Abstract
Increasing plant density is one of the most efficient ways of increasing wheat (Triticum aestivum L.) grain production. However, overly dense plant populations have an increased risk of lodging. We examined lignin deposition during wheat stem development and the regulatory effects of plant density using the wheat cultivars shannong23 and weimai8. Plants were cultivated at densities of 75, 225 and 375 plants per m2 during two growing seasons. Our results showed that decreasing plant density enhanced culm quality, as revealed by increased culm diameter, wall thickness and dry weight per unit length, and improved the structure of sclerenchyma and vascular bundles by increasing lignification. In addition, more lignins were deposited in the secondary cell walls, resulting in strong lodging resistance. The guaiacyl unit was the major component of lignin and there was a higher content of the syringyl unit than that of the hydroxybenzyl unit. Furthermore, we hypothesised that the syringyl unit may correlate with stem stiffness. We describe here, to the best of our knowledge, the systematic study of the mechanism involved in the regulation of stem breaking strength by plant density, particularly the effect of plant density on lignin biosynthesis and its relationship with lodging resistance in wheat.
Stem lodging in wheat (Triticum aestivum L.) is a major agronomic problem that has far-reaching economic consequences1,2. The Green Revolution has effectively increased lodging resistance and the harvest index through the use of semi-dwarf trait3. However, improving the lodging resistance of crops by reducing plant height is only possible up to a certain limit in current crop production. Therefore, determining how to improve stem quality, especially the mechanical strength of the basal culm, has become the main target for increasing crop lodging resistance and thus grain yield.
The plant cell wall provides mechanical support to cells, tissues and the entire plant body4. The composition and structure of plant cell walls are ideally suited to the functions they perform. For example, parenchyma cells, which possess primary walls, provide the main structural support in the growing regions of the plant body. Sclerenchyma cells, which have both primary and thick secondary walls, provide the major mechanical support in the mature regions of the plant body5. During secondary cell wall synthesis, lignins are deposited in the carbohydrate matrix of the cell wall, providing the plant body with strong mechanical support to enable the plant to grow upwards6. Lignin content and composition are important factors that affect the cell wall stiffness and the mechanical strength of the plant body7. The ability to synthesize lignin has been essential in the evolutionary adaptation of plants from an aquatic environment to land8,9. Lignins are irreversible endpoints of a major metabolic pathway in the phenylpropane pathway. Previous studies have demonstrated that lignins are important to the mechanical strength of in other plant species. For example, Sherry et al. conducted a quantitative trait locus (QTL) analysis of stalk strength in four Maize populations. They showed that candidate genes with overlapping QTL confidence intervals included those involved in lignin synthesis10. In addition, in brown midrib mutants of maize, lignin content was extensively reduced with decreasing the stalk mechanical strength11. Significant correlations were similarly found between lignin accumulation and the mechanical strength of wheat (Triticum aestivum L.) and common buckwheat (Fagopyrum esculentum M.)12,13,14.
Lignin deposition in the plant cell walls is not only developmentally regulated but can also be affected by environmental conditions such as biotic and abiotic stresses15. During the early stages of lignification, H and G units and a few S units are incorporated into the polymer. Subsequently, coniferyl alcohol and sinapyl alcohol are incorporated during secondary wall formation to form a fusion of G and S units16. Plants are frequently exposed to different stresses that may change their lignin content and composition. For example, light itself has an effect on lignin biosynthesis17. More photosynthetic products, e.g. glucose, enter the shikimic acid pathway and generate more lignin under sufficient light conditions. Another study also showed that carbon availability related to starch turnover might determine the capacity to synthesise lignin in comparisons of circadian changes in the transcript abundance of lignin biosynthetic genes between wild-type plants and the sex1 mutant, which exhibits impaired in starch turnover18. Lignin composition was also altered in the sex1 mutants, with an increased proportion of syringyl as compared with guaiacyl and p-hydroxyphenyl18. Mechanical ingures induce specific reactions in the plant. Electron microscopy analysis has demonstrated that mechanical injures induce the thickening of the xylem the stem cell wall of poplar19.These findings indicate that the regulation of lignin accumulation in plants is complex and involves various elements, therefore, the molecular mechanisms that determine the mechanical strength are similarly complex.
Mechanical strength is controlled by quantitative trait loci (QTL)20,21,22, and regulated by growth regulators23,24, fertiliser25, and plant density26. Many measures have mainly focused on the morphological characteristics of the crop, including plant height, internode length, and culm diameter. Functional analyses of lignin biosynthetic enzymes, including phenylalanine ammonia-lyase (PAL)27, coumarate 3-hydroxylase (C3H)28, 4-coumarate: CoA ligase (4CL)29, ferulic acid/coniferaldehyde/coniferyl alcohol 5-hydroxylase (F5H)30, caffeic acid-3-o-methytransferase (COMT)31, cinnamoyl CoA reductase (CCR)32, and cinnamyl alcohol dehydrogenase (CAD)33 have been performed in several plants, inculding the model-plants Arabidopsis and tobacco. However, determining the expression pattern of the genes involved in lignin biosynthesis and lignin monomer ratio in winter wheat, and how winter wheat the responds the cultivation conditions remain to be elucidated.
Increasing plant density is the main ways of improving the grain yield of winter wheat. However, overly dense populations may lead to weak stem characteristics and a consequent lodging with strong wind and rain. Studies of the crop lodging response to plant density have reported inconsistent results. Most studies have demonstrated that lodging is more severe at higher plant densities. For example, one study reported that increasing of the plant density caused significant decreases in the stalk crushing strength, dry weight per unit length, and the diameter of the internode decreased significantly34. Other studies also showed that as the plant density increased, the length of the basal internode increased; the mechanical tissue thickeness decreased, the mechanical cell layers and cortical thickness reduced; and the lodging rate increased35,36. While a study of reepseed demonstrated that lodging resistance was elevated with increased plant density37. The response of trees, e.g. poplar, to plant density in terms of diameter and cell wall thickness has been previously reported38,39. Elucidating how plant density regulates lignin biosynthesis in the basal culm, and its relationship with lodging resistance in wheat is of great importance. Furthermore, the investigation of lignin biosynthesis to improve wheat cultivation techniques is an issue worthy to research. In this study, we performed experiment on two extensively cultivated wheat varieties to investigate the effect of three different planting densities on lignin biosynthesis. We reported an in-depth characterisation of the wheat stem, especially regarding lignin biosynthesis and its spatial expression patterns at different plant population densities. We aimed to clarify the regulatory mechanism of plant densities on lodging resistance in wheat from the aspect of lignin biosynthesis. To the best of our knowledge, we provide the first in-depth characterisation of the regulatory role of plant densities in lignin biosynthesis in wheat. A greater understanding of these issues should provide a theoretical basis to enhance the physical strength of the basal part of the culm internode, with the aim of obtaining a higher grain yield.
Results
Effects of plant densities on breaking strength
In both cultivars, from anthesis to the hard dough stage, the breaking strength gradually decreased. Plant density significantly (P < 0.01) influenced the breaking strength of the second basal internodes, which gradually increased with decreasing densities. Compared with the D3 treatment, the D2 treatment significantly increased the breaking strength of the second basal internode by 46.41%, 54.39% and 112.5% from 2012 to 2013 and by 29.97%, 81.64% and 88.14% from 2013 to 2014 (means of the two cultivars) at the anthesis, milk and hard dough stages, respectively. Similarly, the D1 treatment significantly increased the breaking strength by 85.02%, 90.43% and 178.24% and by 65.08%, 163.18% and 178.53% (means of two cultivars) for the respective parameters. The characteristics of the stem second basal internodes of the WM8 plants were increased compared with the SN23 plants in all treatments in both growing seasons (Table 1).
Table 1. Effects of plant densities on the breaking strength of the basal second internode of the two genotypes in the two growing seasons.
| 2012–2013 |
2013–2014 |
||||||
|---|---|---|---|---|---|---|---|
| Cultivar | Treatment | A(N) (DC, 65) | M (N) (DC, 75) | H (N) (DC, 87) | A (N) (DC, 65) | M (N) (DC, 75) | H (N) (DC, 87) |
| D1 | 7.92 ± 0.77b | 6.52 ± 0.60b | 4.77 ± 0.52b | 7.38 ± 0.21b | 6.32 ± 0.25b | 4.65 ± 0.22b | |
| SN23 | D2 | 6.32 ± 0.72c | 5.16 ± 0.34c | 3.84 ± 0.89c | 5.84 ± 0.31c | 4.51 ± 0.18c | 3.46 ± 0.38c |
| D3 | 4.06 ± 0.43e | 3.42 ± 0.53e | 1.48 ± 0.68d | 4.81 ± 0.54d | 2.09 ± 0.34e | 1.65 ± 0.53d | |
| D1 | 9.62 ± 1.05a | 8.01 ± 0.69a | 5.72 ± 0.57a | 9.97 ± 0.39a | 9.05 ± 0.06a | 5.21 ± 0.54a | |
| WM8 | D2 | 7.56 ± 0.61b | 6.62 ± 0.78b | 4.17 ± 0.73bc | 7.82 ± 0.49b | 6.07 ± 0.32b | 3.20 ± 0.13c |
| D3 | 5.42 ± 0.28d | 4.21 ± 0.54d | 2.29 ± 0.86d | 5.70 ± 0.49c | 3.75 ± 0.25d | 1.89 ± 0.21d | |
| Cultivars (C) | 0.0095 | 0.0314 | 0.066 | 0.0709 | 0.0999 | 0.0997 | |
| P-value | Densities (D) | 0.0036 | 0.0123 | 0.0092 | 0.0047 | 0.0077 | 0.0089 |
| C × D | 0.5954 | 0.3262 | 0.5501 | 0.0173 | 0.0001 | 0.0001 | |
Each value represents the means ± SE (n = 15) for the second basal internode of the representative wheat culm. Least significant difference testing (LSD) was performed to compare the means of the three experimental treatments, means followed by the same letters within each column are not significantly different at P < 0.05. N, Newton, A, Anthesis stage; M, Milk stage; H, Hard dough stage; SN23, shannong23; WM8, weimai8; DC, digital code56.
Morphological characteristics of the basal second internode
To evaluate the differences in stem mechanical strength that respond to plant density, the morphological characteristics of the second basal internode were analysed. The second basal internode characteristics were strongly (P < 0.01) affected by plant density (Table 2). Compared with the D3 treatment, the diameter of the second basal internode of the D1 and D2 plants was approximately 15.46% and 9.9% (mean of two cultivars) larger, respectively, from 2012 to 2013 and approximately 13.31% and 5.57% larger, respectively, from 2013 to 2014. Compared with the D3 treatment, D1 and D2 plants exhibited significant increases in wall thickness of 37.76% and 23.78%, respectively, from 2012 to 2013, and 27.11% and 13.25%, respectively, from 2013 to 2014. Plant density also strongly affected the dry weight per unit length of the second basal internode and increased with reduced plant densities (Table 2). Compared with the D3 treatment, the D2 treatment resulted in an increase in the dry weight per unit length of 38.29% and 36.54%, whereas the D1 treatment resulted in an increase in the dry weight per unit length of 91.75% and 97.71% from 2012 to 2013 and from 2013 to 2014, respectively. The culm characteristics of the two cultivars showed similar trends. The morphological features of the WM8 plants were significantly (P < 0.05) increased compared with the SN23 plants.
Table 2. Effects of plant densities on the characteristics of the second basal internode at the hard dough stage (DC, 87).
| 2012–2013 |
2013–2014 |
||||||
|---|---|---|---|---|---|---|---|
| Cultivar | Treatment | D (mm) | WT (mm) | DW (mg cm−1) | D (mm) | WT (mm) | DW (mg cm−1) |
| D1 | 4.48 ± 0.07b | 0.92 ± 0.02b | 28.19 ± 1.28b | 4.65 ± 0.11b | 1.01 ± 0.06ab | 32.03 ± 1.46b | |
| SN23 | D2 | 4.21 ± 0.01bc | 0.88 ± 0.02ab | 21.05 ± 1.49c | 4.37 ± 0.06c | 0.91 ± 0.04bc | 22.26 ± 1.63c |
| D3 | 3.92 ± 0.12c | 0.71 ± 0.03c | 15.46 ± 1.18d | 4.05 ± 0.03d | 0.78 ± 0.04c | 16.91 ± 0.97d | |
| D1 | 5.08 ± 0.12a | 1.05 ± 0.05a | 32.46 ± 1.97a | 5.14 ± 0.05a | 1.10 ± 0.12a | 35.23 ± 2.75a | |
| WM8 | D2 | 4.89 ± 0.17a | 0.89 ± 0.04b | 22.69 ± 1.94c | 4.78 ± 0.09b | 0.97 ± 0.08ab | 24.19 ± 2.16c |
| D3 | 4.36 ± 0.09b | 0.72 ± 0.06c | 16.17 ± 2.08d | 4.59 ± 0.08bc | 0.88 ± 0.07bc | 17.83 ± 1.43d | |
| Varieties (V) | 0.014 | 0.1508 | 0.0125 | 0.0066 | 0.0128 | 0.0049 | |
| P | Densities (D) | 0.0337 | 0.0001 | 0.0001 | 0.0141 | 0.0063 | 0.0001 |
| V × D | 0.6066 | 0.2834 | 0.3158 | 0.7040 | 0.9555 | 0.7815 | |
Each value represents the mean ± the SE (n = 15). The least significant difference test was performed to compare the means of the three treatments. Means followed by the same letters within each column are not significantly different at P < 0.05. D, diameter; WT, wall thickness; DW, dry weight; SN23, shannong23; WM8, weimai8.
Microscopic structural characteristics of the second basal internode
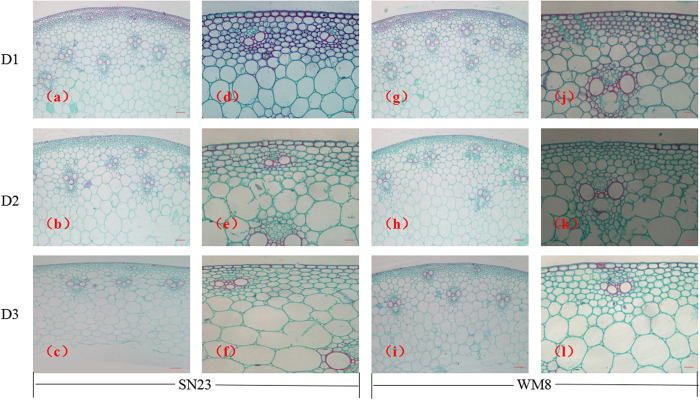
To further identify the factors responsible for the effects of plant densities on the breaking strength, the anatomical structure of the plants was analysed for each of the treatments. In the transverse sections, lignin was observed mainly in the epidermis, sclerenchyma and vascular bundles (Fig. 1). Stain intensity did not differ between the cultivars that received the same treatment. Nevertheless, a greater number of vascular bundles were observed in the WM8 transverse sections than in the SN23 plants in the same visual field. The sclerenchyma of the WM8 plants was also arranged more compactly compared with the SN23 plants. The wheat second basal internodes showed significant differences in their degree of lignification between the treatments. The lignification area in the D1 plants was remarkably larger than that seen in the D2 and D3 plants, and a greater number of vascular bundles were observed in the D1 and D2 plants compared with the D3 plants (Fig. 1).
Figure 1. Effects of plant densities on the degree of lignification of the second basal internode of two wheat cultivars.
Cross-sections of the second basal internodes of two wheat cultivars stained for lignification with safranin O-fast green. The first two columns indicate SN23; the last two columns indicate WM8; first line, D1 treatment (75 plants m−2); second line, D2 treatment (225 plants m−2); third line, D3 treatment (375 plants m−2). Scale bars: first and third columns, 200 μm; second and fourth columns, 20 μm.
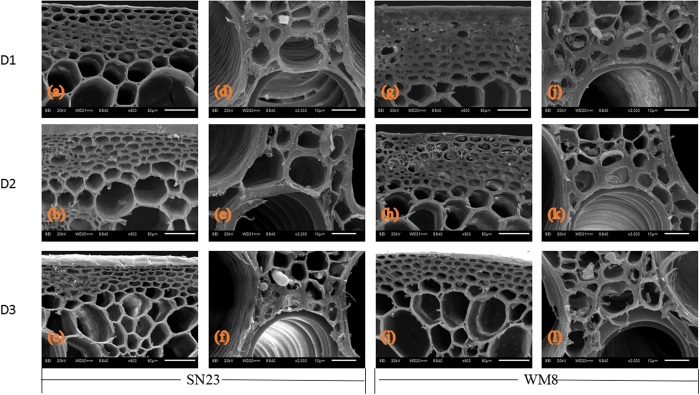
Furthermore, to determine any anatomical changes among the plant density treatments, we analysed basal second internode cross-sections by scanning electron microscopy. Plant densities strongly affected the sclerenchyma and the structure of vascular bundles (Fig. 2). As plant density decreased, the abundance of sclerenchyma cells in the cortex increased, the walls were thicker and the arrangement was more compact. Plant density had a similar effect on the vascular bundles. Compared with the D3 treatment, the vascular bundles of the plants that received the D1 treatment were stronger structures with thicker cell walls and a more compact arrangement of sclerenchyma cells. The structure of the sclerenchyma and vascular bundles in the WM8 plants was slightly superior compared with the SN23 plants.
Figure 2. Scanning electron microscopy of cross-sections of the second basal internode of plants grown at different plant densities.
Transverse sections of the second basal internode were obtained at 10 days after anthesis. The first two columns indicate SN23; the last two columns indicate WM8; first line, D1 treatment (75 plant m−2); second line, D2 treatment (225 plants m−2); third line, D3 treatment (375 plants m−2). The first and third columns reveal details of the sclerenchyma; the second and fourth columns show details of the vascular bundle. Scale bars: first and third columns, 50 μm; second and fourth columns, 10 μm.
Effects of plant densities on lignin accumulation
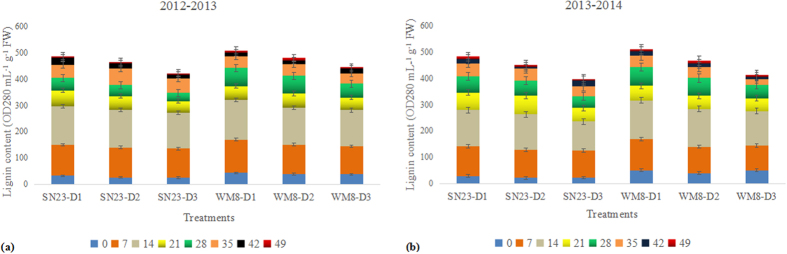
Based on the different effects that the different plant densities had on the degree of lignification, lignin accumulation during wheat stem development was determined. A significant difference in lignin concentration was observed between treatments; lignin accumulation increased with decreasing plant density. The total lignin accumulation of the SN23 plants that received the D1 or D2 treatment was increased by 18.69% and 12.26% (means of two years), respectively, compared with the D3 plants, whereas these values were 18.74% and 10.24%, respectively, in WM8 plants. Furthermore, lignin accumulation during wheat stem development was inconstant (Fig. 3). A relatively faster accumulation rate was noted 0 to 35 days after the formation of the second internode (jointing to early grain filling) compared with 42 to 49 days (later grain filling to hard dough stage). The data also showed that the second basal internode of WM8 plants accumulated more lignin than that of SN23 plants in both growing seasons.
Figure 3. Effect of plant densities on lignin accumulation in the second basal internode of two wheat cultivars.
Different colours represent the days after the second internode formation in the 2012–2013 (a) and 2013–2014 (b) growing seasons. FW, fresh weight.
Effects of plant densities on lignin monomers
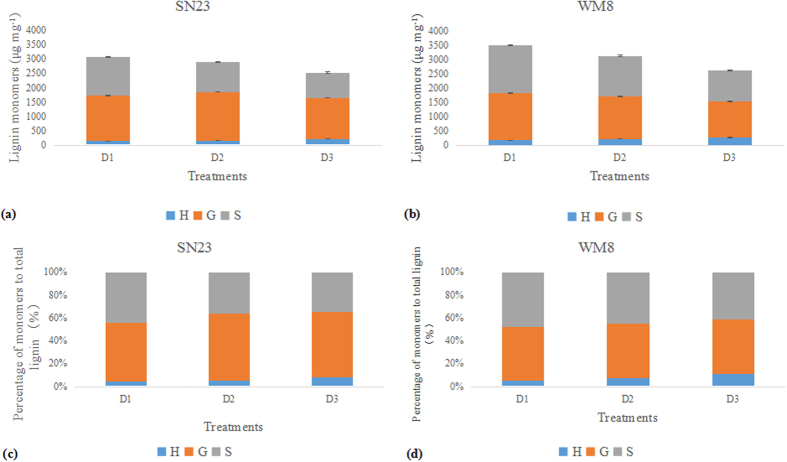
We successfully separated the three lignin monomers using UPLC-MS/MS (Supplementary Fig. S2). In both genotypes, guaiacyl units formed the main structure, followed by syringyl and finally hydrobenzyl units. Plant density had significant effects on both total lignin and monomer contents.
The total lignin monomer contents of the WM8 plants were higher than those of the SN23 plants. In addition, the plants that received the D3 treatment showed the lowest total released monomers. Compared with the D3 treatment, the total monomer contents of the SN23 plants that received the D1 and D2 treatments were 17% and 10% higher, respectively, and the total monomer contents of the WM8 plants were 13% and 8% higher, respectively (Fig. 4a,b). Both cultivars showed a similar variation in the monomers that were released depending on the planting density. Increasing plant density led to the increased production of H monomers at the expense of S monomers, and the G monomer varied slightly (Fig. 4c,d).
Figure 4. Lignin monomer content and percentage.
The lignin building units hydroxyphenyl (blue), guaiacyl (yellow) and sinapyl (grey) were determined in mature wheat by the alkaline nitrobenzene oxidation of purified cell walls. Data of stacked columns are presented as the means ± the SE of three replicates.
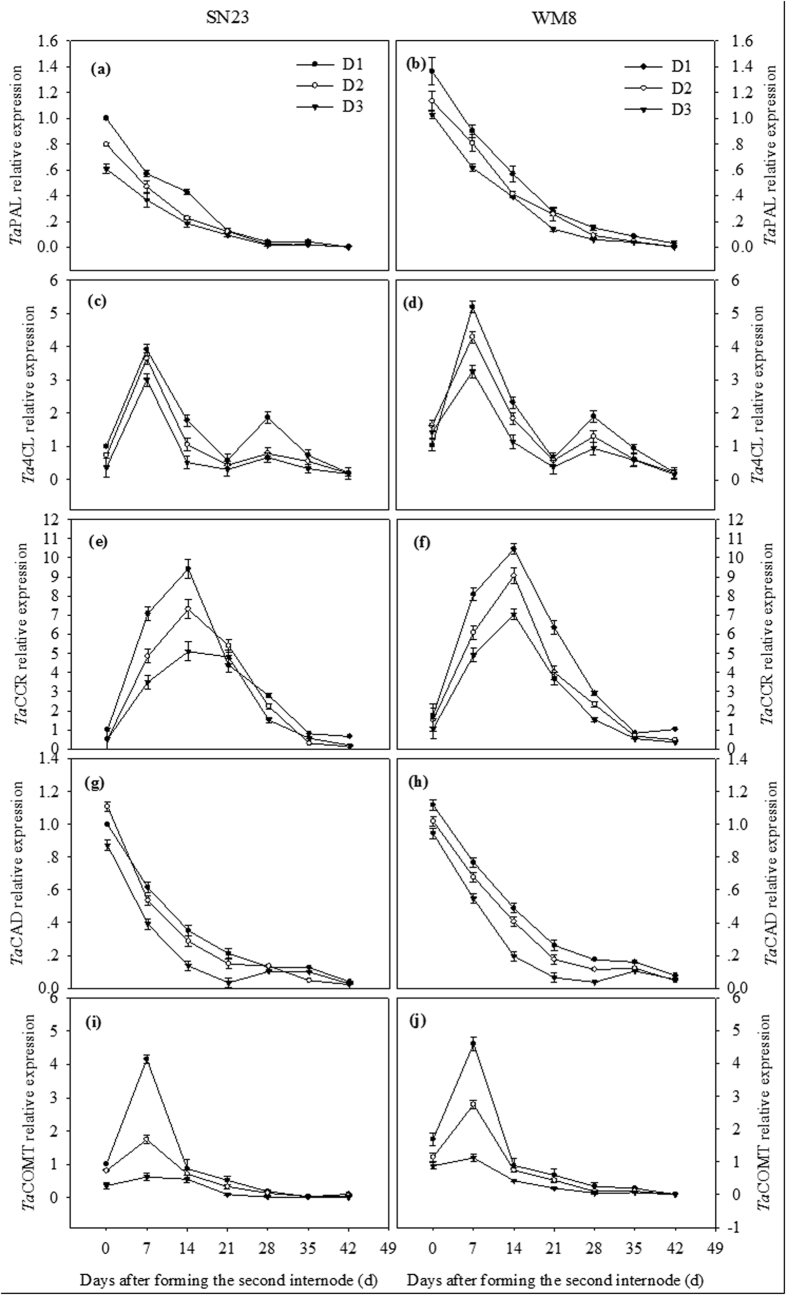
Effects of plant densities on the expression pattern of genes involved in lignin biosynthesis
To illuminate the genes that were likely to be involved in wheat stem lignification, we compared the transcript abundance of the phenylpropanoid gene during wheat stem development by quantitative PCR (Fig. 5). Several distinct spatial expression profiles were observed among the analysed wheat genes. As the stem developed, the relative expression of the TaPAL gene gradually decreased. Ta4CL gene expression reached its peak 14 days after the second internode formation followed by a second peak at 28 days. The relative expression of the TaCCR gene was highest at 0 to 14 days and then gradually decreased. The relative expression of the TaCAD gene decreased to its minimum at 21 days after the formation of the second internode, then slightly increased at 28 days, and decreased again at 42 days. The relative expression of the TaCOMT gene was highest at 0 to 14 days after the formation of the second internode, decreased sharply and was minimally detectable at the late growth stage. The highest expression levels in the second basal internode samples were concomitant with the increase in lignin content during stem development. Plant density has strong regulating effects on the expression of lignin biosynthesis genes. Such genes were expressed at relatively higher levels in the D1 treatment, which might explain the higher levels of lignin monomers in the D1 treatment. Overall, the gene expression levels of the WM8 plants were higher than those of the SN23 plants at all stem developmental stages. The lower expression levels of lignin biosynthesis genes approximately at maturity could be due to their preferential expression in young tissues.
Figure 5. The expression profile of lignin biosynthetic genes analysed by quantitative reverse transcription-PCR.
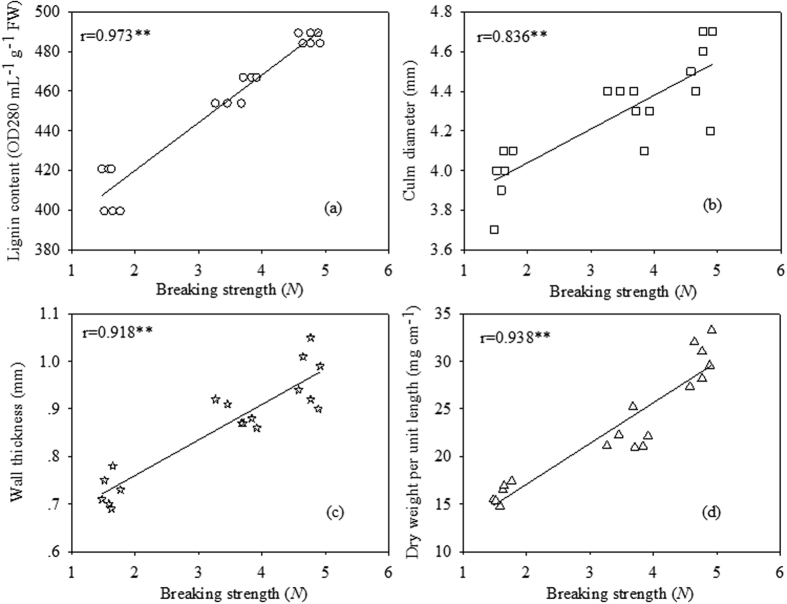
Correlation analysis between breaking strength and morphological characteristics as well as lignin content
There was a significantly positive correlation between the breaking strength and lignin content of the second basal internode at the hard dough stage (Fig. 6a). Correlation analysis also demonstrated that the breaking strength of the second basal internode had a strong positive correlation with various morphological characteristics, including culm diameter, wall thickness and dry weight per unit length (Fig. 6b,c,d).
Figure 6. The relationship between the breaking strength and morphological characteristics of the second basal internode and the lignin content.
(a), lignin content; (b), culm diameter; (c), wall thickness; (d), dry weight per unit length.
Discussion
Lodging control in wheat is mainly determined by genetic factors, but it can also be modulated by plant density34,40. Increasing plant density is an effective cultivation practice to improve wheat grain yield; however, the plants will also be prone to lodging. A reasonable plant density is an important feature associated with stronger culm compared with the culm of overly large populations. Consistent with previous studies, we found that the breaking strength of the wheat second basal internode strongly increased with decreasing plant density34,40, thus decreasing the risk of lodging.
Plant density affects several processes during wheat culm development, such as morphological characteristics, anatomical structure, and the lignin biosynthesis inherent to these structures. In this study, we combined physiology, biochemistry, anatomy and molecular biology to clarify the mechanism through which plant density regulates the breaking strength of wheat culms, focusing on lignin biosynthesis. Our results allowed us to conclude that there is a relationship between plant density and the breaking strength of wheat culms. To understand the mechanism through which plant mechanical strength is controlled in wheat, we performed an in-depth analysis of different plant densities.
Culm morphological traits are related to lodging resistance41. Previous studies have suggested that stem diameter is a key factor in lodging resistance and that increasing the culm diameter in rice breeding programmes would thus improve lodging resistance in this crop32. In our study, we found that plant density had a significant effect on the culm diameter of the wheat second basal internode. With decreasing plant densities, the culm diameter of both cultivars increased in both growing seasons and exhibited a significantly positive (r = 0.836**) correlation with culm breaking strength. Previous reports have also agreed that culm wall thickness also has an important role in improving lodging resistance12. Significant correlations have been detected between resistance to lodging and wall thickness42. In this study, we concluded that decreasing the plant density increased the culm wall thickness. In addition, the dry weight per unit length, which represents the degree of culm filling, had a positive relationship with the culm breaking strength. This finding is similar to results obtained with rice culm, in which the degree of filling also significantly decreased with increasing population size41. Morphological features that provided the best indication of improved lodging resistance included increased culm diameter, wall thickness, and dry weight per unit length. These results support the hypothesis that larger populations have lower culm quality and a consequently increased risk of lodging in the case of wheat.
Anatomical stem characteristics affect lodging in wheat. Significant correlations were detected between lodging resistance and several anatomical features, including the width of the mechanical tissue layers and the width of stem walls42. A reduction in the mechanical strength of culms and negatively affected morphological characteristics in larger populations may reflect alterations in the cell wall structure. Therefore, we examined cell wall morphology using scanning electron microscopy to trace such alterations. In the D3 treatment, the number of mechanical tissues layers, especially those around the peripheral vascular tissues and under the epidermal tissue in culms, were reduced compared with D1 and D2 treatments, thus leading to weaker mechanical support in D3 plants. Scanning electron microscopy observations also revealed sclerenchyma and vascular bundle cells with thin walls in plants that received the D3 treatment. This finding is in striking contrast with that observed in the D1 treatment: the plants had thick-walled sclerenchyma and vascular bundle cells, in which the secondary wall occupied a large proportion of the cell lumen. The physiological factors associated with cell structure are unknown. Further studies are needed to investigate the physiological and genetic aspects of morphogenesis that are associated with the formation of secondary cell walls. Furthermore, staining with safranin O-fast green showed that the degree of lignification in D3 treatment was lower than that in D1 treatment. A high degree of lignification may be responsible for the stiff culm of wheat. Cortical tissues, especially sclerenchyma, are very important for enabling the culm to cope with bending stresses42. Our results suggest that the lower level of mechanical strength recorded for the D3 plants compared with the plants in the other treatments is likely to result from a defect in the cell wall thickening and lignification of the mechanical tissues, such as sclerenchyma, and vascular bundle elements.
A deficiency in the lignin biosynthesis in the secondary cell walls often leads to globally altered plant morphology, cell structure and even sterility43,44,45. In this study, the lignin content showed a significantly positive (r = 0.973**) correlation with the culm breaking strength. Lignin continuously increased between 0 and 35 days after the formation of the second internode in both cultivars, after which considerably reduced accumulation was observed during the later stages of stem development. This pattern of lignification is consistent with previous reports12,46. A similar developmental pattern of lignin deposition was observed in maize, in which a rapid increase in lignin content was observed along the progressive elongation stage47,48. Such a lignin accumulation pattern is consistent with the fact that lignification begins before the cessation of the elongation process. To determine whether the cellular phenotype, the degree of lignification and the reduction in mechanical strength in D3 plants resulted from altered lignin biosynthesis, we compared the lignin content among plant density treatments given that lignin is related to stem stiffness. Compared with the D1 and D2 treatments, the lignin content in D3 was significantly reduced. This result suggests that overly dense populations did not exhibit lignin accumulation.
Lignin accumulation and its composition (i.e., H-, G- and S-type monomers) are important factors influencing the breaking strength of wheat culm. It has been suggested that syringyl biosynthesis provides significant mechanical advantages to angiosperm species. However, the guaiacyl function in these plants involves conduction, not mechanical support45. Our data showed that guaiacyl units were the most abundant monomer in both cultivars. Furthermore, there was a greater abundance of syringyl units was compared with hydroxyphenyl units. Plant density had a strong effect on lignin monomer content and the proportion of each monomer. In this study, we showed that lignin composition also varies with plant density. With increased plant density, the content and proportion of H-type monomers increased at the expense of S-type monomers. It is likely that plants compete for energy in overly large populations; therefore, plants would choose a relatively low-energy monomer pathway, i.e., the H-type. We conclude that the S-type monomer plays an important role in the improved breaking strength of the wheat culm. To the best of our knowledge, this study is the first to determine lignin monomer variation with plant density and to elucidate the relationship between such variation and stem mechanical support.
To further clarify the regulation mechanism of lignin and lignin monomer accumulation according to plant density, transcriptomic data were used to determine several genes that are most likely to be involved in lignin biosynthesis in wheat stems at different developmental stages. Previous studies have reported that PAL, 4CL, CCR, CAD and COMT play key roles in lignin biosynthesis in wheat49,50,51,52,53. Regarding the lignin biosynthetic pathway genes, different transcript abundances were observed at different developmental stages. The relative expressions of TaPAL, Ta4CL, TaCCR, TaCAD and TaCOMT genes were maintained at relatively high levels at approximately the same stage of fast lignin accumulation. Interestingly, the majority of the maximum expression occurred in young internodes followed by a decrease during the later stages of development, whereas lignin content increased at the latest maturity stages. A possible explanation is that a higher level of gene expression occurs in the early stages of stem development and that, even with subsequent down-regulation, lignification is maintained due to the stability of the lignin biosynthesis enzymes54. The S branch-specific gene (i.e., COMT) was down-regulated in the D3 treatment, which is consistent with the decreased S-type monomer content compared with the D1 treatment.
Based on our results, we suggest that a drastic reduction in lignin deposition caused by overly large populations normally impacts plant growth and development. The compression strength and pull strength of wheat stems would be about to change under different plant densities. The mechanical stress would increase under lower plant density. On one hand, this can present as increased the precursor products forming the lignin which provide the stiffness for plant body. On the other hand, due to the responses of plant to stress (e.g. wind stress), plant body would be induced to form defensive substances (lignin). This type of regulatory function in cultivation actually means that induced effect in morphology. A reasonable plant density significantly decreased the risk of lodging occurring, not only by altering the basal stem morphological traits but also by modifying lignin metabolism, especially the mRNA relative expression of genes in the basal internode. It also contributed to lignin accumulation and increasing stem stiffness. It should be noted that this study has examined only a few genes, and other middle pathway enzymes and genes (Supplementary Fig. S1) involved in lignin biosynthesis should be further analysed in subsequent studies.
In conclusion, we have described here, to the best of our knowledge, the first systematic study of the mechanisms regulated by plant density, especially regarding the effect of plant density on lignin biosynthesis and its relation to lodging resistance in wheat. The above mentioned results are valuable for enhancing lodging resistance in wheat. Therefore, we believe that this work can be considered as a reference study for future wheat cultivation.
Methods
Plant material and experimental design
Experiments were performed during two growing seasons from October 2012 to June 2013 and from October 2013 to June 2014 at the Shandong Agricultural University Farm, Tai’an, Shandong Province, China (36°09′ N, 117°09′E). The average content of the organic matter in the tillage layer was 18.11 g kg−1, and the total nitrogen determined by kjeldahl mentod (N). the rapidly available phosphorous determined by colorimetry method (P) and the rapidly available potassium contents determined by flame photometry (K) were 1.23 g kg−1, 17 mg kg−1 and 113 mg kg−1, respectively55. Initially, 120 kg of N ha−1, 100 kg of P2O5 ha−1 and 120 kg of K2O ha−1 were applied as a basal fertiliser before planting. In addition, 120 kg of N ha−1 was applied at the jointing stage (DC 30)56. Pests, diseases and weeds were controlled by appropriate chemical applications during the growing period. We chose two cultivars, shannong23 (SN23) and weimai8 (WM8), which are extensively cultivated. Three plant density levels (75, 225 and 375 plants m−2) corresponding to low (D1), medium (D2) and high (D3) density levels were established for the field experiment. The experimental plots were arranged in a factorial scheme, in a completely randomised block design with three replications in each treatment. The plot size was 3 × 3 m with 10 rows (0.25-m line spacing).
Sampling and measurements
Sampling was performed after the formation of the second basal internode (DC 32). Representative samples of the second basal internode were collected every 7 days from plants growing in the middle of each plot; there were a total of eight collections. The stem sheath was removed from the samples before the samples were plunged directly into liquid nitrogen for at least 30 min, and then stored at −80 °C until analysis.
Morphological characteristics of the second basal internode
Fifteen representative main stems were chosen for the measurement of the culm diameter, wall thickness, and dry weight of the second basal internode, according to the methods described by Wei et al.57. The diameter and wall thickness of the second basal internodes were measured at the internode mid region using a digital calliper with an accuracy of 0.001 mm.
Breaking strength measurement
Breaking strength was measured at anthesis (DC 62), and at the milk (DC 72) and hard dough stages (DC 82) following the methods of Chen et al.58 and Peng et al.12 with slightly modifications. Measurements were obtained using a plant lodging tester (Hangzhou TOP Instrument, China). For these measurements, second basal internodes with the stem sheath removed and were placed on the supporting pillars at a distance of 5 cm. The tester was set perpendicular to the middle of the stem, which lodged gradually, and the breaking strength was measured when the culm internode was pushed to its breaking point. Breaking strength was expressed in Newtons (N).
Histological analysis
Ten 10 days after anthesis, the middle section of the second basal internodes (approximately 0.5 cm in width) from each treatment were fixed in FAA (5 mL of 38% formaldehyde, 5 mL of glacial acetic acid and 90 mL of 70% ethanol) for 24 h, dehydrated with ethanol, and embedded in paraffin. Cross-sections of approximately 4-μm thick were obtained using a microtome (Leica, Germany). To visualise the degree of lignification among the treatments, sections were stained with 1% safranin-O for 1.5 h, washed with distilled water, discoloured in an ethyl series and then counterstained with 0.5% fast green for 1 min. Stained cross-sections were mounted on microscope slides and visualised using a Nikon DS-V3 microscope (Nikon, Japan). Lignified zones were stained red.
The middle sections of the second basal internodes were also fixed in a mixture of 2% paraformaldehyde and 2.5% glutaraldehyde in a 0.1% mol L−1 phosphoric acid buffer for 24 h at 4 °C and post-fixed in 1% OsO4 in a 0.1 mol L−1 phosphoric acid buffer for 30 min. After critical point drying, cross-sectioned samples were sputter-coated with gold and viewed using a scanning electron microscope (JEOL, Japan).
Lignin determination
To assess lignin content, 0.3 g of fresh stem samples free of leaf sheaths were ground in liquid N2, washed five times with 80% ethanol to remove soluble metabolites, washed with acetone and dried in a drying oven. Acetyl bromide analysis was conducted following Peng et al.12. Samples were exposed to a 4:1 (v/v) mixture of acetic acid:acetyl bromide and incubated at 70 °C for 2 h. Then, the samples were cooled to room temperature and transferred to 50-mL volumetric flasks containing 2 M NaOH, acetic acid and 7.5 M hydroxylamine hydrochloride, which were added to terminate the reaction. The constant volume of each sample was completed with acetic acid. Sample absorbance was read at 280 nm by a spectrophotometer (Shimadzu UV-2450, Japan). Lignin content was expressed as OD280 mL−1 g−1 fresh weight (FW).
Determination of lignin monomers
The hard dough developmental stage was chosen for the analysis of lignin monomer yields (H, G and S units), which were determined by alkaline nitrobenzene oxidation. The conventional nitrobenzene oxidation method was used with some modifications59. A total of 20 mg of protein-free cell wall was suspended in 2.5 mL of 2 M NaOH and 0.7 mL of nitrobenzene in a 5-mL vial with an aluminium cap. After mixing, the samples were heated in an autoclave at 127 °C for 2 h. The autoclave reactor was subsequently cooled to 70 °C, and the vials were taken out of the reactor and cooled by placing under running water. The reaction mixture was then centrifuged at 5,000 g for 2 min. The water phase on the top was transferred to a 10-mL centrifuge tube and then extracted with ethyl acetate (2 mL × 3). The organic phase was combined and dried with anhydrous Na2SO4 and the solvent was removed using a Termovap Sample Concentrator. The sample obtained was resuspended with 1.6 mL of the mobile phase (initial conditions) and then subjected to analysis with an Acquity ultra-performance liquid chromatography-electrospray tandem mass spectrometry system (UPLC-MS/MS) (Waters, Milford, MA, USA). Hydroxybenzaldehyde (H), vanillin (G) and syringaldehyde (S) were used as standards to quantify the monomers in the samples. Chromatographic separation was performed on a Waters Acquity BEH C18 column (1.7 μm, 2.1 × 100 mm) with a gradient elution (Supplementary Table S1). The mass spectra method is presented in Supplementary Table S2. The chromatogram graph is presented in Supplementary Fig. S2.
Gene expression analysis
The gene expression encompasses three independent biological replicates of each treatment. For each biological replicate, three technical replicates of each PCR reaction were performed. Briefly, total RNA from the second basal internode was isolated using a modified Trizol extraction method and treated with DNase I to remove any contaminant genomic DNA. First-strand cDNA was synthesised from 1 μg of total RNA using the Prime Script RT Reagent Kit (TIANGEN, China). Quantitative real-time PCR was performed using the SYBR Green Master Mix kit in a Step One Plus instrument (Applied Biosystems, Singapore). The primers used in the present study are presented in Supplementary Table S3. We used the β-actin gene as an endogenous control to normalise the amount of template. The qRT-PCR protocol and the three-step thermal cycling protocol were performed following the manufacturers’ instructions as follows: pre-denaturation at 95 °C for 15 s followed by 40 cycles of denaturation at 95 °C for 10 s, an annealing temperature of 58 °C for 20 s and an extension temperature of 72 °C for 30 s. The baseline and threshold were adjusted according to the manufacturer’s recommendations. Melting curves were used to verify the specificity of amplification. The relative expression levels were calculated with the equation 2−∆∆Ct.
Statistical analysis
Statistical analysis was performed using Data Processing System software 7.05 (DPS). The means and significant differences between treatments were separated using the least significant differences (LSD) test at 5% probability.
Additional Information
How to cite this article: Zheng, M. et al. Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Sci. Rep. 7, 41805; doi: 10.1038/srep41805 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The research was supported by the National Natural Science Foundation of China (NO. 31271661, 31271667), the Shandong Province Mount Tai Industrial Talents Program, the project of Shandong Province Higher Educational Science and Technology Program (J14LF12), the National Basic Research Program of China (973 Program, NO. 2015CB150404), the Special Fund for Agro-scientific Research in the Public Interest of China (No. 201203100), Shangdong Provence key Project for Application Technology Innovation and the National Science and Technology Support Program of China (No. 2012BAD04B05).
Footnotes
The authors declare no competing financial interests.
Author Contributions Z.W. and Y.L. designed the research. W.L., J.N. and Y.W. performed research. Y.L., D.Y. contributed to the practice of field experiments. Y.L. and D.P. performed the morphological observations. M.Z., J.C. and Y.S. wrote the paper and analyzed the data. All authors discussed the data and made comments on the manuscript.
References
- Berry P. M. & Spink J. Predicting yield losses caused by lodging in wheat. Field Crops Res. 137, 19–26 (2012). [Google Scholar]
- Acreche M. M. & Slafer G. A. Lodging yield penalties as affected by breeding in Mediterranean wheats. Field Crops Res. 122, 40–48 (2011). [Google Scholar]
- Khush G. S. et al. Green revolution: preparing for the 21st century. Genome 42, 646–655 (1999). [PubMed] [Google Scholar]
- Keegstra K. Plant cell walls. Plant physiol. 154, 483–486 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. H. et al. Brittle CUM1, which encodes a Cobra-Like protein, affects the mechanical properties of rice plants. Plant Cell 15, 2020–2031 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. et al. Developmental control of lignification in stems of lowland switchgrass variety alamo and the effects on saccharification efficiency. Bioenerg. Res. 2, 233–245 (2009). [Google Scholar]
- Chabannes M. et al. In situ analysis of lignins in transgenic tobacco reveals a differential impact of individual transformations on the spatial patterns of lignin deposition at the cellular and subcellular levels. Plant J. 28, 271–282 (2001). [DOI] [PubMed] [Google Scholar]
- Weng J. K. & Chapple C. The origin and evolution of lignin biosynthesis. New Phytol. 187, 273–285 (2010). [DOI] [PubMed] [Google Scholar]
- Vanholme R., Demedts B., Morreel K., Ralph J. & Boerjan W. Lignin biosynthesis and structure. Plant physiol. 153, 895–905 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry A. et al. Quantitative trait locus analysis of stalk strength in four maize populations. Crop Sci. 43, 13–22 (2003). [Google Scholar]
- Muller L. D., Barnes R. F., Bauman L. F. & Colenbrander V. F. Variations in lignin and other structural components of brown midrib mutants of maize. Crop Sci. 11, 413–415 (1971). [Google Scholar]
- Peng D. L. et al. Lodging resistance of winter wheat (Triticum aestivum L.): lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crops Res. 157, 1–7 (2014). [Google Scholar]
- Wang C. et al. Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 180, 46–53 (2015a). [Google Scholar]
- Wang C. et al. Effects of nitrogen fertilizer and planting density on the lignin synthesis in the culm in relation to lodging resistance of buckwheat. Plant Prod. Sci. 18, 218–227 (2015b). [Google Scholar]
- Moura J. C., Bonine C. A., Dornelas M. C. & Mazzafera P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 52, 360–376 (2010). [DOI] [PubMed] [Google Scholar]
- Grabber J. H. How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Sci. 45, 820–831 (2005). [Google Scholar]
- Syros T. D., Yupsanis T. A. & Economou A. S. Expression of peroxidases during seedling growth in Ebenus cretica L. as affected by light and temperature treatments. Plant Growth Regul. 46, 143–151 (2005). [Google Scholar]
- Rogers L. A. et al. Light, the circadian clock, and sugar perception in the control of lignin biosynthesis. J. Exp. Bot. 56, 1651–1663 (2005). [DOI] [PubMed] [Google Scholar]
- Frankenstein G., Schmitt U. & Koch G. Topochemical studies on modified lignin distribution in the xylem of poplar (Populus spp.) after wounding. Ann. Bot. 97, 195–204 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K. et al. Isolation of a novel lodging resistance QTL gene involved in strigolactone signaling and its pyramiding with a QTL gene involved in another mechanism. Mol. Plant 8, 303–314 (2014). [DOI] [PubMed] [Google Scholar]
- Ookawa T. et al. New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nat. Commun. 1, 132–142 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno A. et al. New approach to increasing rice lodging resistance and biomass yield through the use of high gibberellin producing varieties. PLoS One 9, e86870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador V. H. et al. Cinnamic acid Increases lignin production and inhibits soybean root growth. PLoS One 8, e69105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai J. et al. Paclobutrazol increases canola seed yield by enhancing lodging and pod shatter resistance in Brassica napus L. Field Crops Res. 180, 10–20 (2015). [Google Scholar]
- Zhang J. et al. Lodging resistance characteristics of high-yielding rice populations. Field Crops Res. 161, 64–74 (2014). [Google Scholar]
- Xiao Y. G. et al. Lodging resistance and yield potential of winter wheat: effect of planting density and genotype. Front. Agr. Sci. Eng. 2, 168–178 (2015). [Google Scholar]
- Huang J. et al. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 153, 1526–1538 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornale S. et al. Cell wall modifications triggered by the down-regulation of coumarate 3-hydroxylase-1 in maize. Plant Sci. 236, 272–282 (2015). [DOI] [PubMed] [Google Scholar]
- Steven L. et al. Antisense down-regulation of 4CL expression alters lignification, tree growth, and saccharification potential of field-grown poplar. Plant Physiol. 154, 874–886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaclyn J. et al. The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiol. 150, 621–635 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y. et al. Functional analyses of caffeic acid O-methyltransferase and cinnamoyl-CoA-reductase genes from perennial ryegrass (Lolium perenne). Plant Cell 22, 3357–3373 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T. et al. Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc. Natl. Acad. Sci. USA 103, 230–235 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. H. Functional analysis of a cinnamyl alcohol dehydrogenase involved in lignin biosynthesis in wheat. J. Exp. Bot. 61, 2735–2744 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou L. et al. Effects of population density on stalk lodging resistant mechanism and agronomic characteristics of maize. Acta Agron. Sin. 33, 1688–1695 (2007). [Google Scholar]
- Yao M. N. et al. The effects of different planting densities on the cortex structure of stem and lodging resistance in Maize. Xinjiang Agri. Sci. 50, 2006–2014 (2013). [Google Scholar]
- Feng H. J. et al. Effect of plant density on microstructure of stalk vascular bundle of summer Maize (Zea mays L.) and its characteristics of sap flow. Acta Agron. Sin. 40, 1435–1442 (2014). [Google Scholar]
- Kuai J. et al. The effect of nitrogen application and planting density on the radiation use efficiency and the stem lignin metabolism in rapeseed (Brassica napus L.). Field Crops Res. 199, 89–98 (2016). [Google Scholar]
- Khan G. S. & Chaudhry A. K. Effect of spacing and plant density on the growth of poplar (Populus deltoides) trees under agro-forestry system. Pak. J. Agri. Sci. 44, 321–327 (2007). [Google Scholar]
- Naji H. R., Sahri M. H., Nobuchi T. & Bakar E. S. Clonal and planting density effects on some properties of rubber wood (hevea brasiliensis muell. arg.). Bioresource 7, 189–202 (2012). [Google Scholar]
- Li J. C., Yin J. & Wei F. Z. Effects of planting density on characters of culm and culm lodging resistant in winter wheat. Acta Agron. Sin. 31, 662–666 (2005). [Google Scholar]
- Islam M. S. et al. Lodging-related morphological traits of hybrid rice in a tropical irrigated ecosystem. Field Crops Res. 101, 240–248 (2007). [Google Scholar]
- Kong E. Y. et al. Anatomical and chemical characteristics associated with lodging resistance in wheat. Crop J. 1, 43–49 (2013). [Google Scholar]
- Zhao Q. et al. Loss of function of cinnamyl alcohol dehydrogenase 1 leads to unconventional lignin and a temperature-sensitive growth defect in Medicago truncatula. Proc. Natl. Acad. Sci. USA 110, 13660–13665 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornale S. et al. Altered lignin biosynthesis improves cellulosic biorhtanol production in transgenic maize plants down-regulated for cinnamyl alcohol dehydrogenase. Mol. Plant 5, 817–830 (2012). [DOI] [PubMed] [Google Scholar]
- Anderson N. A. et al. Manipulation of guaiacyl and syring monomer biosynthesis in an Arabidopsis cinnamyl alcohol dehydrogenase mutant results in atypical lignin biosynthesis and modified cell wall structure. The plant Cell 27, 2195–2209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle S. E. & Thomson J. L. Sugarcane internode composition during crop development. BioEnergy Res. 5, 168–178 (2012). [Google Scholar]
- Jung H. G. & Casler M. D. Maize stem tissues: cell wall concentration and composition during development. Crop Sci. 46, 1793–1800 (2006). [Google Scholar]
- Riboulet C. et al. Kinetics of phenylpropanoid gene expression in maize growing internodes: relationships with cell wall deposition. Crop Sci. 49, 211–223 (2009). [Google Scholar]
- Ma Q. H. The expression of caffeic acid 3-O-methyltransferase in two wheat genotypes differing in lodging resistance. J. Exp. Bot. 60, 2763–2771 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. H. Functional analysis of a cinnamyl alcohol dehydrogenase involved in lignin biosynthesis in wheat. J. Exp. Bot. 61, 2735–2744 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven L. et al. Antisense down-regulation of 4CL expression alters lignification, tree growth, and saccharification potential of field-grown poplar. Plant Physiol. 154, 874–886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y. et al. Functional analyses of caffeic acid O-methyltransferase and cinnamoyl-CoA-reductase genes from perennial ryegrass (Lolium perenne). Plant Cell 22, 3357–3373 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewalt V. J. H. et al. Reduced lignin content and altered lignin composition in transgenic tobacco down-regulated in expression of L-phenylalanine ammonia-lyase or cinnamate 4-hydroxylase. Plant Physiol. 115, 41–50 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher A. et al. Lignification in sugarcane: biochemical characterization, gene discovery, and expression analysis in two genotypes contrasting for lignin content. Plant Physiol. 163, 1539–1557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Ren X. J. Soil and fertilizer science. (ed. Wang Y.) 21–26 (Henan science and technology press, 2013). [Google Scholar]
- Zadoks J. C., Chang T. T. & Konzak C. F. A decimal code for the growth stages of cereals. Weed Res. 14, 415–421 (1974). [Google Scholar]
- Wei F. Z. et al. Effects of nitrogenous fertilizer application model on culm lodging resistance in winter wheat. Acta Agron. Sin. 34, 1080–1085 (2008). [Google Scholar]
- Chen X. G. et al. Relationship between lignin metabolism and lodging resistance in wheat. Acta Agron. Sin. 37, 1616–1622 (2011). [Google Scholar]
- Yamamura M., Hattori T., Suzuki S., Shibata D. & Umezawa T. Microscale alkaline nitrobenzene oxidation method for high-throughput determination of lignin aromatic components. Plant Biotechnol. 27, 305–310 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.