Abstract
Although most papillary thyroid carcinomas (PTCs) have a good prognosis, a small but certain fraction shows aggressive behavior. Therefore, a novel and well-performing molecular marker is needed. In the present study, we assessed the impact of the combination of the TERT promoter/BRAF mutations and Ki-67 labeling index (LI) as a prognostic marker in PTC patients. Of 400 PTC samples, 354 were successfully genotyped for both TERT promoter/BRAF and analyzed for Ki-67 LI. Based on the combination of the mutational status and Ki-67 LI, the cases were categorized into three groups: high-, middle-, and low-risk. The recurrence rates of low-, middle-, and high-risk group were 1.9% (6 of 323), 18.2% (4 of 22), and 44.4% (4 of 9), respectively. The Kaplan-Meier curve and log-rank analyses demonstrated that there were statistical differences between any two groups. The hazard ratios for recurrence remained significant after adjustment for age, sex, tumor size, and extrathyroidal extension (low vs. middle: 8.80, 95% CI: 2.35–32.92, p = 0.001; middle vs. high: 6.255, 95% CI: 1.13–34.51, p = 0.035). In conclusion, the combination of the TERT promoter/BRAFV600E mutations and Ki-67 LI performed excellent in predicting PTC recurrence and may be clinically useful.
Papillary thyroid carcinoma (PTC) is the most common malignant tumor in thyroid. Although most PTCs have a good prognosis, a small but certain fraction shows aggressive behavior, and recurrence occurs in 5–20% of the cases1. To discriminate such high-risk cases from others, a number of studies have been conducted to find prognostic molecular markers.
The BRAFV600E mutation is the most prevalent genetic alteration in PTCs, ranging 29–83%, 44% on average2. Many studies have demonstrated that the presence of the BRAFV600E mutation is associated with aggressive characteristics of the disease, such as extrathyroidal invasion, lymph node metastasis, and a poor prognosis3,4,5,6. On the other hand, there are also some studies suggesting no relationship between BRAFV600E and tumor aggressiveness7,8,9. The mutation rates reported from East Asian countries such as Japan and South Korea are generally high (~80%), but PTCs in these countries do not seem to have a worse prognosis. The significance of this mutation as a prognostic marker is still controversial and may be dependent on a population.
Recently, two recurrent somatic mutations, chr5:1,295,228 C>T (C228T) and chr5:1,295,250 C>T (C250T), in the promoter region of the telomerase reverse transcriptase (TERT) gene have been found in various types of cancers including thyroid cancer10,11,12, which represent nucleotide changes of –124 C>T and –146 C>T from the ATG translation start site, respectively. These mutations generate binding sites for E-twenty-six (ETS) transcription factors and have been shown to increase the transcriptional activity of the TERT promoter by two- to four-fold13,14. Telomerase is a ribonucleoprotein enzyme that synthesizes telomeric DNA. Telomerase is not active in most adult tissues. However, it is reactivated in many cancers through the transcriptional regulation of the TERT gene which encodes a key component of the telomerase complex15. The TERT protein is thought to play an important role in human tumorigenesis. Its canonical function is to maintain telomere length, preventing replicative senescence. Recent studies have also suggested that TERT has novel molecular functions including transcriptional regulation of NF-κB and Wnt/β-catenin target genes involved in cell proliferation, resistance to apoptosis, invasion, and metastasis16. In thyroid tumors, the TERT promoter mutations have been shown to be associated with aggressive features in the presence of the BRAF or RAS mutation17,18,19,20,21,22,23,24. Recently, Xing et al. have shown that the coexistence of BRAFV600E and TERTC228T is correlated with high-risk clinicopathological characteristics and a worse prognosis24.
Ki-67 is a nuclear protein that is associated with cellular proliferation. Although little is known about what Ki-67 actually does, it is present during all active phases of the cell cycle but absent in resting (G0) cells. The expression of Ki-67 protein is evaluated as a labeling index (LI) on tissue specimens. A high Ki-67 LI was associated with a poor prognosis in patients with breast cancer and prostate cancer25. In PTCs, it has been reported that the Ki-67 LI is an independent prognostic factor for disease-free and cause-specific survival26,27.
In this study, we set out to clarify the prognostic value of BRAFV600E and the TERT promoter mutations in Japanese PTCs, in which the prevalence of the BRAFV600E mutation is very high. Moreover, we also tried to combine the mutational status with the Ki-67 LI to achieve better prediction.
Results
Mutational status of the BRAF gene and the TERT promoter
DNA samples were first screened for the BRAF mutation. Of 398 samples, 383 (96.2%) were successfully genotyped, of which 313 (81.7%) and 4 (1.04%) were found to carry the BRAFV600E and the BRAFV600delinsYM mutations28, respectively, and 66 (17.2%) were negative for the mutations. We next analyzed the promoter region of TERT. Of 398 samples, 363 samples were available for the test because of limited amount of DNA. Of 363 samples, 357 (98.3%) were successfully genotyped. The TERT promoter mutations were found in 36 (10.1%) samples, among which TERT C228T was more frequent (33 of 36) than C250T (3 of 36), and the two mutations were mutually exclusive. Of these 357 samples, 356 were successfully genotyped for the BRAF status. In our series, all TERT promoter mutation-positive PTC samples harbored the BRAFV600E mutation. There was a significantly higher co-occurrence between the TERT promoter mutation and the BRAFV600E mutation (p < 0.001; Fisher’s exact test, two-sided). Genotyping results were classified into three groups: BRAFV600E alone, 258 patients (72.5%); TERT promoter mutation-positive group (all of these harbored the BRAFV600E mutation), 36 patients (10.1%); and mutation-negative group: 62 patients (17.4%).
Relationship between the mutational status and clinicopathological features
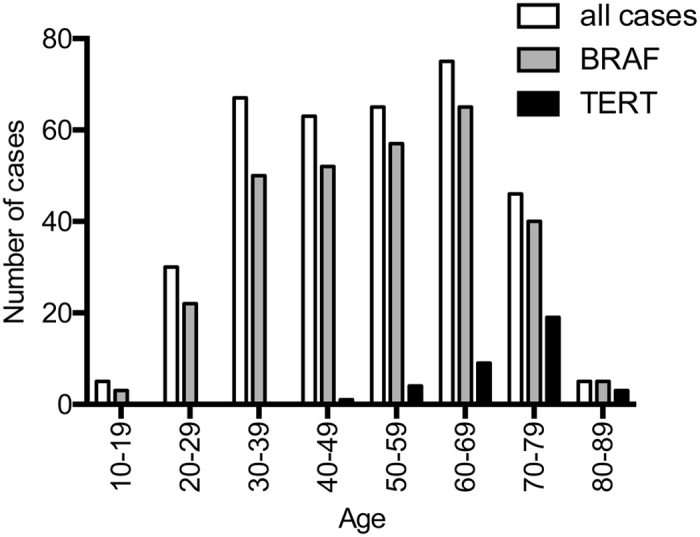
We analyzed the association of BRAFV600E alone and coexistence of BRAFV600E and the TERT promoter mutation with clinicopathological characteristics. Unfortunately, in this series it was not possible to address the effect of the TERT promoter mutation alone due to absence of such cases. As shown in Table 1, the mean age was significantly older in the TERT promoter mutation-positive group (70.1 ± 8.3 years old) than in the BRAFV600E alone (49.7 ± 14.6 years old, p < 0.001) or mutation-negative group (45.8 ± 16.6 years old, p < 0.001). Interestingly, the TERT promoter mutation was not found at all in patients less than 45 years old, and its prevalence was substantially increased with age afterwards (Fig. 1). The tumor size was significantly greater in the TERT promoter mutation-positive group (32.1 ± 15.7 mm) than in the BRAFV600E alone (19.8 ± 11.4 mm, p < 0.001) or mutation-negative group (22.6 ± 15.3 y, p = 0.001) (Table 1). Distant metastasis, advanced stage, and extrathyroidal invasion were also more common in the TERT promoter mutation-positive group while no difference was found for nodal disease frequency among the groups (please see Table 1 for details). These data demonstrate that the TERT promoter mutations were associated with the aggressive clinicopathological characteristics of PTC.
Table 1. Association between mutational status and clinicopathological findings.
| Genotype | No BRAF, no TERT (1) | BRAF mut (2) | BRAF/TERT mut (3) | p-value (1 vs 2) | p-value (2 vs 3) | p-value (1 vs 3) | |
|---|---|---|---|---|---|---|---|
| Number of cases | 62 (17.4%) | 258 (72.5%) | 36 (10.1%) | ||||
| Age (mean ± s.d.) | 45.8 ± 16.6 | 49.7 ± 14.6 | 70.1 ± 8.3 | 0.259a | <0.001a | <0.001a | |
| Sex (F/M, % male) | 57/5 (8.1%) | 218/40 (15.5%) | 28/8 (22.2%) | nsb | nsb | nsb | |
| Tumor size (mean ± s.d.) | 22.6 ± 15.3 | 19.8 ± 11.4 | 32.1 ± 15.7 | 0.445a | <0.001a | 0.014a | |
| LN metastasis | 34/61 (55.7%) | 167/258 (64.7%) | 20/36 (55.6%) | nsb | nsb | nsb | |
| Distant metastasisd | 0 | 1 (0.4%) | 5 (13.9%) | n/p | <0.05b | n/p | |
| Stagec | <0.05b | <0.05b | <0.05b | ||||
| I | 37 | 114 | 2 | ||||
| II | 3 | 5 | 0 | ||||
| III | 16 | 99 | 16 | ||||
| IV | 6 | 40 | 18 | ||||
| Ex | 41 (66.1%) | 189 (73.3%) | 33 (91.7%) | nsb | <0.05b | <0.05b | |
ns: not significant, p ≥ 0.05.
n/p: not performed.
aNon-parametric ANOVA with Dunnett post hoc test.
bMultiple comparison test for proportions (using the COMPROP procedure in SAS http://www2.sas.com/proceedings/sugi31/204-31.pdf).
cCalculations for Stage (I + II) vs. (III + IV).
dAll metastatic sites were the lung.
Figure 1. Age distribution of the BRAF and TERT promoter mutations.
Relationship between Ki-67 LI and clinicopathological features
Of 400 samples, 395 (98.7%) were successfully analyzed for the Ki-67 LI. The patients were categorized into three groups: LI <5%, 304 patients (77.0%); LI 5–10%, 68 patients (17.2%); and LI> 10%, 23 patients (5.8%) (Table 2). Analysis of clinicopathological relevance of Ki-67 LI did not detect significant associations after corrections for multiple comparisons (Table 2). The only statistically significant trend was found for distant metastasis (p = 0.018, the Cochran-Armitage test).
Table 2. Association between Ki67 labeling index and clinicopathological findings.
| Ki67 labeling index | <5% (1) | 5–10% (2) | >10% (3) | p-value (1 vs 2) | p-value (2 vs 3) | p-value (1 vs 3) | |
|---|---|---|---|---|---|---|---|
| Number of cases | 304 (77.0%) | 68 (17.2%) | 23 (5.8%) | ||||
| Age (mean ± s.d.) | 51.2 ± 15.2 | 48.7 ± 17.4 | 52.5 ± 19.5 | 0.622a | 0.793a | 0.985a | |
| Sex (F/M, % male) | 255/47 (15.5%) | 61/7 (10.3%) | 18/5 (21.7%) | nsb | nsb | nsb | |
| Tumor size (mean ± s.d.) | 20.8 ± 12.7 | 22.9 ± 13.1 | 29.5 ± 20.1 | 0.548a | 0.380a | 0.144a | |
| LN metastasis | 190/303 (62.7%) | 43/68 (63.2%) | 16/23 (69.6%) | nsb | nsb | nsb | |
| Distant metastasisd | 3 (1.0%) | 1 (1.5%) | 2 (8.7%) | nsb | nsb | nsb | |
| Stagec | nsb | nsb | nsb | ||||
| I | 128 | 35 | 9 | ||||
| II | 8 | 0 | 0 | ||||
| III | 115 | 23 | 5 | ||||
| IV | 53 | 10 | 9 | ||||
| Ex | 212 (69.7%) | 56 (82.4%) | 17 (73.9%) | nsb | nsb | nsb | |
ns: not significant, p ≥ 0.05.
aNon-parametric ANOVA with Dunnett post hoc test.
bMultiple comparison test for proportions (using the COMPROP procedure in SAS http://www2.sas.com/proceedings/sugi31/204-31.pdf).
cCalculations for Stage (I + II) vs. (III + IV).
dAll metastatic sites were the lung.
Relationship between the mutational status and recurrence
We next assessed the impact of the TERT promoter mutations on disease recurrence. Compared to patients with BRAFV600E (6 of 258, 2.3%) alone and without mutations (2 of 62, 3.2%), those with both BRAFV600E and TERT promoter mutations had a higher recurrence rate (6 of 36, 16.7%). We then performed Kaplan-Meier analysis and log-rank test of recurrence-free survival of the patients. The presence of both BRAFV600E and TERT promoter mutations was significantly associated with recurrence (Fig. 2A, p = 0.017 vs. no mutation, p < 0.001 vs. BRAFV600E only). In contrast, we did not see any difference at all between BRAFV600E alone and no mutation groups (Fig. 2A). The hazard ratio (HR) for tumor recurrence in patients with BRAFV600E and TERT promoter mutations was 6.74 (95% CI: 2.17–20.91, p = 0.001) (Table 3, upper), which remained significant after adjustment for patient age, sex, and lymph node metastasis (HR: 4.17, 95% CI: 1.06–17.7, p = 0.048) (Table 3, upper). However, when tumor size or extrathyroidal extension was included in the adjustment, statistical significance was lost (Table 3, upper). Again, we did not find any significant difference between mutation-negative group and BRAFV600E-positive group (Table 3, upper). In addition, ROC curve analysis demonstrated that, based on the mutation type definition (i.e., no mutation, BRAFV600E only, or TERT promoter/BRAFV600E), the area under curve (AUC) was 0.672 (SD = 0.096, accuracy 28.6%).
Figure 2. Kaplan-Meier curves of recurrence-free survival.
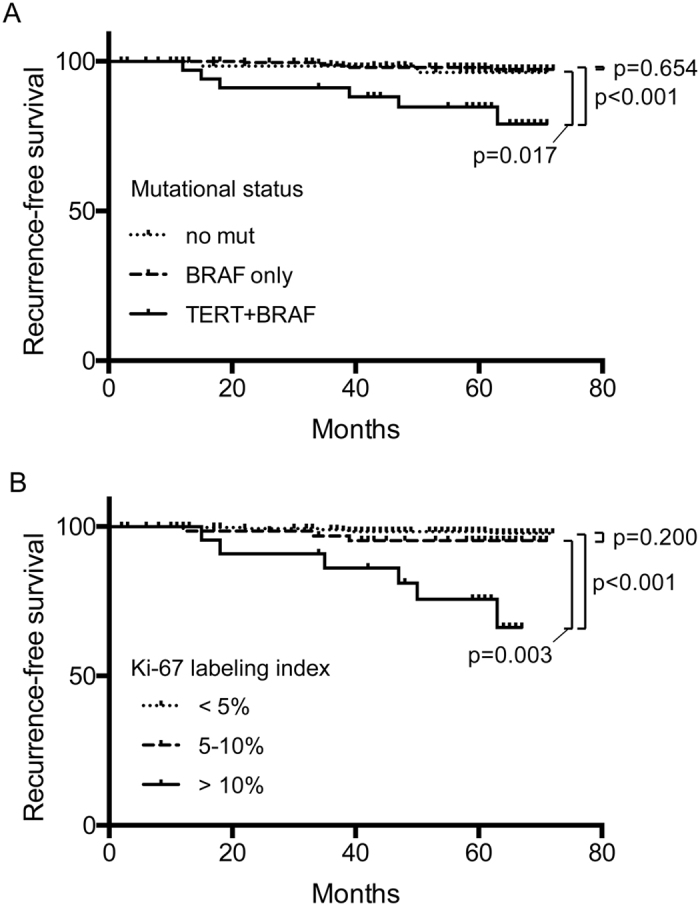
The vertical tick-marks correspond to censored data. p-values of a log-rank test are shown. (A) by mutational status. (B) by Ki-67 labeling index.
Table 3. Hazard ratios of disease recurrence.
| Genotype | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no BRAF, no TERT | 1.000 | 1.000 | 1.000 | 1.000 | ||||||||
| BRAF mut only | 0.696 | 0.141–3.450 | 0.658 | 0.578 | 0.114–2.930 | 0.508 | 0.676 | 0.135–3.398 | 0.635 | 0.626 | 0.125–3.139 | 0.569 |
| BRAF/TERT mut | 6.740 | 2.173–20.905 | 0.001 | 4.172 | 1.011–17.218 | 0.048 | 3.016 | 0.659–13.797 | 0.155 | 4.068 | 0.966–17.129 | 0.056 |
| Adjustment | age, sex, N | age, sex, size | age, sex, Ex | |||||||||
| Ki67 labeling index | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| <5% | 1.000 | 1.000 | 1.000 | |||||||||
| 5–10% | 2.444 | 0.611–9.779 | 0.207 | 2.620 | 0.650–10.563 | 0.176 | 2.247 | 0.547–9.222 | 0.261 | |||
| >10% | 9.871 | 3.422–28.476 | <0.001 | 8.131 | 2.718–24.323 | <0.001 | 5.521 | 1.719–17.737 | 0.004 | |||
| Adjustment | age, sex, N | age, sex, N, size, Ex | ||||||||||
| Risk group | HR | 95% CI | p-value | HR | 95% CI | p-value | ||||||
| Low | 1.000 | 1.000 | ||||||||||
| Low vs. Middle | 10.147 | 2.859–36.012 | 3.36E-04 | 8.795 | 2.349–32.924 | 0.001 | ||||||
| Middle vs. High | 11.762 | 3.636–38.048 | 3.87E-05 | 6.255 | 1.134–34.505 | 0.035 | ||||||
| Adjustment | age, sex, size, Ex | |||||||||||
Relationship between the Ki-67 LI and recurrence
The recurrence rates of the LI <5%, the LI 5–10%, and the LI> 10% groups were 2.0% (6 of 304), 4.4% (3 of 68), and 26.1% (6 of 23), respectively. Kaplan-Meier and log-rank analyses demonstrated that the LI> 10% group showed significantly worse recurrence-free survival (p < 0.001 vs LI <5%, p = 0.003 vs LI 5–10%) (Fig. 2B). However, there was no statistical difference between LI <5% and LI 5–10%. The HR for recurrence in patients with LI> 10% was 9.87 (95% CI: 3.42–28.5, p < 0.001), which remained significant after adjustment for age, sex, lymph node metastasis, tumor size, and extrathyroidal extension (HR: 5.521, 95% CI: 1.72–17.74, p = 0.004) (Table 3, middle). The HRs of those with LI 5–10% were 2.44 (unadjusted; 95% CI: 0.61–9.78, p = 0.207), 2.62 (adjusted for age, sex, and lymph node metastasis; 95% CI: 0.65–10.56, p = 0.176), and 2.25 (adjusted for age, sex, lymph node metastasis, tumor size, and extrathyroidal extension; 95% CI: 0.55–9.22, p = 0.261), all of which were not statistically significant though. ROC curve analysis of recurrence based on the three categories of Ki-67 LI returned AUC of 0.727 (SD = 0.120, accuracy 77.7%).
Combination of the TERT promoter mutations and the Ki-67 LI
As described above, the associations of the TERT promoter mutations with the clinicopathological parameters were stronger as compared to those of the Ki-67 LI. Therefore, we combined both mutational status and LI to re-categorize the patients into three following groups: low-risk group, TERT mutation negative/LI ≤10% and TERT mutation positive/LI <5%; middle-risk group, TERT mutation negative/LI> 10% and TERT mutation positive/LI 5–10%; high-risk group, TERT mutation positive/LI> 10% (Fig. 3A). The recurrence rates of low-, middle-, and high-risk group were 1.9% (6 of 323), 18.2% (4 of 22), and 44.4% (4 of 9), respectively (Fig. 3A). As shown in Fig 3B, the Kaplan-Meier curve of the low-risk group was excellent; that of the high-risk group was the worst; and that of the middle-risk group was in between. There were statistical differences between any two groups (Fig. 3B). The HRs for recurrence were statistically significant (low vs. middle: 10.15, 95% CI: 2.86–36.01, p = 3.36E-04; middle vs. high: 11.76, 95% CI: 3.64–38.05, p = 3.87E-05), which remained significant after adjustment for age, sex, tumor size, and extrathyroidal extension (low vs. middle: 8.80, 95% CI: 2.35–32.92, p = 0.001; middle vs. high: 6.255, 95% CI: 1.13–34.51, p = 0.035) (Table 3, lower). ROC curve analysis based on the three risk categories determined AUC of 0.889 (SD = 0.072, accuracy 91.8%).
Figure 3.
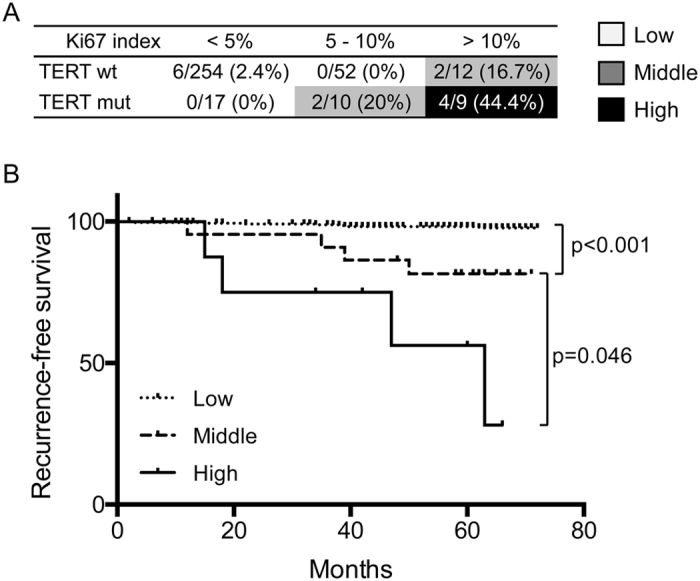
(A) Classification of risk group using the mutational status and the Ki-67 labeling index and recurrence rates. (B) Kaplan-Meier curves of recurrence-free survival by risk groups. The vertical tick-marks correspond to censored data. p-values of a log-rank test are shown.
Discussion
The prevalence of the BRAFV600E mutation in PTCs in East Asian countries including Japan is high. The prevalence of the BRAFV600E mutation was reported to be dependent on the amount of iodine intake29; however, very recently it has been demonstrated that there was no difference in the BRAFV600E prevalence between Japan, an iodine-rich country, and Vietnam, an iodine-deficient country30. Interestingly, in the meta-analysis by Kim et al.31, when they divided analyzed studies into two groups according to the prevalence of the BRAFV600E mutation (≥50% and <50%), the pooled effect sizes of extrathyroidal invasion and lymph node metastasis were about 30% smaller in the BRAFV600E ≥50% group than in the <50% group. The impact of the BRAFV600E mutation on aggressive clinicopathological features might depend on the prevalence of the mutation. In the present study, the association of the BRAFV600E mutation alone with the aggressive clinicopathological features and recurrence was negative. The reason for the difference in the impact of the BRAFV600E mutation remains to be further addressed.
The rate of the TERT promoter mutations (C288T and C250T) in the current series was 10.1%, which is consistent with most of the previous studies 32,33. There seems to be no large difference between populations except for two studies from China reporting 4.1 and 4.4%19,23, perhaps the smallest two among published data. The presence of the TERT promoter mutation only was also reported to be associated with PTC aggressiveness24; however, in the present study we were not able to analyze this because we did not have even one case with the TERT promoter mutation but without BRAFV600E, which is a limitation of our work. To assess the impact of the TERT promoter mutation alone in the BRAFV600E prevalent region such as Japan, it would be necessary to collect a very large number of samples. This is a future issue but cases with TERT promoter mutation alone probably show better prognosis than those with the TERT promoter plus BRAFV600E mutations. A number of studies have demonstrated that the co-existence of the BRAFV600E and TERT promoter mutations are associated with aggressive clinicopathological features and disease recurrence17,18,19,20,21,22,23,24. As far as we know, there is no report against the above finding so far. The result of the present study also supports this. We also observed that the presence of the TERT promoter mutations showed very strong age dependency, and it is noteworthy that the mutations were not detected in any of the patients less than 45 years old. Nevertheless, the HR for recurrence after adjustment for age was still statistically significant.
The TERT promoter mutations were linked to many aggressive clinicopathological features such as large tumor size, distant metastasis, advanced stage, and extrathyroidal extension. Therefore, it was not too surprising that the statistical significance of the increased HR for recurrence was lost after adjustment for tumor size or extrathyroidal extension in addition to age and sex. However, as their effect sizes were still relatively high (3.016 [adjusted for age, sex, and size], 4.068[adjusted for age, sex, Ex]), the number of samples was perhaps not enough to reach statistical significance. Our ROC curve analysis demonstrated that mutation type would not be a good test for predicting recurrence in the series under analysis (AUC = 0.672). This is a first study reporting a clinical significance of the BRAFV600E and TERT promoter mutations in the Japanese PTC cases, which show higher BRAFV600E prevalence (81.7% in this study). This is consistent with the recent report from South Korea where the prevalence of the BRAFV600E mutation is also high20.
It has been demonstrated that the Ki-67 LI is associated with a prognosis of PTC26,27. The current study also confirmed these observations. Indeed, the HR for recurrence of the Ki-67 LI> 10% was significant even after adjustment for a number of parameters, and effect size was greater than that for the TERT promoter/BRAFV600E mutations. Also, ROC curve analysis indicated that Ki-67 LI performed better than mutation type as a test for predicting recurrence (AUC = 0.727).
Interestingly, the associations of the Ki-67 LI with the clinicopathological characteristics were much weaker than those of the TERT promoter/BRAFV600E mutations, suggesting that Ki-67 LI may be a prognostic marker that is associated with higher-risk tumors independently of their mutational status. Indeed, the Ki-67 LI was reported to have an inverse correlation with the thyroglobulin-doubling time, suggesting that it reflects growth velocity of tumor27. On the other hand, the TERT promoter/BRAFV600E mutations are strongly linked to the clinicopathological status. They may be independent factors. We, therefore, combined the mutational status and the Ki-67 LI and divided the cases into three risk groups and found 6–8-fold adjusted differences in effect sizes between the groups. Concordantly, the ROC curve analysis based on the risk groups demonstrated further improvement of the test performance (AUC = 0.889). It should be noted that, despite the presence of the TERT promoter/BRAFV600E mutations, there was no recurrence in the patients with Ki-67 LI <5% (low-risk group). In contrast, 16.7% of the PTCs recurred if Ki-67 LI was >10% even though the tumors did not carry the TERT promoter/BRAFV600E mutations (middle-risk group). Approximately half of the high-risk patients had disease recurrence, although the number of such patients was rather small. Thus, neither mutational status nor Ki-67 LI was the optimal predictor for recurrence. Our statistical calculations showed that the combination of these risk factors performs better, yet ample room for improvement still remains.
In conclusion, the combination of the TERT promoter/BRAFV600E mutations and Ki-67 LI is a promising marker to predict recurrence of PTC. Low-, middle-, or high-risk group showed clear differences in any of the comparisons. The limitations of this method are: 1) The Ki-67 LI can only be obtained using surgical specimens, 2) It may be difficult to compare the Ki-67 LI from different institutions. Therefore, it is desired to find an objective marker which is strongly correlated with Ki-67 LI that can be measured preoperatively.
Methods
PTC samples
A total of 400 adult sporadic PTC samples were collected at Kuma Hospital (Kobe, Japan). All patients received surgical treatment in 2009, and the therapeutic strategy was not changed during whole treatment course. Histological diagnosis was performed by a thyroid pathologist (MH). All patients had no history of radiation exposure. Patients’ age at operation ranged 13–87 years old (mean 51 ± 16, median 52 years old, 15.2% male); follow-up period was 2–72 months (mean 58 ± 14, median 63 months). Disease recurrence was defined as surgically removed and pathologically verified local tumor focus or regional metastasis, or distant metastasis detected by ultrasound or radioisotope imaging not earlier than 12 months after initial treatment. According to this criterion, we excluded one case that had a recurrence within three months. The study protocol was approved by the ethics committees of Nagasaki University and Kuma Hospital. All procedures were performed in accordance with the relevant guidelines and regulations.
DNA extraction and mutation screening
DNA was extracted from formalin-fixed paraffin-embedded (FFPE) PTC tissues using a QIAamp DNA mini kit (QIAGEN) according to the manufacturer’s protocol. DNAs of sufficient quality and quantity for sequencing were obtained from 398 of 400 PTC specimens. BRAF (around V600) and TERT promoter mutations were analyzed by direct DNA sequencing. Primer sequences used for both PCR amplification and sequencing are: BRAF-FW, 5′-ACATACTTATTGACTCTAAGAGGAAAGATGAA-3′; BRAF-RV, 5′-GATTTTTGTGAATACTGGGAACTATGA-3′; TERT-FW, 5′-CAGCGCTGCCTGAAACTC-3′; and TERT-RV, 5′-GTCCTGCCCCTTCACCTT-3′22. First, PCR amplification was done using KOD FX (TOYOBO). PCR products were then treated with ExoSAP-IT PCR clean-up reagent (GE Healthcare), and sequencing was performed with a Big Dye Terminator sequencing kit version 3.1 (Applied Biosystems) on an ABI3730 automated sequencer (Applied Biosystems). We prepared one negative control (without tissue section) per every 23 samples during DNA extraction to ensure contamination-free amplifications.
Ki-67 immunohistochemistry and the LI
Immunostaining was performed using 4-μm-thick FFPE sections of the same PTC cases. Anti-Ki-67 antibody (clone MIB1, Dako) was used as a primary antibody. The staining was carried out using the Dako Cytomation Autostainer Universal System (Dako) and the Envision kit (Dako) according to the manufacturer’s instruction. A single pathologist (MH) evaluated the specimens without clinical information of the patient. To obtain the Ki-67 LI, at least 500 carcinoma cells in hot areas were analyzed under × 400 magnification. Staining results were classified into three groups:<5%, 5%–10%, and >10% of positive cells.
Statistical analysis
Statistical analysis was performed using SPSS software version 21.0.0.0 (IBM), GraphPad Prism version 6.0 (GraphPad Software), and SAS University Edition software (SAS Institute Inc). For multiple comparisons, the nonparametric one-way ANOVA with Dunnett post hoc test or the FREQ and COMPPROP procedures (SAS) were used for continuous variables or group analyses, respectively. For univariate disease-free survival, the log-rank and the Kaplan-Meier estimates were calculated, and the Cox proportional hazard model was applied in multivariate analyses. The Receiver Operating Characteristic (ROC) curve analysis was performed under parametric distribution assumption (Eng J. ROC analysis: web-based calculator for ROC curves. Baltimore: Johns Hopkins University. Available at: http://www.jrocfit.org/, accessed on July 12, 2016). The p value less than 0.05 was regarded as indicating statistical significance.
Additional Information
How to cite this article: Matsuse, M. et al. TERT promoter mutations and Ki-67 labeling index as a prognostic marker of papillary thyroid carcinomas: combination of two independent factors. Sci. Rep. 7, 41752; doi: 10.1038/srep41752 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (#26293222 and #16H02774 for SY, #16H05832 and #26293142 for NM, and #16K09804 for MM).
Footnotes
The authors declare no competing financial interests.
Author Contributions S.Y., A.M., and N.M. designed the study. M.M. performed genetic analysis. M.M., K.S., and N.M. analyzed data. T.Y. and E.N. collected and analyzed clinical data. M. H. performed pathological analysis. V.S. did statistical analysis. M.M., A.M., and N.M. wrote the paper.
References
- Schlumberger M. J. Papillary and follicular thyroid carcinoma. N. Engl. J. Med. 338, 297–306 (1998). [DOI] [PubMed] [Google Scholar]
- Xing M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer 12, 245–262 (2005). [DOI] [PubMed] [Google Scholar]
- Lanzilotta S. G., Grammatica L., Paradiso A. & Simone G. BRAF in papillary thyroid carcinoma. Cell. Oncol. 29, 269–277 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak K., Suh S., Kim S. J. & Kim I. J. Prognostic value of genetic mutations in thyroid cancer: a meta-analysis. Thyroid 25, 63–70 (2015). [DOI] [PubMed] [Google Scholar]
- Xing M. et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 33, 42–50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M. et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309, 1493–1501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y. et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr. J. 56, 89–97 (2009). [DOI] [PubMed] [Google Scholar]
- Kim T. Y. et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin. Endocrinol. (Oxf.) 65, 364–368 (2006). [DOI] [PubMed] [Google Scholar]
- Kim T. Y. et al. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin. Endocrinol. (Oxf.) 63, 588–593 (2005). [DOI] [PubMed] [Google Scholar]
- Landa I. et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J. Clin. Endocrinol. Metab. 98, E1562–1566 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 20, 603–610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinagre J. et al. Frequency of TERT promoter mutations in human cancers. Nat Commun 4, 2185 (2013). [DOI] [PubMed] [Google Scholar]
- Horn S. et al. TERT promoter mutations in familial and sporadic melanoma. Science 339, 959–961 (2013). [DOI] [PubMed] [Google Scholar]
- Huang F. W. et al. Highly recurrent TERT promoter mutations in human melanoma. Science 339, 957–959 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B. Telomerase and cancer therapeutics. Nat. Rev. Cancer 8, 167–179 (2008). [DOI] [PubMed] [Google Scholar]
- Low K. C. & Tergaonkar V. Telomerase: central regulator of all of the hallmarks of cancer. Trends Biochem. Sci. 38, 426–434 (2013). [DOI] [PubMed] [Google Scholar]
- Bullock M. et al. TERT Promoter Mutations Are a Major Indicator of Recurrence and Death due to Papillary Thyroid Carcinomas. Clin. Endocrinol. (Oxf.) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. R. et al. Association of TERT Promoter Mutation, But Not BRAF Mutation, With Increased Mortality in PTC. J. Clin. Endocrinol. Metab. 100, E1550–1559 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L. et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: a study of 653 patients. Oncotarget (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. E. et al. Prognostic Significance of TERT Promoter Mutations in Papillary Thyroid Carcinomas in a BRAFV600E Mutation-Prevalent Population. Thyroid (2016). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 99, E1130–1136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo M. et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 99, E754–765 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. et al. BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Carcinoma in Chinese Patients. PLoS One 11, e0153319 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M. et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J. Clin. Oncol. 32, 2718–2726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T. & Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182, 311–322 (2000). [DOI] [PubMed] [Google Scholar]
- Ito Y. et al. Prognostic significance of ki-67 labeling index in papillary thyroid carcinoma. World J. Surg. 34, 3015–3021 (2010). [DOI] [PubMed] [Google Scholar]
- Miyauchi A. et al. Ki-67 labeling index is a predictor of postoperative persistent disease and cancer growth and a prognostic indicator in papillary thyroid carcinoma. Eur Thyroid J 2, 57–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuse M. et al. Functional characterization of the novel BRAF complex mutation, BRAF(V600delinsYM), identified in papillary thyroid carcinoma. Int. J. Cancer 132, 738–743 (2013). [DOI] [PubMed] [Google Scholar]
- Guan H. et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 94, 1612–1617 (2009). [DOI] [PubMed] [Google Scholar]
- Vuong H. G. et al. Genetic alterations of differentiated thyroid carcinoma in iodine-rich and iodine-deficient countries. Cancer Med (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. H. et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118, 1764–1773 (2012). [DOI] [PubMed] [Google Scholar]
- Alzahrani A. S., Alsaadi R., Murugan A. K. & Sadiq B. B. TERT Promoter Mutations in Thyroid Cancer. Horm. Cancer 7, 165–177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. & Xing M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 23, R143–155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


