Abstract
Fungal diseases in insects are common and widespread and can decimate their populations in spectacular epizootics. Virtually all insect orders are susceptible to fungal diseases, including Dipterans. Fungal pathogens such as Lagenidium, Coelomomyces and Culicinomyces are known to affect mosquito populations, and have been studied extensively. There are, however, many other fungi that infect and kill mosquitoes at the larval and/or adult stage. The discovery, in 1977, of the selective mosquito-pathogenic bacterium Bacillus thuringiensis Berliner israelensis (Bti) curtailed widespread interest in the search for other suitable biological control agents. In recent years interest in mosquito-killing fungi is reviving, mainly due to continuous and increasing levels of insecticide resistance and increasing global risk of mosquito-borne diseases. This review presents an update of published data on mosquito-pathogenic fungi and mosquito-pathogen interactions, covering 13 different fungal genera. Notwithstanding the potential of many fungi as mosquito control agents, only a handful have been commercialized and are marketed for use in abatement programs. We argue that entomopathogenic fungi, both new and existing ones with renewed/improved efficacies may contribute to an expansion of the limited arsenal of effective mosquito control tools, and that they may contribute in a significant and sustainable manner to the control of vector-borne diseases such as malaria, dengue and filariasis.
Keywords: Culicidae, insect-pathogenic fungi, Lagenidium, Coelomomyces, Culicinomyces, Hyphomycetes, biocontrol
Introduction
The world's prime choice to curb nuisance biting by mosquitoes or their transmission of parasitic or arboviral disease continues to be the selective application of residual synthetic insecticides. The public health benefit delivered by these, both in tropical resource-poor settings, as well as in temperate zones, cannot be over-emphasized – they save thousands of lives each year. Powered by a strong industrial lobby, new and more environmentally friendly compounds replace older, more harmful, ones. However, beyond gains in economic and public health terms, the stark reality of environmental impact and ever-developing resistance remains an issue of grave concern (Hemingway and Ranson 2000; Brooke et al., 2002; Chandre et al., 1999). It is therefore not surprising that interest in alternative non-chemical strategies has increased over the last decades. The use of biological control agents such as predatory fish (Legner, 1995), bacteria (Becker and Ascher 1998), protozoa (Chapman 1974; Legner 1995), and nematodes (Kaya and Gaugler 1993) have all shown promise as a means to control mosquito populations, and progress in these fields has recently been reviewed.
The available literature on entomopathogenic fungi for mosquito control, however, is rather scattered and void of recent reviews (Roberts 1974; Ferron et al. 1991). The purpose of the present review is to collate and update the available information about the most important entomopathogenic fungi for mosquitoes. Particular focus is on species belonging to the genera Lagenidium, Coelomomyces, Entomophthora, Culicinomyces, Beauveria, and Metarhizium (see Table 1), discussing their potential and drawbacks to be used as biological control agents in reducing mosquito populations. Table 2 contains a comprehensive list of fungi isolated and/or tested on mosquitoes. Except for the anamorphic fungi, we used the taxonomic nomenclature conform the 9th edition of the dictionary of the Fungi (Kirk et al. 2001).
Table 1.
Overview of fungal taxa (Kingdom to genus) discussed in this review (classification and nomenclature partly after Kirk et al., 2001).
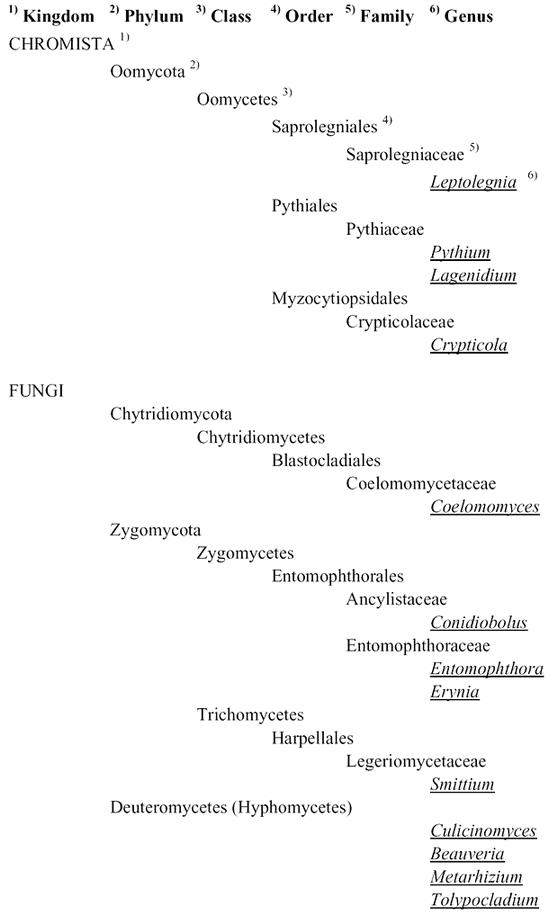
Table 2.
Overview of fungal species found or tested on mosquito species, either in the laboratory* or in the field**.
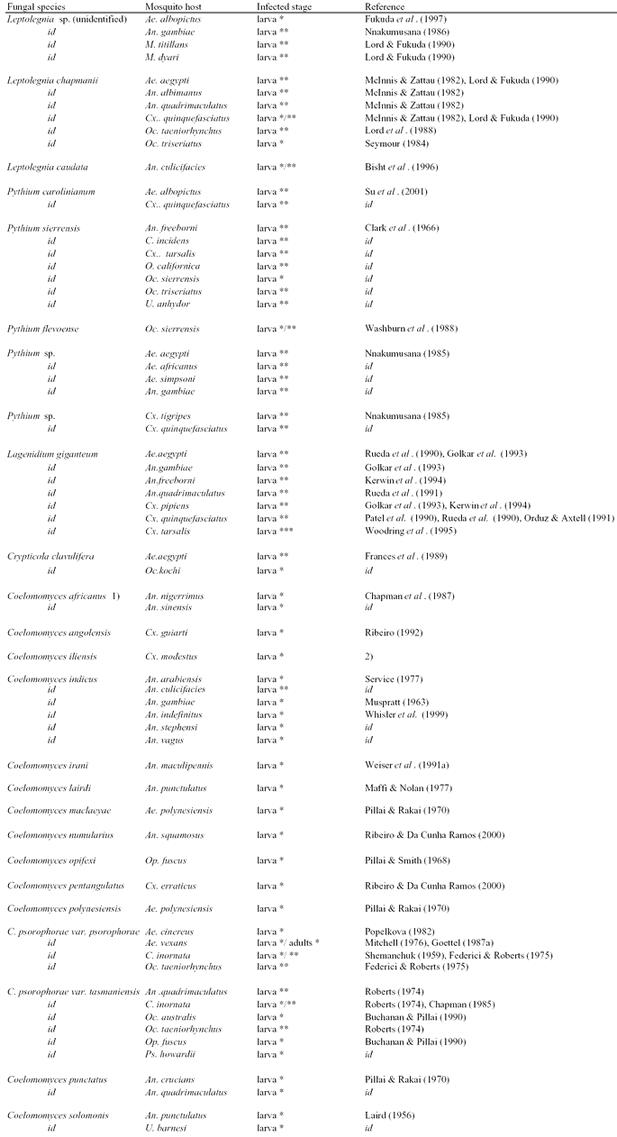
Table 2.
Cont'd
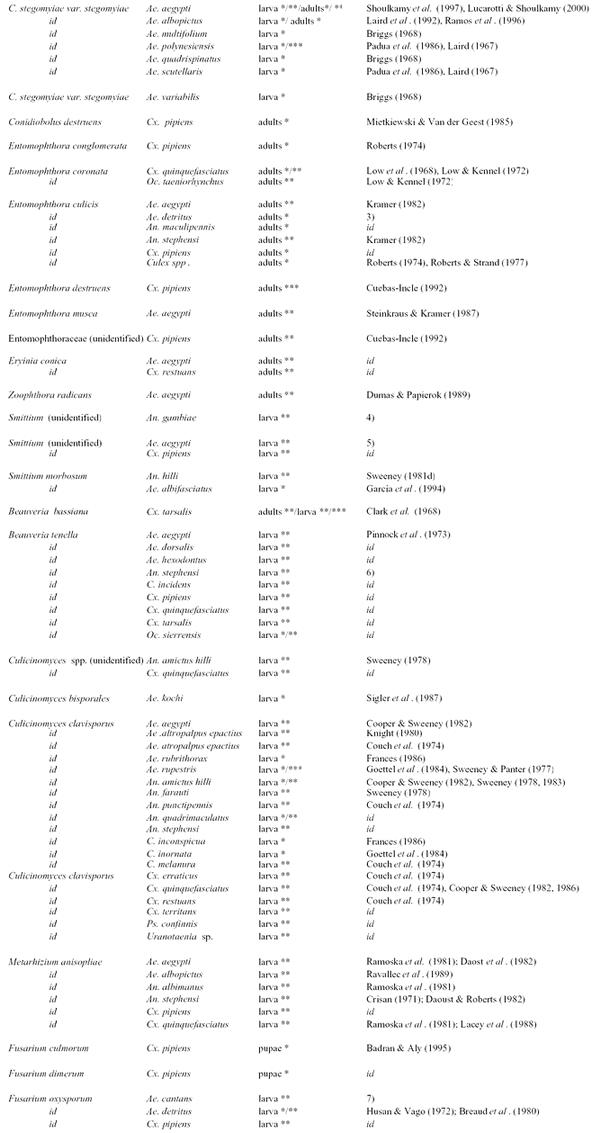
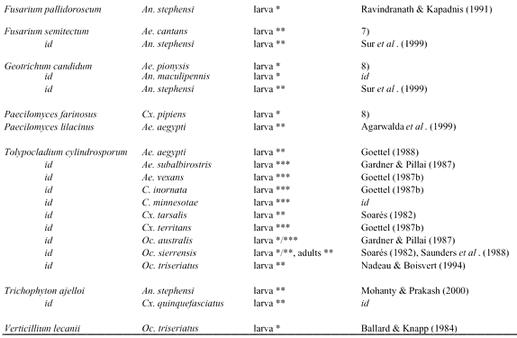
Table 2.
Cont'd
1 Oomycota
The phylogenetic relationship of Oomycetes (watermolds) to fungi has been debated for many years (Kerwin and Peterson 1997). The prevailing view is that Oomycetes belong to the kingdom Chromista, which includes diatoms and brown algae (Sleigh, 1989). Watermolds are aquatic organisms, some of which are facultative parasites of mosquito larvae. Some genera, such as Aphanomyces, surface from time to time in mosquito insectaries and may cause temporal but disruptive epizootics (Seymour and Briggs 1985). Others, like Leptolegnia, Pythium and Cryptiloca, although pathogenic to mosquitoes, have received only limited attention. Lagenidium giganteum Couch is an aquatic species that has been studied extensively and is commercially available as a mosquito control agent.
1.1 Leptolegnia
In the oomycete genus Leptolegnia, only Leptolegnia caudata deBary (Bisht et al. 1996), and Leptolegnia chapmanii R.L. Seymour (McInnis and Zattau 1982) have been isolated from insects. Leptolegnia caudata was isolated from the malaria vector Anopheles culicifacies Giles (Bisht et al. 1996). In laboratory bioassays, a zoospore concentration of 7 × 103 L−1 caused 100% mortality of Anopheles culicifacies larvae after 7 days, and the authors suggested inclusion of this fungus in larval control campaigns to reduce malaria transmission. Leptolegnia chapmanii was first encountered on Ochlerotatus triseriatus (Say) larvae in Louisiana (USA) in 1971 (Seymour 1984). It is a virulent pathogen of first and second instar larvae of Aedes aegypti (L.), which suffer 100% mortality within 24 hrs after exposure. Less than 40% of third and fourth instars were infected after 72 hrs (McInnis and Zattau 1982). The authors reported equal susceptibility of Culex quinquefasciatus Say, Anopheles quadrimaculatus Say and Anopheles albimanus Wiedemann to the fungus. Nnakumusana (1986) found 100% mortality of Anopheles gambiae Giles larvae after 72 hrs. Lord et al. (1988) studied the potential of this fungus against the salt marsh mosquito Ochlerotatus taeniorhynchus (Wiedemann) in Florida, USA. Unfortunately the fungus failed to form zoospores and therefore promised little potential to control mosquito populations in saline environments. McInnis and Schimmel (1985) investigated the host range of Leptolegnia chapmanii by testing it on six different aquatic insect orders, reporting no infections. Leptolegnia spp. are easy to culture in vitro, but tend to loose their larvicidal activity after prolonged culture, albeit that this effect can be reduced by growing the fungus on sterol-rich media (Nnakumusana 1986). For detailed information regarding the Leptolegnia life cycle and infection of mosquito larvae, see Zattau and McInnis (1987) and Seymour (1984).
1.2 Pythium
Most species belonging to the genus Pythium are pathogens of vascular plants, other fungi, and algae (Van der Plaats-Niterink 1981). Some species however, have been found to be mildly, to highly pathogenic to insects. A Pythium sp. caused a high level of mortality in a field collection of the tree hole mosquito Ochlerotatus sierrensis (Ludlow) (Clark et al. 1966). In 1988, Saunders et al. isolated Pythium flevoense Van der Plaats-Niterink from wild populations of Ochlerotatus sierrensis in California, occurring in 42% of the sampled tree holes, although this fungus caused infections in only 14% of larvae during 21 weeks of exposure in laboratory bioassays.
Nnakumusana (1985) mentions that in a laboratory bioassay a non-identified Pythium species proved pathogenic to early instars of Aedes aegypti, Aedes africanus (Theobald), Aedes simpsoni (Theobald), Culex quinquefasciatus, Culex tigripes Grandpré and Charmoy and Anopheles gambiae, reaching mortalities between 50–100%. In other laboratory tests, an unidentified Pythium species selectively killed larvae of Anopheles freeborni Aitken, Ochlerotatus sierrensis, Ochlerotatus triseriatus (Say), Culex tarsalis Coquillet, Culiseta incidens (Thomson), Culiseta inornata (Williston), Orthopodomyia californica Bohart, and Uranotaenia anhydor Dyar that were mechanically punctured with forceps (Clark et al. 1966). The fact that this fungus infected mechanically injured larvae rather than healthy larvae indicates that the fungus is opportunistic rather than strictly entomopathogenic. Even though different mosquito species proved to be susceptible, Clark and colleagues concluded that the conditions under which the infective stage of the fungus could become an important control agent would be hard to achieve, and rather unpractical.
Su et al. (2001) isolated P. carolinianum Matthews from Guizhou province, China, in 1994. In outdoor bioassays the authors found infection levels of 13.3–100% in Culex quinquefasciatus larvae, and mentioned that a population of Aedes albopictus (Skuse) was ‘markedly controlled’, but no infection percentages were given. Notwithstanding the pathogenicity of some Pythium species to mosquitoes, on the whole they are not considered suitable for biocontrol of mosquitoes. For detailed taxonomic information about the genus Pythium, see Van der Plaats-Niterink (1981).
1.3 Lagenidium
Only one species of the genus Lagenidium is known to be a facultative parasite of mosquito larvae, namely Lagenidium giganteum. It consists of two stages: oospores (sexual), and zoospores (asexual) (See Fig. 1). Although this fungus has been named Lagenidium culicidum Umphlett in some publications (Umphlett and Huang 1970; McCray et al. 1973), this was later shown to be Lagenidium giganteum (Couch and Romney 1973).
Figure 1.
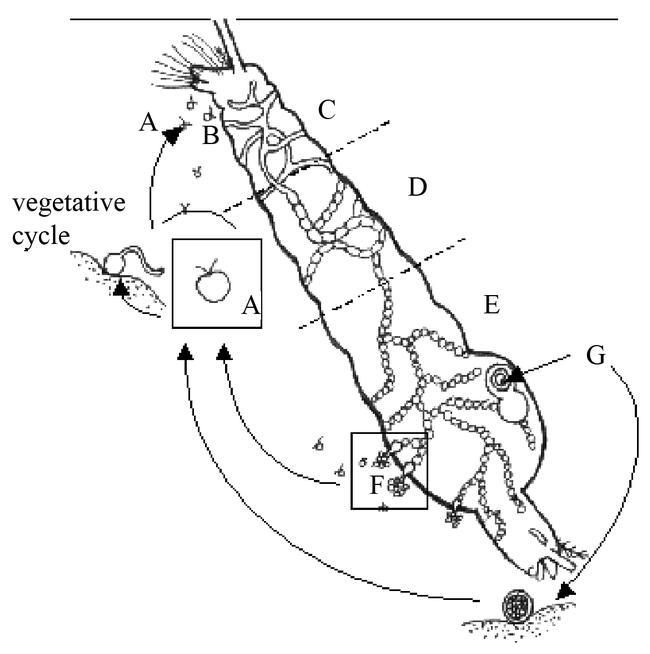
Generalized life cycle for Coelomomyces giganteum. Motile zoospores (A) attach to, and penetrate (B) cuticle of mosquito larva (lateral view). Hyphae grow (C), septate (D), and start forming sporangia (E). Exit vesicles with zoospores (F) (asexual cycle) or oospores (G) (sexual cycle) are formed (drawn by Ernst-Jan Scholte, modified after Fetter-Lasko and Washino (1983)).
Lagenidium giganteum was first described by Couch (1935) from a combined collection of copepods and mosquito larvae (Culex and Anopheles) in North Carolina, USA. The geographical distribution is wide: North America, Europe, Africa, Asia, and even Antarctica (Federici, 1981). The fungus has caused high mortalities in mosquito populations in many laboratories, small- and large-scale field studies (California and North Carolina), especially in Culex (Merriam and Axtell, 1982; Jaronski and Axtell, 1983), Mansonia (Florida) (Cuda et al. 1997) and Anopheles species (Kerwin and Washino, 1987). Laboratory tests by McCray et al. (1973), showed that the fungus could successfully infect and kill larvae of Aedes aegypti, Ochlerotatus triseriatus, Aedes mediovittatus (Coquillett), Ochlerotatus taeniorhynchus Wiedemann, Ochlerotatus sollicitans (Walker), Ochlerotatus taeniorhynchus Theobald, Culex quinquefasciatus and Culex restuans Theobald (Umphlett and Huang, 1970). Anophelines were not found to be susceptible. Also, the fungus is not effective for mosquitoes in brackish or organically rich aquatic habitats (Merriam and Axtell, 1982). The fungus was also ineffective against Ochlerotatus taeniorhynchus Dyar and Knab, Ochlerotatus tormentor Dyar and Knab, Anopheles crucians Wiedemann, Culex peccator Dyar and Knab, Psorophora howardii Coquillett, Uranotaenia sapphirina (Osten Sacken) (Glenn and Chapman 1978), and Aedes albopictus (Becnel et al. 1996). Suh and Axtell (1999) found maximum virulence of Lagenidium giganteum against Culex quinquefasciatus at concentrations of >150 zoospores ml−1 of water, at water temperatures between 20 and 30° C Survival of zoospores as indicated by mosquito larval mortality was greatest at 25° C and was similar at 30, 33, and 35° C. No infection occurred at 17° C and less than 20% larval mortality occurred at 19° C for any age of zoospores. Golkar et al. (1993) studied this variation in susceptibility between different culicines and anophelines in terms of encysting zoospores and host defense reactions. For Anopheles gambiae it was found that, even though a larger number of zoospores attached to its cuticle than would normally be expected in nature, its efficient fast and intense defense (melanization) reaction successfully protected 56% of exposed specimens from death. This immune response was much faster than that observed for Aedes aegypti and Culex pipiens L. Although a very small number of zoospores attach to and penetrate the cuticle of Aedes aegypti and Culex pipiens (compared to the number attached to Anopheles gambiae), approximately 99% of both species succumb to fungal infection. McCray et al. (1973) found 100% mortality of several Aedes and Culex larvae, including Culex quinquefasciatus. Other studies show that 100% mortality occurs when the larvae are very young. Orduz and Axtell (1991) reported high virulence for 1–2 day old larvae, intermediate mortality in 3-day-old larvae and low mortality in 4–5 day old larvae. Kerwin and Washino (1987) supported this finding and suggested that Lagenidium giganteum zoospores might not recognize late instars of otherwise susceptible mosquito species.
As a facultative parasite, Lagenidium giganteum can grow vegetatively both as pathogen on mosquito larvae, or as a saprophyte in aquatic environments (Federici 1981; Service 1983; Legner 1995; Sur et al. 2001). Maintenance of the fungus in vivo, though labor-intensive, is possible (Kerwin and Petersen 1997), but in vitro culture using both defined and complex media (Kerwin et al. 1986; Guzman and Axtell 1986; Sur et al. 2001) is more commonly practiced (Kerwin and Petersen 1997). For small-scale culture of Lagenidium giganteum, solid media are used. For large-scale (10–1000 L) production, liquid cultures of yeast extract-based media (Kerwin and Petersen 1997) are utilized.
Fungal reproduction is both asexual (zoospores) and sexual (oospores) (Federici 1981). In order to infect mosquito larvae, zoospores must be formed. These biflagellate, motile zoospores are the asexual stage of the fungus. They do not have a cell wall, and are therefore too fragile to be used directly for mosquito control (Kerwin et al. 1994). A further disadvantage of the asexual stage is its short shelf life; zoospores survive for only 48 hrs after emerging from an infected larva. Further problems include the need to keep the mycelium completely hydrated, its susceptibility to be overwhelmed by contaminating microorganisms following formulation, lack of stability under extreme temperatures, and special handling required to keep the formulated product from becoming anaerobic (Su et al. 1986; Kerwin and Washino 1986; Kerwin et al. 1994). Several methods have been tried to overcome these issues, for instance by encapsulating the fungus in several types of calcium alginate beads (Rueda et al. 1990, 1991; Patel et al. 1990). This proved to increase retention of infectivity against mosquito larvae and more convenience in storing, handling and application (Rueda et al. 1990). The level of control of anophelines, for instance, was found to increase when 1% ground cork was added to the capsules (Rueda et al. 1991).
Oospores, the sexual stage of Lagenidium giganteum, can also be used as inoculum. They are dormant propagules, resistant to desiccation and mechanical abrasion and stable for at least seven years, which allows multivoltine persistence of the fungus in some habitats (Kerwin and Washino 1986; Kerwin et al. 1994). Unfortunately, mass production yields of oospores remain orders of magnitude below that of the less stable mycelial (asexual, presporangial) stage, and continued problems with spore activation have prevented large-scale field tests (Merriam and Axtell 1982; Kerwin and Washino 1987; Legner 1995, Kerwin and Petersen 1997). Research is continuing to improve oospore yields, which would be much more useful than zoospores in large-scale operational mosquito control programs.
Zoospores of the fungus appear harmless to most aquatic invertebrates (one or more species in the animal groups of Polychaeta, Ostracoda, Copepoda, Cladocera, Diptera, Coleoptera, Hemiptera, and Odonata) (Nestrud and Anderson 1994), and to vertebrates (mallard ducks, mice, rats, and rabbits) (Kerwin et al. 1990). Only Daphnia spp. and copepods reported by Couch (1935), three cladoceran species and a chironomid species reported by Nestrud and Anderson (1994) were found to be susceptible.
In spite of these non-target effects, a Lagenidium giganteum-based product was commercialized under the name Laginex by AgraQuest (California, USA) until 1999. It was claimed to be particularly effective against Culex spp., but the kind of spore used was not mentioned (Khetan 2001). The fungus is compatible with the bacterial agents Bti and Bacillus sphaericus Meyer and Neide when used against Culex quinquefasciatus (Orduz and Axtell 1991), with the fungus having the distinct advantage over Bti in that it is able to recycle in stagnant water, infecting multiple and overlapping generations of mosquitoes (Legner 1995). In field trials in which Laginex 25 was compared with Vectobac-12AS (Bti), Laginex reduced Culex quinquefasciatus larvae by 100% for 22 days whereas Vectobac-12AS required retreatment by the 10th day (Hallmon et al. 2000). Results from a small scale field trial in North Carolina indicated that Lagenidium giganteum recycled for an entire season despite periodic scarcity of hosts and short-term drought, with infections ranging from 0–100% (Jaronski and Axtell, 1983). A large-scale field trial in Californian rice fields, using mycelium from either 20 or 30 liters of fermentation beer per hectare resulted in 40%–90% infection of Culex tarsalis and Anopheles freeborni sentinel larvae (Kerwin and Washino, 1987). For details about culture media, see Kerwin and Petersen (1997) and for detailed information about the life cycle of Lagenidium giganteum, see Kerwin et al. (1994), Kerwin and Peterson (1997), or Woodring et al. (1995).
1.4 Crypticola
Crypticola clavulifera Humber, Frances and Sweeney has been isolated from the midge Forcipomyia marksae Tokunaga (Ceratopogonidae) in Queensland, Australia, in 1984 (Frances et al. 1989). Its biology is similar to that of Lagenidium giganteum. In the laboratory the fungus successfully infected Aedes notoscriptus (Skuse), Anopheles farauti Laveran, Culex annulirostris Skuse, Culex quinquefasciatus (Frances, 1991), and Aedes aegypti (Frances et al. 1989, Frances 1991). Aedes kochi (Dönitz) was not susceptible (Frances 1991). Despite its pathogenicity to several mosquito species no further studies have been published on this fungus.
2 Chytridiomycota
Members of this phylum are aquatic saprobes or parasites growing on decaying and living organic material, both in freshwater and soils (Kirk et al. 2001). They have flagellate zoospores, and chitinous hyphae. The phylum consists of 5 orders, of which the Blastocladiales contains the only mosquito-pathogenic genus of this group: Coelomomyces.
2.1 Coelomomyces
The genus Coelomomyces consists of more than seventy species of obligatory parasitic aquatic fungi that undergo a complex life cycle involving alternating sexual (gametophytic) and asexual (sporophytic) generations (Couch and Bland 1985) (See Fig. 2). The genus has been found in all continents except Antarctica (Roberts 1974). Unlike most other entomogenous fungi, which have rather wide host ranges, involving species in several orders of insects, Coelomomyces is almost entirely restricted to aquatic Dipteran insects, including Culicidae, Psychodidae, Chironomidae, Simuliidae and Tabanidae (Arêa Leão and Carlota Pedroso 1964; Chapman 1974; Roberts 1970). Susceptibility studies indicate that most Coelomomyces spp. are probably not host-specific, but nevertheless have relatively restricted host ranges (Federici 1981).
Figure 2.
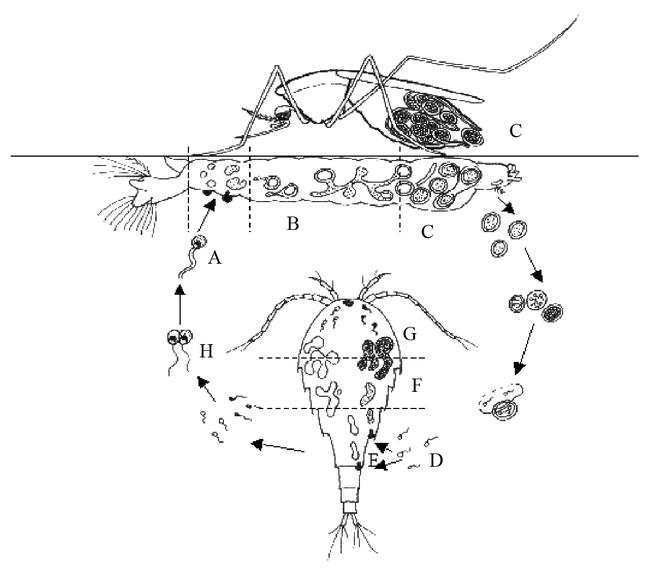
Generalized life cycle for species of Coelomomyces. (A) Biflagellate zygote infects hemocoel of mosquito larvae (lateral view) producing hyphagens, which later form hyphae. (B,C) Hyphae grow in the hemocoel and form resting sporangia. Occasionally, if infected (female) larvae survive to the adult stage, resting spores are formed and dispersed by ‘oviposition’. Ultimately, meiospores (D) are released, which infect a copepod and produce gametophytes (E), each one of each will form gametangia (F) that release gametes of a single-mating type (G). Gametes of opposite mating type fuse inside or outside (H) copepod, forming a biflagellate zygote that completes the cycle by infecting another mosquito larva (drawn by Ernst-Jan Scholte, modified after Federici (1981)).
Because of the high pathogenicity of many Coelomomyces species to mosquito larvae, the fungal genus has interested many researchers. Publications on Coelomomyces and the Oomycete Lagenidium giganteum together compromise the majority of studies published on entompathogenic aquatic fungi that affect mosquito larvae. Chapman and Woodard (1966) listed all hosts for Coelomomyces found in the USA. They list seventeen species belonging to six mosquito genera (Culex, Culiseta, Aedes, Anopheles, Psorophora, and Uranotaenia), with fungi being most common in the genus Anopheles followed by Aedes and Culex (Chapman 1974). Roberts and Strand (1977) listed 22 Coelomomyces species found on 31 mosquito species and Weiser (1988) listed up to 28 Coelomomyces species found on over 60 mosquito species. The basic life cycle has been determined for 11 of the 60 species and subspecies of Coelomomyces recognized by Couch and Bland (1985). Of those 60 species, Coelomomyces indicus Iyengar, a strain with special features and wide distribution has been given much attention. This species has been reported from Africa, the Philippines, India, and Pakistan. The Dipteran hosts include 16 species of anopheline mosquitoes, including several important vectors of malaria, such as Anopheles gambiae (Muspratt 1946). It is known to persist and cause periodic epizootics in rice fields in Southeast Asia (Whisler et al. 1999), Egypt (Gad and Sadek 1968) and Kenya (Service 1977).
The life cycle of Coelomomyces is complex and includes obligatory development in an intermediate microcrustacean host (cyclopoid copepods, harpacticoid copepods, or ostracods) and two mosquito generations for completion (Whisler et al. 1974, 1999; Lucarotti and Andreadis 1995). The fungus survives unfavorable environmental conditions, such as cold or dry periods, as resting sporangia (RS) (Buchanan and Pillai 1990) that develop from diploid hyphae in infected mosquito larvae. Normally, the larvae die in the 4th stage and RS are released as the cadavers decompose. Meiosis occurs in the RS and the posteriorly uniflagellate meiozoospores that emerge from the RS infect the appropriate microscrustacean host and establish the haploid, gametophytic stage, which develops in the hemocoel (Padua et al. 1986; Federici 1995; Lucarotti and Andreadis 1995). At maturation, the gametophyte cleaves forming thousands of uniflagellate gametes. Cleavage results in death of the copepod and escape of the gametes, which fuse forming biflagellate zygotes (zygospores) that seek out to infect another mosquito larva (Lucarotti and Andreadis 1995). The zygotes encyst preferentially on the intersegmental membranes of young and/or recently molted (Lacey and Undeen 1986) mosquito larvae. They enter epithelial cells just below the larval cuticle by means of a penetration tube. From there, the fungus enters the hemocoel where it uses the reserves of the larval fat body to develop into irregularly shaped hyphae without cell walls (Roberts 1974). Sporangia are produced within the hyphae at their tips. These sporangia usually become so numerous that they virtually fill the hemocoel (Roberts 1974). In most cases the larva then dies and forms resting spores (to complete the life cycle). Occasionally, however, the infected larva pupates and ecloses to produce infected adults, an event that Mitchell (1976) credited to be described first by Zharov in 1973 for adult Aedes vexans (Meigen) infected with Coelomomyces psorophorae Couch. The same phenomenon was later described for Coelomomyces stegomyiae var. stegomyiae Keilin infecting adult Aedes albopictus (Laird et al. 1992). Later studies by Lucarotti (1992), Shoulkamy et al. (1997), and Lucarotti and Shoulkamy (2000), describe this process in more detail for adult Aedes aegypti infected with Coelomomyces stegomyiae. In adult female Aedes aegypti, the infection is mostly localized in the ovaries (Lucarotti 1992). During the first 72 hrs following eclosion, as the ovaries enlarge under the influence of juvenile hormone, the hyphae in the hemocoel are transferred to the interstitial spaces of the ovaries. The hyphae in the ovaries will mature to RS in response to changes in host ecdysone levels following a blood meal (Lucarotti 1992). Even though Coelomomyces-infected females will mate, no eggs will develop in the ovaries. Females do, however, attempt to oviposit, but instead of eggs, resting spores are laid (Lucarotti 1987, 1992). Infected adult female mosquitoes therefore play a role in the transmission of the fungus to new habitats, which is especially useful for those species of Coelomomyces that infect mosquitoes occupying small habitats such as tree holes or water containers (Laird et al. 1992; Lucarotti and Andreadis 1995; Shoulkamy et al. 1997).
In contrast to other types of pathogens reported from mosquitoes, many species of Coelomomyces are known to cause significant epizootics, which can persist in larval populations over several years and result in prevalence and mortality rates exceeding 50% and often ranging higher than 90% (Apperson et al. 1992). For an extensive overview of the genus Coelomomyces and its hosts, its epizootics in mosquito populations and control programs, see Couch and Bland (1985). For details on the infection process, see Shoulkamy and Lucarotti (1998), and Couch and Bland (1985).
In a review of data obtained from mosquito populations in Louisiana, Chapman (1985) noted infection rates of 96% for Coelomomyces psorophorae in Psorophora howardii Coquillett and Culiseta inornata (Williston), 97% for Coelomomyces macleayae Laird in Aedes triseriatus, 92 % of Coelomomyces pentagulatus in Culex erraticus (Dyar and Knab), and 100% for Coelomomyces punctatus Couch in Anopheles crucians. Legner (1995) mentions studies in which infection levels exceeding 95% were reported from Culiseta inornata and P. howardii by Coelomomyces psorophorae and in Ochlerotatus triseriatus by Coelomomyces macleayae and 85 percent in Culex peccator Dyar and Knab by Coelomomyces pentangulatus. Infection levels as high as 100% have been reported.
Apperson et al. (1992) cite studies by Deshevykh (1973), who found Coelomomyces iliensis Dubitskij for a two-year period in Culex modestus Ficalbi in Kazachstan, and Muspratt (1963), who reported 100% Coelomomyces infection in some Zambian Anopheles gambiae populations. However, even though many epizootics have been reported, field incidence of mycoses caused by Coelomomyces spp. in mosquito larvae is low (Lacey and Undeen 1986). Infection rates of 24–48% were reported in Anopheles crucians and Anopheles quadrimaculatus in the southeastern USA (Umphlett 1970). The latter infection level was maintained for three years, showing that the fungus did indeed persist, although the infection levels decreased over time to a mere 10% (Umphlett 1969). This decline in mosquito infection rates over a period of several years may be due to reduced copepod populations resulting from parasitisation by Coelomomyces (Apperson et al. 1992). In the southern USA, Umphlett (1970) and Chapman and Glenn (1972) reported the persistence of Coelomomyces punctatus for at least 4 years in populations of anopheline larvae, with infection rates ranging from 12–67%. In 1976, Coelomomyces was introduced on a small Pacific island to control Aedes polynesiensis Marks, a vector of filariasis (Laird 1967). This trial marks one of the few attempts to establish a mosquito pathogen in an area where it did not previously exist. The introduction was successful and the fungus remained active in the new locality for at least seven years. Other successful field trials thus far have been conducted with C. iliensis in the former Soviet Union. High levels of mortality in mosquito larvae over a broad geographical area were reported after inoculation of habitats with infective material (Lacey and Undeen 1986). However, results from such field trials are often less clear-cut, due to large variations in infection levels (Chapman 1974; Federici 1981; Service 1983). For example, following treatment of a rice field in Egypt with sporangia (prior to drying at the end of the growing season) from Anopheles pharoensis Theobald, infection levels in larvae collected the following season varied between 0–94% (Roberts 1974). Couch (1972) introduced sporangia of Coelomomyces punctatus into ditches of North Carolina (USA) along with eggs of Anopheles quadrimaculatus. Infection rates in larvae collected 10–15 days later varied from 0–100% (mean 60%). Additionally, Federici (1981) mentions that Dzerzhinskij et al. (1975) obtained similar fluctuating infection levels with Coelomomyces iliensis against Culex modestus in the former Soviet Union.
These unpredictable infection rates, together with its complicated life cycle make this fungus unsuitable for the control of mosquito populations, according to Service (1983). In contrast, Federici (1981) and Lacey and Undeen (1986), after reviewing the potential of various Coelomomyces species for mosquito control, conclude that the fungus does offer potential. They lay emphasis on the relative specific host range (mainly mosquitoes) and the devastating effects of natural epizootics on larval populations. Kerwin and Petersen (1997) add to this the development of resistance by mosquitoes to available insecticides, and the subsequent need to improve knowledge of alternative methodologies, such as the use of Coelomomyces, to control mosquito populations. The most important reason for the fungus not being used very often for biological control of mosquitoes is the difficulty of mass-production, because its complex life cycle includes micro-crustaceans. Even though some progress has been made in culturing mycelia on synthetic media (Castillo and Roberts 1980; Shapiro and Roberts 1976), no species of Coelomomyces has been successfully cultured in vitro to date. Further application of Coelomomyces as a direct mosquito control agent is dependent on the development of easily cultured inoculum (Legner 1995).
3 Zygomycota
The phylum Zygomycota exists of the two Classes, the Trichomycetes and Zygomycetes. Zygomycetes are characterized by the presence of a coenocytic mycelium, by the absence of flagellate spores, and by sexual reproduction through the formation of zygospores. The most important entomopathogens in the Zygomycota belong to the order Entomophthorales (Humber 1997). There are presently about 200 known species classified in six genera of entomopathogenic fungi in this order. All but one genus (Massospora) are characterized by the production of forcibly discharged spores (Khachatourians 1991) (See Fig. 3). Entomophthorales have been reported from many regions, including Africa, but the majority of the literature is on species from the northern hemisphere. Many epizootics have been reported, most of them in rather cool (1–20° C), moist (44–100% RH) biotopes in middle and northern Europe, the former Soviet Union and North America. They are common, obligate pathogens of a wide range of insects, typically infecting adults (except for Entomopthora aquatica J.F. Anderson and Anagnostakis and Entomopthora conglomerata Sorokin (Keller) that have been found on aquatic stages of insects), and unable to grow saprophytically (Bidochka et al. 2000; Eilenberg 2000). Many species have restricted host ranges and some predominantly infect mosquitoes (Humber 1997).
Figure 3.
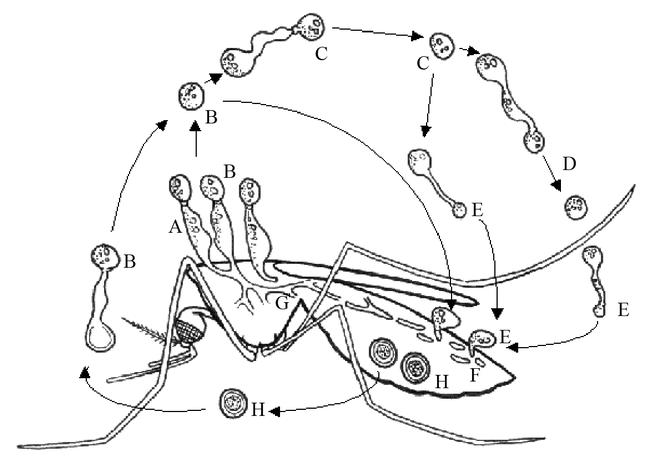
Generalized life cycle of an entomophthorous fungus. Conidiophores (A) on host (in this example a mosquito) forms primary conidium (B), which may form secondary conidium (C), which in turn may form tertiary conidium (D) in absence of a suitable host. Capilliconidia, formed from other conidia, may be formed, infecting a new host. Hyphal bodies (F) are formed and develop into mycelium and stroma (G), which produce resting spores (H) or conidiophores (A). Drawn by Ernst-Jan Scholte, modified after Tanada & Kaya (1993).
The life cycle of an Entomophthorales starts with a spore that infects a host by penetration of the integument. This takes place some 12 hrs after initial contact (Low and Kennel 1972). The fungus develops vegetatively in the host hemocoel by producing small, readily circulated, rod-like hyphal bodies and, depending on the temperature, completes the infection-cycle normally within a few days (Humber 1997). After completing the infection, just before the host dies, the insects are sometimes fixed to a substrate (e.g. leaves) by special hyphae (rhyzoids). After death of the host, the fungus produces sporangiophores, which actively discharge new, primary, spores. These primary spores are short-lived. Roberts (1974) reports that infected adults were no longer useful as inoculum eight days after the first spores were produced. Primary spores that do not contact a suitable host upon discharge have the ability to produce secondary spores, which may also be forcibly dispersed (Humber 1997; Eilenberg 2000). Although spores of Entomophthorales are far bigger than conidia of Hyphomycetes, aerial distribution is not uncommon (Hajek and St. Leger 1994). To survive the winter, they develop thick-walled spores, which are usually referred to as resting spores, although in strict mycological terms they are zygospores (Humber 1997). These resting spores form new primary spores in the spring.
In reviews on Entomophthorales, their potential for biocontrol is often stressed because of high levels of infection observed in nature, and the theory that they can remain active in a site for several years (Hajek and St. Leger 1994; Eilenberg 2000). Release-experiments with Entomopthora maimaiga in Michigan showed that the fungus is able to establish itself easily in new territory and cause epizootics in target species (Smitley et al. 1995). Unfortunately, no commercial formulations are yet available. For more detailed information about the biology, infection and host ranges of the order Entomophthorales, see Eilenberg (2002).
Trichomycetes are fungi that live hidden within the digestive tract of many arthropod species within several orders (including larvae of Diptera, Ephemeroptera and Plecoptera, and adults of some isopods, cladocerans, amphipods, copepods, Collembola, Coleoptera, and diplopods (Lichtwardt et al. 1999). They may be seen as branched or unbranched fungal bodies (thalli) firmly attached to the gut lining and lying within the gut lumen from which they obtain their nutrients (Lichtwardt 1986). The class contains four orders, only one of which, Harpellales, contains a genus, Smittium, with mosquito-pathogenic species. For detailed information on the biology of Trichomycetes, see Lichtwardt (1986).
3.1 Conidiobolus
Lowe et al. (1968) reported Conidiobolus coronatus (Constantin) Batko (formerly Entomohphtora coronata) in Culex quinquefasciatus from a colony of adult mosquitoes maintained in large, outdoor screened cages. The fungus has been found in several orders of insects and in two other classes of invertebrates, and is considered to have the widest host range among the Entomophthorales, but it is a rather weak pathogen (Humber 1997). Adult Ochlerotatus taeniorhynchus and Culex quinquefasciatus treated with Conidiobolus coronatus suffered increased mortality at 7 days post-treatment (Lowe and Kennel 1972). This increase was greater in Ochlerotatus taeniorhynchus than in Culex quinquefasciatus. However, besides infecting insects, this species has been reported as causing facial infection in man (Bras et al. 1965) and horses (Emmons and Bridges 1961), therefore excluding its use as a biocontrol agent. Aside from Conidiobolus coronatus, no infections of vertebrates have been reported for insect-parasitizing Entomophthorales.
3.2 Entomophthora
As in other Entomophthoraleans, Entomophthora infections primarily occur in adult rather than in larval mosquitoes. Entomophthora culicis (Braun) Fresenius was discovered and described from adult Culex pipiens in Germany in 1855 (Braun 1855). Kramer (1982) lists a number of mosquito species on which the fungus occurs in nature, including other Culex species, Ochlerotatus detritus (Haliday), Anopheles maculipennis Meigen, Myzomyia hispaniola Theobald (synonym for Anopheles cinereus Theobald), and an unspecified Asian Aedes species. In a laboratory study 80% infection of adult Aedes aegypti, and 100% of Anopheles stephensi Liston, was achieved from diseased adult midges, Chironomus decorus Johannsen (Kramer 1982). In a study published a year later by the same author, transmission of Entomophthora culicis from the donor Cricotopus similis Goetghebuer (a Chironomid) to the mosquitoes Anopheles stephensi and Culex pipiens was possible, but infection rates differed. 100% of Anopheles stephensi succumbed to infection compared to only 20% of Culex pipiens (Kramer 1983). Mortalities of over wintering mosquitoes infected with Entomophthora spp. (e.g. Entomophthora destruens Weiser and Batko and E. culicis) in damp, relatively cold biotopes such as caves or basements are frequently very high (Chapman 1974; Roberts 1974; Weiser 1988). Mortalities of 85–100% in former Czechoslovakia and 80–90% in the Netherlands have been reported (Teernstra-Eeken and Engel 1967; Mietkiewski and Van der Geest 1985). Roberts (1974) describes a study by Goldberg (1970), where Culex pipiens was infected with an Entomophthora species. Field-collected infected adults were used as inoculum, resulting in 0% infection in 1–3rd instar larvae, 25% in 4th instars, 63–88% in pupae, 33–67% in male adults, and 65–100% in female adults. Roberts (1974) then summarizes several reports of Entomophthora conglomerata, Entomophthora destruens, or unclassified Entomophthora sp. infecting Culex pipiens in nature (yielding infection rates of 49%, 100% and 97%, respectively) suggesting that the fungal isolates involved may be rather species-specific. Entomophthora muscae (Cohn) Fresenius, a well-known Entomophthora species that causes epizootics among pest flies, proved to be able to infect only 3% of exposed Aedes aegypti (Steinkraus and Kramer 1987). Further, Entomophthora conica (Nowakowski) Remaudiére and Hennebert, Entomophthora culicis, Entomophthora destruens, Entomophthora gracilis (Thaxter) Remaudiére and Hennebert, Entomophthora henrici (Molliard) Humber and Ben-Ze'ev, Entomophthora papilliata, Entomophthora radicans (Brefeld) Batko, Entomophthora rhizospora (Thaxter) Remaudiére and Hennebert, Entomophthora schroeteri, Entomophthora thaxteriana Petch, and Entomophthora variabilis (Thaxter) Remaudiére and Hennebert, have also been found to infect mosquitoes belonging to the genera Aedes, Culex, Anopheles, and Culiseta (Anderson and Ringo 1969; Roberts 1974; Eilenberg 2000).
There are probably localities where Entomophthora spp. could be profitably introduced for control of hibernating mosquito populations, but there are no reports of such trials to date. A drawback of using Entomophthora for mosquito control would be that spores are unable to withstand humidities below 75% RH (Wildin, 1973). An option could be to use resting spores, which remain viable for long periods, but prolonged dormancy and asynchronous germination have proven to be obstacles for practical application (Hajek and St. Leger 1994). To use Entomophthora for mosquito control, effective in vitro growth systems need to be developed, because most species are, as yet, unable to grow under mass-production fermentation conditions (Roberts 1974; Papierok and Hajek 1997; Eilenberg 2000). Entomophthora destruens has been cultured on several media, but this fungal material was not infective. However, Entomophthora culicis can be isolated and grown on several media (Kramer 1982; Papierok and Hajek 1997), but to produce quantities needed for large-scale bioassays and field tests, improvements in culturing methods must be made (Eilenberg 2000).
3.3 Erynia
Erynia aquatica (J.F. Anderson and Anagnostakis) Humber is one of the few species within the Entomophthorales that infects aquatic stages of invertebrates. Up to 1981 the species belonged to the genus Entomophthora before Humber relocated the species to the genus Erynia. Anderson and Ringo found the fungus in 1968 on larvae of Ochlerotatus canadensis (Theobald), Culisita morsitans (Theobald) and Ochlerotatus cantator (Coquillett) (Andreadis and Magnarelli 1983) as well as on pupae of Ochlerotatus stimulans (Walker) (Molloy and Wraight 1982). Erynia aquatica is also found on adult mosquitoes, where levels of infection, particularly in over wintering populations of adults, frequently approach 100%. Epizootics have been reported over periods of several years. Erynia aquatica-infected adult Culex pipiens were however capable of flight, taking blood meals, and ovipositing in a study by Anderson and Ringo (1969). The same authors managed to culture Erynia aquatica on an artificial medium, although the fungus grew ‘abnormally’. Erynia conica (Nowakowski) Remaudiére and Hennebert is normally found infecting adults of numerous blackfly (Simulium) species (Nadeau et al. 1996), but it was also able to infect Aedes aegypti in a laboratory study (Cuebas-Incle 1992), killing up to 24% of the adult mosquitoes. Two strains of Erynia radicans (Brefeld) Batko (formerly Zoophthora radicans) proved to be pathogenic to adult Aedes aegypti in a laboratory study (Dumas and Papierok 1989), infecting 100% at the onset of the study, although one strain lost its pathogenicity after several months.
3.4 Smittium
Poisson established the genus Smittium in 1936. Nineteen species have been described of which three originate from Culicid larvae: Smittium culisetae Lichtwardt, Smittium culicis Manier and Smittium morbosum Sweeney. The first two species are not generally thought to be detrimental to their hosts as they are confined to the lumen of the gut and are shed with the cuticle during ecdysis (Lichtwardt and Arenas 1996). There are even indications that mosquitoes, infected with Smittium culisetae or Smittium culicis, may be supplied with certain essential nutrients synthesized by the fungus and consequently have a selective advantage over non-infested individuals (Horn and Lichtwardt 1981).
Smittium morbosum is not shed with gut cuticle at the time of molting and remains within the host, sometimes persisting through the pupal and adult stages (Sweeney 1981d). In the laboratory, infected Anopheles larvae often die because of blockage of the gut by this fungus (Sweeney 1981d). In the same article Sweeney cites two laboratory studies on Smittium spp. in which high mortalities were recorded among Anopheles gambiae (Coluzzi 1966), Aedes aegypti and Culex pipiens molestus (Forskål) (Dubitskij 1978) apparently caused by blockage of the rectum. García et al. (1994) found Smittium morbosum infecting three Aedes, one Anopheles, four Culex, a Mansonia, Psorophora, and one Uranotaenia species in Argentina. Despite the records in which Smittium morbusum caused high larval mortalities in the laboratory, they have never been found causing moderate or high mortalities among mosquitoes in the field. For more detailed information about Smittium spp. and their hosts, see Lichtwardt (1986).
4 Deuteromyces
Within the Class Deuteromycetes a morphological group of fungi known as Hyphomycetes exists. These are filamentous fungi that reproduce by conidia generally formed aerially on conidiophores arising from the substrate. Many genera of entomopathogenic fungi occur in this group of fungi. They have some of the widest of host ranges among entomopathogens, including several mosquito species. The most common route of host invasion is through the external integument, although infection through the digestive tract is possible (Goettel and Inglis 1997). Conidia attach to the cuticle, germinate, and penetrate the cuticle. Once in the hemocoel, the mycelium grows throughout the host, forming hyphal bodies called blastospores. Death of the insect is often due to a combination of the action of fungal toxins, physical obstruction of blood circulation, nutrient depletion and/or invasion of organs. After the host has died, hyphae usually emerge from the cadaver and, under suitable abiotical conditions, conidia are produced on the exterior of the host. These are then dispersed by wind or water (Goettel and Inglis 1997).
4.1 Culicinomyces
In 1972, two separate isolations of mosquito-pathogenic fungi were obtained from laboratory-reared anophelines, one from Anopheles hilli Woodhill and Lee in Sydney, Australia (Sweeney et al. 1973), and the other from Anopheles quadrimaculatus in North Carolina, USA (Couch et al. 1974). The Australian fungus was not identified at the time of its discovery but a new taxonomic status was established for the American fungus, which was described as Culicinomyces clavosporus Couch, Romney and B. Rao. Both fungi produced similar symptoms in diseased mosquito larvae, but it was not until 1982 when, on the basis of morphological comparison, they were considered strains of the same species (Sweeney et al. 1982). In the same year the species name changed from clavosporus to clavisporus Couch, Romney and B. Rao. A third isolate was found in Canada on larvae of Culiseta inornata occurring in a permanent pond (Goettel et al. 1984) and two years later, in 1984, yet another Culicinomyces fungus was found parasitizing larvae of Aedes kochi in Queensland, Australia. Based on several characteristics described by Sigler et al. (1987), it was decided to describe the latter fungus as a new species, namely Culicinomyces bisporalis Sigler, Frances and Panter. Most research to date has focused on Culicinomyces clavisporus.
Like in all Deuteromycota, the life cycle of Culicinomyces is asexual. It usually begins with the ingestion of conidia that adhere to and penetrate through the chitinous wall in the fore- or hindgut (Sweeney 1981a, Federici 1981). This invasion is unusual among parasitic fungi that normally invade insects by penetrating the cuticle. Following invasion the fungus colonizes the body cavity with mycelium of hyaline, septate, branched hyphae may be killed within 2–7 days by growth of hyphae throughout the hemocoel, or within 2 days after ingestion of a high concentration of conidia (>105 conidia ml−1). In the latter case larvae died before the hemocoel was colonized by mycelium. The reason for this rapid death at high concentrations of inoculum is not known, but might be caused by toxic substances associated with the invading hyphae, which only attain a lethal titer when massive invasion originates from large numbers of conidia (Sweeney 1983). After death of the larva, hyphae penetrate through the external cuticle to form a layer of conidiophores on the outside of the cadaver. These conidiophores produce conidia, most frequently at the posterior abdominal segments, which are infective to healthy larvae. Debenham and Russell (1977) demonstrated that an infection of Culicinomyces clavisporus originating in larvae could be carried over into the adult stage. It has been postulated that infection of the adult mosquito may be important in dispersal of the fungus (Goettel et al. 1984).
The fungus is a facultative parasite of a wide range of mosquitoes and related Dipteran larvae (Legner 1995), though in the field only five mosquito species have been found infected, these being Ochlerotatus rupestris Dobrotworsky (Russell et al. 1978), Ochlerotatus rubrithorax (Macquart) (Frances 1986), Anopheles quadrimaculatis (Couch et al. 1974), Culiseta inornata (Goettel et al. 1984), and Culiseta inconspicua (Lee) (Frances 1986). A study on the Australian isolate has shown that within the Nematocera, only members of the Division Culicimorpha were susceptible (families Culicidae, Chironomidae, Ceratopogonidae, and Simuliidae), whereas species of the Division Psychodomorpha (family Psychodidae) and Tipulimorpha (family Tipulidae) were not susceptible (Sweeney 1979). Both laboratory and field studies have shown that the Australian isolate of the fungus is highly lethal to all larvae of the genera Anopheles, Culex and Aedes (Sweeney 1981b). It proved also lethal to the brackish-water species Anopheles farauti and Anopheles amictus hilli Woodhill and Lee when the larvae are reared in fresh water (Sweeney 1978b). The host range of the American strain of Culicinomyces clavisporus includes species of Simuliidae, Chaoborinae, Ceratopogonidae, Chironomidae, Ephydridae, and Syrphidae (Knight 1980). No difference was found in virulence between the American and the Australian strains on Anopheles hilli, Culex quinquefasciatus, and Aedes aegypti (Cooper and Sweeney 1982), but a between-species comparison, with the data pooled for both strains, showed that Aedes aegypti was more susceptible to the fungus than Anopheles hilli (Cooper and Sweeney 1982).
In the laboratory, sporulation of Culicinomyces clavisporus developed on 87–95% of first instar Culex quinquefasciatus larvae within 5 days after exposure to the fungus. Conidia may remain on the cadavers for more than 3 weeks, after which their viability is still 70–90% (Cooper and Sweeney 1986). In the field however, conidia persist and remain infective for only several days (Sweeney and Panter 1977; Frances et al. 1984), up to two weeks (Sweeney 1981b, 1983), suppressing a larval population of Culex australicus Dobrotworsky and Drummond for no more than one generation (Sweeney 1981b). It has been suggested that the application of doses lower than those used in previous tests may increase the proportion of larvae that develop sporulation. Evidence for this was obtained in laboratory observations, which showed that exposure of larvae to 103 conidia ml−1, produced greater external sporulation on larval cadavers than exposure to 106 conidia ml−1 (Cooper and Sweeney 1986). These findings have implications for possible field use of the fungus for biological control, as the latter result would provide more favorable prospects for recycling (Cooper and Sweeney 1986).
The effect of this fungus on mosquito field populations has been shown to be variable and difficult to predict (Service 1983). Several factors influence the level of pathogenicity of Culicinomyces clavisporus to mosquito larvae and species of mosquito larvae have an effect on the degree of susceptibility, with early instar larvae being considerably more susceptible to infection than older instars (Sweeney 1981b, 1983), and anophelines being less susceptible than culicines. Even between different batches of Anopheles larvae, considerable differences in susceptibility to Culicinomyces have been found (Sweeney 1981b), and amongst culicines, Aedes aegypti was found to be far more susceptible than Culex quinquefasciatus (Cooper and Sweeney 1982). Some of these differences may be due to settling of the conidia on the bottom of the assay cups: Conidia of Culicinomyces settle out of suspension at a rate of approximately 8 cm/day (Sweeney 1981b). This suggests that the conidia are more accessible to bottom-feeding Aedes than to surface-feeding Anopheles (Sweeney 1981b; Cooper and Sweeney 1982).
In order to control mosquito populations, conidial suspensions have been effective, but large numbers of conidia are required. In the laboratory, concentrations of 104–106 conidia ml−1 have been found sufficient to cause 100% mortality of mosquito larvae (Sweeney and Panter 1977). In a field experiment, using an aqueous suspension of 106 conidia ml−1, 100% of the larvae were killed within 5 days (Sweeney and Panter 1977). Suspensions of 5 × 109 and 1010 conidia m−2 applied to artificial ponds containing caged, laboratory-reared mosquito larvae produced 100% mortality of Culex quinquefasciatus and 68–100% mortality of Anopheles annulipes Walker larvae (Sweeney 1981b). A field experiment, using a dose of 1010 conidia m−2 applied to a 300 m2 pond produced 90–95% control of late instar Culex australicus during the first week after application. However, the population recovered thereafter, showing no evidence of residual activity of the fungus. Larvae of Anopheles annulipes, which were present in low density, were not controlled by the fungus in this trial (Sweeney 1981b).
Experiments with the Australian isolate have outlined its mode of infection, and abiotic requirements for growth and infection of mosquitoes. Optimum temperatures for germination and for growth are 27.5 and 25° C, respectively. Infection of larvae by this fungus will readily occur at temperatures between 15 and 27.5° C, but not at 30° C (Sweeney 1978a). The fungus may therefore not be effective against larvae occupying water of which the temperature consistently exceeds 30° C (Sweeney 1978a). This optimum temperature range is seen in the field, where the majority of natural infections with Culicinomyces clavisporus occur during the cool season (Lacey and Undeen 1986). High salinity and organic pollution inhibits the ability of Culicinomyces clavisporus to infect larvae (Sweeney 1978b).
Large numbers of conidia may be produced by growing the fungus in surface culture on simple and relatively inexpensive artificial media (Lacey and Undeen 1986; Goettel et al. 1984) and the complex procedures required for Lagenidium and Coelomomyces production are unnecessary. Prolonged subculture on artificial media has apparently not diminished the pathogenicity of Culicinomyces clavisporus to mosquitoes (Cooper and Sweeney 1982), although this was contradicted by the same authors (Cooper and Sweeney 1986) showing in comparative bioassays that conidia produced in vivo were more potent than those produced in vitro in four out of six assays using Aedes aegypti larvae as test insects. Submerged culture in 20–750 liter fermentors has also produced successful inocula for field trials (Sweeney 1981b). However, problems in storage must be overcome if this fungus is to be widely used (Legner 1995). Aqueous suspensions of conidia lose their infectivity for mosquito larvae after several weeks of storage at 25° C (Sweeney 1981c) and after 4 months of storage at −20° C, but not at −70° C. However, according to Lacey and Undeen (1986), storage at −70° C is not economically feasible for large-scale field application, which necessitates the need for the development of a suitable protective medium. Another drawback of Culicinomyces clavisporus is the high dosage required (100–1000 L ha−1) for effective control (Service 1983; Lacey and Undeen 1986), and even though conidiogenesis takes place in infected mosquito larvae, persistence of the conidia in the environment is short. Other problems include the relatively high variability and unpredictability of results in the field (Service 1983), the fact that infected larvae are sometimes able to free themselves of the infection when they molt, and the intolerance of the fungus to high salinities (Service 1983). To enhance the potential of this microbial control agent, improvements should be made to develop new culture media, economic storage conditions that can prolong the stability of infective stages, and to develop formulations that increase the likelihood of contact with target species, such as flotation in Anopheles habitats (Lacey and Undeen 1986). In spite of the selectiveness of Culicinomyces clavisporus to kill mosquito larvae, these problems have led to a reduced interest in this species, and research stopped in the mid-1980's. Only recently Campbell et al. (2002) focused on this species again by studying a genetic transformation system that forms the basis for genetic manipulation of this fungus for potential use in biocontrol of mosquitoes.
4.2 Beauveria
Beauveria is one of the most frequently isolated entomogenous fungal genera and has a cosmopolitan distribution. Although the genus has a very broad host range (Roberts 1974), the natural occurrence of Beauveria on mosquitoes has been reported only four times, three of which were Beauveria bassiana (Balsamo) Vuillemin, two cases were mentioned by Clark et al. (1968) infecting Culex tarsalis, Culex pipiens and Anopheles albimanus, and one by Pinnock et al. (1973), infecting Ochlerotatus sierrensis (the original paper used the synonym Beauveria tenella (Delacroix)). The other species was Beauveria brongniartii (Saccardo) Petch, causing the only known epizootic of this genus in mosquitoes in a field population of Ochlerotatus sierrensis (Pinnock et al. 1973). Conidia of Beauveria bassiana are effective in killing mosquito larvae when applied as a conidial dust to the water surface of breeding sites (Clark et al. 1968). Conidia are hydrophobic, thus floating on the water surface, and contact mosquito larvae that feed below the surface mainly at the tip of the siphon, although Miranpuri and Khachatourians (1991) found the head to be an equally important infection site.
In laboratory tests the fungus proved virulent against larvae of Culex pipiens, Culex tarsalis, Culex tritaeniorhynchus and Anopheles albimanus, but was ineffective against Aedes aegypti, Ochlerotatus sierrensis (Clark et al. 1968; Sandhu et al. 1993; Geetha and Balaraman 1999), and Culex quinquefasciatus (Alves et al. 2002). Susceptible species were prone to infection only shortly after molting. If infection occurred shortly before molting, the mycelium was lost within the molt. In three small-scale outdoor tests with conidia of Beauveria bassiana, reductions of 82, 95 and 69% were found on Culex pipiens larvae and pupae after two weeks (Clark et al. 1968). Studies on tree holes treated with blastoconidia (5 × 103 or 5 × 105 conidia ml−1 water) of Beauveria brongniartii delivered reductions between 53 and 71% of emerging adult Ochlerotatus sierrensis (Pinnock et al. 1973). Laboratory studies showed that besides Ochlerotatus sierrensis, the fungus was pathogenic for larvae of Aedes aegypti, Ochlerotatus dorsalis (Meigen), Ochlerotatus hexodontus Dyar, Culex pipiens, Culex tarsalis and Culiseta incidens (Thomson) (Pinnock et al. 1973). Besides infecting larvae, the fungus proved to be virulent to adult mosquitoes as well, although no Beauveria-infected adult mosquitoes have been reported from the field. In laboratory tests against adult Culex tarsalis, Culex pipiens, Aedes aegypti, Ochlerotatus sierrensis, Ochlerotatus nigromaculis (Ludlow), and Anopheles albimanus, conidia of Beauveria bassiana produced 100% mortality within 5 days after exposure, while less than 50% occurred in corresponding controls (Clark et al. 1968). In laboratory tests 82% of adult Anopheles gambiae were infected, with an LT50 of 3.5 days, against 8.8 days from the control group (Scholte et al. 2003). Outdoor tests against adult Ochlerotatus nigromaculis in screen cages were less successful, yielding only 58% mortality (Clark et al. 1968). In this case, the fact that adults rested on the screen walls of their test cages, rather than in the dusted grass that had been provided, may explain the low mortality. Sections of infected adults, fixed and imbedded in paraffin immediately after death, revealed some mycelium in regions surrounding the main tracheal trunks. It appeared that the conidia entered through the spiracles, germinated, invaded the walls of the tracheae, and subsequently were thought to release a toxin that killed the adults (Clark et al. 1968). Later studies confirmed the existence of toxins produced by species of this genus; Beauvericin, Bassianin, Bassianolide, Beauverolides, and Tenellin from B. bassiana, and Oosporein from Beauveria brongniartii (Ferron 1981; Grove and Pople 1980; Strasser et al., 2000). A dichloromethane extract from mycelium showed activity when assayed against Aedes aegypti larvae at 100 ppm (Gupta et al. 1995). This extract contained Beauvericin and two analogues (Beauvericin A and B). The larvicidal properties of Beauvericin had already been reported by Grove and Pople (1980), who found 86% mortality with Aedes aegypti larvae after 48 hrs at 20 g ml−1, but only 39% when 10 g ml−1 was used. These destruxins pose no obvious risk to humans, and the use of this fungus does not result in toxin levels harmful to the environment (Strasser et al., 2000).
Humidity is considered as one of the critical factors affecting the outcome of both laboratory and field-tests with Beauveria bassiana. Clark et al. (1968) mention a study by Hart and MacLeod (1955), who found optimal germination of Beauveria conidia occurring at relative humidities above 94%. Infection does not appear to be dependent on temperature (Schaerffenberg 1964; Ferron 1981). For conidia however, high temperatures may be harmful, especially in combination with high humidity conditions. The effective stages of the fungus against larvae are conidia and blastoconidia, the latter stage being far more pathogenic (Miranpuri and Khachatourians 1991). Although growing blastoconidia is relatively easy, production has been abandoned because of the difficulties of storing this type of conidium (Ferron 1981). To produce conidiospores, a two-stage technique for mass production can be used. A pilot factory in Krasnodar (former Soviet Union) was able to produce 22 tons of Boverin annually (Beauveria bassiana conidia plus an inert carrier, standardized at 6 × 109 conidia g−1) (Ferron 1981). Other products from Beauveria bassiana include Mycotrol, ESC 170 GH, and Naturalis-O, targeted against whitefly, aphids, thrips, mealy bugs, leaf hoppers and leaf-eating insects, but not mosquitoes or other Dipterans (Khetan 2001). Ago Biocontrol provides a Beauveria brongniartii-product in Colombia that among others also targets Diptera, but fails to specify if Culicidae are susceptible. A problem associated with using conidia is that they have no residual effect. They germinate in mosquito habitats even when not in contact with larvae. This limitation, along with the high dosage needed is serious drawbacks for mosquito larval control. Formulation with oil may overcome some of the problems. Beauveria brongniartii was considered as potential biological control agent since this species is pathogenic for larvae of several tree-hole breeding mosquito species, including Aedes aegypti, Ochlerotatus dorsalis, Ochlerotatus hexodontus, and Ochlerotatus sierrensis. However, the large doses required for significant mortality rates of mosquito larvae was not considered practical (Pinnock et al. 1973; Chapman 1974). Moreover, another problem concerning the use of Beauveria would be its safety regarding vertebrates. Sensitivity has been reported by persons repeatedly exposed to massive amounts of Beauveria bassiana conidia preparations (Ignoffo 1967). However, long-term rodent tests with Beauveria bassiana conidia, including inhalation tests, subcutaneous injection, and oral toxicity tests, did not show adverse health effects.
4.3 Metarhizium
Metarhizium, like Beauveria, is one of the most common entomopathogenic fungi, with a worldwide distribution. The species is soil-borne and infects predominantly soil-dwelling insects. Taxonomy of Metarhizium is not straight-forward. The current classification of the taxon is mainly based on the morphology of conidia and conidiogenous cells. Some authors combine these with biochemical and molecular characteristics (Riba et al., 1986), and/or host pathogenicity, cold-activity and sporulation color (Yip et al., 1992; Rath et al., 1995). Driver et al. (2000) used 10 different clades, based primarily on molecular data, although this leaves room for debate. Metarhizium anisopliae consists of 4 varieties (Driver et al., 2000), two of which are considered important, these being Metarhizium anisopliae var. acridum (previously designated Metarhizium flavoviride) found mainly on Homoptera and Metarhizium anisopliae var. anisopliae (Metschnikoff) Sorokin, the latter being the best known of the two species. Metarhizium anisopliae has a large host-range, including arachnids and five orders of insects (Boucias and Pendland 1998), comprising over 200 species. Mosquitoes are not listed as natural hosts for Metarhizium anisopliae (Veen 1968) but some strains have shown to be virulent against mosquito larvae (Roberts 1967, 1970, 1974; Ramoska 1982; Agudelo-Silva and Wassink 1984; Daoust and Roberts 1983a,b; Ravallec et al. 1989; Sandhu et al. 1993; Alves et al. 2002).
On terrestrial insects, the life cycle begins with a conidium attaching to the host cuticle, forming an appressorium, followed by a penetration peg to enter the cuticle. After entering the hemocoel, hyphae are formed that produce and release toxins, killing the host 4–16 days (depending mainly on the host species) after contamination (Ferron 1981; Khachatourians 1991; Boucias and Pendland 1998). These toxins include Destruxins, Swainsinone, and Cytochalasin C (Strasser et al. 2000). Histopathological studies of elaterid tissues infected by Metarhizium anisopliae suggest that toxins (destruxins) kill the host by inciting degeneration of the host tissues due to loss of the structural integrity of membranes and then dehydration of cells by fluid loss (Ferron 1981). If the conditions are warm and moist, conidiophores will grow through the cuticle to cover the insect with conidia. The cycle in mosquito larvae varies from the above. If floating conidia are applied, larvae contact them when they break the water tension with their perispiracular valves for air intake. The fungus germinates and penetrates into the respirical siphon, blocking the breathing mechanism (Daoust et al. 1982; Lacey et al. 1988). Plugging of the spiracles usually leads to death before significant invasion of the hemocoel has occurred, so hyphal body formation is minimal. Cadavers in the aquatic environment are overrun with bacteria rather than mycelium, and no new conidia are formed. Although much less frequently observed, larvae can also ingest dry conidia (Crisan 1971; Roberts 1974), where they, apparently without germination, release lethal substances into the gut (Crisan 1971; Roberts 1970, 1974). A mixture of these toxins (70% destruxin A and 30% B) proved toxic to mosquito larvae, with LD50 values of 10 to 100 ppm (Roberts 1974).
Many laboratory studies have shown the potential of Metarhizium anisopliae as a mosquito control agent. Roberts (1970) observed effects on larvae of Anopheles stephensi, Anopheles quadrimaculatus, Aedes aegypti, Ochlerotatus atropalpus, Ochlerotatus taeniorhynchus, Culex pipiens, Culex restuans, and Culex salinarius, and found all species susceptible to (unformulated) conidia. In a laboratory experiment reported by Ramoska (1982), the fungus suppressed Culex quinquefasciatus larval populations for nearly a month. On the other hand, the strain used by Alves et al. (2002) had lost its effect on the same mosquito species after only 3 days. Daoust and Roberts (1982), found that over half of 52 strains from a variety of hosts taken from nine countries caused more than 50% mortality of Culex pipiens larvae treated with 1 mg dry conidia per 16 cm2. The strains most virulent to Culex pipiens proved to be highly pathogenic to larvae of Aedes aegypti and Anopheles stephensi as well. In the same study it was shown that virulence of strains towards mosquitoes could increase 1.6 - 2.5 times by passage through mosquito larvae. In small-scale outdoor tests, using 300 or 600 mg of conidia m−2 in small artificial ponds reduced Culex pipiens by 91% and 94% and Ochlerotatus sollicitans by 85% and 98% within three days (Roberts 1974). Besides larvae, also adult mosquitoes proved to be susceptible to the fungus. Recently, adult Culex quinquefasciatus and Anopheles gambiae s.s. were infected in a laboratory study. Both species proved susceptible and succumbed to infection with unformulated dry, and oil-formulated conidia, with LT50 values ranging from 4–6 days (Scholte et al. 2003a,b). A small-scale field study showed that the fungus also infects and kills wild Anopheles gambiae s.l. (Scholte et al. unpublished data)
The optimal growth temperature for most strains is 27–28° C (Ferron 1981), although some exceptions of cold-resistant and heat-resistant strains have been reported (Bidochka et al. 1998; Boucias and Pendland 1998). Conidia normally require a relative humidity of at least 92% to germinate (Ferron 1981). Conidia that are stored under dry conditions show higher germination rates (initially 96%, dropping to 80% after 60 days) than conidia formulated in paraffin oil (from 93% to 73%) (Morley-Davies et al. 1995). Conidia are found to survive longest at a combination of moderate temperatures and high RH (26° C and 97 % RH or 19° C and 97 % RH) or low temperature and low RH (4° C and 0 % RH) (Daoust and Roberts 1983a). The fungus can easily be grown in vitro (Goettel and Inglis, 1997), and storage conditions are more critical to spore survival and virulence than the substrate upon which conidia are produced (Daoust and Roberts 1983b). When relative humidity is high, conidia can be quite tolerant to high temperatures (Zimmerman 1982; Morley-Davies et al. 1995). However, conidial viability decreased rapidly when exposed to UV light (Zimmermann 1982; Morley-Davies et al. 1995). In a conidial viability experiment using a sunlight simulator at 40° C, germination ranged between 10 and 50% after 24 hrs of exposure to UV (Morley-Davies et al. 1995).
Several types of conidia formulations have been developed and tested for mosquito larval control including granulars, dusts, and wettable suspensions (Daoust et al. 1982). Ramoska et al. (1981) compared two formulations, floating and sand, and found that mortality occurred sooner using the sand formulation with below-surface feeding mosquito larvae while the floating preparation worked faster on surface feeders. When suspended in an aqueous suspension containing a surfactant, or when formulated with granular carriers or dust diluents, M. anisopliae conidia tend to lose virulence compared to unformulated conidia against Culex pipiens larvae. In contrast, a diluent derived from dried castor oil significantly enhanced conidial virulence (Daoust et al. 1982).
Metarhizium anisopliae has several characteristics that make it interesting as a microbial mosquito control agent. It causes high mortality of mosquito larvae in laboratory populations, the fungus can be grown in massive amounts on inexpensive artificial media, and conidia can be stored easily. Moreover, its failure to germinate in the mosquito environment until actual exposure to a host and its resulting persistence in the environment, as well as the fact that its effect is not limited to periods of host molting (as for Beauveria bassiana), make this fungus a very promising control agent (Roberts 1970). The fungus is commercially produced by Biocare, Australia, BCP, South Africa, Bayer, Germany (BIO 1020), and several Brazilian companies, as control agents for German cochroaches and termites, black vine weevil, citrus root weevil, sugarcane pests and Aeneolamia varia saccharina Dist., but not mosquitoes (Khetan 2001).
One drawback in the use of Metarhizium anisopliae for mosquito control is that conidia are not produced on submerged, fungus-killed larvae, and it is presumed that inundated releases are to be repeated frequently. This would be less convenient than a single introduction that maintains itself, like Lagenidium and Coelomomyces. Another disadvantage, as with all fungal agents, is that the conidia must contact the larvae physically. Since these are particles rather than solutions, such contact may be difficult in some situations. Zimmermann (1993) claims that because of absence of toxicological or pathological symptoms in birds, fish, mice, rats, and guinea pigs after exposure to conidia of the fungus, Metarhizium anisopliae was safe. Also Strasser et al. (2000) conclude from a risk-assessment study that the fungus poses no obvious risk to humans, or the environment. However, it has been reported that the fungus may cause human keratitis (DeGarcia et al. 1997). Other work has shown that the fungus can cause significant mortality of shrimp, frog and fish embryos exposed to conidia (Genthner et al. 1994, 1998). The latter effects, however, were not revealed in earlier studies where guppies, exposed to massive amounts of conidia, were not affected for periods up to two months (Roberts 1974). Finally, regarding its broad host spectrum, use of conidia for mosquito control should be done with caution to keep the risk of contaminating non-target insects as low as possible.
4.4 Tolypocladium
Tolypocladium cylindrosporum Gams was first isolated from Ochlerotatus sierrensis in California in 1971 (Soarés 1982), and later from Ochlerotatus australis in New Zealand (Weiser and Pillai 1981), and is a pathogen of mosquito larvae. In one study it appeared that also adult Ochlerotatus sierrensis were susceptible to infection (Soarés 1982), killing 50% within 5, and 100% of exposed mosquitoes in 9 days. The fungus caused over 90% larval mortality of Ochlerotatus sierrensis and 67% in Culex tarsalis at 5 × 106 conidia ml−1 in a laboratory study (Soarés 1982). Both blastoconidia and aerial conidia are infectious to mosquito larvae, but blastoconidia are more virulent (Goettel 1988; Nadeau and Boisvert 1994). They invade the host predominantly through the exterior cuticle, but also through ingestion (Soares et al. 1979; Weiser 1988). Nineteen mosquito species were found susceptible to the fungus, including ten Aedes spp., six Culex spp., two Culiseta spp., and one Anopheles sp. (Goettel 1987b). Weiser et al. (1991b) demonstrated greater than 80% mortality in mosquitoes from Tolypocladium terricola Weiser, Matha and Jegorov after 8 days. Soarés (1982) suggested that mosquitoes may die of toxic metabolites excreted by the fungus. The existence of such a metabolite, named tolypin, was described by Weiser and Matha (1988), who found symptoms of intoxication (knock-down) of Culex pipiens larvae during the first hour of exposure to concentrations of 0.026–0.4 mg ml−1 in water.
It was suggested that Tolypocladium cylindrosporum was a candidate for control of mosquito and blackfly larvae in aquatic habitats in temperate areas (Goettel 1987a,b). Soarés (1982), however, suspected that replication of laboratory results with this fungus in the field could be expected only under a narrow range of conditions, which would not make the fungus practical for mosquito control. Field studies in New Zealand (Gardner and Pillai 1987) and in Alberta, Canada (Goettel 1987b), did indeed show evidence that the fungus was not very effective. Moreover, apparent lack of residual activity (Gardner et al. 1986) decreased the interest in this fungus for biocontrol of larval mosquito populations.
Other Anamorphic fungi that have been found on mosquitoes include some species of the genera Aspergillus, Fusarium, Paecilomyces, Penicilium, and Verticillium (Hasan and Vago 1972; Roberts and Strand 1977; Ballard and Knapp 1984; Sur et al. 1999; Agarwala et al. 1999; Scholte et al. 2003a). Fusarium oxisporum Schlecht has been isolated a number of times from Ochlerotatus detritus (Haliday), (Breaud et al. 1980; Hasan and Vago 1972) and proved to be virulent to Culex pipiens in the laboratory, but later reports on the fungus are scarce. However, most of infections were either rare or the particular fungi were not considered highly pathogenic to mosquitoes.
Discussion
The search for effective mosquito pathogens that can be used in mosquito control operations has been ongoing for several decades. Both laboratory and field studies of those fungi that appeared to have potential for operational use, have been evaluated. If promising, this was followed by development of methods for mass production and finally implementation in operational control programs, although few fungal species have reached this latter stage of development. From the studies on fungi reviewed in this article, three common characteristics emerge. First, pathogens are mainly effective against the larval stages of mosquitoes. Second, effective control requires repeated rather than a single application of the agent during the mosquito breeding season, and third, vector control programs can only be cost-effective if the control agent can be produced in vitro (Federici 1995).
Concerning mosquito-pathogenic fungi, three genera are generally considered important; Lagenidium, Coelomomyces, and Culicinomyces (Roberts 1974; Lacey and Undeen 1986; Federici 1995). Each one of these (and others, discussed in this paper) has one or more traits useful for mosquito control, but none of them possesses the full array of properties needed for general applied and cost-effective control. Coelomomyces spp. are very effective in killing many mosquito species, although individual species have narrow host ranges, and have often been reported to cause epizootics, with the major obstacle of dependence on in vivo production, making mass-production difficult. Lagenidium giganteum can probably be regarded as the fungus with the best properties for larval mosquito control, though only for stagnant waters, such as rice fields. The principle advantage of Lagenidium over the bacterium Bacillus thuringiensis israelensis (Bti) is that only a single application is required per season. Even less frequent application may be possible in some habitats, as there are indications that oospores can hibernate, initiating epizootics the following season (Federici 1995). Although low levels of infection are often observed after a Lagenidium giganteum population is established, recurrent epizootics have been reported (Glenn and Chapman 1978). It is the only fungus that has been produced commercially as mosquito control agent (Laginex™). The fungal zoospores have been claimed to infect larvae of all species of mosquitoes but to be especially effective against Culex spp. (Khetan 2001). Culicinomyces clavisporus excited considerable interest initially, but this declined when the high dosages required for effective control and the low persistence of conidia in the environment became apparent (Service 1983; Lacey and Undeen 1986).
Many mosquito-pathogenic fungi were expected to be used for the control of medically important vector species (Service 1983; Lacey and Undeen 1986; Federici 1995). Unfortunately, none of the fungi described above are specifically adapted as larvicidal agents against important vector species such as the African malaria vectors in the Anopheles gambiae complex. The larval habitats of these mosquito species include a variety of transient, mainly sunlit, rain-water pools, such as borrow-pits, drains, brick-pits, car-tracks, foot-and hoof prints around pond and water-holes (Van Someren et al. 1956; Coene 1993; Lyimo 1993). Most of these sites are transient, and in some areas breeding is highly seasonal, following the rainfall pattern of that specific area. It is very unlikely that under normal field conditions, larvae of these mosquito species are in contact with any of the aquatic fungi discussed above, even though some infected females may contaminate a breeding site during oviposition. Lagenidium, Coelomomyces, and Culicinomyces are all aimed at the larval stages of mosquitoes and not at the adult stage. In the field, mortalities of immature mosquitoes can commonly be 95% or more, yet the numbers of emerging adults may still be sufficient for maintaining substantial disease transmission. The important issue is how adult populations are affected (Service 1983).
Some aquatic fungi have been found on adult mosquitoes, including species of Coelomomyces, Culicinomyces and Smittium but this is rare and not considered to be of importance. In these situations the infection takes place on late-instar larvae or pupae, resulting in survival of the mosquito into the adult stage. They probably do not account for considerable adult mortality, but infected adults may disperse the fungus to other habitats (Lucarotti and Andreadis 1995; Shoulkamy et al. 1997). Pathogenic fungi that occur naturally on adult mosquitoes belong mainly to the Entomophthorales. Some species have caused epizootics in hibernating mosquitoes in relatively cool, humid, dark places in the northern hemisphere (Weiser 1988). Unfortunately, spores are short-lived. Moreover, the fungus' inability to grow in vitro makes it an unsuitable control agent. There are, however, some fungi that are normally not associated with mosquitoes, but have proven to be highly pathogenic to adult mosquitoes. The Hyphomycetes Beauveria bassiana (Clark et al. 1968) and Metarhizium anisopliae kill adult mosquitoes in the laboratory (Scholte et al., 2003a) and the latter fungus also killed adult Anopheles gambiae s.l. in the field in Tanzania (Scholte et al., unpublished data). However, practical application methods need to be improved.
We consider the potential for using Hyphomycetes such as Metarhizium or Beauveria for biocontrol of, especially African, vector mosquitoes high: These fungi can be cost-effectively mass-produced, even locally, and many strains are already commercially available, circumventing the time-consuming and costly process of registration, including risk-assessment of ‘new’ fungal control agents. Beauveria (Beauveria bassiana and Beauveria brongniartii) are produced by more than 14, and Metarhizium (Metarhizium anisopliae and Metarhizium anisoplaie var. acridum) by more than 10 companies (including some in Africa), aimed at controlling various insect pests including termites, cockroaches, black vine weevil, white flies, aphids, corn borers, cockchafers, and other insects (Khetan 2001; Strasser et al., 2000; Wraight et al. 2001). Moreover, the development of ULV-CDA formulations of conidia to control locusts in dry climates (Bateman 1992) opens up possibilities for the use of fungi to control mosquito vector species in dry areas in e.g. Sub-Saharan Africa such as Anopheles gambiae and Anopheles funestus (malaria), Aedes aegypti (yellow fever, dengue), and Culex quinquefasciatus (filariasis, viral encephalites).
Concerning the future of myco-insecticides, Burgess (1998) points out: “Improvement in shelf-life duration and formulations is the key to the future and should enable fungi to compete in efficacy with chemical insecticides on nearly equal terms, and should increase projected market size towards industrial viability”. He then continues with “Budgets should cater for adequate research on strain viability, including reaction to formulation requirements. Research should target three areas that need improvement: application, storage, and production”.
In summary, an ideal fungus for mosquito control should have the following characteristics. It a) kills both larval and adult stages, b) requires only one or a few applications per season, c) is actively dispersed by adult females to previously unoccupied breeding sites, d) shows residual activity and persistence in the mosquito population after introduction, e) kills selectively mosquitoes and no other organisms, f) is effective over a large range of salinities, temperatures, relative humidities and breeding sites with variable water quality, g) is easily and cost-effectively mass-produced and formulated, h) retains prolonged activity during storage (long shelf-life), and i) is not harmful to humans and other non-target organisms. None of the mosquito-pathogenic fungi presently known exhibit all these characteristics, but they all exhibit at least some. To determine which fungi could be used for the control of mosquito populations depends on many factors such as the biology of the target mosquito species, its distribution (isolated or uniform), the life-stage targeted, suitability of the ecosystem for survival and viability/virulence of a fungus (biotic and abiotic factors), application methodology and costs thereof, storage facilities, whether or not a fungus can be mass-produced, and whether or not a fungus is registered and available on the market. One question that needs to be addressed is the extent to which it is worthwhile risking infections in non-target organisms, and whether there may be other foreseeable side effects.
In entomopathogenic fungi we find a huge area of partly undiscovered natural pathogens and undeveloped methodologies to apply to mosquito control. Entomogenous fungi, without the assistance of man, have been found affecting substantial proportions of many mosquito populations all over the world. This suggests that manipulation, such as increasing inoculum potential, could make fungi more efficient in mosquito control (Roberts 1970). Facing major problems associated with mosquito-borne human diseases (drug resistance, lack of vaccines) and mosquito control (especially insecticide resistance) it is of utmost importance to search for new/alternative agents and methods. In our view, entomopathogenic fungi, both new and existing ones with renewed/improved efficacies may contribute to an extension of the limited arsenal of effective vector control tools. These new agents and/or methods should preferentially be applied in integrated control strategies in order to gain maximum impact both on larval and adult mosquito populations.
Footnotes
* indicates papers not seen by the authors.
References
- Agarwala SP, Sagar SK, Sehgal SS. Use of mycelial suspension and metabolites of Paecilomyces lilacinus (Fungi: Hyphomycetes) in control of Aedes aegypti larvae. Journal of Communicable Diseases. 1999;31:193–196. [PubMed] [Google Scholar]
- Agudelo-Silva F, Wassink H. Infectivity of a Venezuelan strain of Metarhizium anisopliae to Aedes aegypti larvae. Journal of Invertebrate Pathology. 1984;43:435–436. doi: 10.1016/0022-2011(84)90094-6. [DOI] [PubMed] [Google Scholar]
- Alves SB, Alves LFA, Lopes RB, Pereira RM, Vieira SA. Potential of some Metarhizium anisopliae isolates for control of Culex quinquefasciatus (Dipt., Culicidae) Journal of Applied Entomology. 2002;126:504–509. [Google Scholar]
- Anderson JF, Ringo SL. Entomophthora aquatica sp, n. infecting floodwater mosquitoes. Journal of Invertebrate Pathology. 1969;13:386–393. [Google Scholar]
- Andreadis TG, Magnarelli LA. Erynia (=Entomophthora) aquatica in a salt-marsh mosquito, Aedes cantator. Journal of Invertebrate Pathology. 1983;42:277–279. doi: 10.1016/0022-2011(83)90192-1. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Federici BA, Tarver FR, Stewart W. Biotic and abiotic parameters associated with an epizootic of Coelomomyces punctatus in a larval population of the mosquito Anopheles quadrimaculatus. Journal of Invertebrate Pathology. 1992;60:219–228. doi: 10.1016/0022-2011(92)90002-l. [DOI] [PubMed] [Google Scholar]
- Arêa Leão AE, Carlota Pedroso M. Nova especie do genero Coelomomyces parsito de ovos de Phlebotomus. Mycopathologia Et Mycologia Applicata. 1964;26:305–307. doi: 10.1007/BF02049559. [DOI] [PubMed] [Google Scholar]
- Badran RAM, Aly MZY. Studies on the mycotic inhabitants of Culex pipiens collected from fresh water ponds in Egypt. Mycopathologia. 1995;132:105–110. [Google Scholar]
- Ballard EM, Knapp FW. Occurrence of the fungus Verticillium lecanii on a new host species: Aedes triseriatus (Diptera: Culicidae) Journal of Medical Entomology. 1984;21:6. [Google Scholar]
- Bateman RP. Controlled droplet application of myco-insecticides: an environmentally friendly way to control locusts. Antenna. 1992;16:6–13. [Google Scholar]
- Becker N, Ascher KRS. The use of Bacillus thuringiensis subsp. israelensis (Bti) against mosquitoes, with special emphasis on the ecological impact. Israel Journal of Entomology. 1998;32:63–69. [Google Scholar]
- Becnel JJ, Garcia J, Johnson M. Effects of three larvicides on the production of Aedes albopictus based on removal of pupal exuviae. Journal of the American Mosquito Control Association. 1996;12:499–502. [PubMed] [Google Scholar]
- Bidochka MJ, Kamp AM, and De Croos JNA. 2000 Insect pathogenic fungi: from genes to populations. In: Kronstad JW, editors. Fungal. Pathology: 171–193. Dordrecht, Kluwer Academic Publishers. [Google Scholar]
- Bidochka MJ, Kasperski JE, Wild GAM. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Canadian Journal of Botany/Review Canadien de Botanique. 1998;76:1198–1204. [Google Scholar]
- Bisht GS, Joshi C, Khulbe RD. Watermolds: Potential biological control agents of malaria vector Anopheles culicifacies. Current Science. 1996;70:393–395. [Google Scholar]
- Boucias DR, Pendland JC. 1998 Entomopathogenic fungi; Fungi Imperfecti. In: Boucias DR, Pendland JC, editors. Principles of Insect Pathology. 10:321–359.Dordrecht, Kluwer Academic Publishers. [Google Scholar]
- Bras G, Gordon CO, Emmons CW, Prendegast KM, Sugar M. A case of phycomycosis observed in Jamaica; infection with Entomophthora coronata. American Journal of Tropical Medicine and Hygiene. 1965;14:141–145. doi: 10.4269/ajtmh.1965.14.141. [DOI] [PubMed] [Google Scholar]
- Braun A. 1855 Algarum unicellularium genera nova et minus cognita, praemissis observationibus de algis unicellularibus in genere. Engelmann, Lipsiae. 111. p. [Google Scholar]
- Breaud TP, Crabbe JR, Majori G. The isolation of Fusarium oxysporum from a natural population of Aedes detritus in Italy. Mosquito News. 1980;40:654. [Google Scholar]
- Brooke BD, Hunt RH, Chandre F, Carnevale P, Coetzee M. Stable chromosomal inversion polymorphisms and insecticide resistance in the malaria vector mosquito Anopheles gambiae (Diptera: Culicidae) Journal of Medical Entomology. 2002;39:568–573. doi: 10.1603/0022-2585-39.4.568. [DOI] [PubMed] [Google Scholar]
- Buchanan FC, Pillai JS. Coelomomyces psorophorae var tasmaniensis Couch + Laird (1988) (Coelomomycetaeceae: Blastocladiales), a fungal pathogen of the mosquito Aedes australis. Mycopathologia. 1990;111:25–32. doi: 10.1007/BF02277297. [DOI] [PubMed] [Google Scholar]
- Burgess HD. 1998 Formulation of microbial biopesticides. Dordrecht, Kluwer Academic Publishers. [Google Scholar]
- Campbell EI, Kinghorn JR, Kana'n GJM, Unkles SE, Panter C. Genetic transformation of the mosquito pathogenic fungus Culicinomyces clavisporus. Biocontrol Science and Technology. 2002;12:395–399. [Google Scholar]
- Castillo JM, Roberts DW. In vitro studies of Coelomomyces punctatus from Anopheles quadrimaculatus and Cyclops vernalis. Journal of Invertebrate Pathology. 1980;35:144–157. [Google Scholar]
- Chandre F, Darriet F, Manga L, Akogbeto M, Faye O, Mouchet J, Guillet P. Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bulletin of the World Health Organization. 1999;77:230–234. [PMC free article] [PubMed] [Google Scholar]
- Chapman HC. Biological control of mosquito larvae. Annual Review of Entomology. 1974;19:33–59. doi: 10.1146/annurev.en.19.010174.000341. [DOI] [PubMed] [Google Scholar]
- Chapman HC. 1985 Ecology and use of Coelomomyces species in biological control: A review. In: Couch JN, Bland CE, editors. The Genus Coelomomyces: 82–91. Orlando, Academic Press. [Google Scholar]
- Chapman HC, Glenn FE. Incidence of the fungus Coelomomyces punctatus and C. dodgei in larval populations of the mosquito Anopheles crucians in two Louisiana ponds. Journal of Invertebrate Pathology. 1972;19:256–261. doi: 10.1016/0022-2011(72)90217-0. [DOI] [PubMed] [Google Scholar]
- Chapman HC, Woodard DB. Coelomomyces (Blastocladiales: Coelomomycetaceae) infections in Louisiana mosquitoes. Mosquito News. 1966;26:121–123. [Google Scholar]
- Clark TB, Kellen W, Fukuda T, Lindegren JE. Field and laboratory studies on the pathogenicity of the fungus Beauveria bassiana to three genera of mosquitoes. Journal of Invertebrate Pathology. 1968;11:1–7. doi: 10.1016/0022-2011(68)90047-5. [DOI] [PubMed] [Google Scholar]
- Clark TB, Kellen WR, Lindegren JE, Sanders RD. Pythium sp. (Phycomycetes: Pythiales) pathogenic to mosquito larvae. Journal of Invertebrate Pathology. 1966;8:351–354. [Google Scholar]
- Coene J. Malaria in urban and rural Kinshasa: the entomological input. Medical and Veterinary Entomology. 1993;7:127–137. doi: 10.1111/j.1365-2915.1993.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. *Experimental infections with Rubetella fungi in Anopheles gambiae and other mosquitoes. Proceedings of the First International Congress of Parasitology (Rome) 1966;1:592–593. [Google Scholar]
- Cooper R, Sweeney AW. The comparative activity of the Australian and United States strains of Culicinomyces clavosporus bioassayed in mosquito larvae of three different genera. Journal of Invertebrate Pathology. 1982;40:383–387. doi: 10.1016/0022-2011(82)90177-x. [DOI] [PubMed] [Google Scholar]
- Cooper R, Sweeney AW. Laboratory studies on the recycling potential of the mosquito pathogenic fungus Culicinomyces. Journal of Invertebrate Pathology. 1986;48:152–158. doi: 10.1016/0022-2011(86)90117-5. [DOI] [PubMed] [Google Scholar]
- Couch JN. A new saprofytic species of Coelomomyces, with notes on other forms. Mycologia. 1935;27:376–387. [Google Scholar]
- Couch JN, Romney SV, Rao B. A new fungus which attacks mosquitoes and related diptera. Mycologia. 1974;66:374–379. [PubMed] [Google Scholar]
- Couch JN, Bland CE. 1985 The genus Coelomomyces, 1st edition. Academic Press. [Google Scholar]
- Couch JN, Romney SV. Sexual reproduction in Coelomomyces giganteum. Mycologia. 1973;65:250–252. [Google Scholar]
- Couch JN, Romney SV, Rao B. A new fungus which attacks mosquitoes and related Diptera. Mycologia. 1974;66:374–379. [PubMed] [Google Scholar]
- Crisan EV. Mechanism responsible for release of toxin by Metarhizium spores in mosquito larvae. Journal of Invertebrate Pathology. 1971;17:260–264. doi: 10.1016/0022-2011(71)90101-7. [DOI] [PubMed] [Google Scholar]
- Cuda JP, Hornby JA, Cotterilli B, Cattell M. Evaluation of Coelomomyces for biocontrol of Mansonia mosquitoes in Florida (Diptera: Culicidae) Biological Control. 1997;8:124–130. [Google Scholar]
- Cuebas-Incle EL. Infection of adult mosquitoes by the entomopathogenic fungus Erynia conica (Entomophthorales: Entomophthoraceae) Journal of the American Mosquito Control Association. 1992;8:367–371. [PubMed] [Google Scholar]
- Daoust RA, Roberts DW. Studies on the prolonged storage of Metarhizium anisopliae conidia: effect of temperature and relative humidity on conidial viability and virulence against mosquitoes. Journal of Invertebrate Pathology. 1983a;41:143–150. doi: 10.1016/0022-2011(83)90213-6. [DOI] [PubMed] [Google Scholar]
- Daoust RA, Roberts DW. Studies on the prolonged storage of Metarhizium anisopliae conidia: effect of growth substrate on conidial survival and virulence against mosquitoes. Journal of Invertebrate Pathology. 1983b;41:161–170. doi: 10.1016/0022-2011(83)90215-x. [DOI] [PubMed] [Google Scholar]
- Daoust RA, Ward MG, Roberts DW. Effect of formulation on the virulence of Metarhizium anisopliae conidia against mosquito larvae. Journal of Invertebrate Pathology. 1982;40:228–236. [Google Scholar]
- Debenham ML, Russell RC. The insect pathogenic fungus Culicinomyces in adults of the mosquitoes Anopheles amictis hilli. Journal of Australian Entomological Society. 1977;16:46. [Google Scholar]
- DeGarcia MCC, Arboleda ML, Barraquer F, Grose E. Fungal keratitis caused by Metarhizium anisopliae var. anisopliae. Journal of Medical and Veterinary Mycology. 1997;35:361–363. [PubMed] [Google Scholar]
- Deshevykh ND. 1973 *Seasonal infection of larval Culex modestus by Coelomomyces fungi in the Ill river basin. In: Dubitskij AM editor. Regulators of the Numbers of Blood-Sucking Flies in Southeastern Kazak: 22–26. Kazakh Academy of Science. [Google Scholar]
- Driver F, Milner RJ, Trueman JWH. A taxonomic revision of Metarhizium based on a phylogenitc analysis of rDNA sequence data. Mycological Research. 2000;104:134–150. [Google Scholar]
- Dubitzij AM. 1978 *Biological control of bloodsucking flies in the USSR, 1st edition. Kazachstan Academy of Sciences, Alma Ata. [Google Scholar]
- Dumas J-L, Papierok B. Virulence de l'entomophthorale Zoophthora radicans (Zygomycetes) à l'égard des adultes de Aedes aegypti (Dipt.: Culicidae) Entomophaga. 1989;34:321–330. [Google Scholar]
- Dzerzhinskij VA, Dubitskij AM, and Deshevykh ND. 1975 *The detection of variants of artificial infection of larvae of the blood sucking mosquito Culex modestus with the entomopathogenic fungus Coelomomyces iliensis. Parazitologiya. 9:540–542.(in Russian). [PubMed] [Google Scholar]
- Eilenberg J. 2000 Entomophthorales on Diptera. In: Papp L, Darvas B, editors. Manual of Palaearctic Diptera. 13:521–533.Budapest, Science Herald. Eilenberg J. 2002. Biology of fungi from the order Entomophthorales with emphasis on the genera Entomophthora, Strongwellsea and Eryniopsis: a contribution to insect pathology and biological control. PhD Thesis, Copenhagen: Royal Veterinary and Agricultural University Copenhagen. 407. pp. [Google Scholar]
- Emmons CW, Bridges CH. Entomophtora coronata, the etiologic agent of a phycomycosis of horses. Mycologia. 1961;53:307–312. [Google Scholar]
- Federici BA. 1981 Mosquito control by the Fungi Culicinomyces, Coelomomyces and Coelomomyces. In: Burges HD, editor. Microbial Control of Pests and Plant Diseases 1970–1980. 29:555–572.London, Academic Press. [Google Scholar]
- Federici BA. The future of microbial insecticides as vector control agents. Journal of the American Mosquito Control Association. 1995;11:260–268. [PubMed] [Google Scholar]
- Federici BA, Roberts DW. Experimental laboratory infection of mosquito larvae with fungi of the genus Coelomomyces. 1. Experiments with Coelomomyces psorophorae var. in Aedes taeniorhynchus and Coelomomyces psorophorae var. in Culiseta inornata. Journal of Invertebrate Pathology. 1975;26:21–27. doi: 10.1016/0022-2011(75)90164-0. [DOI] [PubMed] [Google Scholar]
- Ferron P. 1981 Pest control by the fungi Beauveria and Metarhizium. In: Burgess HD, editor. MicrobialControl of Pests and Plant Diseases 1970–1980. 24:465–482.London, Academic Press. [Google Scholar]
- Ferron P, Fargues J, and Riba G. 1991 Fungi as microbial insecticides against pests. In: Arora DK, Ajello L, Mukerji KG editors. Handbook of Applied Mycology. Volume 2: Humans, Animals, and Insects. 18:665–706.New York: Marcel Dekker, Inc. [Google Scholar]
- Fetter-Lasko JL, Washino RK. In situ studies on seasonality and recycling pattern in California of Coelomomyces giganteum Couch, an aquatic fungal pathogen of mosquitoes. Environmental Entomology. 1983;12:635–640. [Google Scholar]
- Frances SP. Record of the mosquito pathogenic fungus Culicinomyces clavisporus Couch, Romney and Rao infecting larvae of Culiseta inconspicua Lee (Diptera: Culicidae) in Victoria. Journal of the Australian Entomological Society. 1986;25:60. [Google Scholar]
- Frances SP. Pathogenicity, host range and temperature tolerance of Crypticola clavifera (Oomycetes: Lagenidiales) in the laboratory. Journal of the American Mosquito Control Association. 1991;7:504–506. [PubMed] [Google Scholar]
- Frances SP, Russell RC, Panter C. Persistence of the mosquito pathogenic fungus Culicinomyces in artificial aquatic environments. Mosquito News. 1984;44:321–324. [Google Scholar]
- Frances SP, Sweeney AW, Humber RA. Crypticola clavulifera gen. et sp. nov. and Coelomomyces giganteum: Oomycetes pathogenic for Dipterans infesting leaf axils in an Australian rain forest. Journal of Invertebrate Pathology. 1989;54:103–111. doi: 10.1016/0022-2011(89)90146-8. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Willis O, Barnard DR. Parasites of the asian tiger mosquito and other container-inhabiting mosquitoes (Diptera: Culicidae) in northcentral Florida. Journal of Medical Entomology. 1997;34:226–233. doi: 10.1093/jmedent/34.2.226. [DOI] [PubMed] [Google Scholar]
- Gad AM, Sadek S. Experimental infection of Anopheles pharoensis larvae with Coelomomyces indicus. Journal of the Egyptian Public Health Association. 1968;43:387–391. [Google Scholar]
- García JJ, Campos RE, Maciá A. Prospección de enemigos naturales de Culicidae (Diptera) de la selva marginal de Punta Lara (Prov. de Buenos Aires, República Argentina) Revista Academia Colombiana Ciencias Exactas Fisicas y Naturales. 1994;19:209–215. [Google Scholar]
- Gardner JM, Pillai JS. Tolypocladium cylindrosporum (Deuteromycotina: Moniliales) a fungal pathogen of the mosquito Aedes australis. III. Field trials against two mosquito species. Mycopathologia. 1987;97:83–88. doi: 10.1007/BF00436842. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Ram RC, Kumar S, Pillai JS. Field trials of Tolypocladium cylindrosporum against larvae of Aedes polynesiensis breeding in crab holes in Fiji. Journal of the Amercian Mosquito Control Association. 1986;2:292–295. [PubMed] [Google Scholar]
- Geetha I, Balaraman K. Effect of entomopathogenic fungus, Beauveria bassiana on larvae of three species of mosquitoes. Indian Journal of Experimental Biology. 1999;37:1148–1150. [PubMed] [Google Scholar]
- Genthner FJ, Foss SS, Fischer WS. Testing of the insect pest control fungus Beauveria bassiana in grass shrimp Palaemonetes pugio. Diseases of Aquatic Organisms. 1994;20:49–57. [Google Scholar]
- Genthner FJ, Chancy CA, Couch JA, Foss SS, Middaugh DP, George SE, Warren MA, Bantle JA. Toxicity and pathogenicity testing of the insect pest control fungus Metarhizium anisopliae. Archives of Environmental Contamination and Toxicology. 1998;35:317–324. doi: 10.1007/s002449900382. [DOI] [PubMed] [Google Scholar]
- Glare TR, O'Callaghan M. 2000 Bacillus thuringiensis: Biology, Ecology and Safety, 1st edition. John Wiley and Sons. [Google Scholar]
- Glenn FE Jr, Chapman HC. A natural epizootic of the aquatic fungus Coelomomyces giganteum in the mosquito Culex territans. Mosquito News. 1978;38:522–524. [Google Scholar]
- Goldberg AM. *Experimental infection with entomophthorosis of mosquitoes of the family Culicidae. Communication I. Species specificity of the fungus of Entomophthora sp. Meditsinskaya Paraziologiya i Parazitarnye Bolezni. 1970;39:472–478. [PubMed] [Google Scholar]
- Goettel MS. Field incidence of mosquito pathogens and parasites in central Alberta. Journal of the American Mosquito Control Association. 1987a;3:231–238. [PubMed] [Google Scholar]
- Goettel MS. Preliminary field trials with the entomopathogenic hypmycete Tolypocladium cylindrosporum in Central Alberta. Journal of the American Mosquito Control Association. 1987b;3:239–245. [PubMed] [Google Scholar]
- Goettel MS. Pathogenesis of the Hyphomycete Tolypocladium cylindrosporum in the mosquito Aedes aegypti. Journal of Invertebrate Pathology. 1988;51:259–274. doi: 10.1016/0022-2011(88)90033-x. [DOI] [PubMed] [Google Scholar]
- Goettel MS, Inglis GD. 1997 Fungi: Hyphomycetes. In: Lacey LA editor. Manual of Techniques in Insect Pathology. 5–3. 213–248.San Diego: Academic Press. [Google Scholar]
- Goettel MS, Sigler L, Carmichael JW. Studies on the mosquito pathogenic hyphomycete Culicinomyces clavisporus. Mycologia. 1984;76:614–625. [Google Scholar]
- Golkar L, LeBrun RA, Ohayon H, Gounon P, Papierok B, Brey PT. Variation of larval susceptibility to Coelomomyces giganteum in three mosquito species. Journal of Invertebrate Pathology. 1993;62:1–8. doi: 10.1006/jipa.1993.1066. [DOI] [PubMed] [Google Scholar]
- Grove JF, Pople M. The insecticidal activity of Beauvericin and the enniatin complex. Mycopathologia. 1980;70:103–105. [Google Scholar]
- Gupta S, Montllor C, Hwang Y-S. Isolation of novel Beauvericin analogues from the fungus Beauveria bassiana. Journal of Natural Products. 1995;58:733–738. [Google Scholar]
- Guzman DR, Axtell RC. Effect of nutrient concentration in culturing three isolates of the mosquito fungal pathogen, Coelomomyces giganteum (Oomycetes: Lagenidiales), on sunflower seed extract. Journal of the American Mosquito Control Association. 1986;2:196–200. [PubMed] [Google Scholar]
- Hajek AE, St. Leger RJ. Interactions between fungal pathogens and insect hosts. Annual Review of Entomology. 1994;39:293–322. [Google Scholar]
- Hallmon CF, Schreiber ET, Vo T, Bloomquist MA. Field trials of three concentrations of Laginex as biological larvicide compared to Vectobac-12AS as a biocontrol agent for Culex quinquefasciatus. Journal of the American Mosquito Control Association. 2000;16:5–8. [PubMed] [Google Scholar]
- Hart MP, MacLeod DM. *An apparatus for determining the effects of temperature and humidity on germination of fungus spores. Canadian Journal of Botany. 1955;33:289–292. [Google Scholar]
- Hasan S, Vago C. The pathogenicity of Fusarium oxysporum to mosquito larvae. Journal of Invertebrate Pathology. 1972;20:268–271. doi: 10.1016/0022-2011(72)90155-3. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Ranson H. Insecticide Resistance in Insect Vectors of Human Disease. Annual Review of Entomology. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- Horn BW, Lichtwardt RW. Studies on the nutritional relationship of larval Aedes aegypti (Diptera: Culicidae) with Smittium culicetae (Trichomycetes) Mycologia. 1981;73:724–740. [Google Scholar]
- Humber RA. 1997 Fungi: Identification. In: Lacey LA editor. Manual of Techniques in Insect Pathology. 5–1. 153–185.San Diego: Academic Press. [Google Scholar]
- Husan S, Vago C. The pathogenicity of Fusarium oxysporum to mosquito larvae. Journal of Invertebrate Pathology. 1973;19:268–271. doi: 10.1016/0022-2011(72)90155-3. [DOI] [PubMed] [Google Scholar]
- Ignoffo CM. 1967 Possibilities of mass-producing insect pathogens. In: Van der Laan PA, editor. Insect Pathology and Microbial ControlAmsterdam: 91–117.North-Holland Publishing Company. [Google Scholar]
- Jaronski S, Axtell RC. Persistence of the mosquito fungal pathogen Coelomomyces giganteum (Oomycetes; Lagenidiales) after introduction into natural habitats. Mosquito News. 1983;43:332–337. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kerwin JL, Petersen EE. 1997 Fungi: Oomycetes and Chytridiomycetes. In: Lacey LA, editor. Manual of Techniques in Insect Pathology. 5–4. 251–268.New York: Academic Press. [Google Scholar]
- Kerwin JL, Dritz DA, Washino RK. Pilot scale production and application in wildlife ponds of Coelomomyces giganteum (Oomycetes: Lagenidiales) Journal of the American Mosquito Control Association. 1994;10:451–455. [PubMed] [Google Scholar]
- Kerwin JL, Dritz DA, Washino RK. Confirmation of the safety of Coelomomyces giganteum (Oomycetes: Lagenidiales) to mammals. Journal of Economic Entomology. 1990;83:374–376. doi: 10.1093/jee/83.2.374. [DOI] [PubMed] [Google Scholar]
- Kerwin JL, Simmons CA, Washino RK. Oosporogenesis by Coelomomyces giganteum in liquid culture. Journal of Invertebrate Pathology. 1986;47:258–270. [Google Scholar]
- Kerwin JL, Washino RK. Ground and aerial application of the sexual andasexual stages of Coelomomyces giganteum (Oomycetes: Lagenidiales) for mosquito control. Journal of the American Mosquito Control Association. 1986;2:182–189. [PubMed] [Google Scholar]
- Kerwin JL, Washino RK. Ground and aerial application of the asexual stage of Coelomomyces giganteum for control of mosquitoes associated with rice culture in the central valley of California. Journal of the American Mosquito Control Association. 1987;3:59–64. [PubMed] [Google Scholar]
- Khachatourians GG. 1991 Physiology and genetics of entomopathogenic fungi. In: Arora DK, Ajello L, Mukerji KG, editors. Handbook of Applied Mycology, Vol.2. Humans, Animals, and Insects. 17:613–663.New York: Marcel Dekker, Inc. [Google Scholar]
- Khetan SK. 2001 Microbial Pest Control, 1st edition. Marcel Dekker. [Google Scholar]
- Kirk PM, Cannon PF, David JC, and Stalpers JA. 2001 Dictionary of the fungi, 9th edition. Cabi Publishing. [Google Scholar]
- Knight AL. Host range and temperature requirements of Culicinomyces clavosporus. Journal of Invertebrate Pathology. 1980;36:423–425. [Google Scholar]
- Kramer JP. Entomophthora culicis (Zygomycetes, Entomophthorales) as a pathogen of adult Aedes aegypti (Diptera, Culicidae) Aquatic Insects. 1982;4:73–79. [Google Scholar]
- Kramer JP. Pathogenicity of the fungus Entomophthora culicis for adult mosquitoes: Anopheles stephensi and Culex pipiens quinquefasciatus. Journal of the New York Entomological Society. 1983;91:177–182. [Google Scholar]
- Lacey CM, Lacey LA, Roberts DR. Route of invasion and histopathology of Metarhizium anisopliae in Culex quinquefasciatus. Journal of Invertebrate Pathology. 1988;52:108–118. doi: 10.1016/0022-2011(88)90109-7. [DOI] [PubMed] [Google Scholar]
- Lacey LA, Undeen AH. Microbial control of black flies and mosquitoes. Annual Review of Entomology. 1986;31:265–296. doi: 10.1146/annurev.en.31.010186.001405. [DOI] [PubMed] [Google Scholar]
- Laird M. A coral island experiment: a new approach to mosquito control. Chronicles of the World Health Organization. 1967;21:18–26. [PubMed] [Google Scholar]
- Laird M, Mogi M, Sota T. Nothernmost occurrences of the protistan pathogen, Coelomomyces stegomyiae var. stegomyiae. Journal of the American Mosquito Control Association. 1992;8:430–432. [PubMed] [Google Scholar]
- Legner EF. Biological control of Diptera of medical and veterinary importance. Journal of Vector Ecology. 1995;20:59–120. [Google Scholar]
- Lichtwardt RW. 1986 The Trichomycetes: fungal associates of arthropods, 1st edition. Springer Verlag. [Google Scholar]
- Lichtwardt RW, Arenas MJ. Trichomycetes in aquatic insects from southern Chile. Mycologia. 1996;88:844–857. [Google Scholar]
- Lichtwardt RW, Ferrington LC Jr, Lópes Lastra C. Trichomycetes in Argentinean aquatic insect larvae. Mycologia. 1999;91:1060–1082. [Google Scholar]
- Lord JC, Fukuda T. A Leptolegnia (Saprolegniales) pathogenic for mosquito larvae. Journal of Invertebrate Pathology. 1990;55:130–132. [Google Scholar]
- Lord JC, Fukuda T, Daniels E. Salinity tolerance of Leptolegnia chapmanii (Oomycetes: Saprolegniales), a fungal pathogen of mosquito larvae. Journal of the American Mosquito Control Association. 1988;4:370–371. [PubMed] [Google Scholar]
- Lowe RE, Kennel EW. Pathogenicity of the fungus Entomophthora coronata in Culex pipiens quinquefasciatus and Aedes taeniorhynchus. Mosquito News. 1972;32:614–620. [Google Scholar]
- Lowe RE, Rumbaugh RG, Patterson RS. Entomophthora coronata as a pathogen of mosquitoes. Journal of Invertebrate Pathology. 1968;11:506–507. doi: 10.1016/0022-2011(68)90201-2. [DOI] [PubMed] [Google Scholar]
- Lucarotti CJ. Coelomomyces stegomyiae infection in adult Aedes aegypti. Mycologia. 1987;79:362–369. doi: 10.1006/jipa.2000.4937. [DOI] [PubMed] [Google Scholar]
- Lucarotti CJ. Invasion of Aedes aegypti ovaries by Coelomomyces stegomyiae. Journal of Invertebrate Pathology. 1992;60:176–184. doi: 10.1006/jipa.2000.4937. [DOI] [PubMed] [Google Scholar]
- Lucarotti CJ, Andreadis TG. Reproductive strategies and adaptations for survival among obligatory microsporidian and fungal parasites of mosquitoes: a comparative analysis of Amblyospora and Coelomomyces. Journal of the American Mosquito Control Association. 1995;11:111–121. [PubMed] [Google Scholar]
- Lucarotti CJ, Shoulkamy MA. Coelomomyces stegomyiae infection in adult female Aedes aegypti following the first, second, and third host blood meals. Journal of Invertebrate Pathology. 2000;75:292–295. doi: 10.1006/jipa.2000.4937. [DOI] [PubMed] [Google Scholar]
- Lyimo EOK. 1993 The bionomics of the malaria mosquito Anopheles gambiae sensu lato in southeast Tanzania. PhD-thesis. Wageningen, the Netherlands: Wageningen University. 142. pp. [Google Scholar]
- Maffi M, Nolan RA. Coelomomyces lairdi, n. sp., a fungal parasite of larvae of the Anopheles (Cellia) punctulatus complex (Diptera: Culicidae) from the highlands of Irian Jaya (Indonesian New Guinea) Journal of Medical Entomology. 1977;14:29–32. doi: 10.1093/jmedent/14.1.29. [DOI] [PubMed] [Google Scholar]
- McCray EM Jr, Umphlett CJ, Fay RW. Laboratory studies on a new fungal pathogen of mosquitoes. Mosquito News. 1973;33:54–60. [Google Scholar]
- McInnis TM, Schimmel LE. Host range studies on a new fungus Leptolegnia, a parasite of mosquito larvae (Diptera: Culicidae) Journal of Medical Entomology. 1985;22:226–227. doi: 10.1093/jmedent/22.2.226. [DOI] [PubMed] [Google Scholar]
- McInnis T Jr, Zattau WC. Experimental infection of mosquito larvae by a species of the aquatic fungus Leptolegnia. Journal of Invertebrate Pathology. 1982;39:98–104. [Google Scholar]
- Merrriam TL, Axtell RC. Evaluation of the entomogenous fungi Culicinomyces clavosporus and Coelomomyces giganteum for control of the salt marsh mosquito, Aedes taeniorhynchus. Mosquito News. 1982;42:594–602. [Google Scholar]
- Mietkiewski R, Van der Geest LPS. Notes on entomophthoraceous fungi infecting insects in the Netherlands. Entomologische Berichten. 1985;45:190–192. [Google Scholar]
- Miranpuri GS, Khachatourians GG. Infection sites of the entomopathogenic fungus Beauveria bassiana in the larvae of the mosquito Aedes aegypti. Entomologia Experimentalis et Aplicata. 1991;59:19–27. [Google Scholar]
- Mitchell CJ. Coelomomyces psorophorae, an aquatic fungus parasitizing Aedes vexans mosquito larvae in Knox County, Nebraska. Mosquito News. 1976;36:501–505. [Google Scholar]
- Mohanty SS, Prakash S. Laboratory evaluation of Trichophyton ajelloi, a fungal pathogen of Anopheles stephensi and Culex quinquefasciatus. Journal of the American Mosquito Control Association. 2000;16:254–257. [PubMed] [Google Scholar]
- Molloy D, Wraight SP. Rediscovery of Erynia aquatica (Entomophthoraceae) in snowpool mosquitoes. Journal of Invertebrate Pathology. 1982;40:142–145. doi: 10.1016/0022-2011(82)90044-1. [DOI] [PubMed] [Google Scholar]
- Morley-Davies J, Moore D, Prior C. Screening of Metarhizium and Beauveria spp. conidia with exposure to simulated sunlight and a range of temperatures. Mycological Research. 1995;100:31–38. [Google Scholar]
- Muspratt J. On Coelomomyces fungi causing high mortality of Anopheles gambiae larvae in Rhodesia. Annals of Tropical Medicine and Parasitology. 1946;40:10–17. doi: 10.1080/00034983.1946.11685258. [DOI] [PubMed] [Google Scholar]
- Muspratt J. Destruction of the larvae of Anopheles gambiae Giles by a Coelomomyces fungus. Bulletin of the World Health Organization. 1963;29:81–86. [PMC free article] [PubMed] [Google Scholar]
- Nadeau MP, Boisvert JL. Larvicidal activity of the entomopathogenic fungus Tolypocladium cylindrosporum (Deuteromycotina: Hyphomycetes) on the mosquito Aedes triseriatus and the black fly Simulium vittatum (Diptera: Simuliidae) Journal of the American Mosquito Control Association. 1994;10:487–491. [PubMed] [Google Scholar]
- Nadeau MP, Dunphy GB, Boisvert JL. Development of Erynia conica (Zygomycetes: Entomophthorales) on the cuticle of the adult black flies Simulium rostratum and Simulium decorum (Diptera: Simuliidae) Journal of Invertebrate Pathology. 1996;68:50–58. doi: 10.1006/jipa.1996.0057. [DOI] [PubMed] [Google Scholar]
- Nestrud LB, Anderson RL. Aquatic safety of Coelomomyces giganteum: effects on freshwater fish and invertebrates. Journal of Invertebrate Pathology. 1994;64:228–233. doi: 10.1016/s0022-2011(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Nnakumusana ES. Laboratory infection of mosquito larvae by entomopathogenic fungi with particular reference to Aspergillus parasiticus and its effects on fecundity and longevity of mosquitoes exposed to sporal infections in larval stages. Current Science. 1985;54:1221–1228. [Google Scholar]
- Nnakumusana ES. Histopathological studies on the progress of infection of Leptolegnia sp (SC-1) in Anopheles gambiae larvae exposed to zoospores in the laboratory. Current Science. 1986;55:633–636. [Google Scholar]
- Orduz S, Axtell RC. Compatibility of Bacillus thuringiensis var. israelensis and Bacillus sphaericus with the fungal pathogen Coelomomyces giganteum (Oomycetes: Lagenidiales) Journal of the American Mosquito Control Association. 1991;7:188–193. [PubMed] [Google Scholar]
- Padua LE, Whisler HC, Gabriel BP, Zebold SL. In vivo culture and life cycle of Coelomomyces stegomyiae. Journal of Invertebrate Pathology. 1986;48:284–288. [Google Scholar]
- Papierok B, Hajek AE. 1997 Fungi: Entomophthorales. In: Lacey LA, editor. Manual of Techniques Series. 5–2. 187–212.San Diego: Academic Press. [Google Scholar]
- Patel KJ, Rueda LM, Axtell RC. Comparisons of different types and concentrations of alginates for encapsulation of Coelomomyces giganteum (Oomycetes: Lagenidiales), a fungal pathogen of mosquito larvae. Journal of the American Mosquito Association. 1990;6:101–104. [PubMed] [Google Scholar]
- Pillai JS, Rakai I. Coelomomyces macleayae Laird, a parasite of Aedes polynesiensis marks in Fiji. Journal of Medical Entomology. 1970;7:125–126. doi: 10.1093/jmedent/7.1.125. [DOI] [PubMed] [Google Scholar]
- Pillai JS, Smith JMB. Fungal pathogens of mosquitoes in New Zealand. I Coelomomyces opifexi sp. n., on the mosquito Opifex fuscus Hutton. Journal of Invertebrate Pathology. 1968;11:316–320. doi: 10.1016/0022-2011(68)90165-1. [DOI] [PubMed] [Google Scholar]
- Pinnock DE, Garcia R, Cubbin CM. Beauveria tenella as a control agent for mosquito larvae. Journal of Invertebrate Pathology. 1973;22:143–147. doi: 10.1016/0022-2011(73)90125-0. [DOI] [PubMed] [Google Scholar]
- Popelkova I. Coelomomyces from Aedes cinereus and a mosquito iridescent virus of Aedes cantans in Sweden. Journal of Invertebrate Pathology. 1982;40:148–149. doi: 10.1016/0022-2011(82)90046-5. [DOI] [PubMed] [Google Scholar]
- Ramos HC, Ribeiro H, Novo T, Bizarro J, Easton ER. First record of genus Coelomomyces in Macau (China): Coelomomyces stegomyiae var. stegomyiae parasitizing Aedes albopictus. Journal of the American Mosquito Control Association. 1996;12:507–509. [PubMed] [Google Scholar]
- Ramoska WA. An examination of the long-term epizootic potential of various artificially introduced mosquito larval pathogens. Mosquito News. 1982;42:603–607. [Google Scholar]
- Ramoska WA, Watts S, Watts HA. Effects of sand formulated Metarhizium anisopliae spores on larvae of three mosquito species. Mosquito News. 1981;41:725–728. [Google Scholar]
- Rath AC, Carr CJ, Graham BR. Characterization of Metarhizium anisopliae strains by carbohydrate utilization (AP150CH) Journal of Invertebrate Pathology. 1995;65:152–161. [Google Scholar]
- Ravallec M, Riba G, Vey A. Sensibilité d'Aedes albopictus (Dipera: Culicidae) à l'hyhomycète entomopathogène Metarhizium anisopliae. Entomophaga. 1989;34:209–217. [Google Scholar]
- Ravindranath G. Isolation and extraction of trichodermin from Fusarium pallidoroseum, a fungal pathogen of Anopheles stephensi. Indian Journal of Microbiology. 1991;31:267–269. [Google Scholar]
- Riba G, Bouvier-Fourcade I, Caudal A. Isozyme polymorphism in Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) entomogenous fungi. Mycopathologia. 1986;96:161–169. [Google Scholar]
- Ribeiro H. Coelomomyces angolensis, new species (Blastocladiales: Coelomomycetaceae): A fungal parasite of the mosquito Culex guiarti (Diptera: Culicidae) from Angola, Africa. Journal of Medical Entomology. 1992;29:30–32. doi: 10.1093/jmedent/29.1.30. [DOI] [PubMed] [Google Scholar]
- Ribeiro H, Da Cunha Ramos H. Coelomomyces numularius sp. nov. (Blastocladiales: Coelomomycetaceae), a new fungal parasite of Anopheles squamosus (Diptera: Culicidae) from Angola, Africa. Journal of Medical Entomology. 2000;37:962–964. doi: 10.1603/0022-2585-37.6.962. [DOI] [PubMed] [Google Scholar]
- Roberts DW. 1967 Some effects of Metarhizium anisopliae and its toxins on mosquito larvae. In: Van der Laan, editor. Insect Pathology and Microbial Control. Amsterdam:243–246. North-Holland Publishing Company. [Google Scholar]
- Roberts DW. Coelomomyces, Entomophtora, Beauveria, and Metarrhizium as parasites of mosquitoes. Miscellaneous Publications of the Entomological Society of America. 1970;7:140–155. [Google Scholar]
- Roberts DW. 1974 Fungal infections of mosquitoes. In: Aubin A, Belloncik S, Bourassa JP, LaCoursière E, Péllissier M, editors. Le contrôle des moustiques/Mosquito control: 143–193. Presses de l'Université du Québec. [Google Scholar]
- Roberts DW, Strand MA. 1977 Pathogens of medically important arthropods. Bulletin of the World Health Organization. 55Suppl.1. [PMC free article] [PubMed] [Google Scholar]
- Rueda LM, Patel KJ, Axtell RC. Efficacy of encapsuled Coelomomyces giganteum (Oomycetes: Lagenidiales) against Culex quinquefasciatus and Aedes aegypti larvae in artificial containers. Journal of the American Mosquito Control Association. 1990;6:694–699. [PubMed] [Google Scholar]
- Rueda LM, Patel KJ, Axtell RC. Comparison of floating and sinking encapsuled formulations of the fungus Coelomomyces giganteum (Oomycetes: Lagenidiales) for control of Anopheles larvae. Journal of the American Mosquito Control Association. 1991;7:250–254. [PubMed] [Google Scholar]
- Russell RC, Debenham MJ, Lee DJ. A natural habitat of the insect pathogenic fungus Culicinomyces in the Sydney area. Proceedings of the Linnaen Society of New South Wales. 1978;103:71–73. [Google Scholar]
- Sandhu SS, Rajak RC, Sharma M. Bioactivity of Beauveria bassiana and Metarhizium anisopliae as pathogens of Culex tritaeniorhynchus and Aedes aegypti: effect of instar, dosages and time. Indian Journal of Microbiology. 1993;33:191–194. [Google Scholar]
- Saunders GA, Washburn JO, Egerter DE, Anderson JR. Pathogenicity of fungi isolated from field-collected larvae of the western treehole mosquito, Aedes sierrensis (Diptera: Culicidae) Journal of Invertebrate Pathology. 1988;52:360–363. doi: 10.1016/0022-2011(88)90148-6. [DOI] [PubMed] [Google Scholar]
- Schaerffenberg B. Biological and environmental conditions for the development of mycoses caused by Beauveria and Metarrhizium. Journal of Insect Pathology. 1964;6:8–20. [Google Scholar]
- Scholte E-J, Takken W, Knols BGJ. Pathogenicity of five East African entomopathogenic fungi to adult Anopheles gambiae s.s. (Diptera: Culicidae) mosquitoes. Proceedings of the Section Experimental Applied Entomology of the Netherlands Entomological Society. 2003a;14:25–29. [Google Scholar]
- Scholte E-J, Njiru BN, Smallegang RC, Takken W, Knols BGJ. Infection of malaria (Anopheles gambiae s.s.) and filariasis (Culex quinquefasciatus) vectors with the entomopathogenic fungus Metarhizium anisopliae. Malaria Journal. 2003b;2:29. doi: 10.1186/1475-2875-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service MW. Mortalities of the immature stages of species B of the Anopheles gambiae complex in Kenya: comparison between rice fields and temporary pools, identification of predators, and effects of insecticidal spraying. Journal of Medical Entomology. 1977;13:535–545. doi: 10.1093/jmedent/13.4-5.535. [DOI] [PubMed] [Google Scholar]
- Service MW. Biological control of mosquitoes, has it a future? Mosquito News. 1983;43:113–120. [Google Scholar]
- Seymour RL. Leptolegnia chapmanii, an Oomycete pathogen of mosquito larvae. Mycologia. 1984;76:670–674. [Google Scholar]
- Seymour RL, Briggs JD. Occurrence and control of Aphanomyces (Saprolegniales: Fungi) infections in laboratory colonies of larval Anopheles. Journal of the American Mosquito Control Association. 1985;1:100–102. [PubMed] [Google Scholar]
- Shapiro M, Roberts DW. Growth of Coelomomyces mycelium in vitro. Journal of Invertebrate Pathology. 1976;27:399–402. doi: 10.1016/0022-2011(76)90106-3. [DOI] [PubMed] [Google Scholar]
- Shemanchuk JA. Note on Coelomomyces psorophorae Couch, a fungus parasitic on mosquito larvae. Canadian Entomologist. 1959;91:743–744. [Google Scholar]
- Shoulkamy MA, Lucarotti CJ. Pathology of Coelomomyces stegomyiae in larval Aedes aegypti. Mycologia. 1998;90:559–564. doi: 10.1006/jipa.2000.4937. [DOI] [PubMed] [Google Scholar]
- Shoulkamy MA, Lucarotti CJ, El-Ktatny MST, Hassan SKM. Factors affecting Coelomomyces stegomyiae infections in adult Aedes aegypti. Mycologia. 1997;89:830–836. [Google Scholar]
- Sigler L, Frances SP, Panter C. Culicinomyces bisporalis, a new entomopathogenic hyphomycete from larvae of the mosquito Aedes kochi. Mycologia. 1987;79:493–500. [Google Scholar]
- Sleigh MA. 1989 Protozoa and other protists. London, Edward Arnold. [Google Scholar]
- Smitley DR, Bauer LS, Hajek AE, Sapio FJ, Humber RA. Introduction and establishment of Entomophaga maimaiga, a fungal pathogen of gypsy moth (Lepidoptera: Lymantriidae) in Michigan. Environmental Entomology. 1995;24:1685–1695. [Google Scholar]
- Soarés GG Jr. Pathogenesis of infection by the hyphomycetous fungus Tolyplcladium cylindrosporum in Aedes sierrensis and Culex tarsalis (Diptera: Culicidae) Entomophaga. 1982;27:283–300. [Google Scholar]
- Soarés GG Jr, Pinnock DE, and Samson RA. 1979 Tolypocladium, a new fungal pathogen of mosquito larvae with promise for use in microbial control. Proceedings of the 47th Annual Conference of the Californian Mosquito Control Association: 51–54. [Google Scholar]
- Steinkraus DC, Kramer JP. Susceptibility of sixteen species of Diptera to the fungal pathogen Entomophthora muscae (Zygomycetes: Entomophthoraceae) Mycopathologia. 1987;100:55–63. [Google Scholar]
- Strasser H, Vey A, Butt TM. Are there any risks in using entomopathogenic fungi for pest control, with particular reference to the bioactive metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Science and Technology. 2000;10:717–735. [Google Scholar]
- Su X, Guzman DR, Axtell RC. Factores affecting storage of mycelial cultures of the mosquito fungal pathogen Coelomomyces giganteum (Oomycetes: Lagenidiales) Journal of the American Mosquito Control Association. 1986;2:350–354. [PubMed] [Google Scholar]
- Su X, Zou F, Guo Q, Huang J, Chen TX. A report on a mosquito-killing fungus, Pythium carolinianum. Fungal Diversity. 2001;7:129–133. [Google Scholar]
- Suh CP, Axtell RC. Coelomomyces giganteum zoospores: effects of concentration, movement, light, and temperature on infection of mosquito larvae. Biological Control. 1999;15:33–38. [Google Scholar]
- Sur B, Bihari V, Sharma A, Basu SK. Survey of termite-inhabited soil and mosquito breeding sites in Lucknow, India for potential mycopathogens of Anopheles stephensi. Mycopathologia. 1999;144:77–80. doi: 10.1023/a:1007072806204. [DOI] [PubMed] [Google Scholar]
- Sur B, Bihari V, Sharma A, Joshi AK. Studies on physiology, zoospore morphology and entomopathogenic potential of the aquatic oomycete: Coelomomyces giganteum. Mycopathologia. 2001;154:51–54. doi: 10.1023/a:1015273516881. [DOI] [PubMed] [Google Scholar]
- Sweeney AW. The effects of temperature on the mosquito pathogenic fungus Culicinomyces. Australian Journal of Zoology. 1978a;26:47–53. [Google Scholar]
- Sweeney AW. The effects of salinity on the mosquito pathogenic fungus Culicinomyces. Australian Journal of Zoology. 1978b;26:55–59. [Google Scholar]
- Sweeney AW. Further observations on the host range of the mosquito fungus Culicinomyces. Mosquito News. 1979;39:140–142. [Google Scholar]
- Sweeney AW. 1981a Fungal pathogens of mosquito larvae. In: Davidson EW, editor. Pathogenesis of Invertebrate Microbial Diseases New Jersey, 14. Allanheld, Osmun Publishers. [Google Scholar]
- Sweeney AW. Preliminary field tests of the fungus Culicinomyces against mosquito larvae in Australia. Mosquito News. 1981b;41:470–476. [Google Scholar]
- Sweeney AW. The effects of low temperature storage on the infectivity of Culicinomyces conidia for mosquito larvae. Journal of Invertebrate Pathology. 1981c;38:294–296. [Google Scholar]
- Sweeney AW. An undescribed species of Smittium (Trichomycetes) pathogenic to mosquito larvae in Australia. Transactions of the BritishMycological Society. 1981d;77:55–60. [Google Scholar]
- Sweeney AW, Couch JN, Panter C. The identity of an Australian isolate of Culicinomyces. Mycologia. 1982;74:162–165. [Google Scholar]
- Sweeney AW. The time-mortality response of mosquito larvae infected with the fungus Culicinomyces. Journal of Invertebrate Pathology. 1983;42:162–166. doi: 10.1016/0022-2011(83)90058-7. [DOI] [PubMed] [Google Scholar]
- Sweeney AW, Lee DJ, Panter C, Burgess LW. A fungal pathogen for mosquito larvae with potential as a microbial insecticide. Search. 1973;4:344–345. [Google Scholar]
- Sweeney AW, Panter C. The pathogenicity of the fungus Culicinomyces for mosquito larvae in a natural field habitat. Journal of Medical Entomology. 1977;4:495–496. doi: 10.1093/jmedent/14.4.495. [DOI] [PubMed] [Google Scholar]
- Tanada Y, Kaya HK. 1993 Insect Pathology. San Diego, Academic Press. [Google Scholar]
- Teernstra-Eeken MH, Engel A. Notes on entomophthorous fungi on Helomyzidae and Culicidae (Diptera) Journal of Invertebrate Pathology. 1967;9:431–432. [Google Scholar]
- Toscano N, Reeves EL. Effect of Apergillus flavus mycotoxin on Culex mosquito larvae. Journal of Invertebrate Pathology. 1973;22:55–59. doi: 10.1016/0022-2011(73)90010-4. [DOI] [PubMed] [Google Scholar]
- Umphlett CJ. Infection levels of Coelomomyces punctatus, an aquatic fungus parasite, in a natural population of the common malaria mosquito, Anopheles quadrimaculatus. Journal of Invertebrate Pathology. 1969;15:299–305. doi: 10.1016/0022-2011(70)90174-6. [DOI] [PubMed] [Google Scholar]
- Umphlett CJ. Infection levels of Coelomomyces punctatus, an aquatic fungus parasite, in a natural population of the common malaria mosquito, Anopheles quadrimaculatus. Journal of Invertebrate Pathology. 1970;15:299–305. doi: 10.1016/0022-2011(70)90174-6. [DOI] [PubMed] [Google Scholar]
- Umphlett CJ, Huang CS. Coelomomyces culicidum as an agent of biological control of mosquitoes. Bulletin Association of Southeastern Biologists. 1970;17:68. [Google Scholar]
- Van der Plaats-Niterink . Monograph of the genus Pythium. Studies in Mycology. 1981;21:1–240. [Google Scholar]
- Van Someren ECC, Teesdale C, Furlong M. The mosquitoes of the Kenya coast; records of occurrence, behaviour and habitat. Bulletin of Entomological Research. 1956;46:463–493. [Google Scholar]
- Veen KH. 1968 Recherches sur la maladie, due a Metharhizium anisopliae chez le criquet Pèlerin. PhD-thesis. Wageningen, the Netherlands: Landbouwhogeschool Wageningen. [Google Scholar]
- Washburn JO, Egerter DE, Anderson JR, Saunders GA. Density reduction in larval mosquito (Diptera: Culicidae) populations by interactions between a parasitic ciliate (Ciliophora: Tetrahymenidae) and an opportunistic fungal (Oomycetes: Pythiaceae) parasite. Journal of Medical Entomology. 1988;25:307–314. doi: 10.1093/jmedent/25.5.307. [DOI] [PubMed] [Google Scholar]
- Weiser J. 1988 Characterization of Main Groups of Pathogens. In: Biological control of vectors, 1st edition. John Wiley Publishers. [Google Scholar]
- Weiser J, Pillai JS. Tolypocladium cylindrosporum (Deuteromycetes: Moniliaceae) a new pathogen of mosquito larvae. Entomophaga. 1981;26:357–361. [Google Scholar]
- Weiser J, Matha V. Tolypin, a new insecticidal metabolite of fungi of the genus Tolypocladium. Journal of the Invertebrate Pathology. 1988;51:94–96. doi: 10.1016/0022-2011(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Weiser J, Zaim M, Saebi E. Coelomomyces irani sp. n. Infecting Anopheles maculipennis in Iran. Journal of Invertebrate Pathology. 1991a;57:290–291. doi: 10.1016/0022-2011(91)90130-i. [DOI] [PubMed] [Google Scholar]
- Weiser J, Matha V, Jegrov A. Tolypocladium terricola sp. n., a new mosquito-killing species of the genus Tolypocladium Gams (Hyphomycetes) Folia Parasitology. 1991b;38:363–369. [PubMed] [Google Scholar]
- Whisler HC, Gabriel PB, Chanpaisaeng J, Zebold SL, Padua LE. Observations on the life cycle of Coelomomyces indicus (Blastocladiales: Coelomomycetaceae) in Anopheline mosquitoes from the Philippines and Thailand. Journal of Medical Entomology. 1999;36:695–701. doi: 10.1093/jmedent/36.6.695. [DOI] [PubMed] [Google Scholar]
- Whisler HC, Zebold SL, Shemanchuk JA. Alternate host for mosquito parasite Coelomomyces. Nature. 1974;251:715–716. doi: 10.1038/251715a0. [DOI] [PubMed] [Google Scholar]
- Wilding N. The survival of Entomophthora spp. in mummified aphids at different temperatures and humidities. Journal of Invertebrate Pathology. 1973;21:309–311. [Google Scholar]
- Woodring J, Kaya HK, Kerwin JL. Coelomomyces giganteum in Culex tarsalis larvae: production of infective propagules. Journal of Invertebrate Pathology. 1995;66:25–32. doi: 10.1006/jipa.1995.1056. [DOI] [PubMed] [Google Scholar]
- Wraight SP, Jackson MA, and de Kock SL. 2001 Production, stabilization and formulation of biocontrol agents. In: Butt TM, Jackson C, Magan N, editors. Fungi as biocontrol agents. 10:253–288.CAB International. [Google Scholar]
- Yip H, Rath AC, Koen TB. Characterization of Metarhizium anisopliae isolates from Tasmanian pasture soils and their pathogenicity on the redheaded cockchafer (Coleoptera: Scarabaeidae: Adoryphorus couloni) Mycological Research. 1992;96:92–96. [Google Scholar]
- Zattau WC, McInnis T Jr. Life cycle and mode of infection of Leptolegnia chapmanii (Oomycetes) parasitizing Aedes aegypti. Journal of Invertebrate Pathology. 1987;50:134–145. doi: 10.1016/0022-2011(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann G. Effect of high temperatures and artificial sunlight on the viability of conidia of Metarhizium anisopliae. Journal of Invertebrate Pathology. 1982;40:36–40. [Google Scholar]
- Zimmermann G. The entomopathogenic fungus Metarhizium anisopliae and its potential as a biocontrol agent. Pesticide Science. 1993;37:375–379. [Google Scholar]


