhans cells. J Exp Med. 2016;213(12):2553–65. 10.1084/jem.20160600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jakubzick C, Gautier EL, Gibbings SL, et al. : Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39(3):599–610. 10.1016/j.immuni.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chow KV, Lew AM, Sutherland RM, et al. : Monocyte-Derived Dendritic Cells Promote Th Polarization, whereas Conventional Dendritic Cells Promote Th Proliferation. J Immunol. 2016;196(2):624–36. 10.4049/jimmunol.1501202 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Blum JS, Wearsch PA, Cresswell P: Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–73. 10.1146/annurev-immunol-032712-095910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu RH, Remakus S, Ma X, et al. : Direct presentation is sufficient for an efficient anti-viral CD8 + T cell response. PLoS Pathog. 2010;6(2):e1000768. 10.1371/journal.ppat.1000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Busche A, Jirmo AC, Welten SP, et al. : Priming of CD8 + T cells against cytomegalovirus-encoded antigens is dominated by cross-presentation. J Immunol. 2013;190(6):2767–77. 10.4049/jimmunol.1200966 [DOI] [PubMed] [Google Scholar]
- 71. Snyder CM, Allan JE, Bonnett EL, et al. : Cross-presentation of a spread-defective MCMV is sufficient to prime the majority of virus-specific CD8+ T cells. PLoS One. 2010;5(3):e9681. 10.1371/journal.pone.0009681 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Heipertz EL, Davies ML, Lin E, et al. : Prolonged antigen presentation following an acute virus infection requires direct and then cross-presentation. J Immunol. 2014;193(8):4169–77. 10.4049/jimmunol.1302565 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Eickhoff S, Brewitz A, Gerner MY, et al. : Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions. Cell. 2015;162(6):1322–37. 10.1016/j.cell.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Alexandre YO, Ghilas S, Sanchez C, et al. : XCR1 + dendritic cells promote memory CD8 + T cell recall upon secondary infections with Listeria monocytogenes or certain viruses. J Exp Med. 2016;213(1):75–92. 10.1084/jem.20142350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Iborra S, Martínez-López M, Khouili SC, et al. : Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1 + Dendritic Cells. Immunity. 2016;45(4):847–60. 10.1016/j.immuni.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Edelson BT, Kc W, Juang R, et al. : Peripheral CD103 + dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207(4):823–36. 10.1084/jem.20091627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Allan RS, Waithman J, Bedoui S, et al. : Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25(1):153–62. 10.1016/j.immuni.2006.04.017 [DOI] [PubMed] [Google Scholar]
- 78. Hor JL, Whitney PG, Zaid A, et al. : Spatiotemporally Distinct Interactions with Dendritic Cell Subsets Facilitates CD4 + and CD8 + T Cell Activation to Localized Viral Infection. Immunity. 2015;43(3):554–65. 10.1016/j.immuni.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 79. Bedoui S, Whitney PG, Waithman J, et al. : Cross-presentation of viral and self antigens by skin-derived CD103 + dendritic cells. Nat Immunol. 2009;10(5):488–95. 10.1038/ni.1724 [DOI] [PubMed] [Google Scholar]
- 80. Kitano M, Yamazaki C, Takumi A, et al. : Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc Natl Acad Sci U S A. 2016;113(4):1044–9. 10.1073/pnas.1513607113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wakim LM, Bevan MJ: Cross-dressed dendritic cells drive memory CD8 + T-cell activation after viral infection. Nature. 2011;471(7340):629–32. 10.1038/nature09863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bennett SR, Carbone FR, Karamalis F, et al. : Induction of a CD8 + cytotoxic T lymphocyte response by cross-priming requires cognate CD4 + T cell help. J Exp Med. 1997;186(1):65–70. 10.1084/jem.186.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang Y, Swiecki M, Cella M, et al. : Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 2012;11(6):631–42. 10.1016/j.chom.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bennett SR, Carbone FR, Karamalis F, et al. : Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–80. 10.1038/30996 [DOI] [PubMed] [Google Scholar]
- 85. Ridge JP, Di Rosa F, Matzinger P: A conditioned dendritic cell can be a temporal bridge between a CD4 + T-helper and a T-killer cell. Nature. 1998;393(6684):474–8. 10.1038/30989 [DOI] [PubMed] [Google Scholar]
- 86. Schoenberger SP, Toes RE, van der Voort EI, et al. : T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–3. 10.1038/31002 [DOI] [PubMed] [Google Scholar]
- 87. Dudziak D, Kamphorst AO, Heidkamp GF, et al. : Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–11. 10.1126/science.1136080 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Gerner MY, Torabi-Parizi P, Germain RN: Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015;42(1):172–85. 10.1016/j.immuni.2014.12.024 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Ferris ST, Carrero JA, Mohan JF, et al. : A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity. 2014;41(4):657–69. 10.1016/j.immuni.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ohta T, Sugiyama M, Hemmi H, et al. : Crucial roles of XCR1-expressing dendritic cells and the XCR1-XCL1 chemokine axis in intestinal immune homeostasis. Sci Rep. 2016;6: 23505. 10.1038/srep23505 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Heng TS, Painter MW: The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9(10):1091–4. 10.1038/ni1008-1091 [DOI] [PubMed] [Google Scholar]