Abstract
The two-spotted leafhopper, Sophonia rufofascia (Kuoh and Kuoh), is an exotic pest from South-East Asia that attacks a wide variety of plant species in Hawaii. Myrica faya Aiton is an aggressive exotic weed that displaces and excludes native plants in Hawaiian forests. It has been argued that because of the high nutritional quality of its foliage, M. faya might facilitate leafhopper invasion of native Hawaiian ecosystems that were originally dominated by the endemic tree Metrosideros polymorpha (Gaudichaud). In the present study, we quantified suitability of M. faya and M. polymorpha as ovipositional hosts for S. rufofascia. Overall, leafhoppers preferred to deposit their eggs into the foliage of M. faya. M. faya presence in the area did not affect leafhopper oviposition on M. polymorpha. Foliar pubescence provided good protection of hirsute morphotypes of M. polymorpha. At the same time, glabrous M. polymorpha morphotypes were quite suitable for leafhopper oviposition. There was no difference in the abundance of leafhopper eggs along a precipitation gradient. Our results confirm that invasion of native Hawaiian forests by the weed M. faya will facilitate their invasion by S. rufofascia. Because of the broad host range characteristic of the two-spotted leafhopper, this build-up may adversely affect a number of endemic plant species growing in native forests.
Keywords: Sophonia rufofascia, Myrica faya, Metrosideros polymorpha, biological invasion, exotic species, oviposition, biotic facilitation
Introduction
The two-spotted leafhopper, Sophonia rufofascia (Kuoh and Kuoh), originally described from southern China (Kouh and Kouh 1983), was first discovered on Oahu in 1987 (Heu and Kumashiro 1989). Since then, it has spread to all of the major Hawaiian islands, where it has been reported to attack over 300 plant species in 83 families (Fukada 1996; Alyokhin et al. 2001). Leafhopper feeding and oviposition cause plant vascular bundle abnormalities, resulting in interveinal chlorosis, vein browning, retarded development of new growth, and even death of affected plants (Jones et al. 2000). In commercial guava, leafhopper injury reduces yields by ≈23% compared to pesticide-protected trees (Jones et al. 1998). Being a highly polyphagous insect, the two-spotted leafhopper poses a threat both to agricultural production and to natural habitat conservation in Hawaii. Among its recorded host plant species, 68% are economically important fruit, vegetable, and ornamental crops; and 22% are endemic to the Hawaiian islands, including 14 rare and endangered species (Fukada 1996). Two-spotted leafhopper damage has been implicated as a cause of severe dieback of several native Hawaiian plants, such as ohia-lehua tree Metrosideros polymorpha (Gaudichaud) and uluhe fern Dicranopteris linearis Underwood (Palmer 1993; Lenz 2000). However, while proving that S. rufofascia feeding is indeed detrimental to D. linearis fern, Jones et al. (2000) failed to find a significant correlation between leafhopper presence and extensive uluhe death in natural habitats. Similarly, Alyokhin et al. (2001) observed wide spread symptoms of leafhopper damage but no significant dieback of surveyed plants. Nevertheless, the two-spotted leafhopper remains an important pest in Hawaii, applying a substantial stress on native and economically important vegetation (Lenz 2000; Lenz and Taylor 2001).
M. polymorpha is the endemic tree that used to dominate a wide variety of Hawaiian ecosystems (Walker and Vitousek 1991). It occurs over a wide ecological range from coast to high-elevation montane habitats, from desert to wet rainfall regions, and from recent lava flows to old, deeply weathered soils (Drake and Mueller-Dombois 1993; Kitayama and Mueller-Dombois 1995; Kitayama et al. 1997). Not surprisingly, this plant displays a high degree of morphological variation, with foliar pubescence being one of the major distinguishing characteristics among infraspecific varieties (Kitayama et al. 1997). This variation is clinal along elevational and rainfall gradients (Corn and Hiesey 1973; Kitayama et al. 1997). Hirsute trees are presumably better adapted to harsh environmental conditions that makes them the more abundant morphotype at higher elevations and in areas prone to drought. For the same reason, they are also predominant in pioneering situations, such as on young lava flows and in disturbed areas along roadsides. Hirsute morphotypes in such places are gradually replaced by glabrous morphotypes, similar to pioneer plant species being replaced by later successional plant species in more floristically diverse continental regions (Stemmermann 1983; Mueller-Dombois 1983; Kitayama et al. 1995; 1997).
M. faya Aiton is an evergreen broad-leafed tree that is listed among the 12 most noxious weeds in the state of Hawaii (Smith 1985). M. faya forms monotypic stands that replace and exclude native vegetation, particularly M. polymorpha. In addition to direct competition with native plants, M. faya also alters ecosystem-level processes indirectly through association with a nitrogen-fixing actinorhizal symbiont (Vitousek et al. 1987; 1989). By increasing the nitrogen content of Hawaiian soils, it facilitates invasion by a broader range of exotic plant species, which are otherwise inadequately adapted to compete with endemic vegetation on nitrogen-poor volcanic soils (Gerrish and Mueller-Dombois 1980; Vitousek et al. 1987; Vitousek and Walker 1989). Furthermore, high nitrogen content in its foliage may favor development of herbivorous insect populations (Mattson 1980; Louda and Collinge 1992; Lenz and Taylor 2001).
An increase in leafhopper abundance on M. faya may result in their subsequent dispersal to other plant species in an area. Therefore, competitive stresses imposed on native vegetation by M. faya invasion might be further aggravated by increased leafhopper herbivory. In a recent survey conducted in Hawaii Volcano National Park, Lenz and Taylor (2001) captured up to 20 times more two-spotted leafhopper adults on traps hung on native plant species within sites invaded by M. faya than in sites where this weed was removed as a part of an ecosystem preservation effort. However, two-spotted leafhopper adults are highly mobile. Therefore, it was not clear if the observed increase in captures was due to the movement of adults that had completed their development on M. faya, to the establishment of higher leafhopper populations on native vegetation, or to the combination of these factors.
Unlike M. polymorpha leaves, M. faya leaves are never pubescent. Foliar pubescence is known to present a physical barrier to leafhopper colonization (Johnson 1975; Tingey 1985). Furthermore, in M. polymorpha pubescence is associated with a thicker cuticle layer (Kitayama et al. 1997), which can provide an additional challenge for both proboscis (especially in younger instars) and ovipositor penetration. Lee (1981) observed lower levels of herbivory on hirsute vs. glabrous trees prior to the leafhopper invasion. Jones et al. (2000) pointed out that feeding damage caused by the two-spotted leafhopper was also more severe on glabrous morphotypes of M. polymorpha and D. linearis when compared to hirsute morphotypes. However, they did not provide any data to quantify their observation. Nothing is known about effects of foliar pubescence on S. rufofascia oviposition. We hypothesized that two-spotted leafhopper eggs would be more abundant on M. faya and glabrous morphotypes of M. polymorpha than on hirsute morphotypes of M. polymorpha. As a result, M. polymorpha forests will be more prone to leafhopper invasion (1) in areas invaded by M. faya, and (2) during late successional stages dominated by glabrous M. polymorpha morphotypes. To test our hypothesis, we quantified the rates of egg infestation of leaves on the two trees in different habitats on the islands of Hawaii and Kauai.
The two-spotted leafhopper has been recorded from a wide variety of habitats (Fukada 1996). In the survey by Alyokhin et al. (2001), leafhoppers appeared to have a preference for wetter, closed habitats rather than drier, open ones. However, the occurrence of different plant species in their survey was itself habitat-dependent. Therefore, it was not clear if the observed difference could be attributed to the humidity itself. Plants suffering from water-stress have been shown to be more prone to attack by phytophagous insects (Mattson and Haack 1987), and leafhopper herbivory by itself increases the level of moisture stress in affected trees (Lenz 2000). Therefore, knowing effects of humidity on leafhopper distribution is important for a better understanding of the consequences of its invasion in different habitats. In the present study, we surveyed the density of leafhopper eggs in floristically very similar habitats located along the precipitation gradient.
Materials and Methods
Site characterization
Natural plant communities were categorized using descriptions and vegetation maps developed by Ripperton and Hosaka (1942), Mueller-Dombois and Fosberg (1974), and Sohmer and Gustafson (1987). Mean annual precipitation for each of the surveyed sites was interpolated from published isohyets based on 67-year rainfall data (Giambelluca et al. 1986). Accordingly, sites were classified as dry if annual precipitation was < 1200 mm, mesic if between 1200 and 2500 mm, and wet if > 2500 mm. Also, sites with canopy cover > 60% were classified as closed, and sites with canopy cover between 25 and 60% were classified as open.
Sampling procedures
Leaves were sampled haphazardly from M. faya and M. polymorpha trees within each survey site in January, March, and April, 2000 (a total of three collection dates). The leaves were sampled at a height of 0.5 – 2 m above the ground from both inner and outer parts of the canopy. No attempt was made to distinguish between sun vs. shade leaves, nor among leaves at different stages of maturity. Collected leaves were brought to the laboratory and checked for the presence of leafhopper eggs using backlighting, as described by Yang et al. (2000). Two-spotted leafhopper eggs were identified using descriptions provided by Culliney (1998). To confirm identification, collected eggs were reared to the nymphal stage by incubating infested leaves at 24 ± 2° C and natural lighting in 1 gallon Ziploc® plastic bags. Voucher leafhopper specimens are stored in the Entomology Museum of the University of Hawaii at Manoa.
The total area of collected leaf material was estimated by measuring 35 leaves of each species with a leaf area meter (LiCor-3100, Lincoln, Nebraska), and then multiplying the mean area of measured leaves by the total number of leaves in a sample (Alyokhin et al. 2001).
Egg abundance in different habitats
In the first study, we surveyed two-spotted leafhopper egg infestation levels in M. faya and M. polymorpha leaves at 9 different sites on the islands of Hawaii and Kauai. A more detailed description of the study sites is provided in Table 1. On Hawaii, which is the youngest Hawaiian island, the sites were located on relatively recent lava flows and represented early stages of plant succession. Therefore, the majority of M. polymorpha trees growing within those sites were hirsute (≈75 : 1 hirsute to glabrous tree ratio). On the geologically and ecologically older island of Kauai, all M. polymorpha trees were glabrous. A total of 23,816 leaves were collected from M. faya trees, and a total of 12,600 leaves were collected from M. polymorpha trees.
Table 1.
Ecological characteristics of the Myrica faya and Metrosideros polymorpha habitats sampled on the islands of Hawaii and Kauai.
Egg abundance in relation to M. faya removal
In the second study, we compared two-spotted leafhopper egg abundance between areas in which M. faya had been mechanically removed, and immediately adjacent areas where the tree was still present. M. faya removal took place from 1986–1988 as part of weed eradication effort within intensively managed special ecological areas of Hawaii Volcano National Park. Presently, these areas remain free of M. faya that has been naturally replaced by M. polymorpha. Our survey was conducted at three sites that were initially covered by an open M. polymorpha-M. faya forest with native shrub understory. The sites were located at Halemaumau, Kulanaokuaiki, and Kipuka Kahalii areas (Table 1). Special ecological areas size varied between 272 and 401 ha. Within each site, an approximately 6 m wide paved road separated special ecological areas from other areas. Samples were taken at least 50 m away from the area border. A total of 8,217 leaves were collected from M. faya trees, 3,517 from M. polymorpha trees growing together with M. faya, and 3,663 leaves from M. polymorpha trees growing in the areas where M. faya had been removed.
Egg abundance in relation to foliar pubescence
In the third study, we compared the infestation rates of leafhopper eggs on glabrous and hirsute M. polymorpha morphotypes. Foliage was collected twice, in May and July of 2000, from glabrous and hirsute M. polymorpha trees growing along Hilina Pali Road (975 – 1067 m elevation, 1500 – 2500 mm annual precipitation), near Pauahi crater (975 m elevation, 2300 mm annual precipitation), and at Kipuka Kahalii area (867 m elevation, 2300 mm annual precipitation) in Hawaii Volcano National Park. Both morphotypes were interspersed in an apparently random manner within each of the three sites. All the sites were covered by an open M. polymorpha-M. faya forest with native shrub understory. A total of 2,611 leaves were collected from glabrous M. polymorpha trees, and a total of 2,882 leaves from hirsute M. polymorpha trees. This time no data on the proportion of leaves infested with leafhopper eggs were taken.
Egg abundance in relation to mean annual precipitation
In the fourth study, we surveyed two-spotted leafhopper egg abundance along a precipitation gradient at ≈1000 m elevation between Mauna Ulu lava shield and Kulanaokuaiki Camp in Hawaii Volcano National Park. Four sites were selected based on their mean annual precipitation, equal to 3000, 2500, 2000, and 1700 mm/year, respectively (Giambelluca et al. 1986). All four sites were covered by an open M. polymorpha-M. faya forest with native shrub understory and separated from each other by a distance of between 1.7 - 3.4 km. A total of 9,850 leaves were collected from M. faya trees, and a total of 4,586 leaves from M. polymorpha trees.
Statistical analysis
Variations in the proportion of sampled leaves containing leafhopper eggs and egg density per m2 of leaf surface area were analyzed by two-way ANOVA (PROC GLM, SAS Institute 1999). Tree species and site of leaf collection were used as the main effects, and all leaves collected at a given site during a given month were considered to comprise a single unit of replication. Prior to the analysis, the data on proportions of infested leaves were transformed using arcsine-square root transformations (Zar 1999). The normality of egg density data was checked using the Wilk-Shapiro test at the 0.05 level of significance (PROC UNIVARIATE, SAS Institute 1999). When necessary, those data were transformed using rank transformations (Conover and Iman 1981). Means and standard errors were calculated from the non-transformed data only. When analyzing impacts of M. faya removal, we used the tree context (e.g., M. faya, M. polymorpha growing together with M. faya, or M. polymorpha growing in the areas where M. faya had been eradicated) instead of tree species as the main effect in ANOVA tests. Those tests were followed by orthogonal multiple contrasts that were designed a priori (Zar 1999) to test the differences between M. faya and M. polymorpha, as well as between M. polymorpha growing together with M. faya and M. polymorpha growing separately from M. faya. The variation between the leaf infestation of glabrous and hirsute M. polymorpha was analyzed by a two-sample t-test (PROC TTEST, SAS Institute 1999).
Results
Egg abundance in different habitats
Overall, 6.0±0.5% (mean ± 1SE) of M. faya leaves and 3.3±0.5% of M. polymorpha leaves contained two-spotted leafhopper eggs. Egg density was equal to 118.1 ± 9.1 eggs/m2 of leaf area for M. faya and 63.9 ± 10.4 eggs/m2 for M. polymorpha. Both proportion of infested leaves and egg density were significantly higher for M. faya than for M. polymorpha (F1,36 = 42.47, P < 0.0001 and F 1,36 = 31.21, P < 0.0001, respectively).
Sampling site had a significant effect on the proportion of infested leaves (F 8,36 = 2.51, P = 0.0280), but not on egg density (F 8,36 = 2.07, P = 0.0654). The interaction between plant species and sampling site was significant for both proportion of infested leaves (F 8,36 = 3.84, P = 0.0023), as well as for egg density (F 8,36 = 2.74, P = 0.0179). At all Hawaii sites, leaf infestation was higher for M. faya than for M. polymorpha trees (Table 2). The same was true for two of the Kauai sites, although the difference was not as great as at the Hawaii sites. At the remaining two Kauai sites, M. polymorpha foliage harbored slightly more eggs than M. faya foliage (Table 2).
Table 2.
Infestation of Myrica faya and Metrosideros polymorpha leaves with the two-spotted leafhopper eggs at the habitats sampled on the islands of Hawaii and Kauai.

Egg abundance in relation to M. faya removal
Leafhopper eggs infested 6.1 ± 0.7% of M. faya leaves, 1.4 ± 0.5% of M. polymorpha leaves collected from the trees growing together with M. faya, and 1.4 ± 0.4% of M. polymorpha leaves collected from the trees growing in the areas where M. faya had been removed. The proportion of infested leaves was significantly different among Halemaumau, Kulanaokuaiki, and Kipuka Kahalii (F 2,18 = 4.61, P = 0.0241). It was also influenced by the tree context (F 2,18 = 21.66, P < 0.0001) (Fig. 1A). However, orthogonal contrasts revealed that while a higher proportion of M. faya leaves contained two-spotted leafhopper eggs compared to M. polymorpha leaves (F = 43.24, P < 0.0001), M. faya presence had no impact on the infestation of M. polymorpha leaves themselves (F = 0.08, P = 0.7774). The interaction between the site and the tree context was not statistically significant (F 4,18 = 1.08, P = 0.3947).
Figure 1.
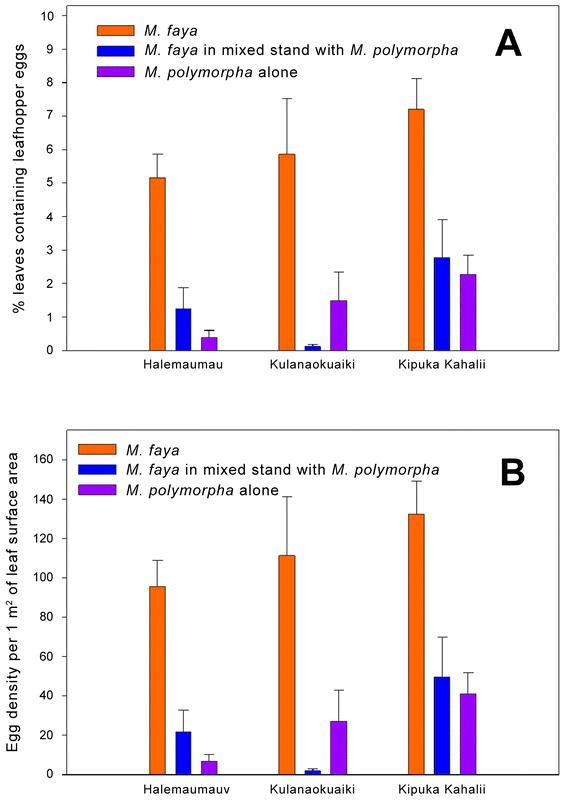
Abundance of Sophonia rufofascia leafhopper eggs in relation to Myrica faya removal in Hawaii Volcano National Park. A – percent leaves containing leafhopper eggs; B – leafhopper egg density/m2 of leaf surface.
Leafhopper egg density was equal to 113.2 ± 11.8 eggs/m2 in M. faya leaves, 24.4 ± 9.6 eggs/m2 in M. polymorpha leaves collected from the trees growing together with M. faya, and 25.1 ± 7.5 eggs/m2 in M. polymorpha leaves collected from the trees growing in the areas where M. faya had been removed. Similar to the proportion of infested leaves, egg density was significantly influenced by sampling site (F 2,18 = 4.52, P = 0.0257) and by tree context (F 2,18 = 27.51, P < 0.0001) (Fig. 1B). Again, the interaction between those two factors was not statistically significant (F 4,18 = 1.08, P = 0.3963). Egg density was higher on M. faya leaves than on M. polymorpha leaves (orthogonal contrast, F = 55.01, P < 0.0001), but M. faya presence had no impact on leafhopper egg density in M. polymorpha leaves (orthogonal contrast, F = 0.01, P = 0.9574).
Egg abundance in relation to foliar pubescence
Glabrous M. polymorpha trees harbored an average of 298.2 ± 59.9 leafhopper eggs/m2 of leaf surface area, while hirsute trees harbored only 11.3 ± 4.2 eggs/m2. The difference between morphotypes was highly significant (t-test, T 10 = −4.78, P = 0.0008).
Egg abundance in relation to mean annual precipitation
Over all leaves collected along the precipitation gradient, approximately 6.7 ± 0.7% of M. faya leaves and 1.7 ± 0.4% of M. polymorpha leaves contained two-spotted leafhopper eggs. Egg density was 126.2 ± 13.4 eggs/m2 for M. faya and 33.4 ± 10.1 eggs/m2 for M. polymorpha. As in the other two surveys, both proportion of infested leaves and egg density were significantly higher for M. faya than for M. polymorpha (F 1,16 = 34.02, P < 0.0001 and F 1,16 = 35.57, P < 0.0001, respectively) (Fig. 2). However, site location along the precipitation gradient influenced neither proportion of infested leaves (F 3,16 = 0.69, P = 0.5624), nor egg density (F 3,16 = 0.34, P = 0.7942). Also, none of the interactions was statistically significant (F 3,16 = 0.18, P = 0.9113 for proportion of infested leaves, and F 3,16 = 0.16, P = 0.9223 for egg density).
Figure 2.
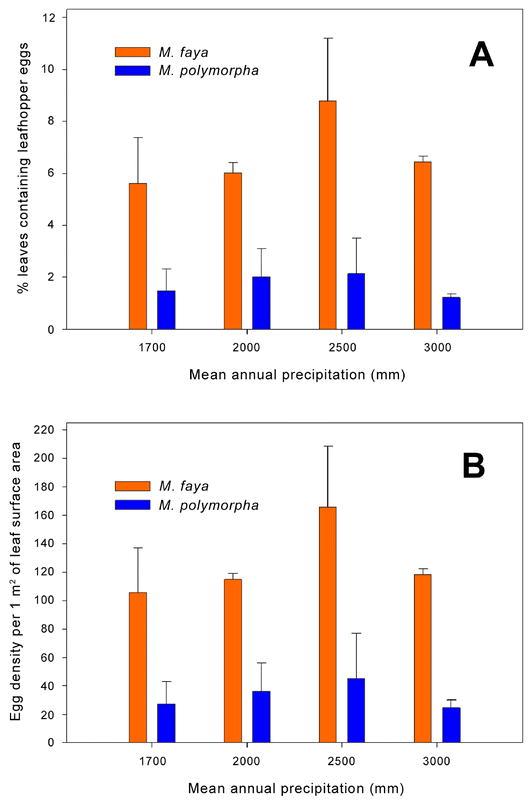
Abundance of Sophonia rufofascia leafhopper eggs in relation to habitat humidity along a precipitation gradient in Hawaii Volcano National Park. A – percent leaves containing leafhopper eggs; B – leafhopper egg density/m2 of leaf surface.
Discussion
Both M. faya and M. polymorpha were suitable oviposition hosts for two-spotted leafhoppers. However, within most surveyed sites leafhoppers laid significantly more eggs on the foliage of M. faya than on M. polymorpha. Therefore, it is probable that invasion of native Hawaiian forests by M. faya creates a more favorable habitat for the two-spotted leafhopper. Our results support the suggestion that damage caused by exotic pests often entails positive interactions, either one-way or two-way, among the introduced species (Howarth 1985; Simberloff and Von Holle 1999). Indeed, in their recent review of the published literature, Simberloff and Von Holle (1999) reported that of 190 pairwise interactions between the alien species tested, 82% consisted of +/– interactions similar to the one described in the present study, 5.3% consisted of +/+ interactions, and only 6.3% consisted of mutually negative −/– interactions.
Oviposition preference of the leafhoppers was strongly influenced by the presence of foliar pubescence on M. polymorpha trees. In all habitats where M. polymorpha trees were mostly hirsute, the number of leafhopper eggs was lower on the endemic tree than on M. faya. Furthermore, M. faya presence did not affect infestation of hirsute M. polymorpha with leafhopper eggs in mixed tree stands. Therefore, we suggest that increased captures of adult leafhoppers on M. polymorpha trees in the areas invaded by M. faya (Lenz and Taylor 2001) probably indicate a general build-up of the leafhopper populations in those areas, not their increase specifically on M. polymorpha trees. In other words, the invasive plant provided a reservoir for the pest to build up upon, but its direct association with the native plant did not appear to be particularly important.
The glabrous morphotype of M. polymorpha was more suitable for leafhopper oviposition than the hirsute morphotype. All M. polymorpha trees surveyed on the island of Kauai lacked foliar pubescence. Accordingly, their infestation with leafhopper eggs was comparable to that of M. faya at two of the Kauai sites. At the remaining two Kauai sites, M. polymorpha harbored fewer eggs than M. faya, possibly because the high nitrogen content of M. faya foliage favored leafhopper development under particular local conditions (Mattson 1980; Louda and Collinge 1992). However, the difference was far less dramatic than at the hirsute morphotype-dominated sites surveyed on the island of Hawaii. When growing within the same geographic area, hirsute morphotypes of M. polymorpha harbored 30 times less eggs than glabrous morphotypes. Also, visible symptoms of leafhopper damage (chlorosis, vein browning) were always more severe on the glabrous trees (Alyokhin, unpublished data). This confirms anecdotal observations of Jones et al. (2000), who reported higher levels of leafhopper damage to the glabrous morphotypes of M. polymorpha and D. linearis when compared to the hirsute morphotypes of the same species.
Foliar trichomes create a mechanical barrier, limiting leafhopper access to the leaf surface, interfering with locomotion and attachment to a plant, and often causing death of entrapped individuals (Broersma et al. 1972; Pillemer and Tingey 1976; Tingey 1985; Elden and Elgin 1992). Therefore, it is not surprising that hirsute M. polymorpha made a poor ovipositional host for two-spotted leafhoppers. Its replacement by glabrous M. faya will result in build-up of leafhopper populations that are otherwise tolerant of dry conditions dominated by the hirsute morphotype. Because of the broad host range characteristic of the two-spotted leafhopper, this build-up may adversely affect a number of endemic plant species growing in native forests.
Acknowledgments
We thank Jonavee Lim and Roxanne Cumming for technical assistance. We also thank the U.S. Department of the Interior and the Hawaii State Department of Land and Natural Resources for allowing us to conduct surveys on their lands. This work was supported in part by USDA-CSREES Special Grant No. 96-34135, Tropical and Subtropical Agriculture Research.
References
- Alyokhin AV, Yang P, Messing RH. Distribution and parasitism of two-spotted leafhopper eggs (Homoptera: Cicadellidae) in Hawaii. Annals of the Entomological Society of America. 2001;94:664–669. [Google Scholar]
- Broersma DB, Bernard RL, Luckmann WH. Some effects of soybean pubescence on populations of the potato leafhopper. Journal of Economic Entomology. 1972;65:78–82. [Google Scholar]
- Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. American Statistician. 1981;35:124–129. [Google Scholar]
- Corn C, Hiesey WM. Altitudinal variation in Hawaiian Metrosideros. American Journal of Botany. 1973;60:991–1002. [Google Scholar]
- Culliney TW. Site of oviposition and description of eggs of Sophonia rufofascia (Homoptera: Cicadellidae: Nirvaninae), a polyphagous pest in Hawaii. Proceedings of the Hawaiian Entomological Society. 1998;33:67–73. [Google Scholar]
- Drake DR, Mueller-Dombois D. Population development of rainforest trees on a chronosequence of Hawaiian lava flows. Ecology. 1993;74:1012–1019. [Google Scholar]
- Elden TC, Elgin JH. Mechanisms of resistance to the potato leafhopper (Homoptera: Cicadellidae) in selected alfalfa clones. Journal of Economic Entomology. 1992;85:576–582. [Google Scholar]
- Fukada M. 1996 Distribution, host range, and seasonal abundance of the two spotted leafhopper, Sophonia rufofascia (Kuoh and Kuoh) in Hawaii. M.S. Thesis, University of Hawaii at Manoa, Honolulu. [Google Scholar]
- Gerrish G, Mueller-Dombois D. Behavior of native and non-native plants in two tropical rainforests on Oahu, Hawaiian islands. Phytocoenologia. 1980;8:237–295. [Google Scholar]
- Giambelluca TW, Nullet MA, and Schroeder TA. 1986 Rainfall Atlas of Hawaii. Honolulu Department of Land and Natural Resources, Division of Water and Land Development. [Google Scholar]
- Heu R, Kumashiro B. Notes and exhibition. Proceedings of the Hawaiian Entomological Society. 1989;29:16–17. [Google Scholar]
- Howarth FG. 1985 Impacts of alien land arthropods and mollusks on native plants and animals in Hawaii. In: Stone CP, Scott JM, editors. Hawaii's Terrestrial Ecosystems: Preservation and Management. University of Hawaii Press, Honolulu, HI, 149–179. [Google Scholar]
- Johnson HB. Plant pubescence: an ecological perspective. Botanical Review. 1975;41:233–258. [Google Scholar]
- Jones VP, Follett PA, Messing RH, Borth WB, Ullman DE, Hu JS. Effect of Sophonia rufofascia (Homoptera: Cicadellidae) on guava production in Hawaii. Journal of Economic Entomology. 1998;91:693–698. [Google Scholar]
- Jones VP, Anderson-Wong P, Follett PA, Yang P, Westcot DM, Hu JS, Ullman DE. Feeding damage of the introduced leafhopper, Sophonia rufofascia (Homoptera: Cicadellidae) to plants in forests and watersheds of the Hawaiian Islands. Environmental Entomology. 2000;29:171–180. [Google Scholar]
- Kitayama K, Mueller-Dombois D. Vegetation changes along gradients of long-term soil development in the Hawaiian montane rainforest zone. Vegetation. 1995;120:1–20. [Google Scholar]
- Kitayama K, Mueller-Dombois D, Vitousek P. Primary succession of Hawaiian montane rain forest on a chronosequence of eight lava flows. Journal of Vegetative Science. 1995;6:211–222. [Google Scholar]
- Kitayama K, Pattison R, Cordell S, Webb D, Mueller-Dombois D. Ecological and genetic implications of foliar polymorphism in Metrosideros polymorpha in a habitat matrix on Mauna Loa, Hawai'i. Annals of Botany. 1997;80:491–497. [Google Scholar]
- Kuoh CI, Kuoh J.I. New species of Pseudonirvana (Homoptera: Nirvanidae) Acta Entomologica Sinica. 1983;26:316–325. [Google Scholar]
- Lee MAB. Insect damage to leaves of two varieties of Metrosideros collina subsp. polymorpha. Pacific Science. 1981;35:89–92. [Google Scholar]
- Lenz LS. 2000 The dieback of an invasive tree in Hawaii: interactions between the two-spotted leafhopper (Sophonia rufofascia) and faya tree (Myrica faya). M.S. Thesis, University of Hawaii at Manoa, Honolulu. [Google Scholar]
- Lenz L, Taylor J. The influence of an invasive tree species (Myrica faya) on the abundance of an alien insect (Sophonia rufofascia) in Hawai'i Volcanoes National Park. Biological Conservation. 2001;102:301–307. [Google Scholar]
- Louda SM, Collinge S.K. Plant resistance to insect herbivores: a field test of the environmental stress hypothesis. Ecology. 1992;73:153–169. [Google Scholar]
- Mattson WJ. Hebivory in relation to plant nitrogen content. Annual Review of Ecological Systems. 1980;11:119–161. [Google Scholar]
- Mattson WJ, Haack RA. 1987 The role of drought stress in provoking outbreaks of phytophagous insects. In: Barbosa P, Schultz J, editors. Insect oubreaks: ecological and evolutionary perspectives. Academic Press, Orlando, FL, 365–407. [Google Scholar]
- Mueller-Dombois D. Canopy dieback and successional processes in Pacific rainforests. Pacific Science. 1983;37:317–325. [Google Scholar]
- Mueller-Dombois D. Forest dynamics in Hawaii. TREE. 1987;2:216–220. doi: 10.1016/0169-5347(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Mueller-Dombois D, Fosberg FR. 1974 Vegetation map of Hawaii Volcano National Park. Technical Report No. 4, Cooperative National Park Resources Studies Unit. [Google Scholar]
- Palmer DD. Hawaiian ferns under attack. Fiddlehead. 1993;20:37. [Google Scholar]
- Pillemer EA, Tingey WM. Hooked trichomes: a physical plant barrier to a major agricultural pest. Science. 1976;193:482–484. doi: 10.1126/science.193.4252.482. [DOI] [PubMed] [Google Scholar]
- Ripperton JC, Hosaka EY. 1942 Vegetation zones of Hawaii. Hawaii Agricultural Experiment Station Bulletin No. 89, Honolulu, HI. [Google Scholar]
- SAS Institute, Inc. 1999 SAS OnLine Doc, Version 8. Cary, NC. [Google Scholar]
- Simberloff D, Von Holle B. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions. 1999;1:21–32. [Google Scholar]
- Smith CW. 1985 Impact of alien plants on Hawaii's native biota. In: Stone CP, Scott JM, editors. Hawaii's Terrestrial Ecosystems: Preservation and Management. Cooperative National Park Research Study Unit, University of Hawai'i, Honolulu, Hawaii, 180–250. [Google Scholar]
- Sohmer SH, Gustafson R. 1987 Plants and flowers of Hawaii. University of Hawaii Press, Honolulu, Hawaii. [Google Scholar]
- Stemmermann L. Ecological studies of Hawaiian Metrosideros in a successional context. Pacific Science. 1983;37:317–325. [Google Scholar]
- Tingey WM. 1985 Plant defense mechanisms against leafhoppers. In: Nault LR, Rodiquez JG, editors. The Leafhoppers and Planthoppers. John Wiley and Sons, New York, 217–231. [Google Scholar]
- Vitousek PM, Walker LR, Whiteaker LD, Mueller-Dombois D, Matson PA. Biological invasion by Myrica faya alters ecosystem development in Hawai'i. Science. 1987;238:802–804. doi: 10.1126/science.238.4828.802. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Shearer G, Kohl DH. Foliar 15N natural abundance in Hawaiian rainforest: patterns and possible mechanisms. Oecologia. 1989;78:383–388. doi: 10.1007/BF00379113. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Walker LR. Biological invasion by Myrica faya in Hawai'i: Plant demography, nitrogen fixation, ecosystem effects. Ecological Monographs. 1989;59:247–265. [Google Scholar]
- Walker LR, Vitousek PM. An invader alters germination and growth of a native dominant tree in Hawai'i. Ecology. 1991;72:1449–1455. [Google Scholar]
- Yang P, Follett PA, Jones VP, Foote D. Oviposition behavior and egg parasitoids of Sophonia rufofascia (Homoptera: Cicadellidae) in Hawaii Volcanoes National Park. Proceedings of the Hawaiian Entomological Society. 2000;34:135–139. [Google Scholar]
- Zar JH. 1999 Biostatistical Analysis. 4th Edition. Upper Saddle River, New Jersey, Prentice Hall. [Google Scholar]


